Abstract
Patients with active tuberculosis (TB) have a stronger humoral but a poorer cellular immune response to the secreted 30-kDa antigen (Ag) of Mycobacterium tuberculosis than do healthy household contacts (HHC), who presumably are more protected against disease. The basis for this observation was studied by examining the Th1 (interleukin 2 [IL-2] and gamma interferon [IFN-γ])- and Th2 (IL-10 and IL-4)-type cytokines produced in response to the 30-kDa Ag by peripheral blood mononuclear cells (PBMC) from patients with active pulmonary TB (n = 7) and from HHC who were tuberculin (purified protein derivative) skin test positive (n = 12). Thirty-kilodalton-Ag-stimulated PBMC from TB patients produced significantly lower levels of IFN-γ (none detectable) than did those from HHC (212 ± 73 pg/ml, mean ± standard error) (P < 0.001). Likewise, 30-kDa-Ag-stimulated PBMC from TB patients failed to express IFN-γ mRNA by reverse transcription-PCR, whereas cells from HHC expressed the IFN-γ gene. In contrast, 30-kDa-Ag-stimulated PBMC from TB patients produced significantly higher levels of IL-10 (403 ± 80 pg/ml) than did those from HHC (187 ± 66 pg/ml) (P < 0.013), although cells from both groups expressed the IL-10 gene. IL-2 and IL-4 were not consistently produced, and their genes were not expressed by 30-kDa-Ag-stimulated cells from either TB patients or HHC. After treatment with antituberculous drugs, lymphocytes from four of the seven TB patients proliferated and three of them expressed IFN-γ mRNA in response to the 30-kDa Ag and produced decreased levels of IL-10.
Tuberculosis (TB) remains an important world health problem. Each year, approximately 8 million people worldwide develop active TB and 3 million die from this disease (20). Despite the severity of this medical problem, however, mechanisms of protective immunity against Mycobacterium tuberculosis in humans have not been clarified. In animal models, a protective immune response against M. tuberculosis depends on the emergence of CD4 T lymphocytes that produce cytokines which activate macrophages to kill intracellular mycobacteria (25). Gamma interferon (IFN-γ) is an essential protective cytokine in mice (1, 6, 10). IFN-γ peaks when protective immunity is maximally expressed and is produced by CD4 lymphocytes when these cells are in contact with macrophages previously infected by live mycobacteria or primed with secreted mycobacterial antigens (Ags) (1). Mice which fail to produce IFN-γ because of disruption of its gene develop a fatal tuberculous infection upon intravenous or aerogenic challenge (6, 10).
In mice, live, but not killed, mycobacteria and culture filtrates of growing mycobacteria induce a protective immune response (37). Thus, secreted Ags are of particular interest as potential targets of the human protective immune response in TB. The Ag 85 complex is a group of three major extracellular Ags of M. tuberculosis encoded by separate genes and secreted by actively proliferating cultures (37). Each of these three proteins is a major secreted product of growing bacilli. Ag 85B is identical to the previously described Ag 6 or α Ag and is now designated the 30-kDa Ag of M. tuberculosis. The 30-kDa Ag induces protective immunity against TB in guinea pigs (13).
We found that the 30-kDa Ag induces lymphocyte proliferation in cells from healthy household contacts (HHC), who presumably have a protective immune response, but does not stimulate blastogenic responses in lymphocytes from patients with active TB (36). Patients with TB, however, have a greater serological response to this Ag than HHC do (36). The 30-kDa Ag and certain of its epitopes directly stimulate IFN-γ production by T cells from tuberculin purified protein derivative (PPD)-positive donors (35). Together, these results suggest that the 30-kDa Ag may stimulate Th1-type protective cytokine responses in HHC but not in TB patients. The pattern of cytokines produced by 30-kDa-Ag-stimulated lymphocytes from HHC as compared with that produced by patients with active TB, however, is not known. In this study, selected Th1 (IFN-γ and interleukin 2 [IL-2])- and Th2 (IL-4 and IL-10)-type cytokine responses (22) to the 30-kDa Ag were examined in peripheral blood mononuclear cells (PBMC) from patients with active pulmonary TB and from HHC. The distribution of immunoglobulin G (IgG) subclasses in antibodies to the 30-kDa Ag in patients with TB also was studied because IgG1 is associated with a Th2 response and IgG2 is associated with a Th1 response in other systems. Results show no differences between levels of IL-2 and IL-4 production by stimulated cells from TB patients and from HHC. No predominant IgG1 or IgG2 subclass was found in the sera of TB patients. Lymphocytes from TB patients, however, showed decreased 30-kDa-Ag-stimulated blastogenesis and production of IFN-γ and increased IL-10 compared to cells from HHC. After 4 months of antituberculous therapy, production and expression of these cytokines were also evaluated.
