Graphical abstract
Keywords: Thyroid hormones, Oxyfluorfen, Sodium iodide symporter, Endocrine
Highlights
-
•
Oxyfluorfen, a newly identified NIS inhibitor, suppressed thyroid hormones in the rat.
-
•
Dose-response suppression of serum T4 was observed following 8-day exposure (LOEL of 3.25 mg/kg).
-
•
Oxyfluorfen accumulated in the thyroid 2 to 3-fold over serum.
-
•
The data collected are useful for IVIVE extrapolations.
-
•
This is the first study to identify oxyfluorfen as an endocrine disrupting chemical.
Abstract
Recently, oxyfluorfen, a pre- and post-emergent diphenyl ether herbicide, was identified in our laboratory as an inhibitor of iodide uptake by the sodium iodide symporter (NIS), the first key step in the synthesis of thyroid hormones (THs). This inhibition was observed in vitro, using both a human NIS engineered cell line (hNIS-HEK293T-EPA) and a rat thyroid follicular cell line (FRTL-5). Oxyfluorfen was found to be a potent inhibitor of NIS activity with an EC50 of approximately 2 µM in both cell lines with no observed cytotoxicity at any concentration tested up to 100 μM. The current research tested the hypothesis that oxyfluorfen alters circulating concentrations of THs. This hypothesis was first tested in a pilot study with both juvenile male and female rats exposed to oxyfluorfen for 4 days at 0, 125, 250 and 500 mg/kg/day. Once we identified that this short-term 4-day oxyfluorfen exposure suppressed both total serum thyroxine (T4) and triiodothyronine (T3) at all doses, we tested seven lower concentrations of oxyfluorfen (0.8125 to 62.5 mg/kg day) in an 8-day exposure paradigm to more closely evaluate the dose–response. We found that oxyfluorfen suppressed serum T4 with a LOEL of 3.25 mg/kg/day and T3 with a LOEL 62.5 mg/kg/day. Analytical chemistry of the serum showed an accumulation over time following oral exposure to oxyfluorfen in both the 4- and 8-day groups. Analytical chemistry of the thyroid glands in the 8-day study revealed higher accumulation in the thyroid as compared to the serum (2 to 3- fold at 62.5 mg/kg). No changes in thyroid weight or serum TSH were observed following the 8-day exposure. This study is the first to demonstrate an effect of oxyfluorfen on serum thyroid hormones in the rat. Additional studies are needed to further evaluate the effects on thyroid homeostasis with extended exposures and the potential implications of the observed effects.
1. Introduction
In the past decade, there has been an increase in the identification and number of effects from environmental endocrine disruptors that can alter the thyroid axis in animals and humans. During critical windows of development, thyroid hormones (TH) regulate an array of physiological processes that are essential for growth and cognitive development, including neural processes in the brain during fetal and post-natal development (Morreale de Escobar et al., 2000, Zoeller and Crofton, 2005, Gilbert et al., 2020). Over the past two decades, several structurally diverse xenobiotics have been shown to interfere with TH homeostasis and result in physiological and morphological perturbations in both humans and wildlife (Brucker-Davis, 1998, Capen and Martin, 1989). More recently, our laboratory was part of a U.S. EPA effort to increase the coverage of molecular initiating events/cellular targets within the thyroid pathway that could be affected by environmental chemicals, including the regulation of circulating TH through feedback mechanisms within the hypothalamic-pituitary-thyroid (HPT) axis, TH synthesis and secretion, TH distribution and transport, TH metabolism, and TH receptor binding and action (Noyes et al., 2019). For this effort, we employed a high throughput (HTP) radioactive iodide uptake assay (RAIU) using a stable human NIS-expressing HEK293T cell line to rapidly screen chemicals that could potentially inhibit the human sodium iodide symporter (NIS) as described in Hallinger et al., 2017. Monovalent anions such as the environmental contaminant perchlorate have been well studied and demonstrate inhibition of the NIS-mediated uptake of iodide (Wolff, 1998), but data on more structurally diverse environmental chemicals was limited. Thyroid function is dependent on an adequate supply of iodide (I−), and the transport of I− by the NIS is the first key step in the synthesis of TH within the thyroid gland in mammals (Eskandari et al., 1997, Carrasco, 1993).
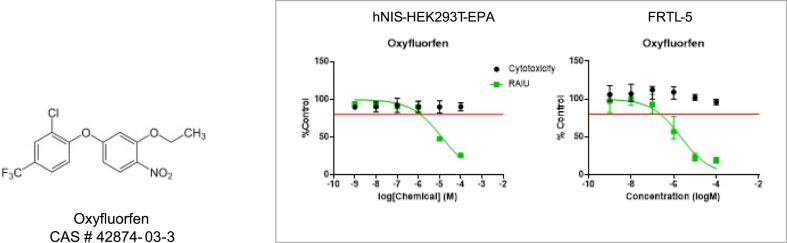
Following this assay development, we tested and prioritized several large environmental chemical libraries under the EPA’s purview (ToxCast Phase 1, 2, E1K and PFAS library) using our human NIS cell line (hNIS-HEK-293T-EPA) and secondary rat follicular FRTL-5 cell line (Wang et al., 2018, Wang et al., 2019, Wang et al., 2021). Utilizing the data collected from these assays, we were able to identify several novel potent in vitro inhibitors of NIS using a unique ranking system of inhibition potency verses cytotoxicity. One of the more potent chemicals identified with no confounding cytotoxicity was the herbicide oxyfluorfen. Oxyfluorfen inhibited NIS in our assay with an effective concentration or EC50 of approximately 2 µM in both the hNIS and rat FRTL5 cell RAIU assays (Fig. 1) (Buckalew et al., 2020). Based on the confirmation of repeat inhibition of NIS in these two cell assays, low oral toxicity, and half-life of approximately 30 h in the rat (2002 RED), our laboratory selected oxyfluorfen as an investigative case study for in vitro to in vivo extrapolation (IVIVE).
