Abstract
Studies of mice infected with Borrelia burgdorferi have indicated that the severity of arthritis is influenced by the genetic composition of the host: the C3H mouse develops severe arthritis while BALB/c and C57BL/6 mice develop mild arthritis. In this study, the effects of increasing infectious dose on the severity of arthritis were determined in these three mouse strains. C3H/He mice developed severe arthritis at all infectious doses, with 100% infection requiring 200 spirochetes. In BALB/cAnN mice, arthritis severity was dependent on infectious dose; symptoms were mild with infection by 200 B. burgdorferi and progressively more severe with increasing infectious dose. Infection of BALB/cAnN mice with 2 × 104 B. burgdorferi resulted in arthritis with severity identical to that in C3H/He mice. Spirochete levels in rear ankle joints of C3H/HeJ and C3H/HeN mice were relatively high, as detected by PCR, and did not increase with infectious dose. Spirochete levels in joints from BALB/cAnN mice increased with increasing infectious dose to levels found in severely arthritic C3H/He mice. Thus, resistance to severe arthritis in BALB/cAnN mice was conditional: it could be overcome by high infectious dose and the arthritis became severe when high levels of B. burgdorferi were present in joints. A unique response to increasing infectious dose was seen in C57BL/6N mice, which displayed mild to moderate arthritis at all doses of B. burgdorferi tested, up to 2 × 105. At all infectious doses, the levels of spirochetes in ankle joints of C57BL/6N mice were high, equivalent to those found in the severely arthritic C3H/He mice. The arthritis observed in infected (C57BL/6N × C3H/HeN)F1 mice was of severity intermediate between those of the two parental strains. The finding that resistance to severe arthritis in C57BL/6N mice could not be overcome by high infectious doses and was independent of spirochete levels in joints suggested that it was mediated by a distinct mechanism from that operating in BALB/cAnN mice.
Lyme disease is caused by infection with the tick-transmitted spirochete Borrelia burgdorferi and is characterized by multisystem involvement (14, 16, 24). Many tissues may display disease involvement, and there is variability in the degree to which patients are affected. This variability could be due to host, microbial, or environmental factors. In fact, infection in Europe by related members of the B. burgdorferi sensu lato group is more frequently associated with chronic skin abnormalities and central nervous system involvement, while infection by B. burgdorferi sensu stricto in the United States is more commonly associated with arthritis (4, 38). Studies using the murine model of Lyme disease, developed by Barthold and colleagues, indicate host factors also influence disease outcome. Arthritis seen in this model is representative of human disease and is characterized by tendonitis, synovial hyperproliferation, and infiltration of neutrophils and other leukocytes (7). Interestingly, a spectrum of arthritis severity has been observed among inbred strains of mice in response to infection by B. burgdorferi. Infected C3H mice develop severe arthritis, whereas infected BALB/c and C57BL/6 mice develop only mild to moderate arthritis (8). Thus, inbred strains of mice provide opportunities to study host influences on disease severity.
The results of several studies using the mouse model suggest the presence of inflammatory and/or anti-inflammatory cytokines can influence disease development and resolution. For example, manipulations of interleukin 12, interleukin 4, and gamma interferon levels by treating infected mice with neutralizing antibodies can influence disease severity and alter its resolution (2, 17, 21). The acquired defenses, particularly antibody production, are clearly involved in disease resolution (9, 30) but do not appear to be required for arthritis and carditis development. Not only does disease develop in scid mice, which lack mature T and B lymphocytes, but the relative differences in severity of arthritis in C3H/He and BALB/c mice is maintained in the presence of the scid mutation (12). Finally, studies with congenic mice expressing distinct major histocompatibility complex haplotypes on resistant or susceptible backgrounds suggest that the major histocompatibility complex itself had little influence on disease severity, but rather, that genes located at distinct chromosomal locations were important determinants of disease (41). These studies suggest that genes independent of acquired defenses play a large role in determining severity of disease in infected mice.
In order to identify host genes that influence disease severity, the phenotypes of severe and mild arthritis must be well characterized. We previously compared B. burgdorferi levels in many tissues of C3H/HeJ and BALB/cJ mice, at several times following infection (42). Quantitative PCR demonstrated that the highest levels of spirochetes were found in the hearts and ankle joints at most time points. C3H/HeJ mice harbored 5- to 10-fold more B. burgdorferi in ankles and hearts than did BALB/cJ mice. This suggested that the severity of arthritis in C3H/HeJ mice was directly related to the high levels of spirochetes in tissues and that the relative resistance in BALB/cJ mice was associated with more restricted growth of the spirochetes.
In this study we report that there are at least two different mechanisms for resistance to severe arthritis in mice. Resistance in BALB/cAnN mice could be overcome by increasing the infectious dose of B. burgdorferi and was associated with low levels of spirochetes in tissues. In contrast, resistance to severe arthritis in C57BL/6N mice was not overcome by increasing infectious dose and did not require the levels of spirochetes in joints to be low. F1 mice from BALB/cAnN × C3H/HeJN mating developed severe arthritis upon infection, suggesting that resistance in BALB/cAnN mice could be masked by alleles from C3H/HeN mice (42). In contrast, infection of F1 mice from a C57BL/6N × C3H/HeN cross resulted in arthritis of intermediate severity, suggesting more equal contribution by C57BL/6N and C3H/HeN genes.
MATERIALS AND METHODS
Bacteria.
The N40 isolate of B. burgdorferi was provided by Stephen Barthold (University of California at Davis) at passage 3 from an infected mouse (8). Passage 4 cultures were maintained as 0.5-ml frozen stocks at −70°C. HB-19 was provided by Alan Barbour (University of California at Irvine) from an infectious clone derived from the original human isolate (5). Fresh aliquots of frozen stocks were seeded in 15 ml of BSK-H medium containing 6% rabbit serum (Sigma, St. Louis, Mo.) and cultured at 32°C for 3 to 5 days prior to injection.
Mice.
Female C3H/HeJNCr, C3H/HeNCr, BALB/cAnNCr, C57BL/6NCr, and B6C3F1 (C57BL/6NCr × C3H/HeNCr) mice were obtained from the National Cancer Institute at 5 weeks of age. During the course of these studies we observed that substrain differences in C57BL/6 mice could influence the degree of arthritis severity. Mice were housed in the Animal Resource Center at the University of Utah Medical Center, according to guidelines of the National Institutes of Health for the care and use of laboratory animals.
