Abstract
Background:
Approval of the adalimumab (ADA) biosimilar ABP 501 for inflammatory bowel disease (IBD) indications was based on the principle of extrapolation, without indication-specific clinical trial data.
Objectives:
To evaluate the real-world treatment patterns of ABP 501 in patients with IBD.
Design:
Retrospective analysis of pharmacy claims data from Germany and France.
Methods:
Continuously insured adult IBD patients who initiated ABP 501 between October 2018 and March 2020 were included. Treatment persistence, adherence, and post-ABP 501 switching patterns were evaluated for two mutually exclusive groups: ADA-naïve patients (i.e. no baseline use of ADA products) and ADA-experienced patients (i.e. previously treated with ADA products).
Results:
A total of 3362 German patients and 733 French patients were included, with 54.4% and 65.3% being ADA-naïve patients, respectively. Median persistence (95% CI) on ABP 501 was 10.9 months (9.8–11.6) in ADA-naïve patients and 14.2 months (12.7–15.2) in ADA-experienced patients in Germany; for the French cohort, ADA-naïve and -experienced patients had median persistence of 12.8 months (10.2–14.7) and 11.5 months (8.8–14.4), respectively. During the first 12 months of ABP 501 initiation, 53.7% of German patients and 51.0% of French patients were adherent to the therapy. About 20% of patients in both countries switched from ABP 501 to another targeted therapy. In the German cohort, ADA-naïve patients most frequently switched to non-tumor necrosis factor inhibitor biologics, but ADA-experienced patients most commonly switched to reference product (RP); in the French cohort, patients most often switched to RP regardless of prior exposure to ADA products.
Conclusion:
About 50% of patients persisted on and were adherent to ABP 501 therapy during the first 12 months after treatment initiation in two large European countries. Post-ABP 501, switching patterns varied between countries, indicating diversified treatment practices warranting further research on reason(s) for switching and potential overall treatment outcomes.
Keywords: ABP 501, adalimumab-atto, adalimumab biosimilar, inflammatory bowel disease, persistence, real-world evidence, treatment pattern
Introduction
Inflammatory bowel disease (IBD) mainly refers to two chronic, immune-mediated inflammatory disorders that primarily affect the intestinal tract: Crohn’s disease (CD) and ulcerative colitis (UC). 1 Both disorders can be debilitating and significantly impair health-related quality of life (HR QoL) and work productivity.2,3 Anti-tumor necrosis factor (TNF) biologics, including adalimumab (ADA) and infliximab, have been shown to significantly improve HR QoL and reduce the need for hospitalization and surgery for patients with IBD4,5 and are the mainstay for treating patients with moderate-to-severe CD or UC.6,7
Biosimilars, a biologic agent highly similar to the licensed reference product (RP, also known as originator), can provide additional treatment options for patients. ABP 501 [AMGEVITA® (EU) or AMJEVITA™ (United States); adalimumab-atto, Amgen Inc., Thousand Oaks, CA, USA) is the first ADA biosimilar approved by the European Medical Association and the US Food and Drug Administration for the treatment of certain immune-mediated inflammatory diseases including IBD. It was marketed in the European Union since October 2018 and in the United States starting January 2023. Biosimilarity between ABP 501 and ADA RP is demonstrated based on the ‘totality-of-the-evidence’ that includes comprehensive analytical and preclinical assessments, a phase I pharmacokinetics equivalence study in healthy volunteers, and two comparative, randomized, double-blind clinical trials in patients with rheumatoid arthritis and patients with psoriasis.8–10 Approval of IBD indications for ABP 501, similar to approvals of other anti-TNF biosimilars for the treatment of IBD to date, was based on the principle of ‘extrapolation’ without IBD-specific clinical trials. Real-world evidence of ABP 501 in patients with IBD is limited, with only a few studies being conducted in small Italian cohorts11–15 that collectively showed the safety and effectiveness of ABP 501 both in patients naïve to ADA and those switched from ADA RP; ABP 501 seemed to be as safe and effective as the RP.11–15
Yet, barriers to biosimilar utilization remain.16,17 A systematic review evaluating studies mainly from Europe and the United States with data collected between 2013 and 2017 revealed that approximately two-thirds of physicians had concerns regarding biosimilars; indication extrapolation and the lack of clinical trial data in IBD were among the most commonly reported reasons for concerns. 18 It is possible that these concerns may even impact the discontinuation of biosimilars after utilization. In addition, several studies have suggested that patients are often reluctant to accept biosimilars as part of their treatment plan, citing unfamiliarity.19–23
Although familiarity and acceptance of biosimilars have significantly increased among IBD specialists over the past years, 24 additional real-world evidence on ADA biosimilars from European countries can provide valuable information upon US market entry to continuously address any potential barriers to utilization. Therefore, in this retrospective study, we aimed to evaluate the real-world treatment patterns of ABP 501 among patients with IBD in Germany and France.
Methods
Study design and data source
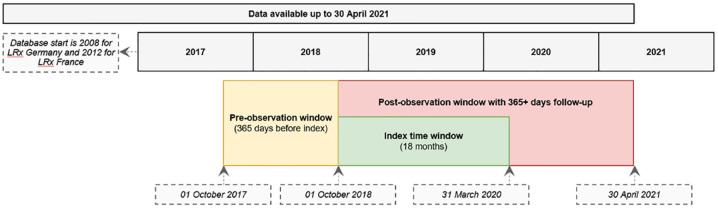
This was a retrospective cohort analysis using the IQVIA German and French pharmacy claims (longitudinal prescription data, LRx) databases that cover data up to 30 April 2021 (at data lock). The study design schema is presented in Figure 1. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 25
Figure 1.
Study design.
LRx, longitudinal prescription data.
