Abstract
Background:
It remains unclear whether patients with asthma and/or chronic obstructive pulmonary disease (COPD) are at increased risk for severe COVID-19.
Objective:
Compare in-hospital COVID-19 outcomes among patients with asthma, COPD, and no airway disease.
Methods:
A retrospective cohort study was conducted on 8,395 patients admitted with COVID-19 between March 2020 and April 2021. Airway disease diagnoses were defined using ICD-10 codes. Mortality and sequential organ failure assessment (SOFA) scores were compared among groups. Logistic regression analysis was used to identify and adjust for confounding clinical features associated with mortality.
Results:
The median SOFA score in patients without airway disease was 0.32 and mortality was 11%. In comparison, asthma patients had lower SOFA scores (median = 0. 15; P < 0.01) and decreased mortality, even after adjusting for age, diabetes and other confounders (odds ratio (OR) = 0.65; P = 0.01). Patients with COPD had higher SOFA scores (median = 0.86; P < 0.01) and increased adjusted odds of mortality (OR = 1.40; P < 0.01). Blood eosinophil count ≥200 cells/μL, a marker of T2 inflammation, was associated with lower mortality across all groups. Importantly, patients with asthma showed improved outcomes even after adjusting for eosinophilia, indicating that non-eosinophilic asthma was associated with protection as well.
Conclusions:
COVID-19 severity was increased in patients with COPD and decreased in those with asthma, eosinophilia, and non-eosinophilic asthma, independent of clinical confounders. These findings suggest that COVID-19 severity may be influenced by intrinsic immunologic factors in patients with airway diseases, such as T2 inflammation.
Keywords: SARS-CoV-2, COVID-19, Asthma, COPD, Eosinophil
INTRODUCTION
Since its emergence in December 2019, coronavirus disease-19 (COVID-19) has claimed nearly 7 million lives worldwide.(1) Early epidemiologic reports identified several clinical features associated with increased risk of acute respiratory distress syndrome (ARDS), multi-organ failure, and death from COVID-19. These features included age >60 years, diabetes, obesity, and chronic respiratory disease.(2, 3)
Subsequent studies have confirmed the risk of severe COVID-19 in individuals with advanced age, diabetes, and obesity.(4) However, the risk to those with chronic respiratory diseases has remained unclear. This is partly attributable to use of the non-specific term ‘chronic lung disease’, which includes a wide array of respiratory conditions with differing pathobiologies that likely affect the severity of COVID-19. Nevertheless, chronic lung disease remains a risk factor on the Centers for Disease Control (CDC) website and in most SARS-CoV-2 guidelines.(5) This leaves significant uncertainty surrounding COVID-19 risk-stratification in patients with chronic respiratory conditions.
Evidence has mounted to suggest that chronic obstructive pulmonary disease (COPD) predisposes to severe COVID-19,(6) but the risk to patients with asthma is less clear. Based on the established link between viral infections and asthma exacerbations, morbidity, and mortality,(7) there was initial concern that COVID-19 outcomes would be worse in patients with asthma.(8, 9) However, the risk of progression to ARDS, as indicated by intensive care unit (ICU) admission or death, has varied widely in studies to date. Indeed, independent meta-analyses have indicated increased risk,(10) no difference,(10) and even protection.(12) These conflicting results are likely attributable to inter-study differences in cohort sizes, degree of ethnic diversity, inclusion of inpatient vs. outpatient populations, and use of statistical adjustments for confounding clinical factors.
Here, we attempt to clarify the relationship between COVID-19 outcomes and airway diseases. To do so, we have analyzed a large and diverse cohort consisting of 8,395 patients within the Yale New Haven Health System (YNHHS) in the Northeastern United States during the first two waves of the pandemic. In order to focus our analysis on severe COVID-19, we included only hospitalized patients. We also performed multiple regression analysis to identify and adjust for previously reported confounders including age, gender, ethnicity, diabetes, nicotine use, and obesity. Finally, to more fully examine the impact of chronic type 2 (T2) inflammation on COVID-19 severity, we independently evaluated the effects of asthma and eosinophilia.
METHODS
Patient cohort
Data on patients admitted with COVID-19 from March 1, 2020 through April 1, 2021 were extracted from the YNHHS electronic medical records software system (Epic EMR) using the Yale Department of Medicine COVID-19 Explorer and Repository (DOM-CovX) tool.(11) The dataset included all patients admitted to YNHHS hospitals, including Yale New Haven Hospital (New Haven, CT), Yale Saint Raphael Campus Hospital (New Haven, CT), Greenwich Hospital (Greenwich, CT), Bridgeport Hospital (Bridgeport, CT), Lawrence & Memorial Hospital (New London, CT), and Westerly Hospital (Westerly, RI). Patients included in the dataset tested positive for SARS-CoV-2 within 14 days of admission by reverse transcriptase-polymerase chain reaction (RT-PCR) assays performed on nasopharyngeal swab specimens. Extracted clinical variables included demographics, past medical history, self-reported race and ethnicity, smoking history (in pack-years), admission vital signs, laboratory tests, medications administered during hospitalization, pre-hospitalization medication lists, sequential organ failure assessment (SOFA) score (mean value during hospitalization), hospitalization-associated diagnoses, and discharge status (to assess in-hospital mortality).
Airway disease classification and comorbidities included in the dataset were based on International Classification of Diseases, Tenth Revision (ICD-10) codes. Diagnoses of airway disease were made if a given patient possessed an asthma-related ICD code (J45.XX) or COPD-related ICD code (J44.XX) in their past medical history or hospitalization-associated diagnoses. Patient groups included No Airway Disease (NAD), Asthma, COPD, and Asthma/COPD (A/COPD). The A/COPD group included patients with ICD-10 diagnoses of asthma and COPD. We further stratified patients with asthma into four subgroups of asthma severity according to medication regimen: intermittent (no use of inhaled corticosteroid (ICS)); mild (only ICS); moderate (low dose ICS + additional controller (i.e. montelukast or long-acting beta agonist or long-acting muscarinic antagonist)), and severe (high dose ICS + additional controller or moderate dose ICS + additional controller or biologic therapy or chronic prednisone therapy).