Infection of mice with B. burgdorferi.
Mice 5 to 6 weeks of age were infected by intradermal injection with the indicated number of B. burgdorferi in the shaven back, a mode of infection reported to require the fewest spirochetes and to most closely mimic tick transmission (6, 25). Spirochete concentrations of 3- to 5-day cultures were determined by darkfield microscopy with a Petroff-Hauser chamber. Dilutions were made with sterile culture medium to allow injection of 20 μl per animal. Control mice were injected with an equal volume of sterile medium.
Measurement of the ankle joints.
Rear ankle joints of mice anesthetized with methoxyflurane (Pitman-Moore) were measured with a metric caliper (Dolla Eastern, Long Island City, N.Y.). Ankle joint measurements have been previously shown to correlate with the histological severity of arthritis and provide a method of monitoring arthritis development without sacrificing the animal (41). Measurements were taken in the anterior and/or posterior position with the ankle extended, which was through the thickest portion of the ankle. Normal ankle joints have a diameter of approximately 3 mm at this position.
Histology of the ankle joints.
The rear ankle joint displaying the greatest swelling at the 4-week sacrifice was taken from each mouse for histological analysis. Samples were fixed in 10% formalin, decalcified, and embedded in paraffin, and sections were stained with hematoxylin and eosin. Slides were viewed in a blinded fashion and given a score for severity. A score of 4+ indicated the greatest severity in tissues of the ankle and tibia and was characterized by a large region of edema with the presence of many neutrophils, thickening of the tendon sheath, and evidence of bone and cartilage abnormalities within the tendon sheath. A score of 1+ displayed slight thickening of the tendon sheath and little edema and neutrophil infiltration, whereas a score of 0 was given to samples indistinguishable from mock-infected controls. Scores of 2 and 3 were assigned to samples with intermediate pathologies.
Preparation of DNA from infected tissues.
Control and infected mice were sacrificed at 4 weeks following infection, and rear ankle joint tissues and hearts were prepared as previously described (32). Tissues were placed individually in 15-ml polypropylene tubes containing 2.5 ml of a 0.1% collagenase A (Boehringer Mannheim) solution in phosphate-buffered saline (pH 7.4). Samples were digested with collagenase for 4 h at 37°C and then mixed with an equal volume of 0.2-mg/ml proteinase K (Boehringer Mannheim) in 200 mM NaCl–20 mM Tris-HCl (pH 8.0)–50 mM EDTA–1% sodium dodecyl sulfate for 16 h at 55°C. DNA was recovered by extraction with an equal volume of phenol:chloroform and precipitation with ethanol. Following digestion with 1-mg/ml DNase-free RNase (Sigma), the DNA samples were subjected to a second extraction and precipitation and finally resuspended in 1.5 ml of distilled water. The average recovery was 200 to 400 μg of DNA from each heart and ankle, as determined by absorbance at 260 nm.
Detection of B. burgdorferi sequences by PCR.
PCR amplification was performed essentially as described previously by using the 1605 Air Thermocycler (Idaho Technologies) (42). Amplification was performed with 150 ng of DNA in a 10-μl final volume containing reaction buffer (50 mM Tris [pH 8.3], 3 mM MgCl2, 20 mM KCl, and 0.5 mg of bovine serum albumin per ml), 70 pmol of each oligonucleotide, 0.2 mM deoxynucleoside triphosphates (GIBCO BRL), 2.5 μCi of [32P]dCTP (New England Nuclear Research Product), and 0.75 U of Taq DNA polymerase (5,000 U/ml; BRL-GIBCO) (37). The oligonucleotide primers used to detect ospA were ospA 149 (5′-TTA TGA AAA AAT ATT TAT TGG GAA T-3′) and ospA 319 (5′-CTT TAA GCT CAA GCT TGT CTA CTG T-3′) (23). The primers used to detect ospB were ospB 219 (5′-CTC CGG CAA ATA TGA TTT AAG AGC A-3′) and ospB 1411 (5′-AGC TTT GAG AGT TTC CTC TGT TAT TGA-3′) (23). In some experiments ospB 146 (5′-TTC CTG CGG TGA CAG AAG AC-3′) was substituted for ospB 219, allowing amplification of a larger region of the ospB gene. The primers for nidogen were 5′-CCA GCC ACA GAA TAC CAT CC-3′ and 5′-GGA CAT ACT CTG CTG CCA TC-3′ (42). Reaction conditions for the 195-bp product from ospA were 35 cycles of denaturing at 94°C for 1 s, annealing at 55°C for 1 s, and elongation at 72°C for 12 s. Reaction conditions for the 238-bp ospB 219-ospB-1411 product were 30 to 31 cycles of denaturing at 94°C for 1 s, annealing at 65°C for 1 s, and elongation at 72°C for 8 s. Reaction conditions for the 311-bp ospB 146-ospB-1411 product were 33 cycles of denaturing at 94°C for 1 s, annealing at 68°C for 1 s, and elongation at 72°C for 8 s. Reaction conditions for the 150-bp nidogen product were 20 to 22 cycles of denaturing at 94°C for 1 s, annealing at 60°C for 1 s, and elongation at 72°C for 6 s. Cycle number variations for some products are due to slight differences in the ages of the radionucleotides.
Reaction products were separated on a 6% polyacrylamide sequencing gel. Products were identified by autoradiography and, in experiments shown in Fig. 3, were quantified by densitometric scanning with a Bio-Rad molecular imager. Once phosphor image analysis became readily available, gels were subjected to phosphor image analysis for quantitation of products by using a Bio-Rad molecular imager. DNA from each group of animals was amplified with nidogen primers and analyzed on a single gel. Samples were adjusted to fall within a twofold range for nidogen product, which was confirmed by analysis of a second set of reactions. This ensured approximately equal loading of tissue DNA for detection of B. burgdorferi product. Amplification of B. burgdorferi sequences was performed with ospA or ospB primers at cycle numbers within the linear range for product. As demonstrated previously, product yields were a direct reflection of the relative amounts of B. burgdorferi in tissues (42). DNA from mock-infected mice was included in each set of reactions to control for reagent and DNA purity. pBR332 DNA which had been digested with MspI and endlabeled with [32P]dCTP was used as a molecular weight ladder for the gels.