IQVIA LRx is a longitudinal pharmacy administrative database that gathers data from retail pharmacies. An anonymized unique patient ID is assigned to each patient, which enables a longitudinal follow-up of these patients and the evaluation of the dynamic treatment pathway over time through medication reimbursed by health insurance and dispensed in retail pharmacies. The German LRx database was created in 2008 and has national coverage of ~84% of Germany’s statutory health insurance patients, with most of the federal states having coverage of 80% or higher. 26 The French LRx was created in 2012, covers a network of more than 9600 retail pharmacies in France (~45% of national coverage), and is representative in terms of geographic spread in continental France and age of population coverage, 27 allowing extrapolation to the overall French population.28–33
Study population
Patients (18 years or older) with documented evidence of IBD who received at least one prescription of ABP 501 between October 2018 (market availability) and March 2020 were included in this analysis. Patients were also required to have at least 365 days of continuous observation of overall pharmacy records in the LRx database both before and after initiation of ABP 501, to allow the evaluation of their baseline characteristics and medication use and treatment patterns during the follow-up period, respectively.
Diagnosis of IBD was not directly documented in the IQVIA LRx pharmacy claims database. For German LRx, the diagnosis was imputed using the machine-learning models developed based on the patient’s treatment/prescription histories and validated in IQVIA electronic medical records databases (Supplemental Material 1). For French LRx, the diagnosis of IBD was imputed based on a rule-based approach (Supplemental Material 2).
Study outcomes and variables
Treatment persistence
Treatment persistence was measured using Kaplan–Meier analysis, which evaluated the cumulative probability of ABP 501 continuation during the follow-up period. Discontinuation of ABP 501 therapy occurred when no additional ABP 501 prescription was detected after the predefined allowable treatment gap of up to 120 days from the end of supply of the previous prescription; or when patients were switched to another targeted therapy during ABP 501 supply or within the predefined gap from the end of the previous prescription. Patients who reached the end of their observation period (e.g. end of record or end of database coverage) were classified as ‘censored’. A sensitivity analysis using a predefined treatment gap of up to 90 days was conducted to evaluate treatment persistence.
Treatment adherence
Adherence was measured using the medical possession ratio (MPR), calculated by the below equation. The prescription durations of all ABP 501 prescriptions from the initiation date of ABP 501 within 365 days of follow-up were summarized as the numerator for calculating the MPR. The denominator was defined as 365 days. The MPR was truncated at 1.0 to prevent the population average from being falsely inflated. Adherence to ABP 501 was considered if MPR was ⩾80% of the days covered.
Initial switching patterns
For patients who switched from ABP 501 to another targeted therapy, the switching patterns immediately post-ABP 501 were evaluated and categorized into the following groups: switching to ADA RP, ADA biosimilars (excluding ABP 501), non-ADA tumor necrosis factor inhibitors (TNFis), non-TNFi biologics, or Janus Kinase inhibitors (JAKis) (Supplemental Material 3 for detailed drug list). The switch was considered when a new targeted therapy was initiated during ABP 501 supply or within the predefined treatment gap (up to 120 days) from the end of the previous prescription of ABP 501.
Covariates
Patient characteristics, including age, sex, treating specialty, treatment setting, comorbidities, prior medication use, and concomitant treatment were reported at baseline (defined as within 12 months prior to initiation of ABP 501). Treatment specialty/setting was defined as the specialty/setting accounting for the highest number of ABP 501 prescriptions, or the initial prescriber/setting in case of an equal number of prescriptions. A detailed drug list for prior medication use and concomitant treatment is provided in Supplemental Material 3. Comorbidities were derived from patients’ prescription records in at least three-quarters during the baseline using the list of European Pharmaceutical Market Research Association Anatomical Classification-Classes. 34
Patient characteristics and outcome measures were stratified by prior use of ADA products (including ADA RP and ADA biosimilars) as follows: (1) ADA-naïve patients, who had not been treated with an ADA product during the baseline period and received ABP 501 as initiating ADA therapy and (2) ADA-experienced patients, who had been treated with an ADA product during the baseline period and switched to ABP 501 therapy. ADA-naïve patients and ADA-experienced patients are mutually exclusive categories.
Statistical analysis
This study was descriptive in nature. No a priori hypotheses were tested and no statistical comparisons were conducted between groups. Data analyses were conducted in each country using the country-specific pharmacy claims (LRx) data. Individual data from Germany and France were not pooled together. Summary statistics, including mean and standard deviation, were calculated for continuous variables. The frequency and percentage were reported for categorical variables. Missing data were not imputed. A threshold of 10 patients is required for presenting aggregated results for French LRx analysis. Below that threshold, results were shown as ‘<10’ due to country-specific data privacy protection guidelines. The analyses were carried out using the statistical software SAS Version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics and baseline medication use
German patient population
A total of 3362 German patients with IBD were included in the final analysis, consisting of 54.4% ADA-naïve patients and 45.6% ADA-experienced patients. Overall, 48.7% of patients were female, the mean age was 40.9 years, and most patients were treated by gastroenterologists (73.4%) and in office-based practices (85.0%). The most commonly observed comorbidities were hypertension (12.2%) and peptic ulcer disease (11.1%) [Table 1(a)].
Table 1a.
Baseline characteristics of German patients with IBD, stratified by prior exposure to an ADA RP or a biosimilar.
