Abstract
Objective:
To determine the feasibility of a digitally automated population-based programme for organised prostate cancer testing (OPT) in Southern Sweden.
Patients and Methods:
A pilot project for a regional OPT was conducted between September 2020 and February 2021, inviting 999 randomly selected men aged 50, 56, or 62 years (y). Risk stratification was based on PSA, PSA density (PSAD), and biparametric prostate MRI. Men with a PSA level of 3–99 ng/mL had an MRI, and men with elevated PSA (≥ 3 ng/mL) had a urological check-up, including a DRE and TRUS. Indications for targeted and/or systematic transrectal prostate biopsies were suspicious lesions on MRI (prostate imaging reporting and data system [PI-RADS] 4–5) and/or PSAD > 0.15 ng/mL/cm3. Additional indications for prostate biopsies were palpable tumours, PSA ratio < 0.1, or cancer suspicion on TRUS. Patient selection, mail correspondence, data collection, and algorithm processing were performed by an automated digital management system. Feasibility is reported descriptively.
Results:
A total of 418 men had a PSA test (42%), with increasing participation rates by age (50y, 38%; 56y, 44%; and 62y, 45%). Among these, 35 men (8%) had elevated PSA (≥ 3 ng/mL: 50y, 1/139; 56y, 10/143; and 62y, 24/146). On MRI, 16 men (48%) had a negative scan (PI-RADS < 3), seven men (21%) had PI-RADS 3, nine men (27%) had PI-RADS 4, and one man (3%) had PI-RADS 5. All men with PI-RADS 4 or 5 underwent prostate biopsies, as well as two men with PI-RADS 3 due to PSAD > 0.15 ng/mL/cm3 or a suspicious finding on TRUS. Prostate cancer was diagnosed in ten men. Six men underwent active treatment, whereas four men were assigned to active surveillance.
Conclusion:
Our OPT model is feasible from an operational point of view, but due to the limited scale of this study no conclusions can be made regarding the efficacy of the diagnostic model or outcome
Keywords: Prostate cancer, screening, prostate-specific antigen, magnetic resonance imaging, algorithm
Introduction
Prostate cancer (PCa) continues to be the leading cause of cancer-related death for males in Sweden, as it has been for decades (1). In the last two decades, improvements in diagnostics and curative and palliative treatments for these patients have improved outcomes regarding PCa-specific morbidity and mortality. However, the incidence and prevalence of PCa are increasing, mainly due to improved diagnostics, a growing elderly population and men living longer with the disease. PCa is a heterogeneous disease with a spectrum from highly aggressive forms that metastasize early to indolent forms that do not necessarily have the potential to metastasize and rarely lead to any symptoms or death. A major challenge is timely diagnoses, to find potentially lethal cancers at a stage when they may be successfully treated while avoiding the detection of cancers that are unlikely to become malignant, thereby preventing anxiety and complications from unnecessary treatments. The European Randomised Screening Study for Prostate Cancer (ERSPC) has demonstrated that screening, based on repeated PSA blood tests, reduces PCa mortality although at the cost of overdiagnoses and overtreatment (2). Recently published STHLM3-MRI and Göteborg-2 trials in Sweden suggest that additional blood tests and MRI in the diagnostic work-up may be included to avoid overdiagnoses and overtreatment (3). The European Association of Urology (EAU) recommends the implementation of organised programmes for risk-stratified early detection of PCa that include MRI (4). In June 2018, the public healthcare services committee in Southern Sweden (Region Skåne [RS]) was commissioned to implement a population-based organised PCa testing (OPT) programme. The focus of this OPT was to improve the availability, quality, and equality of PCa testing within a public healthcare setting. In contrast to current opportunistic PSA testing in primary care, all men in the region within the specific age groups will receive an OPT invitation, available in multiple languages, which will improve accessibility for underserved groups.
The context of regional OPT development, the implementation process, and structure of the digitalised population-based OPT programme in RS and Region Västra Götaland (VGR) have recently been described by Alterbeck et al. (5). Prior to the implementation of the OPT programme in RS, a pilot project was conducted to assess the functionality of an automated digital OPT in terms of invitations, participation rates, and follow-up.
This pilot study was designed to compare participation rates, PSA outcomes, and PCa incidence in men aged 50, 56, and 62 years (y). Our data provide important insights that may inform resource utilisation and allocation for a large-scale programme targeting early detection of PCa. Here we report the outcomes of our pilot project for OPT in Southern Sweden.
Methods
This pilot study was conducted between September 2020 and February 2021 in RS, the Southern County of Sweden, after approval by the Swedish Ethical Review Authority (2020-03923 and 2021-06647-02). Patient selection, mail correspondence, data collection, and algorithm processing were performed by the automated digital management system and supported by the OPT Head Office, as described previously by Alterbeck et al. (5). From the Swedish population registry, a total of 999 men aged 50 (n = 367), 56 (n = 327) or 62 (n = 305) years were randomly selected from 33 municipalities in RS to generate a representative cohort. An invitation letter, detailed information about the potential advantages and disadvantages of participating in PCa testing, a personal referral for PSA testing, and a research consent form were sent to each potential participant by mail. Blood samples for PSA were collected at primary care units or hospitals and sent for analysis at regional laboratories. PSA values were automatically recorded in the administrative system and in each patient’s medical record (5).