Statistical analysis
All statistical analyses were carried out using R (version 4.0.2). Demographic characteristics were compared between Asthma, COPD, A/COPD and NAD patient groups using the Kruskal–Wallis test. The overall mortality rates were calculated for each airway disease group and were compared using a two-proportion z-test. Univariate logistic regression analysis was used to identify demographic, clinical and biological characteristics significantly associated with mortality rate within each patient group separately. Characteristics that were significantly different (P < 0.05) in at least one patient group in the univariate logistic regression analysis (age, body mass index (BMI), nicotine dependence, diabetes and ethnicity) were included as covariates in a multivariate logistic regression model to assess the association between patient group and mortality rate. Regression coefficients in the model represent the log odds ratio between each disease group and NAD group. Based on Ho et al,(12) the absolute eosinophil count on admission was dichotomized using a cutoff value of 200 cells/μL to assess the association between eosinophil count and mortality. The associations between mortality and (i) dichotomized admission eosinophil levels, (ii) pre-hospital use of ICS, (iii) asthma severity, and (iv) in-hospital systemic corticosteroid exposure (SCS) were investigated separately using a logistic regression model adjusted for age, gender, BMI, nicotine dependence, diabetes and ethnicity.(12) In all logistic regression analyses, an odds ratio >1 indicated a positive association with COVID-19 mortality. Wilcoxon rank sum test was applied to compare the mean SOFA score during the hospital stay between patients with airway disease and NAD.
RESULTS
The initial dataset contained 8,661 hospitalizations of patients with COVID-19. Filtering out 266 readmissions, 8,395 unique admissions were included in the analysis. As shown in Figure 1, ICD-10 diagnosis codes and smoking history were utilized to classify patients into four groups: Asthma (969 patients), COPD (1,132 patients), A/COPD (768 patients) and NAD (5,526 patients). The prevalence of asthma in our cohort was 11.5%, consistent with the prevalence in the general population, as reported by the state of Connecticut (10.5%).(13) In contrast, COPD prevalence was higher in our cohort than the general population (13.5% vs 6.1%), indicating increased risk of hospitalization from COVID-19 in patients with COPD.(14)
Figure 1:
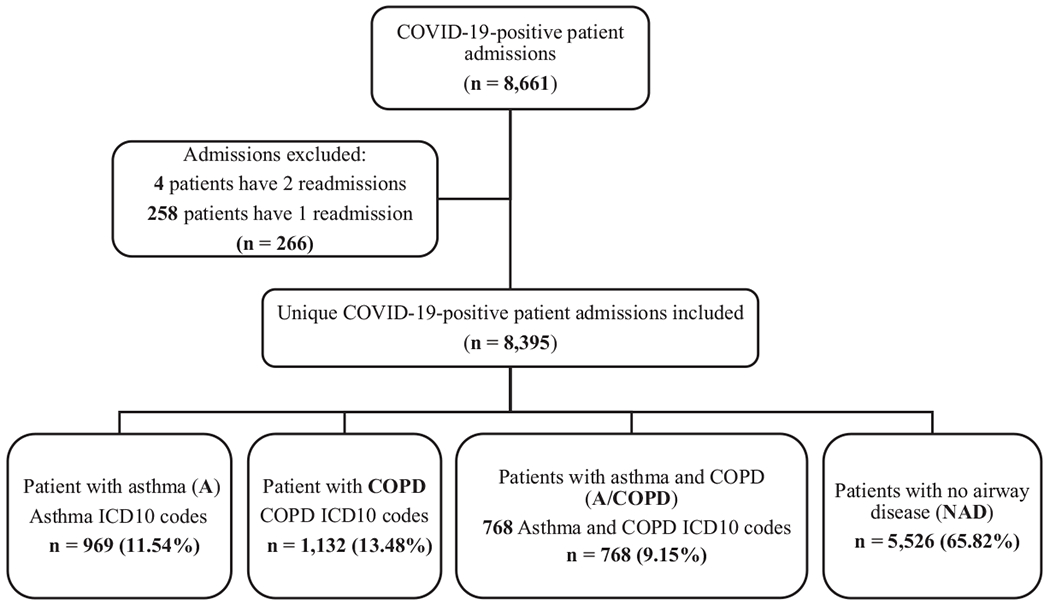
Flow chart of inclusion and exclusion criteria used to select the dataset for analysis. Patients with Asthma, COPD, A/COPD, and NAD were identified using ICD-10 code and smoking history.
Table 1A describes the demographic and clinical characteristics of patients in the dataset, which revealed multiple differences between groups. First, patients with COPD were older compared to those with asthma (75 versus 56 years of age, P < 0.01). Second, there was a higher proportion of females in the groups with asthma: Asthma plus A/COPD versus NAD plus COPD (64.48% versus 46.31%, P < 0.01). Third, mean BMI was higher in patients with asthma: Asthma plus A/COPD versus NAD plus COPD (33.01 kg/m2 versus 29.36 kg/m2, P < 0.01). Fourth, the Asthma and A/COPD group demonstrated more ethnic diversity compared to the other two groups. Finally, nicotine use and diabetes were more prevalent in patients with COPD.
Table 1A:
Demographics and clinical characteristics by airway disease group
| NAD | Asthma | COPD | A/COPD | P-value | |
|---|---|---|---|---|---|
| Sample size, N | 5,526 | 969 | 1,132 | 768 | |
| Mortality, N (%) | 626 (11%) | 52 (5%) | 239 (21%) | 106 (14%) | < 0.01 |
| Age | 64 (49-78) | 56 (40-68) | 75 (65-83) | 69 (59-79) | < 0.01 |
| Female sex, N (%) | 2,549 (46%) | 611 (63%) | 534 (47%) | 509 (66%) | < 0.01 |
| BMI (kg/m2) | 29 (24-33) | 32 (27-38) | 27 (23-33) | 31 (26-37) | < 0.01 |
| Race & Ethnicity | < 0.01 | ||||
| Black, N (%) | 1,101 (20%) | 296 (31%) | 198(17%) | 244 (32%) | |
| White, N (%) | 2,986 (54%) | 439 (45%) | 812 (72%) | 405 (53%) | |
| Other and Unknown, N (%) | 1,439 (26%) | 234 (24%) | 122 (11%) | 119 (15%) | |
| Latino, N (%) | 1,417 (26%) | 266 (27%) | 126 (11%) | 139 (18%) | |
| Nicotine use, N (%) | 583 (11%) | 154 (16%) | 344 (30%) | 232 (30%) | < 0.01 |
| Pack-years | 15 (5 - 30) | 10 (5 - 20) | 35 (20 - 58) | 20 (10 - 45) | < 0.01 |
| Diabetes, N (%) | 2,181 (39%) | 444 (46%) | 595 (53%) | 460 (60%) | < 0.01 |
P-values are determined by the Kruskal–Wallis test, which was performed to identify inter-group differences for each clinical variable.
Values are listed as median (IQR) except otherwise indicated
‘Other’ race includes American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander and other/not listed races.