| Characteristic | All patients, N = 3362 | ADA-naïve patients, n = 1828 | ADA-experienced patients, n = 1534 a |
|---|---|---|---|
| Age, years, mean (SD) | 40.9 (14.4) | 40.1 (14.2) | 41.9 (14.7) |
| Sex b , n (%) | |||
| Female | 1637 (56.1) | 887 (55.3) | 750 (57.1) |
| Male | 1280 (43.9) | 716 (44.7) | 564 (42.9) |
| Region, n (%) | |||
| North | 311 (9.3) | 144 (7.9) | 167 (10.9) |
| West | 1499 (44.6) | 804 (44.0) | 695 (45.3) |
| South | 1160 (34.5) | 656 (35.9) | 504 (32.9) |
| East | 392 (11.7) | 224 (12.3) | 168 (11.0) |
| Treating specialty, n (%) | |||
| Dermatologist | 2 (0.1) | 1 (0.1) | 1 (0.1) |
| Gastroenterologist | 2468 (73.4) | 1346 (73.6) | 1122 (73.1) |
| Rheumatologist | 98 (2.9) | 46 (2.5) | 52 (3.4) |
| Unknown | 794 (23.6) | 435 (23.8) | 359 (23.4) |
| Treatment setting, n (%) | |||
| Hospital based | 506 (15.1) | 284 (15.5) | 222 (14.5) |
| Office based | 2856 (85.0) | 1544 (84.5) | 1312 (85.5) |
| Prior treatment at baseline c , n (%) | |||
| Glucocorticoid | 1410 (41.9) | 994 (54.4) | 416 (27.1) |
| Immunosuppressive drug | 678 (20.2) | 453 (24.8) | 225 (14.7) |
| Non-ADA TNFi | 225 (6.7) | 184 (10.1) | 41 (2.7) |
| Non-TNFi biologic | 104 (3.1) | 88 (4.8) | 16 (1.0) |
| JAKi | 12 (0.4) | 12 (0.7) | 0 (0.0) |
| Concomitant treatment c , n (%) | |||
| Glucocorticoid | 812 (24.2) | 522 (28.6) | 290 (18.9) |
| Immunosuppressive drug | 327 (9.7) | 187 (10.2) | 140 (9.1) |
| Comorbidities, n (%) | |||
| Hypertension | 410 (12.2) | 196 (10.7) | 214 (14.0) |
| Dyslipidemia | 122 (3.6) | 54 (3.0) | 68 (4.4) |
| Diabetes | 87 (2.6) | 50 (2.7) | 37 (2.4) |
| Endocrinological disease | 244 (7.3) | 109 (6.0) | 135 (8.8) |
| Respiratory disease | 87 (2.6) | 49 (2.7) | 38 (2.5) |
| Peptic ulcer disease | 373 (11.1) | 172 (9.4) | 201 (13.1) |
n = 1297 were switched to ABP 501 from ADA RP and n = 237 were switched from other ADA biosimilars.
The percentages of females and males are based on 2917 patients with sex data available.
Categories are not mutually exclusive. Patients were possibly treated with more than one category of drugs.
ADA, adalimumab; IBD, inflammatory bowel disease; JAKi, Janus Kinase inhibitor; RP, reference product; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Prior use of a glucocorticoid, immunosuppressive drugs, non-ADA TNFi, and non-TNFi biologics were 54.4% and 27.1%, 24.8% and 14.7%, 10.1% and 2.7%, and 4.8% and 1.0% for ADA-naive and ADA-experienced patients, respectively. Overall, about one-fourth and 10% of patients received glucocorticoids and immunosuppressive drugs, respectively, along with ABP 501 treatment. The concomitant use of glucocorticoids with ABP 501 was observed in 28.6% of ADA-naïve patients and 18.9% of ADA-experienced patients [Table 1(a)].
French patient population
A total of 733 French patients with IBD were included in the analysis. Of those, 65.3% were ADA-naïve and 34.7% were ADA-experienced patients. Overall, the mean age of patients was 41.9 years, 55.3% were female, and more than half of patients were treated by hospital-based prescribers. Peptic ulcer disease (12.4%) and hypertension (10.5%) were the most commonly observed comorbidities in the patient population [Table 1(b)].
Table 1b.
Baseline characteristics of French patients with IBD stratified by prior exposure to an ADA RP or a biosimilar.
| Characteristic | All patients, N = 733 | ADA-naïve patients, n = 479 | ADA-experienced patients, n = 254 a |
|---|---|---|---|
| Age, years, mean (SD) | 41.9 (15.1) | 41.4 (15.2) | 42.9 (14.9) |
| Sex b , n (%) | |||
| Female | 405 (55.6) | 268 (56.3) | 137 (54.2) |
| Male | 324 (44.4) | 208 (43.7) | 116 (45.8) |
| Region, n (%) | |||
| North East | 221 (30.2) | 146 (30.5) | 75 (29.5) |
| North West | 216 (29.5) | 141 (29.4) | 75 (29.5) |
| South East | 105 (14.3) | 75 (15.7) | 30 (11.8) |
| South West | 79 (10.8) | 43 (9) | 36 (14.2) |
| Parisian region | 112 (15.3) | 74 (15.5) | 38 (15) |
| Treating specialty, n (%) | |||
| Gastroenterologist | 257 (35.1) | 183 (38.2) | 74 (29.1) |
| Rheumatologist | <10 | <10 | <10 |
| Hospital-based prescriber c | 439 (59.9) | 275 (57.4) | 164 (64.6) |
| Unknown | 32 (4.4) | 18 (3.8) | 14 (5.5) |
| Treatment setting, n (%) | |||
| Hospital based | 439 (59.9) | 275 (57.4) | 164 (64.6) |
| Office based | 276 (37.7) | 195 (40.7) | 81 (31.9) |
| Unknown | 18 (2.5) | <10 | <10 |
| Prior treatment at baseline d , n (%) | |||
| Glucocorticoid | 331 (45.2) | 257 (53.7) | 74 (29.1) |
| Immunosuppressive drug | 224 (30.6) | 179 (37.4) | 45 (17.7) |
| Non-ADA TNFi | 12 (1.6) | 10 (2.1) | <10 |
| Non-TNFi biologic | 11 (1.5) | 11 (2.3) | 0 (0.0) |
| JAKi | <10 | <10 | 0 (0.0) |
| Concomitant treatment d , n (%) | |||
| Glucocorticoid | 166 (22.7) | 123 (25.7) | 43 (16.9) |
| Immunosuppressive drug | 144 (19.7) | 118 (24.6) | 26 (10.2) |
| Comorbidities, n (%) | |||
| Hypertension | 77 (10.5) | 41 (8.6) | 36 (14.2) |
| Dyslipidemia | 36 (4.9) | 14 (2.9) | 22 (8.7) |
| Diabetes | 14 (1.9) | <10 | <10 |
| Endocrinological disease | 23 (3.1) | 17 (3.6) | <10 |
| Respiratory disease | 21 (2.9) | 13 (2.7) | <10 |
| Peptic ulcer disease | 91 (12.4) | 57 (11.9) | 34 (13.4) |
n = 246 were switched to ABP 501 from ADA RP and n < 10 were switched from other ADA biosimilars.
