Abstract
Gnotobiotic mice inoculated with an enterohemorrhagic Escherichia coli (EHEC) O157:H7 strain developed a flaccid paresis, usually culminating in death. The bacteria colonized feces at 109 to 1010 CFU per g (inoculum size: 2.0 × 109 CFU/mouse), and Shiga-like toxins (SLTs) were detected in the feces. A microscopic examination of colons showed mild inflammatory cell infiltration, thinning of the intestinal wall, or necrotic foci. Necrosis of tubular cells was noted in these symptomatic mice. Microhemorrhage, thrombosis, and edematous changes of the brain were also seen. Inflammatory cytokines, tumor necrosis factor alpha (TNF-α), interleukin 1α (IL-1α), and IL-6, were detected in the kidney after EHEC infection, but not in the serum. In the brain, only TNF-α was detected. When 2.0 × 102 CFU of EHEC O157:H7 was fed to germ-free mice, the number of bacteria began to rise rapidly on day 1 and was maintained at 108 to 109 CFU/g of feces. SLTs were detected in the feces of the mice. However, the mice showed no histological changes and no cytokine responses, similar to what was found for controls. Treatment with TNF-α modified the clinical neural signs, histopathological changes, and cytokine responses; mice treated with TNF-α developed severe neurotoxic symptoms and had higher frequencies of systemic symptoms and glomerular pathology. Strong cytokine responses were seen in the kidney and brain. Serum cytokines were also detected in this group. In contrast, a TNF-α inhibitor (protease inhibitor) inhibited these responses, especially in the brain. However, local synthesis of the cytokines was observed in the kidney. Thus, TNF-α and the other proinflammatory cytokines could be important in modifying the disease caused by EHEC.
Enterohemorrhagic Escherichia coli (EHEC) strains are important causes of human hemorrhagic colitis, hemolytic uremic syndrome (HUS), and encephalopathy (7, 13). A common histopathological finding in patients is the destruction of endothelial cells lining small blood vessels in the colon, kidneys, and central nervous system (20). The virulence of EHEC has been linked to the production of Shiga-like toxins (SLTs) (12, 14, 16). The SLTs act as an inhibitor of protein synthesis, enzymatically modifying the translational machinery of the host cell (3). Recently, Fujii et al. suggested that SLT-II is toxic to both endothelial cells and neurons in the central nervous system (5, 6). However, the precise contribution of SLT-mediated inhibition of protein synthesis to the development of HUS and encephalopathy remains mysterious.
A variety of animal models have been used to study the symptoms and histopathologic changes associated with human EHEC infection. EHEC strains caused gastrointestinal, neurologic, or systemic symptoms and death in gnotobiotic piglets (4), rabbits (18), and mice (15, 26, 27). Acute tubular necrosis of the kidneys was found in inoculated animals, but glomerular pathology was not observed (26, 27). Recently, Karpman et al. observed that mice inoculated with SLT-II-positive strains developed severe neurotoxic symptoms and a higher frequency of systemic symptoms, glomerular mesangial hypertrophy, and mesangial deposition than did mice inoculated with SLT-II-negative strains (14). However, they did not observe the glomerular vascular lesions characteristic of HUS in humans.
It has been suggested that cooperation between SLT and tumor necrosis factor (TNF) may be important in producing the pathologic changes observed in HUS (2). TNF-α and SLT exhibited synergistic cytotoxic activity toward human endothelial cells (16). Harel et al. suggested that local synthesis of TNF within the kidney may contribute to renal injury induced by SLT (8).
The aim of this study was to assess the relative importance of TNF-α in EHEC infection by using germ-free mice. EHEC infection induced TNF synthesis within the kidney and brain. Treatment with TNF-α or its inhibitor modified the disease for experimental animals.
MATERIALS AND METHODS
Bacterial strains.
EHEC O157:H7 strain EDL 931 (22), which produces both SLT-I and SLT-II, was used for our experiments. Nonpathogenic E. coli MV1184 was also used. The organisms were incubated in brain heart infusion (BHI) medium for 24 h at 37°C. After one passage (incubation for 6 h at 37°C), viable counts were determined by plating on agar media.
Mice.
Germ-free IQI mice, bred from ICR mice, were obtained from Japan Clea Co. Ltd. (Tokyo, Japan). Female and male mice were used at 4 to 5 weeks of age. Specific-pathogen-free ICR mice (male, 4 weeks of age) were used as flora-positive controls.
