Abstract
Waste-water pollution by radioactive elements such as uranium has emerged as a major issue that might seriously harm human health. Graphene oxide, graphene oxide nanoribbons, and sodium alginate nanocomposite aerogels (GO/GONRs/SA) were combined to create a novel nanocomposite using a modified Hummer's process and freeze-drying as an efficient adsorbent. Batch studies were conducted to determine the adsorption of uranium (VI) by aerogel. Aerogels composed of (GO/GONRs/SA) were used as an effective adsorbent for the removal of U (VI) from aqueous solution. Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) were used to describe the structure, morphologies, and characteristics of (GO/GONRs/SA) aerogels. The initial concentration of uranium (VI) and other environmental factors on U (VI) adsorption were investigated, period of contact, pH, and temperature. A pseudo-second-order kinetic model can be employed to characterize the kinetics of U (VI) adsorption onto aerogels. The Langmuir model could be applied to understand the adsorption isotherm, and the maximum adsorption capacity was 929.16 mg/g. The adsorption reaction is endothermic and occurs spontaneously.
Subject terms: Chemistry, Materials science
Introduction
Uranium may infiltrate the environment via uranium mining, production, and usage, posing risks to human health and the natural environment related to its toxicity and radioactivity1. To eliminate uranium from an aqueous solution, a multitude of treatment strategies, including physical, chemical, and biological procedures, have been used2. According to the principle of adsorption, nucleophilic material may chemically adsorb a variety of radioactive elements, and the process is enhanced by the material's porous structure and large specific surface area3.
Due to adsorption's cheap cost, high effectiveness, and abundance of adsorbents, it is often employed to remove radionuclides from wastewater4,5.
The surface and edges of GO are covered with a broad range of functional groups, including hydroxyl, epoxy, carboxyl, carbonyl, etc.6–8. GO has characteristics including excellent dispersion, hydrophilicity, and compatibility because of these oxygen-containing functional groups, which make it an appropriate support material to mix with other composites or chemical active groups9,10. An important part of the uranium recovery and removal was performed by the functional groups on GO, as did the insertion of organic groups on GO to enhance the number of binding sites and the cycloaddition process to activate dormant locations on GO11. The uranium adsorption capabilities of GO-based nanomaterials are highly dependent on experimental circumstances and the effective groups on GO.
Graphene nanoribbons (GONRs), a nanomaterial comprised of six carbon atoms organized as rings, with a theoretical surface area of 2,630 m2/g and good transport features12. GONRs are a viable contender for uranium separation due to their hydrophobicity and large surface area. GONRs may be used to segregate radioactive elements by loading distinct functional groups13,14.
Brown seaweeds contain the linear polysaccharide alginate, also known as alginic acid. Alginates containing monovalent ions (alkali metals and ammonium) are soluble15, which restricts their use in the removal of radionuclides and heavy metals from aqueous solutions. Insoluble hydrogels may be created by ion-exchanging soluble alginate with multivalent metal ions16. For example, uranium recovery from aqueous solutions using calcium alginate beads as an adsorbent for the removal of radionuclides and heavy metal ions17. In this research, the adsorption capacity and hydrophobicity were increased using graphene oxide, GONRs, and sodium alginate. The combination of graphene oxide (GO), graphene oxide nanoribbons (GONRs), and sodium alginate (SA) in this research offers several advantages for the adsorption of uranium from wastewater. Firstly, GO has a large surface area and oxygen-containing functional groups, providing numerous active sites for adsorption. GONRs, with their unique structure and larger surface area compared to GO, further enhance the adsorption capacity. Sodium alginate acts as a scaffold, forming a three-dimensional network structure that increases the available surface area for adsorption. By combining these materials, the nanocomposite aerogels exhibit a higher adsorption capacity than individual components alone. Secondly, the combination enables the creation of a material with balanced hydrophobic-hydrophilic properties. GO is hydrophilic, while GONRs and modified SA contribute to hydrophobic characteristics. This balanced nature is important for selectively adsorbing hydrophobic uranium species while remaining compatible with the aqueous environment. Lastly, the combination ensures structural stability and porosity of the aerogels. GO and GONRs provide stability due to their two-dimensional and nanoribbon structures, respectively. Sodium alginate acts as a binder, forming a three-dimensional network that enhances mechanical strength. The porous structure of the aerogels allows for a high surface area and efficient interaction between uranium ions and active sites. To remove uranium from simulated wastewater, the goal of this work was to synthesize GO/GONRs/SA aerogel via hydrothermal and lyophilization treatment. The form and surface properties of materials were investigated using a variety of techniques, and the aerogel's ability to adsorb aqueous solution uranium was investigated.