Management algorithm, including PSA and MRI
An algorithm for invitations and further management is illustrated in Figure 1. A PSA value below 3 ng/mL was considered negative and resulted in an automated response letter sent by mail; these men were reassigned to the OPT watchlist for new invitations after 2 years (if the PSA level was 1–2.9 ng/mL) or 6 years (if the PSA level was < 1 ng/mL). Men with a PSA level > 100 ng/mL were immediately referred for a urological assessment, with no MRI performed. A PSA value of 3–99 ng/mL resulted in a referral for prostate MRI at the nearest participating radiology department. An OPT workgroup of experienced radiologists agreed on MRI protocol requirements and diagnostic evaluation, in accordance with the prostate imaging reporting and data system (PI-RADS) 2.1 document (6). In addition, two expert radiologists performed a central review of all MRI examinations, for quality assurance. The standard procedure used was biparametric MRI (including T2-weighted and diffusion-weighted imaging sequences), and the radiologists’ reports included prostate volume calculations for PSA density (PSAD), as well as focal lesion characteristics (PI-RADS 1–5) and locations (on a sector-based biopsy map).
Figure 1.
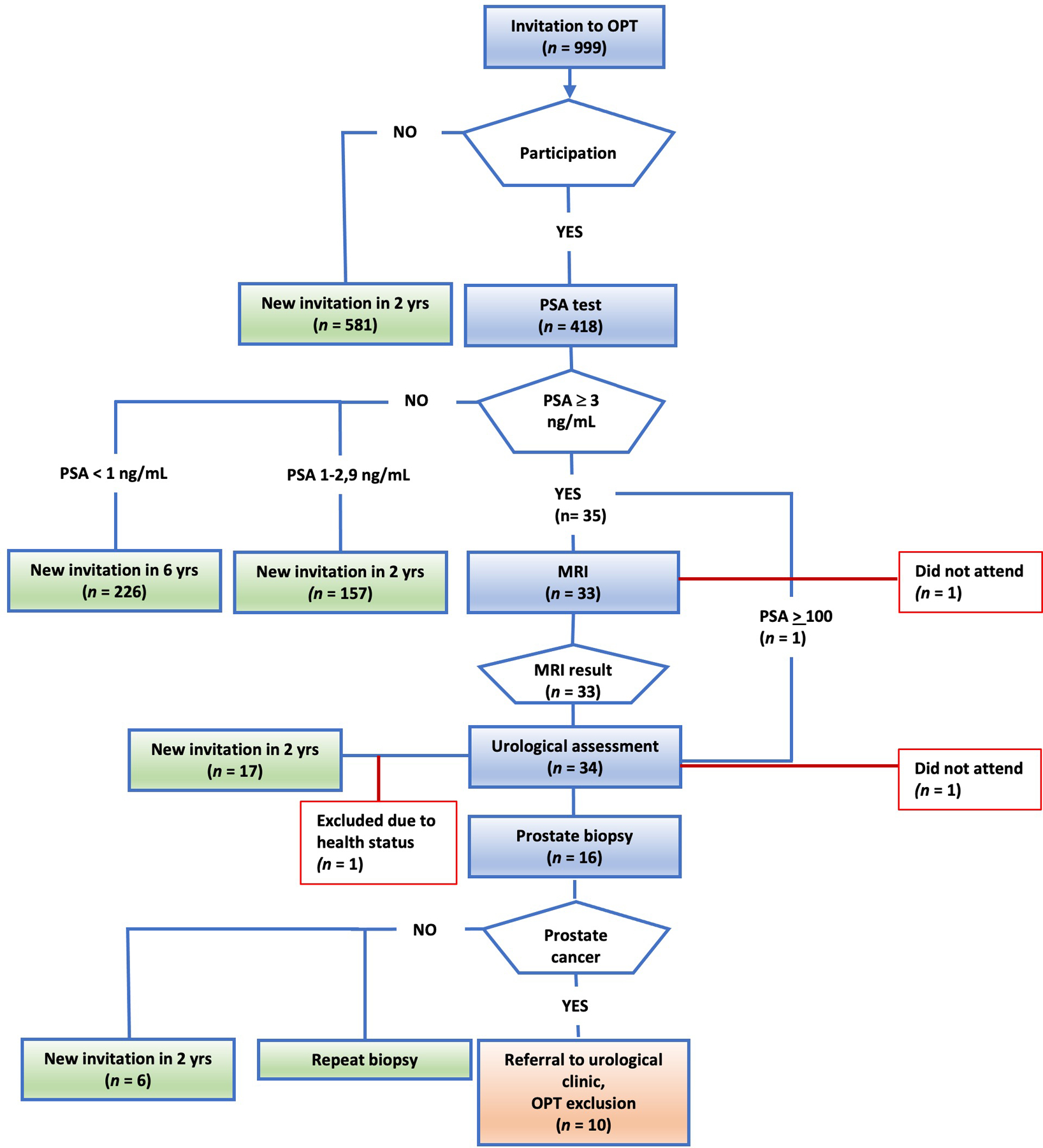
Invitations, participation, and outcomes for the OPT pilot project. Men aged 50, 56, and 62 years were randomly invited to participate in OPT with an initial PSA test. Men with PSA levels of 3–99 ng/mL were automatically referred for biparametric MRI and subsequently for urological assessment with DRE and TRUS. Targeted and/or systematic TRUS-guided biopsies were performed if indicated by the OPT-pilot algorithm (Fig. 2). Men with PSA levels below 3 ng/mL were scheduled for another invitation after 2 years (PSA = 1–2.9 ng/mL) or 6 years (PSA < 1 ng/mL).
OPT: organised prostate cancer testing
Urological examination and prostate biopsy
All men with PSA levels ≥ 3 ng/mL were referred to the nearest participating urology department (Malmö, Helsingborg, Ystad, Kristianstad, Landskrona, or Trelleborg). The urological assessment included a DRE and TRUS, as well as systematic, targeted, or combined prostate biopsies, as indicated by the algorithm (Fig. 2) (5). Participants also completed a brief questionnaire at the urology department before their clinical visit, to provide information about their general health, family history of PCa and use of anticoagulant medication.
Figure 2.
