Abstract
Background
Breast cancer and thyroid cancer are two prevalent malignancies in women, and a potential association between the two diseases has been suggested.
Methods
This retrospective case-control study was conducted involving 97 patients with breast cancer and thyroid cancer (BC-TC group) and 97 age-matched patients with breast cancer alone (BC group). Thyroid hormone levels, including triiodothyronine (T3), thyroxine (T4), free triiodothyronine (FT3), free thyroxine (FT4) and thyroid-stimulating hormone (TSH), were analyzed in healthy controls, BC patients, and BC-TC patients.
Results
BC-TC patients exhibited a higher rate of estrogen receptor (ER) and progesterone receptor (PR) positivity compared to BC patients. Serum T3 levels were significantly decreased in BC and BC-TC patients compared to healthy controls. However, there was no significant difference in T3 levels between BC and BC-TC patients. Serum TSH levels were significantly higher in BC-TC patients compared to BC patients.
Conclusion
ER positivity, PR positivity, and serum TSH levels greater than 4.45 mU/L were independent risk factors for primary thyroid cancer in breast cancer patients.
Keywords: breast cancer, thyroid cancer, estrogen receptor, ER
Introduction
Breast cancer ranks among the most prevalent malignancies affecting women globally, with its incidence steadily on the rise.1 Likewise, thyroid cancer has exhibited an ascending trajectory in most global regions, securing its place as the eighth most common malignancy in women.2 The correlation between breast and thyroid cancers has been a subject of medical scrutiny since the 19th century.3 Prolonged clinical observations have revealed numerous parallels between these two malignancies,4 such as a pronounced gender disparity in incidence, with women experiencing significantly higher rates than men. Furthermore, both diseases manifest their incidence peaks postmenopausally.5,6 Over the past decade, a body of research has substantiated a potential bidirectional causative relationship between breast and thyroid cancers.7 An examination of data from the American Cancer Society revealed a threefold increase in thyroid cancer incidence among women with breast cancer, and a subsequent 19-fold increase among men with breast cancer.8,9
In vitro studies have shown that thyroid hormones promote tumor growth and mimic estrogen-induced breast cell proliferation.10,11 Rodent models have further demonstrated that thyroid hormones stimulate tumor growth and metastasis.12 The synthesis and secretion of thyroid hormones are primarily regulated by thyroid-stimulating hormone (TSH), which acts through TSH receptors to enhance all stages of thyroid hormone synthesis.13,14 Recent investigations have identified significantly elevated serum TSH levels in patients with differentiated thyroid cancer compared to the general population, with TSH expression correlating with tumor aggressiveness.4,15,16 Subsequent subgroup analyses revealed an increased risk of differentiated thyroid cancer development in individuals with normal-range serum TSH and no ultrasound-detected nodules, as TSH levels rose.17
Consequently, this study aims to explore alterations in serum thyroid hormones in breast cancer patients with multiple primary malignancies involving thyroid cancer and examine their association with the occurrence of multiple primary malignancies involving both breast and thyroid cancers.
Methods
Participants
This study constitutes a retrospective case-control investigation. Ninety-seven eligible patients diagnosed with both breast cancer and thyroid cancer (BC-TC group) were retrospectively enrolled from January 2010 to January 2020 at Cangzhou Central Hospital, the largest comprehensive medical center in the city. In parallel, a control group (BC group) consisting of 97 age-matched individuals with isolated breast cancer was randomly selected. Additionally, a cohort of 90 females undergoing routine health examinations served as healthy controls. Approval for this study was obtained from Cangzhou Central Hospital, and written informed consent was obtained from all participants.
Inclusion criteria: patients with both thyroid cancer and breast cancer undergoing surgical treatment in our hospital; patients with complete clinical and pathological data; female patients; patients with no underlying thyroid disease before surgery;
Exclusion criteria: patients with non-primary tumors of breast cancer; patients with non-primary tumors of thyroid cancer; patients with underlying diseases of the hypothalamus or pituitary; patients with autoimmune diseases.
Control group: patients without underlying thyroid disease; patients without breast disease; patients without underlying hypothalamic or pituitary disease; and patients without autoimmune disease.