Associations between presenting vital signs and mortality are shown in Figure E1 (see the Online Repository). As observed in previous studies, elevated respiratory rate and hypoxemia were associated with an increased risk of mortality. Table 1B describes vital signs at the time of admission and mean SOFA scores during hospitalization. Compared to patients with NAD (median = 0.32), those with asthma had significantly lower SOFA scores (median = 0.15, P < 0.01), while those with COPD had higher SOFA scores (median = 0.86, P < 0.01) (Figure 2A). Similarly, patients with asthma had the lowest absolute mortality rate (5%) among the four groups (Figure 2B), whereas patients with COPD had the highest (21%). Compared to patients with NAD, mortality was significantly lower in those with asthma (P < 0.01) and higher in those with COPD (P < 0.01). Absolute mortality rates were similar in the A/COPD and NAD patients (14% and 11%, respectively; P = 0.052), suggesting offsetting effects of asthma and COPD on COVID-19 severity. Notably, we found no significant difference in mortality between patients with asthma taking ICS prior to hospitalization and those not taking ICS (5.21% vs. 4.17%, p = 0.80). All together, these data indicate improved outcomes during COVID-19 in patients with asthma and worse outcomes in those with COPD.
Table 1B:
Mean SOFA score and vital signs during admission.
| NAD | Asthma | COPD | A/COPD | P-value | |
|---|---|---|---|---|---|
| SOFA Score | 0.32 (0.00 - 1.38) | 0.15 (0.00 - 1.00) | 0.86 (0.16 - 2.06) | 0.60 (0.03 - 1.90) | < 0.01 |
| Diastolic BP (mm Hg) | 71.6 (66.4 - 76.9) | 72.3 (67.2 - 77.4) | 69.5 (64.2 - 74.5) | 71.6 (66.3 - 76.2) | < 0.01 |
| Systolic BP (mm Hg) | 124.8 (116.0 - 134.9) | 123.6 (115.1 - 133.6) | 125.7 (115.3 - 136.4) | 126.2 (117.4 - 136.4) | < 0.01 |
| Heart Rate | 79.3 (71.5 - 87.7) | 80.1 (73.0 - 88.8) | 77.0 (69.4 - 85.8) | 78.8 (71.3 - 87.0) | < 0.01 |
| Respiratory Rate | 19.0 (18.3 - 20.6) | 19.0 (18.3 - 20.0) | 19.2 (18.5 - 20.6) | 19.1 (18.4 - 20.4) | < 0.01 |
| Oxygen Saturation (%) | 95.5 (94.6 - 96.9) | 95.7 (94.8 - 97.0) | 95.3 (94.3 - 96.4) | 95.6 (94.6 - 96.7) | < 0.01 |
| Oxygen flow rate | 2.8 (1.9 - 7.9) | 2.5 (1.8 - 4.9) | 3.2 (2.0 - 8.6) | 2.6 (1.9 - 6.5) | < 0.01 |
p-values are determined by the Kruskal–Wallis test, which was performed to identify inter-group differences for each clinical variable.
Reported values are median (IQR). For SOFA score, values represent the median within the subgroup of the mean score during admission for each individual.
Figure 2:
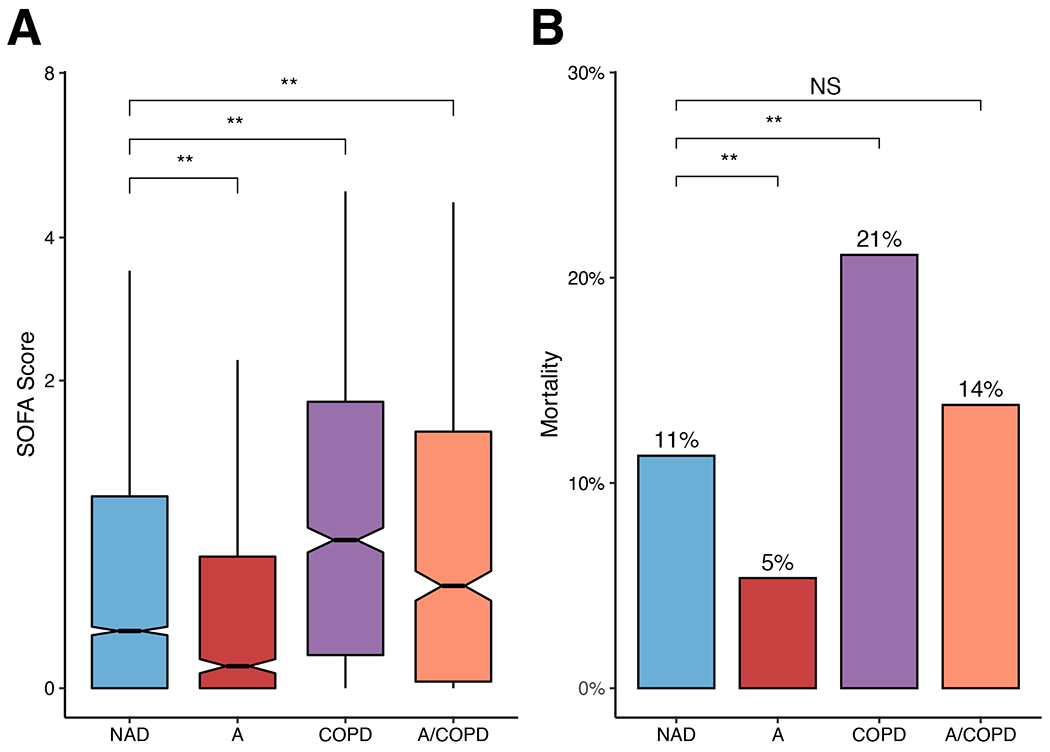
COVID-19 severity in patients with chronic airway disease. (A) Mean SOFA score during hospitalization. Compared to NAD, mean SOFA score is significantly lower for Asthma (P < 0.01) and higher for COPD and A/COPD patients (P < 0.01). The line represents the median value; wedge indicates the 95% CI; box identifies the IQR; whiskers span the 1.5 x IQR values. (B) Absolute mortality rate. Compared to NAD, mortality is significantly different for Asthma and COPD (P < 0.01), but not A/COPD (P = 0.19). Significance: **, P < 0.01; NS, not significant. Abbreviations: A (Asthma), A/COPD (Asthma and COPD), COPD (Chronic Obstructive Pulmonary Disease), and NAD (No Airway Disease).
Given the significant differences in clinical features between groups (Table 1A), we sought to assess their potential confounding effects on mortality using a univariate logistic regression model (see Figure E2 in the Online Repository). Variables included age, gender, BMI, current/past nicotine dependence, current/past diagnosis of diabetes, and ethnicity. This analysis showed that age and diabetes were independently associated with higher odds of mortality from COVID-19, consistent with prior studies.(2, 3) Next, a multivariate logistic regression analysis was performed to determine if the differences in mortality between airway disease groups persisted after adjusting for age and diabetes, as well as gender, BMI, nicotine dependence, and ethnicity (Table 2). In this model, asthma patients had 35% lower adjusted odds of mortality from COVID-19 compared to patients with NAD (OR = 0.65, P = 0.01, 95% CI, 0.48 to 0.89). In contrast, patients with COPD had 40% higher odds of mortality (OR = 1.40, P < 0.01, 95% CI, 1.16 to 1.67). Mortality in the A/COPD group was comparable to that in NAD (OR = 0.99, P = 0.91, 95% CI, 0.77 to 1.26), further indicating that comorbid asthma may offset the risk of severe COVID-19 in patients with COPD. In summary, these data show that the mortality rate was lower in asthma and higher in COPD compared to NAD patients, even after adjusting for confounding clinical variables.
