Highlights
-
•
Threshold-tracking transcranial magnetic stimulation (TMS) was performed before and after at least 24 h sleep deprivation.
-
•
Short- and long interval intracortical inhibition (SICI, LICI), facilitation (SICF, ICF), and short latency afferent inhibition (SAI) were done.
-
•
No effect of sleep deprivation was shown on SICI, LICI, SICF, and ICF while there was a decrease in SAI at 28 ms and 30 ms.
Keywords: Sleep deprivation, Threshold-tracking transcranial magnetic stimulation, Short-interval intracortical inhibition, Intracortical facilitation, Short-interval intracortical facilitation, Long-interval intracortical inhibition, Short-latency afferent inhibition
Abstract
Objective
Insufficient sleep is linked to several health problems. Previous studies on the effects of sleep deprivation on cortical excitability using conventional transcranial magnetic stimulation (TMS) included a limited number of modalities, and few inter-stimulus intervals (ISIs) and showed conflicting results. This study aimed to investigate the effects of sleep deprivation on cortical excitability through threshold-tracking TMS, using a wide range of protocols at multiple ISIs.
Methods
Fifteen healthy subjects (mean age ± SD: 36 ± 3.34 years) were included. The following tests were performed before and after 24 h of sleep deprivation using semi-automated threshold-tacking TMS protocols: short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) at 11 ISIs between 1 and 30 ms, short interval intracortical facilitation (SICF) at 14 ISIs between 1 and 4.9 ms, long interval intracortical inhibition (LICI) at 6 ISIs between 50 and 300 ms, and short-latency afferent inhibition (SAI) at 12 ISIs between 16 and 30 ms.
Results
No significant differences were observed between pre- and post-sleep deprivation measurements for SICI, ICF, SICF, or LICI at any ISIs (p < 0.05). As for SAI, we found a difference at 28 ms (p = 0.007) and 30 ms (p = 0.04) but not at other ISIs.
Conclusions
Sleep deprivation does not affect cortical excitability except for SAI.
Significance
This study confirms some of the previous studies while contradicting others.
1. Introduction
Sleep deprivation (SD) affects the brain in multiple ways, not only on the emotional and cognitive level but it also triggers changes in brain activity and connectivity. Both acute and chronic SD may impact cognitive functioning, learning capacity, and the ability to form new memories, especially the hippocampus-dependent ones (Killgore, 2010). It has been proved that some milder changes can be caused also just by acute SD and that they are of a compensatory nature (Killgore, 2010).
Transcranial magnetic stimulation (TMS) is a non-invasive technique that is based on generating localized magnetic fields that create depolarizing electrical currents (Groppa et al., 2012). Different TMS protocols provide indirect information on various neurotransmitter activities (Ziemann et al., 1996). Short-interval intracortical inhibition (SICI) is a paired-pulse TMS paradigm in which a sub-threshold conditioning stimulus is used to suppress the response evoked by a higher-intensity stimulus, called a test stimulus (Chen et al., 2008). On the chemical level, SICI involves GABAA-related pathways (Ziemann et al., 1996) and intracortical facilitation (ICF) reflects glutamatergic neurotransmission. Short-interval intracortical facilitation (SICF) is, in contrast to SICI and ICF, not associated with a chemical synapse mediator, but it is rather attributed to the indirect transsynaptic excitation of corticospinal neurons (Ziemann, 2020). Long-interval intracortical inhibition (LICI) is proposed to be mediated by GABAB receptors (Florian et al., 2008). Short-latency afferent inhibition (SAI) is elicited by stimulating a peripheral nerve and delivering a TMS pulse ∼2–8 ms after the arrival of the afferent volley in the somatosensory cortex (Chen et al., 2008). SAI is reduced after administering scopolamine, a muscarinic antagonist, suggesting the involvement of cholinergic circuits.
Conventional paired-pulse TMS methods paradigms rely on the motor-evoked potentials (MEP) amplitude, which is a variable parameter (Chipchase et al., 2012, Groppa et al., 2012). To overcome some of the limitations of conventional TMS, the threshold-tracking TMS technique is emerging as a useful clinical and research tool, whereby a constant target MEP response is tracked by a test stimulus (Awiszus et al., 1999, Fisher et al., 2002). Recently, new automated and fast resting motor threshold (RMT), SICI, ICF, SICF, LICI and SAI protocols have been programmed both for threshold-tracking and conventional TMS (Tankisi et al., 2021b).
TMS studies on SD in healthy individuals show variability and sometimes even contradicting results (De Gennaro et al., 2007, Manganotti et al., 2006; Scalise et al., 2006). The paired-pulse TMS protocols used were not consistent and included only a few ISIs. Some studies reported changes after SD in SICI, cortical silent period (CSP), RMT (Kreuzer et al., 2011; Salehinejad et al., 2022, Civardi et al., 2001) and SAI (Salehinejad et al., 2022). However, some other studies failed to find any effect of SD on TMS measures in healthy people (Manganotti et al., 2006).
To date, there is no study that examined a wide range of ISIs, neither for paired-pulse inhibition nor facilitation parameters. Similarly, no study used threshold-tracking TMS, which has been shown to be more sensitive in some conditions, such as amyotrophic lateral sclerosis (Tankisi et al., 2021c). Furthermore, the existing studies exhibit significant variability, yielding contradictory outcomes. Thus, the cortical excitability changes after SD are yet to be further explored. The purpose of this study was to investigate the effect of SD on cortical excitability in healthy subjects using threshold-tracking TMS. Additional parameters, such as the impact on cognitive abilities, have been assessed as well.