The percentages of females and males are based on 729 patients with sex data available.
‘Hospital-based prescribers refer to treating physicians practicing in a hospital setting, including those specializing in gastroenterology or internal medicine.
Categories are not mutually exclusive. Patients were possibly treated with more than one category of drugs.
ADA, adalimumab; IBD, inflammatory bowel disease; JAKi, Janus Kinase inhibitor; RP, reference product; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Baseline use of glucocorticoids was 53.7% and 29.1%, and baseline use of immunosuppressive drugs was 37.4% and 17.7% for ADA-naïve and ADA-experienced patients, respectively. Baseline use of targeted therapies, including non-ADA TNFi, non-TNFi biologics, and JAKis, was low (<2%) in the study population and mainly observed in ADA-naïve patients. About 20% of patients received concomitant glucocorticoids or immunosuppressive drugs with ABP 501, with approximately 25% of ADA-naïve patients and 10–17% of ADA-experienced patients receiving concomitant treatments [Table 1(b)].
Treatment persistence and adherence
German patient population
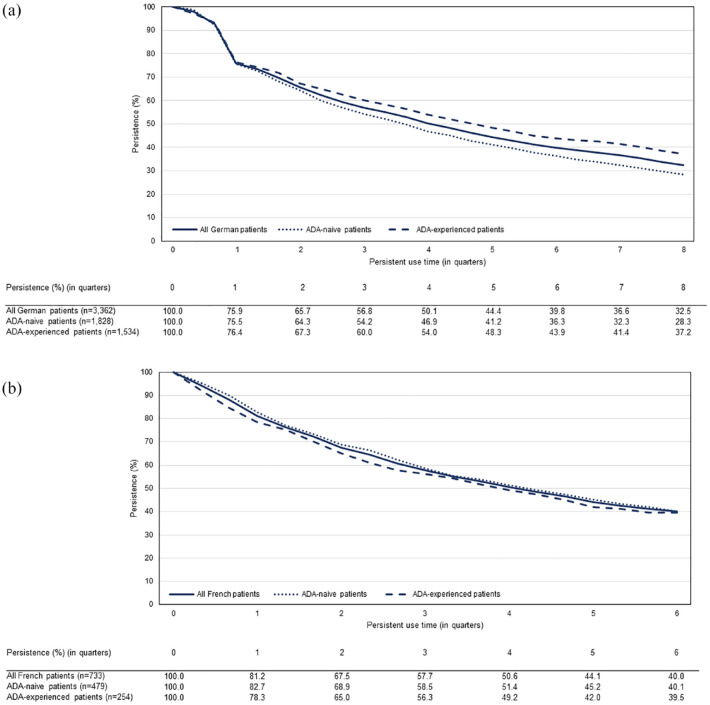
The median persistence on ABP 501 for German patients was 12.1 months [95% confidence interval (CI): 11.3–13.0] overall, 10.9 months (95% CI: 9.8–11.6) in ADA-naïve patients, and 14.2 months (95% CI: 12.7–15.3) in ADA-experienced patients [Figure 2(a)]. After 12 months of treatment initiation, 50.1% (95% CI: 48.4–51.8) of all German patients persisted with ABP 501 therapy [Figure 2(a)].
Figure 2.
Kaplan–Meier curve of treatment persistence of biosimilar ABP 501 among German (a) and French (b) patients with inflammatory bowel disease.
ADA, adalimumab.
The adherence rate to ABP 501 treatment (defined as MPR ⩾ 80%) during the first 12 months of treatment initiation was 53.7% overall, 52.0% in ADA-naïve patients, and 55.7% in ADA-experienced patients.
French patient population
The median persistence on ABP 501 was 12.4 months (95% CI: 10.5–13.9) among the French patient population overall, 12.8 months (95% CI: 10.2–14.7) in ADA-naïve patients, and 11.5 months (95% CI: 8.8–14.4) in ADA-experienced patients [Figure 2(b)]. At the end of the 12 months after treatment initiation, 50.6% (95% CI: 46.9–54.2) of all French patients remained on ABP 501 therapy [Figure 2(b)].
Half (51.0%) of patients were adherent to ABP 501 therapy (MPR ⩾ 80%) during the first 12 months of treatment initiation among the French cohort. The adherence rate was 54.3% in ADA-naïve patients and 44.9% in ADA-experienced patients.
Sensitivity analysis
Sensitivity analysis for treatment persistence was conducted using a permissible treatment gap of up to 90 days. The median persistence of ABP 501 was slightly shorter overall: 10.3 months (95% CI: 9.4–11.0) in German patients and 10.7 months (95% CI: 9.2–12.4) in French patients (Supplemental Figure), but not substantially different from the outcomes in the primary analysis.
Switching patterns
German patient population
Approximately 23% (n = 763) of all patients switched to another targeted therapy within the first 12 months of initiating ABP 501, most frequently to non-TNFi biologics [mainly interleukin (IL) 12/23 inhibitor and integrin antagonists]. When analyzing ADA-naïve and ADA-experienced patients separately, the most frequent switch was to non-TNFi biologics among ADA-naïve patients, and to ADA RP among ADA-experienced patients [Table 2(a)].
Table 2a.
