Abstract
Study design
Systematic Review and Meta-analysis.
Objective
To compare the complication rates associated with anterior and posterior approaches for the surgical treatment of unstable hangman's fractures.
Methods
A systematic review and meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in PubMed, Web of Science, and Scopus databases to identify comparative studies reporting complications of anterior versus posterior approaches for the treatment of unstable hangman's fractures.
Results
The search yielded 1163 papers from which 5 studies were fully included. One hundred fifteen (115) patients were operated on using an anterior approach versus 65 through a posterior approach. The average complication rates for the anterior and posterior approaches were 26.1 % and 13.8 %, respectively. No complications following the anterior approach required pharmacological or surgical intervention (Clavien-Dindo, Grade 1), while 88.9 % of complications following the posterior approach did (Clavien-Dindo, Grade 2).
Conclusion
No significant differences in the complication rates were found when comparing anterior versus posterior surgery for treating a C2 traumatic spondylolisthesis. However, most of the complications presented in the posterior surgery group were more severe.
Keywords: Hangman's fracture, Systematic review, Anterior approach, Posterior approach, Complications, C2 traumatic spondylolisthesis
Abbreviations list
- MVAs =
Motor vehicle accidents
- ACDF =
Anterior cervical discectomy and fusion
- ROBINS-I =
Risk of bias in non-randomized studies of interventions
- PRISMA =
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
1. Introduction
Cervical spine injuries have been observed in up to 4 % of patients presenting to emergency departments as trauma activations following blunt injuries.1 Motor vehicle accidents (MVAs), older age (>65 years), and falls are predictive factors that increase the risk of suffering from a fracture/dislocation of the cervical spine.2 As the average age of the world's population continues to rise,3,4 it is likely that an increase in the prevalence of cervical spine injuries will be observed in the future.5 “Hangman's fractures,” a term coined by Schneider in 1965, was used to describe a distinct fracture pattern of the upper cervical spine following MVAs that were similarly seen following judicial hangings.6 Nowadays, a hangman's fracture, or traumatic spondylolisthesis of the axis, is defined as a bilateral fracture of the pars interarticularis of the C2 vertebra resulting in a traumatic spondylolisthesis of C2 over C3.7 These injuries account for 4%–7% of all cervical spine fractures and 20%–22 % of axis fractures.8,9 It is the second most common fracture pattern of the C2 vertebrae following odontoid fractures, and its incidence increases with the patient's age. The wide diameter of the spinal canal at the C2 level and the typically centrifugal burst pattern of the fracture fragments explain why hangman's fractures are rarely associated with neurologic deficits and carry an excellent clinical prognosis after treatment.7
In 1993, Starr and Eismont10 divided these lesions into typical and atypical fracture types depending on the symmetry of the fracture. A more descriptive Hangman's fracture classification was proposed in 1981 by Effendi et al11 and further modified by Levine and Edwards12 in 1985 with the addition of a subclassification of type II fractures. This is the most widely used fracture classification system for typical Hangman's fractures, and it divides fractures based on the mechanism of injury into 4 types. Type I fractures are considered stable and thus are successfully managed nonoperatively with a short period (6–12 weeks) of immobilization in a hard cervical orthosis.13 In contrast, type II, IIA, and type III fracture patterns are inherently unstable and typically require operative intervention due to the predominant flexion force imparted in these injuries and the frequently associated injury to the C2–C3 disc space.12 Similarly, the atypical hangman's fracture, with a fracture passing through the vertebral body, may require operative intervention due to the greater potential for neurological injury.10
When internal fixation is required, the surgeon must decide whether an anterior or posterior approach is necessary. Anatomical familiarity, the evolution of implants, and better quality of intraoperative imaging have driven posterior techniques (C2 transpedicular screw, C2–C3 fixation, arthrodesis from C1 to C3, and occipitocervical fixation) to become the preferred approach by many authors.11,14, 15, 16, 17 However, certain conditions such as a C2 body fracture, C2–C3 disc herniation or small C2 pedicles demand an anterior approach.9,18,19 The use of a combined approach with a C2–C3 anterior cervical discectomy and fusion (ACDF) and posterior cervical fusion is mainly reserved for fractures that have significant displacement and the C2 vertebral body dislocated anterior to C3, generally Type III fractures.20
Different surgical complications have been described following both anterior and posterior approaches. Some of them are potentially catastrophic, such as vascular and neurological injuries, infection, and other approach-related injuries like esophagus lacerations. However, previously published reviews regarding C2 traumatic spondylolysis have not found any significant difference between approaches.7,21
Despite all this knowledge, standard treatment strategies for unstable hangman's fractures are still controversial and remain poorly standardized.22 There is no clear clinical advantage of one approach over the other in outcomes or complication rates between the various types of anterior and/or posterior fusion techniques,22 and as such, this decision is often based on surgeon preference and should optimally incorporate injury and patient-specific factors. The decision between anterior and posterior approaches in treating unstable Hangman's fractures should not be arbitrary. The present study aims to compare the complication rates associated with anterior versus posterior approaches for the surgical treatment of hangman's fractures.
2. Material and methods
Institutional Review Board approval and informed consent were not required since, unlike primary researchers, systematic reviewers do not collect deeply personal, sensitive or confidential information from participants. Systematic reviewers use publicly accessible documents as evidence and are seldom required to seek institutional ethics approval before commencing a systematic review.