MATERIALS AND METHODS
Study groups.
Seven patients with active pulmonary TB in radiographically advanced stages were studied. Each of the patients had acid-fast bacilli in his sputum and a positive sputum culture for M. tuberculosis. A PPD skin test was positive for six of the seven patients before treatment. All of the TB patients had a negative human immunodeficiency virus serologic test. Patients were studied before treatment and at 4 months after initiation of treatment. Twelve PPD-skin-test-positive HHC of TB patients were also studied. Each of these PPD-positive HHC had received Mycobacterium bovis BCG vaccination as a child. Active pulmonary TB was excluded from these HHC by chest roentgenogram and sputum smears for acid-fast bacilli. Seven PPD-skin-test-negative HHC who had received BCG as children were also studied.
Preparation of 30-kDa Ag.
The 30-kDa Ag was purified from the H37Ra strain of M. tuberculosis as described previously (31) and was a gift of Thomas Daniel (Case Western Reserve University, Cleveland, Ohio). In brief, culture filtrates of H37Ra were fractionated by DEAE-cellulose ion-exchange chromatography. The isolated product was identified as a single band by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with the 30-kDa-Ag-specific monoclonal antibody TB-C-27 (31).
DNA synthesis (blastogenesis) assay.
PBMC were separated on Ficoll-Hypaque (Nycomed Pharma, Oslo, Norway) and suspended in growth medium which consisted of RPMI 1640 (Sigma, St. Louis, Mo.), 10% fetal calf serum (Gibco BRL, Grand Island, N.Y.), 4 mM l-glutamine, 25 mM HEPES buffer, and 100 U of penicillin per ml. A total of 2 × 105 cells/well were cultured in triplicate with the 30-kDa Ag (2 μg/ml) at 37°C with 5% CO2. This concentration of 30-kDa Ag was used because preliminary studies showed that this level stimulated peak responses by PBMC. After 5 days, the cells were pulsed with [methyl-3H]thymidine (1 μCi/well; specific activity of 3H, 185 GBq/mmol); 16 h later, the cells were harvested and [3H]thymidine incorporation was measured by liquid scintillation spectroscopy. Incorporation of [3H]thymidine into DNA was expressed as the following stimulation index (SI): (counts per min [cpm] of triplicate wells with antigen)/(cpm of the triplicate wells without antigen). An index of >3 was considered a positive response. The baseline count without antigen was <1,500 cpm.
IgG assay.
For detection of antibodies against the 30-kDa Ag, an enzyme-linked immunosorbent assay (ELISA) technique was used as published previously (30). In brief, round-bottom ELISA plates (Falcon, Oxnard, Calif.) were coated with the 30-kDa Ag (0.25 μg/well). Serum (diluted 1:75) from study subjects was added in triplicate to the wells. A second anti-IgG antibody conjugated to alkaline phosphatase was then added, followed by p-nitrophenyl as a substrate. The optical density of the plates was read at 410 nm. For characterization of the different subclasses of IgG, the plates were coated with the 30-kDa Ag and serum was added (diluted 1:75). A 1:2,000 dilution of a mouse monoclonal antibody against either human IgG1, IgG2, IgG3, or IgG4 (Calbiochem, La Jolla, Calif.) was then added, followed by anti-mouse IgG (1:10,000) conjugated to biotin (Sigma). Streptavidin peroxidase and the substrate were then added to each well, and the optical density of the plates was read at 492 nm.
Immunoassays for cytokines.