Fig. 1.
Oxyfluorfen chemical structure and in vitro screening results for Oxyfluorfen effects on iodide uptake in hNIS and FRTL-5 cell RAIU screening assays. Data points are medians with upper and lower ranges and the red line indicates 20% inhibition. Solid line indicates percent iodide uptake in the RAIU, and circles indicates percent cell viability at each concentration.
Oxyfluorfen, whose brand names include Goal and GoalTender[2-chloro-1-(3-ethoxy-4-nitrophenoxy)-4-(trifluoromethyl) benzene], is a diphenyl-ether herbicide used for the control of broadleaf and grassy weeds by disrupting the final step in chlorophyll synthesis (Camadro et al., 1995) and first registered in the U.S. in 1979(U.S. EPA, 2002). It is primarily used for controlling the growth of weeds in a variety of tree fruits, vines, field crops, but also for non-agricultural ornamental applications such as residential landscaping (U.S. EPA, 2019). Human exposure to oxyfluorfen can occur from both occupational and residential applications, contaminated surface and groundwater sources of drinking water, and limited exposure in food (40 CFR 180.381).
The purpose of this study is to evaluate a recently identified NIS inhibitor, oxyfluorfen, for in vivo effects on circulating thyroid hormones using two short-term juvenile rat models. This evaluation of thyroid hormones in the rat model with parallel serum and thyroid gland chemical analysis will provide quantitative data for IVIVE to inform adverse outcome pathway (AOP) development for potential health outcomes.
2. Material and methods
2.1. Chemicals
Oxyfluorfen, 2-chloro-1-(3-ethoxy-4-nitrophenoxy)-4(trifluoromethyl)benzene (CAS 42874–03-3; Toronto Research Chemicals, Toronto, Ontario) with a purity of 98 %, was received and stored protected from light at 4 °C. For each study, oxyfluorfen was prepared in a suspension of 1 % methyl cellulose, placed in an amber dosing bottle and stirred throughout the treatment period. Body weights were taken daily prior to oral gavage with a daily dose of 0.005 ml/g BW.
2.2. General Animal information
This study was conducted with prior approval from the U.S. Environmental Protection Agency’s Institutional Animal Care and Usage Committee (IACUC) and was carried out in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facility. Sprague-Dawley Rats were shipped from Charles Rivers (Raleigh, NC) on PND21 and acclimated upon arrival under controlled temperature (20–24 °C), humidity (40–50 %), and light (12:12 light/dark cycle) with Purina AIN-93 food (iodide sufficient diet, 225 ng/g) and water available ad libitum. This level of iodine is <25 % of that found in typical rodent chows but is more than sufficient for thyroid hormone production, assuring that the NIS inhibition can be identified in vivo (Gilbert et al., 2013). Polycarbonate cages with heat-treated pine shavings were used for housing. The day before treatment, weanling rat were weighed to the nearest 0.1 g and weight ranked and assigned so that treatment groups (n = 6) had similar body weight means and CV (<2.5 %). After assignment to treatment groups, rats were housed two per cage and the day before necropsy placed in a holding room adjacent to the necropsy room. This room was maintained under the same lighting and temperature conditions as described above but allowed rats to be euthanized (via decapitation) within 15 s after removal from their home cage to minimize stress.
2.3. Four-day exposure group
Male and female weanlings were dosed daily by oral gavage from PND 27–30 in a 4-day dosing scenario with 0, 125, 250 or 500 mg/kg (n = 6 per dose group in each sex) of oxyfluorfen solubilized in a 1 % methyl cellulose suspension (continually stirred). Body weights were recorded daily prior to exposure with doses adjusted accordingly. The rats were euthanized by decapitation within 15 s of removal from holding room approximately 2 hr after the 4th dose on PND 30 to prevent any stress related effects on hormonal responses. Trunk blood was collected in serum separation tubes (BD Vacutainers, Fisher Scientific) by centrifugation at 1260g for 30 min, serum harvested, aliquoted and stored frozen at −80 °C for subsequent evaluation of thyroid hormones and chemical analysis.
2.4. Eight-day exposure study
Next, we exposed additional male weanling rats for a period of eight days (PND27-PND34) by oral gavage to seven concentrations of oxyfluorfen (0, 0.8125, 1.625, 3.25, 7.5, 15, 31.25, 62.5 mg/kg; n = 6–12 per treatment). Trunk blood was collected for serum as described above for subsequent thyroid hormone assays and chemical analysis. In this 8-day study the thyroids with bracketed tracheas were also removed and frozen for later dissection, weighing, and chemical analysis.