Switch rates and patterns among German patients with IBD who switched from biosimilar ABP 501 to another targeted therapy during the first 12 months after initiating ABP 501.
| Evaluated variable | All patients | ADA-naïve patients | ADA-experienced patients |
|---|---|---|---|
| All patients, n | 3362 | 1828 | 1534 |
| Total switched patients, n (%) | 763 (22.7) | 374 (20.5) | 389 (25.4) |
| Initial switching patterns post-ABP 501, n (%) | |||
| ADA reference product | 221 (29.0) | 38 (10.2) | 183 (47.0) a |
| ADA biosimilars (excluding ABP 501) | 126 (16.5) | 35 (9.4) | 91 (23.4) b |
| Non-ADA TNFi | 74 (9.7) | 57 (15.2) | 17 (4.4) |
| Non-TNFi biologics | 314 (41.2) | 220 (58.8) | 94 (24.2) |
| IL1 inhibitor | 1 (0.1) | 1 (0.3) | 0 (0.0) |
| IL12/23 inhibitor | 168 (22.0) | 110 (29.4) | 58 (14.9) |
| IL17 inhibitors | 2 (0.3) | 0 (0.0) | 2 (0.5) |
| IL6 inhibitors | 1 (0.1) | 0 (0.0) | 1 (0.3) |
| Integrin antagonists | 142 (18.6) | 109 (29.1) | 33 (8.5) |
| JAKi | 28 (3.7) | 24 (6.4) | 4 (1.0) |
ADA, adalimumab; IBD, inflammatory bowel disease; IL, interleukin; JAKi, Janus Kinase inhibitor; RP, reference product; TNFi, tumor necrosis factor inhibitor.
Included patients who were treated with RP (n = 165, 90.2%) or other ADA biosimilars (n = 18, 9.8%) prior to initiation of ABP 501.
Included patients who were treated with RP (n = 65, 71.4%) or other ADA biosimilars (n = 26, 28.6%) prior to initiation of ABP 501.
French patient population
The switch rate (~20%) within the first 12 months of ABP 501 initiation in French patients overall was generally consistent with the observation in German patients but varied between ADA-naïve and ADA-experienced patients. About 15% of ADA-naive patients and 28% of ADA-experienced patients switched to another targeted therapy. Of all switched patients (n = 145), the majority (81.4%) switched to ADA RP regardless of prior exposure to ADA products [ADA-naïve patients: 76.7%, ADA-experienced patients: 86.1%; Table 2(b)].
Table 2b.
Switch rates and patterns among French patients with IBD who switched from biosimilar ABP 501 to another targeted therapy during the first 12 months after initiating ABP 501.
| Evaluated variable | All patients | ADA-naïve patients | ADA-experienced patients |
|---|---|---|---|
| All patients, n | 733 | 479 | 254 |
| Total switched patients, n (%) | 145 (19.8) | 73 (15.2) | 72 (28.3) |
| Initial switching patterns post-ABP 501, n (%) | |||
| ADA reference product | 118 (81.4) | 56 (76.7) | 62 (86.1) a |
| ADA biosimilars (excluding ABP 501) | <10 c | <10 c | <10b,c |
| Non-ADA | <10 c | <10 c | <10 c |
| Non-TNFi biologics | 13 (9.0) | 11 (15.3) | <10 c |
| IL1 inhibitor | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IL12/23 inhibitor | 13 (9.0) | 11 (15.3) | <10 c |
| IL17 inhibitors | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IL6 inhibitors | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Integrin antagonists | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| JAKi | <10 c | <10 c | 0 (0.0) |
Included patients who were treated with RP (n = 61, 98.4%) or other ADA biosimilars (n = 1, 1.6%) prior to initiation of ABP 501.
Included patients who were treated with RP (85.7%) or other ADA biosimilars (14.3%) prior to initiation of ABP 501.
Actual patient numbers cannot be presented due to country-specific privacy protection guidelines.
ADA, adalimumab; JAKi, Janus Kinase inhibitor; IBD, inflammatory bowel disease; IL, interleukin; RP, reference product; TNFi, tumor necrosis factor inhibitor.
Discussion
The introduction of biosimilars has offered more affordable treatment options to patients, but initial clinician hesitancy in prescribing and patients’ reluctance to accept biosimilar treatment poses barriers to utilization in the field of gastroenterology. 35 This may be because approval of IBD indications was based on data extrapolated from comparative clinical trials in other disease type(s), such as rheumatoid arthritis and psoriasis. In recent years, increased knowledge of the rigorous approval pathway for biosimilars and supportive randomized controlled trials,36,37 and real-world efficacy and safety data (mainly from infliximab biosimilars)11–14,38–40 have helped increase confidence and comfort level with the use of biosimilars in clinical gastroenterology. A 2015 study examining 118 survey responses of IBD-treating clinicians across many European countries reported that familiarity and confidence in the use of biosimilars had improved substantially, with 19.5% stating a lack of confidence in using biosimilars in 2015, down from 63% in 2013.24,41 However, continuous provision of real-world evidence of biosimilar use among patients with IBD is still of importance to help address concerns and build confidence for both healthcare providers and patients. Real-world studies of ADA biosimilar ABP 501 are particularly warranted as data are scarce and mainly from relatively small cohorts in Italy.11–15 Therefore, in the current study, we leveraged the nationally representative pharmacy claims databases of German and French populations to evaluate treatment persistence, adherence, and switching patterns of ABP 501 among patients with IBD. Despite the differences between the United States and European healthcare delivery systems, such real-world experience from European countries could inform the US medical community upon ADA biosimilars entering the US market starting in January 2023.
