Abstract
An analysis of 13 immunoglobulin A1 (IgA1) protease genes (iga) of strains of Streptococcus pneumoniae, Streptococcus oralis, Streptococcus mitis, and Streptococcus sanguis was carried out to obtain information on the structure, polymorphism, and phylogeny of this specific protease, which enables bacteria to evade functions of the predominant Ig isotype on mucosal surfaces. The analysis included cloning and sequencing of iga genes from S. oralis and S. mitis biovar 1, sequencing of an additional seven iga genes from S. sanguis biovars 1 through 4, and restriction fragment length polymorphism (RFLP) analyses of iga genes of another 10 strains of S. mitis biovar 1 and 6 strains of S. oralis. All 13 genes sequenced had the potential of encoding proteins with molecular masses of approximately 200 kDa containing the sequence motif HEMTH and an E residue 20 amino acids downstream, which are characteristic of Zn metalloproteinases. In addition, all had a typical gram-positive cell wall anchor motif, LPNTG, which, in contrast to such motifs in other known streptococcal and staphylococcal proteins, was located in their N-terminal parts. Repeat structures showing variation in number and sequence were present in all strains and may be of relevance to the immunogenicities of the enzymes. Protease activities in cultures of the streptococcal strains were associated with species of different molecular masses ranging from 130 to 200 kDa, suggesting posttranslational processing possibly as a result of autoproteolysis at post-proline peptide bonds in the N-terminal parts of the molecules. Comparison of deduced amino acid sequences revealed a 94% similarity between S. oralis and S. mitis IgA1 proteases and a 75 to 79% similarity between IgA1 proteases of these species and those of S. pneumoniae and S. sanguis, respectively. Combined with the results of RFLP analyses using different iga gene fragments as probes, the results of nucleotide sequence comparisons provide evidence of horizontal transfer of iga gene sequences among individual strains of S. sanguis as well as among S. mitis and the two species S. pneumoniae and S. oralis. While iga genes of S. sanguis and S. oralis were highly homogeneous, the genes of S. pneumoniae and S. mitis showed extensive polymorphism reflected in different degrees of antigenic diversity.
Bacterial immunoglobulin A1 (IgA1) proteases are highly specific endopeptidases that cleave the heavy chain of human IgA1, including its secretory form (S-IgA1), in the hinge region. Such proteases are secreted by a small number of bacteria associated with humans (reviewed in references 20 and 28). IgA1 protease-producing bacteria include the mucosal pathogens Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus influenzae, Streptococcus pneumoniae, and Ureaplasma urealyticum as well as some members of resident oral and pharyngeal microfloras. Among the latter are species of Prevotella and Capnocytophaga, Streptococcus sanguis, Streptococcus oralis, and Streptococcus mitis biovar 1.
Three different classes of proteinases are represented among the IgA1 proteases, illustrating that cleavage of human IgA1 is a property that has evolved among bacteria through convergent evolution following at least three independent lines. Molecular characterizations have revealed that the IgA1 proteases of Haemophilus and Neisseria are genetically related serine proteinases (1, 25, 29, 30) and that those of S. sanguis and S. pneumoniae are metalloproteinases (8, 32, 40). Studies using specific inhibitors indicate that the IgA1 protease produced by Prevotella melaninogenica is a cysteine proteinase (26). IgA1 proteases are postproline endopeptidases, and those of streptococcal origin cleave one and the same Pro-Thr peptide bond at positions 227 to 228 in the human IgA1 heavy chain (18, 34).
The enzymes have been shown to be active in vivo, and by cleaving human IgA1 they interfere with the protective functions of the principal mediator of specific immunity of the upper respiratory tract (reviewed in reference 20). Consequently, these proteases are thought to be important for the ability of the bacteria to colonize human mucosal surfaces in the presence of specific S-IgA1 antibodies. Furthermore, they may constitute an important factor in the pathogenesis of invasive infections (20). Besides, it has been proposed that increased colonization of the pharynges of infants with IgA1 protease-producing bacteria, in particular S. mitis biovar 1, may compromise S-IgA-mediated protection against allergens and lead to atopic sensitization (17).
The iga genes encoding IgA1 protease from S. sanguis and S. pneumoniae have been characterized previously (8, 32, 40). Here we report on the cloning and sequencing of iga genes from the other two Streptococcus species, S. oralis and S. mitis, known to produce IgA1-cleaving activity. Furthermore, we have sequenced iga genes from an additional seven strains of S. sanguis representing the four different biovars of this species.
MATERIALS AND METHODS
Bacterial strains and cloning vectors.
The 27 streptococcal strains included in this study, all of which produce IgA1 protease activities, were identified as S. sanguis biovar 1 SK1 (ATCC 10556T) and SK85; S. sanguis biovar 2 SK4 and SK115; S. sanguis biovar 3 SK161 and SK162; S. sanguis biovar 4 SK49 and SK112; S. mitis biovar 1 SK141, SK286, SK564, SK595, SK597, SK599, SK601, SK603, SK605, SK609, and SK610; S. oralis SK2 (ATCC 10557), SK23 (NCTC 11427), SK39, SK100, SK105, SK153, and SK155; and S. pneumoniae PK81 by using identification principles described previously (19). In addition to the 11 strains of S. mitis listed, 44 isolates of that species were included in the study (see Results). The streptococci were grown at 37°C in an atmosphere of air plus 5% CO2 on blood agar and in Todd-Hewitt broth (Difco, Detroit, Mich.) or, for protein analyses, 2× YT medium (35). The latter medium is devoid of high-molecular-weight proteins. Bacteriophage λL47.1 (22) was used as a BamHI substitution vector, and recombinant phages were plated on Escherichia coli K802 (42). E. coli XL1-Blue (Stratagene, La Jolla, Calif.) was used as a host for propagation of recombinant forms of the phages M13mp18 and M13mp19 (43) as well as for derivatives of pUC18. E. coli XL1-Blue clones were grown in 2× YT medium supplemented with antibiotics when appropriate. DNA fragments generated by PCR amplification were cloned into plasmid pCRII with a TA cloning kit (R&D Systems Europe Ltd., Abingdon, United Kingdom).
Southern blot analyses.