Analysis of Thyroid Hormone Levels
Peripheral venous blood was obtained from all patients in the morning of the next day before operation, and the serum was separated and frozen at −80°C. Triiodothyronine (T3), free triiodothyronine (FT3), thyroxine (T4), free thyroxine (FT4) and TSH were detected on a Cobas e601 electrochemiluminescence immunoassay analyzer.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software. The comparison of measurement data between two groups was done by t test, and the comparison of count data between two groups was done by χ2 test or Fisher’s exact probability method. Multivariate analysis of factors affecting breast cancer combined with thyroid cancer was performed using Logistic stepwise regression analysis. Significance tests were all two-sided tests, and the test level was α = 0.05.
Results
Clinicopathological Factors for the Patients
Table 1 provides a comprehensive examination of clinicopathological factors in patients with exclusive primary breast cancer (BC) and those with concurrent primary breast cancer and primary thyroid cancer (BC-TC). Age did not exhibit a statistically significant difference between the two cohorts (p = 0.376). The presence or absence of a family history of breast cancer (p = 0.630) or thyroid cancer showed no significant disparity between the two groups (p = 0.497). The distribution of patients across post-menopausal, pre-menopausal, and perimenopausal categories demonstrated similarity between the BC and BC-TC groups (p = 0.589).
Table 1.
Comparisons of Clinicopathological Factors of Breast Cancer Between Patients with Only Primary Breast Cancer (BC) and Patients with Both Primary Breast Cancer and Primary Thyroid Cancer (BC-TC)
| Factors | BC (n=97) | BC-TC (n=97) | p value |
|---|---|---|---|
| Age (years) | |||
| < 55 | 56 (57.7%) | 63 (64.9%) | 0.376 |
| ≥ 55 | 41 (42.3%) | 34 (35.1%) | |
| Family history of breast cancer | |||
| Yes | 8 (8.2%) | 11 (11.3%) | 0.630 |
| No | 89 (91.8%) | 86 (88.7%) | |
| Family history of thyroid cancer | |||
| Yes | 3 (3.1%) | 6 (6.2%) | 0.497 |
| No | 94 (96.9%) | 91 (93.8%) | |
| Menstrual state | |||
| Post-menopause | 54 (55.7%) | 47 (48.4%) | 0.589 |
| Pre-menopause | 36 (37.1%) | 41 (42.3%) | |
| Perimenopause | 7 (7.2%) | 9 (9.3%) | |
| Pathological type | |||
| Invasive ductal carcinoma | 71 (73.2%) | 67 (69.1%) | 0.694 |
| Invasive lobular carcinoma | 11 (11.3%) | 13 (13.4%) | |
| Medullary carcinoma | 5 (5.2%) | 3 (3.1%) | |
| Carcinoma in situ | 10 (10.3%) | 14 (14.4%) | |
| Histological grade | |||
| I | 4 (4.1%) | 1 (1.0%) | 0.108 |
| II | 62 (63.9%) | 53 (54.6%) | |
| III | 31 (32.0%) | 43 (44.4%) | |
| Lymph node metastasis | |||
| Positive | 34 (35.1%) | 25 (25.8%) | 0.212 |
| Negative | 63 (64.9%) | 72 (74.2%) | |
| Tumor size (cm) | |||
| ≤ 2 | 49 (50.5%) | 58 (59.8%) | 0.248 |
| > 2 | 48 (49.5%) | 39 (40.2%) | |
| Estrogen receptor | |||
| Positive | 44 (45.4%) | 66 (68.1%) | 0.002 |
| Negative | 53 (54.6%) | 31 (31.9%) | |
| Progesterone receptor | |||
| Positive | 50 (51.5%) | 69 (71.1%) | 0.008 |
| Negative | 47 (48.5%) | 28 (28.9%) | |
| Human epidermal growth factor receptor | |||
| Positive | 36 (37.1%) | 42 (43.3%) | 0.464 |
| Negative | 61 (62.9%) | 55 (56.7%) | |
| Metastasis classification | |||
| M0 | 91 (93.8%) | 89 (91.8%) | 0.783 |
| M1 | 6 (6.2%) | 8 (8.2%) | |
Notes: The data were shown as n (percentage). The comparisons of data between the two groups were done by Fisher’s exact test or Chi-square test.