2. Material and methods
The study was carried out in accordance with the Declaration of Helsinki and the local ethical committee at Central Region of Denmark approved the study (1-10-72-171-22). Written informed consent was obtained from all participants prior to the investigations.
2.1. Participants
Fifteen healthy volunteers (7 females, 8 males, mean age ± SD: 36 ± 3.34 years, range: 20–59) were included. Two subjects were sleep-deprived at their home on their own, the other subjects were under the observation of one of the co-authors. The participants have been instructed not to drink alcohol 48 h before the measurement and not to consume coffee at least 12 h before the measurement. The participants were not taking any medication that could influence the TMS parameters and there were no other contraindications to magnetic stimulation. All subjects were examined at baseline and after at least 24 h of sleep deprivation by the same examiners (MM or ADG). Verbal instruction and clinical supervision minimized possible biases due to vigilance fluctuations during the measurements so that all subjects were awake during the measurement. The baseline and SD measurements were performed in the morning between 6:00 and 8:00 to minimalize the influence of circadian variability. The baseline measurements were performed after the usual hours of sleep. Subjects were randomized for whether the sleep-deprived session was the first or second examination.
2.2. Cognitive testing
The individuals performed the computerized Sternberg Memory Task (SMT), available at the following website https://rpadgett.butler.edu/nw221/sternberg_lab/index.html, as cognitive test on both examination days. SMT evaluates short-term memory and is widely used to assess a mnemonic capacity in clinical studies. SD impacts both overall performance and reaction time assessed with SMT and its modified version after short-term (Lim and Dinges, 2010) and long-term sleep deprivation (Tucker et al., 2010). Reaction time and the number of errors were analysed for 200 passages. For the subjects (6 subjects) that filled in 50 passages only, the result was standardized for 200 (multiplied by 4). The results of SMT were also used to control if the subjects were sleep deprived as SMT.
2.3. TMS methodology
The recordings were obtained from the abductor pollicis brevis (APB) muscle.
A figure-of-eight coil (Magstim® D70 Remote Coil) connected to two Magstim®200 stimulators was positioned at approximately 4 cm left in the binauricular line from vertex, with the handle pointing 45° to the parasagittal plane (Ørskov et al., 2021). Once the hotspot was located, the outline of the coil was drawn on a cap to enable constant coil positioning, and the automated stimulation protocols were initiated. Stimulus delivery and data acquisition were controlled by QtracW software (© University College London) using QTMSG‐12 recording protocols (QTMS Science Ltd.).
The MEP was amplified (1000 × gain) and filtered (3 Hz to 3 kHz) using a D440‐2 Channel Isolated Amplifier (Digitimer Ltd). A 50/60 Hz HumBug Noise Eliminator was used to remove line frequency contamination, and the amplified signals were digitized (NI USB‐6251, National Instruments).
2.3.1. Resting motor threshold (RMT)
Resting motor threshold (RMT) for a 200 µV (RMT200) peak‐to‐peak response was detected by a 4 → 2 → 1 tracking rule, as described elsewhere (Tankisi et al., 2021a, Tankisi et al., 2022). RMTs and all further thresholds, whether conditioned or unconditioned, were estimated from the stimuli and responses by weighted logarithmic regression.
2.3.2. Paired-pulse threshold-tracking TMS protocols
SICI, ICF, SICF, LICI and SAI protocols were performed in random order.
For each protocol, after the hot spot was identified and RMT200 was measured, the threshold was tracked for a 200 μV MEP amplitude at different ISIs in pseudo‐random order. The ISIs for each protocol are shown in Table 1.
Table 1.
Interstimulus intervals (ISIs) for each paired-pulse threshold-tracking transcranial magnetic stimulation (TMS) protocol.
| Paired-pulse TMS protocol | ISIs (ms) |
|---|---|
| SICI/ICF | 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 7, 15, 30 |
| SICF | 1, 1.3, 1.6, 1.9, 2.2, 2.5, 2.8, 3.1, 3.4, 3.7, 4.0, 4.3, 4.6, 4.9 |
| LICI | 50, 100, 150, 200, 250, 300 |
| SAI | 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, 28, 30 |
Short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), short-interval intracortical facilitation (SICF), long-interval intracortical inhibition (LICI), short-latency afferent inhibition (SAI), inter-stimulus intervals (ISIs).
For T‐SICI/ICF, the parallel tracking method previously designated as T‐SICIp was used, in which 200 µV MEP amplitude was tracked independently at each ISI from 1 to 30 ms (Tankisi et al., 2021b). Conditioning stimulus intensity was set to 70 % of RMT200. Test‐alone stimuli were delivered after each of three conditioning + test stimuli combinations, with the ISIs presented in pseudo‐random order. Each of the eleven paired stimuli was delivered 10 times, making a total of 146 stimuli with the test‐alone stimuli.