Infection protocol.
Each E. coli strain was prepared by washing the bacterial pellet twice in phosphate-buffered saline (PBS; pH 7.4). Bacterial suspensions (0.1 ml in PBS; EHEC EDL 931: 2.0 × 103/ml or 2.0 × 1010/ml; E. coli MV1184: 2.4 × 1010/ml) were deposited intragastrically through a soft polyethylene catheter. Immediately after inoculation, the catheter was removed, and no further manipulations were performed. The controls received 0.1 ml of PBS.
Mouse colonization experiments.
Mice were maintained in a level 3 environment. Food and drinking water for mice were autoclaved before use. After bacterial inoculation, fecal samples were collected from each mouse. They were suspended at a concentration of 100 mg/ml in BHI medium, homogenized, and plated on Chromagar O157 (chromogenic medium for the isolation and differentiation of EHEC O157; CHROMagar Microbiology, Paris, France) and BHI agar. In this investigation, colonizing ability was assessed by determining the level at which a strain persisted in mouse feces.
SLT antigen level determination.
SLT antigen levels were determined with an enzyme-linked immunosorbent assay (ELISA) kit (Novapath EHEC; Japan Bio-Rad Laboratories, Tokyo, Japan). This immunoassay is for the detection of SLT-I and -II in stool specimens and cultures. An E. coli verotoxin detection kit for reversed passive latex agglutination (RPLA; Denka Seiken Co. Ltd., Tokyo, Japan) was also used.
TNF-α and protease inhibitor.
To determine the role of TNF-α in the pathogenesis of EHEC O157:H7, a TNF-α-treated mouse group and a TNF-α-inhibited group were prepared. We used recombinant TNF-α (R & D Systems Europe Ltd., Abingdon, Oxon, England) for intraperitoneal injections (10 ng per mouse). The dose was determined in a study previously reported (9). The first injection of TNF-α was done 3 h before EHEC feeding. TNF-α was injected at 2, 4, and 6 days postinfection. Nafamostat mesilate (NM; 6-amidino-2-naphthyl p-guanidinobenzoate dimethanesulfonate) was provided by Torii Pharmaceutical Co. Ltd. (Tokyo, Japan) and was used as a TNF-α inhibitor (0.02 mg/mouse; intraperitoneal injections 3 h before and every day after EHEC feeding). NM is a synthetic protease inhibitor that inhibits the various serine proteases during the coagulation cascade as well as during the inflammatory process (1). NM, at a concentration of 10−5 M, inhibited the production of TNF-α by lipopolysaccharide-stimulated monocytes in vitro (24).
Cytokine assay.
Cytokine assays were done by a method previously described (10). TNF-α, interleukin 1α (IL-1α), and IL-6 levels were quantified with ELISA kits (Genzyme, Cambridge, Mass.).
Hematology.
Blood was obtained from mice 1 and 7 days after inoculation. Blood cell counts and hemoglobin concentrations were determined by using a Sysmex microcell counter (model F-800; Toa Medical Electronics Co. Ltd., Kobe, Japan).
Histological examinations.
Seven days postfeeding or when signs of disease were first evident, animals colonized with E. coli O157:H7 were sacrificed and subjected to full necropsy. Tissue specimens were collected for histological examination. Specimens were fixed in 10% buffered neutral Formalin and processed by standard procedures. Sections of paraffin-embedded tissue were stained with hematoxylin and eosin.
SEM.
Intestinal tissue was obtained from mice after 1 and 7 days of inoculation. Specimens were treated by a routine method described previously (11), dried to the critical point in an HPC-2 critical point dryer (Hitachi Co. Ltd., Ibaragi, Japan), coated with gold-palladium metal with an Eiko IB-3 coating machine, and examined in a Hitachi X-650 scanning electron microscope (SEM).
Statistics.
Geometric mean CFUs from each group of three or more animals were calculated, and the significance of differences between means of the groups was determined by an unpaired t test. Probability values <0.05 were considered significant.
RESULTS
Symptoms of disease in gnotobiotic mice inoculated with EHEC O157:H7.
