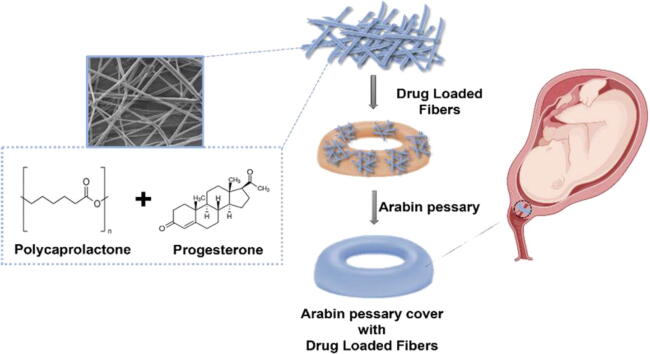
Graphical abstract
Keywords: Arabin pessaries, Vaginal delivery, Nanofibers, Electrospinning, Progesterone, Preterm labor
Abstract
Preterm labor is a growing health problem that causes newborn death, and safe and effective therapy is significantly needed. Arabin pessaries and progesterone are preventive and therapeutic approaches that can be applied to managing the short cervix; hence, reducing the risk of preterm labor. The main goal of current work is to fabricate a novel nanofiber formulation based on polycaprolactone (PCL) and loaded with progesterone to coat for Arabin pessaries to be used as dual preventive and therapeutic approaches for local vaginal delivery. Several important criteria were considered in this study to assess the prepared nanofibers (i.e.; nanofiber diameter, progesterone loading efficiency, progesterone release profiles and in vitro cytotoxicity assessment). The results showed a dimeter of 397 ± 88 nm, drug loading of 142 ± 3 µg/mg and encapsulation efficiency of 99 ± 2 % for the progesterone-loaded nanofibers. Approximately, 17 % of progesterone was released from the nanofibers after 90 days. The in vitro assessment showed that the application of progesterone is safe upon 24 and 48-hours incubation on HFF-1 cell line at concentrations ≤ 32 µg/mL and within 72-hours at a dose of ≤ 8 µg/mL. To conclude, the data recommended that progesterone-loaded nanofibers can coat the Arabin pessaries with the potential of being a safe and effective dual preventive and therapeutic tool for preterm labor.
1. Introduction
Preterm labor, opening of pregnant cervix between 20 and 37 weeks of pregnancy, is considered to be underlying the principal cause of newborn mortality and morbidity, accounting for 5 – 12 % of all pregnancies (Romero et al., 2014); (Stricker et al., 2016). Number of research have struggled to identify a pathogenic cause for preterm labor. However, they have indicated multiple contributing factors, including precocious cervical ripening, infection, uterine distension, low progesterone action, and a short cervix (Romero et al., 2014);(The P5 Working Group et al., 2019). The management of a short cervix includes the administration of progesterone or the usage of an Arabin (cervical) pessary (Stricker et al., 2016).
Progesterone, a steroidal hormone naturally secreted from the ovaries during early pregnancy, plays a major role in the reproduction cycle (Czyzyk et al., 2017); (Arikan et al., 2011). It prepares the endometrium for the embryo's implantation and modulates the immune system during pregnancy, promoting a healthier and competent microenvironment (Arikan et al., 2011);(Navathe and Berghella, 2016);(Coomarasamy et al., 2019). Alternatively, Arabin pessaries were first introduced in 1959 as a preventive tool for preterm labor (Stricker et al., 2016). The convex-shaped ring provided physical support to the cervix, resulting in lower preterm labor occurrences (Melcer et al., 2020);(Goya et al., 2012). Combined vaginal progesterone and Arabin pessaries therapy have been recently used as prevention and management tools to reduce preterm labor occurrence in cases diagnosed with a short cervix (Stricker et al., 2016); (The P5 Working Group et al., 2019). It was reported that this combined therapy showed promising results in reducing preterm labor probabilities in high-risk patients by acting on two routes of preterm labor causes; biochemical and mechanical (Melcer et al., 2020), (The P5 Working Group et al., 2019).
The technology of electrospinning has emerged recently as a functional and reliable technique for fabricating fibers in the submicron range. It employs a high electric voltage field pointed toward a metallic needle that ejects a polymeric solution. As the needle ejects a steamed jet of the solution, it gets charged with high voltage, leading to the formation of a continuous stream of fibers (Agarwal et al., 2008);(Subbiah et al., 2005). Due to its simplicity, flexibility, and biocompatibility, electrospinning has been used for many biomedical applications, including wound healing, implants, drug delivery, and tissue engineering (Agarwal et al., 2008); (Rostamabadi et al., 2020). Using biodegradable and biocompatible polymers in the fabrication of nanofibers, for instant polyvinylpyrrolidone (PVP), poly(lactic-co-glycolic acid) (PLGA), polyvinyl alcohol (PVA), and polylactic acid (PLA), offers a broader range of pharmaceutical advantages such as a structured drug release mechanism, controlled degradation behaviors, and high drug encapsulation efficiencies, making electrospun fibers excellent candidates for different drug delivery applications (Subbiah et al., 2005); (Rostamabadi et al., 2020). Polycaprolactone (PCL) has been a heavily studied polymer, and utilized for different applications in biomedical field, including cell culture, drug targeting, and medical devices (Cameron and Kamvari-Moghaddam, 2012a)(Azimi et al., 2014). PCL is known as a synthetic biodegradable polyester with a partially crystalline structure and has a low melting point of around 60 °C with the property of a glass transition temperature of −60 °C (Nair et al., 2017). It is a hydrophobic polymer with good water resistance, thus lead to longer degradation times 2–5 years (Cameron and Kamvari-Moghaddam, 2012b). The crystalline nature of this polymer enables formability at low temperatures which has an exceptional blend-compatibility to be used in biomedical applications (Mohamed and Yusoh, 2015). This polymer shows a good solubility in many organic solvents such as chloroform, dichloromethane, carbon tetrachloride, benzene, toluene, cyclohexanone and 2-nitropropane at room temperature. Due to its biocompatibility, biodegradability, and drug-loading efficiency, it attracted much attention for preparation of drug-loaded nanofibers (Dash and Konkimalla, 2012).