Moreover, the distribution of various pathological types, such as invasive ductal carcinoma, invasive lobular carcinoma, medullary carcinoma, and carcinoma in situ, did not significantly differ between BC and BC-TC patients (p = 0.694). No statistically significant variance was observed in the histological grade of breast cancer between the two groups (p = 0.108). The presence or absence of lymph node metastasis displayed no significant difference between BC and BC-TC patients (p = 0.212). Similarly, the distribution of tumor sizes (≤ 2 cm and > 2 cm) did not significantly differ between the two groups (p = 0.248).
Notably, there was no significant distinction in the status of human epidermal growth factor receptor between BC and BC-TC patients (p = 0.464). However, BC-TC patients exhibited a significantly higher rate of estrogen receptor (ER) positivity compared to BC patients (p = 0.002). Similarly, BC-TC patients displayed a significantly higher rate of progesterone receptor (PR) positivity compared to BC patients (p = 0.008).
Comparisons of Serum T3, T4, FT3, FT4 and TSH Levels Among Healthy Controls, BC Group and BC-TC Group
The study involved the analysis and comparison of T3 (Figure 1A), FT3 (Figure 1B), T4 (Figure 1C), FT4 (Figure 1D), and TSH (Figure 1E) levels among three distinct cohorts: 90 healthy controls (HC), 97 patients with breast cancer only (BC), and 97 patients with the dual diagnosis of breast cancer and thyroid cancer (BC-TC). The findings indicate a noteworthy decrease in serum T3 levels among both breast cancer patients and those with breast cancer and thyroid cancer when compared to the HC group. However, no significant disparity in T3 levels was observed between the BC and BC-TC groups.
Figure 1.
Comparisons of serum triiodothyronine (T3) (A), thyroxine (T4) (B), free triiodothyronine (FT3) (C), free thyroxine (FT4) (D) and thyroid-stimulating hormone (TSH) (E) among healthy controls (n = 90), only primary breast cancer (BC, n = 97) and patients with both primary breast cancer and primary thyroid cancer (BC-TC, n = 97). Data were shown with box plot. ns indicates no statistical significance. ***p < 0.001.
Contrastingly, there were no notable differences in T4, FT3, and FT4 levels across the three groups. TSH levels exhibited no significant variance between the HC and BC groups. Nevertheless, the BC-TC group demonstrated a substantial increase in TSH levels, establishing a statistically significant difference when compared to both the HC and BC groups.
Table 2 shows the multivariate analysis between 97 patients with breast cancer and thyroid cancer and 97 patients with breast cancer alone. The variables with significant differences in Table 1 are used as independent variables. Whether combined with primary thyroid cancer was the dependent variable (BC=0, BC-TC=1). Our data showed that ER, PR positive and serum TSH > 4.45 mU/L were independent risk factors for primary thyroid cancer in breast cancer patients.
Table 2.
Multivariate Logistic Analysis of Clinicopathological Factors for Complicated Primary Thyroid Cancer in Patients with Primary Breast Cancer
| OR | 95% CI | p value | |
|---|---|---|---|
| ER positive | 2.74 | 1.63 to 4.82 | 0.016 |
| PR positive | 2.28 | 1.39 to 5.71 | 0.021 |
| Serum TSH > 4.45 mU/L | 1.86 | 1.24 to 3.35 | 0.029 |
Abbreviations: OR, odds ratio; ER, Estrogen receptor; PR, Progesterone receptor; TSH, Thyroid stimulating hormone.