IQI mice fed the strain of EHEC (2.0 × 109 CFU) became lethargic, occasionally exhibited hind-limb paralysis, stopped eating, retained urine, and died within 7 days of infection (Table 1). The lethality was 60%. In TNF-treated mice, severe neurologic symptoms developed within 4 to 7 days after inoculation; all of them showed convulsions and died. The clinical features of TNF-treated mice were similar to those observed in humans except for the absence of hemorrhagic diarrhea. NM treatment decreased lethality after EHEC infection. However, IQI mice treated with both TNF-α and NM showed neurologic symptoms and died. Retention of urine was associated with renal failure (renal tubular necrosis).
TABLE 1.
Virulence of EHEC O157:H7 for orally inoculated IQI and ICR mice and effect of TNF-α
| Mouse type (treatment) | E. coli strain | Inoculum size (CFU/mouse) | No. of mice that died/total no. examined | No. of mice with indicated neurological sign/total no. examined
|
No. of mice showing retention of urine/total no. examined | |
|---|---|---|---|---|---|---|
| Paralysis | Convulsions | |||||
| IQI | EDL931 | 2.0 × 109 | 6/10 | 6/10 | 0/10 | 6/10 |
| IQI (TNF-α) | EDL931 | 2.0 × 109 | 6/6 | 0/6 | 6/6 | 6/6 |
| IQI (NM) | EDL931 | 2.0 × 109 | 2/6 | 3/6 | 0/6 | 3/6 |
| IQI (TNF-α and NM) | EDL931 | 2.2 × 109 | 5/6 | 0/6 | 5/6 | 5/6 |
| IQI | EDL931 | 2.0 × 102 | 0/10 | 0/10 | 0/10 | 0/10 |
| IQI | MV1184 | 2.4 × 109 | 0/6 | 0/6 | 0/6 | 0/6 |
| ICR | EDL931 | 2.0 × 109 | 0/6 | 0/6 | 0/6 | 0/6 |
| Controlsa | ||||||
| IQI | 0/6 | 0/6 | 0/6 | 0/6 | ||
| IQI (TNF-α) | 0/3 | 0/3 | 0/3 | 0/3 | ||
| IQI (NM) | 0/3 | 0/3 | 0/3 | 0/3 | ||
| IQI (NM and TNF-α) | 0/5 | 0/5 | 0/5 | 0/5 | ||
Controls received 0.1 ml of PBS.
IQI mice inoculated intragastically with 2.0 × 102 CFU of either EHEC or control strain MV1184 did not develop gastrointestinal, neurologic, and systemic symptoms. ICR mice (specific-pathogen-free, flora-positive controls) also did not develop any symptoms. Control mice receiving 0.1 ml of PBS intragastically did not develop any symptoms.
Hematology.
The hematology results showed significant alterations in mean platelet and leukocyte counts on day 1 after EHEC infection (P < 0.01). Platelet and leukocyte counts were (76.5 ± 24.1) × 104/mm3 and (53.0 ± 3.6) × 102/mm3, respectively, in the group subjected to EHEC infection, whereas they were (129.5 ± 26.4) × 104/mm3 and (22.5 ± 2.1) × 102/mm3, respectively, in the controls. TNF treatment was effective in increasing the leukocyte count ([85.3 ± 14.4] × 102/mm3; P < 0.01; significantly higher than controls). The majority of the other hematologic parameters were not significantly different for the three treatments.
Concerning the hematology of surviving asymptomatic mice, there were no significant alterations with only one exception: leukocyte count. The mean leukocyte count for mice subjected to EHEC infection and not treated with NM ([52.0 ± 2.8] × 102/mm3) was two times higher than that of controls; for mice infected with EHEC and treated with NM, the mean leukocyte count ([80.7 ± 28.3] × 102/mm3) was three times higher than that of controls.
Histology.
A macroscopic examination of colons showed mild edemas in all sick mice. A microscopic examination revealed mild polymorphonuclear leukocyte infiltration, thinning of the intestinal wall, or necrotic foci involving the entire intestinal wall. The destruction of the mucous layer was observed in these mice by SEM examinations.