Table 2:
Adjusted odds ratios of COVID-19 mortality for airway disease groups.
| Odds ratio of mortality | 95% CI | P-value | |
|---|---|---|---|
| Asthma | 0.65 | (0.48, 0.89) | 0.01 |
| COPD | 1.40 | (1.16, 1.67) | < 0.01 |
| A/COPD | 0.99 | (0.77, 1.26) | 0.91 |
Odds ratios compare the mortality risk of each airway disease group to the No Airway Disease control group.
All odds ratios are adjusted for patient age, gender, BMI, nicotine dependence, diabetes and ethnicity.
P-values are determined by the logistic regression model.
We next conducted a univariate logistic regression analysis of laboratory values on admission to identify additional factors associated with mortality. This analysis showed that mortality was significantly associated with higher total white blood cell count, immature granulocyte count, and neutrophil count in all disease groups (Table 3, Figure E3 in the Online Repository). Conversely, we found that elevated blood eosinophil count (a biomarker of T2 inflammation) was associated with a lower risk of mortality in all disease groups, with the strongest association observed in patients with asthma.
Table 3:
Univariate analysis of the association between COVID-19 mortality and peripheral blood cell counts at admission.
| NAD | Asthma | COPD | A/COPD | ||
|---|---|---|---|---|---|
| Eosinophil | Odds Ratio | 0.06 | < 0.01 | 0.02 | 0.18 |
| P-value | < 0.01 | 0.04 | < 0.01 | 0.15 | |
| CI | (0.01, 0.28) | (< 0.01, 0.60) | (< 0.01, 0.23) | (0.02,1.84) | |
| Monocyte | Odds Ratio | 1.02 | 0.95 | 1.67 | 1.18 |
| P-value | 0.84 | 0.91 | < 0.01 | 0.59 | |
| CI | (0.84, 1.23) | (0.40, 2.26) | (0.17, 2.38) | (0.64, 2.17) | |
| Lymphocyte | Odds Ratio | 1.01 | 1.02 | 0.87 | 1.02 |
| P-value | 0.72 | 0.12 | 0.24 | 0.22 | |
| CI | (0.98, 1.03) | (0.99, 1.04) | (0.68, 1.10) | (0.99, 1.06) | |
| Neutrophil | Odds Ratio | 1.08 | 1.18 | 1.12 | 1.10 |
| P-value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| CI | (1.06, 1.11) | (1.10, 1.26) | (1.08, 1.16) | (1.05, 1.16) | |
| Total White Blood Cell Count | Odds Ratio | 1.04 | 1.09 | 1.07 | 1.06 |
| P-value | < 0.01 | < 0.01 | < 0.01 | 0.01 | |
| CI | (1.03, 1.06) | (1.03, 1.15) | (1.04, 1.11) | (1.01, 1.11) | |
| Immature Granulocyte | Odds Ratio | 2.81 | 13.02 | 7.73 | 13.28 |
| P-value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| CI | (1.84, 4.28) | (3.61, 46.87) | (2.47, 24.15) | (3.03, 58.19) |
Odds ratios represent the change of mortality odds of each patient group as the peripheral blood cell counts increased by 1.
P-values are from logistic regression model.
CI = Confidence Interval
Since higher blood eosinophil counts favored survival, we further examined the effect of blood eosinophils on mortality using univariate analysis. This approach showed that patients with asthma (Asthma and A/COPD) had higher levels of eosinophils on admission (see Figure E4 in the Online Repository). After adjusting for confounders (age, diabetes, gender, BMI, nicotine dependence, ethnicity, and airway disease), patients with an absolute eosinophil count ≥200 cells/¼L still had lower odds of mortality (OR = 0.52, P < 0.01, 95% CI, 0.33 to 0.80) compared to patients with <200 cells/¼L (Table 4). In addition, differences in mortality between airway disease groups persisted even after adjusting for eosinophil levels (Asthma: OR = 0.67, P = 0.01, 95% CI, 0.49 to 0.92; COPD: OR = 1.38, P < 0.01, 95% CI, 1.15 to 1.66), indicating that peripheral eosinophilia does not fully account for the protection associated with asthma.
Table 4:
Effect of eosinophilia on the adjusted odds ratios of COVID-19 mortality for airway disease groups.
| Odds ratio of mortality | 95% CI | P-value | |
|---|---|---|---|
| Asthma | 0.67 | (0.49, 0.92) | 0.01 |
| COPD | 1.38 | (1.15, 1.66) | < 0.01 |
| A/COPD | 0.99 | (0.77, 1.28) | 0.96 |
| ≥200 eosinophils/μL | 0.52 | (0.33, 0.80) | < 0.01 |
| Interaction term | 95% CI | P-value | |
| Asthma × eosinophilia | 0.43 | (0.06, 3.38) | 0.42 |
| COPD × eosinophilia | 1.05 | (0.48, 2.29) | 0.90 |
| A/COPD × eosinophilia | 1.63 | (0.70, 3.77) | 0.25 |
Odds ratios compare the mortality risk of each airway disease group to the No Airway Disease control group; the eosinophilia group is compared to patients without eosinophilia.
All odds ratios are adjusted for patient age, gender, BMI, nicotine dependence, diabetes, and ethnicity. Odds ratios for the airway disease groups are additionally adjusted for admission blood eosinophil levels. Odds ratio for eosinophilia is additionally adjusted for disease status.
Interaction terms represent a scalar factor that defines the effect of eosinophilia on the odds ratio of mortality for each airway disease.
P-values are determined by the logistic regression model.
Lastly, we examined the potential effects of asthma severity and in-hospital steroid exposure on COVID-19 outcomes. Table E1 (see the Online Repository) summarizes our analysis of the association between asthma severity and COVID-19 mortality. Similar to prior reports,(15, 16) we found no such association, and in fact observed a trend towards improved outcomes at all levels of asthma severity. Tables E2-E3 (see the Online Repository) evaluate whether in-hospital treatment with SCS could have confounded the changes in mortality observed in patients with asthma, COPD, and eosinophilia. As shown in Table E2 (see the Online Repository), we identified significant differences in exposure to SCS between groups, and an overall increase in mortality associated with intravenous (IV) methylprednisolone therapy (OR = 4.77, P < 0.01, 95% CI, 3.69 to 6.17), which was expected given its selective use in patients with severe COVID-19. However, a multiple regression analysis including IV methylprednisolone and oral prednisone as covariates (see Table E3 in the Online Repository) showed that mortality was still significantly increased in patients with COPD and decreased in patients with asthma and eosinophilia, even after adjusting for SCS exposure.
