Abstract
Background
Structural disorders of hemoglobin are a group of rare and fatal genetic diseases that disrupt the transport and exchange of oxygen in the blood, causing tissue damage and ultimately leading to chronic conditions. The hemoglobin (Hb) S variant predominantly impacts individuals of Afro-descendant heritage. A significant concentration of the Afro-descendant population in Colombia, notably 12.5 %, is found in the city of Cali. Previous research has identified this city's structural hemoglobin disorders prevalence rate of 3.78 %. The aim of this study was to determine the prevalence of HbC, HbS, HbF, and HbA2 variants within a population who underwent HbA1c testing, as well as the prevalence of chronic diseases among patients with these hemoglobin alterations, at a high-complexity hospital in the city of Cali from 2015 to 2019.
Methods
A descriptive observational study was conducted, involving a study population that comprised patients with both suspected and monitored diagnoses of diabetes. The cohort was selected from a high-complexity hospital in Cali. A total of 15,608 patients were included in the analysis, all of whom underwent HbA1C measurement through capillary electrophoresis, which also offers an indirect diagnosis of certain structural disorders of hemoglobin. Bayesian methods were employed for frequency analysis.
Results
Among the 15,608 patients assessed, 63.6 % (n = 9920) were women. The overall prevalence of structural hemoglobin disorders was 1.98 % (n = 287, 95 % CI = 1.77 %–2.21 %). The co-occurrence of diabetes and kidney disease emerged as the most prevalent combination of pathologies observed in individuals with HbC, for both men and women across various age groups: 18–42 (58.3 % and 50.0 % respectively), 43–55 (50.0 % for both), 56–65 (50.0 % and 37.5 % respectively), and >65 years (66.7 % and 57.1 % respectively).
Conclusions
The observed prevalence of the studied variants exceeded 1 %, a threshold underscored by the World Health Organization (WHO) as epidemiologically significant. Among HbC and HbS-positive patients, the elevated prevalence of diabetes and kidney disease is a guiding factor in developing proactive prevention strategies.
Keywords: HbC, HbS, HbF, HbA2, Chronic disease, Bayesian analysis, Frequency, Prevalence
1. Introduction
Hemoglobin (Hb) is a pivotal protein for oxygen transport, encoded on human chromosomes 11 and 16. In adults, consistent concentrations of three Hb types (HbA, HbA2, and HbF) exist [1]. Genetic disorders can lead to defective hemoglobin, causing structural changes in the hemoglobin molecule (structural disorders of hemoglobin) or production amount changes (thalassemias, a subset of hemoglobin variants) [2].
HbS, HbE, and HbC are the main structural hemoglobin variants, whereas α- and β-thalassemia are the main types of thalassemia. HbS prompts sickle cell disease. These deviations result in oxygen transport deficits, infarction-like tissue damage, and severe conditions such as pulmonary hypertension, heart failure, kidney damage, and diabetes, ultimately culminating in mortality [3].
Structural disorders of hemoglobin were declared the most common genetic diseases [3]. The World Health Organization (WHO) states that any hemoglobin variant with a frequency greater than 1 % is considered regionally endemic [4,5]. By 2012, projections indicated that approximately 330,000 newborns were carriers of structural hemoglobin variants globally, with 275,000 being carriers of HbS [6], and that this number was set to rise to 400,000 annually by 2050 [7]. These disorders claim the lives of 75 % of rural children before reaching the age of 5.5, with two-thirds of cases occurring in Africa [8].
Sickle cell anemia is widespread in regions such as sub-Saharan Africa, the Mediterranean, the Middle East, and the Indian subcontinent. The independent emergence of the sickle cell gene in Africa and other regions is remarkable, potentially occurring multiple times due to gene conversion events. Hemoglobin sickle cell disease prevails in western and northern Africa, while HbS β thalassemia is found in parts of sub-Saharan Africa, the Middle East, and the Indian subcontinent [6].
Thalassemic patients can also be encountered in the Mediterranean region, where 4 distinct hemoglobin disorder subgroups prevail including beta thalassemia major (TM), beta thalassemia intermedia (TI), sickle cell disease (SCD) and hemoglobin H disease (alpha thalassemia). Their clinical spectrum ranges from mild to severe, responding to the complex interactions between genes and environmental factors [9].
Milder forms of α thalassemia exist in tropical regions spanning sub-Saharan Africa, the Mediterranean, the Middle East, and Asia. Despite their prevalence in these areas, structural hemoglobin variants have spread worldwide through population migrations, with the sickle cell gene appearing in the Caribbean Islands, North America, and other countries [6].
In developed countries, 99 % of newborn hemoglobin variant carriers survive, yet most patients die before age 25 [10], with 42 % dying before age 15 due to treatment failures, leading to a life expectancy that barely exceeds 55 years [11]. To address these challenges, countries like the United States, the Netherlands, France, Germany, and India have implemented genetic counseling and neonatal screening for various hemoglobin variants including HbF, HbE, HbD, and HbC [12]. These programs intend to reduce healthcare costs, expand vaccinations, improve primary care, and offer prophylactic antibiotics [13,14].