Whole-cell DNA was isolated as described previously (12). Approximately 1 μg was digested with EcoRI or MspI, treated with RNase, and electrophoresed in a 1% agarose gel, and the DNA fragments were transferred and fixed by UV radiation (UV Stratalinker; Stratagene) onto a Nytran nylon membrane (Schleicher & Schuell, Keene, N.H.). Hybridizations were performed according to the method of Sambrook et al. (35) with modifications as described previously (32). Notably, the low-stringency hybridization protocol included a gradual decline in hybridization temperature which presumably favors specific annealing. Two hybridization probes, each representing half of the iga gene of S. sanguis strain SK1 (ATCC 10556) (bp 571 to 3395 and 3369 to 5702, respectively, in the published iga gene sequence [8]), have been described previously (32). The hybridization probe representing the S. pneumoniae PK81 iga gene contained bp 1 to 4722 of the published sequence (32) previously cloned into pBluescriptII, and the S. oralis SK2 iga gene probe consisted of the 6.0-kb insert of plasmid pTB1 isolated in this study. Vector sequences were removed from the DNA probes by digestion with appropriate restriction enzymes followed by agarose gel electrophoresis and elution with a GeneClean kit (Bio 101, Vista, Calif.). The two S. mitis SK141 iga gene probes were the hybridizing DNA restriction fragments of λSK141iga1 described in this study divided into the gene’s 5′ and 3′ parts at the XbaI site. The DNA fragments used for hybridization probes were labelled with [α-32P]dATP by nick translation (35).
Construction and screening of genomic libraries.
For S. mitis SK141 a Sau3A partial digest of whole-cell DNA was fractionated by agarose gel electrophoresis. Fragments in the size range 10 to 17 kb were isolated by electroelution and used to construct a genomic library with λL47.1 as a BamHI substitution vector. Recombinant phages packaged in vitro with Gigapack II Packing Extracts (Stratagene) were plated on E. coli K802 as described previously (35), and positive plaques were identified by low-stringency hybridization (32). For S. oralis SK2 the genomic library was prepared from partial MboI-digested whole-cell DNA ligated with BamHI-restricted plasmid pUC18 and transformed into competent E. coli XL1-Blue. We used MboI because the genomic DNA was resistant to cleavage with Sau3A. Recombinant plasmids carrying genes encoding IgA1 protease activities were identified among the transformants by the agar overlay method as described previously (2). Briefly, plates with the growing transformants were overlaid with agar containing insolubilized human IgA1 and then Fab fragments released by IgA1 protease activity were captured for subsequent detection on nitrocellulose discs coated with anti-light-chain antibody.
PCR.
For amplification of genomic DNA sequences, we used Taq DNA polymerase (Life Technologies, Roskilde, Denmark) as recommended by the supplier and 19- to 27-mer oligonucleotides (DNA technology, Aarhus, Denmark) as primers. The thermocycling program consisted of denaturation at 94°C for 5 min and 30 cycles of 94°C for 1 min, 55 or 60°C for 1 min, and 72°C for 2 min followed by an extension at 72°C for 6 min. For cloning of PCR products, we used the pCRII vector.
DNA sequencing.
As the DNA template for the sequencing reactions we used either single-stranded DNAs prepared from M13 phages or double-stranded DNAs from plasmids, PCR products, or lambda phage. DNAs from M13 phages were prepared as described previously (35), whereas plasmid DNAs were prepared as recommended by the supplier of the sequencing kit. Lambda phage DNA prepared as described previously (35) and PCR products were purified with Wizard Minicolumns (Promega, Madison, Wis.). Individual sequence reactions were done with a Taq DyeDeoxy-Terminator cycle sequencing kit (Perkin-Elmer, Foster City, Calif.) and analyzed with an Applied Biosystem DNA sequencer (Perkin-Elmer). As sequencing primers, we used the universal sequencing primers as well as synthetic oligonucleotides (DNA technology) designed on the basis of preceding sequences. The iga gene sequences were determined by sequencing of both strands. Computer analysis of the sequences was performed with the program manual for the Wisconsin Package (Genetics Computer Group, Madison, Wis.) and PILEUP for multiple sequence analysis, GAP for calculation of pairwise homologies, and FASTA for database searching.
IgA1 protease assay.
Proteins in the supernatant of an overnight culture in 2× YT medium were concentrated 10- to 100-fold by size exclusion centrifugation with Centriprep concentrators (Amicon, Beverly, Mass.) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). IgA1 protease activity in the gel after electrophoresis was detected as described previously (32). Briefly, inhibition of SDS was overcome and proteins were renatured by incubation of the gel for 1 h in 50 mM Tris-HCl (pH 7.4) containing 0.154 M NaCl, 1% Triton X-100, and 0.5% bovine serum albumin. Subsequently, the gel was laid on a polyvinylidene difluoride membrane onto which human myeloma IgA1 had been immobilized via its Fc part in advance by the following procedure: the membrane was incubated first with rabbit antibody directed against mouse Ig, next with a murine monoclonal antibody recognizing the Fc fragment of human IgA1, and finally with purified human myeloma IgA1 (with κ light chains). Proteins in the gel were allowed to react with IgA1 on the membrane for 2 h at 37°C. After the membrane was washed, cleavage of immobilized IgA1 was visualized by incubation with peroxidase-conjugated rabbit antibody to human κ light chains, followed by incubation with a chromogenic substrate. In this assay, IgA1 protease activity in the gel is reflected on the membrane as a loss of light chains (as part of Fab fragments) and detected on the membrane as a lack of staining.
Nucleotide sequence accession numbers.
The nucleotide sequences of the streptococcal iga genes reported here have been deposited in the EMBL nucleotide sequence database. The iga genes of the listed strains have been assigned the following accession numbers: S. sanguis SK4, Y13459; S. sanguis SK49, Y13460; S. sanguis SK85, Y13461; S. sanguis SK112, Y13455; S. sanguis SK115, Y13456; S. sanguis SK161, Y13457; S. sanguis SK162, Y13458; S. mitis SK141, Y10285; S. oralis SK2, Y10286; and S. oralis SK23, Y13224.
RESULTS
Cloning, isolation, and sequencing of streptococcal iga genes.