Many efforts have been deployed to utilized polymeric electrospun fibers for intra-vaginal localized drug delivery systems to prevent or treat various diseases (Sharma et al., 2016) (Tuğcu-Demiröz et al., 2020). Given their high biocompatibility and drug-loading efficiency, polymeric fibers were developed to address several conditions, including Sexually transmitted diseases (STDs) prevention, vaginal microbial infections, and hormonal therapy for preterm labor (Sharma et al., 2016); (Tuğcu-Demiröz et al., 2020);(Cam et al., 2020), exhibiting high vaginal drug concentrations when compared with other dosage forms (Nunes et al., 2021). Progesterone-loaded fibers have been recently recognized as a potential localized progesterone drug delivery approach to prevent preterm labor (Cam et al., 2020). A recent study indicated that progesterone-loaded fibers may offer higher bioavailability than oral dosage form, leading to a reduction in the frequency of dosage and side effects (Cam et al., 2020).
The current study is aimed to prepare progesterone-loaded PCL electrospun fibrous to coat Arabin pessaries with the ultimate goal of having a local delivery of progesterone in the vaginal cavity with the physical support derived from the Arabin pessaries. The prepared fibers will be characterized for their diameter, progesterone-loading, drug release and in vitro cytotoxicity assessment. This dual preventive and therapeutic approach for preterm labor can lead to an increase of the local drug uptake and accumulation and, therefore, bioavailability, and potentially decrease the preterm labor occurrence.
2. Materials and methods
2.1. Materials
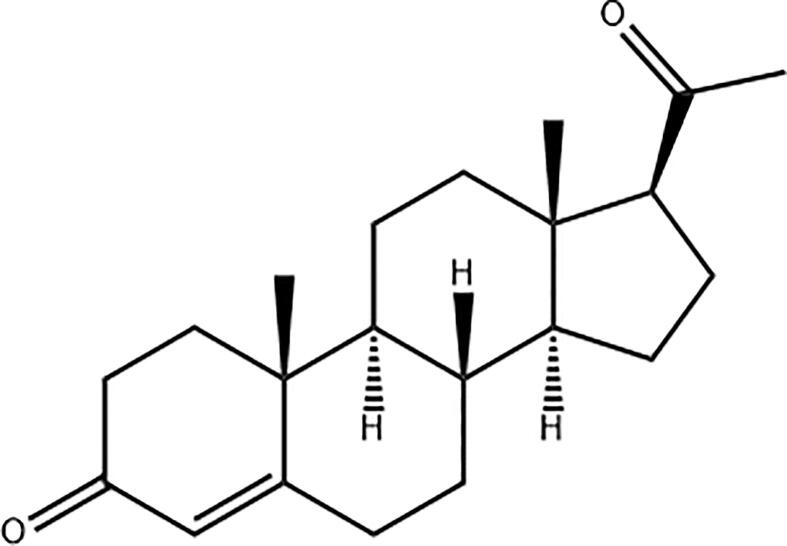
The biodegradable and biocompatible synthetic polymer employed in the entire work, PCL with average molecular weight of 80,000 Da, the HPLC grade of dimethylformamide (DMF), methanol, ethanol (≥99.5 %), acetonitrile, Chloroform, hydrochloric acid (HCl) and tablets of phosphate buffer saline (PBS) were all purchased from Sigma-Aldrich (St. Louis, MO, USA). The solution of PBS was prepared by mixing 400 µL of distilled water with 2 tablets of PBS, and adjusted to the proposed pH (4.5) using 5 M HCl. Progesterone (CAS No. 57–83-0) which is a white or creamy white crystalline powder, with a melting point of the progesterone is 121 °C that exists as a polymorph, was obtained from Merck (Darmstadt, Germany). It is a hydrophobic in nature which is soluble in alcohol and vegetable oils (solubility in ethanol is 10 mg/mL), and Fig. 1 illustrated its chemical structure. The distilled water used in this work was produced through Milli Q, Millipore (Billerica, MA, USA).
Fig. 1.
The chemical structure of progesterone drawn by ACD/ChemSketch.
2.2. Preparation of the electrospun nanofibers
The Spraybase® electrospinning setup (Dublin, Ireland) was used in the current work for the preparation of progesterone-loaded PCL nanofibers, following the previous study (Alshaya et al., 2022). The PCL polymer solution was prepared by dissolving the 12 % (w/v) of PCL powder in 70 % (v/v) chloroform and 30 % (v/v) DMF. The solution was kept under stirring for 2 h at 24 °C, until PCL polymer dissolved completely. The solution of PCL was then mixed with the drug solution in a concentration of 2 % (w/v) to obtain a 6:1 PCL: progesterone ratio and the mixture was stirred again for an additional hour to assure the complete homogeneity between the two components. Blank samples were prepared by making a 12 % (w/v) PCL solution alone without any drug. The nanofibers were fabricated at a relative humidity of 30–45 % and room temperature. The flow rate of the syringe pump used in electrospinning setup was optimized at 800 μL/hour, with a distance of 20 cm between the needle and collector, and 0.55 mm a diameter of inner needle. A voltage of 9–––10 kV was applied to achieve a stable jet.
2.3. Scanning electron microscopy morphology determination of the nanofibers
To evaluate the morphology of nanofibers surface and measure the actual diameter of achieved nanofibers, scanning electron microscope (SEM - JSM-IT500HR SEM, JEOL Inc., Peabody, MA, USA) was applied, with average measurements of at least 30 fibers for each fibrous system and at a 5 kV accelerated voltage. Aluminum foil was used for the collection of the formulated nanofibers, and then the formulations were coated with 2 nm platinum following the collection in a JEC-3000FC auto fine coater (JEOL Inc., Peabody, MA, USA). ImageJ software (National Institute of Health, Bethesda, MD, USA), was used for the analysis of nanofibers diameter. The progesterone-loaded fibers were also assessed during the release study. After day 6, the fibers were removed and initially dried using filter paper and then left in a 37 °C incubator for 24 h to remove any remaining release medium, i.e., PBS. The dried fibers were evaluated using the abovementioned SEM method.
2.4. X-ray diffraction (XRD) analysis
Rigaku Miniflex 300/600 (Tokyo, Japan) was employed in the current study to perform the XRD analysis. This instrument was equipped with current of 15 mA, voltage of 40 kV, and Cu Kα radiation (λ = 1.5148 227 Å). The XRD analysis was performed for progesterone and PCL, their physical mixture (PM) as raw powder, and both nanofibrous systems, i.e. the blank and progesterone-loaded nanofibers. All assessed nanofibers were collected on glass holders and analyzed at 2θ from 2° to 50° with a scan speed of 5°/min.