T-SICF was measured at 14 ISIs from 1 to 4.9 ms, and the threshold was tracked with 10 paired pulses at each ISI (Tankisi et al., 2021b). Conditioning stimuli intensity was set to 95 % of RMT200. Test-alone stimuli were delivered as every 4th stimulus, and the 14 conditioning + test stimuli were delivered in pseudorandom order. Each of the fourteen paired stimuli was delivered 10 times, making a total of 186 stimuli with the test-alone stimuli.
For T-LICI, 10 paired pulses at the same 6 ISIs were delivered, and the thresholds for RMT200 were tracked while conditioning stimulus was set to 120 % of the tracked RMT200 (Tankisi et al., 2021b). Test-alone stimuli were delivered as every 4th stimulus, and conditioning + test stimuli were delivered 10 times, making a total of 80 stimuli with the test-alone stimuli.
For the T-SAI, the electrical stimulus intensity for a 1-mV compound muscle action potential was first determined (EMT1000). Then, stimulation switched to the magnetic stimulus, and the hotspot was determined in the usual way. The program then determined RMT200 in a way similar to the other tracking protocols. Furthermore, the program proceeded straight into tracking SAI, with the ISI between electrical stimulus and magnetic test stimulus increased in 1 ms steps between 16 and 30 ms at 12 ISIs (Cengiz et al., 2022). Test-alone stimuli were delivered as every 4th stimulus, and conditioning + test stimuli were delivered 10 times at 12 ISIs, making a total of 160 stimuli with the test-alone stimuli.
2.4. Data analysis
QtracP software was used for data analysis and producing the figures. Depending on whether the data were normally distributed or not, a parametric or non-parametric paired samples test was applied to compare the results before and after SD within the same individuals. For the analysis of cognitive testing SPSS program was used.
2.5. Literature review
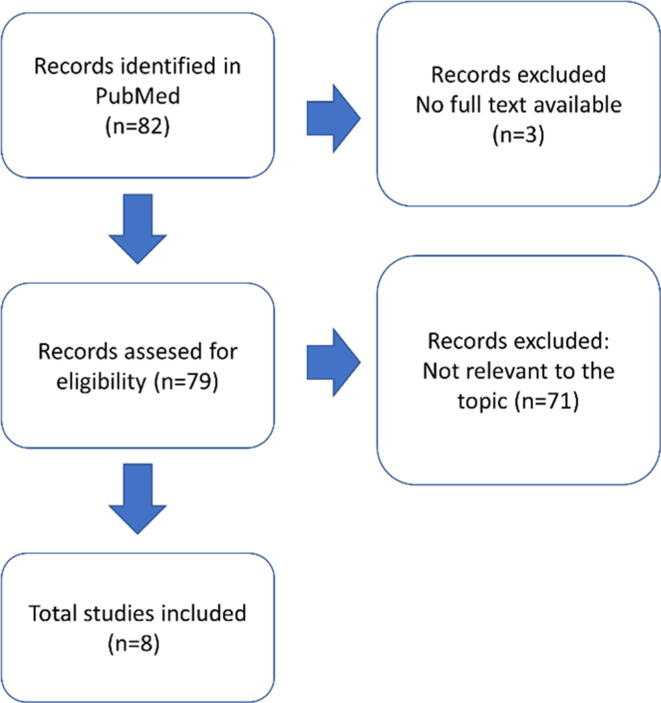
For the literature review on TMS after SD in healthy participants, the PubMed database has been searched on February 2023 using the terms: “TMS” AND “sleep deprivation” or “cortical excitability” AND “sleep deprivation” as keywords. The manuscripts on healthy participants have been selected manually. We excluded the sleep studies focusing on the REM phase only and the ones using a combined TMS-EEG method, which highlighted mainly EEG changes or were focused only on a particular stage of sleep (Placidi et al., 2013, Del Felice et al., 2011, Gaggioni et al., 2019, Manganotti et al., 2013). The workflow representing manuscript selection is shown in Fig. 1.
Fig. 1.
Workflow illustrating manuscripts selection.
3. Results
3.1. Cognitive testing
The results of cognitive testing at baseline and after SD were available for 11 participants. Both reaction time and the number of errors were significantly higher in the SD group. The baseline number of errors was 26.72 vs. 43.73 after SD (p < 0.01). The mean reaction time was 819.18 ms at baseline vs. 902.54 ms in the SD group (p = 0.01).
3.2. TMS protocols
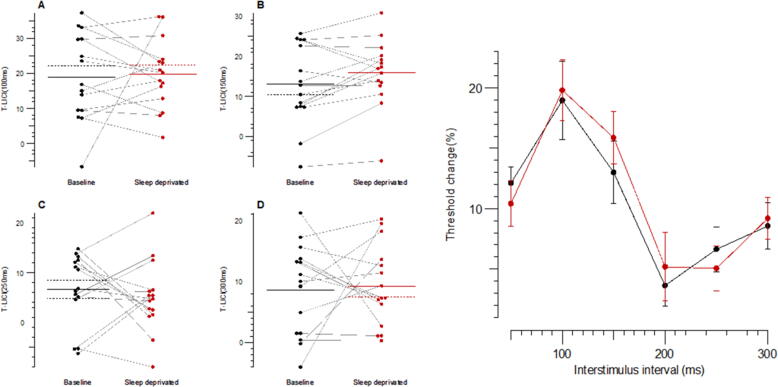
There was no significant difference in SICI, ICF, SICF or LICI at any of the ISIs. There was a significant difference in the SAI at 28 ms and 30 ms, which fall outside the typical range of analysis (18–22 ms). No differences were seen in SAI at other ISIs. Detailed data are illustrated in Fig. 2, Fig. 3, Fig. 4, Fig. 5 and provided in the Supplementary Tables 1–4.