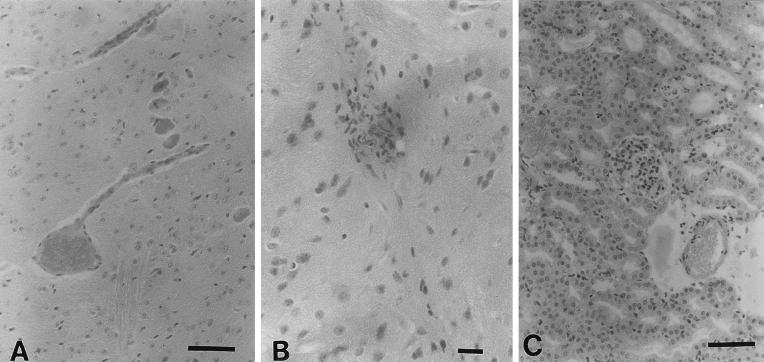
Microhemorrhages, thrombosis, and edematous changes of the capillary endothelia were observed in the brains of moribund mice which were showing neurological symptoms, especially in the group subjected to EHEC plus TNF-α (Fig. 1A). Several neural cells looked globular, and a strong degenerative change was observed in these mice. Sometimes, endothelial-like cell proliferation was observed in the brains of mice in this group (Fig. 1B). The pathological changes were mild in symptomatic mice subjected to EHEC infection only. Edemas of the brain were seen in these mice. It seemed that NM treatment was effective in inhibiting neural pathological changes after EHEC infection.
FIG. 1.
Histopathological changes in a symptomatic mouse with EHEC infection and TNF-α treatment. Bars: 10.0 μm. (A) Edematous change of the brain with microthrombosis at 7 days after infection. The capillary endothelial cells also show edematous changes. In several neural cells, globular, spindled, and degenerative changes are observed. (B) Proliferation of endothelial-like cells in the brain. (C) Microhemorrhages, thrombosis, and proliferation of glomerular mesangial cells. The necrosis of tubular cells seen in this mouse is a common histological change after EHEC infection, regardless of treatment.
The kidneys of symptomatic mice with EHEC infection were pale and swollen. Histopathologic changes in the kidney sections from TNF-treated mice included focal proliferation of glomerular mesangial cells and increased deposition of mesangial matrix. The changes of the kidney in these experiments were similar to those described by Karpman et al. (14). Slight proliferations of glomerular mesangial cells were seen in mice before the appearance of clinical signs (day 1 after infection). The proliferation of glomerular mesangial cells, hemorrhages, and microthrombosis in the TNF-treated mice was observed on day 7 after infection (Fig. 1C). Focal proliferation of glomerular mesangial cells was mild in symptomatic mice subjected to EHEC infection only. In NM-treated mice, glomerular pathology was not observed. Necrosis of tubular cells was noted in all symptomatic mice.
Histopathologic changes were not observed in the colon, kidney, and brain tissue from asymptomatic mice inoculated with the strain or from controls (PBS-treated mice and E. coli MV1184-inoculated mice).
In vivo colonization.
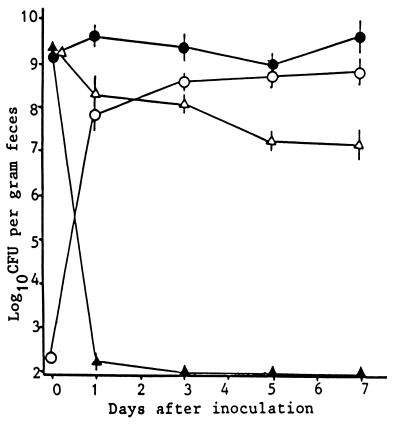
When 2.0 × 109 CFU of EHEC strain EDL 931 was fed individually to germ-free mice, the bacterium colonized the feces of the groups equally well (109 to 1010 CFU/g of feces) for the experimental period (Fig. 2). There were no significant differences in colonization among the three groups: EHEC infection only, EHEC infection plus TNF treatment, and EHEC infection plus NM treatment (data not shown). In contrast, the bacteria dropped to undetectable numbers (<102 CFU/g of feces) by 3 days postinoculation in flora-positive ICR mice. When 2.0 × 102 CFU of strain EDL 931 was fed to germ-free IQI mice, the number of CFU of the strain in feces began to rise rapidly on day 1 until a stable level of colonization (108 to 109 CFU/g of feces) was maintained. Intestinal colonization was examined by SEM. EHEC O157:H7 was randomly seen in the feces at various sites. No aggregation of EHEC was observed.
FIG. 2.