We have previously shown by hybridization that the iga genes encoding IgA1 protease produced by S. sanguis, S. pneumoniae, S. oralis, and S. mitis biovar 1 are all related (32). Therefore, each of two fragments of the S. sanguis iga gene was used as a radioactively labelled probe to screen a λL47.1 phage library of whole-cell DNA from S. mitis SK141. Two positive recombinant phages, λSK141iga1 and λSK141iga2, were isolated, and their DNAs were extracted. Sites for the restriction enzymes EcoRI, HindIII, and BamHI were mapped within DNA from each of the recombinant phages. The location and orientation of the iga gene were determined by Southern blot analyses of phage DNA restricted with the same enzymes and with the two S. sanguis iga gene fragments as probes (Fig. 1). Among the two positive phages, only λSK141iga1 was found to encode IgA1-cleaving activity as detected in the E. coli K802 culture lysate. Overlapping restriction fragments of the hybridizing 5.5-kb part of this phage were subcloned into phages M13mp18 and M13mp19 and sequenced. Comparison with the published iga gene sequences from S. sanguis and S. pneumoniae (8, 32, 40) suggested that the resulting sequence lacked 113 bp in the 5′ ends, which was not contained within λSK141iga1, and at least 46 bp in the 3′ ends of the genes. Since λSK141iga1 lacked the 5′ part of the iga gene, the observed expression of IgA1 protease activity was presumably driven by an in-frame promoter in the λL47.1 vector. Alternatively, a sequence within the iga gene incidentally resembled a gram-negative gene promoter and was recognized as such by E. coli as previously described for the S. sanguis iga gene (8). The sequence of the 5′ end of the S. mitis SK141 iga gene was obtained by direct sequencing of a PCR fragment generated from SK141 genomic DNA by a primer based on the sequences from λSK141iga1 combined with one based on sequences upstream of the S. oralis SK2 iga gene determined in parallel (see below). The 3′-end sequence of the S. mitis SK141 iga gene was completed by direct sequencing of λSK141iga1 DNA with a primer based on the established sequence.
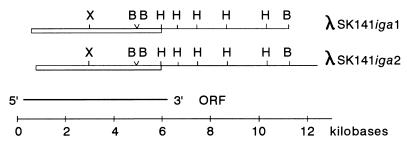
FIG. 1.
Structure of the S. mitis SK141 iga gene. Restriction maps of the two analyzed recombinant lambda phage inserts with homology to the S. sanguis iga gene probes are shown. The culture supernatant of E. coli K802 infected with λSK141iga1 possessed IgA1 cleaving activity. Symbols: H, HindIII; B, BamHI; X, XbaI. The presence of two closely located BamHI sites was revealed by sequence analysis only. Restriction fragments hybridizing to the two iga gene probes from S. sanguis are boxed. The deduced orientation of the iga gene is indicated by 5′ and 3′. The location of the large ORF is indicated below. Note that in the 5′ end of the iga gene, this region extends beyond the S. mitis SK141 genomic sequences cloned in the lambda phages as described in the text.
An unsuccessful attempt was made to amplify by PCR the iga genes from an additional six strains of S. mitis with several different combinations of the oligonucleotides synthesized for sequencing of the S. mitis SK141 and S. oralis SK2 iga genes. This result suggests that iga genes are highly heterogeneous among strains of S. mitis.
The S. oralis SK2 (ATCC 10557) iga gene was isolated from a library of whole-cell DNA cloned into plasmid pUC18 and transformed into E. coli XL1-Blue. Recombinant plasmids encoding IgA1 protease activity were identified among the transformants by the agar overlay method. A single positive clone, pTB1, containing an insert of 6.0 kb was selected for further analyses. The nucleotide sequence of the pTB1 insert was determined and revealed that this plasmid contained the complete iga gene from S. oralis SK2. The observed expression of IgA1 protease activity from the S. oralis iga gene in E. coli host cells harboring plasmid pTB1 was not examined further. It is not likely that the streptococcal iga gene promoter is recognized in gram-negative bacteria. Presumably, a promoter different from the authentic one functioned in E. coli, as was observed for the S. sanguis iga gene (8).
Both the S. oralis SK2 and the S. mitis SK141 iga gene sequences revealed a large open reading frame (ORF) starting with an ATG triplet and having the potential of encoding polypeptides of 1,839 and 1,854 amino acids, respectively, with extensive homology to the other known streptococcal IgA1 proteases (see below). In the 50-bp region preceding the ORF, these two novel iga gene sequences were 95% identical whereas they had no significant homology with the corresponding sequences from S. sanguis and S. pneumoniae (Fig. 2A). All the streptococcal iga genes analyzed contained a plausible ribosome binding site just upstream of the initiation codon, whereas a conserved −10 promoter sequence element was not evident (Fig. 2A). The authentic transcription start site of the iga genes has not been determined (8). In the nucleotide sequences downstream of the ORF, the S. oralis and S. mitis strains analyzed also displayed homologies not found among S. sanguis and S. pneumoniae strains (Fig. 2B). This region included an inverted repeat structure which may constitute a transcription terminator.
FIG. 2.
Comparison of sequences upstream and downstream of different streptococcal iga genes. Abbreviations: oralis, S. oralis SK2 (this study); mitis, S. mitis SK141 (this study); pneu2, S. pneumoniae P110 (40); pneu1, S. pneumoniae PK81 (32); sanguis, S. sanguis ATCC 10556 (8). Dashes indicate nucleotides identical to nucleotides of the S. oralis sequence. (A) Upstream sequences. The ATG initiation codons are indicated by boldface italic type. The putative ribosome binding site in the S. oralis and S. mitis sequences is overlined. The dot in the S. oralis sequence marks a gap introduced for the alignment. (B) Downstream sequences. The stop codons of the respective iga genes are indicated by boldface italic type. The putative transcription terminators in the S. oralis and S. mitis sequences are indicated by divergent arrows.
The nucleotide sequence of the iga gene from an additional strain of S. oralis, SK23 (NTCT 11427), was obtained by direct sequencing of overlapping DNA fragments generated by PCR amplification of whole-cell DNA with, as primers, different combinations of the oligonucleotides synthesized for sequencing of the two iga genes described above.
The iga gene sequence from S. sanguis ATCC 10556 (SK1) has been published previously (8). To evaluate the diversity of the iga gene in S. sanguis, we sequenced the genes from an additional seven strains of the species. Together with SK1, these strains represented each of the four biovars of S. sanguis by two strains. For the sequencing, we amplified by PCR each of the seven iga genes in three DNA fragments corresponding to bp 1 to 716, 571 to 3395, and 3369 to 5702 in the published iga gene sequence of ATCC 10556 (8). These three overlapping fragments cover the ORF of the iga gene. For each strain, the DNA fragment corresponding to bp 1 to 716 was sequenced directly with the PCR product as the template in the sequencing reactions, whereas the other two DNA fragments were sequenced after cloning into plasmid pCRII and with DNA from the resulting recombinant plasmids as the template. A few errors in the sequences of the last two fragments might have occurred during the PCR if the Taq DNA polymerase made mistakes that were copied in the cloning procedure. Three single-base differences were detected when fragments containing a total of 5 kb were resequenced directly from PCR-amplified templates. Thus, the expected frequency of PCR-generated errors in the sequences was less than 1 in 1,000.
Homologies among streptococcal IgA1 proteases.