Materials and methods
Graphite Powder (99% Purity, Chemical Reagent from China) was utilized as a precursor to create graphene oxide (GO). Carbon nanotubes with multiple walls were used to create graphene oxide nanoribbons (GONRs) (MWCNTs, Cheap Tube Inc., USA). In the synthesis of GONRs and GO, potassium permanganate (KMnO4, BDH, England) functioned as an oxidant. Uranyl nitrate (UO2(NO3)2.6H2O, solid 98–102% BDH, England) was used to create 1 g/L stock solutions of uranium. 2.109 g of Uranyl nitrate (UO2(NO3)2.6H2O) were dissolved in 1 mL of concentrated HNO3-containing deionized water. Add deionized water to the solution in a 1-L standard flask until the mark is achieved. The manufacturer of sodium alginate (SA), is Sigma-Aldrich, Germany. All other reagents utilized in this experiment were of analytical purity, and deionized water was used to make all solutions.
Preparation of GO/GONRS/SA aerogels
With a volume ratio of 9:1, H2SO4 and H3PO4 were combined to create graphene oxide (GO) (180:20 mL). After 15 min of stirring, 1.5 g of powdered graphite was added. 9.0 g of KMnO4 was then slowly added while the mixture was continually swirled. For 12 h, the mixture was regularly mixed. 200 mL of freezing deionized water and 4 ml of 30% hydrogen peroxide (H2O2) were used to terminate the reaction after 12 h, and the solution was then colored bright yellow. For purification, the mixture was divided among numerous centrifuge tubes. Hydrochloric acid (HCl) 10% and deionized water were alternated with centrifugation at a speed of 5000 rpm for 15 min at each washing step to complete the cleaning. The finished item was then dried in an oven for 24 h at 80 °C18.
To produce GONRs, multi-walled carbon nanotubes were unzipped. A typical process included pre-oxidizing 1 g of MWCNTs with sulfuric acid (150 mL) at room temperature for six hours with stirring. The reaction mixture was then added with 500% weight of KMnO4 and swirled for one hour at room temperature. For 30 min, and to 55 °C the reaction mixture was heated, to 70 °C, and the reaction temperature was increased, where it was stabilized for some time before being allowed to return to room temperature. Before being filtered by (0.5µm) a PTFE membrane, the mixture was added to 400 mL of ice that had been combined with 5 mL 10% v/V H2O2. Following dispersion in deionized water (120 mL), for 30 min the substance was sonicated. By using a PTFE membrane, the mixture was then filtered and the filtrate was dried at 60 °C for 24 h19–21.
The dried GO of 0.32 g (8 mg/mL) and GONRs of 0.32 g (8 mg/mL) were ultrasonically dispersed in 20 mL deionized water for 1 h before being combined with 20 mL of (1 g) SA solution (25 mg/mL). The uniform solution was then dropped into the CaCl2 (2 wt%) solution to produce hydrogel beads, which were then cross-linked with Ca2+ for 6 h22. Hydrogel beads formed of GO/GONRs/SA were five times rinsed with deionized water before being vacuum freeze-dried for 72 h to create aerogels23.
Characterization
The materials were studied using transition electron microscopy TEM, scanning electron microscopy SEM, Fourier transform infrared spectroscopy FT-IR, and X-ray diffraction XRD, to produce SEM images. The French-made TESCAN MIRA3 scanning electron microscope with a 15 kV electron beam was used to carry out the SEM experiments. The compounds' FT-IR spectra were captured in pressed KBr pellets (Aldrich, 99%, analytical reagent) at room temperature using a BRUKER TENSOR 35 spectrophotometer 65. At 25 °C, the crystal phases were verified using an X-ray diffractometer (Xpert, PANalytical Philips, Holland), with (Cu K radiation line of wavelength 1.54 A° in 2 range from 10° to 80°. Operating parameters for the CuK radiation source were 40 kV voltage, 30 mA current, and a 10°/min scanning speed. Nanocomposite TEM pictures were captured at 100 kV using a Phillips EM 208S microscope.
Experiments of adsorption
A specified quantity of GO/GONRs/SA aerogel was introduced to the uranium solution for the uranium adsorption studies. Centrifugation was then used to remove the adsorbent, and the supernatant was analyzed by Energy dispersion X-ray fluorescence ED-XRF (Rigaku, USA). Lee et al.24 used a high-resolution ED-XRF to determine the concentration of uranium-contaminated soil in a similar study using this analytic method. Balaji Rao et al.25 evaluated the ED-XRF method, which was developed and standardized for the routine assessment of uranium in various process stream solutions ranging from 0.1 to 400 g L−1, from a Uranium Extraction Plant at the Nuclear Fuel Complex. Kumar et al.26 investigated the possibility of using Rh K scattered peaks: Compton and Rayleigh, for minimizing matrix effect and determining uranium in different matrices using ED-XRF spectrometry. Using the following equation, get the removal rate (p) and adsorption capacity (qe).
| 1 |
| 2 |
where uranium volume V (mL), and M (mg) represent the weight of GO/GONRs/SA aerogel, Ce (mg/L) and Co (mg/L) represent the equilibrium and initial adsorption concentrations of uranium in the solution, respectively.