Pattern of colonization of germ-free mice by EHEC O157:H7 strain EDL 931. At the times indicated, fecal samples were plated on Chromagar. The bacterial detection limit for assessment of initial colonization was 102 CFU/g of feces. Each point represents the mean CFU per gram ± the standard deviation. •, IQI mice inoculated with EHEC 157:H7 (2.0 × 109 CFU/mouse); ○, IQI mice inoculated with EHEC 157:H7 (2.0 × 102 CFU/mouse); ▴, ICR mice inoculated with EHEC 157:H7 (2.0 × 109 CFU/mouse); ▵, IQI mice inoculated with E. coli MV1184 (2.4 × 109 CFU/mouse).
Positive cultures were obtained from the stomach, small intestines, and large intestines of infected mice. Blood cultures were negative for both symptomatic and asymptomatic mice. Cultures from the kidney and brain were also negative for these mice.
SLT level in the feces.
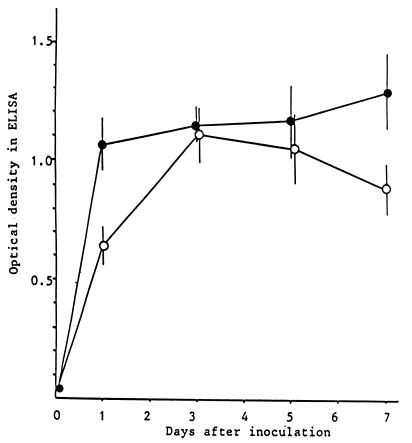
SLT was detected in the feces but not in the serum. As shown in Fig. 3, SLT was detected by ELISA at levels of more than 10 ng/g of feces, because optical densities were 0.409 and 0.947 for standard SLT-I (1,000 pg/ml) and SLT-II (1,000 pg/ml), respectively. SLT levels in feces of mice inoculated with 2.0 × 109 CFU of EHEC were significantly higher than those in the feces of mice inoculated with 2.0 × 102 CFU of EHEC on days 1 and 7 after inoculation. SLT levels in the feces of IQI mice at 5 to 7 days after inoculation with EHEC were similar to those in the feces of TNF-treated mice (Table 2). Lower SLT levels were detected in NM-treated IQI mice and IQI mice inoculated with 2.0 × 102 CFU of EHEC than in IQI mice inoculated with 2.0 × 109 CFU of EHEC. There were no significant differences in SLT level between symptomatic and asymptomatic mice in each group. Both SLT-I and SLT-II were detected by RPLA when the samples were positive by ELISA. There was no detection of SLT in control groups.
FIG. 3.
Changes of SLT level in the feces of experimental IQI mice. •, EHEC O157:H7 inoculation at 2.0 × 109 CFU/mouse; ○, EHEC O157:H7 inoculation at 2.0 × 102 CFU/mouse.
TABLE 2.
SLT levels in the feces of experimental mice after inoculation with EHEC O157:H7
| Mouse type | E. coli strain | Inoculum size | Treatment | SLT level at 5–7 days after inoculation by:
|
||
|---|---|---|---|---|---|---|
| ELISAa | RPLAb
|
|||||
| SLT-I | SLT-II | |||||
| IQI | EDL 931 | 2.0 × 109 | —c | 1.299 ± 0.162 | 448 ± 78 | 512 ± 64 |
| IQI | EDL 931 | 2.0 × 109 | TNF-α | 1.072 ± 0.143 | 512 ± 78 | 576 ± 64 |
| IQI | EDL 931 | 2.0 × 109 | NM | 0.730 ± 0.143 | 448 ± 78 | 448 ± 78 |
| IQI | EDL 931 | 2.0 × 102 | — | 0.891 ± 0.110 | 320 ± 88 | 448 ± 78 |
| ICR | EDL 931 | 2.0 × 109 | — | 0.042 ± 0.006 | <20 | <20 |
| IQI | MV1184 | 2.4 × 109 | — | 0.054 ± 0.001 | <20 | <20 |
| IQI | — | 0.020 ± 0.001 | <20 | <20 | ||
Optical densities of 10-fold-diluted samples ± standard deviations.
Final dilutions ± standard deviations.
—, no treatment.
Cytokine responses in the serum, kidneys, and brains.
Serum cytokines TNF-α and IL-6 were detected in TNF-treated mice at 7 days, but not in the mice at 1 day, after inoculation of EHEC. The levels of TNF-α and IL-6 were 179 ± 53 and 240 ± 90 pg/ml, respectively. They were significantly higher than those for negative controls. In the EHEC-only group, the EHEC plus NM group, and the EHEC plus TNF-α plus NM group, no proinflammatory cytokines were detectable in the serum at days 1 and 7 after inoculation of EHEC.