The clinical question was formulated following the PICOT criteria to guide our review: “Does an anterior approach have a higher, lower, or similar complication rate than a posterior approach when treating an unstable hangman's fracture?
2.1. Eligibility criteria for study selection
The selection criteria were as follows:
-
•
Articles published in English;
-
•
Articles published in a peer-reviewed scientific journal;
-
•
Prospective or retrospective randomized or not clinical trials;
-
•
Case-control or cohort (prospective or retrospective) studies;
-
•
Articles published in the last 20 years;
-
•
Articles comparing anterior and posterior approaches as treatments for Hangman's fracture in adults (patients >18 years old);
-
•
Articles reporting the complications related to each approach as an outcome.
The exclusion criteria were:
-
•
Other systematic reviews, case reports, letters to the editor, book chapters, and commentaries;
-
•
Articles not reporting complications within the results;
-
•
Articles including a double approach (anterior + posterior);
-
•
Articles in languages other than English;
-
•
Articles with a follow-up < 1 year.
2.2. Participants
Participant patient's eligibility criteria were: adult patients >18 years old, diagnosed with an unstable hangman's fracture (Levine and Edwards II, IIa, and III), and a minimum follow-up of one year postoperatively.
2.3. Interventions
The comparison was carried out between anteriorly approached patients who had undergone ACDF at C2–C3 with or without plate versus posteriorly approached patients treated with posterior instrumented stabilization (C2-3 fusion, C1–C3 fusion, or C2 osteosynthesis).
2.4. Literature search strategy
The following databases were screened: PubMed.gov (http://www.ncbi.nlm.nih.gov/pubmed), Web of Science (https://webofknowledge.com), and Scopus (www.scopus.com) to identify published articles comparing complications between anterior versus posterior approaches for the treatment of unstable hangman's fractures.
The following combined search terms were used: “(hangman fracture OR hangman's fracture OR traumatic spondylolisthesis OR traumatic spondylolisthesis of axis OR traumatic spondylolisthesis of C2 OR C2 pars interarticularis fracture) AND (anterior approach OR anterior surgery OR anterior cervical fusion OR anterior arthrodesis OR anterior fixation OR ACDF) AND (posterior approach OR posterior surgery OR posterior fixation OR posterior fusion OR posterior arthrodesis) AND (complications OR complication)”.
Titles of studies found in the databases were compared. Duplicate records were removed, and the remaining listings were screened for inclusion by title and abstract review. All papers included both (anterior and posterior approaches) comparative groups. In addition, full-text manuscripts of all papers included were reviewed to ensure that only relevant papers were captured, as were all cross-referenced articles. Eligibility assessments were performed independently in a standardized manner by two reviewers (MPD and MG). Discrepancies among the two reviewers’ assessments were discussed with an independent, blinded third reviewer (AG) until a consensus was reached.
2.5. Primary and secondary outcomes
The primary outcome of this study was to determine the rate of postoperative complications among surgical approaches for the treatment of unstable hangman's fractures. The complication rate was assessed considering the number of complications over the number of participants in each group (risk ratio). Complications included were postoperative infections (wound infection, pulmonary, urinary, and others), non-union, reoperation, implant failure, neurological complications, deep venous thrombosis and pulmonary embolism, dysphagia, dysphonia, vertebral artery injury, bleeding from posterior venous plexus, need for transfusion and death.
Complications were graded by the Clavien-Dindo classification.23 This classification is graded from 1 to 5 according to increasing severity and the type of intervention required to treat the complication24, 25, 26 (Table 1).
Table 1.
Clavien-Dindo Classification.23
| Grade | Description |
|---|---|
| I | Any deviation from the normal postoperative course without the need for pharmacologic treatment or surgical, endoscopic, and radiographic interventions. |
| II | Requiring pharmacologic treatment with drugs (blood transfusions and total parenteral nutrition are also included). |
| IIIA | Requiring surgical, endoscopic, or radiographic intervention not/under general anesthesia |
| IIIB | Requiring surgical, endoscopic, or radiographic intervention under general anesthesia |
| IVA | Life-threatening complication requiring IC/ICU management - Single-organ dysfunction |
| IVB | Life-threatening complication requiring IC/ICU management - Multiorgan dysfunction |
| V | Death of a patient |
Secondary outcomes included intraoperative blood loss (the volume of blood loss throughout the procedure), which was analyzed as a continuous variable expressed in milliliters. Surgical time was measured in minutes and was also considered a continuous variable.
2.6. Data extraction and analysis
After excluding all ineligible papers, the full texts of the remaining articles were reviewed in detail. Baseline characteristics extracted from each paper included first author, year of publication, journal, study design, demographic data from included patients (age, gender), classification of fractures according to Levine-Edwards,12 complications, and other available perioperative and postoperative data, such as hospital length of stay, estimated blood loss and surgical time. Data was compiled and organized using Microsoft Excel 2013. Meta-analysis was carried out for outcomes that had complete information details within the paper using Revman®.27
2.7. Missing data, publication bias, and heterogeneity assessment
Articles were excluded from the final analysis if outcome data was unavailable for both primary endpoints. Statistical heterogeneity was assessed utilizing visual inspection of graphics and using the I2 test. The authors considered substantial statistical heterogeneity when the value of I2 was above 50 %, and in those situations, an assessment of reasons for that heterogeneity was performed.