Correlation Between TSH Levels and ER and PR
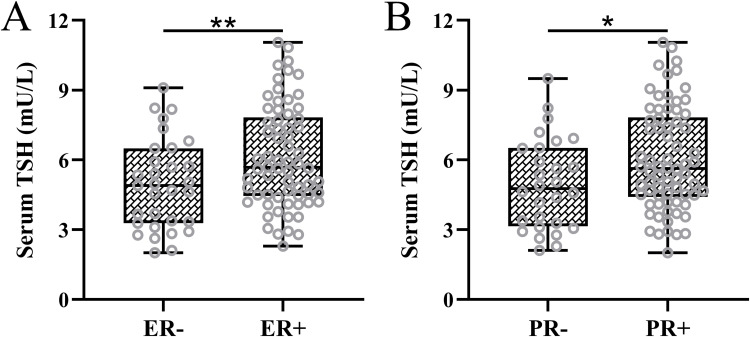
Moreover, we conducted a detailed analysis of the association between serum TSH levels and the presence of ER and PR positivity among the cohort of 97 patients diagnosed with both breast cancer and thyroid cancer. The results revealed elevated serum TSH levels in individuals with ER-positive or PR-positive breast cancer (Figure 2A and B). This observation provides additional insight into the correlation between serum TSH levels and the identified risk factors associated with the co-occurrence of breast cancer and thyroid cancer.
Figure 2.
(A) Comparisons of serum thyroid-stimulating hormone (TSH) between estrogen receptor negative (ER-, n = 31) and positive (ER+, n = 66) in patients with both primary breast cancer and primary thyroid cancer. (B) Comparisons of serum TSH between progesterone receptor negative (PR-, n = 28) and positive (PR+, n = 69) in in patients with both primary breast cancer and primary thyroid cancer. Data were shown with box plot. *p < 0.05, **p < 0.01.
Discussion
Breast cancer and thyroid cancer are two common malignancies in women, and there is growing evidence suggesting a potential association between the two diseases.18 A 2001 Japanese study showed that women with breast cancer had a higher incidence of thyroid disease.19 In 2002, Sctrlis carefully reviewed the details of previously published studies and pointed out that previous studies on the relationship between thyroid and breast diseases may be due to selection bias in the collection of clinical data.20 In a Korean study of 518 breast cancer patients, a total of 13 (2.5%) patients were diagnosed with papillary carcinoma after thyroidectomy.21 Tumors had a mean longest diameter of 9.9 mm (range 1–30 mm). Six cases (6/13, 46.2%) were diagnosed as co-occurring breast and thyroid cancers, and the remaining thyroid cancers were detected 6 to 33 months (mean 16.5 months) after breast cancer surgery.22 A high incidence of thyroid cancer (2.5%) in breast cancer patients has been proposed, and ultrasound screening can be used for early detection of thyroid cancer.22 Another study in South Korea in 2014 showed that the incidence of thyroid cancer in breast cancer patients increased, and the detection stage was earlier than that of patients with simple thyroid cancer, which may be related to more timely screening of breast cancer patients. However, the prognosis of these patients is not better than that of simple breast cancer. A meta-analysis conducted by Kyle.R et al in 2015 included 26 studies and showed that both the probability of developing breast cancer in patients with thyroid cancer and the probability of developing thyroid cancer in breast cancer patients were higher than that of the general population.23–25 Patients with thyroid cancer are more likely to later develop thyroid cancer than patients with thyroid cancer to later develop breast cancer. In 2018, a retrospective study of 13,978 breast cancer patients showed that 247 (1.8%) of them had co-occurred thyroid cancer at the same time or successively, and 98.0% of them were papillary thyroid cancer.24 The standardized incidence ratio was 4.48. Co-occurrence of thyroid and breast cancers was an independent prognostic factor for shorter disease-free survival.
The present study delves into the intricate relationship between breast cancer and thyroid cancer, offering a nuanced clinicopathological perspective. The findings contribute significantly to our understanding of the interplay between these two malignancies, shedding light on potential implications for clinical practice. The clinical importance of this investigation lies in its ability to unravel distinctive patterns and correlations, fostering a more comprehensive approach to patient care.