Overall, we found that slightly more than half of the study population persisted on and remained adherent to ABP 501 therapy 12 months after treatment initiation in both German and French patients. Our findings were consistent with a US claims-based study evaluating real-world treatment persistence of five marketed biological medicines, including ADA originator, in patients with IBD during 2008–2015, which reported that persistence of ADA originator was 50.9% in patients with CD and 45.4% in patients with UC in the first year. 42 A previously published claims analysis of the German population using the same database as our analysis, IQVIA LRx, reported a comparable 1-year persistence rate of ~50% for ADA originators in patients with IBD. 43 Evidence from two smaller retrospective cohort studies of German patients with IBD showed slightly better persistence rates, with one reporting that 63.5% of patients persisted with ADA originator after 12 months, 44 and the other showing that the survival rate of ADA originator used as initial biologic treatment for IBD was 65.4% at 12 months. 45 It is important to note that many factors can impact treatment persistence. Previous studies showed that patients with CD appeared to stay on the initial biological treatment longer than patients with UC.42,46 Prior and concomitant medication use across studied patient populations may also affect persistence results. For example, the use of ADA with steroids was reported to be associated with an increased risk of non-persistence.42,44 When interpreting our results of the biosimilar ABP 501 in relevance to data of the ADA originator published prior to the market availability of biosimilars, some additional facts need to be taken into consideration, including the availability of more treatment options and patient cohort including those already treated with the originator before receiving biosimilars. Finally, our study duration included the COVID-19 pandemic period, which has been reported to have significantly impacted clinical practice and treatment persistence. 47
When analyzing treatment persistence stratified by prior use of ADA products, we observed a larger proportion of persistent users in ADA-experienced patients than in ADA-naïve patients in the German patient population, which was consistent with a previous line of evidence from a real-world study for ABP 501. 11 In part, this may be due to ADA-experienced patients being more likely to be stable on, responsive to, and tolerant of ADA therapy, in turn leading to better persistence than patients who were new starters to ADA therapy. However, it is important to note that baseline clinical characteristics of patients were not available in the LRx database, and baseline medication use was different between ADA-naïve and ADA-experienced patients in our cohort – which could both have an impact on treatment persistence and adherence. In our French cohort, we did not observe substantial differences in ABP 501 persistence between ADA-naïve and -experienced patients. This could be due to the initiation of biologics, including biosimilars, in France being restricted to hospital-based specialists—a setting in which patients are more closely managed and followed if they initiate a new medicine.
Regarding initial switching patterns post-ABP 501 therapy, we observed an interesting finding in that the German cohort showed varied switching patterns between ADA-naïve and ADA-experienced patients. Of the patients who switched from ABP 501 to another targeted therapy, ADA-naïve patients most commonly switched to non-TNFi biological treatments, whereas ADA-experienced patients most frequently switched back to ADA RP. This may be attributable to the nocebo effect, which has been documented as a more negative effect of an intervention induced by negative perceptions or expectations, 48 as this pattern was not observed in the ADA-naïve patients who were not previously treated with RP. A recent systematic review assessed more than 30 studies in which patients were switched from infliximab RP to infliximab biosimilar. They found that median discontinuation rates were 14.7% in open-label studies, compared with 6.95% in double-blind trials, supporting the idea that the nocebo effect may influence biosimilar acceptance in patients. 49 Interestingly, the difference in switch patterns was not observed in French patients, where patients most often switched to ADA RP regardless of prior exposure to ADA products. One of the reasons might be the timing of the availability of non-TNFi drugs in the French market. In Germany, most of the patients switched from ABP 501 to either IL12/23 inhibitor or integrin antagonist. In France, however, these two classes of drugs were largely unavailable during the study period (e.g. vedolizumab, the only drug of the integrin antagonist class, was first available in French retail pharmacies in March 2021; ustekinumab, the only IL12/23 inhibitor in France, had its UC indication endorsed for reimbursement by French health authorities in July 2020). However, as the reasons for switching were not captured in this IQVIA LRx claims database, future studies are necessary to further understand differences in switching patterns between ADA-naïve and -experienced patients and across different healthcare delivery systems.
Our study has a few limitations. First, patient diagnoses were not documented in the LRx database, but were imputed using a rule-based (for French LRx) or a well-validated machine-learning model (for German LRx) (see Supplemental Materials 1 and 2). In Germany, for example, the models have been trained on the German IQVIA Disease Analyzer database, including prescriptions, diagnoses, and patient demographics from electronic medical records, and have 95% accuracy for correctly predicting IBD diagnosis. Second, the IQVIA LRx is a retail pharmacy claims database that does not include over-the-counter medication or medication dispensed through hospital pharmacies; thus, treatment histories may be incomplete in some cases. The share of ABP 501 prescriptions dispensed in a hospital setting makes up only ~1% of all dispensed supply in both Germany and France; thus, this limitation can be expected to carry little significance for the analyses performed overall. However, this limitation may impact the analysis of switch patterns since a follow-up treatment received in the hospital setting could be missing in LRx data (e.g. infliximab is only dispensed in a hospital setting during the study period and 71% of supply for Remicade is dispensed in a hospital setting in Germany). Third, with the nature of this study being a claims-based analysis, patient clinical characteristics data are not available. When interpreting the findings, potential differences in patient baseline characteristics, prior use of biologics and other targeted therapies (e.g. JAKis), and concomitant use with steroids or immunosuppressants need to be taken into consideration. Lastly, as discussed above, the study period overlaps with the COVID-19 pandemic during which some patients were forced to quarantine and may have had limited access to their medication. Physician practice patterns were impacted and modified accordingly, thus influencing persistence, adherence, and switching patterns. 47
In conclusion, approximately half of the study population of IBD patients remained on ABP 501 therapy in both Germany and France after 12 months. The switch rate from biosimilar ABP 501 to another targeted therapy was about 20% during the first 12 months of initiating ABP 501, and was consistent between German and French patients; but switching patterns differed between countries and by prior use of ADA products. Further research is warranted to understand the patterns of biosimilar utilization for treating patients with IBD in a real-world setting.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231222332 for Treatment persistence and switching patterns of ABP 501 in European patients with inflammatory bowel disease by Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Greg Kricorian, James L. O’Kelly and Walter Reinisch in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848231222332 for Treatment persistence and switching patterns of ABP 501 in European patients with inflammatory bowel disease by Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Greg Kricorian, James L. O’Kelly and Walter Reinisch in Therapeutic Advances in Gastroenterology
Acknowledgments
This study was sponsored by Amgen Inc., Thousand Oaks, CA, USA. Pranali Pathare Mangat, PhD (BioScience Communications, New York, NY, USA) provided writing and editorial support, which was funded by Amgen Inc.
Footnotes
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ran Jin, Amgen Inc., 1 Amgen Center Dr, Thousand Oaks, CA 91320, USA.
Silvia Kruppert, IQVIA Real World Solutions, Frankfurt, Germany.