DISCUSSION
Clinical outcomes in COVID-19 range dramatically, from asymptomatic infection to fulminant ARDS with multi-organ failure and death. It is critical to identify the populations that are at risk for severe COVID-19, as they require more intensive mitigation strategies such as social distancing, vaccine boosters, and early outpatient pharmacotherapy. Epidemiologic studies since the start of the pandemic have demonstrated elevated risk in patients with advanced age, diabetes, and obesity. However, it has remained unclear which chronic respiratory conditions may predispose to severe disease.
In an effort to address this question, we conducted one of the largest retrospective studies to date, including more than 8,000 patients hospitalized with COVID-19 during the first two waves of the pandemic. We found that asthma was independently associated with a 35% decrease in mortality, similar to the 40% reduction observed by Bucholc et al in a cohort from Northern Ireland.(17) We also found that peripheral eosinophilia, a common biomarker for T2 inflammation, was associated with protection regardless of the underlying airway disease. Finally, we showed that patients with COPD were at higher risk for severe COVID-19, and that co-morbid asthma appeared to offset this risk, as demonstrated by the improved outcomes of patients with A/COPD (Figure 2). Importantly, each of these differences persisted even after adjusting for known risk factors, including age, sex, obesity, and diabetes.
These data fit with a provocative theme emerging in the literature – that T2 inflammation may be protective in COVID-19. Along these lines, peripheral eosinophilia has been linked to protection in prior studies,(12, 18, 19) as shown here. Reciprocally, eosinopenia has been identified as a strong risk factor for severe disease.(20–23) Notably, our analyses also demonstrate that patients with asthma have improved outcomes independent of eosinophilia (Table 4). Thus, there appear to be additional immunologic mechanisms in asthma that confer protection from COVID-19 beyond those identified by commonly measured T2 biomarkers, such as eosinophil count.
These results beg the question of how asthma and eosinophilia may be linked mechanistically to protection from COVID-19. In principle, T2 inflammation could impact one or both of the pathogenic phases of COVID-19: (i) early failure to control viral replication; and/or (ii) late development of ARDS due to immunopathologic lung injury.(24) In support of the former mechanism, multiple studies have shown that the T2 cytokine, IL-13, down-regulates the SARS-CoV-2 co-receptor ACE2 in airway epithelial cells, which could lead to diminished replication in the lower airways of patients with asthma.(25–27). Conversely, lung explant studies have shown that active smoking and COPD are associated with increased epithelial ACE2 expression.(28) As such, patients with COPD may be more susceptible to SARS-CoV-2 infection and therefore worse outcomes, as observed in the present study.
A second, nonexclusive hypothesis is that T2 inflammation confers protection from COVID-19 through modulation of immunopathologic host responses. The T2 cytokine IL-4, for instance, is known to inhibit lung injury by promoting polarization of alveolar macrophages towards an anti-inflammatory M2-like phenotype and suppressing histotoxic neutrophil effector functions.(29, 30) In addition, cellular mediators of type 2 immune responses, including Th2 and type 2 innate lymphoid cells (ILC2), suppress their type 1 counterparts (Th1 and ILC1 cells),(31) which are linked to tissue damage in COVID-19.(32)
This pathophysiologic model may also account for the poorer outcomes in COPD. Work by Wunderink and colleagues has defined an immune circuit driven by Th1 cells and SARS-CoV-2 infected M1-like alveolar macrophages, leading to prolonged generation of inflammatory cytokines including TNF-α and IFN-γ.(33) In turn, macrophage hyperstimulation leads to inflammasome activation, IL-1β and IL-18 production, and immunopathologic lung injury.(34–36) Patients with COPD have a well-established T1 immune diathesis that may predispose to establishment of these circuits, or a failure to terminate them.(37–44) With the establishment of translational biobanks and sophisticated animal models,(36) these hypotheses can be readily tested.
Strengths of this study include the size of the cohort, the comparison of multiple airway disease phenotypes within a single cohort, and the multivariate analyses to examine the effect of potential confounders. Limitations include the retrospective nature of the study, which necessitated the use of ICD10 codes for airway disease diagnosis. In addition, restriction of our cohort to hospitalized patients prevented us from formally assessing whether patients with airway disease are at higher risk of hospital admission. However, the similar rates of asthma in our cohort and the general population would suggest no increased risk of hospitalization in patients with asthma, consistent with certain prior reports;(9) COPD patients did appear at higher risk by this analysis. A final consideration is that our study does not distinguish between COVID-19 pneumonia (due to T1 immunopathology and alveolar exudates) and asthma exacerbations induced by SARS-CoV-2 infection (due to T2 inflammation, mucus hypersecretion and bronchoconstriction). It is conceivable that patients with asthma may be protected from the former, but more susceptible to the latter.
In summary, our results show that eosinophilia, asthma, and non-eosinophilic asthma are associated with improved disease severity and survival in COVID-19, while COPD is associated with worse outcomes. Thus, T2 inflammation may confer protection during SARS-CoV-2 infection. Further clinical and mechanistic studies will be needed to explore this possibility more fully.
Highlights box.
1. What is already known about this topic?
Advanced age, diabetes, and obesity are established risk factors for developing severe COVID-19. However, the risk of severe COVID-19 in patients with chronic respiratory diseases is less clear.
2. What does this article add to our knowledge?
This large-scale retrospective clinical study shows that COVID-19 disease severity and mortality are worse in COPD patients, but markedly improved in patients with conditions associated with T2 inflammation, such as asthma and eosinophilia.
3. How does this study impact current management guidelines?
Whereas current guidelines indicate that patients with asthma are at increased risk from COVID-19, our findings indicate they may in fact be protected from severe disease and death.
Acknowledgements:
We would like to thank the Yale Department of Medicine Covid Explorer (DOM-CovX) data repository for the data.
Funding information:
Supported by the Department of Medicine, the George M. O’Brien Kidney Center at Yale grant P30DK079310 (DOM-CovX, M.S., F.P.W.); UM1 (AI114271), U23 (HL138998), R01 (HL153604), HL153911 (G.L.C.); R01 (HL153604) (J.G.); R21 (LM012884) and NIH grant R01LM014087-01S1 (X.Y.); Prostate Cancer Foundation (JMV) and COVID-19 Early Treatment Fund (J.M.V.); K08 (HL159422), CFF Fellowship (GAUTAM20D0), PBF Fellowship, Patterson Award, YCCI Scholar Award, O’Brien Center Pilot Award, and Doris Duke FRCS at Yale Award (#2021266) (S.G.);Prime: (NHLBI)/NIH/DHHS (#21-004125) (H.R.)