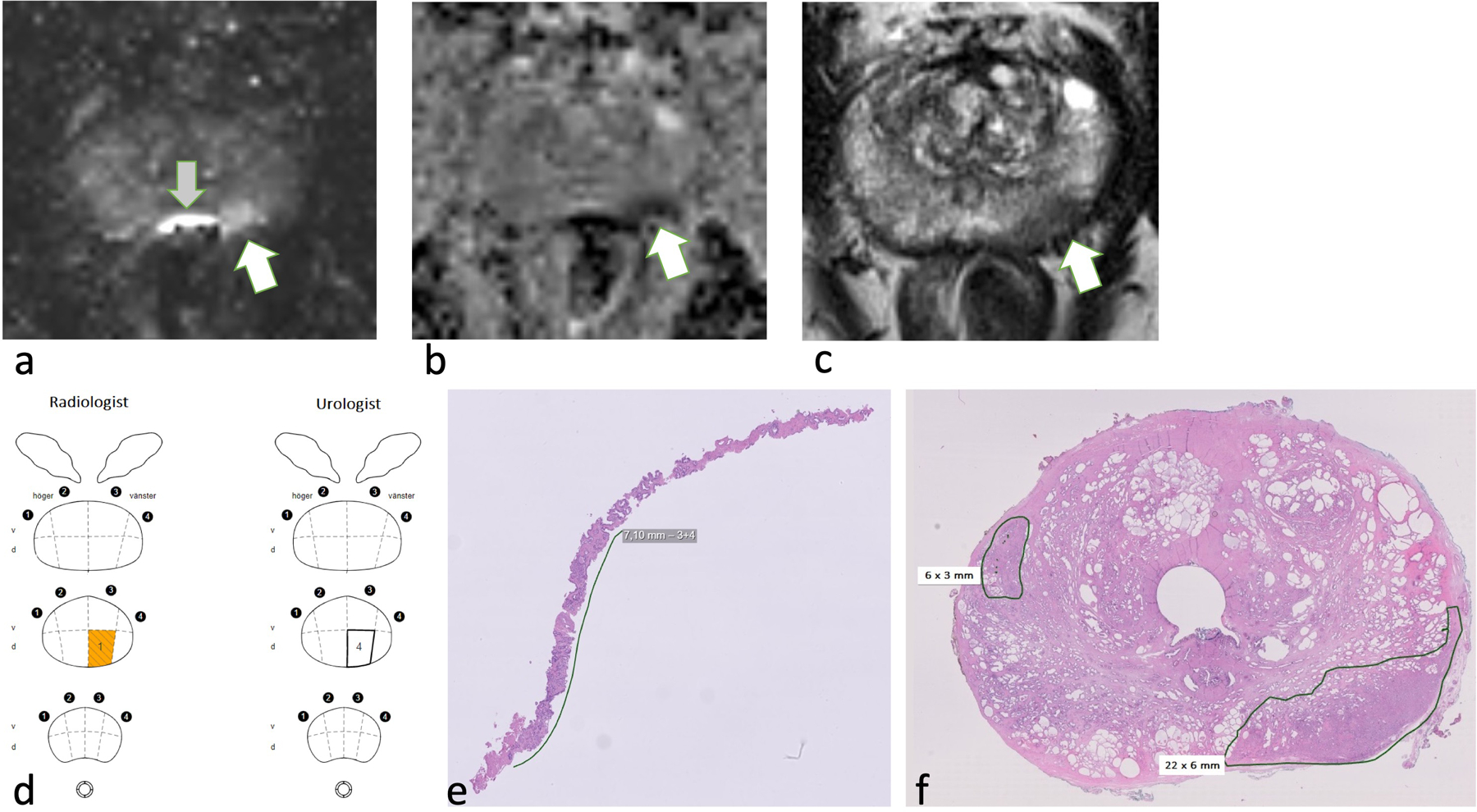
The OPT pilot algorithm for men with elevated PSA levels. Men with elevated PSA levels (≥ 3 ng/mL, but less than 100 ng/mL) underwent biparametric prostate MRI scans, which were assessed using PI-RADS. MRI was followed by urological assessments using DREs and TRUS. Targeted (3–4 per lesion) and/or systematic (10–12) transrectal biopsies were performed when indicated by the PIRADS assessments, PSAD (ng/mL/cm3), or suspicious findings on TRUS or DREs. Men with PSA levels of 100 ng/mL or greater were immediately referred for urological assessments and prostate biopsies without prior MRI.
OPT: organised prostate cancer testing; PI-RADS: prostate imaging reporting and data system; PSAD: PSA density; PSA ratio: Free PSA/total PSA.
Pathology
All biopsies were sent to regional pathology departments in Malmö, Helsingborg, or Kristianstad for histological evaluation, based on the International Society of Urological Pathology (ISUP) 2014 consensus and the ISUP Gleason grade group (GG) classification (7).
Management of prostate cancer/further investigation
Patients with PCa-positive prostate biopsies were transferred to urology departments in RS for further evaluation and treatment, and by default excluded from the OPT programme. Biopsy-negative men were informed of their results by mail and returned to the OPT watchlist for a new invitation after 2 years with the following exceptions: 1) men with PI-RADS > 4 underwent targeted biopsies with the TRUS/MRI fusion technique; 2) men with PI-RADS < 3 and PSAD > 0.3 ng/mL/cm3 had another PSA test after 3 months and if their PSA level was unchanged or increased, they had systematic and, if possible (i.e., PI-RADS 3), targeted biopsies with the TRUS/MRI fusion technique.
Statistical analysis
Data were retrieved from the administrative system and medical records, including the municipal registry, number of invitations, participation rate, lead times within the OPT pathway, PSA values, MRI results, PSAD values, cancer detection rates on prostate biopsy, and treatment choice. Descriptive statistics were reported as means, medians, interquartile ranges (IQRs), and percentages with binomial exact 95% confidence intervals.