Fig. 2.
Threshold-tracking short-interval intracortical inhibition (T-SICI) for the following interstimulus intervals (ISIs): (a) 1 ms; (b) 3 ms; (c) 1–3.5 ms; (d) 1–7 ms; (e) mean values for all ISIs, black plots and line indicate baseline whereas red plots and line indicate SD; B-baseline; SD-sleep deprivation; RMT-resting motor threshold. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Short interval intracortical facilitation (SICF) for the following interstimulus intervals (ISIs): (a) 1.6 ms; (b) 2.5 ms; (c) 3.4 ms; (d) 4.3 ms; (e) mean values for all ISIs, black plots and line indicate baseline whereas red plots and line indicate SD; B-baseline; SD-sleep deprivation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Long-interval intracortical inhibition (LICI) for the following interstimulus intervals (ISIs): (a) 100 ms; (b) 150 ms; (c) 250 ms; (d) 300 ms; (e) mean values for all ISIs, black plots and line indicate baseline whereas red plots and line indicate SD; B-baseline; SD-sleep deprivation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Short-latency afferent inhibition (SAI) for the following interstimulus intervals (ISIs): (a) 20 ms; (b) 23 ms; (c) 28 ms; (d) 30 ms; (e) mean values for all ISIs, black plots and line indicate baseline whereas red plots and line indicate SD; B-baseline; SD-sleep deprivation. *p < 0.05*, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Literature review
The literature search using “TMS” AND “sleep deprivation” as keywords in PubMed database yielded 47 hits (7 studies included) while the search “cortical excitability” AND “sleep deprivation” gave 35 hits (2 additional studies included). The TMS protocols performed in previous studies investigating SD include MT, SICI, ICF, and cortical silent period (CSP); few studies incorporated additional protocols, such as input/output (I–O) curve, SAI, LICI, and assessment of the I wave (Table 2).
Table 2.
Studies that investigated the effects of SD on transcranial magnetic stimulation.
| Reference | Number of subjects | Details | Changed | Unchanged |
|---|---|---|---|---|
| Civardi et al., 2001 | 8 HC | ≥24 h of SD | ↓SICI at ISI = 2–3 ms ↓ICF at ISI = 14–16 ms |
RMT and AMT CSP duration |
| Manganotti et al., 2001 | 7 HC | ≥24 h monitoring. Recordings every 6 h (daytime) and every 3 h (night) |
↑RMT (only night) ↑CSP duration (only night) ↑SICI at ISI = 1–4 ms (only night) |
MEP amplitude ICF at ISIs = 10–15 ms |
| Manganotti et al., 2006 | 10 HC | Partial SD (∼9 h from midnight to 9am) | None | RMT MEP amplitude CSP duration SICI at ISI = 1–4 ms ICF at ISI = 10–15 ms |
| 10 patients with JME | Partial SD (∼9h from midnight to 9am) | ↓RMT ↓SICI at ISI = 1–4 ms ↑ICF at ISI = 10–15 ms |
MEP amplitude CSP duration |
|
| Scalise et al., 2006 | 7 HC | ≥24 h of SD | ↓CSP duration ↓SICI at ISIs = 1 and 2 ms |
RMT MEP amplitude SICI at ISI = 3, 4, 5, 6 ms |
| Badawy et al., 2006 | 13 HC | ≥20 h of wakefulness | ↓LICI at ISI = 250 ms | SICI/ICF at ISI = 1, 2, 5, 10, 15 ms LICI at ISI = 200 and 300 ms |
| 15 patients with generalised epilepsy | ≥20 h of wakefulness | ↓SICI at ISI = 2 ms ↑ICF at ISI = 5, 10, 15 ms LICI at ISI = 200, 250 and 300 ms |
SICI at ISI = 1 | |
| 15 patients with focal epilepsy | ≥20 h of wakefulness | ↑ICF at ISI = 5, 10, 15 ms LICI at ISI = 200, 250 and 300 ms |
SICI at ISI = 1 and 2 ms | |
| De Gennaro et al., 2007 | 33 HC | ≥40 h of SD | ↑RMT ↑LT* and ↑UT** ICF at ISI = 7, 10, 12, 15 ms (females) |
SICI/ICF at ISI = 1 and 3 ms (males and females) ICF at ISI = 7, 10, 12, 15 ms (males) |
| Kreuzer et al., 2011 | 15 HC | ≥24 h of SD | ↓SICI at ISI = 2 ms | RMT CSP ICF at ISI = 15 ms |
| Salehinejad et al., 2022 | 30 HC | ≥24 h of SD | ↓SICI at ISI = 2 and 3 ms ↑ICF at ISI = 10 and 15 ms ↓SAI at ISI = 20 and 40 ms ↑I-wave facilitation (SICF) at ISI = 1–4.4 ms I-O curve$ |
RMT/AMT MEP amplitude SI1mv |
| Mroczek et al. (This study) | 15 HC | ≥24 h of SD | ↓SAI at ISI = 28 and 30 ms | SICI/ICF at ISI = 1–30 ms SICF at ISI = 1–4.9 ms LICI at ISI = 50–300 ms SAI at ISI = 16–30 ms |
Resting motor threshold (RMT), active motor threshold (AMT).