In Colombia, the population mainly comprises three ethnic groups: Caucasians, Blacks, and Asians [5,6]. Structural disorders of hemoglobin have been included in the National Information System for Patients with Orphan Diseases since 2012. Mandatory reporting of prevalence started in 2016 in the National System of Public Health Surveillance (Sistema Nacional de Vigilancia en Salud Pública), along with its inclusion in the high-cost account [8].
A neonatal screening program began in 2019, covering structural hemoglobin disorders and tests for congenital hypothyroidism, phenylketonuria, galactosemia, cystic fibrosis, congenital adrenal hyperplasia, and biotinidase deficiency. The goal was to enhance education, promotion, prevention, and genetic counseling networks, reducing births of carrier children and related treatment expenses. Prevalence studies are limited, with estimated low event reporting, with 224 cases reported in 2016–2019 [[14], [15], [16], [17]].
The department of Valle del Cauca has 1.6 % of Colombia's indigenous and 25.3 % of its African-descended population. Cali, its capital, has 12.5 % of Colombia's African-descended population, with 26.2 % born there [18], and 55 % residing in urban areas [19]. A 2004 neonatal study in Cali found a structural hemoglobin variant prevalence of 3.78 % (4), including carriers of HbS, HbAC, and HbAD [5,20]. A 2013 publication noted that 98.1 % of mothers of affected children were unaware of this condition [20].
Given Cali's large African-descendant population [19], this study assessed structural hemoglobin variants prevalence in a health institution using an indirect diagnosis. Data from 2015 to 2019, including glycated hemoglobin tests via capillary electrophoresis on suspected diabetes cases and from follow-up patients for this disease, were analyzed. The prevalence of specific structural hemoglobin variants was estimated using Bayesian inference, and findings were stratified by age and type of variants, which included HbS, HbC, changes at HbF, and changes at HbA2. The association with comorbidities like diabetes, heart disease, kidney issues, and hypertension was explored.
2. Material and methods
An observational study was conducted to determine the prevalence of structural hemoglobin variants in patients from a hospital in Cali, Colombia, using an indirect diagnosis obtained by the HbA1C test via capillary electrophoresis on suspected diabetes cases or under follow-up for this pathology. This test is confined to detecting the existence or absence of alterations in structural hemoglobin variants; nevertheless, it does not possess the capacity to identify other hemoglobin disorders, mutations in alleles, or individuals with distinct traits.
Patient age was grouped into quartiles for frequency analysis. Within each study group (age, sex, HbS, HbC, HbF, HbA2 variants), 16 pathologies were analyzed using frequentist methods. Noncommunicable chronic disease frequency was determined using Bayesian methods, considering age and sex, assuming a priori conjugate distribution.
3. Data
Data was obtained from a clinical laboratory's HbA1c test by electrophoresis database. Initially, 61,941 records were identified between 2015 and 2019. Among these, there were 15,608 unique records of HbA1c tested using capillary electrophoresis. Of these, only 287 subjects met the inclusion criteria, being residents of Cali over 18 years of age, tested between April 1, 2015, and April 30, 2019, without medullary pathologies [21]. The records included information about the type of structural hemoglobin variant (HbC, HbS, altered HbA2, altered HbF) and the presence of pathologies including diabetes, heart disease, kidney disease, and hypertension.
4. Statistical analysis
For each of the groups obtained from the combinations of the categories of sex, age, and HbC, HbS, HbF, or HbA2, the prevalence was estimated from Bayes's formula. The prior distribution was assumed as a due to non-specialist information; therefore, a prevalence value between 0 and 1 was assumed, with the same probability for all values. The posterior distribution obtained was a , where refers to the number of patients with HbC, HbS, HbF or HbA2 inside of group r; and is the number of patients in the same group. Chains of 10,000 random values were simulated for each distribution, using an absolute loss function, estimating the prevalence by group, and its 95 % credibility region (2.5th and 97.5th percentiles).
Prevalence for 16 groups with four base pathologies (diabetes, kidney disease, heart disease, and hypertension) and their combinations was estimated. For the 287 patients with structural hemoglobin variants, data was obtained only in 21 groups (of 32 possible) with a small sample size. Prevalence was estimated similarly to general prevalence but with informative prior distributions. Bayesian empirical methods [22] and Tovar's approach [18] were employed to establish prior distribution parameters.
5. Results
A total of 15,608 records were considered and analyzed during the study period, of which 63.56 % (n = 9920) were women (Table 1). The search for patients with altered HbC, HbS, HbF, or HbA2 yielded 287 positive cases that met the inclusion criteria (Supplementary Table 1). These patients represented a prevalence of 1.98 % (95 % CI = 1.77 %–2.21 %) for any of the structural variants of hemoglobin found. The prevalence in women was 1.68 % (95 % CI = 1.43 %–1.94 %), while in men, it was 2.51 % (95 % CI = 2.11 %–2.92 %). The most frequent variant was HbS, accounting for 73.2 % of women and 62.7 % of men. Conversely, men exhibited a higher frequency of HbC at 34.3 %, as opposed to women with a frequency of 26.1 %. Regarding the grouping by age, Table 1 shows that the highest prevalence for all types of variants occurred in the subgroup of men older than 65 years (2.93 %, 95 % CI = 1.79 %–2.80 %). The highest prevalence in women occurred between 43 and 55 years (2.14 %, 95 % CI: [1.54–2.74]).