2.5. Radioimmunoassay
Total T4 and T3 radioimmunoassays (RIAs) were used to analyze all the serum collected using kits from In Vitro Diagnostic Technologies (IVD, Santa Ana, CA). The level of detection (LOD) for the T4 RIA was 1.24 ng/ml and 0.50 ng/ml for T3 (using a low-end standard of 0.25 ng/ml). The RIAs were measured on a Wizard 1479 gamma counter (Perkin Elmer, Waltham MA). Intra-assay coefficient of variation (CV) for T4 was 3. 0 ng/ml and 5.5 ng/ml for T3 based on quality control samples distributed across the assay. Serum samples from the male 8-day multi-concentration study were also analyzed for thyroid stimulating hormone (TSH) by RIA as previously described in Stoker et al., 2006. TSH was measured using materials from the National Hormone and Pituitary Agency including iodination preparation I-9, reference preparation RP-3 and antisera S-6. The I-9 preparation (rTSH, FP11542B) was obtained from Golden West BioSolutions (Temecula, CA) and radiolabeled with 125I (Perkin Elmer, Boston, MA) by a modification of the chloramine-T method of Greenwood et al. (1963). Labeled antigen was separated from unreacted iodide gel filtration chromatography as described previously (Goldman et al., 1986). The intra-assay CV for the TSH RIA was 3.4 %.
2.6. Analytical chemistry
2.6.1. Serum T3/T4 comparison of LC/MS/MS to RIA
We tested a subset of our serum samples for verification of IVD RIA serum T3 and T4 results with liquid chromatography mass spectrometry (LC/MS/MS) to confirm our IVD assay for use in this study. For the comparison of hormone content by LC/MS/MS analysis an AB Sciex (Framingham, Massachusetts) Exion AC UHPLC-Qtrap 6500þ Linear Ion Trap system was used as previously described (Hornung et al., 2015, Hassan et al., 2017). Selected serum samples from each group were processed using solid phase extraction (SPE). Solvent-based calibration standards were used for quantitation over a range of 10–10,000 pg/ml in a 100 µL standard curve volume. The percent recovery for T4 and T3 was acceptable at 71–82 % and 85–107 %, respectively. Comparison of a set of biological replicates run in both RIA (IVD kit) and LC/MS/MS for T3 and T4 showed good correlation (r2 87 % for T4 and 76.2 % for T3) justifying use of this cost-effective method for all serum samples in this study.
2.6.2. Oxyfluorfen quantitation
Oxyfluorfen concentrations were analyzed in both 4-day and 8-day serum and 8-day thyroids. Qualitative and quantitative determination of oxyfluorfen in serum was evaluated using protein precipitation for sample preparation and liquid chromatography/tandem mass spectrometry (LC/MS/MS) on an AB Sciex (Framingham, MA) 4000 Qtrap linear ion trap mass spectrometer for analysis. Quantitation of oxyfluorfen in serum was achieved by using the method of isotope dilution and matrix-matched calibration standards.
Oxyfluorfen calibration standards were prepared in methanol for quantitation. The matrix matched (serum) calibration curve used a minimum of 5 points over a range of 2.5–5000 ng/ml. A second source standard known as the independent calibration verification standards (ICV) was prepared in serum at concentrations of 10 ng/mL and 100 ng/ml with recoveries of 75 % and 107 %, respectively (acceptance range 70–130 %). The concentration with the lowest calibration standard met the requirements for qualitative identification of oxyfluorfen set at the limit of quantitation (25 ng/mL in serum and 2.5 ng/g for thyroid gland). The calibration curve had a CV of ≥0.995 and calculated concentrations of oxyfluorfen were within 80–120 % of actual spike amount. Analyte detections below the LOQ were reported as not detected (ND).
Briefly, all calibrators, method blanks, and serum samples (60 µL) were spiked with 6 µL of 3000 ng/mL internal standard (Oxyfluorfen-(ethyoxy-d5, Sigma Aldrich), vortexed for 5 s and allowed to equilibrate at room temperature for 30 min. Acetonitrile (450 µL) was added to an Isolute PPT+ (protein precipitation plate), followed by addition of 50 µL of the sample mixture to the wells of the PPT + plate and vortexed for 2 min at 2000 rpm. Positive pressure was used to filter the samples to a clean 96-well collection plate. Protein precipitation supernatants were analyzed on an AB Sciex 6500 + QTRAP Linear Ion Trap mass spectrometer using electrospray ionization in positive ion, multiple reaction monitoring mode. Chromatographic separation was performed on a Kinetex C18 column (2.1 m × 50 mm, 2.6 µm) with mobile phase components of 0.2 % formic acid in water and acetonitrile.
A continuing calibration verification (CCV) standard was analyzed at the start of each sample batch and at the end of the analytical sequence and the percent recovery was acceptable for all batches (same acceptance of 70–130 %). A method blank (MB) free of the target analyte was included with each sample batch and was prepared from water spiked with internal standard analyzed prior to sample analysis. In addition, a Laboratory Control Sample (LCS) and Laboratory Control Sample Duplicate (LCSD) were made in serum spiked with oxyfluorfen at concentrations of 10 ng/mL and 500 ng/ml with acceptable percent recoveries between 82 and 108 %.
