Abstract
While regulation of nociceptive processes in the dorsal horn by deep brain structures has long been established, the role of cortical networks in pain regulation is minimally explored. The medial prefrontal cortex (mPFC) is a key brain area in pain processing that receives ascending nociceptive input and exerts top-down control of pain sensation. We have shown critical changes in mPFC synaptic function during neuropathic pain, controlled by endocannabinoid (eCB) signaling. This study tests whether mPFC eCB signaling modulates neuropathic pain via descending control. Intra-mPFC injection of cannabinoid receptor type 1 (CB1R) agonist WIN-55,212–2 (WIN) in the chronic phase transiently alleviates the pain-like behaviors in spared nerve injury (SNI) rats. In contrast, intra-mPFC injection of CB1R antagonist AM4113 in the early phase of neuropathic pain reduces the development of pain-like behaviors in the chronic phase. SNI reduced the mechanical threshold to induce action potential firing of dorsal horn (DH) wide dynamic range neurons, but this was reversed in rats by WIN in the chronic phase of SNI and by mPFC injection of AM4113 in the early phase of SNI. Elevated DRG neuronal activity after injury was also diminished in rats by mPFC injection of AM4113, potentially by reducing antidromic activity and subsequent neuronal inflammation. These findings suggest that depending on the phase of the pain condition, both blocking and activating CB1 receptors in the mPFC can regulate descending control of pain and affect both dorsal horn neurons and peripheral sensory neurons, contributing to changes in pain sensitivity.
Introduction:
Chronic pain is an inadequately addressed public health challenge [57]. It is well recognized that pain activates numerous brain areas [44], which underlies affective aspects of the pain experience. The medial prefrontal cortex (mPFC) is a critical brain area in ascending and descending pain control [23; 39]. The rat and human mPFC receive innervation from the thalamus [3; 4; 9; 19; 33], which receives nociceptive input from the spinal cord dorsal horn (DH), making the mPFC an area subjected to peripheral noxious input during pain [37]. Supporting this view, rats with chronic arthritis develop disordered neurotransmitter levels in the frontal cortex [40] and elevated extracellular signal-regulated kinase 1/2 activation in the PFC [6]. Peripheral nerve injury is accompanied by sustained transcriptome-wide gene expression changes in the mouse PFC [1]. Our previous study reveals a dynamic change of endocannabinoid (eCB)-dependent mPFC activity after painful peripheral nerve injury [37].
The mPFC is also a crucial center in the descending pain control system that links the brain and spinal cord to provide potent and targeted regulation of nociceptive processing [23; 39]. The periaqueductal gray (PAG) in the midbrain is the primary relay center for this descending pain modulation. Projections from the mPFC are the main cortical input to the PAG, and mPFC activity modulates PAG activity with subsequent pain regulation [16; 23; 31; 34; 39]. Following an acute injury, mPFC activity is increased, activating descending inhibition to inhibit spinal DH circuits and thus reduce afferent nociceptive activity and moderate central sensitization [22; 37; 50]. Lowering mPFC activity in the acute phase of painful nerve injury resulted in an increase in sensitivity to mechanical and cold stimuli [23]. Reduced activity in the mPFC was observed in both patients with chronic pain and preclinical models of chronic pain [2; 37], while PFC activation alleviates chronic pain in patients [7; 56] and in rats [23]. Thus, the PFC controls spinal nociceptive processing.
eCB signaling regulates mPFC activity via the cannabinoid receptor type 1 (CB1Rs) [37], which is primarily located in the inhibitory gamma-aminobutyric acid (GABA)-ergic presynaptic terminals [28]. Our previous findings revealed that CB1R activation suppresses inhibitory input to the mPFC, and this signaling pathway becomes hyperactive after spared nerve injury (SNI). Over time, CB1R-dependent disinhibition of the mPFC is lost, and mPFC activity becomes hypoactive in the chronic phase of the neuropathic pain [37]. Since the dynamic change of eCB signaling after neuropathic pain contributes to the changes in mPFC neuronal activity and function, it is possible that manipulating mPFC eCB signaling in different phases of neuropathic pain may alter descending inhibition of peripheral processes, providing a site for therapeutic intervention. To test whether mPFC eCB signaling controls neuropathic pain by this descending mechanism, we employed pharmacological, behavioral, and electrophysiological approaches to evaluate the involvement of mPFC eCB signaling in the modulation of descending pain control.