Results
Participation rate
Among the 999 men who were invited, 418 (42%) participated and had a PSA test. In men aged 50, 56, and 62 years, the participation rates were 139/367 (38%), 143/327 (44%), and 136/305 (45%), respectively (Table 1).
Table 1.
The participation rates, PSA results in ng/mL (%) and number of PCa diagnoses for men in the different age groups, as well as the number of men who return to OPT and are scheduled for another invitation after 2 years (PSA = 1–2.9 ng/mL) or 6 years (PSA < 1 ng/mL).
| 50 years | 56 years | 62 years | All men | |
|---|---|---|---|---|
| Participation rate, n (%) | 139/367 (38%; 95% CI, 33–43 ) | 143/327 (44%; 95% CI, 38–49) | 136/305 (45%; 95% CI, 39–50) | 418/999 (42%; 95% CI, 39–45) |
| PSA < 1 ng/mL, n (%) | 93 (67% ; 95% CI, 58–75) | 77 (54%; 95% CI, 45–62) | 56 (41%; 95% CI, 33–50) | 226 (54%; 95% CI, 49–59 ) |
| PSA = 1–2.9 ng/mL, n (%) | 45 (32%; 95% CI, 25–41) | 56 (39%; 95% CI, 31–48 ) | 56 (41%; 95% CI, 33–50) | 157 (38%; 95% CI, 33–42) |
| PSA ≥ 3 ng/mL, n (%) | 1 (0,7%; 95% CI, 0,02–4) | 10 (7%; 95% CI, 3–12) | 24 (18%; 95% CI, 12–25) | 35 (8%; 95% CI, 6–11) |
| Prostate cancer, n | 0 | 1 | 9 | 10 |
| Return to OPT after: | ||||
| 2 years, n (%) | 45 (32%; 95% CI, 25–41) | 56 (39%; 95% CI,31–48 ) | 56 (41% ; 95% CI, 33–50) | 157 (38%; 95% CI, 33–42) |
| 6 years, n (%) | 93 (67%; 95% CI, 58–75) | 77 (54%; 95% CI, 45–62) | 56 (41%; 95% CI,33–50%) | 226 (54%; 95% CI, 49–59 ) |
| Total, n (%) | 138 (99%; 95% CI, 96–100%) | 133 (93%; 95% CI, 88–97%) | 112 (82% ; 95% CI, 75–88%) | 383 (92%; 95% CI, 89–94%) |
OPT: organised prostate cancer testing; PCa: prostate cancer.
Automated digital management system
All 999 invitations were managed by the digital management system and OPT head office, as were data retrieval (PSA, MRI, and biopsy results) from the 418 participants, adherence to the algorithm, and management of all response letters and future invitations.
Distribution of PSA values
The PSA levels among the 418 participants are shown in Table 1. In total, 35 participants (8%; 95% CI, 6–11) had PSA levels ≥ 3 ng/mL with the following distribution among the age groups: 0,7%; 95% CI, 0,02–4) (1/139) of men aged 50 years, 7%; 95% CI, 3–12 (10/143) of men aged 56 years, and 18%; 95% CI, 12–25 (24/136) of men aged 65 years. Men with elevated PSA (≥ 3 ng/mL) had a median PSA concentration of 4 ng/mL (IQR, 3.2–5.8), and men who were diagnosed with PCa had a median PSA level of 5.6 ng/mL (IQR, 3.43–36.5). The number of men with low (1–2.9 ng/mL) and very low (< 1 ng/mL) PSA levels decreased in the older age groups.
MRI
In total, 33 men with elevated PSA had an MRI scan. Among these, 16 men (48%; 95% CI, 31–66) had a negative MRI result (PI-RADS ≤ 2) and 17 men (52%; 95% CI, 34–69) had a positive MRI result. Among the men with positive MRI results, seven examinations (21%; 95% CI, 9–39) were classified as PI-RADS 3, nine (27%; 95% CI, 13–46) as PI-RADS 4 and one as PI-RADS 5. Two men did not have MRI scans despite having elevated PSA: one man did not respond to his invitation for an MRI or for a urological examination despite multiple reminders, and one man had a PSA level of > 100 ng/mL and was immediately referred for urological assessment and a prostate biopsy. The median time from the PSA test to the MRI report was 24 days (IQR, 13.5–29), and the median time from the MRI report to the urological visit was 16 days (IQR, 12–20). The median time between an invitation and a visit to a urological centre was 65 days (IQR, 52–76).
Prostate biopsy results and treatments
A total of 16 men had prostate biopsies. In total, 13 of the 33 men who had MRI scans were biopsied based on PSAD values > 0.15 ng/mL/cm3 and/or PI-RADS > 3. Two men with PSAD values < 0.15 ng/mL/cm3 and PI-RADS 3 lesions (i.e., no indications for biopsies, based on the OPT algorithm) had targeted biopsies due to suspicious findings on TRUS, and one man had biopsies without a prior MRI scan due to a PSA level > 100 ng/mL.
Systematic prostate biopsies were performed on four participants, targeted biopsies on seven participants, and combined biopsies on five participants. The mean number of biopsies retrieved per participant was 10.8 for systematic biopsies, 4.5 for targeted biopsies, and 12.6 for combined biopsies.
Significant cancer, defined as ISUP GG ≥ 2, was detected in seven of ten men with PCa. The overall detection rates of PCa were 1% (10/999) and 2.4% (10/418) among men who were invited to participate and who had a PSA test, respectively. Clinically significant PCa was found in 7 of 418 participants (1.7%), whereas three participants (0.7%) were diagnosed with clinically insignificant PCa (ISUP GG < 2).