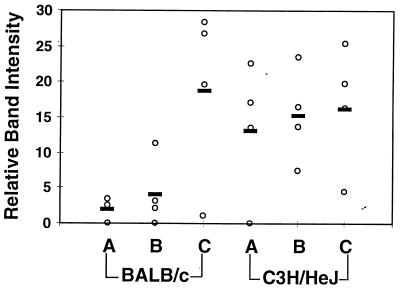
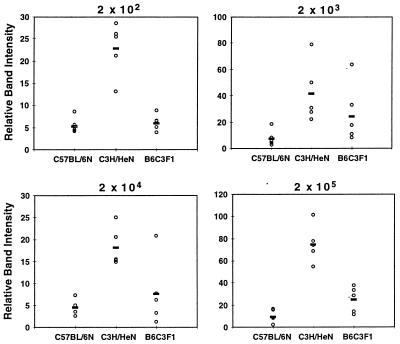
FIG. 3.
Detection of spirochete DNA in tissues of mice infected with increasing concentrations of B. burgdorferi. DNA from the mice from Fig. 1 was prepared at sacrifice, and semiquantitative PCR was used to assess the quantity of B. burgdorferi persisting in the rear ankle joint. Samples were amplified with primers for nidogen to ensure loading equivalence and with ospA primers to detect B. burgdorferi DNA, as described in Materials and Methods. The relative intensities of bands were determined by densitometric scanning. Relative densitometric units were determined for ospA products from each infected mouse (three to four mice per group) and are indicated by the open circles while the bold bars indicate the averages for each group. The means and standard deviations for the relative band intensities at the 2 × 102 infection dose were 2.0 ± 1.8 and 13.3 ± 9.6 for the BALB/c and C3H/HeJN mice, respectively. The corresponding results for the other doses were 4.1 ± 4.9 and 15.3 ± 6.6 (2 × 103) and 18.9 ± 12.5 and 16.48 ± 8.9 (2 × 104) for the BALB/c and C3H/HeJN mice, respectively.
Standard curve for B. burgdorferi DNA.
A standard curve for detection of B. burgdorferi DNA in mouse tissue was generated by spiking a constant number of freshly isolated mouse splenocytes with increasing numbers of B. burgdorferi. The ability to detect B. burgdorferi sequences in these spiked mouse tissues was determined by PCR, which indicated that this technique provides efficient recovery of B. burgdorferi DNA in the presence of mouse tissues. The nidogen contents of standard curve samples were equilibrated with those used for ankles and joints and then amplified with primers for ospB. The quantity of product was determined by phosphor image analysis and was found to be linear for standards in the range of 6 to 690 B. burgdorferi equivalents per amplification reaction. Comparison of product from tissue samples with the standard curve allowed estimation of the quantity of B. burgdorferi DNA sequences present in infected mouse tissues.
Quantification of B. burgdorferi-specific IgM and IgG.
Serum samples from infected and control mice were taken from each mouse at the 4-week sacrifice and analyzed for B. burgdorferi-specific antibody by a quantitative enzyme-linked immunosorbent assay described previously (32). Sera from uninfected mice had B. burgdorferi-specific immunoglobulin G (IgG) levels less than 100 ng/ml, whereas sera from infected mice had specific IgG levels greater than 20 μg/ml. By this criterion, all animals injected with greater than 200 B. burgdorferi were infected. Further, B. burgdorferi-specific PCR products were only detected in tissues from mice positive for B. burgdorferi-specific antibody.
Statistical analysis.
The degree of statistical significance of the quantitative differences between sample groups was determined by application of Student’s t test.
RESULTS
Effects of B. burgdorferi inoculum on arthritis development in BALB/cAnN and C3H/HeJN mice.
Our initial studies comparing mildly arthritic and severely arthritic mice infected with B. burgdorferi focused on two strains of inbred mice: C3H/He and BALB/c (32, 41, 42). C3H/He mice develop severe arthritis 3 to 4 weeks following intradermal injection of spirochetes, whereas BALB/c mice infected with the same isolate of B. burgdorferi develop mild arthritis. We observed a sudden increase in virulence of the N40 isolate of B. burgdorferi maintained in our laboratory that occurred following a change in growth medium to the commercially prepared BSK-H. This was characterized by a 50-fold decrease in the number of B. burgdorferi required for 50% infection (a reduction from 1,000 spirochetes to 20 spirochetes) and by an increase in severity of disease. This was most obvious in BALB/c mice, which developed severe arthritis when infected with 2 × 105 spirochetes, a dose which previously was consistently associated with mild arthritis (41, 42). For all three strains of inbred mice used in this study infection rates of 25 to 75% were obtained in groups inoculated with 20 spirochetes, while 100% infection was found in mice receiving 200 B. burgdorferi, similar to reports of others (6).