2.7. Data analysis
All data were analyzed by GraphPad Prism (v8.4.3) for one-way ANOVA followed by Dunnett’s Multiple Comparison Test for comparison of the treated groups with controls (acceptable significance level set at p > 0.05). All data are shown as mean ± SEM in the figures for each study. NOELs were defined as the lowest dose without a significant effect and the lowest observed effects levels (LOELs) were defined as the lowest dose with a significant effect for each thyroid hormone.
3. Results
3.1. Weight and general toxicity
Oxyfluorfen exposure did not significantly affect final body weight or percent growth (weight gain over treatment divided by initial BW) following the 4-or 8-day treatment at any dose in the male or female juvenile rats (Supplemental Tables 1 and 2). In addition, no visible signs of toxicity were observed in any of the treated animals in this study based on daily observations before and during dosing.
3.2. Hormones
3.2.1. Four-day exposure
After 4 days of oral exposure to oxyfluorfen in the female rat, serum T4 was significantly suppressed in a dose-responsive manner by approximately 60 to 70 % from 125 to 500 mg/kg (Fig. 2A). Serum T3 was also significantly suppressed at 125 and 500 mg/kg in the female, but the 250 mg/kg group mean was not significantly suppressed when compared to the control mean (Fig. 2B).
Fig. 2.
Effects of a 4-Day exposure to oxyfluorfen on serum thyroid hormones and chemical analysis in the female juvenile rat. (A) Bars represent the mean (± SEM) serum T4 concentrations (left y-axis, ng/ml) (*P < 0.05) and the mean (± SEM) serum oxyfluorfen levels (solid line) for each treatment group (right y-axis, ng/ml) (n = 6). (B) Bars represent the mean (± SEM) serum T3 concentrations (ng/ml) for each treatment group (*P < 0.05; n = 6).
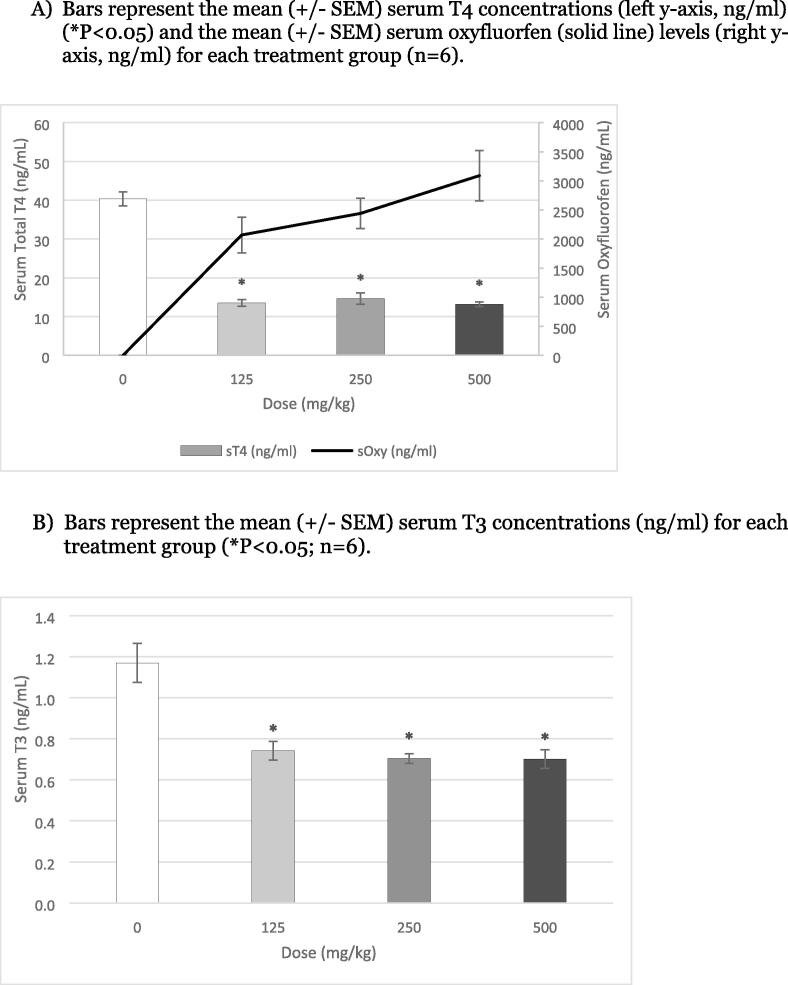
In the male rats, 4 days of exposure to oxyfluorfen resulted in a 70 % significant suppression of serum T4 at all doses (with a flat dose response across treatments) (Fig. 3A). In addition, serum T3 was significantly suppressed approximately 30 % following exposure to 125 to 500 mg/kg (Fig. 3B). The increased effect of oxyfluorfen on TH in the male rats was likely due to the higher observed concentrations in the serum and thyroid gland as compared to the female.
Fig. 3.