Together with the three streptococcal iga genes published previously (8, 32, 40), the results from this study enabled us to deduce and compare amino acid sequences of the IgA1 proteases from eight strains of S. sanguis, one strain of S. mitis, two strains of S. oralis, and two strains of S. pneumoniae. The resulting alignment is shown in Fig. 3A. These 13 streptococcal IgA1 proteases were highly homologous except for a region in their N-terminal thirds which differed in both length and sequence. None of the streptococcal iga genes showed significant homology to the serine-type IgA1 proteases from Neisseria and Haemophilus or to other sequences in the GenBank and EMBL databases.
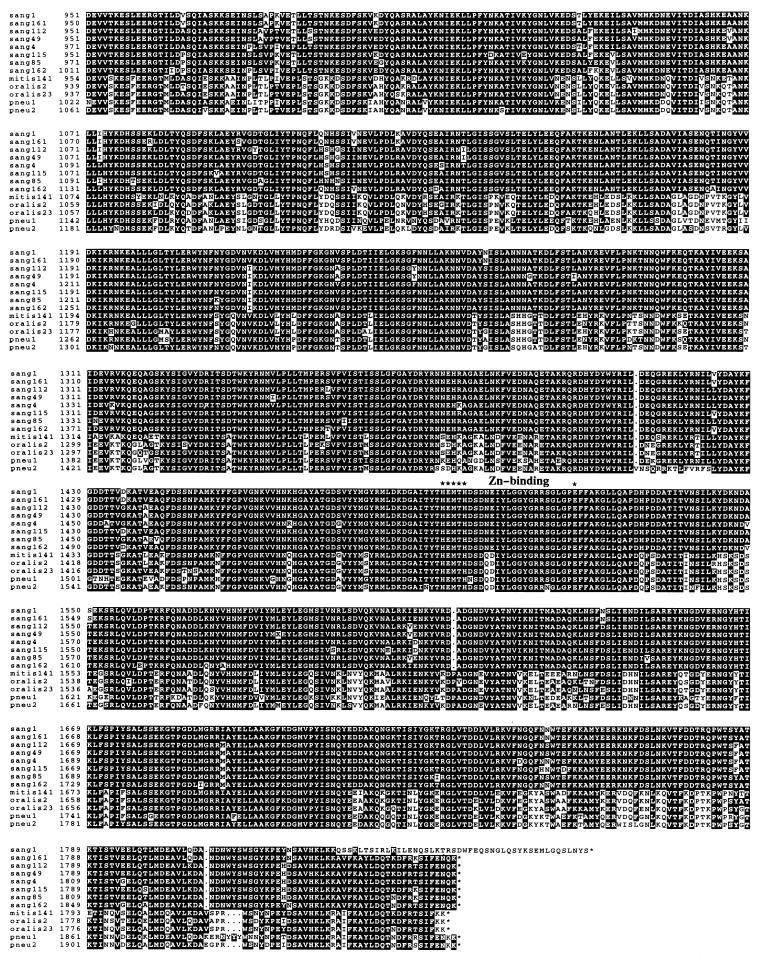
FIG. 3.
Comparison of streptococcal IgA1 protease sequences. The amino acid sequences were deduced from the iga gene sequences of S. sanguis ATCC 10556 from reference 8 (sang1), S. sanguis SK161, SK112, SK49, SK4, SK115, SK85, and SK162 from this study (sang161, sang112, sang49, sang4, sang115, sang85, and sang162, respectively), S. mitis SK141 from this study (mitis141), S. oralis SK2 and SK23 from this study (oralis2 and oralis23), S. pneumoniae PK81 from reference 32 (pneu1), and S. pneumoniae P110 from reference 40 (pneu2). (A) Alignment of the 13 IgA1 protease sequences. Gaps, indicated by dots, were introduced by the PILEUP program. Identical amino acids are in white letters on a black background. The proposed signal peptidase cleavage site, the cell wall anchor motif, and the putative zinc-binding sequence are indicated. (B) Schematic, consensus structure of the streptococcal IgA1 proteases. Black boxes indicate hydrophobic regions proposed to serve as transmembrane domains in the signal peptide and in combination with the cell wall anchor motif.
All the streptococcal IgA1 proteases contained the sequence HEMTH (at amino acid positions 1495 to 1499 in the S. sanguis SK1 IgA1 protease sequence) followed by an E residue 20 amino acids downstream (Fig. 3). These features are characteristic of the active sites of bacterial metalloproteinases (38), and site-directed mutagenesis of this motif in the S. sanguis SK1 IgA1 protease has shown that it is essential for enzyme activity (8). Thus, all the streptococcal IgA1 proteases share this catalytic mechanism. Based on its exclusive use in other bacterial metalloproteinases, Zn is the most likely ligand.
At the N terminus, a peptide of 42 amino acids with typical features of a signal sequence (39) was present in all the streptococcal IgA1 proteases analyzed. Taking this signal peptide into account, the molecular masses of the deduced proteases were approximately 200,000 Da. As with the IgA1 protease activity secreted by S. pneumoniae (32), IgA1-cleaving activities in culture supernatants of strains representing S. sanguis, S. mitis, and S. oralis could be ascribed to several molecular species with apparent molecular masses ranging from 130 to 200 kDa (Fig. 4). For the S. sanguis and S. mitis strains tested, electrophoretically determined Mrs of the high-molecular-weight form were in agreement with the calculated molecular masses, whereas for S. oralis SK2, the largest protein detected with IgA1 cleaving activity was below 200 kDa. Upon prolonged storage, conversion of the larger molecular forms of active IgA1 protease into the smaller forms was observed (data not shown).
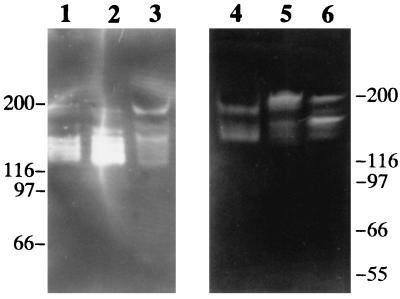
FIG. 4.
Examples of IgA1 protease profiles of different streptococcal species. Proteins in culture supernatants concentrated 10- to 100-fold were separated according to size by SDS-PAGE, renatured in the gel, and allowed to react for 2 h with human IgA1 bound via its Fc part to a filter. IgA1 cleaving activity diffusing out of the gel was visualized on the filter by lack of reaction with anti-light-chain antibodies (bands of reduced or no staining). Lane 1, S. sanguis SK4; lane 2, S. sanguis SK115; lane 3, S. sanguis SK162; lane 4, S. oralis SK2; lane 5, S. mitis SK141, lane 6, S. pneumoniae PK81. Molecular mass markers in kilodaltons are indicated for the two gels.