Materials and Methods
Animals
Male and female Sprague Dawley rats weighing 170–200g were obtained from Taconic Biosciences (Hudson, NY) (https://www.taconic.com/rat-model/sprague-dawley). They were maintained and used according to the NIH Guide for the Care and Use of Laboratory Animals, and in compliance with federal, state, and local laws. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (AUA5615). Animals were housed in a pathogen-free facility, two animals per ventilated cage, in a room maintained at 25±1 °C at 35 to 45% humidity, with a 12/12-hour (hr) day/night cycle. Animals had free access to food and water. At the termination of the study, euthanasia was performed by decapitation during deep isoflurane anesthesia. A total of 136 rats were used in this study. Simple randomization was used in this study to allocate animals to different groups. The sample sizes for behavioral tests and electrophysiology are determined based on our previous reports [10; 13; 37].
Injury model preparation
Some rats were subjected to spared nerve injury (SNI) surgery [25]. Briefly, during anesthesia by inhalation of isoflurane (1–3%), an incision (~2 cm) was made on the lateral mid-thigh, and the underlying muscles separated to expose the sciatic nerve at its trifurcation, and the tibial and common peroneal branches were individually ligated with 6.0 sutures and cut distally to the ligature, and 2–3 millimeter (mm) of each nerve was removed distal of the ligation. The preservation of the sural nerve was ensured, and efforts were made to avoid contact with it. Muscle and skin were closed using 4.0 monofilament nylon sutures. Sham control rats received sham surgery in the form of skin incision, nerve exposure, and closure only. Animals were randomly assigned to two groups either with SNI or sham surgery.
Intra-mPFC injection
Rats were anesthetized with 2–3% isoflurane and placed in a stereotaxic device (David Kopf Instruments). Bilateral guide cannulae (26 gauge; Plastics One) were implanted with a 10° angle from the middle line targeting the layer V of prelimbic mPFC and avoiding damage to the dorsal mPFC (Supple. Fig. 1), using the following stereotaxic coordinates: from bregma, anteroposterior, ±3.2 mm; mediolateral, ±1.0 mm; dorsoventral, 3.5 mm [41]. Obturators were placed in the guide cannula and extended 1 mm beyond it and were left there at all times except during microinjections. On the day of injection, the rats were anesthetized, and the obturator was removed from one of the guide cannulas, and a stainless-steel injector tube (30 gauge; Plastics One) was inserted to a depth 1 mm beyond the end of the guide cannula. The injector tube was connected through polyethylene tubing to a 10 μl Hamilton microsyringe, which was mounted on a single-syringe infusion pump (Masterflex). The drugs were injected at a speed of 0.5 μl/minute. The injector was kept in the guide cannula for an additional 60s to ensure adequate diffusion from the injector tip. Similar microinjection was made on the contralateral side.
After completion of all experiments, cannulae placements were anatomically verified. The animals were anesthetized with 5% isoflurane and then perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were cut into 30 μm sections and stained with cresyl violet and examined with light microscopy. Some brain sections were stained with hematoxylin/eosin.