The distribution of cancers in each PI-RADS group is shown in Table 2. One man had a high-grade prostatic intraepithelial neoplasia in 4/4 targeted biopsies from a PI-RADS 3 lesion and a PSAD value < 0.15 ng/mL/cm3; he was reassigned to the OPT watchlist for a new invitation after 2 years. Men with no cancer in biopsy specimens were also reassigned to receive new invitations after 2 years.
Table 2.
Distribution of men with elevated PSA, PSAD, the number of men who underwent biopsies, and the cancer-specific outcomes for those in each PI-RADS group and for those who did not undergo MRI.
| Total | PI-RADS < 3 | PI-RADS 3 | PI-RADS 4 | PI-RADS 5 | MRI not performed | |
|---|---|---|---|---|---|---|
| PSA ≥ 3 ng/mL, n (%) | 35 | 16 (48% ; 95% CI, 31–66) | 7 (21%; 95% CI, 9–39) | 9 (27%; 95% CI,13–46) | 1 (3%; 95% CI, 0,08–16) | 2* |
| PSAD > 0.15 ng/mL/cm3 | 6 | 3 (19%; 95% CI, 4–45) | 0 (0%) | 2 (22%; 95% CI, 3–60) | 1 (100% ; 95% CI,3–100) | 1 |
| Biopsies, n (%) | 16 | 3 (19% ; 95% CI, 4–45) | 2 (29%; 95% CI, 4–71) | 9 (100%; 95% CI, 66–100) | 1 (100% ; 95% CI,3–100) | 1 |
| Prostate cancer, n (%) | 10 | 1 (6% ; 95% CI, 0,2–30) | 1 (14%; 95% CI, 0,4–58) | 6 (67%; 95% CI, 30–93) | 1 (100% ; 95% CI,3–100) | 1 |
| PSAD > 0.15 ng/mL/cm3 in cancer, n | 6 | 1 | 0 | 1 | 1 | 1 |
| GG > 2, n | 7 | 1 | 1 | 3 | 1 | 1 |
One participant did not attend for their MRI scan or urological check-up.
GG: Gleason grade group; PI-RADS: prostate imaging reporting and data system; PSAD: PSA density.
Men diagnosed with PCa were managed according to national guidelines, with active surveillance (n = 4), robot-assisted radical prostatectomy (RARP; n = 3), and external beam radiation therapy (n = 2), as well as androgen deprivation therapy (n = 1) for a case of metastasized disease. OPT associated characteristics for PCa positive cases are shown in table 3. According to the final pathology reports, all three men who underwent RARP had significant tumours that corresponded to the MRI lesions, in agreement with the biopsy findings. In addition, two of the men who started active surveillance underwent RARP within 2 years, and in both cases, pathology reports showed GG2 and GG3 tumours corresponding to initial PI-RADS 4 lesions.
Table 3.
OPT characteristics of prostate cancer positive individuals including PSA levels, PSA ratios (free/total), PSAD values, and T-stage. The table also indicates whether positive biopsies were found in MRI-positive lesions (PI-RADS > 3), the number of positive cores/total cores, and the total length of cancer-positive cores/total length of all cores, as well as subsequent treatment decisions.
| Age (years) | PSA (ng/mL) |
PSA ratio (Free/Total) | PSAD (ng/mL/cm3) |
PI-RADS | T-stage | Biopsies | GG | Positive biopsy in lesion | Positive/Total cores | Cancer/Total length | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 62 | 4 | 0.24 | 0.06 | 4 | T1c | Sbx | 1 | Yes | 12-Mar | 4.7/140 | Active surveillance |
| 62 | 5.6 | 0.1 | 0.18 | 4 | T2a | SBx + TBx | 1 | Yes | 16-Feb | 0.8/180 | Active surveillance* |
| 62 | 5 | 0.17 | 0.08 | 4 | T1c | TBx | 1 | Yes | 7-Jan | 5/135 | Active surveillance |
| 62 | 3.2 | 0.11 | 0.11 | 4 | T2b | Tbx | 2 | Yes | 12-Feb | 16.7/127 | Active surveillance* |
| 62 | 3.5 | 0.09 | 0.11 | 3 | T1c | SBx + TBx | 2 | Yes | 11-Mar | 14/210 | Radical prostatectomy |
| 62 | 38 | n/a | 0.29 | < 3 | T1c | SBx | 2 | -** | 12-Jan | 2/173 | Radical prostatectomy |
| 62 | 5.8 | 0.12 | 0.14 | 4 | T1c | TBx | 2 | Yes | 4-Mar | 7/64 | EBRT |
| 56 | 3.1 | 0.12 | 0.1 | 4 | T1c | TBx | 2 | Yes | 4-Apr | 25/74 | Radical prostatectomy |
| 62 | 36 | n/a | 0.2 | 5 | T1c | TBx | 5 | Yes | 6-Jun | 43/110 | EBRT |
| 62 | 266 | n/a | 5.7 | -*** | T2 | Sbx | 5 | -*** | 9-Sep | 155/178 | Hormonal treatment |
GG: Gleason grade group; TBx: targeted biopsies; SBx: systematic biopsies; PI-RADS: prostate imaging reporting and data system; EBRT: external beam radiation therapy; PSAD: PSA density.
Both cases underwent radical prostatectomy within 2 years.
No lesion to target due to PI-RADS < 3
The participant did not have an MRI scan due to PSA > 100 ng/mL
Discussion
This pilot project is the first study of risk-adapted OPT for early detection of PCa conducted in a public healthcare setting within Sweden. The project logistics worked well, and the number of participants and men diagnosed with PCa were as expected. Our study results should help to estimate participation rates and allocate necessary resources before launching a continuous regional or national OPT programme.
Recently, we published details of the design of our OPT programme, which was developed in parallel with a similar programme in VGR (5). The VGR programme is a 3-year pilot project and the results have yet to be reported.