2.8. Methodologic quality evaluation
All the studies were graded for level of evidence, in accordance with the Oxford Centre for Evidence-Based Medicine.28 For risk of bias assessment, the ROBINS-I (risk of bias in non-randomized studies of interventions) tool was used.29
2.9. Protocol registration, quality, and evidence assessment
The protocol was registered at PROSPERO on the 24th of September 2022, ID 362201. The review was conducted as protocol, except for changes in the participating authors. AMSTAR 2 checklists to evaluate the study quality was used. The report was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.28
Global certainty of evidence was assessed using the GRADE framework and software (GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2021. Available from gradepro.org).
3. Results
3.1. Results of the search
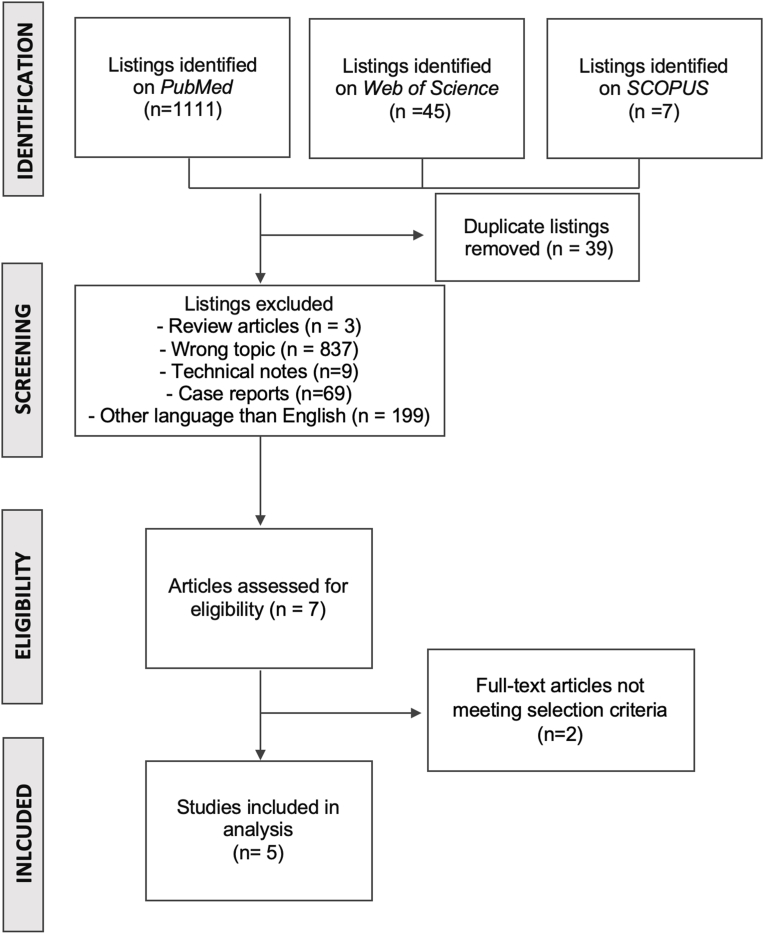
Among the three databases, we identified a total of 1163 papers. After an initial screening, application of inclusion and exclusion criteria, and removal of duplicates, five studies were fully included and evaluated (Fig. 1). Four studies were retrospective cohort studies comparing outcomes with anterior versus posterior approaches for Hangman's fracture; one paper was a retrospective analysis of a multicentric prospectively collected database of Hangman's fracture. A total of 115 patients were operated on using an anterior approach versus 65 through a posterior approach in the five studies included.
Fig. 1.
Systematic review flowchart, including inclusion and exclusion criteria.
The study design, the grade for the level of evidence for each paper, and the number of patients in each group are reported in Table 2.
Table 2.
Level of evidence and number of patients of the 5 included studies.
| First Author (year of publication) | Type of Study | Number of Patients | Level of Evidence |
|---|---|---|---|
| Yang et al (2018)30 | Retrospective | Anterior 53 | III |
| Posterior 10 | |||
| Prost et al (2019)22 | Prospective | Anterior 6 | III |
| Posterior 7 | |||
| Patel et al (2020)31 | Retrospective | Anterior 12 | III |
| Posterior 9 | |||
| Jin et al (2020)32 | Retrospective | Anterior 20 | III |
| Posterior 25 | |||
| Ge et al (2015)9 | Retrospective | Anterior 24 | III |
| Posterior 14 |
3.2. Risk of bias assessment using the Cochrane ROBINS-I tool
All five studies were rated at moderate risk of confounding due to the retrospective nature of data collection and the inability to randomly or strategically assign patients to treatment groups. The two interventions and the differences between groups (anterior vs. posterior approaches) were reasonably described in all the papers included. Hence, we rated both intervention risks as low. All studies were of low risk for bias among certain measurements that were objective enough that they should not be influenced by who the assessor was (e.g., duration of surgery), while others were considered of moderate bias because they were assessor dependent (e.g., presence of dysphagia or dysphonia). Overall, we considered them all low to moderate risk. We cannot find instances of missing data or biased reporting. Assessment of each subject can be found in Table 3.