Behavioral tests
Sensory testing of the plantar skin included evoking reflexive behaviors by punctate mechanical stimulation at threshold intensity (von Frey test) and at noxious intensity (pin test), by dynamic non-noxious mechanical stimulation (brush test), and by cold stimulation (acetone test). Sensitivity to heat was not tested as we have previously observed no consistent changes after SNL [20]. The open-field test was used to determine whether intra-cranial injection of AM4113 has effects on locomotor activity and anxiety-like behavior. The experimenters conducting these experiments were blinded to the animal’s treatment, including the type of injury and the drug used.
von Frey test:
The von Frey test was performed using calibrated monofilaments (Patterson Medical, Bolingbrook, IL). Beginning with the 2.8-gram filament, the tip of the filament was applied perpendicularly to the glabrous skin on the central portion of the plantar aspect of the hind paw for 1s, with just enough force to bend the fiber. If a paw removal response was observed, then the next weaker filament was applied, and if no response was observed, then the next stiffer fiber was applied, until a reversal in the sequence occurred. After a reversal event, 4 more stimulations were performed following the same pattern. Applications were separated by intervals of at least 10s. The forces of the filaments before and after the reversal, and the 4 filaments applied after the reversal, were used to calculate the withdrawal threshold [14]. Rats not responding to any filament were assigned a score of 25 g.
Noxious punctate mechanical stimulation (pin test):
Noxious punctate mechanical stimulation was performed using the point of a 22-gauge spinal anesthesia needle that was applied to the central portion of the hind paw with enough force to indent the skin but not puncture it. This was repeated for 5 times, with an inter-stimulus interval of at least 10s. This stimulation protocol was repeated after 1min, for a total of 10 stimuli. Each application evoked one of two types of behavior. One type of behavior, typically observed in uninjured rats, consists of a very brief (<1s) withdrawal, with immediate return of the foot to the cage floor. The second type of behavior, which we term a hyperalgesic response, consists of a complex event with sustained elevation of at least 1s, variably combined with grooming that includes licking and chewing of the paw, and shaking of the limb [29]. We have shown that this hyperalgesic behavior is specifically associated with the place avoidance [53], indicating that it represents an aversive experience. Hyperalgesia was quantified by tabulating the number of hyperalgesic responses as a percentage of the 10 stimuli delivered.
Cold stimulation (acetone test):
The acetone test was assessed using application of acetone, which was expelled through tubing to form a convex meniscus on the end of the tubing, which was touched to the lateral plantar skin without contact of the tubing with the skin [17]. The response was scored as positive if the paw was removed, and 3 repetitions were spaced at least 1min apart.
Dynamic mechanical stimulation (brush test):
A camel hair brush was applied to the lateral plantar skin of the hind paw by light stroking in the direction from heel to toe over the span of 2s [29]. The response was scored as either positive if the paw was removed or none in the absence of movement. The test was applied three times to each paw, separated by intervals of at least 10s.
Open field:
The behavior of a rat in a new environment (an open field) contains sufficient complexity and sensitivity to a wide range of neurological disorders. The Behavioral Spectrometer is a newly developed device that is capable of automatically identifying 23 unique behaviors and providing a complete, real-time profile of animal behavior in an open field scenario [8; 24]. The apparatus (Biobserve Inc, Bonn, Germany) consists of a 40 cm by 40 cm square area enclosed in a cube box with an edge length of 45 cm. A camera is mounted in the ceiling above the arena to monitor the animal’s position and posture. A row of 32 infrared transmitter and receiver pairs is embedded in the walls to monitor the rat behaviors. Accelerometers embedded in the floor are used to capture the rat’s vibrations for detecting the rear behaviors. A combination of these sensors and detailed descriptions of rat behavior can be generated. Each behavior produces a distinct pattern of sensor data, such that multiple patterns of sensor readings are detected and recorded by the software (Viewer3, Biobserve).
In vivo electrophysiological recording
Electrophysiological signals were collected with an Axoclamp 900 A microelectrode amplifier (Molecular Devices, San Jose, CA) and the headstage (HS-9A-x0.1U with feedback resistance of 100MΩ) was served as a preamplifier with gain setting of 500 or higher, filtered at 1 kHz, and sampled at 10 kHz using a digitizer (DigiData 1440 A, Molecular Devices). Individual action potentials were isolated and amplified using either a window discriminator (Clampfit 11, Molecular Devices) or by template matching using Spike2 (Cambridge Electronic Design Limited, Cambridge, UK).