Results and discussion
Characterization of GO/GONRS/SA aerogels
Figure 1 displays GO, GONRs, and GO/GONRs/SA aerogels FT-IR spectra. GO's FT-IR Fig. 1a spectrum reveals distinctive peaks at 1619 cm−1 (graphitic C = C) and 1341 cm-1 (indicating the bending vibrations of a group –CH and –CH2) In addition, GO contains distinctive bands at 1720, 1619, and 1046 cm−1, which correspond to the stretching vibrations of carbonyl C=C, carboxyl C=O and epoxy C–O, respectively. While the large peak between 3165 and 3403 cm−1 suggested –OH for the carboxylic group27,28. As seen in Fig. 1b, FT-IR spectrum data for GONRs likewise revealed a variety of vibrational frequencies. GONRs exhibited distinctive peaks at 1532, 1560 cm–1 (graphitic C=C), 2941 cm–1 –CH alkane (stretching vibration), 1098, 1109 cm–1 to C–O (stretching vibration), and 1663 cm–1 to C=O (stretching vibration)29.
Figure 1.
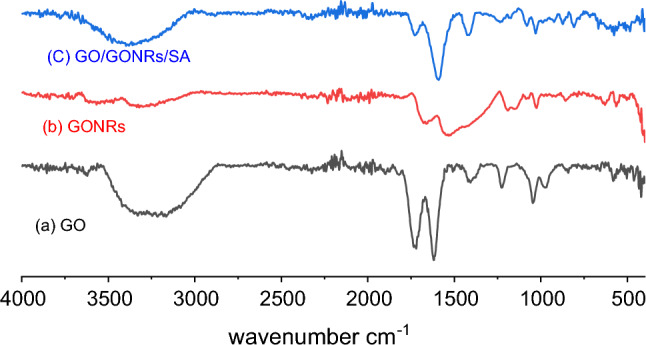
Spectrum FT-IR of GO (a), GONRs (b), GO/ GONRs /SA (c) aerogels.
The possible interactions between SA chains and GO/GONRs nanosheets were examined using FT-IR. According to Fig. 1c, the broad and powerful OH stretching vibration corresponds to the unique peak of GO at 3208 cm-1. OH stretching vibration may be responsible for the large peak at 3208 cm−130. Due to the strong hydrogen bonds between sodium alginate chains, a noticeable shoulder peak is seen.
Figure 2 displays X-ray diffraction (XRD) spectra of the GO, GONRs, and GO/GONRs/SA aerogels. The X-ray diffraction (XRD) spectra of GO are shown in Fig. 2a; the diffraction peaks were visible at an angle of 12.22°, which corresponded to the peak that characterizes GO. The GO peak at 2 θ° = 12.22°, which was GO's reflection in the crystal plane (001). Another peak, which is part of the crystal plane, was seen at the 2 θ = 43°. (100)21. Figure 2b displays the results of the XRD structural characterization. The (002) and (100) planes of the GONRs correspond to the two diffraction peaks at 25.0° and 43.0°, respectively31. The XRD patterns of the GO/GONRs/SA aerogel are displayed in Fig. 2c. The diffraction peak for GO is at 2 θ° = 12.22°. The amorphous character of SA in general is shown by the large peak of SA at 2 θ° = 13.6°. The absence of a GO peak in the GO/GONRs/SA aerogel shows that the small addition of GO has no appreciable impact on the crystallinity. Additionally, Intermolecular interactions may enhance the homogeneity of the components and give high miscibility for the creation of the required aerogels32,33.
Figure 2.
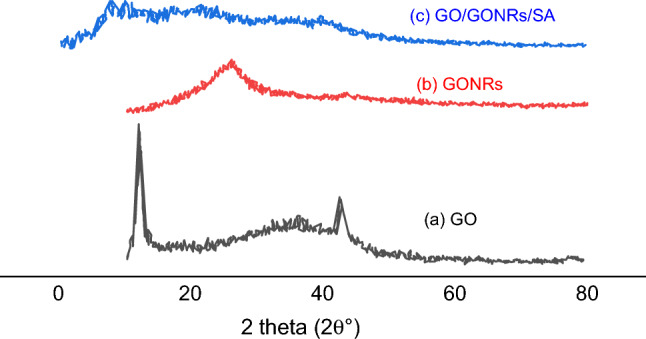
The XRD patterns of GO (a), GONRs (b), and GO/GONRs/SA (c) aerogels.
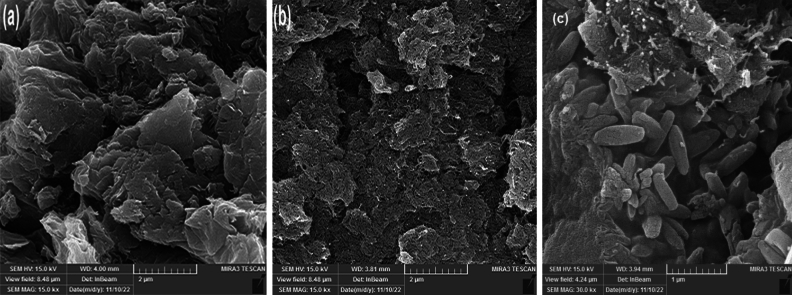
GO possesses a two-dimensional, sheet-like structure, as seen in Fig. 3a. The SEM images amply demonstrate the many lamellar layers and distinct sheet borders that characterize the GO structure34. The films are folded in parts and stacked one on top of the other. It's also important to note that the borders of the GO sheets were thicker. This is a result of the oxygen-containing functional groups being linked mostly at the GO's boundaries. The SEM images of the GONR sheets are shown in Fig. 3b and reveal a much rougher surface with a larger ribbon structure. Figure 3c demonstrates that the GO/GONRs/SA aerogel had a lamellar and network structure, showing that the GO sheets were compatible with the (SA) and efficiently integrated.