Table 3.
Risk of bias assessment using the Cochrane ROBINS-I tool.
| First Author (year of publication) | Confounders | Subjet Selection | Classification of intervention | Deviation in intervention | Missiong Data | Biased Measurements | Biased Reporting | Overall Bias Rating |
|---|---|---|---|---|---|---|---|---|
| Yang et al (2018) | Moderate | Moderate | Low | Low | Low | Low/Moderate | Low | Moderate |
| Prost et al (2019) | Moderate | Low | Moderate | Low | Low | Low/Moderate | Low | Moderate |
| Patel et al (2020) | Moderate | Moderate | Low | Low | Low | Low/Moderate | Low | Moderate |
| Jin et al (2020) | Moderate | Moderate | Low | Low | Low | Low/Moderate | Low | Moderate |
| Ge et al (2015) | Moderate | Moderate | Low | Low | Low | Low/Moderate | Low | Moderate |
The study of Yang et al30 specifically searched for dysphagia and dysphonia, this augmented complication rate significantly in anterior approaches compared with posterior ones, maybe the real incidences were sub-diagnosticated in the other studies since the vast majority were classified as mild. At the same time, there is no report of other possible complications in this cohort of patients.
Demographic data extracted from each study are shown in Table 4. This data was not discriminated against according to the type of approach selected for treatment in the studies conducted by Yang et al30 and Prost et al22 These studies also described a significant number of patients with Levine-Edwards Type I fractures that were mostly non-operatively treated.
Table 4.
Demographic data.
| Yang et al (2018) |
Prost et al (2019) |
Patel et al (2020) |
Jin et al (2020) |
Ge et al (2015) |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | PA | AA | PA | AA | PA | AA | PA | AA | PA | AA | PA | ||
| Number of patients | 53 | 10 | 6 | 7 | 12 | 9 | 20 | 25 | 24 | 14 | 115 | 65 | |
| Age (years) | 47 (18–73) | 59.1 ± 23 (19–94) | 39.8 ± 4.5 | 41.3 ± 6.7 | 44.7 ± 9.1 (25–58) | 48.5 ± 11.3 (30–70) | 36.5 ± 7.8 (19–52) | 39.6 ± 8.1 (22–65) | |||||
| Sex (%) | |||||||||||||
| Male | 68 (73.1 %) | 22 (64.7 %) | 10 (83.3 %) | 8 (88.8 %) | 12 (60 %) | 15 (60 %) | 16 (66.7 %) | 9 (64.3 %) | 38 | 32 | |||
| Female | 25 (26.9 %) | 12 (35.3 %) | 2 (16.7 %) | 1 (11.2 %) | 8 (40 %) | 10 (40 %) | 8 (33.3 %) | 5 (35.7 %) | 18 | 16 | |||
| Levine-Edward Classification (%) | |||||||||||||
| I | 26 (27.9 %) | 23 (68 %) | – | – | |||||||||
| II | 46 (49.5 %) | 10 (29 %) | 6 (50 %) | 5 (55.5 %) | 17 (85 %) | 16 (64 %) | 13 (54.2 %) | 8 (57.1 %) | 36 | 29 | |||
| IIA | 11 (11.8 %) | – | 4 (33.3 %) | 3 (33.3 %) | 3 (15 %) | 6 (24 %) | 9 (37.5 %) | 6 (42.9 %) | 16 | 15 | |||
| III | 10 (10.8 %) | 1 (3 %) | 2 (16.7 %) | 1 (11.2 %) | – | 3 (12 %) | 2 (8.3 %) | – | 4 | 4 | |||
| Follow-up (months) | 12 | 12 | 36.2 ± 8.8 | 36.1 ± 8.9 | 20 (18–21) | 19 (18–20) | 42 (24–60) | ||||||
| Patients Included Non-operatively treated | 30 | 21 | – | – | – | 51 | |||||||
AA = Anterior Approach; PA = Posterior Approach.
3.3. Effects of interventions
Complications reported by each study are summarized in Table 5. The average complication rate for the anterior approach was 26.1 % and 13.8 % for the posterior. Two-thirds of the reported complications in these studies involved dysphagia and dysphonia in the study by Yang et al.30 Patel et al31 did not publish the total number of patients with postoperative dysphagia, although they claimed that a “significant number” of them presented with it.
Table 5.
Reported Complications According to each Approach.
| Yang et al (2018) |
Prost et al (2019) |
Patel et al (2020) |
Jin et al (2020) |
Ge et al (2015) |
Total |
Percentage |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | PA | AA | PA | AA | PA | AA | PA | AA | PA | AA | PA | AA | PA | |
| Number of Patients | 53 | 10 | 6 | 7 | 12 | 9 | 20 | 25 | 24 | 14 | 115 | 65 | ||
| Deep Wound Infection | 1 | 0 | 1 | – | 1.5 | |||||||||
| Dysphagia | 17 | 1 | Significant Number | 17 | 1 | 14.8 | 1.5 | |||||||
| Dysphonia | 13 | 13 | 0 | 11.3 | – | |||||||||
| Bleeding from Posterior venous Plexus | 4 | 3 | 0 | 7 | – | 10.8 | ||||||||
| Complication Rate (%) | 56.6 | 10 | 0 | 14.3 | 0 | 44.4 | 0 | 0 | 0 | 21.4 | 30 | 9 | 26.1 | 13.8 |
AA = Anterior Approach; PA = Posterior Approach.