Animal preparation:
Anesthesia was induced with 2% isoflurane, after which rats were given urethane (1000mg/kg, subcutaneous injection), followed 30 min later by progressively reducing isoflurane to 0.2% such that the rats remained immobile but metabolically and hemodynamically stable, confirmed by arterial blood analysis with a blood gas analysis (ABL800FLEX, Radiometer, Copenhagen, Denmark), and continuous intra-arterial blood pressure monitoring via a carotid artery cannula. A heating pad was used to maintain body temperature at 37 °C.
DH neuron single unit recording:
A laminectomy was performed from the T13 to the L3 vertebrae to expose the mid-lumbar spinal cord, and a stabilizing stereotaxic clamp was applied to the spinal process rostral to the exposure. The dura was opened, and the cord was covered in warm mineral oil (36°C). A single-barreled glass micropipette filled with a solution containing 1 M NaCl (with a resistance of 15–20 MΩ) was advanced into the spinal cord at the L4 to L5 level using a microdrive (David Kopf Instruments, Tujunga, CA) at 2μm per step. Wide dynamic range neurons (WDR) of lamina IV to VI at depths of 400–700 μm from the cord surface were targeted, as those neurons encode the discrimination of noxious from non-noxious stimuli and represent an important component in central sensitization in pain [21; 26]. Action potentials were isolated by setting the threshold above background noise, and individual units were identified by template matching using Spike2 (Cambridge Electronic Design Limited, Cambridge, UK) or pClamp 11 (Molecular Devices). At 50 μm intervals of electrode advancement, the ipsilateral paw was mechanically stimulated to identify units with RFs in this area. Specifically, sequential mechanical stimuli were applied to the ipsilateral hind paw using a standard set of graded von Frey fibers applied for 1 s to determine the threshold (i.e. weakest force capable of producing recorded activity) for response to punctate mechanical stimulation, then stroking with a brush to evoke dynamic mechanical stimulation, then application of von Frey fibers (8, 16, 29g) for 10 s each to determine firing rates (averaged over 10s) at these specified stimulation intensities, then application of a 16 g modified von Frey fiber with a tungsten tip for 10 s to determine firing rate. Inclusion criteria for the WDR type were responses to innocuous (brush, von Frey filament) and noxious (modified von Frey filament with 200 μm tungsten tip) stimulation in a graded manner [11].
Teased dorsal root single unit recording:
The spinal cord was exposed by a laminectomy at the level of vertebrae T13 to L3 and covered with warm mineral oil (36°C), and bone wax was used to stop bleeding from the bone. The dura was removed, rats were mounted on a spinal frame, and the vertebral column was stabilized by vertebral clamps applied to the spinal process rostral/caudal to the exposure. The loosened skin from the midline incision was sutured to a metal rectangle ring (3 × 4 cm) to form a basin that was filled with warm mineral oil. A fine glass probe with a rounded tip was used to gently release the L4 dorsal root from connective tissues, after which they were transected at the point where they were divided into rootlets. The dorsal root was then placed on a glass platform, upon which the distal portion of the dorsal root was repeatedly teased to create fine neuronal bundles. These were placed on a platinum/iridium recording electrode for recording single-unit activity. A reference electrode was attached to the adjacent muscle tissue. Data were obtained using electronic systems comparable to that described above for dorsal horn recording. Initially, spontaneous activity (SA) was sought during a 3min observation period and recorded for 3–4 min if present.
For both DH and dorsal root recordings, the unit’s receptive field (RF) was identified by low-intensity mechanical stimulation of the glabrous plantar skin of the hind paw with a small glass probe (with a 1 mm round tip), and a von Frey monofilament (29g). Only units with cutaneous rather than deep RFs were used for the study, which was confirmed by the induction of APs when the skin in the RF was gently pinched with forceps. This allows comparison with the pain behaviors we evoked by plantar stimulation, and damage of these cutaneous units has been linked with the development of neuropathic pain [5; 30]. Muscle spindles or joint receptor units that responded to slow extension/flexion of the ankle or knee joint were also excluded.