Table 1.
Frequency of hemoglobin variants by age and sex.
|
Age group |
Women |
Men |
||
|---|---|---|---|---|
| n (%) | Frequency of hemoglobin variant [95 % CI] | n (%) | Frequency of hemoglobin variant [95 % CI] | |
| 18–42 | 2694 (27,16) | 1,60 [1,12–2,07] | 1424 (25,00) | 2,25 [1,48–3,02] |
| 43–55 | 2241 (22,59) | 2,14 [1,54–2,74] | 1445 (25,40) | 2,35 [1,57–3,13] |
| 56–65 | 2459 (24,79) | 1,50 [1,02–1,99] | 1623 (28,50) | 2,59 [1,82–3,36] |
| >65 | 2526 (25,46) | 1,54 [1,06–2,02] | 1196 (21,00) | 2,93 [1,97–3,88] |
| Total | 9920 | 1,68 [1,43–1,94] | 5688 | 2,51 [2,11–2,92] |
When considering the relationships between structural hemoglobin variants, age, sex, and comorbidity, diabetes appeared across all age-gender groups. Both sexes displayed combinations of diabetes with kidney disease and diabetes with heart disease. These combinations were observed across a broader age range for females, reaching 80 years, compared to 70 years in males. The combination of diabetes, hypertension, and kidney disease consistently appeared among women of all ages, with a median age of 35. In men, it emerged after age 45, with a median age of 55.
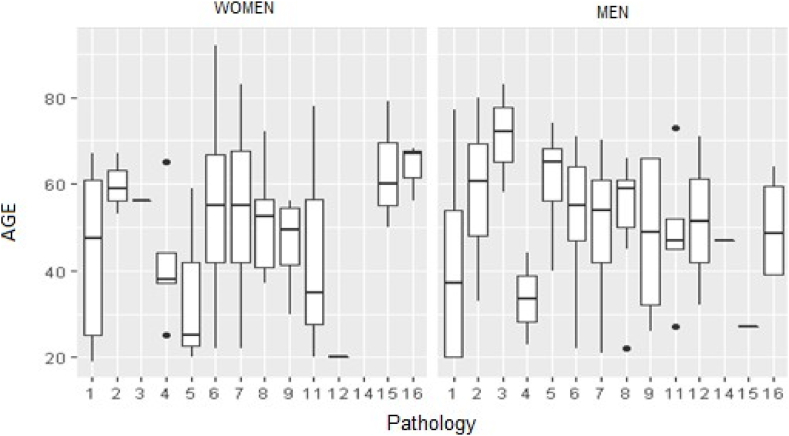
From the frequency analysis of the 16 pathologies within each study group (age, sex, structural hemoglobin variant), the following observations were made: Among men, the most common combination was diabetes with kidney disease (>50 % across all ages). HbC exhibited a higher prevalence in combination with diabetes and kidney disease in men, whereas HbS prevailed except within the 18–42 age group. Approximately 3.85 % of men between ages 43 and 55 exhibited comorbidities related to four structural hemoglobin variants. Only two men with diabetes plus kidney disease showed changes in fetal hemoglobin concentration. Similarly, diabetes with kidney disease and diabetes with heart disease were common comorbidities in women. However, the lowest prevalence was 37.5 % for women with HbC aged 56–65 (Fig. 1).
Fig. 1.
Age by pathology and sex in patients with hemoglobin variants
Pathology: 1: diabetes; 2: hypertension; 3: kidney disease; 4: heart disease; 5: diabetes and hypertension; 6: diabetes and kidney disease; 7: diabetes and heart disease; 8: hypertension and kidney disease; 9: hypertension and heart disease; 10: kidney disease and heart disease; 11: diabetes, hypertension, and kidney disease; 12: diabetes, hypertension, and heart disease; 13: diabetes, kidney disease, and heart disease; 14: hypertension, kidney disease, and heart disease; 15: diabetes, hypertension, kidney disease, and heart disease; 16: none of the four pathologies.
Table 2, Table 3 present the Bayesian estimation results for the 16 pathologies within each study group. These tables provide expected frequencies for each pathology within the respective groups. For instance, the probability of finding diabetes and kidney disease in HbC-carrying men aged 18–42 was 57.39 % (95 % CI = 37.53 %–76.68 %) (Table 2). Furthermore, Table 2, Table 3 demonstrate that within population 1, which corresponds to diabetes, the highest prevalence of HbS occurred in women older than 65 years, with a prevalence of 7.79 % (95 % CI = 1.97 %–16.46 %).
Table 2.