None of the studies selected for this systematic review reported urinary, pulmonary, or other postoperative infections other than wound infections (1 patient, Prost et al22). There was no report of reoperations, implant failures, neurological complications, deep venous thrombosis or pulmonary embolisms, vertebral artery injuries or deaths.
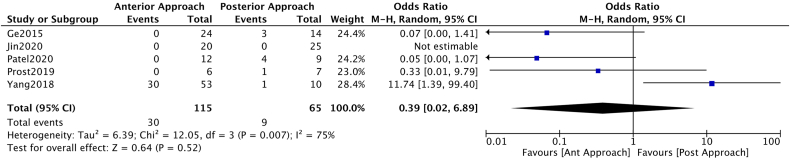
Considering complications as a dichotomous variable, the analysis could not establish a significant benefit from the anterior or posterior approach, OR 0.39 [CI 0.02–6.89] (Fig. 2).
Fig. 2.
Forest plot representation of the Odds Ration when considering the presence of complications regarding the number of patients included in each study.
When classifying complications according to Clavien-Dindo23 (Table 6), 100 % were classified as type I for the anterior approach, while 88.9 % were type II for the posterior approach. The present systematic review classified none of the reported complications as type III, IV, or V.
Table 6.
Complications according to Clavien-Dindo classification.
| Classification | AA |
PA |
||
|---|---|---|---|---|
| N | Percentage | N | Percentage | |
| I | 30 | 100 | 1 | 11.1 |
| II | – | – | 8 | 88.9 |
| IIIA | – | – | – | – |
| IIIB | – | – | – | – |
| IVA | – | – | – | – |
| IVB | – | – | – | – |
| V | – | – | – | – |
AA = Anterior Approach; PA = Posterior Approach.
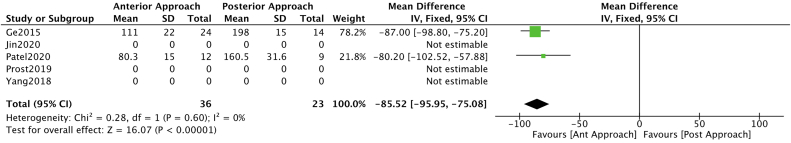
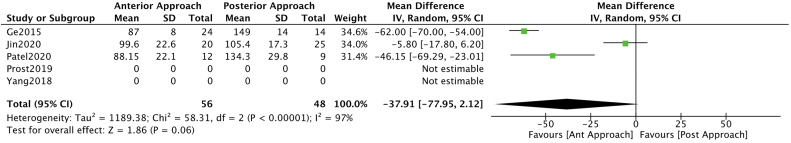
A meta-analysis of available data was possible for blood loss and surgical time. Blood loss analysis resulted in a benefit towards the anterior approach OR -85.52 (CI -95.95 – (−75.08)) (Fig. 3). While no clear benefit was found for any approach regarding surgical time OR -73.91 (CI -77.95 – 2.12) (Fig. 4).
Fig. 3.
Forest plot and a meta-analysis carried out for blood loss segregated by approach type.
Fig. 4.
Forest plot and a meta-analysis carried out for surgical time segregated by approach type.
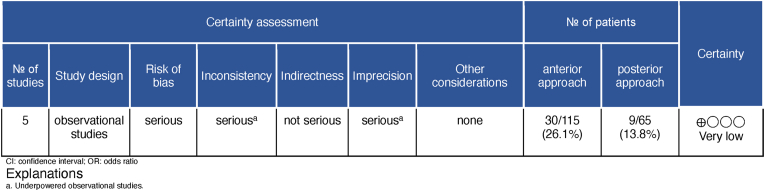
Analysis of the evidence using the GRADE methodology demonstrated a very low level of certainty that the actual effect was similar to the estimated for the occurrence of adverse effects after surgery in patients diagnosed with Hangman fractures (Fig. 5).
Fig. 5.
Global certainty of evidence using the GRADE framework. Outcome: Complication.
4. Discussion
This systematic review shows no difference in the complication rates when treating unstable hangman's fractures according to the selected approach (anterior versus posterior). However, all complications that presented after the anterior approach were classified as grade I regarding the Clavien-Dindo classification (any deviation from the normal postoperative course without the need for pharmacologic or surgical treatment), while 88.9 % (8 of 9) of the complications observed after the posterior approach, were grade II complications, requiring pharmacologic treatment, including blood transfusions, hence, more severe. At this moment, we are unaware of any available evidence stating that this distinction in complication grading is clinically relevant for the patient. However, the requirement of pharmacologic treatment, including transfusion, could impact the hospital length of stay, the need for additional analysis and laboratory or imaging studies, the increased cost of hospitalization, and possible secondary complications related to the installed treatment.