PBMC were cultured at 106 cells/well in 24-well plates with or without the 30-kDa Ag (2 μg/ml). Preliminary studies indicated that the peak production of IFN-γ, IL-4, and IL-10 was at 48 h after stimulation. IL-2 production peaked at 24 h and was comparable to this peak level at 48 h. Therefore, supernatants were collected routinely at 48 h after initiation of culture. IFN-γ, IL-2, IL-4, and IL-10 levels in culture supernatants were determined by ELISA with commercial kits for human cytokines (Endogen [Cambridge, Mass.] for IFN-γ, IL-4, and IL-2; R&D [Minneapolis, Minn.] for IL-10). The cytokine sensitivities for these assays were as follows: IFN-γ, 2 pg/ml; IL-2, 31.3 pg/ml; IL-4, 7.8 pg/ml; IL-10, 3 pg/ml.
Reverse transcription (RT)-PCR for cytokines.
PBMC (106 cells/well) were stimulated for 48 h with or without the 30-kDa Ag. The cells were lysed, and mRNA was extracted by using RNAzol (Biotecx Laboratories, Houston, Tex.) and chloroform as described by Chirgwin et al. (5). Any residual DNA was removed by treatment with DNase 1 (Gibco BRL). Reverse transcriptase reactions of total RNA were performed with 200 U of the enzyme Moloney murine leukemia virus reverse transcriptase and the oligo(dT)12–18 primer (Gibco BRL). Samples were incubated for 50 min at 42°C in the presence of 25 mM MgCl2 and 2 mM deoxynucleoside triphosphate (dNTP) (Pharmacia, Piscataway, N.J.). Amplification of cDNA by PCR was performed by using specific primers for β actin, IL-4, IFN-γ, and IL-10 deduced from published sequences (39) and with the following conditions: 2.5 mM MgCl2, 0.2 mM dNTP, 200 nM 5′ and 3′ primers, and 1 U of Taq DNA polymerase (Gibco BRL). A DNA thermocycler 480 (Perkin-Elmer Cetus, Norwalk, Conn.) was used to amplify DNA for 40 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 2 min, and extension at 72°C for 1.5 min for both IFN-γ and IL-10. The same conditions were used for IL-4 except that annealing was performed at 65°C. The PCR products were electrophoresed on 2% agarose gels and detected by ethidium bromide staining.
Statistical analysis.
Differences in the responses between groups were calculated by using analysis of variance (ANOVA) for the DNA synthesis and antibody assays. For differences between levels of DNA synthesis before and after treatment in the group of patients with TB, a nonparametric Kruskal-Wallis ANOVA and a Fridman two-way ANOVA were used. All of the statistical analyses were performed with a statistical packet for personal computers (SYSTAT: the System for Statistics, 1990; SYSTAT, Inc., Evanston, Ill.).
RESULTS
Cellular and humoral responses to the 30-kDa Ag.
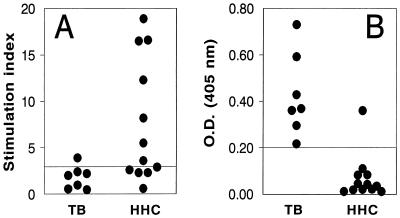
First, DNA synthesis was determined in 30-kDa-Ag-stimulated PBMC from patients with active TB and from PPD skin test-positive HHC without TB (Fig. 1A). Similar to our previous findings (36), the mean response to the 30-kDa Ag of TB patients was significantly lower than that of HHC (P < 0.05). In addition, PBMC from only 1 of 7 TB patients showed a significant proliferative response to this Ag (SI, >3.0), whereas PBMC from 7 of 12 HHC responded significantly. The response of PBMC from PPD-negative HHC was minimal, 2,601 ± 814 cpm (mean ± standard error [SE]; data not shown). Figure 1B also shows the results of the ELISA used for detection of antibodies against the 30-kDa Ag. An OD of >0.2 was used as the positive cutoff value based on the positive predictive value for this assay determined in previous studies of patients with TB and other diseases (30). The OD for 30-kDa-Ag-specific antibodies in the sera of PPD-negative HHC was 0.2 (SE, 0.17) (data not shown). Each of the seven patients with active TB had antibodies against the 30-kDa Ag. In addition, the mean 30-kDa-Ag-specific antibody level in sera from TB patients was significantly higher than that in sera from HHC (P < 0.001). Thus, in these subjects, consistent with previous results, the serologic response to the 30-kDa Ag, but not the blastogenic response of PBMC, was higher in TB patients than in HHC (36).
FIG. 1.