Table 3 shows cytokine levels of the kidneys on days 1 and 7 after infection. A TNF-α response, but not IL-1α and IL-6 responses, was seen at 1 day after EHEC infection. At 7 days after infection, TNF-α, IL-1α, and IL-6 were detected in the kidneys of mice with systemic clinical signs. In the EHEC plus TNF-α group, proinflammatory cytokines were clearly detected in the kidneys at 1 and 7 days after infection. In the EHEC plus NM group, cytokine induction was different than it was in the other two groups. The EHEC plus TNF-α plus NM group showed lower cytokine levels than the EHEC plus TNF-α group, although mice in this group had severe clinical signs.
TABLE 3.
Cytokine levels in the kidneys of mice infected with EHEC
| Group | Level of indicated cytokine in the kidney (pg/0.1 g)a at:
|
|||||
|---|---|---|---|---|---|---|
| 1 day after infection
|
5–7 days after infection (symptomatic mice) and/or treatment
|
|||||
| TNF-α | IL-1α | IL-6 | TNF-α | IL-1α | IL-6 | |
| EHEC | 216 ± 60 | ND | ND | 498 ± 83 | 184 ± 58 | 476 ± 110 |
| EHEC + TNF-α | 465 ± 72 | 214 ± 19 | 642 ± 51 | 662 ± 70 | 103 ± 25 | 460 ± 38 |
| EHEC + NM | ND | 138 ± 10 | ND | 199 ± 83 | ND | 904 ± 44 |
| EHEC + TNF-α + NM | NT | NT | NT | 209 ± 23 | ND | 412 ± 58 |
| MV1184 controls | ND | ND | ND | ND | ND | ND |
| Controls + TNF-α | NT | NT | NT | ND | ND | ND |
| Controls + NM + TNF-α | NT | NT | NT | ND | ND | ND |
| Controls without treatment | ND | ND | ND | ND | ND | ND |
Data were expressed as means ± standard deviations. ND, <30 pg/0.1 g; NT, not tested.
As shown in Table 4, brain cytokines were detected in IQI mice with or without TNF-α after EHEC infection but not in NM-treated mice. In the EHEC-only group, only TNF-α was detectable in the brain. Levels of TNF-α, IL-1α, and IL-6 in the EHEC plus TNF-α group were significantly higher than those in other groups at 1 day after EHEC infection (P < 0.01). TNF-α and IL-6 were detected in the brains of mice in the EHEC plus TNF-α group at 7 days after EHEC infection.
TABLE 4.
Brain cytokine levels in mice infected with EHEC
| Group | Brain level of indicated cytokine (pg/0.1 g)a at:
|
|||||
|---|---|---|---|---|---|---|
| 1 day after infection
|
5–7 days after infection (symptomatic mice) and/or treatment
|
|||||
| TNF-α | IL-1α | IL-6 | TNF-α | IL-1α | IL-6 | |
| EHEC | 68 ± 31 | ND | ND | 71 ± 26 | ND | ND |
| EHEC + TNF-α | 808 ± 98 | 337 ± 85 | 963 ± 97 | 175 ± 12 | ND | 98 ± 26 |
| EHEC + NM | ND | ND | ND | ND | ND | ND |
| EHEC + TNF-α + NM | NT | NT | NT | ND | ND | ND |
| MV1184 controls | ND | ND | ND | ND | ND | ND |
| Controls + TNF-α | NT | NT | NT | ND | ND | ND |
| Controls + NM + TNF-α | NT | NT | NT | ND | ND | ND |
| Controls without treatment | ND | ND | ND | ND | ND | ND |
Data were expressed as means ± standard deviations. ND, <30 pg/0.1 g; NT, not tested.
No cytokines were detected in the serum, kidneys, and brains of MV1184 controls and flora-positive controls. IQI mice inoculated with 2.0 × 102 CFU of EHEC also showed no cytokine responses, similar to the controls.
DISCUSSION
TNF-α treatment causes severe damage to the target organs in the EHEC mouse model. In contrast, a protease inhibitor had an inhibitory effect on pathological symptoms in the model. Therefore, the inhibition of TNF processing could act as an effective therapeutic agent in vivo but did not eliminate EHEC and the organ failure associated with EHEC infection (direct action of SLTs). The combination of an SLT-specific inhibitor with a TNF inhibitor will be required to prevent HUS and severe neurologic symptoms.