Abbreviations:
- A/COPD
Asthma and COPD
- BMI
Body Mass Index
- COPD
Chronic Obstructive Pulmonary Disease
- COVID-19
Coronavirus disease – 19
- DOM-CovX
Yale Department of Internal Medicine COVID Explorer
- Epic EMR
Epic Electronic Medical Records
- ICS
Inhaled Corticosteroids
- NAD
No Airway Disease
- SCS
Systemic Corticosteroids
- RT-PCR
Real Time-Polymerase Chain Reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SOFA Sore
Sequential Organ Failure Assessment Score
- O2
Oxygen
- T2
Type 2
- YNHHS
Yale New Haven Health System
Online Repository
Figure E1:
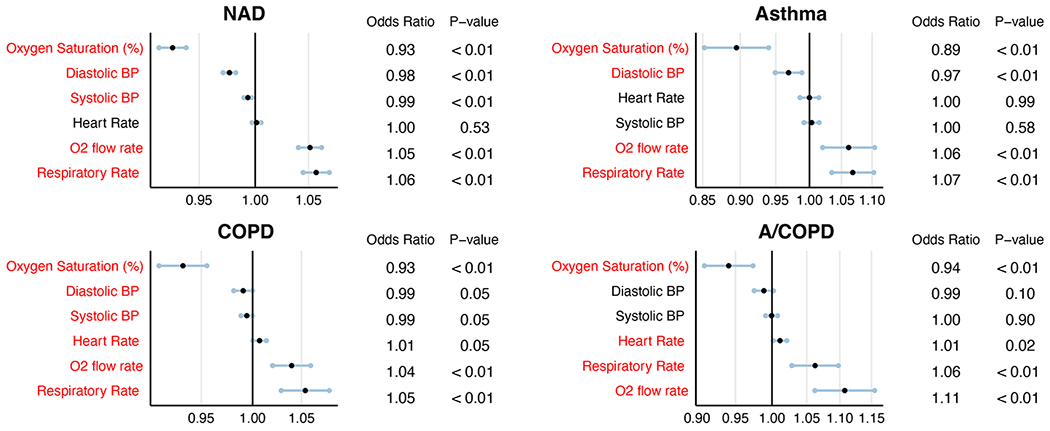
Clinical risk factors associated with mortality among patients hospitalized with COVID-19. (A) NAD (B) Asthma (C) COPD and (D) A/COPD. Statistically significant variables are represented in red.
Figure E2:
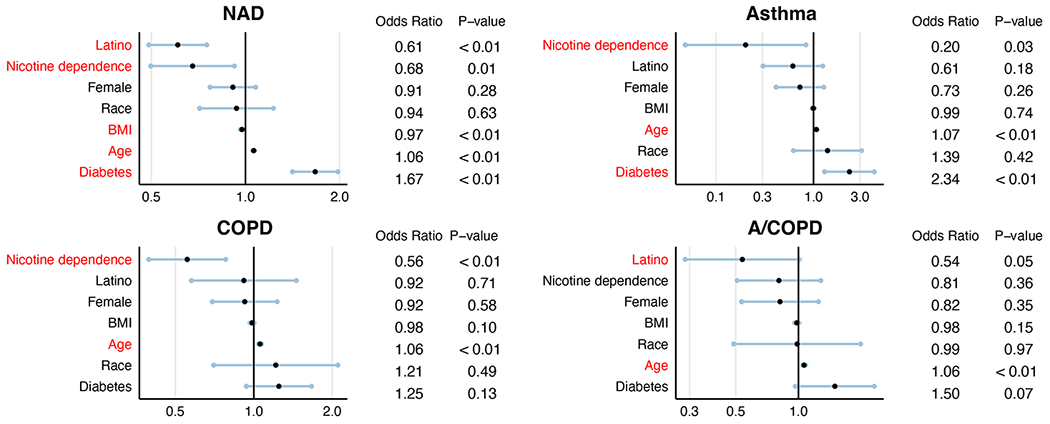
Analysis of comorbidities associated with mortality among hospitalized COVID-19 patients. (A) NAD (B) Asthma (C) COPD and (D) A/COPD. Statistically significant variables are represented in red.
Figure E3:
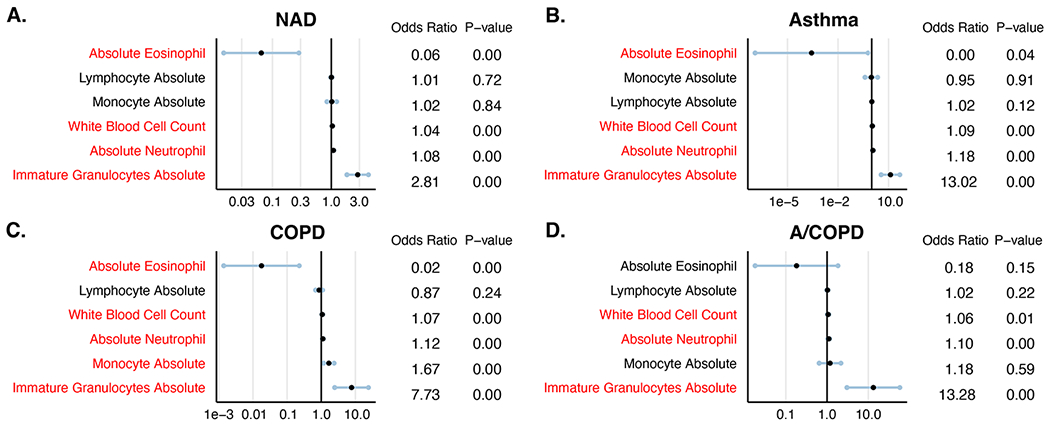
Analysis of white blood cell counts associated with mortality among hospitalized COVID-19 patients. (A) NAD (B) Asthma (C) COPD and (D) A/COPD. Statistically significant variables are represented in red.