Blastogenic (A) and serologic (B) responses to the 30-kDa Ag in TB patients and HHC. (A) PBMC from patients with active TB and HHC that were PPD skin test positive were stimulated with the 30-kDa Ag (2 μg/ml) for 6 days, and incorporation of [3H]thymidine was measured. Results are expressed as SIs. An SI of >3 was considered a positive response. The baseline counts without Ag were 660 ± 398 cpm for TB patients and 678 ± 271 cpm for HHC. (B) ELISA was performed on serum samples from TB patients and PPD-positive HHC for reactivity to the 30-kDa Ag. Results are expressed in OD units. An OD of >0.2 was considered a positive result.
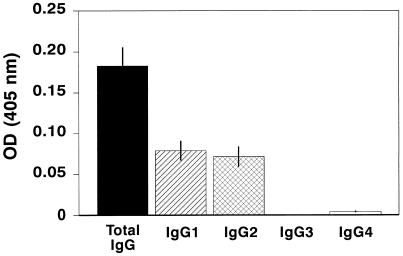
Since IgG1 is associated with Th2 responses and IgG2 is associated with Th1 responses in other systems, IgG subclasses directed against the 30-kDa Ag were further analyzed for serum from the TB patients (Fig. 2). No IgG3 or IgG4 subclasses were detected. Approximately 50% of total IgG corresponded to IgG1, and 50% corresponded to IgG2. Thus, there was no preferential expression of IgG1 and IgG2 subclasses reactive with the 30-kDa Ag among the TB patients.
FIG. 2.
Detection of IgG subclasses directed against the 30-kDa Ag. Sera from patients with active TB were analyzed by ELISA for specific IgG subclass reactivity to 30-kDa Ag. Results are presented as mean OD units ± SE.
Induction of cytokines by the 30-kDa Ag in TB patients and HHC.
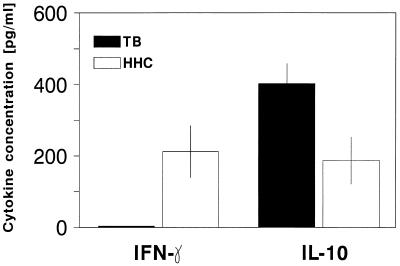
Production of IFN-γ, IL-2, IL-4, and IL-10 by PBMC in response to the 30-kDa Ag was determined by ELISA. Neither unstimulated cells from TB patients nor those from PPD-positive HHC produced detectable levels of any of the cytokines measured (data not shown). IL-4 was produced by 30-kDa-Ag-stimulated PBMC from only one of the patients with active TB (15 pg/ml) and was not detectable in cell supernatants from any of the HHC. IL-2 was produced by 30-kDa-Ag-stimulated PBMC from 3 of 6 TB patients (131 ± 30 pg/ml [mean ± SE]) and from 1 of 12 PPD-positive HHC (198 pg/ml). Overall, there were no significant differences in the production of IL-4 and IL-2 by stimulated cells from TB patients and HHC. The results for IFN-γ and IL-10 production are shown in Fig. 3. The mean concentration of IFN-γ in supernatants of 30-kDa-Ag-stimulated PBMC from TB patients was significantly lower than that of HHC (P < 0.01). In addition, there was no detectable IFN-γ produced by stimulated cells from any of the TB patients, but stimulated cells from 7 of 12 HHC produced detectable levels (212 ± 73 pg/ml; range, 6 to 535 pg/ml). Stimulated PBMC from PPD-negative HHC produced a low level of IFN-γ (31 ± 6 pg/ml; data not shown). In contrast, PBMC from all patients with active TB produced IL-10 after stimulation with the 30-kDa Ag (403 ± 80 pg/ml), and mean levels of this group were significantly higher than those of PPD-positive HHC (P < 0.005), of whom only 4 of 12 produced a detectable level of IL-10 (187 ± 66 pg/ml).
FIG. 3.
IFN-γ and IL-10 production in response to the 30-kDa Ag. PBMC from TB patients and PPD-positive HHC were stimulated with the 30-kDa Ag for 48 h. Supernatants of cells were assayed for IFN-γ and IL-10 by ELISA. Results are expressed as mean ± SE in picograms per milliliter.
Steady-state expression of cytokine mRNA in TB patients and HHC.