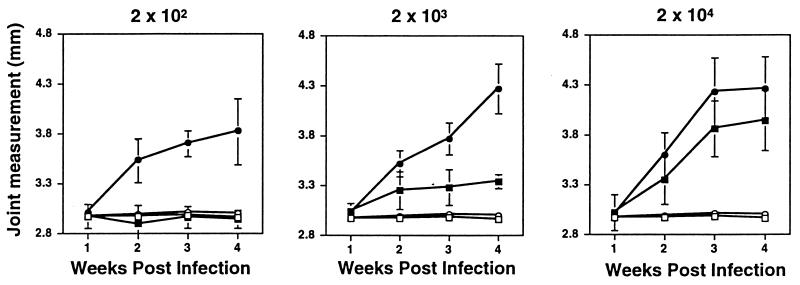
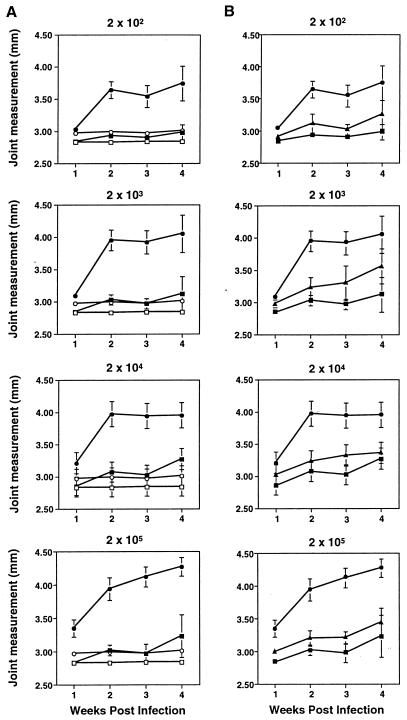
We investigated the effects of increasing the inoculum of B. burgdorferi from 20 to 2 × 104 on the development of arthritis in BALB/cAnN and C3H/HeJN mice. Groups of three to four mice were injected with each dose of B. burgdorferi, and rear ankle measurements were taken at weekly intervals from each mouse to monitor disease progression (Fig. 1). As not all mice injected with 20 spirochetes became infected, they were not included in further analysis. All C3H/HeJN mice injected with 200 spirochetes became infected, as determined by ankle swelling, PCR detection of B. burgdorferi DNA in tissues, and enzyme-linked immunosorbent assay detection of elevated anti-B. burgdorferi IgG in serum (described in Materials and Methods). BALB/cAnN mice injected with 200 B. burgdorferi did not display ankle measurements greater than those of mock-infected control mice but did have elevated IgG levels and detectable B. burgdorferi DNA, indicative of infection. BALB/cAnN mice infected with 2 × 103 or 2 × 104 B. burgdorferi developed progressively increasing ankle swelling. The mice which were used to obtain the results shown in Fig. 1 were sacrificed at 4 weeks following infection, and the most severely swollen rear ankle from each mouse was taken for histological analysis. The remaining rear ankle of each mouse was used for preparation of DNA.
FIG. 1.
Effects of increasing infectious dose on B. burgdorferi-induced ankle swelling in BALB/cAnN and C3H/HeJN mice. Groups of three to four C3H/HeJN (circles) or BALB/cAnN (squares) mice were infected by intradermal injection with the indicated numbers of the N40 strain of B. burgdorferi (filled symbols). Uninfected control mice were injected with sterile medium (open symbols). Measurements of the most severely swollen ankle were collected weekly for each animal, and the data shown are average values at each time point for each treatment group. Error bars indicate standard deviations within the groups. The same group of mock-infected mice is shown in each panel for comparison. Mice were sacrificed at 4 weeks postinfection and used for the analyses shown in Fig. 2 and 3.
The caliper measurements of ankle swelling are reflective of the amount of edema associated with the region directly proximal to the ankle and provide a gross measurement of the inflammatory response. A more complete determination of arthritis severity is revealed by histological analysis. Figure 2 shows hematoxylin-and-eosin-stained slides prepared from BALB/cAnN mice representative of the groups infected with 2 × 104 B. burgdorferi shown in Fig. 1. The histological abnormalities of joints from BALB/cAnN mice infected with 2 × 104 B. burgdorferi were severe and similar to those seen with C3H/He mice (7) (see also Fig. 5C). Severe arthritis was characterized by tendonitis and hyperproliferation of the tendon sheath (Fig. 2). Neutrophils were abundant in the inflammatory infiltrate, along with a prevalence of mononuclear cells, plasma cells, and mast cells. Figure 2B, from a BALB/cAnN mouse, shows another abnormal feature commonly seen in severe B. burgdorferi-induced arthritis in C3H mice: a conspicuous area of chondrocyte development, adjacent to a region of newly formed bone within the tendon sheath of the tibiotarsal region (42). Histological assessment of rear ankle joints from the BALB/cAnN mice revealed an increase in severity associated with increasing infectious dose, with mice inoculated with 200 B. burgdorferi receiving a histopathological score of 1 ± 0.5, those inoculated with 2 × 103 B. burgdorferi receiving a score of 1 ± 0.82, and those inoculated with 2 × 104 B. burgdorferi receiving a score of 3.75 ± 0.5. The average score for C3H/HeJ mice was 3.08 ± 0.79. The findings of a dose-dependent increase in arthritis severity in BALB/cAnN mice have been repeated in a second experiment with the N40 isolate and have also been found with a second B. burgdorferi isolate, HB19 (data not shown).
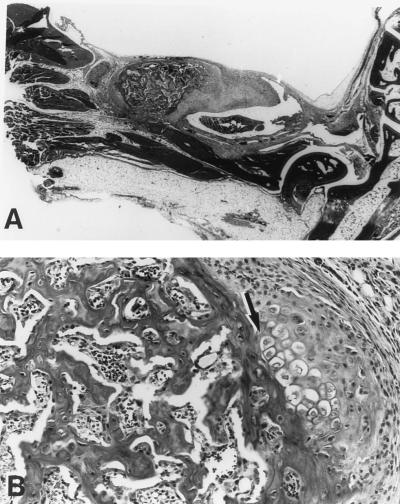
FIG. 2.
Histological analysis of severely arthritic BALB/cAnN mice. (A) Hematoxylin-and-eosin-stained slide from a representative BALB/cAnN mouse from Fig. 1 infected with 2 × 104 B. burgdorferi. Note the thickening of the tendon sheath in the region of the ankle joint. Magnification, ×5.75. (B) Area within the tendon sheath displaying abnormalities of chondrocyte and bone proliferation (arrow). Magnification, ×11.5. For comparison, severe arthritis in C3H/He mice can be seen in Fig. 5C.
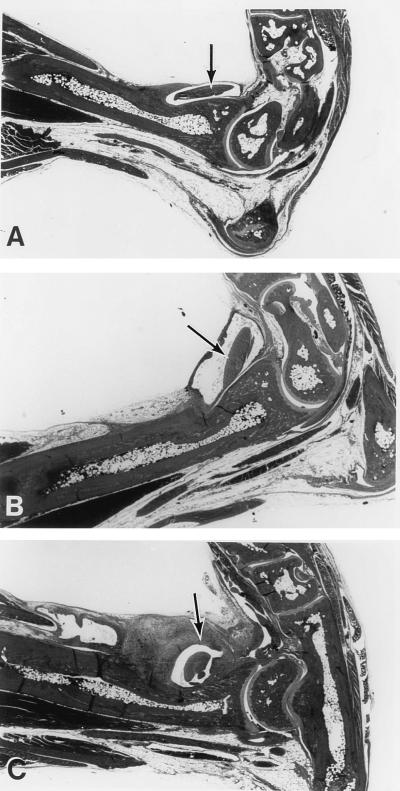
FIG. 5.