Figure 3.
SEM images of GO (a), GONRs (b), and GO/GONRs/SA (c)aerogels.
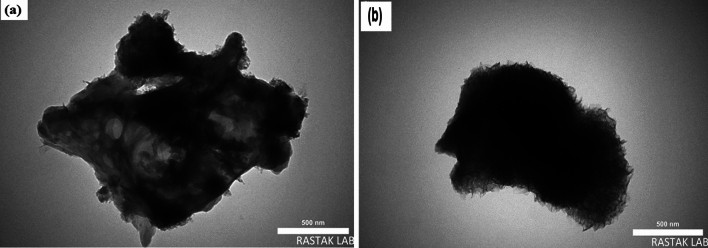
Figure 4 illustrates how the TEM images clearly showed the formation of graphene oxide sheets in addition to graphene oxide ribbons, as well as how these materials were made using alginate and how a triple network was constructed. In addition to plainly seeing the aerogels' porous structure, it is also possible to view graphene oxide nanoribbons emerging from the nanocomposite's surface like antennas.
Figure 4.
The TEM images of GO/GONRs/SA.
Adsorption of uranium(VI) on GO/GONRS/SA aerogels pH effect
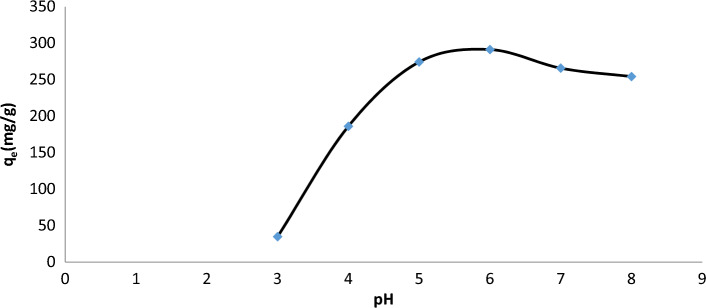
The solution pH is one of the most important factors for the sorption of U(VI) because it influences the speciation of U(VI), surface binding sites, and surface charge. Figure 5 demonstrates the considerable reliance on the pH solution adsorption, the adsorption rises abruptly from pH 2.0 to pH 4.0 and slowly from pH 4.0 to pH 8.0, U(VI) is largely present in the solution as UO22+, and sorption is minimal because H+ ions compete for the GO–aerogels binding sites. At pH 5.0–7.0, where UO2+, UO2(OH)+, (UO2)2(OH)22+, and UO3(OH)5+ predominate, electrostatic attraction causes the sorption to reach its maximum. Due to the decreased precipitation constant at pH ≥ 8.0, the amount of U(VI) adsorption on GO/GONRs/SA aerogels is especially attributable to the precipitation of UO2(OH)2(s). By examining this variable and analyzing the results of the studies, it was determined that a pH = 6 is optimal for this adsorption.
Figure 5.
Effect of pH on adsorption capacity at 313 K, 24 h, mass of GO/GONRs/SA = 15.2 mg, conc. U = 100 ppm.
Contact time effect and studies of adsorption kinetics
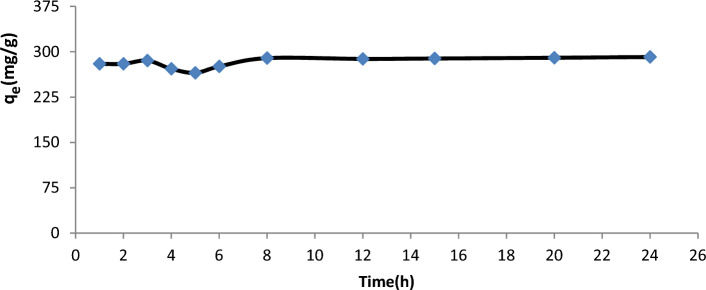
Contact time is another crucial factor that might reveal the adsorption kinetics. As demonstrated in Fig. 6, the sorption capacity of U(VI) changes as a function of contact time. The quantity of U(VI) adsorbate rose quickly in comparison at the beginning of 1 h before gradually reaching equilibrium at 8 h. The U(VI) adsorption of GO-based nanomaterials may quickly approach equilibrium due to the huge surface area and abundant effective groups on the surface of GO-based nanoparticles, which significantly increase the rate of U(VI) adsorption.