It has been reported that TNF treatment of human vascular endothelial cells leads to enhanced biosynthesis of the SLT receptor, thereby sensitizing cells to the cytotoxic action of SLT (25). Several hours’ exposure to TNF-α was enough to enhance the number of SLT receptors (10- to 100-fold) on the endothelial cells (25). NM treatment was not effective in decreasing the severity of pathological changes in the target organs and lethality for IQI mice treated with TNF-α. NM could inhibit the synthesis of TNF-α but could not inhibit the action of inoculated TNF-α.
Purified SLTs induced expression of proinflammatory cytokines from peritoneal macrophages (23). Harel et al. showed that SLT acts to induce TNF synthesis within the kidney and at the same time increases renal sensitivity to the toxic effects of TNF (8). We agree that local synthesis of TNF within the kidney may contribute to renal injury. These observations would suggest an interaction between SLT and cells capable of responding to the toxins by synthesizing TNF-α.
When EHEC was inoculated into gnotobiotic mice, no circulating TNF could be detected by ELISA of serum samples. In contrast, TNF production occurred within specific tissues such as the kidney and brain. In the mice treated with TNF, strong cytokine responses were recognized in the kidney before and after symptoms.
Cytokine levels in the brains of TNF-treated mice were significantly higher than those in the brains of mice of other groups at day 1. At 7 days after EHEC infection, all of the groups showed local TNF responses. Brain lesions were severe in TNF-treated mice but mild in mice not treated with TNF. It has been suggested that the state of cell differentiation or activation (including the cell type) is important in determining the cellular response to SLT (19). In the response to inflammatory stimuli, the presence of microbial products such as SLT and host factors resulted in further proinflammatory cytokine synthesis and tissue injury in the central nervous systems.
The results of this study suggest the following pathogenesis. The bacteria are established in the alimentary tract. The destruction of the mucous layer and/or focal necrosis of the gut wall may allow bacterial components to cross the damaged intestinal wall and reach the bloodstream. Whether EHEC O157:H7 strains can invade through an intact intestinal mucosal barrier has been discussed (17). However, we could not observe bacteremia in our mouse model. Actually, positive blood cultures are rarely found for patients with HUS. Furthermore, IQI mice with intact mucous layers were healthy after inoculation with 2.0 × 102 CFU of EHEC. Thus, bacteremia did not appear to be essential for the development of HUS and neurologic symptoms, and the destruction of the mucous layer could be important for the entry of SLTs and bacterial components.
Symptoms and pathology in the target organs may be caused by the spread of bacterial components. SLT is most important because pathological events similar to those in experimental animals inoculated with EHEC can occur in animals to which the toxin is administered (5, 15, 21). We found the highest frequency of symptoms in TNF-treated mice infected with EHEC. This group of mice also developed the most severe combination of renal and neural symptoms, as in human cases, suggesting that SLT and TNF have an additive or synergistic effect. Presumably, in HUS and encephalopathy, TNF levels are elevated in response to circulating SLT and/or endotoxin. TNF produced in response to toxins may then act to upregulate toxin receptor levels in target (vascular endothelial) cells.