Histological analysis of tendonitis development in C57BL/6N, C3H/HeN, and B6C3F1 mice infected with 2 × 104 B. burgdorferi. (A) Representative ankle joint from a C57BL/6N mouse from the experiment shown in Fig. 4. There is very little edema or change in the tendon sheath evident in this section. (B) Ankle joint from a representative B6C3F1 mouse from Fig. 4 showing evidence of arthritis. Note the presence of edema surrounding the tendon crossing the ankle. (C) Ankle joint from a representative C3H/HeN mouse from Fig. 4 showing evidence of severe arthritis. Note the predominant thickening of the tendon sheath in the tibiotarsal region. Arrows identify prominant tendons. Magnification, ×5.75.
Effects of increasing inoculum on spirochete presence in BALB/cAnN and C3H/HeJN mice.
We previously reported that mildly arthritic BALB/cJ mice possessed 5- to 10-fold fewer spirochetes in joint tissues and hearts than did those tissues from severely arthritic C3H/HeJ mice (42). This was determined for a large number of samples by using the semi-quantitative PCR technique also used in this study and was confirmed for representative samples by a truly quantitative competitive target procedure. For the experiment whose results are shown in Fig. 1, we wished to determine whether the increase in severity of arthritis in BALB/cAnN mice receiving high doses of spirochetes was correlated with increased numbers of spirochetes in infected tissues. For each mouse included in this study, DNA was isolated from tissue taken from the rear ankle joint not collected for histology and subjected to semi-quantitative PCR for detection of B. burgdorferi sequences. Data in Fig. 3 indicate that increasing the infectious dose of B. burgdorferi given to C3H/HeJN mice had only a minor effect on the level of B. burgdorferi DNA in rear ankle joint tissue. In contrast, infected BALB/cAnN mice displayed a trend associating increasing dose with increasing amounts of B. burgdorferi DNA in joints. BALB/cAnN mice inoculated with 200 B. burgdorferi had significantly less B. burgdorferi DNA in ankle tissues than did C3H mice (P = 0.015). This difference was less significant in BALB/cAnN infected with 2,000 B. burgdorferi (P = 0.067). BALB/cAnN mice inoculated with the highest dose of B. burgdorferi (2 × 104) had levels of spirochetes in joints similar to those found in severely arthritic C3H/HeJN mice (P = 0.46). These results indicate that doses of B. burgdorferi that overcome resistance to arthritis in BALB/cAnN mice are correlated with the presence of high numbers of spirochetes in joint tissues.
Effects of B. burgdorferi dose on arthritis development in C57BL/6N and C3H/HeN mice.
The effect of increasing infectious dose was also tested in C57BL/6N mice, which have been reported to develop mild arthritis when infected with B. burgdorferi (8, 10). Since we wished to assess arthritis severity in F1 mice, C3H/HeN mice were used as positive controls for arthritis development rather than C3H/HeJN mice because the B6C3F1 mice (C3H/HeN × C57BL/6N) were available from the vendor only with the C3H/HeN parent. The endotoxin hyporesponsive mutation of the C3H/HeJ mouse has been shown to have no effect on any measurable parameter of B. burgdorferi-induced disease (11, 42). The degree of arthritis development in groups of four to five mice infected with doses of B. burgdorferi ranging from 200 to 2 × 105 was monitored by weekly ankle measurements (Fig. 4A). The degree of ankle swelling was high in C3H/HeN mice at all infectious doses. C57BL/6N mice were resistant to severe arthritis even at the highest infectious dose, 2 × 105. Grouped by the degree of rear ankle swelling, C3H/HeN and C57BL/6N mice formed two nonoverlapping groups at each time point and each infectious dose.
FIG. 4.
Effects of infectious dose on ankle swelling in C57BL/6N and C3H/HeN mice. (A) Groups of four to five C57BL/6N (squares) and C3H/HeN (circles) mice were infected by intradermal injection with the indicated numbers of the N40 strain of B. burgdorferi (filled symbols). Uninfected control mice were injected with sterile medium (open symbols). Weekly measurements of the most severely swollen ankle were made for each animal, and the values were used to determine the means and standard deviations (error bars) within each treatment group at each time point. The same mock-infected mice from each mouse strain are included in each panel for comparison. Mice were sacrificed at 4 weeks postinfection and were used for the analyses shown in Fig. 5 to 7. (B) B6C3F1 mice (triangles) were infected at the same time and with the same doses as the parental mice shown in panel A. Rear ankle measurements from infected B6C3F1 (triangles) are shown in comparison with infected parental strains (same symbols as for panel A). Measurements for uninfected B6C3F1 mice were similar to those for parental mice shown in panel A.
The rear ankle joint displaying the highest degree of swelling in each mouse was assessed for histopathology. There was much less inflammatory involvement in C57BL/6N than in C3H/HeN mice (Fig. 5). Most joints from C57BL/6N mice had little synovial hyperproliferation or tendonitis, even those from mice inoculated with 2 × 105 B. burgdorferi. A range of arthritis severity was observed in C57BL/6N at each inoculum dose, with one or two mice developing moderate synovial hyperproliferation (1+ to 2+) and the remaining three or four displaying very limited abnormality (0 to 1+). The average histopathological score for all C57BL/6N mice shown in Fig. 4 was 0.78 ± 0.79, for C3H/HeN mice it was 3.58 ± 0.49, and for the B6C3F1 mice it was 1.53 ± 0.75. In a second experiment, in which arthritis severity in C57BL/6N mice was compared with that in C3H/HeJN mice, similar results to those shown in Fig. 4 and 5 were obtained (data not shown).
Effects of inoculum size on B. burgdorferi persistence in tissues of C57BL/6N and C3H/HeN mice.