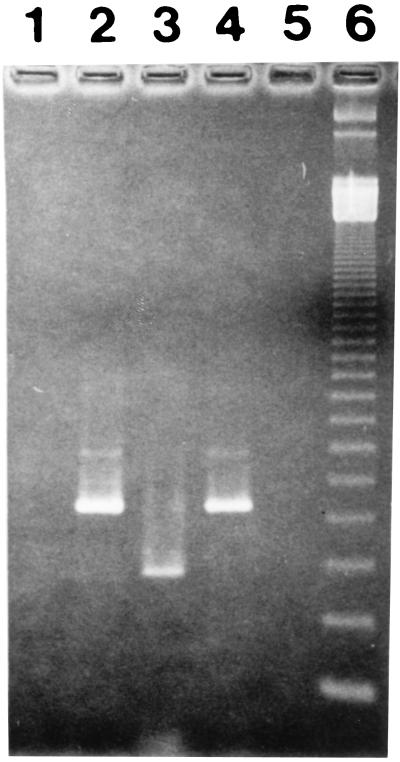
Unstimulated PBMC from the TB patients and HHC did not express IFN-γ or IL-4 mRNA as determined by RT-PCR. The IFN-γ gene was not expressed by 30-kDa-Ag-stimulated cells from any of the patients with active TB. In contrast, stimulated cells from each of the 12 HHC expressed IFN-γ mRNA. A representative experiment is shown in Fig. 4. IFN-γ mRNA was expressed by 30-kDa-Ag-stimulated cells from two of seven PPD-negative HHC (data not shown). IL-4 was not expressed by the 30-kDa-Ag-stimulated cells from any of the HHC and was expressed by cells from only 1 of the TB patients (data not shown). Thirty-kilodalton-Ag-stimulated cells from both TB patients and HHC expressed IL-10 mRNA.
FIG. 4.
Expression of IFN-γ mRNA by 30-kDa-Ag-stimulated PBMC. PBMC from HHC and patients with TB were stimulated with the 30-kDa Ag, and RNA was extracted after 48 h. RT-PCR was performed as described in Materials and Methods. RT-PCR products were visualized by staining with ethidium bromide. Lanes: 1, negative control for the extraction (all reagents without DNA from cells); 2, lysates of PBMC from HHC amplified for β actin; 3, lysates of PBMC from HHC stimulated with the 30-kDa Ag and amplified for IFN-γ; 4, lysates of PBMC from TB patients amplified for β actin; 5, lysates of PBMC from a TB patient stimulated with the 30-kDa Ag and amplified with IFN-γ; 6, molecular weight markers.
Production of cytokines after antituberculous therapy.
Of the original seven patients with active tuberculosis, follow-up data are reported for six. Blastogenic responses of 30-kDa-Ag-stimulated PBMC from TB patients were increased 4.5-fold (range, 1.5- to 7.5-fold; P < 0.025) after 4 months of treatment as compared to responses observed at initiation of therapy (Table 1). Thirty-kilodalton-Ag-stimulated cells from three of six patients studied also expressed IFN-γ mRNA as determined by RT-PCR after 4 months of treatment (data not shown). PBMC from two of these three patients expressing IFN-γ mRNA also secreted IFN-γ, although at very low levels (4 and 17 pg/ml); these two patients did not produce detectable IFN-γ before treatment (Fig. 3). PBMC from three patients with a negative RT-PCR also did not produce detectable IFN-γ in the culture supernatants. IL-10 production by 30-kDa-Ag-stimulated PBMC from patients with active TB was decreased after 4 months of treatment (403 ± 80 pg/ml before treatment versus 146 ± 56 pg/ml after treatment; P = 0.013).
TABLE 1.
Blastogenic responses to the 30-kDa Ag by PBMC from TB patients before and after treatmenta
| Patient no. | Before treatment
|
After treatment
|
||
|---|---|---|---|---|
| [3H]thymidine (cpm) | SI | [3H]thymidine (cpm) | SI | |
| 1 | 950 | 2.2 | 1,638 | 3.39 |
| 2 | 340 | 0.96 | 2,689 | 3.5 |
| 3 | 436 | 0.46 | 5,151 | 5.6 |
| 4 | 2,426 | 0.56 | 3,910 | 4.2 |
| 5 | 468 | 2.4 | 1,030 | 1.9 |
| 6 | 1,530 | 3.9 | 560 | 2.02 |
PBMC from patients with TB were stimulated with the 30-kDa Ag (2 μg/ml) before treatment and at 4 months after treatment. Blastogenic responses were measured after 6 days as the incorporation of [3H]thymidine.