One of the noteworthy aspects of our study is the meticulous analysis of clinicopathological factors among patients with exclusive primary breast cancer and those concurrently diagnosed with primary breast and thyroid cancers. The absence of significant differences in age, family history, menopausal status, pathological types, histological grade, lymph node metastasis, tumor size, and human epidermal growth factor receptor status underscores the importance of recognizing the shared clinical characteristics between these patient groups. This knowledge is instrumental in refining diagnostic and prognostic strategies, enabling healthcare professionals to tailor their approach based on the specific clinical profile of each patient. The investigation into serum thyroid hormone levels among healthy controls and patients with breast cancer alone or in conjunction with thyroid cancer provides novel insights into the potential systemic implications of this dual diagnosis. Notably, the significant decrease in serum T3 levels among breast cancer patients, regardless of thyroid cancer coexistence, prompts a reconsideration of the metabolic dynamics in these individuals. The observed elevation in TSH levels in the breast cancer and thyroid cancer cohort further underscores the intricate relationship between thyroid function and breast cancer. This finding holds paramount clinical significance as it suggests a potential avenue for further research into targeted therapeutic interventions that may influence thyroid hormone levels and, consequently, impact the progression of breast cancer.
Moreover, multivariate analysis revealed that ER positivity, PR positivity, and serum TSH levels greater than 4.45 mU/L were independent risk factors for primary thyroid cancer in breast cancer patients. This further supports the notion that hormone receptor expression and elevated TSH levels play a role in the development of multiple primary malignancies of breast and thyroid cancers. Finally, we explored the correlation between TSH levels and hormone receptor expression. Our data demonstrated that serum TSH levels were higher in both ER-positive and PR-positive breast cancer patients with concomitant thyroid cancer. This finding suggests a potential relationship between elevated TSH levels and hormone receptor status in the context of breast cancer complicated by thyroid cancer.
Conclusions
In conclusion, this study significantly advances our understanding of the clinicopathological nuances and systemic implications associated with the coexistence of breast and thyroid cancers. The observed correlations between clinicopathological factors and serum thyroid hormone levels underscore the importance of a multidisciplinary approach to patient management. The findings not only contribute to the academic discourse but also hold promising implications for the development of targeted therapeutic strategies, potentially improving outcomes for patients navigating the complex terrain of breast cancer complicated by thyroid cancer. Further research is warranted to elucidate the underlying mechanisms and validate the clinical utility of these observations, paving the way for more personalized and effective interventions in this challenging patient population.
Data Sharing Statement
The raw data will be made available upon reasonable request to the corresponding author (Jie Li).
Ethical Approval
Cangzhou Central Hospital approved this study, and written informed consent was obtained from the participants. The study was performed in strict accordance with the Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects.
Consent for Publication
Not applicable.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Zhang YN, Xia KR, Li CY, Wei BL, Zhang B. Review of Breast Cancer Pathologigcal Image Processing. Biomed Res Int. 2021;2021:1994764. doi: 10.1155/2021/1994764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi: 10.1016/S0140-6736(16)30172-6 [DOI] [PubMed] [Google Scholar]
- 3.Bolf EL, Sprague BL, Carr FE. A Linkage Between Thyroid and Breast Cancer: a Common Etiology? Cancer Epidemiol Biomarkers Prev. 2019;28(4):643–649. doi: 10.1158/1055-9965.EPI-18-0877 [DOI] [PubMed] [Google Scholar]
- 4.Yuan S, Kar S, Vithayathil M, et al. Causal associations of thyroid function and dysfunction with overall, breast and thyroid cancer: a two-sample Mendelian randomization study. Int J Cancer. 2020;147(7):1895–1903. doi: 10.1002/ijc.32988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen SM, White MG, Hong S, et al. The Breast-Thyroid Cancer Link: a Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25(2):231–238. doi: 10.1158/1055-9965.EPI-15-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph KR, Edirimanne S, Eslick GD. The association between breast cancer and thyroid cancer: a meta-analysis. Breast Cancer Res Treat. 2015;152(1):173–181. doi: 10.1007/s10549-015-3456-6 [DOI] [PubMed] [Google Scholar]