The overall participation rate in this pilot study was 42%, with increasing participation rates among older age groups. Our participation rate was somewhat lower than in earlier PCa screening studies in Sweden, such as the Göteborg-1 trial (76%; participant age, 50–64 years) and the Göteborg-2 trial (46%; participant age, 50–60 years) (8, 9). However, lower participation rates have been noted in recent trials, such as the STHLM3-MRI study (26%; for men aged 50–74 years) and the Finnish ProScreen Pilot study (41%; for 65-year-old men) (3, 10). Because our pilot project was initiated in the autumn of 2020, restrictions due to the coronavirus disease 2019 pandemic may have affected the participation rate.
Details about the invitation to the OPT program, and informed decision-making procedure, has been reported in detail previously. Participants were informed about the pros and cons of screening, in multiple available languages, and about the voluntary nature of participation.(5) One of the foundations of OPT is the decision of well-informed men to participate. As such, the participation rate reflects the rate of informed decision making, where choosing to undergo or forgo screening are both similarly accepted options, based on a man’s values and preferences. However, if a low participation rate is due to structural or informational inadequacies of the program, then future investigations must examine the motives of non-attenders to improve the program.
A strength of our study is the design involving three age groups, which provides information about the resources needed for screening men of different ages in terms of participation rates, PSA levels, number of MRI examinations, and the need for prostate biopsies. For example, in the 50-year-old age group, the participation rate was only 38%, and less than 1% of these participants had PSA levels ≥ 3 ng/mL. By contrast, in the 62-year-old age group, the participation rate was 44%, and 18% of these participants had elevated PSA levels. Among the ten men diagnosed with PCa, nine were detected in the 62-year-old age group and one in the 56-year-old age group. There is a tendency for the proportion of men with PSA levels ≥ 3 ng/mL to increase with age, necessitating further examinations and re-invitations. These differences are of the utmost importance when planning and implementing an OPT programme because they affect the resources needed for MRI, prostate biopsies, subsequent treatments, and re-invitations.
Furthermore, because the PSA-level has a tendency to fluctuate and many men return to “normal” PSA-levels on subsequent measurement(11), it is possible that a second reflex-PSA could reduce unnecessary MRIs and prostate biopsies for men with temporarily elevated PSA due to e.g., inflammation or /infection. However, there are practical difficulties challenges with such a reflex test in an automated OPT program, mainly due to patient compliance, resource constraints and lead times issues
In this pilot study, we found that 8% of the participants (all age groups) had elevated PSA levels ≥ 3 ng/mL, which is consistent with the results reported for the Göteborg-2 trial, in which 7% of participants (aged 50–60 years) had elevated PSA levels (9). In the ProScreen trial, 17% of the men in the study group (aged 64–65 years) had elevated PSA levels, which is similar to our cohort, in which 18% of 62-year-old men had elevated PSA levels (10). Based on our risk-stratified algorithm, which included PSAD and MRI assessments, approximately half of the men with elevated PSA (19/35) could be spared prostate biopsies.
Importantly, our PI-RADS frequencies should be interpreted with caution because there were comparatively few MRI examinations in our study. However, compared to other MRI screening studies, we observed a lower percentage (48%; 16/33) of negative MRI results (PI-RADS < 3) than in the Göteborg-2 (65%; 487/755) and Stockholm 3 (62%; 521/846) trials (3, 9). A significant advantage of incorporating MRI into a screening algorithm is avoiding unnecessary biopsies and overdiagnoses of indolent cancers, which in turn relies on avoiding false positive MRI results. The proportion of PI-RADS 4 lesions in our pilot study was greater (27%; 9/33) than in the Göteborg-2 (20%; 150/755) or STHLM3-MRI (10%; 85/846) trials. Furthermore, we found only 33% (3/9) of our PI-RADS 4 lesion biopsies had significant cancers, compared with 76% (65/85) of the PI-RADS 4 lesion biopsies in the Stockholm 3 trial. Notably, three other screening studies (Göteborg-2, STHLM2, and ProScreen) implemented centralised MRI reading by experienced radiologists, whereas in our pilot project, MRI reading was decentralised and performed by many readers with varying levels of experience, as would be the case in large population-based programmes. Further studies are needed to determine how MRI reading may be optimised in future screening programmes. Our research group plan to study the value of centralised MRI reading by expert radiologists in OPT in a future study.
Our PCa detection rate was 1% (10/999) among all men invited to participate in the study and 2.4% (10/418) among those men who did participate and had a PSA test. Clinically significant PCa was found in 7 of 418 participants (1.7%), whereas three men (0.7%) were diagnosed with clinically insignificant PCa (defined as GG1). Our findings may be compared with those of the Göteborg-2 study, which identified 0.6% insignificant and 0.9% significant cancers in the experimental group (9). The higher incidence of clinically significant cancers in our cohort may be because we invited a group of 62-year-old men and found 9 of 10 cancers in this group, whereas the men in the Göteborg-2 trial were aged 50–60 years.
Three of ten cancers (30%) detected were insignificant (GG1), compared with 29% (2/7) in the initial report from the ProScreen trial and 38% (66/176) in the Göteborg-2 study. By contrast, 18% (41/233) of cancers detected in the STHLM3-MRI trial were insignificant (3, 9, 10). However, relatively few cancers were detected in either the ProScreen trial or in our study.
Previous studies have shown the importance of re-invitations in a screening programme (12). Our algorithm includes re-invitations after 2 or 6 years, based on the findings of previous screening studies (2, 8). Delaying detection of localised PCa may not always be harmful, and offering active surveillance for localised PCa instead of immediate treatment may be a safe option, as demonstrated by the SPCG4 and ProtecT studies (13, 14).