Benefits and complications related to the anterior and posterior approaches are well known. It has been proposed that the anterior approach avoids the time and risk involved in flipping the patient to the prone position and the associated muscular morbidity of the posterior approach. Previous literature has indicated that the anterior approach provides better dynamic stabilization and prevents delayed neurological compromise related to post-traumatic disc herniation. Other advantages include a lower risk of intraoperative vertebral artery injury and preservation of C1–C2 mobility.22 It also allows for reconstructing cervical lordosis. Patel et al31 hypothesized that an ACDF is a simpler procedure with advantages such as minimal soft‐tissue damage, reduced intra‐operative blood loss, shorter operative time, early pain-free status, and reduced hospital stay. However, the anterior approach has disadvantages like injuries to the facial and hypoglossal nerves, branches of the external carotid, the superior laryngeal nerve, and the esophagus.33
On the other hand, the posterior approach is familiar to all spinal surgeons and avoids the potential neurovascular risk associated with the anterior approach. It allows for the direct fixation of the pars fracture of C2.15 However, direct pars repair does not address instability at the disc space and the disruption of the anterior and/or posterior longitudinal ligament and therefore, it is reserved for cases with minimal or no injury to the C2–C3 disc. The posterior approach is associated with dissection of the posterior cervical muscles, excessive bleeding due to injury to the venous plexuses, longer operative time, and longer use of postoperative drainage. Liu et al20 believed that posterior fixation for a highly unstable hangman's fracture might aggravate the forward displacement of C2 because of the intraoperative prone position and the forward thrust of the C2 screw. Finally, the lack of the medullary canal in C2 pedicles with too narrow and deformed C2 pedicles renders screw placement difficult and such cases should be considered for longer posterior fusions (occiput or C1) or anterior fixation.34
Regarding complications related to these fractures, a previously published systematic review conducted by Murphy et al21 concluded that they did not find statistically significant differences in complication rate (p = 0.28) after analyzing 200 patients treated by a C2–C3 ACDF versus 193 patients resolved by posterior cervical fusion. They did not classify complications following any grading system. Similarly, in our study, none of the five comparative papers included a severity scale of complications.
Murphy et al21 described 13 complications in the ACDF group (3 wound infections, 3 neurologic deficits, 2 hematomas, 4 prolonged iliac crest donor site pain, and 1 loose or broken hardware), and 4 complications in the posterior fusion group (1 wound infection, 1 implant failure, 1 vertebral artery injury, and 1 death). Interestingly, they reported one case of a grade V complication, a death following a posterior approach. The cause of death was not specified, whether it was a direct complication of the selected treatment or a consequence of another associated lesion or comorbidity. None of the five comparative papers included in this systematic review accounted for the severity of complications.
In relation to pseudoarthrosis, Murphy et al21 describe four cases of non-union in the ACDF group and two in the posterior group. However, these were not considered complications. This review did not find any cases of non-union. Moreover, studies that analyzed fusion rate within their outcomes,31,32 stated that all patients achieved a solid fusion at the last follow-up, independent of the selected approach, concluding that both an anterior and a posterior approach resulted in a high fusion rate.
Anterior C2–C3 surgery was expected to result in a higher incidence of postoperative dysphagia due to the special location and difficult exposure of the upper cervical spine, which often requires a much more powerful traction strength during surgery. The incidence of dysphagia reported by Yang et al30 after an anterior approach (32 %) was similar to the incidence of general anterior cervical surgeries. Even when these patients often suffered from edema of the esophagus and prevertebral soft tissue which might contribute to the increased incidence of this complication. Other reported risk factors associated with a higher possibility of dysphagia after anterior cervical surgery are: female patients, older patients, C4–C5 surgery, anterior plating, longer operative time, multi-level surgery, and use of bone morphogenetic protein.30 Yang et al30 also demonstrated that the incidence of dysphagia after the posterior surgical approach in hangman's fracture patients was 10 % (1 of 10 patients), which was similar to previous studies.8 Possible reasons for dysphagia after posterior cervical surgery could be pain from posterior neck dissection, immobilization from a cervical collar, and tracheal intubation.
Yang et al30 published a 23.2 % rate of dysphonia in the 53 patients treated with ACDF, while none of the other included studies reported dysphonia as a complication. They concluded that both complications (dysphonia and dysphagia) were mild and gradually decreased during the subsequent three months following surgery with no additional treatment.30
Excessive bleeding due to injury to the venous plexus was reported by Ge et al9 in 3 patients during the posterior approach, while no other surgical complication was detected in the ACDF. Similarly, Patel et al31 reported 4 cases of excessive bleeding in their series of posterior cervical instrumented fusion. These studies were also the only ones that described blood loss as an outcome. Both studies concluded that ACDF was associated with minimal soft‐tissue damage, reduced intraoperative blood loss, and shorter operative times. These conclusions are in accordance with the results of our meta-analysis.
Evidently, the authors believe that selecting the appropriate surgical approach for these complex lesions does not depend only on statistics of complication rates, hospital length of stay, blood loss, or surgical time. The patient's characteristics are of paramount importance, such as dimensions of the neck, associated injuries, specific previous surgeries or treatments (radiation therapy) at the neck level, and most importantly, the patient's anatomy. Not every patient is indifferently approachable anteriorly or posteriorly. There are contraindications that surgeons must have in mind before choosing the appropriate surgical technique for a patient. For instance, contraindications of posterior C2–C3 spinal fusion are the lack of the medullary canal in C2 pedicles, too small and deformed C2 pedicles, or specific vertebral artery malformations.34 In these cases, surgeons will need to utilize another surgical strategy, such as an anterior approach or a longer posterior fusion (C1–C3).