At amino acid positions 96 to 100, all 13 streptococcal IgA1 proteases analyzed contained a typical gram-positive cell wall anchor motif, i.e., the sequence LPNTG followed by a hydrophobic stretch (positions 103 to 125) with the potential of spanning the cytoplasmic membrane and a charged region rich in lysines (Fig. 3) (6, 15). As noted previously (8, 40), the charged lysine-rich region was followed by another hydrophobic region (positions 133 to 162) (Fig. 3).
In the N-terminal third, the IgA1 proteases were quite different. In this region (corresponding to amino acid positions 350 to 546 in the S. sanguis SK1 sequence) all the proteases contained repeat structures. S. sanguis strains SK161, SK112, SK49, SK4, SK115, SK85, and SK162; S. mitis strain SK141; and S. oralis strains SK2 and SK23 contained 10, 10, 10, 11, 10, 11, 13, 10, 9, and 9 tandem repeats, respectively, of a sequence motif similar to the 20-amino-acid repeat sequence represented by 10 copies in the S. sanguis SK1 IgA1 protease (8). The two pneumococcal IgA1 protease sequences contained three tandemly arranged copies of a different sequence of 17 amino acids in addition to several short duplicated sequence elements with homology to the repeat structures found in the other streptococcal IgA1 proteases (32, 40). Another notable feature of the N-terminal thirds of the IgA1 proteases was the high proportion of proline residues.
At the C terminus, the IgA1 proteases analyzed were very similar except for that of S. sanguis SK1, which differed markedly. This difference is caused by deletion of a single nucleotide in the SK1 iga gene sequence (at position 5538 in the published sequence [8]), resulting in a frameshift mutation. We confirmed this difference by sequencing the SK1 iga gene in this region.
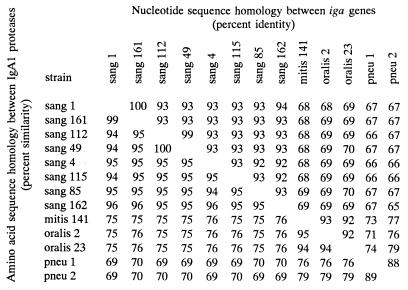
The degree of nucleotide sequence homology among iga genes representing the four different streptococcal species varied from 65 to 93% identity. Correspondingly, the amino acid sequence similarity, i.e., allowing for differences, including functionally conserved amino acids, among the deduced IgA1 protease sequences varied from 69 to 94% (Fig. 5). The highest degree of interspecies identity (92 to 93%) was displayed by the iga genes from S. mitis SK141 and the two strains of S. oralis analyzed. In contrast, the iga genes from strains of S. sanguis, which were very homogeneous within the species (92 to 100% identity), were more distantly related to those of the other species (65 to 70% identity). The two S. pneumoniae iga genes were 88% identical and showed homologies to the iga genes of the other species ranging from 65 to 79%. In the region upstream of the iga gene the two published pneumococcal sequences were almost identical. This identity extended 1.3 kb into the iga gene except for two nucleotides. The remaining part of the S. pneumoniae iga gene was highly heterogeneous. This difference in homology within the gene presumably reflects a recent recombinational event.
FIG. 5.
Percent homologies between different streptococcal iga genes and between the deduced IgA1 proteases. Species and strain designations are described in the legend to Fig. 3. One hundred percent homology means ≥99.5% homology. Abbreviations are as defined in the legend to Fig. 3.
Restriction fragment length polymorphism analyses.
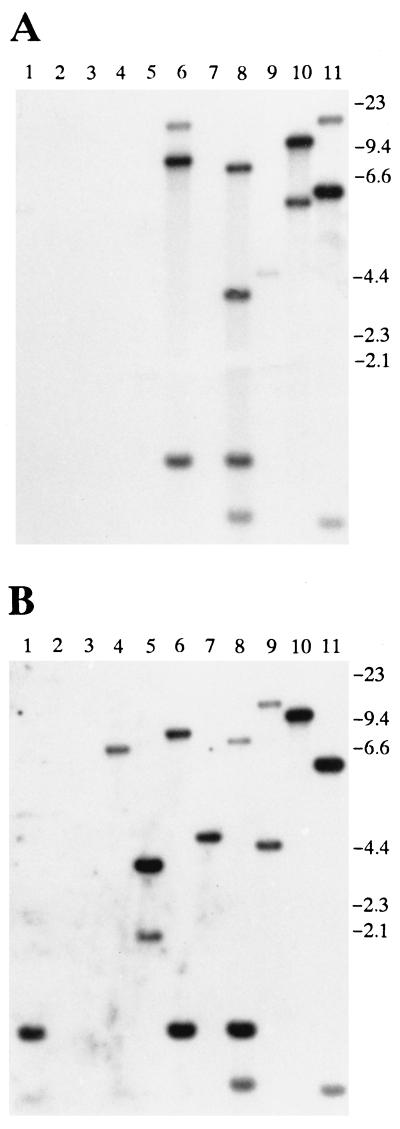
To compare iga genes among S. oralis and S. mitis strains, we performed Southern blot analyses on MspI-digested whole-cell DNA from 6 strains of S. oralis and 11 strains of S. mitis using as probes each of the iga genes from S. oralis SK2 and S. pneumoniae PK81 under stringent hybridization conditions. For each of the S. oralis strains analyzed, a unique pattern formed when MspI restriction fragments hybridized strongly with the S. oralis SK2 iga gene probe. At least one fragment from each strain hybridized also with the S. pneumoniae PK81 probe (data not shown). The 11 strains of S. mitis showed an extremely diverse pattern of MspI fragments recognized by the two probes. Five of the strains hybridized with the S. oralis SK2 iga gene probe, and nine hybridized with the S. pneumoniae PK81 probe, whereas two strains hybridized with neither of the probes (Fig. 6). In the genomes of some of the hybridizing strains (SK141, SK286, SK610, and SK595), there were MspI fragments that were recognized by the S. oralis SK2 probe only, whereas in other genomes (SK564, SK599, SK597, SK601, and SK609), there were fragments hybridizing exclusively with the S. pneumoniae PK81 iga probe.
FIG. 6.
Southern blot analysis of S. mitis biovar 1 strains. Whole-cell DNA was restricted with MspI, electrophoresed in an agarose gel, transferred to a nylon membrane, and hybridized with the iga gene probe from S. oralis SK2 (A) or S. pneumoniae PK81 (B). Lane 1, SK609; lane 2, SK605; lane 3, SK603; lane 4, SK601; lane 5, SK597; lane 6, SK595; lane 7, SK599; lane 8, SK610; lane 9, SK564; lane 10, SK286; lane 11, SK141. Molecular size markers (in kilobases) are indicated to the right.