To characterize response properties, the mechanical threshold was examined with graded von Frey monofilaments. The mechanical threshold was defined as the minimal von Frey hair force that evoked firing.
Chemicals
All drugs were prepared as concentrated stock solutions and stored at −20 or −80°C before use. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX), tetrodotoxin, WIN-55212–2 (WIN), and AM4113 were obtained from Tocris Bioscience. Lidocaine was obtained from Sigma-Aldrich. WIN and AM4113 were dissolved in dimethyl sulfoxide (DMSO). The drugs were diluted to 20% DMSO/normal saline when administered to rats in vivo, as this is the minimum amount of DMSO needed to dissolve AM4113. The 20% DMSO/normal saline mixture did not cause noticeable harm to the mPFC as seen through histological examination (Supple. Fig. 2A, B).
Statistical analysis
Significance testing was performed with SPSS 29 (IBM, SPSS Inc. Chicago, IL), and the figures were prepared with Prism 9 (GraphPad Software, La Jolla, CA). To identify differences induced by two factors, i.e. injury state (SNI vs. Sham) and drug administration (agent vs. vehicle), a 2-way ANOVA design was used to evaluate effects on measured outcomes, with post hoc comparisons using Tukey’s test. Comparisons for repeated measures data were performed by calculating the area under the curve (AUC) with the reference baseline for calculation being the timepoint prior to the intervention. P<0.05 was considered significant. Data are reported as mean ± SEM. For all experiments, detailed statistical data are presented in Supplementary Table. 1.
Results:
Pain-like behaviors are alleviated by the activation of CB1Rs in the mPFC in the chronic phase of neuropathic pain.
In our previous report, we found that mPFC eCB/CB1R signaling was diminished in the chronic phase of neuropathic pain [37], suggesting that activating CB1Rs in this phase of neuropathic pain could provide analgesia. To examine this possibility, we activated CB1Rs in the mPFC during the chronic phase via bilateral infusions of the CB1R agonist WIN (1μg/0.5μl/side) on day 28th post-SNI and assessed sensory behavior responses over the subsequent 3hr period (Fig. 1A). As expected, sham rats with and without WIN administration (Sham+vehicle and Sham+WIN) did not show pain-like behavior phenotypes, while SNI rats without WIN infusions (SNI + vehicle) showed lower thresholds for withdrawal from mechanical stimuli (von Frey) and increased frequencies of hyperalgesic responses to noxious stimuli (pin) (Figs. 1B–E, Supple. Table. 1A and B), indicating a neuropathic pain state. Upon intra-mPFC injection of WIN (SNI+WIN), thresholds for withdrawal from mechanical stimuli increased, which lasted up to 90 min post-injection, and the frequency of hyperalgesic responses to noxious stimuli decreased (peak at 30 min and lasting 60 min post-injection) (Figs. 1B–E, Supple. Table. 1A and B). These findings suggest that activation of CB1Rs in the mPFC in the chronic phase of neuropathic pain can palliatively reverse pain-like behaviors.
Figure 1.
Effects of intra-mPFC injection of CB1R agonist, WIN-55,212–2 in the chronic phase of neuropathic pain on evoked pain-like behaviors. (A) Timeline for the intra-mPFC injection of WIN-55,212–2 (WIN) and sensory behavior tests in rats with SNI. The time course of sensory behavior tests is shown in the left panels and AUC analysis in the right panels of intra-mPFC injection of CB1R agonist, WIN-55,212–2 acutely alleviate mechanical allodynia (von Frey, B, C) and hyperalgesia (pin, D, E) after SNI (n=8 rats/group). Each dot represents one rat. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001 compared to baseline at 0 minute with two-way repeated measure ANOVA followed by Dunnett post hoc analysis. ##p<0.01, ###p<0.001 with two-way repeated measure ANOVA followed by Tukey post hoc analysis.