DISCUSSION
This study focused on the immune response to the 30-kDa Ag of M. tuberculosis by PBMC from patients with active TB and by PBMC from HHC. As we found previously (36), patients with active TB had strong humoral and weak cellular proliferative responses to the 30-kDa Ag, whereas, inversely, HHC had weak humoral and strong cellular responses. These results are consistent with those of Havlir et al. (12). To define the specific profile of cytokines produced in response to the 30-kDa Ag, IFN-γ and IL-2 production levels were examined as representative of Th1 responses and those of IL-4 and IL-10 were examined as representative of Th2 responses (33, 39). Results show that 30-kDa-Ag-stimulated PBMC from TB patients fail to produce IFN-γ but produce high levels of IL-10. These results contrasted with the cytokine responses of cells from HHC which produced high levels of IFN-γ and low levels of IL-10. IL-4 and IL-2 were not produced consistently by stimulated PBMC from either TB patients or HHC, and responses were not different between the two groups.
There is unequivocal evidence that IFN-γ is a protective cytokine in animal models of TB (6, 10). The role of IFN-γ in protection against TB in humans is less certain (7, 29). IFN-γ production by PBMC from patients with active pulmonary TB is, however, clearly decreased in response to PPD or M. tuberculosis, suggesting a relationship between low IFN-γ levels and lack of protection (26, 40). In contrast, lymphocytes obtained from the pleural fluid of patients with TB pleurisy produce IFN-γ after being cultivated with the Erdman strain of M. tuberculosis (2). Since patients with active pleural TB have localized disease, it was proposed that IFN-γ confers protection in this clinical situation (2).
Our results further show a failure of PBMC from TB patients to produce IFN-γ in response to the 30-kDa Ag and a strong response to this antigen by cells from HHC, suggesting a protective role of IFN-γ in these HHC. Others have shown a decreased IFN-γ response to the 32-kDa Ag in TB patients (17), but ours is the first to concurrently show high IFN-γ levels produced by cells from HHC to the 30-kDa Ag. Boesen et al. (3) and Launois et al. (21), however, found that M. tuberculosis Ag obtained by culture filtrate or the 85A Ag induces production of IFN-γ in TB patients. An explanation for these differences in results may be the extent of disease: our TB patients had advanced disease and were studied before receiving treatment, whereas the patients of the study of Boesen et al. had minimal disease and those of the study of Launois et al. received treatment before the study. In fact, the patients with advanced disease studied by Boesen et al. also had decreased production of IFN-γ similar to our results (3).
The decrease in production of IFN-γ during TB might be related to lack of production of IL-12, which induces a Th1 response (32). Another possibility is that IFN-γ-producing cells may be compartmentalized to the lung during TB. Robinson et al. (28) demonstrated by in situ hybridization that cells obtained by bronchoalveolar lavage from patients with TB express the IFN-γ gene, which supports the possibility that IFN-γ-producing lymphocytes are sequestered in the lung during disease.
Orme et al. (24) found that in mice infected with M. tuberculosis, IFN-γ is produced initially and IL-4 production follows later. In the current study, IL-4 was produced by stimulated PBMC from neither HHC nor TB patients. These results are consistent with the human studies of others (2, 40). Therefore, the role of IL-4 in human TB is not clear.
IL-10 is a potent suppressor of IFN-γ synthesis by helper T cells (9) and by NK cells (15) and inhibits antigen presentation to Th1 cells (14). In leprosy, IL-10 inhibits T-cell responses as well as release of IFN-γ (34). It is possible, therefore, that the increase in IL-10 production by PBMC from TB patients in our study might be responsible for both the decreased blastogenic response to the 30-kDa Ag during active tuberculosis and the decrease in the production of IFN-γ. Our finding of high IL-10 production is of particular interest because, recently, Murray et al. (23) have shown that in transgenic mice, secretion of IL-10 by T cells induces a negative effect on BCG infection through antagonism of macrophage function.