The high degree of diversity of the iga genes observed in S. mitis strains was confirmed by EcoRI restriction fragment length polymorphism typing with two iga gene probes from S. mitis SK141 representing the 5′ and 3′ parts of the gene. A total of 48 strains of S. mitis biovar 1, of which 26 showed IgA1 protease activities, and seven strains of S. mitis biovar 2, all devoid of IgA1 protease activity, were included. Except for one strain (SK272, biovar 1), only strains producing detectable IgA1 protease activities showed hybridization with the two probes. Of the 26 IgA1 protease-positive strains, 12 hybridized with both the 5′ and 3′ parts of the SK141 iga gene, 6 hybridized with the 3′ part only, and 8 were not recognized by either of the two probes. All hybridizing strains showed a distinct pattern of EcoRI fragments recognized by the two probes (data not shown).
DISCUSSION
Results from this study together with published iga gene sequences (8, 32, 40) allowed us to deduce and compare the amino acid sequences of IgA1 proteases from 13 streptococcal strains representing the four species of the genus known to possess IgA1 cleaving activity, i.e., S. sanguis, S. oralis, S. mitis, and S. pneumoniae. The IgA1 proteases of the strains were found to be homologous enzymes, and the comparison revealed conserved as well as variable areas. Among the conserved areas, all the IgA1 proteases contained a zinc-binding consensus sequence, indicating that they are Zn metalloproteinases.
In gram-positive bacteria many surface proteins are anchored to the cell wall via a structure close to the C terminus consisting of an LPXTG sequence motif followed by a membrane-spanning hydrophobic region and a charged sequence (6, 15, 27). The amino acid sequence comparison confirmed the observation previously made by Wani et al. (40) that in the streptococcal IgA1 proteases such a structure is found in the N-terminal part. Its exceptional location implies that it cannot function in the traditional way as a cell wall anchor for surface exposure. However, in the IgA1 proteases the anchor structure is followed by another hydrophobic region and a large area rich in prolines (Fig. 3). For surface-exposed proteins, a sequence containing a high proportion of proline residues is commonly associated with the cell wall (15). Provided that the cell wall anchor motif combined with two membrane-spanning regions followed by a wall-associated region rich in prolines are functional, one may speculate that the protease molecule is coupled to the cell wall via its N terminus, traverses the cytoplasmic membrane twice, and spans the cell wall, ending up on the surface of the bacterium, from where it is released by proteolysis.
Another characteristic common to the streptococcal IgA1 proteases was tandemly repeated sequence elements in their N-terminal parts. Repeat structures are found in most surface-exposed and secreted proteins of streptococci and may provide a means for variation through intragenic homologous recombination (15). Variation in number and sequence of repeats may contribute to antigenic diversity and, hence, to immune evasion (15). However, studying the S. sanguis IgA1 protease, Gilbert and coworkers (9) demonstrated that the repeat region is not essential for enzyme activity. This finding is of interest, since by SDS-PAGE we found that active IgA1 proteases in streptococcal culture supernatants varied in Mr from ∼200,000 (∼200K) to 130K, 200K being the Mr calculated for the entire translation product. Although nonenzymatic hydrolysis of Asp-Pro bonds has been observed after extensive boiling of certain streptococcal proteins in acidic sample buffer (21, 41), this phenomenon is not a relevant explanation in the present case, because the preparation did not involve acidic conditions and, moreover, the location of Asp-Pro peptide bonds in the IgA1 proteases, in S. sanguis in particular, could not account for the observed sizes. Thus, the multiple molecular forms indicate posttranslational, possibly postsecretional, processing of the proteases.
Although the sites cleaved during processing have not been identified, the N-terminal part is likely to be involved. The smallest molecular version of the active protease results from removal of portion(s) corresponding to ∼70 kDa from the primary translation product. If such processing involved the C-terminal part only, it would deprive the enzyme of its active site (Fig. 3). As previously suggested (32), processing at the N terminus might involve autoproteolysis, as occurs in the processing of the serine-type IgA1 proteases produced by N. gonorrhoeae, N. meningitidis, and H. influenzae (25, 29, 30). Autoproteolytic cleavage of the latter enzymes is known to involve also post-proline bonds different from the Pro-Ser or Pro-Thr peptide bond cleaved in human IgA1 (20). By analogy, autoproteolysis of streptococcal IgA1 proteases, if it occurs, might involve not only Pro-Thr, as in the cleavage of the human α1 chain, but also Pro-Ser and other post-proline bonds, which are abundant in the N-terminal thirds of the streptococcal enzymes (Fig. 3A).
Gilbert and coworkers also found that the repeat region of the S. sanguis protease was immunogenic when it was injected in recombinant form into rabbits and that antibodies reacting with this region of the protease were present in normal human saliva in the form of S-IgA (9). Notably, such antibodies did not inhibit enzyme activity (9). Based on these observations and the overall structural similarity of the streptococcal IgA1 proteases, epitopes located C-terminally to the repeat region are likely to be the targets of neutralizing antibodies to these enzymes. Cross-inhibition experiments with such antibodies, obtained from rabbits immunized with concentrated culture supernatants or from individuals recovering from streptococcal disease (34), have revealed considerable serological diversity of IgA1 proteases in S. pneumoniae (23) and S. mitis (32a). In contrast, very limited diversity was found in S. sanguis and S. oralis (34). These results are in agreement with the structural diversity of IgA1 protease genes in each of the species. Thus, nucleotide sequence comparison combined with Southern blot analysis revealed that in S. sanguis and S. oralis the iga gene is relatively conserved whereas in S. mitis it is extremely heterogeneous. In S. pneumoniae strains, we have previously shown that iga genes are diverse (32), though not to the degree observed in S. mitis strains.
In a previous study of IgA1 protease-inhibiting antibodies (33), sera and saliva of most healthy subjects were found to be devoid of activities inhibiting the protease of S. sanguis, which is a lifelong member of the oral flora. This finding suggests that the human host responds rarely, if at all, to the activity-relevant structures located C-terminally to the repeat region of the S. sanguis protease. Yet, as mentioned above, antibodies to the repeat region are present in saliva (9). Comparable immunological data have been obtained for other microbial, including streptococcal, proteins containing repeat regions identified as immunodominant (7, 37). For these proteins it is believed that an exaggerated response to the immunodominant repeats suppresses responses to other epitopes (7, 37), as with the immunodominance demonstrated for certain epitopes in other proteins (3, 4). Assuming, however, that N-terminal processing, as observed in vitro, occurs also during colonization of the human host, it remains to be explained why the enzymatically active part of the S. sanguis protease molecule devoid of the repeat structures rarely induces neutralizing secretory and systemic antibodies.