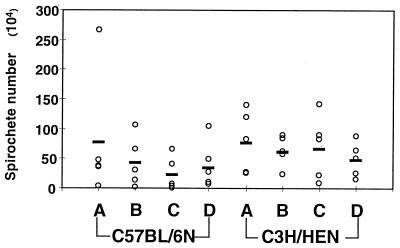
Our findings with BALB/c mice (42) (Fig. 3) suggested that the mild pathology seen in C57BL/6N mice would be associated with lower levels of spirochetes in the joint tissues than those in the severely arthritic C3H/HeN mice. Semiquantitative PCR revealed that spirochete levels did not increase with increasing infectious dose in either C3H/HeN or C57BL/6N mice and that C57BL/6N mice harbored levels as high as those found in C3H/HeN mice (Fig. 6). To demonstrate that relative differences would have been detected by this protocol, a standard curve spanning the range of B. burgdorferi DNA detected in tissue samples was included with each set of reaction. This allowed normalization of samples run on separate gels and allowed estimation of actual B. burgdorferi numbers in infected ankle joints. Because results from several individual gels were pooled, the data presented in Fig. 6 are expressed as spirochete numbers rather than as relative band intensities. At all B. burgdorferi doses, rear ankle joint tissue from C3H/HeN and C57BL/6N mice possessed approximately equal amounts of spirochete DNA. (The P values for the comparisons of the various doses were as follows: infectious dose at 200 B. burgdorferi, P = 0.98; dose of 2 × 103, P = 0.39; dose of 2 × 104, P = 0.13; dose of 2 × 105, P = 0.70; pooling of data from all C57BL/6N and C3H/HeN mice, P = 0.25.) The standard curve was linear for samples containing 6 to 690 B. burgdorferi in the preamplified reaction mixture and spanned the range found in samples from infected tissues. This demonstrates that the technique would have allowed the distinction of 5- to 10-fold differences in spirochete numbers in the C57BL/6N and C3H/HeN samples, as were previously observed with BALB/cJ mice and C3H/HeJ mice. The estimated average number of spirochetes in C3H/HeN ankle joints analyzed in Fig. 6 was 6.4 × 105, similar to our previous estimate of 4.3 × 105 for C3H/HeJ mice, which was also obtained by using this technique (42). PCR analysis of tissues collected in the second dose-response experiment, in which C57BL/6N mice were compared with C3H/HeJN mice, gave similar results for spirochete levels in ankles (data not shown). These results indicate that spirochete numbers in mildly arthritic joints of C57BL/6N mice were as high as in joints from severely arthritic C3H/HeN mice.
FIG. 6.
Effects of B. burgdorferi infectious dose on DNA levels in ankle joints from C57BL/6N and C3H/HeN mice. The concentrations of DNA in the samples were adjusted based on products generated with nidogen primers, as described in Materials and Methods. Samples were amplified with primers for ospB, and products were quantified by phosphor image analysis. The estimation of total B. burgdorferi numbers in each sample of joint DNA was made by comparison with a standard curve, generated as described in Materials and Methods, and calculated from the fraction of total DNA amplified in the PCR. Open circles indicate spirochete numbers from each mouse, and bold bars indicate average numbers for the groups of 4 to 5 mice. Spirochete numbers (means ± standard deviations) per tissue are given for each infectious dose with group A receiving 2 × 102 (C57BL/6, [7.7 ± 10.6] × 105; C3H/HeN, [7.9 ± 5.2] × 105), group B receiving 2 × 103 (C57BL/6, [4.3 ± 4.2] × 105; C3H/HeN, [6.3 ± 2.6] × 105), group C receiving 2 × 104 (C57BL/6, [2.3 ± 2.9] × 105; C3H/HeN, [6.9 ± 5.4] × 105), and group D receiving 2 × 105 B. burgdorferi (C57BL/6, [3.9 ± 4.0] × 105; C3H/HeN, [4.8 ± 2.9] × 105).
The heart is a second tissue which is heavily infected with B. burgdorferi in the mouse, and cardiac involvement has been demonstrated in C3H/He, BALB/c, and C57BL/6 mice (8, 12, 29). We previously reported that infected C3H/HeJ mice possessed higher levels of B. burgdorferi DNA in hearts than did BALB/cJ mice, at several time points following infection (42). Levels of spirochete-specific DNA in hearts of the BALB/cAnN mice shown in Fig. 1 did increase with increasing infectious dose of B. burgdorferi, with hearts from BALB/cAnN mice infected with 2 × 104 B. burgdorferi having levels equivalent to those in C3H/HeJN mice (data not shown but the trends were very similar to those shown for ankles in Fig. 3). PCR amplification was also performed on DNA prepared from the hearts of the C3H/HeN and C57BL/6N mice shown in Fig. 4. Increased inoculating dose did not cause an increase in the numbers of spirochetes in hearts from either C3H/HeN or C57BL/6N mice (data not shown), consistent with the findings with the ankle joints from these mice, shown in Fig. 6. However, direct comparison of the PCR products generated from heart samples of C57BL/6N and C3H/HeN mice at each infectious dose of B. burgdorferi revealed significantly higher spirochete levels in C3H/HeN mice than in C57BL/6N mice (P < 0.05) (Fig. 7).
FIG. 7.
Detection of B. burgdorferi DNA in hearts collected from infected C3H/HeN, C57BL/6N, and B6C3F1 mice. Hearts were collected from the mice in Fig. 4, and DNA was prepared for PCR analysis. Samples were determined to contain equivalent amounts of DNA by amplification with nidogen primers, as described in Materials and Methods. Samples for mice infected with each indicated dose of B. burgdorferi were amplified with ospB primers. Phosphor image determination of relative quantities of product were made from samples run on a single gel. Open circles indicate relative band intensities from individual mice and bold bars reflect averages for the groups of 4 to 5 mice. Although means and standard deviations cannot be compared between samples run on different gels, comparisons between C3H/HeN and C57BL/6N mice at each dose of B. burgdorferi were made and indicated significant differences as follows: dose of 200, P = 0.007; dose of 2 × 103, P = 0.032; dose of 2 × 104, P = 0.001; dose of 2 × 105, P = 0.002.
Assessment of arthritis severity and spirochete persistence in B6C3F1 mice.