It is of interest that treatment of patients with active TB changes the pattern of the immune response to the 30-kDa Ag in some of them. Lymphocytes from four of seven subjects unable to proliferate before treatment proliferated after treatment. In addition, cells from three of these four patients also expressed the IFN-γ gene and two of them produced low levels of IFN-γ after treatment. Our results are in agreement with those of Carlucci et al. (4), who also observed that PBMC from some but not all patients with TB proliferate in response to different mycobacterial antigens after treatment (4), and those of Wilkinson et al. (38), who showed that the production of IFN-γ increases during treatment of TB patients in response to the 16- and 38-kDa Ags of M. tuberculosis. Thus, it can be hypothesized that the initial failure to produce IFN-γ is a transitory phenomenon in some patients and possibly genetically related in others in whom no change is observed after treatment. We also demonstrated that there was a decrease in IL-10 production in cells from all of the TB patients after treatment. Thus, these changes in IFN-γ production and decreases in IL-10 production suggest that there may be a modification of cytokine expression during different stages of TB. We can speculate that these changes are related to the antigenic load, which decreases after treatment.
BCG has not been demonstrated convincingly to prevent TB (8), and there are no other alternative effective vaccines. The search for purified mycobacterial Ags useful for vaccines has thus been extensive (11, 27). In animal models, secreted Ags of mycobacteria induce a protective immune response (1). The 30-kDa Ag is a major secreted protein of M. tuberculosis (11). In mice, the 85A Ag and some peptides of this Ag induce the production of IFN-γ (16), and in guinea pigs, the 30-kDa Ag confers protection against TB (13). Epitope mapping of the 30-kDa Ag has elucidated distinct peptides of this protein that stimulate human T cells, suggesting specific sites that may induce protection (35). A recent study of mice by Huygen et al. demonstrated that a DNA vaccine encoding the 85A and 85B Ags of the Ag 85 complex (including the 30-kDa Ag) induced a Th1 response (tumornecrosis factor, IFN-γ, and IL-2), cytotoxic activity against purified native 85 Ag, and protection against challenge with BCG (18, 19). Our findings that HHC presumably protected against TB, but not patients with active TB, produce IFN-γ in response to the 30-kDa Ag further suggest that the 30-kDa Ag is a target of the human protective immune response. The basis for such protection is speculative but likely involves secretion of this protein by M. tuberculosis early during active intracellular infection. Lymphocytes previously sensitized against this Ag then may start to produce IFN-γ, which, in turn, may activate macrophages to kill mycobacteria by various intermediates and efficiently prevent the development of active TB.
ACKNOWLEDGMENTS
We thank Luis Llorente for his advice on the setup of PCR assays and Rogelio Perez Padilla for help in performing the statistical analyses.
This work was supported by grants from CONACYT Mexico (0628-M9108) and the National Institutes of Health, Bethesda, Md. (HL51630).
REFERENCES
- 1.Andersen P, Herron I. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect Immun. 1993;61:844–851. doi: 10.1128/iai.61.3.844-851.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes P F, Lu S, Abrams J S, Wang E, Yamamura M, Modlin R L. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boesen H, Jensen B N, Wilcke T, Andersen P. Human T-cell response to secreted antigen fraction of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlucci S, Beschin A, Tuosto L, Ameglio F, Gandolfo G M, Cocito C, Fiorucci F, Saltini C, Piccolella E. Mycobacterial antigen complex A60-specific T-cell repertoire during the course of pulmonary tuberculosis. Infect Immun. 1993;61:439–447. doi: 10.1128/iai.61.2.439-447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirgwin J M, Przybyla A E, McDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from a source enriched in ribonuclease. Biochemistry. 1979;18:5294. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douvas G S, Looker D L, Vatter A E, Crowle A J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine, P. E. 1989. The BCG story: lesson from the past and implications for the future. Rev. Infect. Dis. 11(Suppl. 3):53–59. [DOI] [PubMed]
- 9.Fiorentino D F, Bond M W, Mossman T R. Two types of mouse T helper cells. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harboe M H, Wilker G, Nagai S. Protein antigens of mycobacteria studied by quantitative immunological techniques. Clin Infect Dis. 1992;14:313–319. doi: 10.1093/clinids/14.1.313. [DOI] [PubMed] [Google Scholar]
- 12.Havlir D, Wallis R, Boom H, Daniel T, Chervenak K, Ellner J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991;59:665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hortwitz M A, Lee B E, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular protein of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard M, O’Garra A. Biological properties of IL 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 15.Hsu D H, Moore K W, Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992;4:563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- 16.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, De Bruyn J, Van Vooren J-P, DeLeys R. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Vanvooren J P, Turner M, Bosmans R, Dierck P, De Bruyn J. Specific lymphoproliferation, gamma interferon production and serum immunoglobulin G directed against a purified 32-kD antigen P32 in patients with tuberculosis. Scand J Immunol. 1988;27:187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 18.Huygen K, Content J, Denis O, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 19.Huygen, K., J. Content, O. Denis, et al. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex, abstr. O23. Third International Conference on the Pathogenesis of Mycobacterial Infections, 1996.