2.5. Fourier transform infrared (FTIR) analysis
To measure FTIR spectra of each sample, an Agilent Cary 630 ATR-FTIR analyzer (Agilent Technologies Inc., Santa Clara, USA) was used. The analysis was conducted at spectral resolution of 1 cm − 1 over the range of 4000 to 650 cm − 1 and 4 scans/sample.
2.6. High-performance liquid chromatography (HPLC) quantification of progesterone
Waters e2695 HPLC system (Waters Technologies Corporation, Milford, USA) was used for the quantification of progesterone. The drug was separated at 20 °C by an isocratic elution of 1 % mobile phase (pH of 3.9 adjusted using formic acid), composed of triethanolamine (60 %), methanol (5 %), and acetonitrile (35 %). 20 μL, 1.5 mL/min, and 243 nm were employed as the injection volume, flow rate of mobile phase and detection wavelength, respectively. The preparation of progesterone stock solution was conducted by mixing the progesterone with acetonitrile until complete dissolving, and then further diluted into acetonitrile: PBS (30:70). The calibration curve of progesterone was plotted by performing several serial dilutions (200 to 0.5 μg/mL), and 4.1 min was calculated as the progesterone retention time (Rt).
2.7. Encapsulation efficiency % (EE%), drug loading (DL) and drug release of the progesterone encapsulated nanofibers
The quantification of progesterone DL into nanofibers was conducted by using 20 mL of acetonitrile and adding three pieces of the formulated fibers (6.5 ± 0.1 mg) into solution. Then, solution was kept overnight at room temperature to ensure that tested nanofibers is dissolved completely. The HPLC instrument was used to identify and quantify the concentration and amount of loaded progesterone, and the following equations were used to calculate the EE% and DL:
| (1) |
| (2) |
The determination of the drug release profile of the progesterone-loaded nanofibers was conducted by adding three pieces of the formulated fibers (6.2 ± 0.1 mg) into vials containing 10 mL of pre-warmed PBS with pH 4.5. To identify the progesterone drug release from the fabricated nanofibers, a shaking incubator (Excella E24 Incubator Shaker Series, New Brunswick Scientific Co., Enfield, CT, USA) was employed with a shaking rate and temperature of 100 RPM and 37 °C, respectively. One mL of the assessed preparation was aspirated at a time-point from 30 min up to 90 days and replaced with one mL of PBS (pH 4.5). The quantification of progesterone amount was conducted using HPLC, and the following equation was applied to calculate the cumulative release percentage as a function of time:
| (3) |
The results demonstrated as average ± SD of independent triplicates.
2.8. In vitro cytotoxicity evaluation of progesterone
The evaluation of in vitro cellular viability following the application of progesterone was conducted by applying the colorimetric MTS reagent (Promega, Southampton, UK) following 24, 48 and 72-hour cells exposure of progesterone to HFF-1 cell line (ATCC- SCRC-1401), which is human skin fibroblast cells. The assessment of progesterone metabolic activity was accomplished in vitro using the modified method (Alamer et al., 2023). HFF-1 cells were normally growing in a complete Dulbecco's modified eagle medium (DMEM).
Following the confluency of HFF-1, the detachment solution (trypsin) was applied and incubated for several minutes, and then cells were collected, and were then cultured into the proper plate with a density of seeding of 1.5 × 104 cells per well. The seeded cells were then returned to incubator for 24-hour at 37 °C and 5 % CO2. 0.1 mL of increasing concentration of progesterone, ranging from 8 to 1000 µg/mL, was then applied to the human skin fibroblast cells for 24, 48 and 72-hours. DMEM and 0.2 % Triton X-100 were applied to cells and considered as a negative and positive controls, respectively. Following the proposed incubation time-point, 20 μL of the MTS solution was mixed with 100 μL of cell culture media and then was added to each well. After 3-hours, the plate was placed into normal microplate reader to measure the absorbance of formazan color produced from the cells upon the living at 492 nm, the calculation of cellular viability (%) was conducted following the below equation (Aburayan et al., 2022):
| (4) |
where S is the human skin fibroblast cell line absorbance following incubation with progesterone, T is the measurement of cells treated with Triton X-100, and H is the measurement of cells treated with fresh media only. The data were plotted as average ± SD of independent triplicates.
2.9. Statstical analysis
OriginPro® 2021 software (OriginLab Corporation, Northampton, USA) was used to present the XRD, FTIR, HPLC, and release profile data.
3. Results
3.1. Scanning electron microscopy morphology determination of the nanofibers
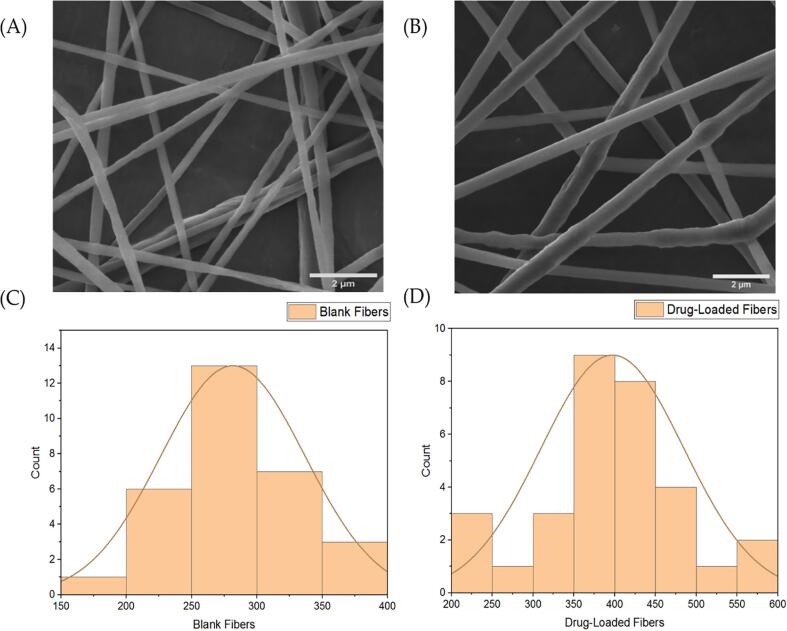
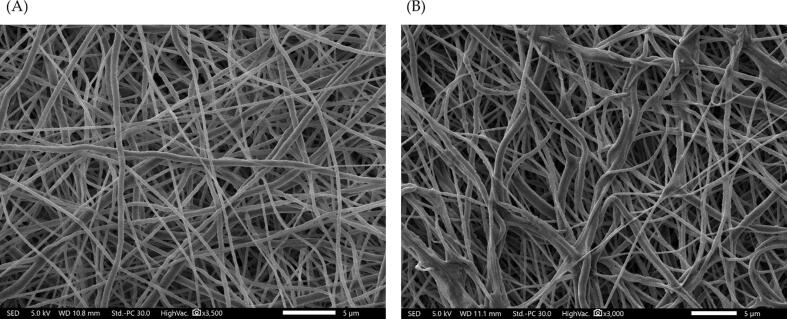
The SEM results revealed that the formulated electrospun fibers lacked of surface pores and beaded, as presented in Fig. 2 (A) and (B). The average measured diameter of drug-loaded nanofibers measurement demonstrated average blank nanofiber diameter of 282 ± 55 nm and drug-loaded nanofiber diameter of 397 ± 88 nm as shown in Fig. 2.