5. Limitations
There are significant limitations to this study, including all of those inherent to any systematic review. Specifically, the results of this analysis are only as accurate as the existing literature, and none of the five studies included had an overall low risk of bias.
Another significant limitation is the lack of individual patient data reported in the literature, and this prevented a meaningful subgroup analysis of how fracture type or specifications from surgical treatment affect the complication rate. Furthermore, there are two studies (Yang et al30 and Patel et al31) that included in their analysis a non-operative group of patients, and the demographic data does not discriminate between treatment groups.
Many different complications have been reported following unstable hangman's fractures, mainly published as case reports or short case series, without a formal comparison between anterior versus posterior approaches. It is a limitation that all those reports are not included due to the low evidence provided. Another potential real and frequent limitation is the underreporting of complications,35 such as grade I complications of Clavien-Dindo Classification. However, this could be compensated by studies like Yang et al,30 in which these minor complications were the focus of the research.
With the lack of high-level clinical studies and no clear benefit of one treatment over another shown from our systematic review, evidence-based management decisions have to consider multiple factors that include the best level of available evidence including expert opinion, relative risk, benefit, and burden of the intervention(s), and patient preference. The latter is of greater importance in scenarios of clinical equivalence between two treatment options. Clearly, better-designed high-quality prospective trials are needed to determine the optimal treatment for this condition. Due to the logistic difficulties of such studies for a relatively uncommon surgical condition, it is unlikely that such data will ever be readily available.13
6. Conclusion
According to this very low level of certainty and moderate risk of bias systematic review and meta-analysis no significant differences in the complication rates were found when comparing anterior versus posterior surgery for treating a C2 traumatic spondylolisthesis. However, most of the complications presented in the posterior surgery group were more severe according to Clavien-Dindo Classification. Hence, prospective randomized studies, dividing patients according to the type of fracture, are needed to fully understand the main differences between each approach regarding the severity of the lesion.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Matias Pereira-Duarte: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Martin Gagliardi: Data curation, Investigation, Methodology, Writing - review & editing. Charles André Carazzo: Data curation, Investigation, Writing - review & editing. Gaston Camino-Willhuber: Methodology, Supervision, Validation, Writing - review & editing. Alberto Gotfryd: Supervision, Writing - review & editing. Michael Rogers: Methodology, Supervision. Alfredo Guiroy: Conceptualization, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was organized by the AO Spine Latin America Research Study Group. AO Spine is a clinical division of the AO Foundation, which is an independent, medically guided not-for-profit organization. Study support was provided directly through AO Spine Latin America regarding proofreading.
References
- 1.Milby A.H., Halpern C.H., Guo W., Stein S.C. Prevalence of cervical spinal injury in trauma. Neurosurg Focus. 2008;25(5):E10. doi: 10.3171/FOC.2008.25.11.E10. [DOI] [PubMed] [Google Scholar]
- 2.Hasler R.M., Exadaktylos A.K., Bouamra O., et al. Epidemiology and predictors of cervical spine injury in adult major trauma patients: a multicenter cohort study. J Trauma Acute Care Surg. 2012;72(4):975–981. doi: 10.1097/TA.0b013e31823f5e8e. [DOI] [PubMed] [Google Scholar]
- 3.Lutz W., Sanderson W., Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 4.Knickman J.R., Snell E.K. The 2030 problem: caring for aging baby boomers. Health Serv Res. 2002;37(4):849–884. doi: 10.1034/j.1600-0560.2002.56.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devivo M.J. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50(5):365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 6.Schneider R.C., Livingston K.E., Cave A.J., Hamilton G. Hangman's Fracture of the cervical spine. J Neurosurg. 1965;22:141–154. doi: 10.3171/jns.1965.22.2.0141. [DOI] [PubMed] [Google Scholar]
- 7.Turtle J., Kantor A., Spina N.T., France J.C., Lawrence B.D. Hangman's fracture. Clin Spine Surg. 2020;33(9):345–354. doi: 10.1097/BSD.0000000000001093. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mahfoudh R., Beagrie C., Woolley E., et al. Management of typical and atypical hangman's fractures. Global Spine J. 2016;6(3):248–256. doi: 10.1055/s-0035-1563404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge C., Hao D., He B., Mi B. Anterior cervical discectomy and fusion versus posterior fixation and fusion of C2-3 for unstable hangman's fracture. J Spinal Disord Tech. 2015;28(2):E61–E66. doi: 10.1097/BSD.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 10.Starr J.K., Eismont F.J. Atypical hangman's fractures. Spine. 1993;18(14):1954–1957. doi: 10.1097/00007632-199310001-00005. [DOI] [PubMed] [Google Scholar]
- 11.Effendi B., Roy D., Cornish B., Dussault R.G., Laurin C.A. Fractures of the ring of the axis. A classification based on the analysis of 131 cases. J Bone Joint Surg Br. 1981;63-B(3):319–327. doi: 10.1302/0301-620X.63B3.7263741. [DOI] [PubMed] [Google Scholar]
- 12.Levine A.M., Edwards C.C. The management of traumatic spondylolisthesis of the axis. J Bone Joint Surg Am. 1985;67(2):217–226. [PubMed] [Google Scholar]
- 13.Vaccaro AR, Fehlings MG, Dvorak MF. Spine and spinal cord trauma. Evidence-based management. THIEME. Chapter 26: management of C2 traumatic spondylolisthesis (Hangman's Fracture) and Other Variants. Y. Raja Rampersaud and S. Samuel Bederman. Vol 1., p. 248-264.