Lower threshold (LT), defined as the maximum intensity at which 10 stimuli all produced no response was found by decreasing intensity in 1 % steps.
Upper threshold (UT), defined as minimum intensity at which 10 stimuli all produced a positive response found by increasing the intensity in 1 % steps from the lowest level which so far had not resulted in a “no response”, short-interval intracortical inhibition (SICI), long-interval intracortical inhibition (LICI), short-interval intracortical facilitation (SICF), Short-latency afferent inhibition (SAI), cortical silent period (CSP), intracortical facilitation (ICF), inter-stimulus intervals (ISIs), input–output curve (I–O curve), MEP amplitude of 1 mV (SI1mV).
I–O curve showed a marginally significant interaction of sleep condition × TMS intensity and significant main effects of sleep condition and TMS intensity.
4. Discussion
This is the first study on sleep-deprived individuals performed using threshold-tracking TMS, which has been shown to be a more sensitive measure than the conventional paired-pulse TMS in ALS (Tankisi et al., 2021c, Tankisi et al., 2023). Additionally, this is the first study using such a wide range of ISIs for SICI, ICF, SICF, LICI and SAI. However, we did not find any effect of SD on cortical excitability measurements in any of the TMS measures, apart from SAI, at few ISIs.
The literature search yielded eight studies on the effects of SD on cortical excitability using conventional TMS and amplitude measurement (Table 2). Most of the studies were based on a small number of patients. The protocols and ISIs applied in these studies varied widely, making any direct comparison difficult. RMT and SICI/ICF were performed in all eight studies (Civardi et al., 2001, Manganotti et al., 2001, Manganotti et al., 2006, Scalise et al., 2006, Badawy et al., 2006, De Gennaro et al., 2007, Kreuzer et al., 2011, Salehinejad et al., 2022) while cortical silent period was only assessed in five (Civardi et al., 2001, Manganotti et al., 2001, Manganotti et al., 2006, Scalise et al., 2006, Kreuzer et al., 2011). The literature on the other paired-pulse protocols is sparse. LICI was performed only in one study (Badawy et al., 2006) and so were SAI and SICF (Salehinejad et al., 2022). Although in two other studies (Manganotti et al., 2006, De Gennaro et al., 2007), the authors reported that they examined SICF, it was obvious from the methodology description that the paired-pulse TMS method used was ICF at ISIs 10–15 ms, suggesting a mistake in the terminology.
Civardi et al. (2001) reported a significant reduction in both SICI and ICF in eight healthy subjects after SD using a conventional paired-pulse TMS protocol. Decreased ICF in this study is in contrast with the other studies which found either increased or unchanged ICF. Similarly, Scalise et al. found reduced SICI in seven healthy subjects at 1 and 2 ms ISIs but not at 3 ms, an ISI where the most prominent inhibition is expected. This study did not evaluate the facilitation (Scalise et al., 2006). In one more study, no differences in SICI and ICF were emphasized (de Gennaro et al., 2007) whereas in two other studies (Salehinejad et al., 2022, Kreuzer et al., 2011) the authors reported a facilitation in SICI. RMT was found to be unaffected after SD in most studies (Manganotti et al., 2006, Kreuzer et al., 2011, Salehinejad et al., 2022) and increased in only one study (de Gennaro et al., 2007). The data on CSP are also unequivocal: in one study the CSP duration was shortened (Scalise et al., 2006) whereas in most studies it was unchanged (Civardi et al., 2001, Manganotti et al., 2006, Kreuzer et al., 2011). Unfortunately, we could not add CSP to this study.
We did not find any changes in LICI or SICF. As stated before, LICI was previously examined only in one study which showed a decrease in inhibition at 250 ms but not at other ISIs (Badawy et al., 2006). (Table 2). The limited number of healthy subjects and different methodologies may explain the discrepancy between this study and ours. Our SICF results are also in contrast with the study by Salehinejad et al. (2022). In this case, the methodological difference (threshold-tracking versus conventional amplitude measurements) may account for the conflicting results. Additionally, Salehinejad et al. (2022) examined 30 healthy subjects, finding changes in all paired-pulse TMS measures (SICI, ICF, SICF, and SAI). As for SAI, it has been systematically investigated only in this study, and it was found to be reduced at 20 ms and 40 ms ISIs (Salehinejad et al., 2022). We found reduced SAI as well, but only at 28 ms and 30 ms with no change at 20 ms. The significant decrease obtained in both reaction time and in the number of errors after SD in our study shows that our participants were, in fact, sleep-deprived, and these findings support the genuineness of the alteration we found in the SAI. However, we do not know whether the significant change in SAI at such late ISIs holds a particular meaning. SAI is known to be reduced in most forms of dementia, including Parkinson’s disease, Lewy Body Disease, Alzheimer’s disease (AD), mild cognitive impairment due to AD and Huntington’s disease (Vucic et al., 2023), whereas it remains unchanged in frontotemporal dementia (FTD) and therefore it has been proposed as a tool to differentiate AD from FTD (Benussi et al., 2017). However, in these conditions, maximum SAI reduction has been reported to occur 20–22 ms after median nerve stimulation at the wrist (Rossini et al., 2015) so that later ISIs (28 ms and 30 ms) are rarely included in the protocols. Few available studies report conflicting results regarding the decrease in SAI at 28 ms and 30 ms in AD dementia (Di Lorenzo et al., 2013, Benussi et al., 2020). So far, only one study where repetitive TMS was used to treat patients with drug-resistant depression reported a decrease in SAI at 30 ms (Kallioniemi et al., 2018). To the best of our knowledge, there is no data regarding SAI at 28 ms and 30 ms ISIs after SD so no direct comparison with our study can be made.