Figure 6.
Represents the relationship between the adsorption capacity and contact time at 313 K, pH = 6.02, mass of GO/GONRs/SA = 15.2 mg, conc. U = 100 ppm.
To explore the mechanism of adsorption, two alternative kinetic models—pseudo-first and second-order models were used. The models may be expressed using the relevant Eqs. (3) and (4) below:
| 3 |
| 4 |
where the amount of U(VI) sorbed at equilibrium time is qe (mg/g), and the amount at any given time is qt (mg/g) (h). k1 (h−1) and k2 are the abbreviations for the pseudo-first-order and pseudo-second-order sorption rate constants, respectively (g mg−1 h−1).
The essential kinetics parameters for the equations were calculated, and the values for k1 and k2 are shown in Table 1. As can be observed, the pseudo-second-order was better suited to represent the adsorption process of U(VI) on GO/GONRs/SA aerogels since it had the greatest correlation coefficient (R2) when compared to other kinetic models and the estimated qe, cal value was so closer to the experimental qe,exp.
Table 1.
The kinetic parameters of U(VI) sorption on GO/GONRs/SA aerogel.
| qe,exp/(mg/ g) | Pseudo-first-order kinetic | ||
|---|---|---|---|
| k1/(h−1) | qe,cal/(mg/g) | R2 | |
| 0.125 | 16.8 | 0.6948 | |
| 286.7105 | Pseudo-second-order kinetic | ||
|---|---|---|---|
| k2/(g mg−1 h−1) | qe,cal/(mg/g) | R2 | |
| 0.0158 | 293.19 | 0.9996 | |
The idea that the rate-determining step may be thought of as chemisorption served as the foundation for the pseudo-second-order model.
Graphene oxide (GO) has a large specific surface area due to its two-dimensional structure and the presence of oxygen-containing functional groups, such as hydroxyl, epoxy, carboxyl, and carbonyl groups. These functional groups provide binding sites for adsorption35. Graphene oxide nanoribbons (GONRs) further enhance the adsorption capacity due to their unique structure and larger surface area compared to GO. The theoretical surface area of GONRs of about 2630 m2/g36. Sodium alginate (SA), when combined with GO and GONRs, can form nanocomposite aerogels. SA acts as a scaffold, providing a three-dimensional network structure that increases the overall surface area available for adsorption. By combining GO, GONRs, and SA, the adsorption capacity is expected to be increased compared to using any individual component alone. The synergistic effect of these materials' properties, such as high surface area, presence of functional groups, and three-dimensional network structure, enhances the adsorption capacity for uranium removal37.
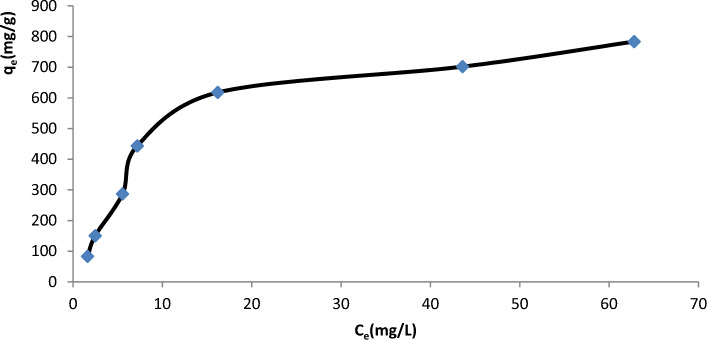
Effect of initial U(VI) concentration and isotherm studies
The equilibrium experiments were carried out at various initial U(VI) concentrations ranging from 50 to 350 mg/L to better understand the effects of the initial U(VI) concentrations. The amount of U(VI) adsorption on GO/GONRs/SA aerogels increased as the equilibrium concentration of U(VI) rose, as seen in Fig. 7.
Figure 7.
Effect of initial conc. of adsorbate on adsorption capacity at 313 K, 24 h, pH = 6.02, mass of GO/GONRs/SA = 15.2 mg.
The equilibrium sorption isotherms were simulated using the Freundlich and Langmuir isotherm models. According to the Langmuir isotherm model, monolayer adsorption takes place on a homogenous surface without any interactions between the closer binding sites of the adsorbates38. It may be written as Eq. (5).
| 5 |
where qe is the sorbed amount at equilibrium (mg/g), qmax is the Langmuir monolayer sorption capacity, KL (L/ mg) is the equilibrium constant, and Ce is the equilibrium concentration (mg/ L).
The Freundlich isotherm model explains the sorption of solutes from a liquid to a solid surface using an empirical relation. It assumes the participation of several sites with different sorption energies, and its linear form is given by the following Eq. (6):
| 6 |
where Freundlich constants: KF [(mg/g) (L/mg)1/n] and n correspond to the sorbent's adsorption capacity and adsorption intensity, respectively. The relevant parameters were calculated using the slopes and intercepts of the plots of Ce/qe vs Ce and lnqe versus lnCe (Table 2).
Table 2.