Effects of a 4-Day exposure to Oxyfluorfen on serum thyroid hormones in the male juvenile rat. A) Bars represent the mean (± SEM) serum T4 concentrations (left y-axis, ng/ml) (*P < 0.05) and the mean (± SEM) serum oxyfluorfen (solid line) levels (right y-axis, ng/ml) for each treatment group (n = 6). B) Bars represent the mean (± SEM) serum T3 concentrations (ng/ml) for each treatment group (*P < 0.05; n = 6).
3.2.2. Eight-day exposure
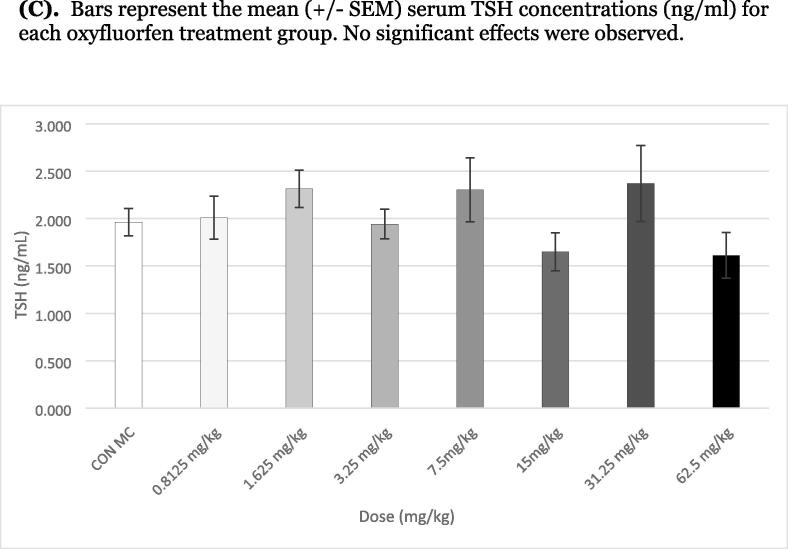
Serum T4 was significantly suppressed from 3.25 to 62.5 mg/kg in the male rats with a clear dose–response decrease of 30–80 % suppression (Fig. 4A). The NOEL for serum T4 was 1.625 mg/kg and the low effect level LOEL was 3.25 mg/kg based on this daily 8-Day oral exposure. Serum T3 was only significantly suppressed at 62.5 mg/kg, although a non-significant decrease was observed at both 15 and 31.25 mg/kg (Fig. 4B). There were no significant differences in thyroid weights (data not shown) or serum TSH between the controls and any of the treatment groups after 8 days of exposure to oxyfluorfen (Fig. 4C).
Fig. 4.
Effects of an 8-Day exposure to oxyfluorfen on serum thyroid hormones and chemical analysis (serum and thyroid gland) in the male juvenile rat. (A) Bars represent the mean (± SEM) (*P < 0.05) serum T4 concentrations (left y-axis, ng/ml) and mean (± SEM) serum oxyfluorfen (solid line) levels (right y-axis, ng/ml) for each treatment group. (B) Bars represent the mean (± SEM) (*P < 0.05) serum T3 concentrations (left y-axis, ng/ml) and thyroid gland oxyfluorfen (right y-axis, ng/gl) levels for each treatment group. (C). Bars represent the mean (± SEM) serum TSH concentrations (ng/ml) for each oxyfluorfen treatment group. No significant effects were observed.
3.3. Analytical chemistry of oxyfluorfen levels
Serum oxyfluorfen was detected in all treated serum samples in both the 4- and 8- days studies (Fig. 2 A, 3A and 4A) and appeared to correlate with the observed decrease in serum T4. There was a greater concentration of oxyfluorfen in the serum of males compared to the females following the same 4-day exposure to oxyfluorfen. In the female exposures (125 to 500 mg/kg), there was an increase of ∼800 to 1600 ng/ml of oxyfluorfen, while the same exposures in the male increased from ∼2200 to 3000 ng/ml two hours following the fourth exposure (Fig. 2A and 3A). In addition to the levels of chemical in the serum, the thyroids of the 8-day males were also evaluated for the concentration of oxyfluorfen in the gland. There was an increase in thyroid oxyfluorfen levels as compared to the serum levels (ng/g or ppm), with a sharp increase starting at 7.5 mg/kg and continuing to 62.5 mg/kg with a 4- to 5-fold increase (Fig. 4A and B). Based on in vitro potency of oxyfluorfen for NIS inhibition in the human and rat NIS iodine uptake assays, the IC50 of 2 μM is equivalent to 723.4 ng/ml. The effective dose levels for TH suppression (3.25 to 62.5 mg/kg) range from approximately 250 to 5300 ng/g of oxyfluorfen in the thyroid gland after 8 days of daily exposure to the herbicide. This demonstrates the accumulation of oxyfluorfen at the target site (thyroid gland) with sufficient concentrations for expected NIS inhibition based on in vitro potency.