Bayesian estimation of the frequencies of pathologies by age group and type of hemoglobin variant in male patients.
| Age | Hba | P1 (95 % CR) | P2 (95 % CR) | P3 (95 % CR) | P4 (95 % CR) | P5 (95 % CR) | P6 (95 % CR) | P7 (95 % CR) | P8 (95 % CR) |
|---|---|---|---|---|---|---|---|---|---|
| 18–42 | HbC | 6.12 (0.09–17.78) | 12.45 (1.7–27.03) | 0 (0–0.02) | 0 (0–0.01) | 4.06 (0–14.46) | 57.39 (37.53–76.68) | 2.67 (0–11.79) | 0.65 (0–7.32) |
| 18–42 | HbS | 0.68 (0–5.64) | 16.37 (5.79–29.62) | 6.62 (0.36–16.24) | 0.3 (0–4.38) | 4.52 (0.1–13.07) | 22.03 (9.51–36.74) | 22.53 (9.29–37.43) | 5.17 (0.23–14.52) |
| 18–42 | HbF | 0 (0–0.02) | 0 (0–0.03) | 0 (0–0.02) | 0 (0–0.03) | 0 (0–0.04) | 100 (99.97–100) | 0 (0–0.02) | 0 (0–0.02) |
| 43–55 | HbC | 8.43 (0.13–23.84) | 10.76 (0.41–27.51) | 0 (0–0.02) | 0 (0–0.01) | 0.21 (0–6.79) | 54.16 (31.69–76.64) | 3.69 (0–16.18) | 0.85 (0–9.72) |
| 43–55 | HbS | 5.22 (0.41–13.07) | 5.61 (0.49–13.47) | 0.49 (0–4.33) | 2.44 (0.01–8.54) | 3.62 (0.04–10.32) | 29.32 (16.03–43.08) | 25.11 (13.09–38.79) | 4.05 (0.07–11.03) |
| 56–65 | HbC | 1.47 (0–8.51) | 6.66 (0.33–17.74) | 0 (0–0.01) | 0 (0–0.01) | 0.13 (0–4.02) | 52.69 (34.07–70.43) | 17.43 (5.5–33.18) | 4.22 (0.01–13.66) |
| 56–65 | HbS | 3.06 (0.02–10.01) | 11.51 (3.22–22.7) | 0.53 (0–4.94) | 2.7 (0–9.28) | 1.36 (0–6.85) | 16.32 (6.16–28.9) | 14.33 (4.66–25.99) | 4.49 (0.08–12.05) |
| 56–65 | HgA2 | 0 (0–0.02) | 0 (0–0.03) | 0 (0 - <0.001) | 0 (0–0.02) | 0 (0–0.03) | 0 (0–0.02) | 100 (100–100) | 0 (0–0.02) |
| >65 | HbC | 3.18 (0–17.44) | 5.75 (0–21.26) | 0 (0–0.01) | 0 (0–0.01) | 0.27 (0–8.87) | 58.45 (38.66–76.7) | 12.13 (0.24–31.1) | 8.91 (0.03–27.63) |
| >65 | HbS | 1.11 (0–9.27) | 6.17 (0.06–18) | 1.05 (0–9.28) | 0.51 (0–7.38) | 12.39 (1.87–28.33) | 48.17 (30.61–65.22) | 36.43 (19.22–55.07) | 12.95 (1.69–28.36) |
| >65 | HbF | 0 (0–0.27) | 0 (0–0.37) | 0 (0–0.3) | 0 (0–0.56) | 0 (0–0.3) | 53.03 (31.56–74.33) | 0 (0–0.31) | 0 (0–0.23) |
| >65 | HgA2 | 0 (0–0.31) | 0 (0–0.34) | 100 (100–100) | 0 (0–0.21) | 0 (0–0.31) | 0 (0–0.31) | 0 (0 - <0.001) | 0 (0–0.39) |
| Age | Hba | P9 (95 % CR) | P10 (95 % CR) | P11 (95 % CR) | P12 (95 % CR) | P13 (95 % CR) | P14 (95 % CR) | P15 (95 % CR) | P16 (95 % CR) |
|---|---|---|---|---|---|---|---|---|---|
| 18–42 | HbC | 0 (0–0.01) | 0 (0–0.01) | 0.45 (0–6.56) | 6.05 (0.12–17.77) | 0 (0–0.01) | 0 (0–0.01) | 0 (0–0.01) | 0.88 (0–7.94) |
| 18–42 | HbS | 8.16 (1.06–18.77) | 0 (0–0.01) | 3.86 (0.01–12.01) | 0 (0–0.01) | 0 (0–0.01) | 0.04 (0–2.85) | 0.02 (0–2.39) | 0.24 (0–4.09) |
| 18–42 | HbF | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.03) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) |
| 43–55 | HbC | 0 (0–0.01) | 0 (0–0.01) | 0.61 (0–8.75) | 8.3 (0.1–23.29) | 0 (0–0.01) | 0 (0–0.01) | 0 (0–0.02) | 1.17 (0–10.62) |
| 43–55 | HbS | 6.46 (0.88–14.83) | 0 (0–0.01) | 3.07 (0.01–9.33) | 0 (0–0.01) | 0 (0–0.01) | 0.03 (0–2.22) | 2.02 (0–7.61) | 4.77 (0.36–12.23) |
| 56–65 | HbC | 0 (0–0.01) | 0 (0–0.01) | 7.67 (0.47–19.45) | 1.47 (0–8.67) | 0 (0–0.01) | 0 (0–0.01) | 0 (0–0.01) | 0.74 (0–6.69) |
| 56–65 | HbS | 4.44 (0.26–12.22) | 0 (0–0.01) | 3.34 (0.03–10.37) | 0 (0 - <0.001) | 0 (0 - <0.001) | 2.31 (0–8.56) | 0.02 (0–2.27) | 0.21 (0–3.72) |
| 56–65 | HgA2 | 0 (0–0.02) | 0 (0–0.03) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) |
| >65 | HbC | 0 (0–0.02) | 0 (0–0.01) | 0.83 (0–11.86) | 3.12 (0–17.26) | 0 (0–0.02) | 0 (0–0.01) | 0 (0–0.02) | 17.4 (1.87–39.48) |