An important aspect of SD is the impact of the circadian phase on cortical excitability. Examination of the subjects at different times of the day may account, at least partially, for the conflicting results. Manganotti et al. showed the circadian effect on cortical excitability by monitoring TMS measures (SICI/ICF, MEP amplitude, RMT, CSP) for 24 h (Manganotti et al., 2001). This study showed a temporary decrease in cortical excitability during the night, which returned to “normality” in the morning; the authors attributed this decrease to the subjects’ drowsiness at night. The same research group proposed a reduction in cortical excitability during sleep stages using both TMS and EEG methods in another study (Manganotti et al., 2004). Later, they compared healthy subjects with patients with juvenile myoclonic epilepsy (JME); examinations performed at 9 AM revealed no changes in SICI/ICF, MEP amplitude, RMT and CSP in healthy subjects after SD while sleep-deprived patients with JME showed decreased RMT and SICI as well as increased ICF, a finding consistent with cortical hyperexcitability (Manganotti et al., 2006, Manganotti et al., 2001).
Moreover, it has been shown, using TMS-EEG method, that cortical excitability increases with sleep deprivation over 29 h. However, the pattern increase seems to be not linear (Ly et al., 2016, Chellappa et al., 2016): after an initial increase, the cortical excitability returns to baseline between 9:00 and 11:00 PM and then reaches its peak in the morning, around 6:00–8:00 (Ly et al., 2016). In our study, we tried to minimize the impact of circadian fluctuations by performing all the studies early in the morning, between 6:00 and 8:00 AM (the period of maximal cortical excitability). However, like Manganotti et al., 2001, Manganotti et al., 2006, we did not find any changes in RMT, SICI or ICF.
Another important consideration is that TMS can provide information only on selectively investigated excitability of the primary motor cortex that is altered after sleep deprivation (Oroz et al., 2021). Recently, it was shown that different parts of the cortex displayed a different reaction to the TMS stimulation, reflecting a connectivity profile (Castrillon et al., 2020). The study of Castrillon et al. (2020) reported that identical low-frequency stimulation may have opposite effects on cognitive and sensory brain regions, depending on the different cortical regions stimulated. In the frontal cortex, the low-frequency stimulation decreased local inhibition and disrupted feedforward and feedback connection, whereas the same stimulation in the occipital region increased inhibition and enhanced signalling. Furthermore, during sleep, especially at sleep onset, more pronounced changes occur in the occipital region (Chia et al., 2021, Gorgoni et al., 2019). Therefore, results obtained examining the motor cortex may not be representative of the whole brain as we cannot exclude that other parts of the cortex exhibit different changes after sleep deprivation. Also, the influences of transitory drowsiness on the measurement in our subjects are not to be excluded, despite verbal instructions aimed at minimalizing them.
There are also some technical considerations related to the method that should be mentioned. The use of the 4 → 2 → 1 tracking rule may have influenced the results because the MEP amplitude varies considerably from stimulus to stimulus. We have shown in previous studies that SICI using conventional amplitude measurements had less variability compared to threshold-tracking SICI at ISIs of 1–7 ms (Tankisi et al., 2021a, Tankisi et al., 2022). In these studies, the reliability has been assessed using the coefficient of variations and standard deviations. In an earlier study, threshold tracking SICI was found to be more reliable with higher intraclass correlation coefficient values than conventional amplitude measurements (Samusyte et al., 2018). However, in this study, the study design was different and 2.5 ms was the only ISI investigated using different conditioning stimulus intensities between 60 and 80 % RMT. Also, it may be discussed whether the proportional tracking method is a true tracking method, provided that is applies a fixed number of stimuli in contrast to the serial tracking that targets the point when the condition is met. However, we showed in our previous studies several limitations of serial tracking (Tankisi et al., 2021a). Therefore, we introduced a parallel tracking approach.
Finally, although it consists of 15 individuals and is comparable with other TMS SD studies, the main limitation of our study is the small sample size. We cannot rule out the possibility that the limited sample size has affected our results and that certain parameters would have reached statistical significance with a larger cohort.
In conclusion, our findings do not show any effect of SD on cortical excitability assessed through TMS, with the exception of some alterations in late SAI, despite the broad number of ISIs investigated and the several protocols applied. Therefore, our study lends support to the existing literature that shows no impact of SD on the neurophysiological level. Further studies with larger cohorts and comparing threshold-tracking with conventional amplitude measurement techniques are needed.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘HT is a shareholder of QTMS Science Ltd., which licences the QTMSG-12 recording protocols used. Other authors have no potential conflict of interest to declare’.