- 7.Shim SR, Kitahara CM, Cha ES, Kim SJ, Bang YJ, Lee WJ. Cancer Risk After Radioactive Iodine Treatment for Hyperthyroidism: a Systematic Review and Meta-analysis. JAMA Network Open. 2021;4(9):e2125072. doi: 10.1001/jamanetworkopen.2021.25072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halada S, Casado-Medrano V, Baran JA, et al. Hormonal Crosstalk Between Thyroid and Breast Cancer. Endocrinology. 2022;163(7). doi: 10.1210/endocr/bqac075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakos B, Kiss A, Arvai K, et al. Co-occurrence of thyroid and breast cancer is associated with an increased oncogenic SNP burden. BMC Cancer. 2021;21(1):706. doi: 10.1186/s12885-021-08377-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauthier BR, Sola-Garcia A, Caliz-Molina MA, et al. Thyroid hormones in diabetes, cancer, and aging. Aging Cell. 2020;19(11):e13260. doi: 10.1111/acel.13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meirovitz A, Nisman B, Allweis TM, et al. Thyroid Hormones and Morphological Features of Primary Breast Cancer. Anticancer Res. 2022;42(1):253–261. doi: 10.21873/anticanres.15480 [DOI] [PubMed] [Google Scholar]
- 12.Petranovic Ovcaricek P, Verburg FA, Hoffmann M, et al. Higher thyroid hormone levels and cancer. Eur J Nucl Med Mol Imaging. 2021;48(3):808–821. doi: 10.1007/s00259-020-05018-z [DOI] [PubMed] [Google Scholar]
- 13.Kopp P. The TSH receptor and its role in thyroid disease. Cell Mol Life Sci. 2001;58(9):1301–1322. doi: 10.1007/pl00000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galofre JC, Chacon AM, Latif R. Targeting thyroid diseases with TSH receptor analogs. Endocrinol Nutr. 2013;60(10):590–598. doi: 10.1016/j.endonu.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 15.Cho A, Chang Y, Ahn J, Shin H, Ryu S. Cigarette smoking and thyroid cancer risk: a cohort study. Br J Cancer. 2018;119(5):638–645. doi: 10.1038/s41416-018-0224-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grani G, Ramundo V, Verrienti A, Sponziello M, Durante C. Thyroid hormone therapy in differentiated thyroid cancer. Endocrine. 2019;66(1):43–50. doi: 10.1007/s12020-019-02051-3 [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Rusiecki J, Zhao N, et al. Thyroid-Stimulating Hormone, Thyroid Hormones, and Risk of Papillary Thyroid Cancer: a Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1209–1218. doi: 10.1158/1055-9965.EPI-16-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nisman B, Allweis TM, Carmon E, et al. Thyroid Hormones, Silencing Mediator for Retinoid and Thyroid Receptors and Prognosis in Primary Breast Cancer. Anticancer Res. 2020;40(11):6417–6428. doi: 10.21873/anticanres.14663 [DOI] [PubMed] [Google Scholar]
- 19.Evans HS, Lewis CM, Robinson D, Bell CM, Moller H, Hodgson SV. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer. 2001;84(3):435–440. doi: 10.1054/bjoc.2000.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wapnir IL, van de Rijn M, Nowels K, et al. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab. 2003;88(4):1880–1888. doi: 10.1210/jc.2002-021544 [DOI] [PubMed] [Google Scholar]
- 21.Tazebay UH, Wapnir IL, Levy O, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6(8):871–878. doi: 10.1038/78630 [DOI] [PubMed] [Google Scholar]
- 22.Cho JY, Leveille R, Kao R, et al. Hormonal regulation of radioiodide uptake activity and Na+/I- symporter expression in mammary glands. J Clin Endocrinol Metab. 2000;85(8):2936–2943. doi: 10.1210/jcem.85.8.6727 [DOI] [PubMed] [Google Scholar]
- 23.Berger AC, Korkut A, Kanchi RS, et al. A Comprehensive Pan-Cancer Molecular Study of Gynecologic and Breast Cancers. Cancer Cell. 2018;33(4):690–705 e9. doi: 10.1016/j.ccell.2018.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricketts CJ, De Cubas AA, Fan H, et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018;23(1):313–326 e5. doi: 10.1016/j.celrep.2018.03.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung J, Atherton I, Kyle RG, Hubbard G, McLaughlin D. Psychological distress, optimism and general health in breast cancer survivors: a data linkage study using the Scottish Health Survey. Support Care Cancer. 2016;24(4):1755–1761. doi: 10.1007/s00520-015-2968-2 [DOI] [PubMed] [Google Scholar]