4. Discussion
This study confirms that the diphenyl-ether herbicide oxyfluorfen, recently identified as a novel NIS inhibitor in both human and rat NIS high throughput in vitro RAIU assays, decreases circulating concentrations of serum thyroid hormones T4 and T3 following oral exposure in the juvenile rat. This is the first known report of an effect on serum thyroid hormones by this broad-spectrum herbicide. The suppression of serum THs was observed following both a 4- and 8-day oral exposure with a correlation between the suppression of T4 with the increasing doses and concentrations of oxyfluorfen in the serum and thyroid gland. There did appear to be a sex difference both in the magnitude of effect on serum THs and in the accumulation of oxyfluorfen in the serum, with greater effects in the male. For example, 125 mg/kg oxyfluorfen resulted in an approximate 55 % decrease in females as opposed to a 68 % decrease in the male serum T4. For serum T3, the males again displayed a greater effect, with a 35 % decrease in T3 at 125 mg/kg as compared to an 18 % decrease in the females. This higher serum oxyfluorfen in the male following a 4-day exposure suggests a clear difference in ADME (absorption, distribution, metabolism, and excretion) as compared to the females.
Although a feedback increase in the secretion of pituitary TSH was expected with marked reductions in serum T4, there were no significant changes in serum TSH following the 4- or 8-day exposure. Future investigations will address this feedback following a more extended exposure to oxyfluorfen in the rat.
Previously, an acute exposure to oxyfluorfen (4 or 320 mg/kg) in adult rats reported a peak of C14-labelled oxyfluorfen between 6 and 24 h with a half-life between 26 and 32 h (U.S. EPA, 2019), thus agreeing with the observed increased accumulation in this study. This same report also found that the lung, thyroid, liver, ovaries, kidneys, adrenals and fat had the highest levels of oxyfluorfen following acute exposure (U.S. EPA, 2019). In the current study thyroid gland oxyfluorfen concentrations are sufficient to inhibit NIS, with an in vitro IC50 concentration of 2 μM was shown to inhibit NIS by 50 % in FRTL5 cells (Buckalew et al., 2020) that is equivalent to 0.8 mg/g in the tissue.
The higher purity (>98 %) most currently registered oxyfluorfen has low acute oral, dermal, and inhalation toxicity (RED 2002; 2019) as compared to the older legacy studies that used 71 % or 85 % pure technical grade oxyfluorfen. The older oxyfluorfen studies found numerous adverse effects, including induced hepatic PPAR α (U.S. EPA, 2002, Butler et al., 1988 () anemia, and liver adenomas in mice. However, this less pure oxyfluorfen was discontinued in 1997 and replaced with the current 98 % pure technical grade which has not shown liver tumor incidence up to 73 mg/kg (U.S. EPA, 2021) and only weaker effects related to specific hepatic changes (Stagg et al., 2012).
All of the older guideline assessment studies (1978 to 1997) used the lower technical grade oxyfluorfen with a few that included measurement of thyroid weight, but most questioned toxicological implications due to study quality or lack of corresponding histopathology measures (U.S. EPA, 2019). A few of these studies did observe significant thyroid weight changes, including a chronic dog feed study with increased relative thyroid weight at the highest dose tested (∼125 mg/kg) (1981, MRID 00078767) and chronic rat oral study with decreased relative thyroid weight at the highest dose tested (∼60 mg/kg) (1990, MRID 00083445). Based on the lack of sufficient corresponding data using the lower purity chemical, the current study and additional future research using the 98 % oxyfluorfen in use today will be key to elucidate the mechanisms and biological implications of the suppression of serum thyroid hormones. Interestingly, a recent California human epidemiology study reported that oxyfluorfen showed an increased odds ratio with the occurrence of thyroid cancer (Omidakhsh et al., 2022) suggesting possible human implications of exposure.
Although we found that oxyfluorfen was a potent inhibitor of both human and rat NIS in our earlier in vitro RAIU assays, the current study cannot definitively rule out the involvement of other targets of thyroid disruption, including receptor, transport proteins or hepatic clearance of THs. Other ToxCast in vitro assays have tested the potential effects of oxyfluorfen on thyroid targets, including the thyroid peroxidase and deiodinase inhibition assays (Olker et al., 2019, Paul Friedman et al., 2016) with no reported thyroid activity. However, other assays indicate potential bioactivity across multiple in vitro assays including hepatic and endocrine targets (U.S. EPA, 2023) indicating the need for a closer evaluation of the effects of oxyfluorfen.
5. Conclusion
This study demonstrated that oxyfluorfen dose-dependently suppressed serum T4, with thyroid gland concentrations at or above the level that can inhibit NIS based on our earlier in vitro potency levels. This work also identified a lower NOEL and LOEL for in vivo effects following exposure to this herbicide. The current NOEL for T4 in the 8-day exposure is 1.625 mg/kg with a LOEL of 3.25 mg/kg. These effect levels are below the current guideline NOELs and LOELs (33 mg/kg) based on mouse and rabbit studies (U.S. EPA, 2021).
The confirmation of the effect of oxyfluorfen as an endocrine disrupting chemical in this study acknowledges that approaches for screening and prioritization for potential NIS inhibitors can be used to help identify candidate chemicals that may impact thyroid homeostasis in mammals. The hormone and chemical data from these in vivo studies and ADME information were used to develop quantitative IVIVE predictive models for extrapolation to humans (Decrane et al., 2023), providing information on oxyfluorfen and structurally related chemicals with the potential to inhibit NIS function. Future work investigating feedback effects on TSH and thyroid follicular hypertrophy following a longer exposure and additional mechanistic evaluations following oxyfluorfen exposure are certainly warranted.