Fig. 2.
SEM images of that (A) blank nanofibers and (B) progesterone-loaded nanofibers as smooth, non-porous, and non-beaded, with average diameters of, (C) blank nanofibers diameter distribution and (D) progesterone-loaded nanofibers diameter distribution. The diameter of the blank and progesterone-loaded nanofibrous systems were measured as 282 ± 55 nm and 397 ± 88 nm, respectively (n = 30).
3.2. X-ray diffraction (XRD) analysis
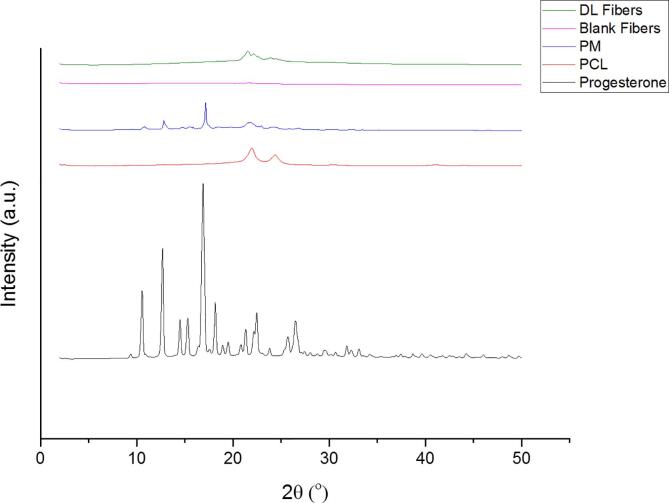
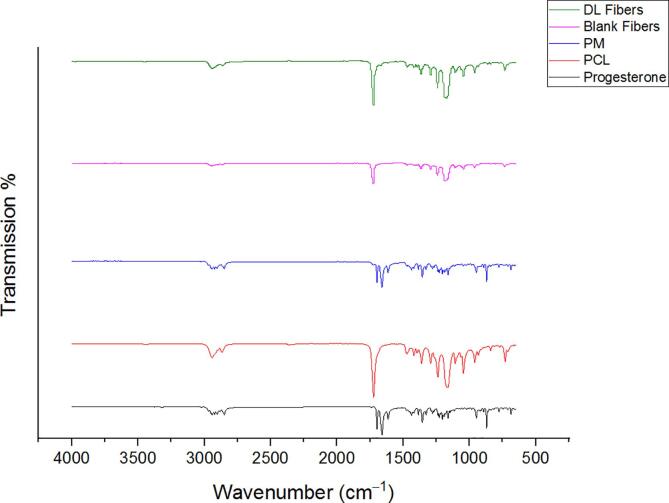
Fig. 3 exhibits an intense reflections pattern of progesterone at 2θ: 10.5°, 12.6°, and 16.3°, while the PCL polymer characteristic peaks were shown at 2θ: 21.84° and 24.26°. The PM demonstrated blend peaks of PCL at 2θ: 21.32° and 24.1°, and progesterone at 2θ: 10.4°, 14.97°, and 16.4°. The blank and drug-loaded nanofibers showed a similar diffraction pattern to PCL (i.e., 2θ: 21.3° to 24.3°).
Fig. 3.
XRD Bragg reflection patterns of progesterone and PCL, their PM, and the blank and progesterone-loaded nanofibrous systems. The patterns showed that progesterone was in the crystalline form as a pure drug and the PM, while the characteristic reflections of progesterone disappeared in the drug-loaded nanofibers due to its molecular dispersion. DL: drug-loaded, PM: physical mixture.
3.3. Fourier transform infrared (FTIR) analysis
The FTIR results presented in Fig. 4 showed peaks at 2865 cm−1, symmetric stretching of C–H; 1726 cm−1, stretching vibration of C = O (carboxyl); and 1186 cm−1, stretching vibrations of the ether groups (C-O-C) of the pure PCL. The progesterone FTIR spectrum showed characteristic peaks at 1660 and 1698 cm−1, carbonyl stretching bands at C3 and C30; 2860 cm−1, C–H asymmetrical stretching; and 870 cm−1, C = C–H stretching. The PM FTIR spectrum showed a mix of the stretching vibration of the function groups presented in the PCL and progesterone, which presented in the drug-loaded nanofibers, with a slight shift, and lacked from the blank nanofibers.
Fig. 4.
FTIR transmissions of progesterone and PCL, their PM, and the blank and progesterone-loaded nanofibers. The spectra showed that the function groups presented in the progesterone and PCL also presented in the drug-loaded nanofibers, with a slight shift, but lacked in the blank nanofibers. PM: physical mixture, DL: drug-loaded.