- 14.ElMiligui Y., Koptan W., Emran I. Transpedicular screw fixation for type II Hangman's fracture: a motion preserving procedure. Eur Spine J. 2010;19(8):1299–1305. doi: 10.1007/s00586-010-1401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma W., Xu R., Liu J., et al. Posterior short-segment fixation and fusion in unstable Hangman's fractures. Spine. 2011;36(7):529–533. doi: 10.1097/BRS.0b013e3181d60067. [DOI] [PubMed] [Google Scholar]
- 16.Muthukumar N. C1-C3 lateral mass fusion for type IIa and type III Hangman's fracture. J Craniovertebr Junction Spine. 2012;3(2):62–66. doi: 10.4103/0974-8237.116541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying Z., Wen Y., Xinwei W., et al. Anterior cervical discectomy and fusion for unstable traumatic spondylolisthesis of the axis. Spine. 2008;33(3):255–258. doi: 10.1097/BRS.0b013e31816233d0. [DOI] [PubMed] [Google Scholar]
- 18.Boullosa J.L., Colli B.O., Carlotti C.G., Jr., Tanaka K., dos Santos M.B. Surgical management of axis' traumatic spondylolisthesis (Hangman's fracture) Arq Neuropsiquiatr. 2004;62(3B):821–826. doi: 10.1590/s0004-282x2004000500015. [DOI] [PubMed] [Google Scholar]
- 19.Tuite G.F., Papadopoulos S.M., Sonntag V.K. Caspar plate fixation for the treatment of complex hangman's fractures. Neurosurgery. 1992;30(5):761–765. [PubMed] [Google Scholar]
- 20.Liu J., Li Y., Wu Y. One-stage posterior C2 and C3 pedicle screw fixation or combined anterior C2-C3 fusion for the treatment of unstable hangman's fracture. Exp Ther Med. 2013;5(3):667–672. doi: 10.3892/etm.2013.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy H., Schroeder G.D., Shi W.J., et al. Management of Hangman's Fractures: A Systematic Review. J Orthop Trauma. 2017;31(Suppl 4):S90–S95. doi: 10.1097/BOT.0000000000000952. [DOI] [PubMed] [Google Scholar]
- 22.Prost S., Barrey C., Blondel B., et al. Hangman's fracture: Management strategy and healing rate in a prospective multi-centre observational study of 34 patients. Orthop Traumatol Surg Res. 2019;105(4):703–707. doi: 10.1016/j.otsr.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Camino Willhuber G., Elizondo C., Slullitel P. Analysis of Postoperative Complications in Spinal Surgery, Hospital Length of Stay, and Unplanned Readmission: Application of Dindo-Clavien Classification to Spine Surgery. Global Spine J. 2019;9(3):279–286. doi: 10.1177/2192568218792053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clavien P.A., Barkun J., de Oliveira M.L., et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 25.Clavien P.A., Sanabria J.R., Strasberg S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111(5):518–526. [PubMed] [Google Scholar]
- 26.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934. ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Review Manager Web (revman.cochrane.Org). Version 5.4. The Cochrane Collaboration. Available at: revman.cochrane.org.
- 28.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. Published 2015 Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. Published 2016 Oct 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Dai L., Ma L., Gao X., Liu H. Incidence of dysphagia and dysphonia after Hangman's fractures: Evidence from 93 patients. Medicine (Baltim) 2018;97(49) doi: 10.1097/MD.0000000000013552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel J.Y.K., Kundnani V.G., Kuriya S., Raut S., Meena M. Unstable Hangman's fracture: Anterior or posterior surgery? J Craniovertebr Junction Spine. 2019;10(4):210–215. doi: 10.4103/jcvjs.JCVJS_112_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin C., Xie N., Ren Y., et al. How Does Cervical Sagittal Balance Change After Hangman Fracture Treated with Anterior or Posterior Approach Surgery? World Neurosurg. 2020;138:e767–e777. doi: 10.1016/j.wneu.2020.03.070. [DOI] [PubMed] [Google Scholar]
- 33.McAfee P.C., Bohlman H.H., Riley L.H., Jr., Robinson R.A., Southwick W.O., Nachlas N.E. The anterior retropharyngeal approach to the upper part of the cervical spine. J Bone Joint Surg Am. 1987;69(9):1371–1383. [PubMed] [Google Scholar]
- 34.Ludwig S.C., Kramer D.L., Balderston R.A., Vaccaro A.R., Foley K.F., Albert T.J. Placement of pedicle screws in the human cadaveric cervical spine: comparative accuracy of three techniques. Spine. 2000;25(13):1655–1667. doi: 10.1097/00007632-200007010-00009. [DOI] [PubMed] [Google Scholar]
- 35.Camino-Willhuber G., Cabrera J.P., Carazzo C., et al. Reporting Complications in Spinal Surgery-a Systematic Literature Review. World Neurosurg. 2021;150:e765–e770. doi: 10.1016/j.wneu.2021.03.143. [DOI] [PubMed] [Google Scholar]