Disclaimer
This paper was reviewed by the Center for Public Health and Environmental Assessment, Public Health and Integrated Toxicology Division, Office of Research and Development, U.S. Environmental Protection Agency for publication. Approval doesn’t necessarily indicate the views of the Agency, nor does mention of any trade names of commercial products constitute endorsement for use.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was conducted and funded by the U.S. EPA in the Neurological and Endocrine Toxicology Branch of the Center for Public Health and Environmental Assessment in the Office of Research and Development in Research Triangle Park, N.C. The authors thank Drs. Hisham El-Masri and Andrew Johnstone for their careful review of a previous draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2023.100146.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Brucker-Davis F. Effects of environmental synthetic chemicals on thyroid function. Thyroid. 1998;8(9):827–856. doi: 10.1089/thy.1998.8.827. [DOI] [PubMed] [Google Scholar]
- Buckalew A.R., Wang J., Murr A.S., Deisenroth C., Stewart W.M., Stoker T.E., Laws S.C. Evaluation of potential sodium-iodide symporter (NIS) inhibitors using a secondary Fischer rat thyroid follicular cell (FRTL-5) radioactive iodide uptake (RAIU) assay. Arch. Toxicol. 2020;94:873–885. doi: 10.1007/s00204-020-02664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler E.G., Tanaka T., Ichida T., Maruyama H., Leber A.P., Williams G.M. Induction of hepatic peroxisome proliferation in mice by lactofen, a diphenyl ether herbicide. Toxicology and applied pharmacology. 1988;93(1):72–80. doi: 10.1016/0041-008x(88)90026-9. [DOI] [PubMed] [Google Scholar]
- Camadro J.M., Matringe M., Thome F., Brouillet N., Mornet R., Labbe P. Photoaffinity Labeling of Protoporphyrinogen Oxidase, the Molecular Target of Diphenylether‐Type Herbicides. European journal of biochemistry. 1995;229(3):669–674. doi: 10.1111/j.1432-1033.1995.tb20512.x. [DOI] [PubMed] [Google Scholar]
- Capen C.C., Martin S.L. The effects of xenobiotics on the structure and function of thyroid follicular and C-cells. Toxicol. Pathol. 1989;17(2):266–293. doi: 10.1177/019262338901700205. [DOI] [PubMed] [Google Scholar]
- Carrasco N. Iodide transport in the thyroid gland. Biochim. Biophys. Acta (BBA)-Rev. Biomembranes. 1993;1154(1):65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- Decrane, R., Stoker, T., Murr, A., Ford, J. and El-Masri, H., 2023. Cross species extrapolation of the disruption of thyroid hormone synthesis by oxyfluorfen using in vitro data, physiologically based pharmacokinetic (PBPK), and thyroid hormone kinetics models. Curr. Res. Toxicol., p.100138. https://doi.org/10.1016/j.crtox.2023.100138. [DOI] [PMC free article] [PubMed]
- Eskandari S., Loo D.D., Dai G., Levy O., Wright E.M., Carrasco N. Thyroid Na+/I− symporter: mechanism, stoichiometry, and specificity. J. Biol. Chem. 1997;272(43):27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- Gilbert M.E., Hedge J.M., Valentín-Blasini L., Blount B.C., Kannan K., Tietge J., Zoeller R.T., Crofton K.M., Jarrett J.M., Fisher J.W. An animal model of marginal iodine deficiency during development: the thyroid axis and neurodevelopmental outcome. Toxicol. Sci. 2013;132(1):177–195. doi: 10.1093/toxsci/kfs335. [DOI] [PubMed] [Google Scholar]
- Gilbert M.E., O’Shaughnessy K.L., Axelstad M. Regulation of thyroid-disrupting chemicals to protect the developing brain. Endocrinology. 2020;161(10) doi: 10.1210/endocr/bqaa106. bqaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J.M., Cooper R.L., Rehnberg G.L., Hein J.F., McElroy W.K., Gray L.E., Jr Effects of low subchronic doses of methoxychlor on the rat hypothalamic-pituitary reproductive axis. Toxicol. Appl. Pharmacol. 1986;86(3):474–483. doi: 10.1016/0041-008X(86)90375-3. [DOI] [PubMed] [Google Scholar]
- Greenwood C., Hunter W.M., Glover J.S. The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochem. J. 1963;89(1):114. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallinger D.R., Murr A.S., Buckalew A.R., Simmons S.O., Stoker T.E., Laws S.C. Development of a screening approach to detect thyroid disrupting chemicals that inhibit the human sodium iodide symporter (NIS) Toxicol. in Vitro. 2017;40:66–78. doi: 10.1016/j.tiv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Hassan I., El-Masri H., Kosian P.A., Ford J., Degitz S.J., Gilbert M.E. Neurodevelopment and thyroid hormone synthesis inhibition in the rat: quantitative understanding within the adverse outcome pathway framework. Toxicol Sci. 2017;160(1):57–73. doi: 10.1093/toxsci/kfx163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung M.W., Kosian P.A., Haselman J.T., Korte J.J., Challis K., Macherla C., Nevalainen E., Degitz S.J. vitro, ex vivo, and in vivo determination of thyroid hormone modulating activity of benzothiazoles. Toxicological sciences. 