We previously reported that the (C3H/HeJ × BALB/cJ)F1 mouse developed severe arthritis following infection with the N40 strain of B. burgdorferi and harbored high levels of spirochetes in tissues (42). This result has been confirmed in (C3H/HeJN × BALB/cAnN)F1 mice, with an inoculating dose of 200 spirochetes of the highly virulent N40 cultures used in the present study (data not shown). The severity of arthritis and the prevalence of spirochetes in tissues was assessed in the F1 generation of a cross between C3H/HeN and C57BL/6N mice (B6C3F1). As shown in Fig. 4B, B6C3F1 mice develop rear ankle swelling intermediate to that observed in the parental strains at all infectious doses. Histological analysis also reflected pathology that was intermediate to that observed with C3H/HeN and C57BL/6N mice (Fig. 5). Spirochete levels in rear ankle joint tissues taken at 4 weeks postinfection were similar to those found in the two parental strains (data not shown). Interestingly, spirochete levels in hearts were intermediate between those of the two parental strains (Fig. 7). Unfortunately, the relevance to carditis development could not be assessed in this experiment, as all hearts were used for DNA preparation.
DISCUSSION
This study of arthritis development in mice infected with increasing doses of B. burgdorferi suggests that disease outcomes may be influenced at several stages. Resistance to severe arthritis in BALB/cAnN mice is dependent on a low initial infectious dose and appears to depend on the mouse’s ability to control the expansion of spirochetes within tissues. Infectious doses of 2 × 104 and higher result in severe ankle swelling and rear ankle arthritis in BALB/cAnN mice. Severe arthritis in the BALB/cAnN mouse is associated with higher levels of spirochetes in ankle joints. In contrast, the C57BL/6N mouse is resistant to severe arthritis development even at very high infectious doses of B. burgdorferi. Of particular interest is that the mildly arthritic C57BL/6N mice harbor high levels of B. burgdorferi in tissues of the rear ankle joint. These findings indicate that two distinct mechanisms can be responsible for arthritis resistance. One, as seen in BALB/cAnN mice, is dependent on controlling spirochete levels in tissues and can be overcome by high infectious dose. The second, exemplified by the C57BL/6N mouse, is independent of infectious dose and is not strictly tied to minimizing the presence of spirochetes in tissues.
These studies imply that many aspects of host-pathogen interactions contribute to the development of arthritis in B. burgdorferi-infected mice. Clearly, the bacterium is highly invasive, and it has been shown by culture, silver staining, immunochemistry, in situ hybridization, and PCR to be present in many tissues including the joints (3, 11, 42). B. burgdorferi can transcytose endothelial monolayers in vitro, suggesting direct mechanisms for tissue invasion (15, 36). The spirochete expresses products of different lipoprotein genes at different stages of infection of ticks and mammals (1, 18, 28, 31, 35, 39). The lipid-modified amino-terminal regions of these lipoproteins are potent activators of mouse and human cells and are capable of stimulating cytokine and nitric oxide production by macrophages (19, 20, 27), adhesion molecule and chemokine expression by endothelial cells (13, 33, 34, 40), and adhesion molecule expression and release of granule contents by neutrophils (22). Thus, the presence of lipoproteins in infected tissues could participate in the inflammatory processes. The comparison between BALB/cAnN and C57BL/6N mice is interesting in that it suggests that BALB/cAnN mice are just as susceptible to severe arthritis as are C3H/HeJN mice but that they are better at controlling B. burgdorferi dissemination or growth. In contrast, the rear ankle joint tissues of C57BL/6N mice harbor as many spirochetes as do C3H/HeN mice, yet arthritis severity is much less. This suggests that the C57BL/6N mice are truly resistant to the development of severe arthritis, even in the presence of the high numbers of spirochetes and their inflammatory lipoproteins that are associated with severe arthritis in C3H/HeN mice. Whether this reflects greater regulation of inflammatory responses by infected C57BL/6N mice or alteration in inflammatory potential by B. burgdorferi infecting this host is not known.
The finding that C3H/HeN mice harbor higher levels of spirochetes in hearts than do C57BL/6N mice suggests that there is variability in the level of B. burgdorferi present in various tissues of C57BL/6N mice. Thus, studies designed to test the influence of manipulations of immune defenses on control of spirochetes may need to sample more than one type of tissue. C3H mice are reported to have more severe carditis than C57BL/6 mice, suggesting that pathology in hearts may be related to spirochete levels in this tissue (3, 8, 12). Demonstration that B. burgdorferi DNA levels in the hearts of B6C3F1 mice are intermediate between those of the two parental strains indicates that this trait is genetically regulated and maintained over a wide range of infectious doses. Further experiments will be required to assess the effects of infectious dose on carditis in C3H/HeN, C57BL/6N, and B6C3F1 mice. Others have reported a correlation between spirochete numbers and joint and neurological pathology in scid mice infected with the relapsing fever agent Borrelia turicatae, consistent with our results with BALB/c and C3H mice (26).
Identification of a mouse strain which is highly resistant to B. burgdorferi-induced severe arthritis will permit elucidation of the genetic contribution to this process of infection. The intermediate arthritis phenotype identified in the B6C3F1 mice implies that homozygosity at a particular C3H genetic allele(s) is required for development of the most severe arthritis. This is in contrast to infection of the (BALB/c × C3H)F1 mice, which develop severe arthritis, and suggests that the resistance of BALB/c mice can be more easily overcome by the presence of a single C3H allele for a crucial gene(s) than can the resistance of C57BL/6 mice. Important genes could include regulatory molecules for inflammatory events, inflammatory genes themselves, and genes that influence the emigration of leukocytes into tissues. Studies of mice which express a granulocyte deficiency (beige) and of mice treated with cytokine-neutralizing antibodies support the suggestion that allelic differences in inflammatory pathways could alter disease outcome (10, 17, 21). The finding that the relative degree of severity of arthritis in BALB/c and C3H/HeN mice is maintained in animals with the scid mutation (12) further suggests that genes involved in innate host defenses may make important contributions to severity phenotypes.