| >65 | HbS | 3.34 (0–13.73) | 0 (0–0.01) | 1.53 (0–10.55) | 0 (0–0.01) | 0 (0–0.01) | 0.08 (0–5.02) | 0.04 (0–4.13) | 0.42 (0–6.94) |
| >65 | HbF | 0 (0–0.27) | 0 (0–0.38) | 0 (0–0.45) | 0 (0–0.34) | 0 (0–0.38) | 0 (0–0.33) | 0 (0–0.5) | 0 (0–0.37) |
| >65 | HgA2 | 0 (0–0.25) | 0 (0–0.4) | 0 (0–0.3) | 0 (0–0.28) | 0 (0–0.38) | 0 (0–0.43) | 0 (0–0.25) | 0 (0–0.46) |
Hb a: hemoglobin.
Table 3.
Bayesian estimation of the frequencies of pathologies by age group and type of hemoglobin variant in female patients.
| Age | Hba | P1 (95 % CR) | P2 (95 % CR) | P3 (95 % CR) | P4 (95 % CR) | P5 (95 % CR) | P6 (95 % CR) | P7 (95 % CR) | P8 (95 % CR) |
|---|---|---|---|---|---|---|---|---|---|
| 18–42 | HbC | 1.42 (0–12.27) | 7.68 (0.02–23.78) | 0 (0–0.01) | 0.49 (0–9.14) | 0 (0–0.01) | 48.15 (24.12–72.22) | 13.35 (1.25–32.34) | 2.2 (0–14.02) |
| 18–42 | HbS | 4.52 (0.54–10.52) | 0.25 (0–2.81) | 0.27 (0–2.9) | 0.63 (0–3.82) | 0.84 (0–4.38) | 27.39 (16.67–39.04) | 23.8 (13.74–34.86) | 4.79 (0.56–10.81) |
| 43–55 | HbC | 13.05 (1.22–30.63) | 1.05 (0–10.14) | 0 (0–0.01) | 6.07 (0–20.31) | 0 (0–0.01) | 42.01 (19.87–64.81) | 18.2 (3.5–37.37) | 7.96 (0.06–22.86) |
| 43–55 | HbS | 5.6 (1.26–11.8) | 0.23 (0–2.52) | 0.23 (0–2.53) | 3.54 (0.36–8.73) | 3.76 (0.41–8.9) | 24.47 (14.92–35.38) | 21.22 (12.57–31.8) | 4.3 (0.63–9.94) |
| 56–65 | HbC | 1.42 (0–12.26) | 7.74 (0.01–24.39) | 0 (0–0.01) | 0.53 (0–9.16) | 0 (0–0.01) | 41.37 (17.73–65.47) | 20.55 (3.76–42.06) | 15.95 (1.64–36.17) |
| 56–65 | HbS | 6.61 (1.55–13.77) | 0.26 (0–3.06) | 0.26 (0–3.01) | 2.44 (0.01–7.24) | 0.91 (0–4.62) | 28.93 (17–40.62) | 17.84 (8.98–28.69) | 3.26 (0.12–8.78) |
| >65 | HbC | 5.65 (0.01–17.81) | 0.84 (0–8.11) | 0 (0–0.01) | 4.9 (0.01–16.58) | 0 (0–0.01) | 53.43 (32.51–73.16) | 14.38 (2.86–30.91) | 1.56 (0–10.04) |
| >65 | HbS | 7.79 (1.97–16.46) | 2.36 (0–7.81) | 2.32 (0.01–7.82) | 0.8 (0–5.04) | 3.19 (0.03–9.21) | 51.82 (38.06–65.79) | 21.27 (10.82–33.72) | 1.79 (0–6.95) |
| >65 | HgA2 | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) |
| Age | Hba | P1 (95 % CR) | P2 (95 % CR) | P3 (95 % CR) | P4 (95 % CR) | P5 (95 % CR) | P6 (95 % CR) | P7 (95 % CR) | P8 (95 % CR) |
|---|---|---|---|---|---|---|---|---|---|
| 18–42 | HbC | 1.42 (0–12.27) | 7.68 (0.02–23.78) | 0 (0–0.01) | 0.49 (0–9.14) | 0 (0–0.01) | 48.15 (24.12–72.22) | 13.35 (1.25–32.34) | 2.2 (0–14.02) |
| 18–42 | HbS | 4.52 (0.54–10.52) | 0.25 (0–2.81) | 0.27 (0–2.9) | 0.63 (0–3.82) | 0.84 (0–4.38) | 27.39 (16.67–39.04) | 23.8 (13.74–34.86) | 4.79 (0.56–10.81) |
| 43–55 | HbC | 13.05 (1.22–30.63) | 1.05 (0–10.14) | 0 (0–0.01) | 6.07 (0–20.31) | 0 (0–0.01) | 42.01 (19.87–64.81) | 18.2 (3.5–37.37) | 7.96 (0.06–22.86) |
| 43–55 | HbS | 5.6 (1.26–11.8) | 0.23 (0–2.52) | 0.23 (0–2.53) | 3.54 (0.36–8.73) | 3.76 (0.41–8.9) | 24.47 (14.92–35.38) | 21.22 (12.57–31.8) | 4.3 (0.63–9.94) |
| 56–65 | HbC | 1.42 (0–12.26) | 7.74 (0.01–24.39) | 0 (0–0.01) | 0.53 (0–9.16) | 0 (0–0.01) | 41.37 (17.73–65.47) | 20.55 (3.76–42.06) | 15.95 (1.64–36.17) |
| 56–65 | HbS | 6.61 (1.55–13.77) | 0.26 (0–3.06) | 0.26 (0–3.01) | 2.44 (0.01–7.24) | 0.91 (0–4.62) | 28.93 (17–40.62) | 17.84 (8.98–28.69) | 3.26 (0.12–8.78) |
| >65 | HbC | 5.65 (0.01–17.81) | 0.84 (0–8.11) | 0 (0–0.01) | 4.9 (0.01–16.58) | 0 (0–0.01) | 53.43 (32.51–73.16) | 14.38 (2.86–30.91) | 1.56 (0–10.04) |