REFERENCES
- 1.Aoyama T, Ino Y, Ozeki M, Oda M, Sato T, Koshiyama Y, Suzuki S, Fujita M. Pharmacological studies of FUT-175, nafamostat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments. Jpn J Pharmacol. 1984;35:203–227. doi: 10.1254/jjp.35.203. [DOI] [PubMed] [Google Scholar]
- 2.Barrett T J, Potter M E, Wachsmuth I K. Bacterial endotoxin both enhances and inhibits the toxicity of Shiga-like toxin II in rabbits and mice. Infect Immun. 1989;57:3434–3437. doi: 10.1128/iai.57.11.3434-3437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donohue-Rolfe A, Acheson D W K, Keusch G T. Shiga toxin: purification, structure, and function. Rev Infect Dis. 1991;13:S293–S297. doi: 10.1093/clinids/13.supplement_4.s293. [DOI] [PubMed] [Google Scholar]
- 4.Francis D H, Collins J E, Duimstra J R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986;51:953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii J, Kinoshita Y, Kita T, Higure A, Takeda T, Tanaka N, Yoshida S. Magnetic resonance imaging and histopathological study of brain lesions in rabbits given intravenous verotoxin 2. Infect Immun. 1996;64:5053–5060. doi: 10.1128/iai.64.12.5053-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii J, Kita T, Yoshida S-I, Takeda T, Kobayashi H, Tanaka N, Ohsato K, Mizuguchi Y. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H− in mitomycin-treated mice. Infect Immun. 1994;62:3447–3453. doi: 10.1128/iai.62.8.3447-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin P M, Tauxe R V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 8.Harel Y, Silva M, Giroir B, Weinberg A, Cleary T B, Beutler B. A reporter transgene indicates renal-specific induction of tumor necrosis factor (TNF) by shiga-like toxin. J Clin Invest. 1993;92:2110–2116. doi: 10.1172/JCI116811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isogai E, Isogai H, Kimura K, Fujii N, Takagi S, Hirose K, Hayashi M. In vivo induction of apoptosis and immune responses in mice by administration of lipopolysaccharide from Porphyromonas gingivalis. Infect Immun. 1996;64:1461–1466. doi: 10.1128/iai.64.4.1461-1466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isogai E, Isogai H, Kimura K, Hayashi S, Kubota T, Nishikawa T, Nakane A, Fujii N. Cytokines in the serum and brain in mice infected with distinct species of Lyme disease Borrelia. Microb Pathog. 1996;21:413–419. doi: 10.1006/mpat.1996.0072. [DOI] [PubMed] [Google Scholar]
- 11.Isogai E, Isogai H, Sawada H, Ito N. Bacterial adherence to gingival epithelial cells of rats with naturally occurring gingivitis. J Periodontol. 1986;57:225–230. doi: 10.1902/jop.1986.57.4.225. [DOI] [PubMed] [Google Scholar]
- 12.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali M A, Petric A M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 14.Karpman D, Connell H, Svenson M, Scheutz F, Alm P, Svanborg C. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J Infect Dis. 1997;175:611–620. doi: 10.1093/infdis/175.3.611. [DOI] [PubMed] [Google Scholar]
- 15.Lindgren S W, Melton A R, O’Brien A D. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect Immun. 1993;61:3832–3842. doi: 10.1128/iai.61.9.3832-3842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louise C B, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1β, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect Immun. 1991;59:4173–4179. doi: 10.1128/iai.59.11.4173-4179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee M L, O’Brien A D. Investigation of enterohemorrhagic Escherichia coli O157:H7 adherence characteristics and invasion potential reveals a new attachment pattern shared by intestinal E. coli. Infect Immun. 1995;63:2070–2074. doi: 10.1128/iai.63.5.2070-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai C H, Kelly J K, Meyers G L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986;51:16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramegowda B, Tesh V L. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect Immun. 1996;64:1173–1180. doi: 10.1128/iai.64.4.1173-1180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson S E, Karmali M A, Becker L E, Smith C R. The histopathology of the hemolytic uremic syndrome (HUS) associated with verocytotoxin-producing Escherichia coli (VTEC) infections. Hum Pathol. 1988;19:1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- 21.Richardson S E, Rotman T A, Jay V, Smith C R, Becker L E, Petric M, Olivieri N F, Karmali M A. Experimental verocytotoxemia in rabbits. Infect Immun. 1992;60:4154–4164. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley L W, Remis R S, Helgerson S D, McGee H B, Wells J G, Davis B R, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 23.Tesh V L, Ramegowda B, Samuel J E. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect Immun. 1994;62:5085–5094. doi: 10.1128/iai.62.11.5085-5094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchiba M, Okajima K, Murakami K, Okabe H, Takatsuki K. Effect of nafamostat mesilate on pulmonary vascular injury induced by lipopolysaccharide in rats. Am J Respir Care Med. 1997;155:711–718. doi: 10.1164/ajrccm.155.2.9032217. [DOI] [PubMed] [Google Scholar]
- 25.Van der Kar N C A J, Monnens L A H, Karmali M A, van Hinsbergh V W M. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood. 1992;80:2755–2764. [PubMed] [Google Scholar]
- 26.Wadolkowski E A, Snug L M, Burris J A, Samuel J E, O’Brien A D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:959–965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadolkowski E A, Burris J A, O’Brien A D. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58:2438–2445. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]