Figure E4:
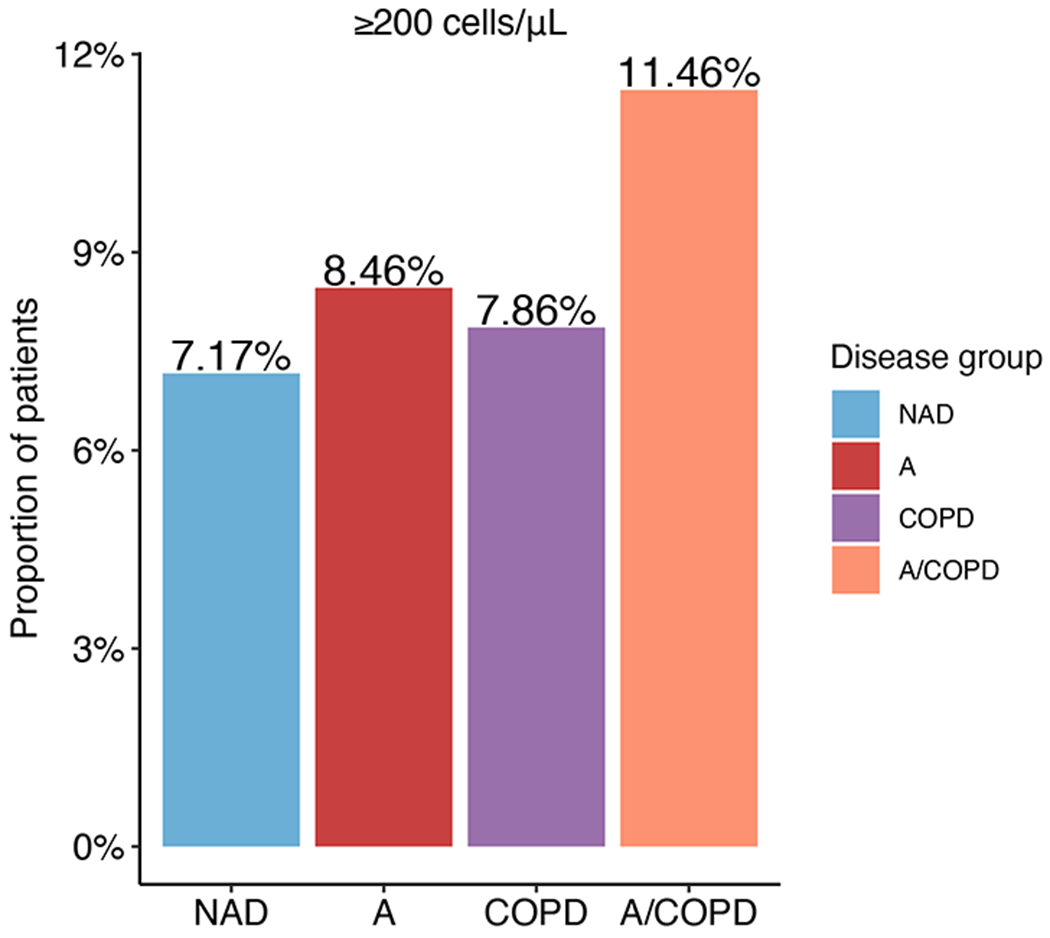
Proportion of patients hospitalized with COVID-19 with blood eosinophil count ≥200 cells/μL on admission.
Table E1:
Association between asthma severity and COVID-19 outcomes.
| Odds ratio of mortality | 95% CI | P-value | |
|---|---|---|---|
| Intermittent asthma | 0.58 | (0.25, 1.38) | 0.22 |
| Mild asthma | 0.62 | (0.42, 0.90) | 0.01 |
| Moderate asthma | 0.92 | (0.21, 3.98) | 0.91 |
| Severe asthma | 0.81 | (0.42, 1,59) | 0.54 |
Odds ratios compare the mortality risk of each asthma severity subgroup to the No Airway Disease control group.
All odds ratios are adjusted for patient age, gender, BMI, nicotine dependence, diabetes ethnicity, and admission blood eosinophil levels.
P-values are determined by the logistic regression model.
Table E2:
Administration of systemic corticosteroids during admission.
| NAD | Asthma | COPD | A/COPD | P-value | |
|---|---|---|---|---|---|
| Methylprednisolone | 435 (8%) | 116 (12%) | 157 (14%) | 152 (20%) | < 0.01 |
| Prednisone | 237 (4%) | 81 (8%) | 136 (12%) | 146 (19%) | 0.01 |
P-values are determined by the Kruskal–Wallis test, which was performed to identify inter-group differences for exposure to each corticosteroid.
Table E3:
Effect of corticosteroid exposure on the adjusted odds ratios of COVID-19 mortality for airway disease groups
| Odds ratio of mortality | 95% CI | P-value | |
|---|---|---|---|
| Asthma | 0.64 | (0.44, 0.93) | 0.02 |
| COPD | 1.37 | (1.11, 1.70) | < 0.01 |
| A/COPD | 0.82 | (0.59, 1.12) | 0.21 |
| ≥200 eosinophils/¼L | 0.56 | (0.36, 0.87) | 0.01 |
| Methylprednisolone | 4.77 | (3.69, 6.17) | < 0.01 |
| Prednisone | 0.79 | (0.52, 1.21) | 0.28 |
| Interaction term | 95% CI | P-value | |
| Asthma × eosinophilia | 0.44 | (0.06, 3.41) | 0.43 |
| COPD × eosinophilia | 1.00 | (0.46, 2.21) | 0.99 |
| A/COPD × eosinophilia | 1.65 | (0.70, 3.90) | 0.25 |
| Asthma × methylprednisolone | 0.86 | (0.41, 1.80) | 0.69 |
| COPD × methylprednisolone | 0.65 | (0.40, 1.05) | 0.08 |
| A/COPD × methylprednisolone | 0.80 | (0.46, 1.41) | 0.45 |
| Asthma × prednisone | 1.17 | (0.38, 3.59) | 0.78 |
| COPD × prednisone | 1.12 | (0.59, 2.13) | 0.72 |
| A/COPD × prednisone | 1.29 | (0.64, 2.59) | 0.47 |
Odds ratios compare the mortality risk of each airway disease group to the No Airway Disease control group; the eosinophilia group is compared to patients without eosinophilia; the corticosteroids groups are compared to patients without exposure to corticosteroids.
All odds ratios are adjusted for patient age, gender, BMI, nicotine dependence, diabetes, and ethnicity. Odds ratios for the airway disease groups are additionally adjusted for eosinophilia and steroid exposure. The odds ratio for the eosinophilia group is additionally adjusted for steroid exposure. Odds ratios for the corticosteroid groups are additionally adjusted for eosinophilia.
Interaction terms represent a scalar factor that defines the effects of eosinophilia or corticosteroid exposure on the odds ratio of mortality for each airway disease.