GO/GONRs/SA aerogels isotherm parameters for U adsorption.
| Sorbent | Langmuir sorption isotherm | Freundlich sorption isotherm | ||||
|---|---|---|---|---|---|---|
| GO/GONRs/SA | qmax (mg g−1) | KL (L mg−1) | R2 | KF [(mg g−1) (L mg−1)1/n] | n | R2 |
| 929.16 | 0.083 | 0.9836 | 93.3 | 1.74 | 0.8785 | |
Upon comparison of R2 values, Langmuir isotherm was determined to be more appropriate than Freundlich isotherm for describing the features of U(VI) adsorption on GO/GONRs/SA aerogels. A monolayer mechanism was most likely involved in the adsorption of U(VI) on GO/GONRs/SA aerogels, according to the results. The results showed that the GO/SA composite had a considerable adsorption capacity for U(VI) of 929.16 mg/g−1, which was higher than previous studies that used GO/SA composite beads, which had a capacity of 149.76 mg/L22, and L-Lysine-GO/SA composite, which had a capacity of 704.22 mg/g37.
Thermodynamic studies
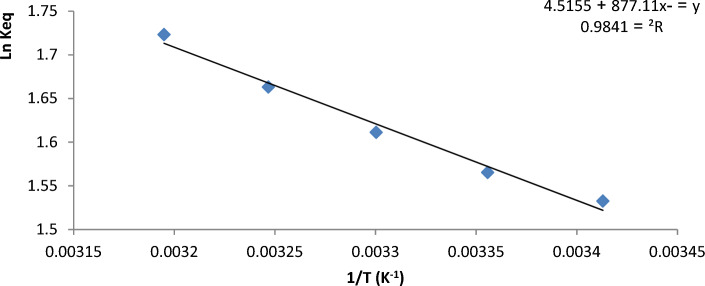
To comprehend the change in energy and establish whether the process can be spontaneous or not, sorption thermodynamics is an important factor. Table 3 provides the ΔG, ΔS, and ΔH thermodynamic characteristics of the U(VI) adsorption in hybrid aerogel. Figure 8 depicts the thermodynamics diagram as well as the link between adsorption temperature and adsorption capacity. According to Table 3, the adsorption process was spontaneous and endothermic and the reaction was promoted by high temperatures because of the positive values of ΔS°, ΔH° and negative value of ΔG°.
Table 3.
The thermodynamic parameters of U(VI) sorption on GO/GONRs/SA aerogel.
| ΔH (KJ mol−1) | ΔS (J mol−1 K−1) | ΔG (KJ mol−1) | |
|---|---|---|---|
| 7.292 | 37.54 | – 3.706 | 293 (K) |
| − 3.894 | 298 (K) | ||
| − 4.082 | 303 (K) | ||
| − 4.270 | 308 (K) | ||
| − 4.457 | 313 (K) | ||
Figure 8.
Plots between equilibrium constant and temperature inverse to calculate Thermodynamic functions for uranium(VI) on GO/GONRs/SA aerogel.
Use the following Eq. (7) to compute throughout the adsorption process the change in thermodynamic parameters.
| 7 |
Keq represents the distribution coefficient (mL g−1), whereas R represents the constant of gas (8314 J mol−1 K−1) and T represents the absolute temperature (K). ΔH is the enthalpy change (KJ mol−1) and ΔS is the entropy change (J mol−1 K−1). Equation (8) represents the change in Gibbs free energy (ΔG) values (KJ mol-1).
| 8 |
The values of enthalpy ΔH and entropy ΔS given in Table 3 were calculated using the intercept and slope of the plots of ln Keq vs T−1 (Fig. 8), whereas Eq. (7). Positive values of ΔH and ΔS indicate the endothermic character of the sorption process and the increasing randomness at the solid-solution interface during sorption, respectively. The ΔG value decreased by negativity values with increasing temperature, indicating that the adsorption process was spontaneous at all conditions examined.
Conclusions
Aerogel materials as adsorbents are very appealing because of their diverse chemical composition, high porosity, and a variety of pore sizes, including micro-, meso–, and macropores. These features lead to efficient and affordable processes, and frequently also to selectivity towards the desired adsorbates. In the current study, freeze-drying was used to create GO/GONRs/SA hybrid aerogels, which were then used to eliminate uranium(VI) from simulated wastewater solutions. Aerogels produced from GO/GONRs/SA have a porous structure 3D network and a high surface area. The sorption of U(VI) onto GO/GONRs/SA aerogels was affected by pH. The pseudo-second-order model could be utilized to describe the sorption kinetics, whereas the Langmuir isotherm could effectively define the sorption process. The maximum monolayer sorption capacity feasible is 929.16 mg g−1. Adsorption technique was found to be effective, spontaneous, and endothermic. These investigations revealed that the synthesized GO/GONRs/SA aerogels might serve as an effective sorbent for effluents from the nuclear industry and other important water sources.
Acknowledgements
The authors are thankful to the Deanship of Scientific Research at the University of Bisha for supporting this work through the Fast-Track Research Support Program.