| >65 | HbS | 7.79 (1.97–16.46) | 2.36 (0–7.81) | 2.32 (0.01–7.82) | 0.8 (0–5.04) | 3.19 (0.03–9.21) | 51.82 (38.06–65.79) | 21.27 (10.82–33.72) | 1.79 (0–6.95) |
| >65 | HgA2 | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) | 0 (0–0.02) |
Hb a: hemoglobin.
6. Discussion
A prevalence of 1.98 % (95 % CI: 1.77 %–2.21 %) for the considered variants in this selected sample was found, emphasizing the need for broader prevalence studies using randomized screening in the city's general population. The sample was composed of patients with glucose metabolism alterations, who underwent glycated hemoglobin tests performed using capillary electrophoresis. This facilitated the identification and enrollment of patients with certain hemoglobin variants. Our findings highlight a significant public health concern for the region, in line with the WHO criteria that define endemism as having a prevalence of over 1 % [5].
The results indicate a higher prevalence of hemoglobin variant expression in men, regardless of age, which challenges Mendelian inheritance expectations [23]. However, it should be noted that random sampling was not employed, and these results depend on the characteristics of this specific population, which could explain the discrepancy with expected outcomes.
To draw scientifically robust conclusions, larger-scale studies controlling for epidemiological variables are vital. Satizabal's study [4] on Cali's structural disorders of hemoglobin prevalence showed HbC as the most common variant, followed by HbS. However, our study revealed HbS as the most frequent variant in both men and women, due to the city's ethnic composition, being more common in women than in men. HbC, on the other hand, was more prevalent in men.
Analyzing the data matrix for pathology frequency by age, sex, and type of hemoglobin variant, combinations of diabetes plus kidney disease and diabetes plus heart disease were prevalent. This is consistent with the used database, as diabetes is a common comorbidity due to metabolic conditions, environmental factors, and genetic convergence with structural hemoglobin disorders [24]. Both heart and kidney diseases, within diabetes and normal hemoglobin contexts, relate to permanent oxygen transport deficiency in this condition [25].
7. Strengths
This study represents the first investigation conducted in Cali, among adults, that addresses the prevalence of chronic diseases in patients with structural hemoglobin disorders. The methodological rigor of the Bayesian approach enables the establishment of valid conclusions for this population, owing to its analytical nature. Despite being focused on a specific population, this study will provide compelling grounds to advocate for broader prevalence studies in the general Cali population.
8. Limitations
This study collected information from a database in a single healthcare institution; therefore, it is not possible to generalize the findings to the entire city. The study focused on a specific population of individuals suspected of having diabetes or undergoing treatment. As a result, it is not possible to ensure external validity and the extrapolation of the results obtained to patients without alterations in glucose metabolism or the general population. Furthermore, due to the inherent characteristics of the subjects, it is expected that the most frequent comorbidity is diabetes, and this could lead to differences when conducting the study with appropriate sampling techniques.