3.4. High-performance liquid chromatography (HPLC) quantification of progesterone
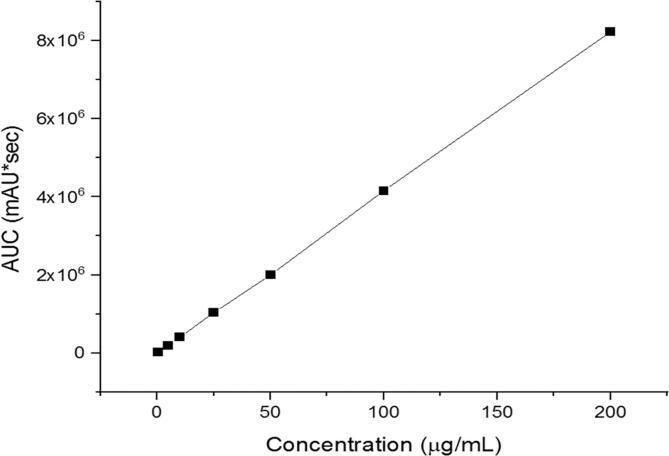
A developed HPLC method was implemented for the quantification of progesterone as shown in Fig. 5, where the regression equation was determined as y = 41206x −7882.3 with coefficient of determination (R2 = 0.9999). The successful drug separation has been determined after 4.1 min (i.e., Rt). The calibration curve exhibits the excellent linearity of this developed method and its successful drug separation.
Fig. 5.
The developed HPLC calibration curve of progesterone showed an excellent linearity (R2 = 0.9999).
The HPLC was used to calculate the progesterone, after being encapsulated into the PCL fibers, to measure its EE%, DL and release rate. The amount of progesterone was calculated first by using the linear equation ‘y = 41206x − 7882.3′ to measure the concentration in μg/mL, then the concentration was multiplied by the volume used to dissolve the fibers, either acetonitrile for the purpose of measuring the EE and DL or PBS for the release study. After that, the drug amount ‘actual amount’ was used in the Equations (1), (2), whereas, the cumulative drug amount was used in Equation (3).
3.5. Encapsulation efficiency % (EE%), drug loading (DL) and drug release of the progesterone encapsulated nanofibers
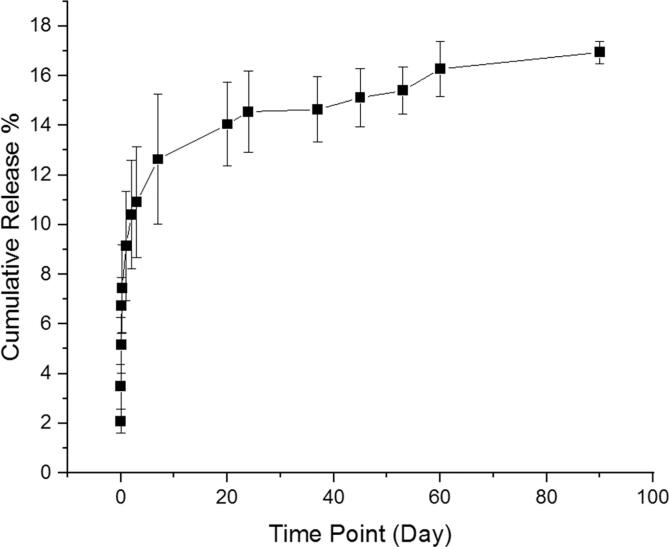
The EE%, DL and cumulative release of the progesterone-loaded nanofibers were calculated using the developed HPLC method as 99 ± 2 % and 142 ± 3 µg/mg, respectively. A sustained release of progesterone was demonstrated through 90 days, with a 2 % drug release was obtained after 30 min followed by 9 % progesterone release profile after 24 h, as presented in Fig. 6. A steady increment of the drug release of 12.6 % was obtained at day 7, followed by 14 % drug release after 20 days, then 15.1 % after 45 days, and finally, 17 % after 90 days (Fig. 6). An SEM image of the progesterone-loaded fibers before and during the release study was illustrated in Fig. 7.
Fig. 6.
Progesterone-loaded nanofibers release profile. The result shows a sustained release profile for progesterone released after 90 days is presented as the mean ± SD (n = 3).
Fig. 7.
SEM images showing the morphology of the progesterone-loaded fibers (A) before the release, and (B) 6 days after the release in PBS.
3.6. In vitro cytotoxicity evaluation of progesterone
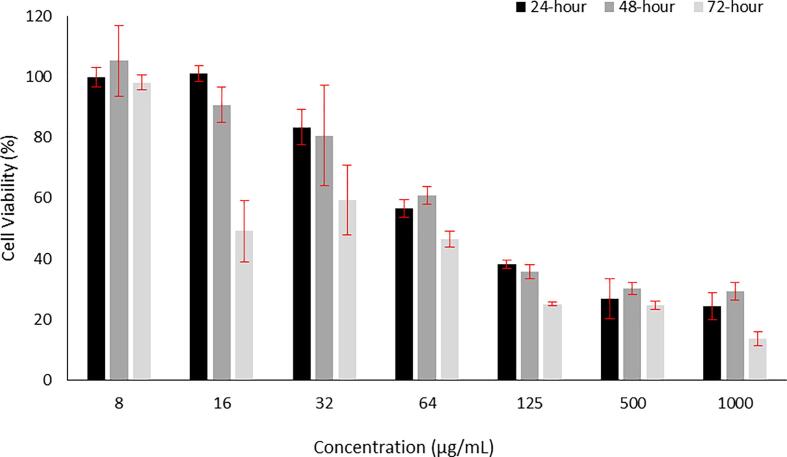
Different concentrations of progesterone incubated with human skin fibroblast cell line were assessed over 24, 48 and 72-hours, and the data is presented in Fig. 8. Progesterone concentrations ≥ 64 µg/mL showed a cellular viability below 60 % over 24 and 48-hour exposure time, while incubating progesterone for 72-hours reduced the cell viability below 60 % at a lower dose, i.e. 16 µg/mL. Progesterone concentrations of ≤ 32 µg/mL exhibited a cellular viability of ≥ 80 % over 24 and 48-hours incubation. Progesterone at 8 µg/mL demonstrated the highest percentage of cellular viability (≈ 100 %) over the three incubation time points.
Fig. 8.
Cell viability of progesterone upon 24, 48 and 72-hours treatment of the human skin fibroblast cells (HFF-1). The figure revealed that progesterone is not toxic at ≤ 32 µg/mL upon 24 and 48-hour exposure and ≤ 8 µg/mL upon exposure for 72-hour. Results are plotted as mean ± SD (n = 3).