Even if our algorithm misses some small medium-risk PCa, new assessments, which occur by default after 2 or 6 years, will probably detect any cancers that progress. Therefore, continuous evaluation of OPT outcomes and the occurrence of interval cancers is necessary to adapt the OPT algorithm and improve the detection of medium and high-risk tumours.
Previous screening trials, such as the ERSPC, have shown that PCa screening programmes can increase the risk of overdiagnoses and overtreatment (2). The risk of overdiagnoses can be reduced if algorithms for the diagnostic procedure include MRI, PSAD measurements, targeted biopsies, and other biomarker assessments. To reduce the risk of overtreatment, active surveillance programmes may also be implemented. Active surveillance programmes have been successfully implemented in Sweden, where approximately 90% of patients with low-risk disease are managed (15). We detected three ISUP GG1 tumours, which were managed by active surveillance; one GG2 tumour was also managed by active surveillance. However, two of four patients assigned to active surveillance underwent RARP within 2 years, due to tumour upgrades at subsequent check-ups.
We included two precautionary procedures in our pilot study that would not be included in a full-scale OPT programme. First, all men with PSA levels ≥ 3 ng/mL who underwent MRI also had a urological assessment, regardless of MRI findings. Second, the urologist could recommend biopsies based on the TRUS findings, DRE, or free/total PSA ratio < 0.1. These precautionary procedures were implemented in the pilot study as quality assurance steps to ensure the integrity of the automated system and MRI evaluations. In total, 2 of 35 men with elevated PSA levels underwent biopsies due to these precautionary procedures. In one case, we found a small GG 7 tumour; in the other case, we found a high-grade prostatic intraepithelial neoplasia. These two patients would not have been referred for biopsies under the full-scale OPT programme. Instead, they would have been reassigned to the OPT watchlist and given a new invitation after 2 years. These cases illustrate the trade-off between a sensitive testing algorithm and an effective but economical testing programme.
Due to the limited sample size (n = 999) and the short follow-up times, we cannot draw any conclusions regarding the efficacy of the OPT programme in reducing morbidity and mortality. However, the pilot study does provide valuable insights into the functionality of an automated and digitalised programme, as well as information on the resources needed to implement a full-scale OPT programme.
Conclusions
Our study showed that the proposed model and setup for a community-based, automated, risk-adapted OPT programme is feasible from an operational point of view. Our results suggest that an increasing age for invited men may impact participation rates, PSA levels, and PCa detection rates, affecting the resources needed for a full-scale OPT programme. Due to the limited scope of this study, it is not possible to make any assertions about the efficacy of OPT regarding the diagnostic algorithm, prostate cancer mortality, morbidity or cost-effectiveness.
Figure 3.
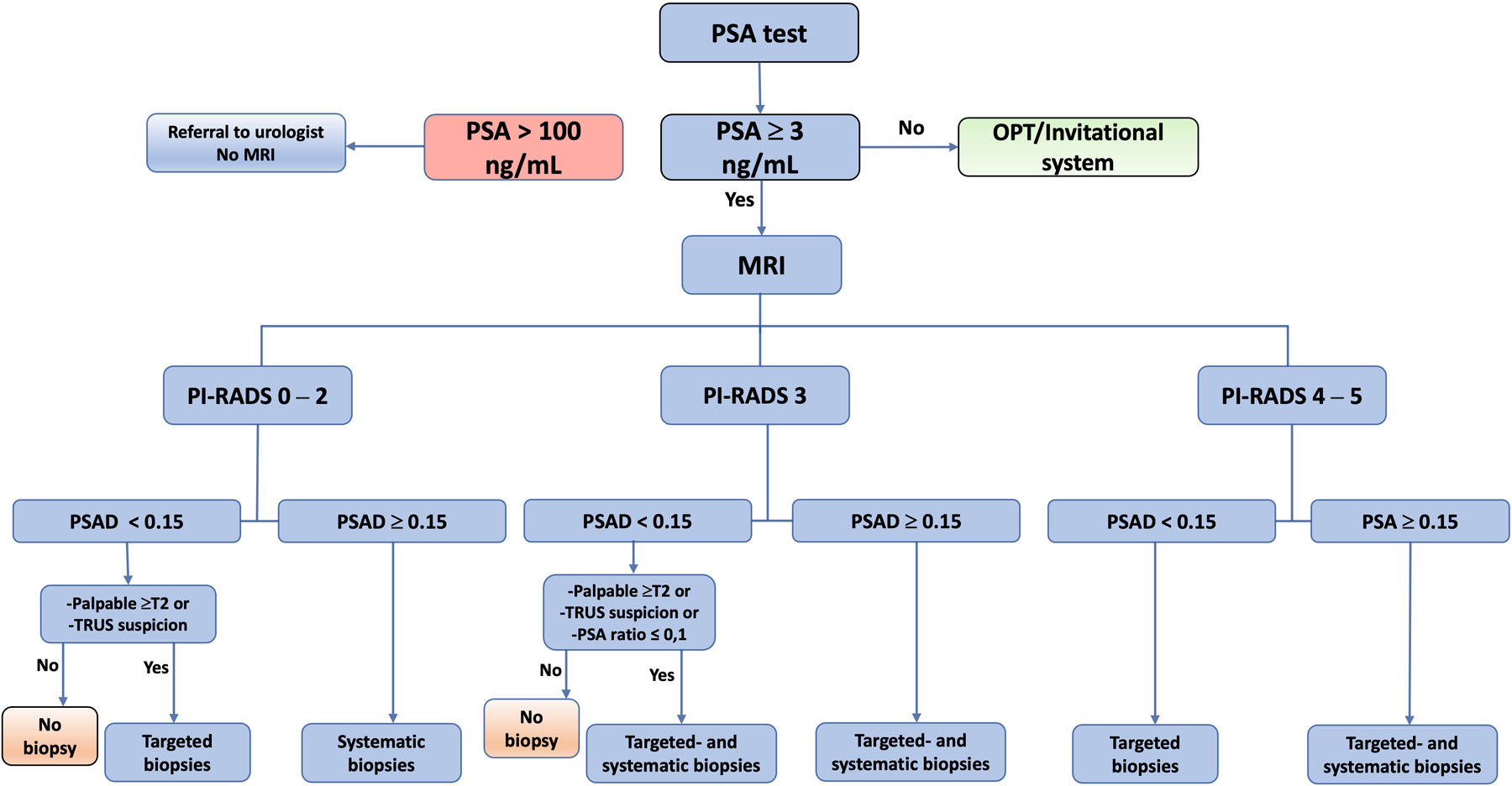
56-year-old man, PSA = 3.1 ng/mL, PSAD = 0.10 ng/mL/cm3. The MRI shows a PI-RADS 4 lesion in the dorsal portion of the mid-gland PZ. Targeted biopsies (n = 4) with cancer in 4/4 biopsies, Gleason 3+4=7. RARP with the index tumour corresponding to a PI-RADS 4 lesion.