9. Conclusions
The general prevalence of hemoglobin variants including HbC or HbS, or alterations in HbF or HbA2, was 1.98 % (95 % CI 1.77 %–2.21 %) by frequentist methods. This rate is above the 1 % value suggested by the WHO as a threshold to declare the condition as endemic for a localized population. Therefore, the hemoglobin variant condition is endemic in the studied population.
In the population suspected of diabetes or under follow-up for this pathology, who underwent HbA1c testing via capillary electrophoresis, the presence of the studied hemoglobin variants revealed prevalence differences by sex, with men being the most affected. HbS was the most frequent genetic variant in both sexes within each study group. The combination of diabetes and kidney disease is a common finding in patients with HbS and HbC.
Ethics statement
This manuscript was written in compliance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration. We have the approval of the Ethics Committee in Biomedical Research from Fundación Valle del Lili. This is supported in letter No. October 21, 2019, which is available with the Corresponding Author if needed.
Consent for publication
Not applicable.
Data availability statement
The data related to our study has not been deposited in a publicly available repository. Nevertheless, all data and materials will be available upon request by contacting the correspondence author (Liliana Fernández-Trujillo, liliana.fernandez@fvl.org.co).
Funding
No funding sources were used.
CRediT authorship contribution statement
Maryory Galvis: Writing - review & editing, Writing - original draft, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Juan D. Díaz: Writing - review & editing, Formal analysis. Daniel E. Cuartas: Writing - review & editing, Validation, Supervision. José R. Tovar: Writing - review & editing, Validation, Supervision, Formal analysis. Liliana Fernandez-Trujillo: Writing - review & editing, Writing - original draft, Supervision, Conceptualization. Luz F. Sua: Writing - review & editing, Writing - original draft, Validation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23855.
List of abbreviations
- Hb
Hemoglobin
- HbC
Hemoglobin C
- HbS
Hemoglobin S
- HbF
Hemoglobin F
- HbA2
Hemoglobin A2
- HbA1c
Glycated hemoglobin
- WHO
World Health Organization
- HbA
Hemoglobin A
- HbE
Hemoglobin E
- TM
Beta thalassemia major
- TI
Beta thalassemia intermedia
- SCD
Sickle cell disease
- HbD
Hemoglobin D
- CI
Credible interval
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Elsayid M., Al-Shehri M.J., Alkulaibi Y.A., Alanazi A., Qureshi S. Frequency distribution of sickle cell anemia, sickle cell trait and sickle/beta-thalassemia among anemic patients in Saudi Arabia. J. Nat. Sci. Biol. Med. 2015;6:S85–S88. doi: 10.4103/0976-9668.166093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angastiniotis M., Lobitz S. Thalassemias: an Overview. Int J Neonatal Screen. 2019;5:16. doi: 10.3390/ijns5010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohne E. Hemoglobinopathies. Dtsch Ärztebl Int. 2011;108:532–540. doi: 10.3238/arztebl.2011.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satizabal Soto J.M., Neuta Arciniegas P.A., Somoyar Ordosgoiti P., Torres Muñoz J. Tamizaje de hemoglobinopatías en neonatos de Cali. 2013. https://bibliotecadigital.univalle.edu.co/entities/publication/1cfe3728-bcba-484d-adac-1db1137a4747 Colombia.
- 5.Satizabal Soto J.M., Neuta Arciniégas P.A., Torres Muños J., Samoyar Ordosgoitia A. Incidencia de hemoglobinopatías en neonatos de Cali. Salud Uninorte. 2004;18:71–73. https://go.gale.com/ps/i.do?p=IFME&sw=w&issn=01205552&v=2.1&it=r&id=GALE%7CA149165761&sid=googleScholar&linkaccess=abs [Google Scholar]
- 6.Williams T.N., Weatherall D.J. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2:a011692. doi: 10.1101/cshperspect.a011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrose E.E., Makani J., Chami N., Masoza T., Kabyemera R., Peck R.N., et al. High birth prevalence of sickle cell disease in Northwestern Tanzania. Pediatr. Blood Cancer. 2018;65 doi: 10.1002/pbc.26735. 10.1002/pbc.26735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premawardhena A., Allen A., Piel F., Fisher C., Perera L., Rodrigo R., et al. The evolutionary and clinical implications of the uneven distribution of the frequency of the inherited haemoglobin variants over short geographical distances. Br. J. Haematol. 2017;176:475–484. doi: 10.1111/bjh.14437. [DOI] [PubMed] [Google Scholar]