Florian Scholz, IQVIA Real World Solutions, Frankfurt, Germany.
Isabelle Bardoulat, IQVIA Real World Solutions, Paris, France.
Khalil Karzazi, IQVIA Real World Solutions, Paris, France.
Greg Kricorian, Amgen Inc., Thousand Oaks, CA, USA.
James L. O’Kelly, Amgen Inc., Thousand Oaks, CA, USA
Walter Reinisch, Division of Gastroenterology and Hepatology, Medical University of Vienna, Vienna, Austria.
Declarations
Ethics approval and consent to participate: IQVIA LRx is a fully de-identified database and therefore does not require approval from an institutional review board or ethics committee or patient consent to access patient treatment histories.
Consent for publication: Not applicable.
Author contributions: Ran Jin: Conceptualization; Writing – original draft; Writing – review & editing.
Silvia Kruppert: Formal analysis; Writing – review & editing.
Florian Scholz: Conceptualization; Writing – review & editing.
Isabelle Bardoulat: Formal analysis; Writing – review & editing.
Khalil Karzazi: Formal analysis; Writing – review & editing.
Greg Kricorian: Conceptualization; Writing – review & editing.
James L. O’Kelly: Conceptualization; Writing – review & editing.
Walter Reinisch: Conceptualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Amgen Inc., Thousand Oaks, CA, USA.
R. Jin, G. Kricorian, and J. L. O’Kelly are employees and stockholders of Amgen. S. Kruppert, F. Scholz, I. Bardoulat, and K. Karzazi are employees of IQVIA Real World Solutions. W. Reinishch has received research grants from Takeda, AbbVie, Pfizer, Janssen, consulting fees from AbbVie, AOP Orphan, Bioclinica, Bristol Myers Squibb, Calyx, Eli Lilly, Galapagos, Gilead, Index Pharma, Janssen, Landos Biopharma, Microbiotica, MSD, Pfizer, Protagonist, Seres Therapeutics, Takeda, Teva Pharma, and speakers bureau fees from AbbVie, Celltrion, Janssen, Galapagos, MSD, Takeda.
Availability of data and materials: Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/.
References
- 1. Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med 2009; 60: 97–110. [DOI] [PubMed] [Google Scholar]
- 2. De Boer AG, Bennebroek Evertsz F, Stokkers PC, et al. Employment status, difficulties at work and quality of life in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol 2016; 28: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 3. Argyriou K, Kapsoritakis A, Oikonomou K, et al. Disability in patients with inflammatory bowel disease: correlations with quality of life and patient’s characteristics. Can J Gastroenterol Hepatol 2017; 2017: 6138105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol 2009; 2: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lowe SC, Sauk JS, Limketkai BN, et al. Declining rates of surgery for inflammatory bowel disease in the era of biologic therapy. J Gastrointest Surg 2021; 25: 211–219. [DOI] [PubMed] [Google Scholar]
- 6. Siegel CA, Yang F, Eslava S, et al. Treatment pathways leading to biologic therapies for ulcerative colitis and Crohn’s disease in the United States. Clin Transl Gastroenterol 2020; 11: e00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jean-Frédéric Colombel WR, Armuzzi A. The emerging treatment landscape of inflammatory bowel disease: role of innovator biologics and biosimilars. EMJ Gastroenterol 2018; 7: 50–57. [Google Scholar]
- 8. Markus R, McBride HJ, Ramchandani M, et al. A review of the totality of evidence supporting the development of the first adalimumab biosimilar ABP 501. Adv Ther 2019; 36: 1833–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2017; 177: 1562–1574. [DOI] [PubMed] [Google Scholar]
- 10. Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017; 76: 1093–1102. [DOI] [PubMed] [Google Scholar]
- 11. Macaluso FS, Cappello M, Busacca A, et al. SPOSAB ABP 501: a sicilian prospective observational study of patients with inflammatory bowel disease treated with adalimumab biosimilar ABP 501. J Gastroenterol Hepatol. 2021; 36: 3041–3049. [DOI] [PubMed] [Google Scholar]
- 12. Cingolani L, Barberio B, Zingone F, et al. Adalimumab biosimilars, ABP501 and SB5, are equally effective and safe as adalimumab originator. Sci Rep 2021; 11: 10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ribaldone DG, Caviglia GP, Pellicano R, et al. Effectiveness and safety of adalimumab biosimilar ABP 501 in Crohn’s disease: an observational study. Rev Esp Enferm Dig 2020; 112: 195–200. [DOI] [PubMed] [Google Scholar]
- 14. Barberio B, Zingone F, Melatti P, et al. Effectiveness and safety of ABP501, an adalimumab biosimilar, in naive patients with IBD and in those undergoing switching from adalimumab originator: a multicenter North Italian study. Gastroenterology 2020; 158: S410. Abstract Sa1755. [Google Scholar]
- 15. Tursi A, Mocci G, Allegretta L, et al. Comparison of performances of adalimumab biosimilars SB5, ABP501, GP2017, and MSB11022 in treating patients with inflammatory bowel diseases: a real-life, multicenter, observational study. Inflamm Bowel Dis 2023; 29: 376–383. [DOI] [PubMed] [Google Scholar]
- 16. Fiorino G, Allocca M, Danese S. Adalimumab biosimilar in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2021; 6: 775–776. [DOI] [PubMed] [Google Scholar]
- 17. Nabhan C, Valley A, Feinberg BA. Barriers to oncology biosimilars uptake in the United States. Oncologist 2018; 23: 1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarnola K, Merikoski M, Jyrkkä J, et al. Physicians’ perceptions of the uptake of biosimilars: a systematic review. BMJ Open 2020; 10: e034183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan E, Piercy J, Waller J, et al. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One 2017; 12: e0175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen AJ, Gascue L, Ribero R, et al. Uptake of infliximab biosimilars among the medicare population. JAMA Intern Med 2020; 180: 1255–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Food and Drug Administration. Biosimilar and interchangeable biologics: more treatment choices, https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices (2020, accessed 3 January 2024).