Author contributions
A.A.J., D.H.H., K.H.L., performed the experiments and data analysis, S.A., A.K.J., G.M.S., M.M.A., prepared and characterized samples, S.A., G.M.S., A.G.A. writing-review and editing the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors in Reference 10 and the Author Contributions section. Full information regarding the corrections made can be found in the correction for this Article.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-024-79455-3
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/19/2024
A Correction to this paper has been published: 10.1038/s41598-024-53952-x
Change history
11/12/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-024-79455-3
Contributor Information
Ali A. Jabbar, Email: alialwasti@uomustansiriyah.edu.iq
Salim Albukhaty, Email: albukhaty.salim@uomisan.edu.iq.
Adel Kareem Jasim, Email: Adelkarimmm@gmail.com.
Ghassan M. Sulaiman, Email: ghassan.m.sulaiman@uotechnology.edu.iq
References
- 1.Srivastava, R. R., Pathak, P. & Perween, M. Environmental and health impact due to uranium mining. In Uranium in Plants and the Environment (eds Gupta, D. K. & Walther, C.) 69–89 (Springer Nature, 2020). [Google Scholar]
- 2.Li, L. N. et al. A combined experimental and theoretical study on the extraction of uranium by amino-derived metal–organic frameworks through post-synthetic strategy. ACS Appl. Mater. Interfaces8, 31032–31041. 10.1021/acsami.6b11332 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Gunathilake, C., Górka, J., Dai, S. & Jaroniec, M. Amidoxime-modified mesoporous silica for uranium adsorption under seawater conditions. J. Mater. Chem. A3, 11650–11659 (2015). [Google Scholar]
- 4.Crini, G. et al. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett.17, 195–213. 10.1007/s10311-018-0786-8 (2019). [Google Scholar]
- 5.Chakraborty, A., Pal, A. & Saha, B. B. A critical review of the removal of radionuclides from wastewater employing activated carbon as an adsorbent. Materials15(24), 8818. 10.3390/ma15248818 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jihad, M. A. et al. Polyethylene glycol functionalized graphene oxide nanoparticles loaded with Nigella sativa extract: A smart antibacterial therapeutic drug delivery system. Molecules26(11), 3067. 10.3390/molecules26113067 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai, J. et al. Synthesis of amidoximated polyacrylonitrile nanoparticle/graphene composite hydrogel for selective uranium sorption from saline lake brine. Chem. Eng. J.391, 123553. 10.1016/j.cej.2019.123553 (2020). [Google Scholar]
- 8.Imarah, A. A. et al. Graphene oxide-induced, reactive oxygen species-mediated mitochondrial dysfunctions and apoptosis: high-dose toxicity in normal cells. Nanomedicine (Lond).18(11), 875–887. 10.2217/nnm-2023-0129 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Wei, L. & Mao, Y. Enhanced hydrogen storage performance of reduced graphene oxide hybrids with nickel or its metallic mixtures based on spillover mechanism. Int. J. Hydrogen Energy41(27), 11692–11699. 10.1016/j.ijhydene.2016.04.030 (2016). [Google Scholar]
- 10.Mohammed, H. A. et al. Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol. Rev.12(1), 20220517 (2023). [Google Scholar]
- 11.Yang, P. et al. Nano-sized architectural design of multi-activity graphene oxide (GO) by chemical post-decoration for efficient uranium(VI) extraction. J. Hazard. Mater.375, 320–329. 10.1016/j.jhazmat.2019.05.005 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Higginbotham, A. L., Kosynkin, D. V., Sinitskii, A., Sun, Z. & Tour, J. M. Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano4, 2059–2069. 10.1021/nn100118m (2010). [DOI] [PubMed] [Google Scholar]
- 13.Wu, P. et al. Synthesis of magnetic graphene oxide nanoribbons composite for the removal of Th(IV) from aqueous solutions. J. Radioanal. Nucl. Chem.319, 1111–1118. 10.1007/s10967-018-6375-2 (2019). [Google Scholar]
- 14.Lim, J. et al. Open porous graphene nanoribbon hydrogel via additive-free interfacial self-assembly: fast mass transport electrodes for high-performance biosensing and energy storage. Energy Storage Mater.16, 251–258 (2019). [Google Scholar]
- 15.Davis, T. A., Volesky, B. & Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res.37, 4311–4330 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Zia, F. et al. Alginate-based hybrid nanocomposite materials. In Algae Based Polymers, Blends, and Composites Chemistry, Biotechnology and Materials Science Vol. 17 (ed. Zia, F.) 603–648 (Elsevier, 2017). 10.1016/B978-0-12-812360-7.00017-3. [Google Scholar]