- 9.Diamantidis M.D., Karanikola R.-A., Polyzoudi C., Delicou S., Manafas A., Savera H., et al. Clinical significance of mutational variants in beta and alpha genes in patients with hemoglobinopathies from two large Greek centers: a complex interplay between genotype and phenotype. J Mol Med Berl Ger. 2023 doi: 10.1007/s00109-023-02342-3. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar Martinez P., Angastiniotis M., Eleftheriou A., Gulbis B., Mañú Pereira M.D.M., Petrova-Benedict R., et al. Haemoglobinopathies in Europe: health & migration policy perspectives. Orphanet J. Rare Dis. 2014;9:97. doi: 10.1186/1750-1172-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadhim K.A., Baldawi K.H., Lami F.H. Prevalence, incidence, trend, and complications of thalassemia in Iraq. Hemoglobin. 2017;41:164–168. doi: 10.1080/03630269.2017.1354877. [DOI] [PubMed] [Google Scholar]
- 12.Ding Z.-Y., Shen G.-S., Zhang S., He P.-Y. Epidemiology of hemoglobinopathies in the huzhou region, zhejiang province, southeast China. Hemoglobin. 2016;40:304–309. doi: 10.1080/03630269.2016.1200988. [DOI] [PubMed] [Google Scholar]
- 13.Grosse R., Lukacs Z., Cobos P.N., Oyen F., Ehmen C., Muntau B., et al. The prevalence of sickle cell disease and its implication for newborn screening in Germany (hamburg metropolitan area) Pediatr. Blood Cancer. 2016;63:168–170. doi: 10.1002/pbc.25706. [DOI] [PubMed] [Google Scholar]
- 14.Teawtrakul N., Jetsrisuparb A., Pongudom S., Sirijerachai C., Chansung K., Wanitpongpun C., et al. Epidemiologic study of major complications in adolescent and adult patients with thalassemia in Northeastern Thailand: the E-SAAN study phase I. Hematol Amst Neth. 2018;23:55–60. doi: 10.1080/10245332.2017.1358845. [DOI] [PubMed] [Google Scholar]
- 15.Romero-Sánchez C., Gómez Gutiérrez A., Duarte Y., Amazo C., Manosalva C., Chila M.L., et al. Variantes de hemoglobina en una población con impresión diagnóstica positiva para hemoglobinopatías en Colombia. Rev Médica Chile. 2015;143:1260–1268. doi: 10.4067/S0034-98872015001000004. [DOI] [PubMed] [Google Scholar]
- 16.INS . 2016. Comportamiento epidemiológico de las enfermedades huérfanas. Colombia.https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2019%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%205.pdf hasta semana epidemiológica 05 de 2019 2019. [Google Scholar]
- 17.Chaturvedi S., DeBaun M.R. Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: the last 40 years. Am. J. Hematol. 2016;91:5–14. doi: 10.1002/ajh.24235. [DOI] [PubMed] [Google Scholar]
- 18.Torres G.A., Ceballos L.E.F., Arbeláez L.C.F. Aplicación de la econometría espacial para el análisis de la miseria en los municipios del departamento de Antioquia. Semest Económico. 2015;18:103–128. doi: 10.22395/seec.v18n37a4. [DOI] [Google Scholar]
- 19.Urrea F., Viafara C., Arias W., Carabali B., Correa J., Meza M.L. 2011. Cuantos somos Como vamos: Diagnóstico Sociodemográfico de Cali y 10 municipios del Pacífico nariñense.https://www.dane.gov.co/files/censo2005/etnia/sys/cuantos_somos.pdf [Google Scholar]
- 20.Alvear C.C., Barboza M., Viola M., Moneriz C., Araque L.M. Pilot study of hemoglobinopathies in newborns of the Rafael Calvo maternity clinic of Cartagena, Colombia. Colomb Médica CM. 2012;43:196. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4001955/ [PMC free article] [PubMed] [Google Scholar]
- 21.Greer J.P., Rodgers G.M., Glader B., Arber D.A., Means R.T., Jr., List A.F., et al. fourteenth ed. Wolters Kluwer; 2019. Wintrobe's Clinical Hematology. CHAPTER 35 Thalass. Syndr. Quant. Disord. Globin Chain Synth; pp. 865–914. [Google Scholar]
- 22.Prieto V.H., Quintero C., Rodríguez I. Análisis de Bayes empírico mediante un ejemplo. Rev Colomb Estad. 1995;16 https://revistas.unal.edu.co/index.php/estad/article/view/10080 [Google Scholar]
- 23.Sankaran V.G., Lettre G., Orkin S.H., Hirschhorn J.N. Modifier genes in Mendelian disorders: the example of hemoglobin disorders. Ann. N. Y. Acad. Sci. 2010;1214:47–56. doi: 10.1111/j.1749-6632.2010.05821.x. [DOI] [PubMed] [Google Scholar]
- 24.Bouzid K., Ahmed H.B., Kalai E., Blibeche S., Couque N., Khiari K., et al. Prevalence of hemoglobin variants in a diabetic population at high risk of hemoglobinopathies and optimization of HbA1c monitoring by incorporating HPLC in the laboratory workup. Libyan J. Med. 2014;9 doi: 10.3402/ljm.v9.25768. 10.3402/ljm.v9.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehdi U., Toto R.D. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32:1320–1326. doi: 10.2337/dc08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data related to our study has not been deposited in a publicly available repository. Nevertheless, all data and materials will be available upon request by contacting the correspondence author (Liliana Fernández-Trujillo, liliana.fernandez@fvl.org.co).