Contrary to the results for the S. sanguis IgA1 protease, sera and saliva of healthy humans contain neutralizing antibodies to the IgA1 proteases of S. oralis and S. mitis (33). The immunogenicities of distinct regions of these proteases have not been examined. However, the different result for S. sanguis compared to results for S. oralis and S. mitis is remarkable, considering that the three species are closely related and have a common, commensal relationship with the host. In view of the closer relationship of the proteases of S. oralis and S. mitis than that of the proteases of S. sanguis and S. pneumoniae (discussed below), we speculate that neutralizing antibodies to the proteases of S. oralis and S. mitis may be raised by cross-reactive IgA1 proteases of S. pneumoniae clones consecutively colonizing humans during childhood and adolescence (10). The results of our previous (24) and ongoing studies of the serological relationship between streptococcal IgA1 proteases corroborate this hypothesis.
Species within Streptococcus are naturally competent, and evidence for horizontal DNA transfer within as well as between species has been reported (5, 13, 16, 23, 36). Our results on hybridization between restriction fragments of iga genes from the different species strongly indicate that iga gene sequences have been transferred between S. mitis and two other species, S. oralis and S. pneumoniae. In some strains of S. mitis we found genomic DNA restriction fragments that were highly homologous to the iga gene of S. oralis SK2 and different from that of S. pneumoniae PK81; in other strains we found fragments that were homologous to the iga gene of S. pneumoniae PK81 and different from that of S. oralis SK2, whereas in still others the iga gene did not hybridize with that of either of the strains representing the two other streptococcal species. Thus, in S. mitis the IgA1 protease gene is a complex mosaic of sequences closely related to each of the two other species, S. oralis and S. pneumoniae, and sequences only distantly related to these. Such a mosaic structure is most adequately explained by a process of horizontal transfer in vivo between iga genes of the different species.
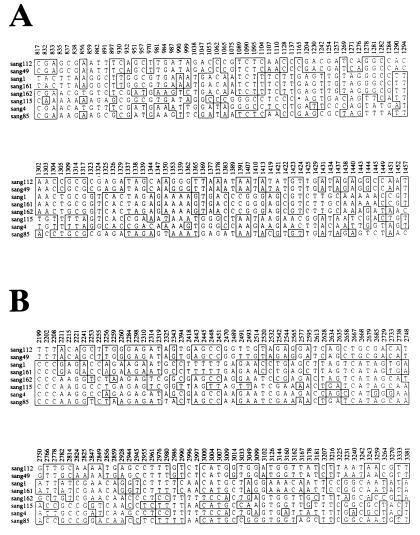
In S. sanguis strains iga genes were very homogeneous, with only relatively low homology to the other streptococcal iga genes sequenced. A comparison of the eight S. sanguis iga gene sequences provides information on the evolutionary origin of the limited variation within the species. The homologies reveal a mosaic-like organization strongly indicative of horizontal genetic exchange between iga genes of different clones of S. sanguis. Examples of two regions depicting such an organization are shown in Fig. 7, in which the sequence comparison has been condensed to include only informative polymorphic sites, i.e., sites with at least two alleles, each represented by more than one strain. Remnants of recombination between iga gene sequences of individual S. sanguis strains are illustrated by the patched organization of the homologies to strain SK162: at the informative sites from bp 1038 to 1443, strain SK162 is identical to strains SK1 and SK161, whereas from bp 2945 to 3144, strains SK162 and SK4 are identical. A similar mosaic-like organization has been previously found among the iga genes encoding serine-type IgA1 proteases in N. gonorrhoeae, H. influenzae, and N. meningitidis (11, 25, 31). Lack of hybridization between some of the streptococcal iga genes, e.g., between those of S. sanguis and those of the other three species (32), may constitute a barrier for horizontal transfer of iga gene sequences.
FIG. 7.
Informative polymorphic nucleotide sites in the eight S. sanguis iga genes in the regions corresponding to nucleotides 817 to 1457 and 2199 to 3381 in the published S. sanguis ATCC 10556 iga gene sequence (8). Each site is numbered according to the ATCC 10556 iga sequence. Abbreviations are as defined in the legend to Fig. 3.
The overall relationships among the IgA1 protease gene sequences from the different streptococcal species included in this study are in accordance with the phylogeny of the genus previously suggested on the basis of 16S rRNA sequences (14). However, in our analysis three of the species were represented by only one or two strains. Together with the high degree of intraspecies diversity of the gene, in S. mitis in particular, this implies that the sequence comparison presented here does not necessarily reveal in full the phylogenetic relationships of IgA1 proteases among streptococci.
ACKNOWLEDGMENTS
This work was supported by Danish Medical Research Council grant 12-1615, the Velux Foundation, Aarhus Universitets Forskningsfond, and Research Career Development Award DE-00236 from the National Institute for Dental Research to T.A.B.
We thank Søren H. Thomassen, Latha Pathangey, and Ella Brandt for technical assistance.