4. Discussion
The progesterone fibers were successfully prepared, with the drug being encapsulated into PCL fibers for a sustained release purpose. The analysis of blank (PCL nanofibers) and the progesterone-loaded nanofibers morphology was conducted following the modified method (Alamer et al., 2023). The SEM images showed a successful fibrous preparatory criteria, i.e., lack of surface pores and beads, with mean nanofiber diameters of 282 ± 55 nm and 397 ± 88 nm for the blank and progesterone-loaded nanofibers, respectively (Fig. 2). The variation in nanofibers' average diameter between the two measured formulations might be attributed to the concentration of loaded progesterone that led to an increase in the size of drug-loaded nanofibers. Moreover, this increase indicated the successful encapsulation of progesterone. No observed drug crystals have been detected on the outer surfaces of progesterone-loaded nanofibers. All mentioned morphological properties confirmed the successful fabrication and preparation of fibers which is consistent with previous PCL nanofiber systems (Yaseri et al., 2023).
The XRD instrument was employed to evaluate the physical state of the raw materials (i.e., progesterone, PCL and their PM), and the blank and progesterone-loaded nanofibers. This was to confirm progesterone molecular dispersion owing to the electrospinning processing. An intense reflections pattern (sharp peaks) of progesterone observed at 2θ: 10.5°, 12.6°, and 16.3°, which confirms the crystallinity nature of the pure progesterone (Fig. 3). A previous study done by (Brako et al., 2018) showed a similar distinctive reflection of progesterone at 2θ: 12.7°, and 16.9°. The PCL polymer characteristic pattern demonstrated low-intensity peaks obtained at 2θ: 21.84° and 24.26°, hence PCL was in the crystalline nature as reported by (Gautam et al., 2013). For the PM, XRD results showed blend peaks of PCL at 2θ: 21.32° and 24.1°, while the progesterone demonstrated low-intensity peaks at 2θ: 10.4°, 14.97°, and 16.4° (Fig. 3). The low intensity of progesterone in the drug-polymer mixture might be attributed to the low drug concentration in the prepared nanofibers. The diffraction pattern seen in the blank fibers is similar to the XRD pattern of PCL, i.e., the characteristic of amorphous material. There were some reflections showed in the drug-loaded fibers at 2θ angles, specifically at 16.4°, but in less intensity than the pure progesterone. Intense peaks of PCL ranging from 21.3° to 24.3° were also observed, indicating the crystallinity of progesterone and PCL.
To identify the molecular structure of both polymer and drug and also to evaluate the interaction degree and the compatibility between PCL and progesterone within the nanofibers, FTIR spectroscopy was used. This is an important indicator of confirming the high quality and stability of fabricated nanofibers. The results indicated that the peak at 1726 cm−1 showed the starching vibration of C = O (carboxyl), which only existed in PCL (Fig. 4). The stretching vibrations of the ether groups (C-O-C) at 1186 cm−1 and the symmetric stretching of C–H at 2865 cm−1 were found in the spectra of pure PCL (Shoja et al., n.d.). Furthermore, the FTIR spectrum of progesterone exhibited characteristic peaks at 1660 and 1698 cm−1, which indicates the carbonyl stretching bands at C3 and C30. Other peaks present C–H asymmetrical stretching at 2860 cm−1 and C = C–H at 870 cm−1 (Fig. 4). Progesterone-loaded PCL nanofibers revealed both functional groups of PCL and progesterone with a slight shift, and this confirms the successful interaction between progesterone and PCL.
The developed method of HPLC method was employed to quantify the progesterone EE%, DL and release rate after its encapsulation in PCL fibers using the electrospinning technique. The EE% and DL result were calculated as 99 ± 2 % and 142 ± 3 µg/mg, respectively. Electrospun nanofibers show a sustained release behavior from PCL during 90 days (Fig. 6). After 30 min, the drug-loaded nanofibrous system was able to release 2 % of the loaded drug, while after 24 h, a burst release of the drug (≈ 9 %) was obtained. Then, a steady increment of the drug release until the seventh day, with a cumulative drug release % of ≈ 12.6 %. The initiative release is due to the rapid release of the surface of drug molecules followed by steady release, where the release is controlled by both diffusion and erosion mechanisms (Rubert et al., 2014)(Volokhova et al., 2021);(Manoukian et al., 2018), (Abdullah et al., 2020). Progesterone release was enhanced to ≈ 14 % after 20 days, then reached ≈ 15.1 % after 45 days showing an excellent sustained drug release rate. Approximately 17 % of the drug was released after 90 days. This result of the sustained release rate of progesterone is due to the hydrophobicity and crystallinity of both PCL and progesterone, which reduced the release rate of the drug from nanofibers in the PBS (pH 4.5). Another reason that may slowed the release of progesterone is that due to the entanglement and the fusion of some nanofibers together, the release of progesterone might have hindered (Fig. 7). This was consistent with a study by Tawfik et al, who reported a similar observation using another hydrophobic polymer, i.e., PLGA (Tawfik et al., 2020). The release study showed a promising result of selecting PCL as a polymer to control the drug release in the vaginal environment, as the intended final product (i.e. Arabin pessaries coated by progesterone-loaded nanofibers) will be placed in the vagina for a few months. A further in vivo vaginal model is required to demonstrate the effect of the vaginal environment on the release bahaivor of the progesterone from the fabricated formulation.
The evaluation of tested formulation activity on relevant human cells is a crucial stage to demonstrate its appropriateness for biomedical applications (Alamer et al., 2023). Increasing the dose of progesterone demonstrated a high reduction in the HFF-1 cells metabolic activity over time (Fig. 8). The dose ≥ 64 µg/mL revealed a cellular viability below 60 % over 24 and 48-hour exposure time, while the incubation of progesterone with the cells for 72 h reduced the cell viability below 60 % at a lower dose of 16 µg/mL. The lowest applied concentrations 8, 16 and 32 µg/mL following the incubation for 24 and 48 h and 8 µg/mL following the incubation for 72 h exhibited a high percentage of cellular viability (≥80 %). The result of progesterone cytotoxicity assessment shows it was not affecting the cellular viability negatively upon 24 and 48-hour treatment of tested cells at the used concentrations of ≤ 32 µg/mL and at a concentration of ≤ 8 µg/mL for 72-hour exposure. The in vitro data recommends that progesterone can be considered as not toxic, especially with the sustained release behavior of the drug-loaded fibers which favored its application due to the small amount of the drug being exposed to the cells daily. It should be noted that is important to perform additional in vitro and in vivo assessments to ensure its compatibility for vaginal delivery.