a. MRI DWI b1500, white arrow indicates the lesion, grey arrow indicates artifact from rectal gas.
b. MRI ADC, arrow indicates the lesion.
c. MRI T2-weighted, arrow indicates the lesion.
d. Swedish nationwide web-based register platform for cancer patient data (INCA) database information from radiologist (lesion location is shown in yellow) and urologist (number and locations of targeted biopsies).
e. Digitalised pathology image of targeted biopsy shows 7-mm tumour, Gleason 3+4.
f. Digitalised image of a prostatectomy specimen. Green demarcation shows the index lesion (22 × 6 mm), dorsal portion of the mid-gland PZ. The second tumour, Gleason 3+3 (6 × 3 mm) anterior right side of the TZ, was not detected by MRI.
PSAD: PSA density, PIRADS: prostate imaging reporting and data system. PZ: peripheral zone. TZ: transition zone. RARP: robot-assisted radical prostatectomy. DWI: diffusion-weighted imaging, ADC: apparent diffusion coefficient.
Acknowledgements
The authors are grateful to NS Anna-Karin Börjedahl at the OPT office and to research administrator Ms. Anna Holst for administrative work.
Funding
This work was supported by grants from the following: The Swedish Cancer Society [#CAN 2018/522]; The Cancer Foundation at Skåne University Hospital Malmö; governmental funding (ALF) through The Faculty of Medicine at Lund University; a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30-CA008748) to Memorial Sloan Kettering Cancer Center; and donations from Gösta Jönssons Fond, Hillevi Fries Fond and Prostatacancerförbundet.
Footnotes
Conflicts of interest
Sigrid Carlsson has received travel reimbursements from Ipsen, unrelated to the current manuscript.
Disclosure statement
Sigrid Carlsson has received honorarium and travel reimbursements from Ipsen, unrelated to the current manuscript.
References
- 1.Welfare NBoHa. Cause of death statistics National Board of Health and Welfare: National Board of Health and Welfare; 2022. [Available from: https://sdb.socialstyrelsen.se/if_dor/val.aspx.
- 2.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360(13):1320–8. [DOI] [PubMed] [Google Scholar]
- 3.Nordström T, Discacciati A, Bergman M, Clements M, Aly M, Annerstedt M, et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol 2021;22(9):1240–9. [DOI] [PubMed] [Google Scholar]
- 4.Van Poppel H, Hogenhout R, Albers P, van den Bergh RCN, Barentsz JO, Roobol MJ. Early Detection of Prostate Cancer in 2020 and Beyond: Facts and Recommendations for the European Union and the European Commission. Eur Urol 2021;79(3):327–9. [DOI] [PubMed] [Google Scholar]
- 5.Alterbeck M, Järbur E, Thimansson E, Wallström J, Bengtsson J, Björk-Eriksson T, et al. Designing and Implementing a Population-based Organised Prostate Cancer Testing Programme. Eur Urol Focus 2022;8(6):1568–74. [DOI] [PubMed] [Google Scholar]
- 6.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 2019;76(3):340–51. [DOI] [PubMed] [Google Scholar]
- 7.van Leenders G, van der Kwast TH, Grignon DJ, Evans AJ, Kristiansen G, Kweldam CF, et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am J Surg Pathol 2020;44(8):e87–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol 2010;11(8):725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugosson J, Månsson M, Wallström J, Axcrona U, Carlsson SV, Egevad L, et al. Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only. N Engl J Med 2022;387(23):2126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rannikko A, Leht M, Mirtti T, Kenttämies A, Tolonen T, Rinta-Kiikka I, et al. Population-based randomized trial of screening for clinically significant prostate cancer ProScreen: a pilot study. BJU Int 2022;130(2):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastham JA, Riedel E, Scardino PT, Shike M, Fleisher M, Schatzkin A, et al. Variation of serum prostate-specific antigen levels: an evaluation of year-to-year fluctuations. Jama 2003;289(20):2695–700. [DOI] [PubMed] [Google Scholar]
- 12.Arnsrud Godtman R, Holmberg E, Lilja H, Stranne J, Hugosson J. Opportunistic testing versus organized prostate-specific antigen screening: outcome after 18 years in the Goteborg randomized population-based prostate cancer screening trial. Eur Urol 2015;68(3):354–60. [DOI] [PubMed] [Google Scholar]
- 13.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364(18):1708–17. [DOI] [PubMed] [Google Scholar]
- 14.Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, Turner EL, et al. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 2023. [DOI] [PubMed]
- 15.Sweden Tnpcro. NPCR: The national prostate cancer register of Sweden; 2022. [Available from: https://statistik.incanet.se/npcr/.