- 20.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–3. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 21.Launois P, DeLeys R, Niang M N, Drowart A, Andrien M, Dierckx P, Cartel J-L, Sarthou J-L, Van Vooren J-P, Huygen K. T-cell-epitope mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossman T, Cherwinski M, Bond M, Giedlin M, Coffman R. Two types of murine helper T cell clone according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 23.Murray P J, Wang L, Onufryk C, Tepper R, Young R. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 24.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 25.Orme I M, Andersen P, Boom H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 26.Owngubalili J K, Scott G M, Robinson J A. Deficient immune interferon production in tuberculosis. Clin Exp Immunol. 1985;59:405–413. [PMC free article] [PubMed] [Google Scholar]
- 27.Rich E A, Ellner J J. Pathogenesis of tuberculosis. In: Friedman L N, editor. Tuberculosis: a comprehensive update. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 27–50. [Google Scholar]
- 28.Robinson D, Ying S, Taylor I, Wangoo A, Mitchell D, Kay B, Hamid Q, Shaw R. Evidence for a Th1-like bronchoalveolar T-cell subset and predominance of interferon-gamma gene activation in pulmonary tuberculosis. Am J Respir Crit Care Med. 1994;149:989–993. doi: 10.1164/ajrccm.149.4.8143065. [DOI] [PubMed] [Google Scholar]
- 29.Rook G A W, Steele U, Fraher L, Barker S, Karmali S R, O’Riordan J, Stanfor J. Vitamin D3, gamma interferon and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 30.Sada E, Ferguson L, Daniel T. An enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of tuberculosis using a 30,000-dalton native antigen. J Infect Dis. 1990;162:928–931. doi: 10.1093/infdis/162.4.928. [DOI] [PubMed] [Google Scholar]
- 31.Salata R A, Sanson A J, Malotra I J, et al. Purification and characterization of the 30,000-dalton native antigen of Mycobacterium tuberculosis and characterizations of 6 monoclonal antibodies reactive with a major epitope of this antigen. J Lab Clin Med. 1991;118:589–598. [PubMed] [Google Scholar]
- 32.Scott P. IL12: initiation of cytokine for cell-mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 33.Scott P, Kaufmann S. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12:346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 34.Sieling P A, Abrams J S, Yamamura M, Salgame P, Bloom B R, Rea T H, Modlin R L. Immunosuppressive roles for IL10 and IL4 in human infection. In vitro modulation of T-cell responses in leprosy. J Immunol. 1993;150:5501–5510. [PubMed] [Google Scholar]
- 35.Silver R F, Wallis R S, Ellner J J. Mapping of T-cell epitopes of the 30-kDa antigen of Mycobacterium bovis strain bacillus Calmette-Guerin in purified protein derivative (PPD)-positive individuals. J Immunol. 1995;154:4665–4674. [PubMed] [Google Scholar]
- 36.Torres M, Mendez P, Jimenez L, Teran L, Camarena A, Quezada R, Ramos E, Sada E. Comparison of the immune response against Mycobacterium tuberculosis antigens between a group of patients with active pulmonary tuberculosis and healthy household contacts. Clin Exp Immunol. 1994;96:75–78. doi: 10.1111/j.1365-2249.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson, R. J., H. M. Vordemeir, K. de Smet, G. Pasvol, C. Moreno, and J. Ivany. 1996. Chemotherapy for human tuberculosis is accompanied by epitope spreading and cytokine changes, abstr. O17. Third International Conference on the Pathogenesis of Mycobacterial Infections, 1996.
- 39.Yamamura M, Uyemura K, Deans R, Weinberg K, Rea T, Bloom B, Modlin R. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, Lin Y, Iyer D K, Gong J, Abrams J S, Barnes P F. T-cell cytokine responses in human infections with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]