5. Conclusions
In summary, this study showed that progesterone-loaded PCL electrospun nanofibers can successfully coat the Arabin pessaries. The fabricated electrospun fibers lacked any surface pores and beads and had a mean diameter of 397 ± 88 nm. The drug loading of progesterone-loaded nanofibers was 142 ± 3 μg/mg while the EE% was 99 ± 2 %. A sustained release behavior of progesterone was demonstrated with about 17 % released after 90 days. The in vitro cell viability study showed a high cellular viability of human dermal fibroblasts (i.e., HFF-1) of progesterone at concentration of ≤ 32 µg/mL and ≤ 8 µg/mL for 24- and 48-hour, and 72-hour cellular exposure, respectively. This work offers a dual-delivery approach for progesterone nanofibers that can coat Arabin pessaries to be used in the vaginal route of administration. This route has the advantage of local delivery and sustained release of progesterone. Using relevant in vivo model is crucial to evaluate and approve the activity and suitability of the fabricated nano formulation.
Funding
This work was funded by the National Industrial Development and Logistics Program (NIDLP) through the Health Initiative and the Technology Leader Program Initiative, projects numbers 20–0103 and 20–0051.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Aliyah Almomen, Email: alalmomen@KSU.EDU.SA.
Essam A. Tawfik, Email: etawfik@kacst.gov.sa.
References
- Abdullah T., Gauthaman K., Mostafavi A., Alshahrie A., Salah N., Morganti P., Chianese A., Tamayol A., Memic A. Sustainable drug release from polycaprolactone coated chitin-lignin gel fibrous scaffolds. Sci. Rep. 2020;10:20428. doi: 10.1038/s41598-020-76971-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aburayan W.S., Alajmi A.M., Alfahad A.J., Alsharif W.K., Alshehri A.A., Booq R.Y., Alsudir S.A., Alsulaihem F.M., Bukhary H.A., Badr M.Y., Alyamani E.J., Tawfik E.A. Melittin from Bee Venom encapsulating electrospun fibers as a potential antimicrobial wound dressing patches for skin infections. Pharmaceutics. 2022;14:725. doi: 10.3390/pharmaceutics14040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Wendorff J.H., Greiner A. Use of electrospinning technique for biomedical applications. Polymer. 2008;49:5603–5621. doi: 10.1016/j.polymer.2008.09.014. [DOI] [Google Scholar]
- A.A. Alamer, N.B. Alsaleh, A.H. Aodah, A.A. Alshehri, F.A. Almughem, S.H. Alqahtani, H.A. Alfassam, E.A. Tawfik, 2023. Development of Imeglimin Electrospun Nanofibers as a Potential Buccal Antidiabetic Therapeutic Approach. Pharmaceutics 15, 1208. https://doi.org/10.3390/pharmaceutics15041208. [DOI] [PMC free article] [PubMed]
- Alshaya H.A., Alfahad A.J., Alsulaihem F.M., Aodah A.H., Alshehri A.A., Almughem F.A., Alfassam H.A., Aldossary A.M., Halwani A.A., Bukhary H.A., Badr M.Y., Massadeh S., Alaamery M., Tawfik E.A. Fast-dissolving nifedipine and atorvastatin calcium electrospun nanofibers as a potential buccal delivery system. Pharmaceutics. 2022;14:358. doi: 10.3390/pharmaceutics14020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikan I., Barut A., Harma M., Harma I.M. Effect of progesterone as a tocolytic and in maintenance therapy during preterm labor. Gynecol. Obstet. Invest. 2011;72:269–273. doi: 10.1159/000328719. [DOI] [PubMed] [Google Scholar]
- Azimi B., Nourpanah P., Rabiee M., Arbab S. Poly (∊-caprolactone) fiber: An overview. J. Eng. Fibers Fabr. 2014;9 doi: 10.1177/155892501400900309. [DOI] [Google Scholar]
- Brako F., Raimi-Abraham B.T., Mahalingam S., Craig D.Q.M., Edirisinghe M. The development of progesterone-loaded nanofibers using pressurized gyration: A novel approach to vaginal delivery for the prevention of pre-term birth. Int. J. Pharm. 2018;540:31–39. doi: 10.1016/j.ijpharm.2018.01.043. [DOI] [PubMed] [Google Scholar]
- Cam M.E., Hazar-Yavuz A.N., Cesur S., Ozkan O., Alenezi H., Turkoglu Sasmazel H., Sayip Eroglu M., Brako F., Ahmed J., Kabasakal L., Ren G., Gunduz O., Edirisinghe M. A novel treatment strategy for preterm birth: Intra-vaginal progesterone-loaded fibrous patches. Int. J. Pharm. 2020;588 doi: 10.1016/j.ijpharm.2020.119782. [DOI] [PubMed] [Google Scholar]
- R.E. Cameron, A. Kamvari-Moghaddam, 2012a. Synthetic bioresorbable polymers, in: Durability and Reliability of Medical Polymers. Elsevier, pp. 96–118. https://doi.org/10.1533/9780857096517.1.96.
- R.E. Cameron, A. Kamvari-Moghaddam, 2012b. Synthetic bioresorbable polymers, in: Durability and Reliability of Medical Polymers. Elsevier, pp. 96–118. https://doi.org/10.1533/9780857096517.1.96.