Acknowledgements
This study has been supported by Lundbeck Foundation, Grant numbers (R346-2020-1946, R392-2022-699 and R359-2020-2620) GROSSERER L.F. FOGHTS Foundation, Aase og Ejnar Danielsen Foundation, and Dagmar Marshalls Foundation.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cnp.2023.12.001.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
References
- Awiszus F., Feistner H., Urbach D., Bostock H. Characterisation of paired-pulse transcranial magnetic stimulation conditions yielding intracortical inhibition or I-wave facilitation using a threshold-hunting paradigm. Exp. Brain Res. 1999;129:317–332. doi: 10.1007/s002210050901. [DOI] [PubMed] [Google Scholar]
- Badawy R.A., Curatolo J.M., Newton M., Berkovic S.F., Macdonell R.A. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Neurology. 2006;67(6):1018–1022. doi: 10.1212/01.wnl.0000237392.64230.f7. [DOI] [PubMed] [Google Scholar]
- Benussi A., Di Lorenzo F., Dell'Era V., Cosseddu M., Alberici A., Caratozzolo S., et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89(7):665–672. doi: 10.1212/WNL.0000000000004232. [DOI] [PubMed] [Google Scholar]
- Benussi A., Grassi M., Palluzzi F., Koch G., Di Lazzaro V., Nardone R., et al. Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. Ann. Neurol. 2020;87(3):394–404. doi: 10.1002/ana.25677. [DOI] [PubMed] [Google Scholar]
- Castrillon G., Sollmann N., Kurcyus K., Razi A., Krieg S.M., Riedl V. The physiological effects of noninvasive brain stimulation fundamentally differ across the human cortex. Sci. Adv. 2020;6(5) doi: 10.1126/sciadv.aay2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz B., Boran H.E., Alaydın H.C., Tankisi H., Samusyte G., Howells J., et al. Short latency afferent inhibition: comparison between threshold-tracking and conventional amplitude recording methods. Exp. Brain Res. 2022;240(4):1241–2127. doi: 10.1007/s00221-022-06327-5. [DOI] [PubMed] [Google Scholar]
- Chellappa S.L., Gaggioni G., Ly J.Q., Papachilleos S., Borsu C., Brzozowski A., et al. Circadian dynamics in measures of cortical excitation and inhibition balance. Sci. Rep. 2016;6 doi: 10.1038/srep33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Cros D., Curra A., Di Lazzaro V., Lefaucheur J.P., Magistris M.R., et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2008;119(3):504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Chia C.H., Tang X.W., Cao Y., Cao H.T., Zhang W., Wu J.F., et al. Cortical excitability signatures for the degree of sleepiness in human. Elife. 2021;10 doi: 10.7554/eLife.65099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipchase L., Schabrun S., Cohen L., Hodges P., Ridding M., Rothwell J., et al. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clin. Neurophysiol. 2012;123(9):1698–1704. doi: 10.1016/j.clinph.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civardi C., Boccagni C., Vicentini R., Bolamperti L., Tarletti R., Varrasi C., et al. Cortical excitability and sleep deprivation: a transcranial magnetic stimulation study. J. Neurol. Neurosurg. Psychiatry. 2001;71(6):809–812. doi: 10.1136/jnnp.71.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L., Marzano C., Veniero D., Moroni F., Fratello F., Curcio G., et al. Neurophysiological correlates of sleepiness: a combined TMS and EEG study. Neuroimage. 2007;36(4):1277–1287. doi: 10.1016/j.neuroimage.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Del Felice A., Fiaschi A., Bongiovanni G.L., Savazzi S., Manganotti P. The sleep-deprived brain in normals and patients with juvenile myoclonic epilepsy: a perturbational approach to measuring cortical reactivity. Epilepsy Res. 2011;96(1–2):123–131. doi: 10.1016/j.eplepsyres.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo F., Martorana A., Ponzo V., Bonnì S., D'Angelo E., Caltagirone C., et al. Cerebellar theta burst stimulation modulates short latency afferent inhibition in Alzheimer's disease patients. Front. Aging Neurosci. 2013;5 doi: 10.3389/fnagi.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.J., Nakamura Y., Bestmann S., Rothwell J.C., Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp. Brain Res. 2002;143(2):240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Florian J., Müller-Dahlhaus M., Liu Y., Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J. Physiol. 2008;586(2):495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioni G., Ly J.Q.M., Muto V., Chellappa S.L., Jaspar M., Meyer C., et al. Age-related decrease in cortical excitability circadian variations during sleep loss and its links with cognition. Neurobiol. Aging. 2019;78:52–63. doi: 10.1016/j.neurobiolaging.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Gorgoni M., Bartolacci C., D’Atri A., Scarpelli S., Marzano C., Moroni F., et al. The spatiotemporal pattern of the human electroencephalogram at sleep onset after a period of prolonged wakefulness. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V., et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123(5):858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi E., Määttä S., Könönen M., Mervaala E., Viinamäki H., Valkonen-Korhonen M. F137. Effects of repetitive transcranial magnetic stimulation on short-latency afferent inhibition: A study in treatment-resistant depression. Clin. Neurophysiol. 2018 [Google Scholar]
- Killgore W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- Kreuzer P., Langguth B., Popp R., Raster R., Busch V., Frank E., et al. Reduced intra-cortical inhibition after sleep deprivation: a transcranial magnetic stimulation study. Neurosci. Lett. 2011;493(3):63–66. doi: 10.1016/j.neulet.2011.02.044. [DOI] [PubMed] [Google Scholar]