- 17.Gok, C. & Aytas, S. Biosorption of uranium (VI) from aqueous solution using calcium alginate beads. J. Hazard. Mater.168, 369–375 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Mura, S. et al. Graphene oxide/iron oxide nanocomposites for water remediation. ACS Appl. Nano Mater.1, 6724–6732. 10.1021/acsanm.8b01540 (2018). [Google Scholar]
- 19.Ding, Y. J. et al. Multifunctional three-dimensional graphene nanoribbons composite sponge. Carbon104, 133–140. 10.1016/j.carbon.2016.03.058 (2016). [Google Scholar]
- 20.Wang, Y. et al. Uranium(VI) sorption on graphene oxide nanoribbons derived from unzipping of multiwalled carbon nanotubes. J. Radioanal. Nucl. Chem.304, 1329–1337. 10.1007/s10967-015-3981-0 (2015). [Google Scholar]
- 21.Hu, X. et al. Synthesis of graphene oxide nanoribbons/chitosan composite membranes for the removal of uranium from aqueous solutions. Front. Chem. Sci. Eng.14, 1029–1038. 10.1007/s11705-019-1898-9 (2020). [Google Scholar]
- 22.Li, D. et al. U(VI) adsorption by sodium alginate/graphene oxide composite beads in water. J. Radioanal. Nucl. Chem.327, 1131–1141. 10.1007/s10967-021-07598-y (2021). [Google Scholar]
- 23.Tao, E., Ma, D., Yang, S. & Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Comp.832, 154833 (2020). [Google Scholar]
- 24.Lee, H. K. et al. Design and applicability of a water recycling system to treat wastewater generated from real uranium-contaminated soil. Chem. Eng. J.472, 144927 (2023). [Google Scholar]
- 25.Balaji Rao, Y. et al. Determination of uranium in process stream solutions from uranium extraction plant employing energy dispersive X-ray fluorescence spectrometry. J. Radioanal. Nucl. Chem.294, 371–376. 10.1007/s10967-011-1477-0 (2012). [Google Scholar]
- 26.Kumar, S. S. & Dhara, S. Energy dispersive X-ray fluorescence determination of uranium in different uranates using Rh Kα scattered peaks for matrix correction. Spectrochim. Acta B193, 106427 (2022). [Google Scholar]
- 27.Sudesh, K. N., Das, S., Bernhard, C. & Varma, G. D. Effect of graphene oxide doping on superconducting properties of bulk MgB2. Supercond. Sci. Technol.26, 095008–095015. 10.1088/0953-2048/26/9/095008 (2013). [Google Scholar]
- 28.Sun, Z., Fan, W. & Liu, T. Graphene/graphene nanoribbon aerogels as tunable three-dimensional framework for efficient hydrogen evolution reaction. Electrochim. Acta250, 91–98 (2017). [Google Scholar]
- 29.Wang, W. et al. Formation mechanism of 3D macroporous graphene aerogel in alcohol-water media under gamma-ray radiation. Appl. Surf. Sci.427, 1144–1151. 10.1016/j.apsusc.2017.09.058 (2018). [Google Scholar]
- 30.Nie, L. et al. Effects of surface functionalized graphene oxide on the behavior of sodium alginate. Carbohydr. Polym.117, 616–623 (2015). [DOI] [PubMed] [Google Scholar]
- 31.AlMalki, F. A. et al. Eco-Friendly synthesis of carbon nanoparticles by laser ablation in water and evaluation of their antibacterial activity. J. Nanomater.10.1155/2022/7927447 (2022). [Google Scholar]
- 32.Maharana, H. S., Rai, P. K. & Basu, A. Surface-mechanical and electrical properties of pulse electrodeposited Cu–graphene oxide composite coating for electrical contacts. J. Mater. Sci.52, 1089–1105. 10.1007/s10853-016-0405-7 (2017). [Google Scholar]
- 33.Teles, R. et al. Understanding the influence of the biomass-derived alcohols on the activity and stability of Pt nanoparticles supported on graphene nanoribbons. Electrocatalysis8, 151–163. 10.1007/s12678-016-0349-3 (2017). [Google Scholar]
- 34.Sun, L. & Fugetsu, B. Graphene oxide captured for green use: Influence on the structures of calcium alginate and macroporous alginic beads and their application to aqueous removal of acridine orange. Chem. Eng. J.240, 565–573. 10.1016/j.cej.2013.10.083 (2014). [Google Scholar]
- 35.Khine, Y. Y., Wen, X., Jin, X., Foller, T. & Joshi, R. Functional groups in graphene oxide. Phys. Chem. Chem. Phys.24(43), 26337–26355. 10.1039/d2cp04082d (2022). [DOI] [PubMed] [Google Scholar]
- 36.Knerelman, E. I. et al. Highly porous materials as potential components of natural gas storage systems: Part 2 (a review). Pet. Chem.62, 677–713. 10.1134/S0965544122050127 (2022). [Google Scholar]
- 37.Gan, J. et al. Synergistic action of multiple functional groups enhanced uranium extraction from seawater of porous phosphorylated chitosan/coal-based activated carbon composite sponge. Desalination545, 116154. 10.1016/j.desal.2022.116154 (2023). [Google Scholar]
- 38.Alam, S. N., Sharma, N. & Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO)*. Graphene06, 1–18 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.