- Coomarasamy A., Devall A.J., Cheed V., Harb H., Middleton L.J., Gallos I.D., Williams H., Eapen A.K., Roberts T., Ogwulu C.C., Goranitis I., Daniels J.P., Ahmed A., Bender-Atik R., Bhatia K., Bottomley C., Brewin J., Choudhary M., Crosfill F., Deb S., Duncan W.C., Ewer A., Hinshaw K., Holland T., Izzat F., Johns J., Kriedt K., Lumsden M.-A., Manda P., Norman J.E., Nunes N., Overton C.E., Quenby S., Rao S., Ross J., Shahid A., Underwood M., Vaithilingam N., Watkins L., Wykes C., Horne A., Jurkovic D. A randomized trial of progesterone in women with bleeding in early pregnancy. N. Engl. J. Med. 2019;380:1815–1824. doi: 10.1056/NEJMoa1813730. [DOI] [PubMed] [Google Scholar]
- Czyzyk A., Podfigurna A., Genazzani A.R., Meczekalski B. The role of progesterone therapy in early pregnancy: from physiological role to therapeutic utility. Gynecol. Endocrinol. 2017;33:421–424. doi: 10.1080/09513590.2017.1291615. [DOI] [PubMed] [Google Scholar]
- Dash T.K., Konkimalla V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- Gautam S., Dinda A.K., Mishra N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C. 2013;33:1228–1235. doi: 10.1016/j.msec.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Goya M., Pratcorona L., Merced C., Rodó C., Valle L., Romero A., Juan M., Rodríguez A., Muñoz B., Santacruz B., Bello-Muñoz J.C., Llurba E., Higueras T., Cabero L., Carreras E. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomised controlled trial. Lancet. 2012;379:1800–1806. doi: 10.1016/S0140-6736(12)60030-0. [DOI] [PubMed] [Google Scholar]
- Manoukian O.S., Arul M.R., Sardashti N., Stedman T., James R., Rudraiah S., Kumbar S.G. Biodegradable polymeric injectable implants for long-term delivery of contraceptive drugs: ARTICLE. J. Appl. Polym. Sci. 2018;135:46068. doi: 10.1002/app.46068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcer Y., Kovo M., Maymon R., Bar J., Wiener I., Neeman O., Pekar-Zlotin M., Zimerman A. Arabin cervical pessary with vaginal progesterone versus vaginal progesterone for preventing preterm delivery. J. Matern. Fetal Neonatal Med. 2020;33:3439–3444. doi: 10.1080/14767058.2019.1573894. [DOI] [PubMed] [Google Scholar]
- Mohamed R.M., Yusoh K. A review on the recent research of polycaprolactone (PCL) AMR. 2015;1134:249–255. doi: 10.4028/www.scientific.net/AMR.1134.249. [DOI] [Google Scholar]
- N.R. Nair, V.C. Sekhar, K.M. Nampoothiri, A. Pandey, 2017. Biodegradation of Biopolymers, in: Current Developments in Biotechnology and Bioengineering. Elsevier, pp. 739–755. https://doi.org/10.1016/B978-0-444-63662-1.00032-4.
- Navathe R., Berghella V. Progesterone as a tocolytic agent for preterm labor: a systematic review. Curr. Opin. Obstet. Gynecol. 2016;28:464–469. doi: 10.1097/GCO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- Nunes R., Bogas S., Faria M.J., Gonçalves H., Lúcio M., Viseu T., Sarmento B., Das Neves J. Electrospun fibers for vaginal administration of tenofovir disoproxil fumarate and emtricitabine in the context of topical pre-exposure prophylaxis. J. Control. Release. 2021;334:453–462. doi: 10.1016/j.jconrel.2021.05.003. [DOI] [PubMed] [Google Scholar]
- Romero R., Dey S.K., Fisher S.J. Preterm labor: One syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostamabadi H., Assadpour E., Tabarestani H.S., Falsafi S.R., Jafari S.M. Electrospinning approach for nanoencapsulation of bioactive compounds; recent advances and innovations. Trends Food Sci. Technol. 2020;100:190–209. doi: 10.1016/j.tifs.2020.04.012. [DOI] [Google Scholar]
- Rubert M., Dehli J., Li Y.-F., Taskin M.B., Xu R., Besenbacher F., Chen M. Electrospun PCL/PEO coaxial fibers for basic fibroblast growth factor delivery. J. Mater. Chem. B. 2014;2:8538–8546. doi: 10.1039/C4TB01258E. [DOI] [PubMed] [Google Scholar]
- Sharma R., Garg T., Goyal A.K., Rath G. Development, optimization and evaluation of polymeric electrospun nanofiber: A tool for local delivery of fluconazole for management of vaginal candidiasis. Artif. Cells Nanomed. Biotechnol. 2016;44:524–531. doi: 10.3109/21691401.2014.966194. [DOI] [PubMed] [Google Scholar]
- M. Shoja, K. Shameli, M.B. Ahmad, Z. Zakaria, n.d. PREPARATION AND CHARACTERIZATION OF POLY (ε- CAPROLACTONE)/TiO2 MICRO-COMPOSITES.
- Stricker N., Timmesfeld N., Kyvernitakis I., Goerges J., Arabin B. Vaginal progesterone combined with cervical pessary: A chance for pregnancies at risk for preterm birth? Am. J. Obstet. Gynecol. 2016;214:739.e1–739.e10. doi: 10.1016/j.ajog.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Subbiah T., Bhat G.S., Tock R.W., Parameswaran S., Ramkumar S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005;96:557–569. doi: 10.1002/app.21481. [DOI] [Google Scholar]
- Tawfik E.A., Craig D.Q.M., Barker S.A. Dual drug-loaded coaxial nanofibers for the treatment of corneal abrasion. Int. J. Pharm. 2020;581 doi: 10.1016/j.ijpharm.2020.119296. [DOI] [PubMed] [Google Scholar]
- The P5 Working Group, R.C. Pacagnella, B.W. Mol, A. Borovac-Pinheiro, R. Passini, M.L. Nomura, K.C. Andrade, N. Ellovitch, K.G. Fernandes, T.G. Bortoletto, C.M. Pereira, M.J. Miele, M.S. França, J.G. Cecatti, 2019. A randomized controlled trial on the use of pessary plus progesterone to prevent preterm birth in women with short cervical length (P5 trial). BMC Pregnancy Childbirth 19, 442. https://doi.org/10.1186/s12884-019-2513-2. [DOI] [PMC free article] [PubMed]
- Tuğcu-Demiröz F., Saar S., Tort S., Acartürk F. Electrospun metronidazole-loaded nanofibers for vaginal drug delivery. Drug Dev. Ind. Pharm. 2020;46:1015–1025. doi: 10.1080/03639045.2020.1767125. [DOI] [PubMed] [Google Scholar]
- Volokhova A.A., Kudryavtseva V.L., Spiridonova T.I., Kolesnik I., Goreninskii S.I., Sazonov R.V., Remnev G.E., Tverdokhlebov S.I. Controlled drug release from electrospun PCL non-woven scaffolds via multi-layering and e-beam treatment. Mater. Today Commun. 2021;26 doi: 10.1016/j.mtcomm.2021.102134. [DOI] [Google Scholar]
- Yaseri R., Fadaie M., Mirzaei E., Samadian H., Ebrahiminezhad A. Surface modification of polycaprolactone nanofibers through hydrolysis and aminolysis: a comparative study on structural characteristics, mechanical properties, and cellular performance. Sci. Rep. 2023;13:9434. doi: 10.1038/s41598-023-36563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]