- Lim J., Dinges D.F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 2010;136(3):375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly J.Q.M., Gaggioni G., Chellappa S.L., Papachilleos S., Brzozowski A., Borsu C., et al. Circadian regulation of human cortical excitability. Nat. Commun. 2016;7 doi: 10.1038/ncomms11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P., Palermo A., Patuzzo S., Zanette G., Fiaschi A. Decrease in motor cortical excitability in human subjects after sleep deprivation. Neurosci. Lett. 2001;304(3):153–156. doi: 10.1016/s0304-3940(01)01783-9. [DOI] [PubMed] [Google Scholar]
- Manganotti P., Fuggetta G., Fiaschi A. Changes of motor cortical excitability in human subjects from wakefulness to early stages of sleep: a combined transcranial magnetic stimulation and electroencephalographic study. Neurosci. Lett. 2004;362(1):31–34. doi: 10.1016/j.neulet.2004.01.081. [DOI] [PubMed] [Google Scholar]
- Manganotti P., Bongiovanni L.G., Fuggetta G., Zanette G., Fiaschi A. Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: a combined transcranial magnetic stimulation and EEG study. J. Neurol. Neurosurg. Psychiatry. 2006;77(1):56–60. doi: 10.1136/jnnp.2004.041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P., Formaggio E., Del Felice A., Storti S.F., Zamboni A., Bertoldo A., et al. Time-frequency analysis of short-lasting modulation of EEG induced by TMS during wake, sleep deprivation and sleep. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroz R., Kung S., Croarkin P.E., Heung J. Transcranial magnetic stimulation therapeutic applications on sleep and insomnia: a review. Sleep Sci. Pract. 2021;5(3) [Google Scholar]
- Ørskov S., Bostock H., Howells J., Pugdahl K., Fuglsang-Frederiksen A., Nielsen C.S., et al. Comparison of figure-of-8 and circular coils for threshold tracking transcranial magnetic stimulation measurements. Neurophysiol. Clin. 2021;51(2):153–160. doi: 10.1016/j.neucli.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Placidi F., Zannino S., Albanese M., Romigi A., Izzi F., Marciani M.G., et al. Increased cortical excitability after selective REM sleep deprivation in healthy humans: a transcranial magnetic stimulation study. Sleep Med. 2013;14(3):288–292. doi: 10.1016/j.sleep.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehinejad M.A., Ghanavati E., Reinders J., Hengstler J.G., Kuo M.F., Nitsche M.A. Sleep-dependent upscaled excitability, saturated neuroplasticity, and modulated cognition in the human brain. Elife. 2022;11 doi: 10.7554/eLife.69308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samusyte G., Bostock H., Rothwell J., Koltzenburg M. Short-interval intracortical inhibition: Comparison between conventional and threshold-tracking techniques. Brain Stimul. 2018;11(4):806–817. doi: 10.1016/j.brs.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalise A., Desiato M.T., Gigli G.L., Romigi A., Tombini M., Marciani M.G., et al. Increasing cortical excitability: a possible explanation for the proconvulsant role of sleep deprivation. Sleep. 2006;29(12):1595–1598. doi: 10.1093/sleep/29.12.1595. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Cengiz B., Howells J., Samusyte G., Koltzenburg M., Bostock H. Short-interval intracortical inhibition as a function of inter-stimulus interval: Three methods compared. Brain Stimul. 2021;14(1):22–32. doi: 10.1016/j.brs.2020.11.002. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Howells J., Cengiz B., Samusyte G., Koltzenburg M., Bostock H. Conventional and threshold-tracking transcranial magnetic stimulation tests for single-handed operation. J. Vis. Exp. 2021;174 doi: 10.3791/62787. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Nielsen C.S., Howells J., Cengiz B., Samusyte G., Koltzenburg M., et al. Early diagnosis of amyotrophic lateral sclerosis by threshold tracking and conventional transcranial magnetic stimulation. Eur. J. Neurol. 2021;28(9):3030–3309. doi: 10.1111/ene.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankisi H., Cengiz B., Samusyte G., Howells J., Koltzenburg M., Bostock H. Short interval intracortical inhibition: Variability of amplitude and threshold-tracking measurements with 6 or 10 stimuli per point. Neurophysiol. Clin. 2022;52(2):170–173. doi: 10.1016/j.neucli.2021.11.006. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Pia H., Strunge K., Howells J., Cengiz B., Samusyte G., et al. Three different short-interval intracortical inhibition methods in early diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2023;24(1–2):139–147. doi: 10.1080/21678421.2022.2101926. [DOI] [PubMed] [Google Scholar]
- Tucker A.M., Whitney P., Belenky G., Hinson J.M., Van Dongen H.P. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33(1):47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S., Stanley Chen K.H., Kiernan M.C., Hallett M., Benninger D.H., Di Lazzaro V., et al. Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee. Clin. Neurophysiol. 2023;150:131–175. doi: 10.1016/j.clinph.2023.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. I-waves in motor cortex revisited. Exp. Brain Res. 2020;238:1601–1610. doi: 10.1007/s00221-020-05764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U., Lönnecker S., Steinhoff B.J., Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann. Neurol. 1996;40(3):367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.