- 22. Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther 2017; 33: 2160–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leonard E, Wascovich M, Oskouei S, et al. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm 2019; 25: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danese S, Fiorino G, Michetti P. Changes in biosimilar knowledge among European Crohn’s Colitis Organization [ECCO] members: an updated survey. J Crohns Colitis 2016; 10: 1362–1365. [DOI] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 26. Richter H, Dombrowski S, Hamer H, et al. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci 2015; 13: Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vilcu A-M, Blanchon T, Sabatte L, et al. Cross-validation of an algorithm detecting acute gastroenteritis episodes from prescribed drug dispensing data in France: comparison with clinical data reported in a primary care surveillance system, winter seasons 2014/15 to 2016/17. BMC Med Res Methodol 2019; 19: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ilgin Y, Staus A, Chauvin F, et al. Statistical models to predict indication of respiratory patients between asthma and COPD. Value Health 2016; 19: PA381. [Google Scholar]
- 29. Richette P, Allez M, Descamps V, et al. Identification des maladies inflammatoires chroniques traitées en ville par biothérapie ou traitement synthétique ciblé. Revue du Rhumatisme 2020; 87: A46–A47. [Google Scholar]
- 30. Richette P, Allez M, Descamps V, et al. Impact de la COVID-19 sur l’initiation des prescriptions des biothérapies dans les maladies inflammatoires chroniques. Rev Rhum Ed Fr 2022; 89: 313–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allez M, Richette P, Descamps V, et al. P.301: Identification des patients ayant une maladie inflammatoire chronique intestinale sous biothérapie sous cutanée en France à partir des données de dispen ation : étude de faisabilité et analyse de l’impact de la COVID-19. 2021. [Google Scholar]
- 32. Larger E, Alexandre-Heymann L, Pilet S, et al. Polypharmacy in diabetes: a nation-wide, pharmacy-based, observational study. Diabet Epidemiol Manag 2022; 8: 100088. [Google Scholar]
- 33. Joumaa H, Sigogne R, Maravic M, et al. Artificial intelligence to differentiate asthma from COPD in medico-administrative databases. BMC Pulm Med. 2022; 22: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. European Pharmaceutical Market Research Association. EPHMRA Anatomical Classification Guidelines 2023, https://www.ephmra.org/sites/default/files/2023-01/2023%20ATC%20Guidelines%20Final_0.pdf (2023, accessed January 3, 2024).
- 35. Kaida-Yip F, Deshpande K, Saran T, et al. Biosimilars: Review of current applications, obstacles, and their future in medicine. World J Clin Cases. 2018; 6: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017; 389: 2304–2316. [DOI] [PubMed] [Google Scholar]
- 37. Hanauer S, Liedert B, Balser S, et al. Safety and efficacy of BI 695501 versus adalimumab reference product in patients with advanced Crohn’s disease (VOLTAIRE-CD): a multicentre, randomised, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021; 6: 816–825. [DOI] [PubMed] [Google Scholar]
- 38. Komaki Y, Yamada A, Komaki F, et al. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases. Alimentary Pharmacol Ther 2017; 45: 1043–1057. [DOI] [PubMed] [Google Scholar]
- 39. Bernard EJ, Fedorak RN, Jairath V. Systematic Review: Non-medical switching of infliximab to CT-P13 in inflammatory bowel disease. Dig Dis Sci 2020; 65: 2354–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Derikx L, Dolby H, Plevris N, et al. Long-term outcomes following a switch from originator adalimumab to the biosimilar SB5 (Imraldi) in IBD. J Crohns Colitis 2021; 15: S353–S354 Abstract P330. [Google Scholar]
- 41. Danese S, Fiorino G, Michetti P. Viewpoint: knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn’s and Colitis organization. J Crohns Colitis 2014; 8: 1548–1450. [DOI] [PubMed] [Google Scholar]
- 42. Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis 2019; 25: 1417–1427. [DOI] [PubMed] [Google Scholar]
- 43. Helwig U, Kostev K, Schmidt C. Comparative analysis of 3-year persistence with vedolizumab compared with antibodies against tumor necrosis factor-alpha in patients with inflammatory bowel disease in Germany: retrospective analysis of a large prescription database. J Clin Gastroenterol 2021; 55: e1–e7. [DOI] [PubMed] [Google Scholar]
- 44. Mevius A, Brandes A, Hardtstock F, et al. Persistence with biologic treatment in patients with inflammatory bowel disease: a German claims data analysis. Digestion 2021; 102: 216–226. [DOI] [PubMed] [Google Scholar]
- 45. Mahlich J, May M, Feig C, et al. Persistence with biologic therapy and associated costs of patients with inflammatory bowel disease: a German retrospective claims data analysis. Crohn’s and Colitis 360 2021; 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huynh L, Hass S, Peyrin-Biroulet L, et al. Real-world treatment patterns and physician preferences for biologics in moderate-to-severe inflammatory bowel disease: retrospective chart review in Europe. Crohns Colitis 360. 2022; 4: otac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Agrawal M, Brenner EJ, Zhang X, et al. Physician practice patterns in holding inflammatory bowel disease medications due to COVID-19, in the SECURE-IBD registry. J Crohns Colitis 2021; 15: 860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim H, Alten R, Avedano L, et al. The Future of biosimilars: maximizing benefits across immune-mediated inflammatory diseases. Drugs 2020; 80(2): 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Odinet JS, Day CE, Cruz JL, Heindel GA. The biosimilar Nocebo effect? A systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm 2018; 24: 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231222332 for Treatment persistence and switching patterns of ABP 501 in European patients with inflammatory bowel disease by Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Greg Kricorian, James L. O’Kelly and Walter Reinisch in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848231222332 for Treatment persistence and switching patterns of ABP 501 in European patients with inflammatory bowel disease by Ran Jin, Silvia Kruppert, Florian Scholz, Isabelle Bardoulat, Khalil Karzazi, Greg Kricorian, James L. O’Kelly and Walter Reinisch in Therapeutic Advances in Gastroenterology