2015;146(2):254–264. doi: 10.1093/toxsci/kfv090. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G., Obregón M.J., Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J. Clin. Endocrinol. Metab. 2000;85(11):3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- Noyes P.D., Friedman K.P., Browne P., Haselman J.T., Gilbert M.E., Hornung M.W., Barone S., Jr, Crofton K.M., Laws S.C., Stoker T.E., Simmons S.O. Evaluating chemicals for thyroid disruption: opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ. Health Perspect. 2019;127(9):95001. doi: 10.1289/EHP5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olker J.H., Korte J.J., Denny J.S., Hartig P.C., Cardon M.C., Knutsen C.N., Kent P.M., Christensen J.P., Degitz S.J., Hornung M.W. Screening the ToxCast phase 1, phase 2, and e1k chemical libraries for inhibitors of iodothyronine deiodinases. Toxicol. Sci. 2019;168(2):430–442. doi: 10.1093/toxsci/kfy302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidakhsh N., Heck J.E., Cockburn M., Ling C., Hershman J.M., Harari A. Thyroid cancer and pesticide use in a central California agricultural area: a case control study. J. Clin. Endocrinol. Metab. 2022;107(9):e3574–e3582. doi: 10.1210/clinem/dgac413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul Friedman K., Watt E.D., Hornung M.W., Hedge J.M., Judson R.S., Crofton K.M., Houck K.A., Simmons S.O. Tiered high-throughput screening approach to identify thyroperoxidase inhibitors within the ToxCast phase I and II chemical libraries. Toxicol. Sci. 2016;151(1):160–180. doi: 10.1093/toxsci/kfw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg N.J., LeBaron M.J., Eisenbrandt D.L., Gollapudi B.B., Klaunig J.E. Assessment of possible carcinogenicity of oxyfluorfen to humans using mode of action analysis of rodent liver effects. Toxicol. Sci. 2012;128(2):334–345. doi: 10.1093/toxsci/kfs157. [DOI] [PubMed] [Google Scholar]
- Stoker T.E., Ferrell J.M., Laws S.C., Cooper R.L., Buckalew A. Evaluation of ammonium perchlorate in the endocrine disruptor screening and testing program's male pubertal protocol: ability to detect effects on thyroid endpoints. Toxicology. 2006;228(1):58–65. doi: 10.1016/j.tox.2006.08.026. [DOI] [PubMed] [Google Scholar]
- U.S. EPA, 2002. Oxyfluorfen: Human health risk assessment. Health Effects Division (HED) chapter for the Reregistration Eligibility Decision (RED) document. Reregistration Case No. 2490. C.F.R. §111.601. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-111601_1-Aug-02.pdf.
- U.S. EPA, 2021. Oxyfluorfen: Addendum to the 2019 Draft Human Health Risk Assessment for Registration Review (D445742) D458758, 40 C.F.R. §180.381.https://www.regulations.gov/document/EPA-HQ-OPP-2014-0778-0067.
- U.S. EPA, 2019. Oxyfluorfen: Draft Human Health Risk Assessment for Registration Review. 40 C.F.R.§180.381. https://www.regulations.gov/document/EPA-HQ-OPP-2014-0778-0025.
- U.S. EPA. , 2023. ToxCast Summary Files from invitrodb_v2.3.03. Retrieved from https://comptox.epa.gov/dashboard/chemical. Assessed in November, 2023.
- Wang J., Hallinger D.R., Murr A.S., Buckalew A.R., Simmons S.O., Laws S.C., Stoker T.E. High-throughput screening and quantitative chemical ranking for sodium iodide symporter (NIS) inhibitors in ToxCast Phase I chemical library. Enviro. Sci. Technol. 2018;52:5417–5426. doi: 10.1021/acs.est.7b06145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Hallinger D.R., Murr A.S., Buckalew A.R., Lougee R.R., Richard A.M., Laws S.C., Stoker T.E. High-throughput screening and chemotype-enrichment analysis of ToxCast phase II chemicals evaluated for human sodium-iodide symporter (NIS) inhibition. Environ. Int. 2019;126:377–386. doi: 10.1016/j.envint.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Richard A.M., Murr A.S., Buckalew A.R., Lougee R.R., Shobair M., Hallinger D.R., Laws S.C., Stoker T.E. Expanded high-throughput screening and chemotype-enrichment analysis of the phase II: e1k ToxCast library for human sodium-iodide symporter (NIS) inhibition. Arch. Toxicol. 2021;95:1723–1737. doi: 10.1007/s00204-021-03006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacol. Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- Zoeller R.T., Crofton K.M. Mode of action: developmental thyroid hormone insufficiency—neurological abnormalities resulting from exposure to propylthiouracil. Crit. Rev. Toxicol. 2005;35(8–9):771–781. doi: 10.1080/10408440591007313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.