P-values are determined by the logistic regression model.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Geoffrey Chupp, MD – Speakers Bureau / Advisory Board – AstraZeneca, Glaxo Smith-Kline, Boehringer Ingelheim, Sanofi, Regeneron, Genentech
Samir Gautam, MD PhD – Advisory Board – AstraZeneca
Hector Ortega, MD – Employed by Nexstone Immunology
Joseph M. Vinetz, MD – LeptoX BioPharma, Inc., co-Founder
REFERENCES:
- 1.NCDC. Coronavirus disease (COVID-2019) situation reports. World Health Organisation; 2020; [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA - Journal of the American Medical Association 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y dong, Ding M, Dong X, Zhang J jin, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy: European Journal of Allergy and Clinical Immunology 2021. ;76:428–455. [DOI] [PubMed] [Google Scholar]
- 5.Centers of Diseases and Control Prevention. People with Certain Medical Conditions. Centers for Disease Control and Prevention; 2022;at <https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html>. [Google Scholar]
- 6.Gerayeli FV, Milne S, Cheung C, Li X, Yang CWT, Tam A, et al. COPD and the risk of poor outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine 2021. ;33:100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse WW, Lemanske RF, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. The Lancet 2010;376:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. People with Moderate to Severe Asthma. Centers for Disease Control and Prevention; 2020; [Google Scholar]
- 9.Gao YD, Agache I, Akdis M, Nadeau K, Klimek L, Jutel M, et al. The effect of allergy and asthma as a comorbidity on the susceptibility and outcomes of COVID-19. Int Immunol 2022;34:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunjaya AP, Allida SM, Tanna GL Di, Jenkins CR. Asthma and COVID-19 risk: a systematic review and meta-analysis. European Respiratory Journal 2022;59:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Medicine Covid explorer (DOM-CovX). 2021;at <https://spinup-0011f4.spinup.yale.edu/domcovx/>.
- 12.Ho KS, Howell D, Rogers L, Narasimhan B, Verma H, Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Annals of Allergy, Asthma and Immunology 2021;127:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Most Recent Asthma Data | CDC. at <https://www.cdc.gov/asthma/most_recent_data.htm>. [Google Scholar]
- 14.State Estimates - Chronic Obstructive Pulmonary Disease (COPD) | CDC. at <https://www.cdc.gov/copd/data-and-statistics/state-estimates.html>. [Google Scholar]
- 15.Lee B, Lewis G, Agyei-Manu E, Atkins N, Bhattacharyya U, Dozier M, et al. Risk of serious COVID-19 outcomes among adults and children with moderate-to-severe asthma: a systematic review and meta-analysis. Eur Respir Rev 2022;31:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zein JG, Mitri J, Bell JM, Lopez D, Strauss R, Attaway AH. The relationship of asthma severity to COVID-19 outcomes. J Allergy Clin Immunol Pract 2022;10:318–321.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucholc M, Bradley D, Bennett D, Patterson L, Spiers R, Gibson D, et al. Identifying pre-existing conditions and multimorbidity patterns associated with in-hospital mortality in patients with COVID-19. Scientific Reports 2022. 12:1 2022;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferastraoaru D, Hudes G, Jerschow E, Jariwala S, Karagic M, de Vos G, et al. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. Journal of Allergy and Clinical Immunology: In Practice 2021;9:1152–1162.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zein JG, Strauss R, Attaway AH, Hu B, Milinovich A, Jawhari N, et al. Eosinophilia Is Associated with Improved COVID-19 Outcomes in Inhaled Corticosteroid-Treated Patients. Journal of Allergy and Clinical Immunology: In Practice 2022;10:742–750.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J Med Virol 2020;92:1902–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: A retrospective observational study. Am J Respir Crit Care Med 2020;201:1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. Journal of Allergy and Clinical Immunology 2020;146:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggert LE, He Z, Collins W, Lee AS, Dhondalay G, Jiang SY, et al. Asthma phenotypes, associated comorbidities, and long-term symptoms in COVID-19. Allergy: European Journal of Allergy and Clinical Immunology 2022;77:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science (1979) 2022;375:1122–1127. [DOI] [PubMed] [Google Scholar]
- 25.Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. Journal of Allergy and Clinical Immunology 2020;146:80–88. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson DJ, Busse WW, Bacharier LB, Kattan M, O’Connor GT, Wood RA, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. Journal of Allergy and Clinical Immunology 2020;146:203–206.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camiolo M, Gauthier M, Kaminski N, Ray A, Wenzel SE. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. Journal of Allergy and Clinical Immunology 2020;146:315–324.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs M, van Eeckhoutte HP, Wijnant SRA, Janssens W, Brusselle GG, Joos GF, et al. Increased expression of ACE2, the SARS-CoV-2 entry receptor, in alveolar and bronchial epithelium of smokers and COPD subjects. Eur Respir J 2020;56:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Alessio FR, Craig JM, Singer BD, Files DC, Mock JR, Garibaldi BT, et al. Enhanced resolution of experimental ards through il-4-mediated lung macrophage reprogramming. Am J Physiol Lung Cell Mol Physiol 2016;310:L733–L746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris AJ, Mirchandani AS, Lynch RW, Murphy F, Delaney L, Small D, et al. IL4RA signaling abrogates hypoxic neutrophil survival and limits acute lung injury responses in vivo. Am J Respir Crit Care Med 2019;200:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butcher MJ, Zhu J. Recent advances in understanding the Th1/Th2 effector choice. Fac Rev 2021;10:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med 2021;9:622–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant RA, Morales-Nebreda L, Markov NS, Swaminathan S, Querrey M, Guzman ER, et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature 2021;590:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junqueira C, Crespo Â, Ranjbar S, de Lacerda LB, Lewandrowski M, Ingber J, et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022. 606:7914 2022;606:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vora SM, Lieberman J, Wu H. Inflammasome activation at the crux of severe COVID-19. Nature Reviews Immunology 2021. 21:11 2021;21:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022;606:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol 2014;5:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell REK, Culpitt SV, DeMatos C, Donnelly L, Smith M, Wiggins J, et al. Release and Activity of Matrix Metalloproteinase-9 and Tissue Inhibitor of Metalloproteinase-1 by Alveolar Macrophages from Patients with Chronic Obstructive Pulmonary Disease. https://doi.org/101165/ajrcmb2654685 2012;26:602–609. [DOI] [PubMed] [Google Scholar]
- 39.Eapen MS, Hansbro PM, McAlinden K, Kim RY, Ward C, Hackett TL, et al. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci Rep 2017;7:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaykhiev R, Krause A, Salit J, Strulovici-Barel Y, Harvey B-G, O’Connor TP, et al. Smoking-Dependent Reprogramming of Alveolar Macrophage Polarization: Implication for Pathogenesis of Chronic Obstructive Pulmonary Disease. The Journal of Immunology 2009;183:2867–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bazzan E, Turato G, Tinè M, Radu CM, Balestro E, Rigobello C, et al. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir Res 2017;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii W, Kapellos TS, Baβler K, Händler K, Holsten L, Knoll R, et al. Alveolar macrophage transcriptomic profiling in COPD shows major lipid metabolism changes. ERJ Open Res 2021;7:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Q, Wang Y, Zhang L, Qian W, Shen S, Wang J, et al. Single-cell transcriptomics highlights immunological dysregulations of monocytes in the pathobiology of COPD. Respir Res 2022;23:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapellos TS, Bassler K, Aschenbrenner AC, Fujii W, Schultze JL. Dysregulated Functions of Lung Macrophage Populations in COPD. J Immunol Res 2018;2018:. [DOI] [PMC free article] [PubMed] [Google Scholar]