REFERENCES
- 1.Bachovchin W W, Plaut A G, Flentke G R, Lynch M, Kettner C A. Inhibition of IgA1 proteinases from Neisseria gonorrhaeae and Haemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990;265:3738–3743. [PubMed] [Google Scholar]
- 2.Brown T A, Leak I G. A solid-phase immunoassay for detection of IgA1 protease activity on agar plates. J Immunol Methods. 1989;123:241–247. doi: 10.1016/0022-1759(89)90228-7. [DOI] [PubMed] [Google Scholar]
- 3.Delves P J, Lund T, Roitt I M. Can epitope-focused vaccines select advantageous immune responses? Mol Med Today. 1997;3:55–60. doi: 10.1016/s1357-4310(96)20036-x. [DOI] [PubMed] [Google Scholar]
- 4.Deng H, Ohmen J, Fosdick L, Gladstone B, Bang H, Tsai D Y-T, Guo J, Sercarz E. Site-directed mutagenesis of HEL (hen egg lysozyme) and alteration in immunodominance patterns. J Cell Biochem. 1994;18D:289. [Google Scholar]
- 5.Dowson C G, Coffey T J, Spratt B G. Origin and molecular epidemiology of penicillin-binding-protein-mediated resistance to beta-lactam antibiotics. Trends Microbiol. 1994;2:361–366. doi: 10.1016/0966-842x(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 6.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from Gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 7.Fischetti V A, Windels M. Mapping the immunodeterminants of the complete streptococcal M6 protein molecule. J Immunol. 1988;141:3592–3599. [PubMed] [Google Scholar]
- 8.Gilbert J V, Plaut A G, Wright A. Analysis of the immunoglobulin A protease gene of Streptococcus sanguis. Infect Immun. 1991;59:7–17. doi: 10.1128/iai.59.1.7-17.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert J V, Ramakrishna J P, Wright A, Plaut A G. Streptococcal IgA protease tandem repeat influences antigenicity but not activity. J Dent Res. 1993;72:327. [Google Scholar]
- 10.Gray B M, Converse III G M, Dillon H C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 11.Halter R, Pohlner J, Meyer T F. Mosaic-like organization of IgA protease genes in Neisseria gonorrhoeae generated by horizontal genetic exchange in vivo. EMBO J. 1989;8:2737–2744. doi: 10.1002/j.1460-2075.1989.tb08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohwy J, Kilian M. Clonal diversity of the Streptococcus mitis biovar 1 population in the human oral cavity and pharynx. Oral Microbiol Immunol. 1995;10:19–25. doi: 10.1111/j.1399-302x.1995.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 13.Kapur V, Kanjilal S, Hamrick M R, Li L L, Wittam T S, Sawyer S A, Musser J M. Molecular population genetic analysis of the streptokinase gene of Streptococcus pyogenes: mosaic alleles generated by recombination. Mol Microbiol. 1995;16:509–519. doi: 10.1111/j.1365-2958.1995.tb02415.x. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura Y, Hou X-G, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 15.Kehoe M A. Cell-wall-associated proteins in Gram-positive bacteria. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. New York, N.Y: Elsevier Science B.V.; 1994. pp. 217–261. [Google Scholar]
- 16.Kehoe M A, Kapur V, Whatmore A M, Musser J M. Horizontal gene transfer among group A streptococci: implications for pathogenesis and epidemiology. Trends Microbiol. 1996;4:436–443. doi: 10.1016/0966-842x(96)10058-5. [DOI] [PubMed] [Google Scholar]
- 17.Kilian M, Husby S, Høst A, Halken S. Increased proportions of bacteria capable of cleaving IgA1 in the pharynx of infants with atopic disease. Pediatr Res. 1995;38:182–186. doi: 10.1203/00006450-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kilian M, Mestecky J, Kulhavy R, Tomana M, Butler W T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis and Streptococcus sanguis: comparative immunological studies. J Immunol. 1980;124:2596–2600. [PubMed] [Google Scholar]
- 19.Kilian M, Mikkelsen L, Henriksen J. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Nìven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906) Int J Sys Bacteriol. 1989;39:471–484. [Google Scholar]
- 20.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen E V G. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 21.Landon M. Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- 22.Loenen W A M, Brammar W J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980;10:249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- 23.Lomholt H. Evidence of recombination and an antigenically diverse immunoglobulin A1 protease among strains of Streptococcus pneumoniae. Infect Immun. 1995;63:4238–4243. doi: 10.1128/iai.63.11.4238-4243.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomholt H, Kilian M. Antigenic relationships among immunoglobulin A1 proteases from Haemophilus, Neisseria, and Streptococcus species. Infect Immun. 1994;62:3178–3183. doi: 10.1128/iai.62.8.3178-3183.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomholt H, Poulsen K, Kilian M. Comparative characterization of the iga gene encoding IgA1 protease in Neisseria meningitidis, Neisseria gonorrhoeae and Haemophilus influenzae. Mol Microbiol. 1995;15:495–506. doi: 10.1111/j.1365-2958.1995.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 26.Mortensen S B, Kilian M. Purification and characterization of an immunoglobulin A1 protease from Bacteroides melaninogenicus. Infect Immun. 1984;45:550–557. doi: 10.1128/iai.45.3.550-557.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in Gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 28.Plaut A G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- 29.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 30.Poulsen K, Brandt J, Hjorth J P, Thøgersen H C, Kilian M. Cloning and sequencing of the immunoglobulin A1 protease gene (iga) of Haemophilus influenzae serotype b. Infect Immun. 1989;57:3097–3105. doi: 10.1128/iai.57.10.3097-3105.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen K, Reinholdt J, Kilian M. A comparative genetic study of serologically distinct Haemophilus influenzae type 1 immunoglobulin A1 proteases. J Bacteriol. 1992;174:2913–2921. doi: 10.1128/jb.174.9.2913-2921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulsen K, Reinholdt J, Kilian M. Characterization of the Streptococcus pneumoniae immunoglobulin A1 protease gene (iga) and its translation product. Infect Immun. 1996;64:3957–3966. doi: 10.1128/iai.64.10.3957-3966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Reinholdt, J. Unpublished data.
- 33.Reinholdt J, Kilian M. Titration of inhibiting antibodies to IgA1 proteases of oral streptococci in human serum, saliva, and colostral S-IgA. J Dent Res. 1993;73:957. [Google Scholar]
- 34.Reinholdt J, Tomana M, Mortensen S B, Kilian M. Molecular aspects of immunoglobulin A1 degradation by oral streptococci. Infect Immun. 1990;58:1186–1194. doi: 10.1128/iai.58.5.1186-1194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Sánchez-Beato A R, García E, López R, García J L. Identification and characterization of IS1381, a new insertion sequence in Streptococcus pneumoniae. J Bacteriol. 1997;179:2459–2463. doi: 10.1128/jb.179.7.2459-2463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, Gwadz R W, Schlesinger D H, Godson G N. Immunogenicity of the repetitive and nonrepetitive peptide regions of the divergent CS protein of Plasmodium knowlesi. J Immunol. 1986;137:357–361. [PubMed] [Google Scholar]
- 38.Vallee B L, Auld D S. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci USA. 1990;87:220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Mol Microbiol. 1989;3:1825–1831. doi: 10.1111/j.1365-2958.1989.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 40.Wani J H, Gilbert J V, Plaut A G, Weiser J N. Identification, cloning, and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect Immun. 1996;64:3967–3974. doi: 10.1128/iai.64.10.3967-3974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wastfelt M, Stalhammar-Carlemalm M, Delisse A M, Cabezon T, Lindahl G. Identification of a family of streptococcal surface proteins with extremely repetitive structure. J Biol Chem. 1996;271:18892–18897. doi: 10.1074/jbc.271.31.18892. [DOI] [PubMed] [Google Scholar]
- 42.Wood W B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affected the restriction and modification of DNA. J Mol Biol. 1966;16:118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- 43.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]