In summary, this study provides evidence that distinct mechanisms can limit arthritis severity in mice. In a population of patients with Lyme disease, there is observed a spectrum of disease severity and differences in the organs that become involved (24). Some of this variety is likely to reflect different abilities of patients to effectively respond to B. burgdorferi infection. Additionally, the characteristics of the infecting pathogen and the number of infecting bacteria may also influence pathological outcome. Understanding host factors that influence resistance to arthritis in mice infected with B. burgdorferi may have important implications for humans.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AR43521 (to J.J.W. and C.T.) and AI24158 (to J.H.W.) from the National Institutes of Health and grant 5P30-CA-42014 to the University of Utah. The project described was also supported in part by an award from the American Lung Association (J.H.W.).
We acknowledge the help of Nancy Chandler in the University of Utah Health Sciences Research Microscopy Facility and the helpful suggestions of Stephen Barthold, University of California at Davis.
REFERENCES
- 1.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 2.Anguita J, Persing D H, Rincon M, Barthold S W, Fikrig E. Effect of anti-interleukin 12 treatment on murine lyme borreliosis. J Clin Invest. 1996;97:1028–1034. doi: 10.1172/JCI118494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 4.Balmelli T, Piffaretti J C. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol. 1995;146:329–340. doi: 10.1016/0923-2508(96)81056-4. [DOI] [PubMed] [Google Scholar]
- 5.Barbour A G, Tessier S L, Todd W J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983;41:795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W. Infectivity of Borrelia burgdorferi relative to route of inoculation and genotype in laboratory mice. J Infect Dis. 1991;163:419–420. doi: 10.1093/infdis/163.2.419. [DOI] [PubMed] [Google Scholar]
- 7.Barthold S W. Lyme borreliosis in the laboratory mouse. J Spirochetal Tick-borne Dis. 1996;3:22–44. [Google Scholar]
- 8.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 9.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barthold S W, de Souza M. Exacerbation of Lyme arthritis in beige mice. J Infect Dis. 1995;172:778–784. doi: 10.1093/infdis/172.3.778. [DOI] [PubMed] [Google Scholar]
- 11.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 12.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 13.Boggemeyer E, Stehle T, Schaible U E, Hahne M, Vestweber D, Simon M M. Borrelia burgdorferi upregulates the adhesion molecules E-selectin, P-selectin, ICAM-1 and VCAM-1 on mouse endothelioma cells in vitro. Cell Adhesion Commun. 1994;2:145–157. doi: 10.3109/15419069409004433. [DOI] [PubMed] [Google Scholar]
- 14.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease: a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 15.Comstock L E, Thomas D D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989;57:1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson R C, Schmid G P, Hyde F W, Steigwalt A G, Brenner D J. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int J Syst Bacteriol. 1984;34:496–497. [Google Scholar]
- 17.Keane-Myers A, Nickell S P. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–2028. [PubMed] [Google Scholar]
- 18.Lam T T, Nguyen T P, Fikrig E, Flavell R A. A chromosomal Borrelia burgdorferi gene encodes a 22-kilodalton lipoprotein, P22, that is serologically recognized in Lyme disease. J Clin Microbiol. 1994;32:876–883. doi: 10.1128/jcm.32.4.876-883.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Seiler K P, Tai K F, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison T B, Weis J H, Weis J J. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J Immunol. 1997;158:4838–4845. [PubMed] [Google Scholar]
- 23.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 24.Nocton J J, Steere A C. Lyme disease. Adv Intern. 1995;40:69–117. [PubMed] [Google Scholar]
- 25.Pachner A R, Delaney E, Ricalton N S. Murine Lyme borreliosis: route of inoculation determines immune response and infectivity. Reg Immunol. 1992;4:345–351. [PubMed] [Google Scholar]
- 26.Pennington P M, Allred C D, West C S, Alvarez R, Barbour A G. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radolf J D, Norgard M V, Brandt M E, Isaacs R D, Thompson P A, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis: analysis using a CAT reporter construct. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 28.Ramamoorthy R, Povinelli L, Philipp M T. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of Borrelia burgdorferi. Infect Immun. 1996;64:1259–1264. doi: 10.1128/iai.64.4.1259-1264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruderman E M, Kerr J S, Telford III S R, Spielman A, Glimcher L H, Gravallese E M. Early murine Lyme carditis has a macrophage predominance and is independent of major histocompatibility complex class II-CD4+ T cell interactions. J Infect Dis. 1995;171:362–370. doi: 10.1093/infdis/171.2.362. [DOI] [PubMed] [Google Scholar]
- 30.Schaible U E, Wallich R, Kramer M D, Nerz G, Stehle T, Museteanu C, Simon M M. Protection against Borrelia burgdorferi infection in SCID mice is conferred by presensitized spleen cells and partially by B but not T cells alone. Int Immunol. 1994;6:671–681. doi: 10.1093/intimm/6.5.671. [DOI] [PubMed] [Google Scholar]
- 31.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiler K P, Vavrin Z, Eichwald E, Hibbs J, Jr, Weis J J. Nitric oxide production during murine Lyme disease: lack of involvement in host resistance or pathology. Infect Immun. 1995;63:3886–3895. doi: 10.1128/iai.63.10.3886-3895.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sellati T J, Abrescia L D, Radolf J D, Furie M B. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect Immun. 1996;64:3180–3187. doi: 10.1128/iai.64.8.3180-3187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sellati T J, Burns M J, Ficazzola M A, Furie M B. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect Immun. 1995;63:4439–4447. doi: 10.1128/iai.63.11.4439-4447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szczepanski A, Furie M B, Benach J L, Lane B P, Fleit H B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990;85:1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan S S, Weis J H. Development of a sensitive reverse transcriptase PCR assay, RT-RPCR, utilizing rapid cycle times. PCR Appl Methods. 1992;2:137–143. doi: 10.1101/gr.2.2.137. [DOI] [PubMed] [Google Scholar]
- 38.van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 39.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wooten R M, Modur V R, McIntyre T M, Weis J J. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-κB and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 41.Yang L, Ma Y, Schoenfeld R, Griffiths M, Eichwald E, Araneo B, Weis J J. Evidence for B-lymphocyte mitogen activity in Borrelia burgdorferi-infected mice. Infect Immun. 1992;60:3033–3041. doi: 10.1128/iai.60.8.3033-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]