The incidence of SA in WDR neurons in the spinal cord DH is diminished by intra-mPFC injection of WIN-55,212–2 in the chronic phase of neuropathic pain.
To directly assess the effects of mPFC CB1R activity on the cephalad transmission of neuronal activity in the spinal nociceptive pathway, we recorded activity in DH WDR neurons, which transmit nociceptive information from sensory neurons to the brain [21; 26]. WIN (1μg/0.5μl/side) was injected into the mPFC to activate CB1Rs in that region selectively. During the recording sessions, a second injection into the mPFC was performed 2hr following the first to ensure continued action (Fig. 2A–C). We found that SNI increased the incidence of SA compared to Sham groups, but WIN treatment of SNI animals (SNI+WIN group) reduced the incidence of SA compared to the level of non-injured rats (Fig. 2D, Supple. Table. 1C). There was no difference in SA firing rates across all groups (Fig. 2E). These findings illustrate that intra-mPFC injection of WIN modulates descending control of DH neurons in the chronic phase of neuropathic pain. As the two largest branches of sciatic nerves were cut in rats with SNI, the number of recorded WDR neurons with RF in the hind paw was lower compared to rats that underwent sham injury (Supple. Fig. 3A). The rats used in this study were those that underwent the behavioral tests shown in Figure 1. Three rats died during the laminectomy surgeries.
Figure 2.
Effects of intra-mPFC injection of WIN-55,212–2 on the incidence of SA and SA firing rates of WDR neurons in the spinal cord DH in the chronic phase of pain. (A) Timeline for the intra-mPFC injection of WIN-55,212–2 (WIN) and dorsal horn recording. (B) Time course of intra-mPFC injection of WIN-55,212–2 during dorsal horn recording. (C) In SNI and sham rats, vehicle (saline+DMSO) or WIN-55,212–2 was injected via intra-mPFC, and DH WDR neurons’ activity was recorded. (D) The incidence of SA in recorded WDR neurons was categorized by injury (SNI vs. sham SNI without treatment or with WIN-55,212–2) and averaged for each animal. Each dot represents the number of animals. (E) SA firing rates were recorded in different injury conditions with and without WIN-55,212–2 administration, shown for individual neurons. Each dot represents an individual neuron. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001 compared to SNI+vehicle with two-way repeated measure ANOVA followed by Tukey post hoc analysis.
AP firing of DH WDR neurons evoked by plantar mechanical stimulation is diminished by intra-mPFC injection of WIN-55,212–2 in the chronic phase of neuropathic pain.
We additionally examined the effect of intra-mPFC injection of WIN on evoked AP firing of DH WDR neurons in the chronic phase of neuropathic pain (Fig. 3A–C). We found that the force threshold to induce AP firing of individual neuronal units was reduced in SNI rats, which was reversed by intra-mPFC WIN (Fig. 3D). Also, AP firing rates of DH neurons in response to 8 g, 16 g, and 29 g standard von Frey fibers and 16 g mvF were increased in SNI rats, which was reserved with WIN administration (Fig. 3E–I, Supple. Table. 1D). These findings suggest CB1R activation in the mPFC in the chronic phase of neuropathic pain can acutely suppress spinal cord DH activity via descending control mechanisms. The rats in this study were the same group used for experiments in Figure 2, and only neurons without spontaneous activity were used for this purpose.
Figure 3.
Effects of intra-mPFC injection of WIN-55,212–2 on the firing of DH WDR neurons evoked by plantar mechanical stimulation. (A) Timeline for the intra-mPFC injection of WIN-55,212–2 (WIN) and dorsal horn recording. (B) Time course of intra-mPFC injection of WIN-55,212–2 during dorsal horn recording. (C) In SNI and sham rats, vehicle (saline+DMSO) or WIN-55,212–2 was injected via intra-mPFC as AP firing was evoked by plantar von Frey application, and the activity of WDR neurons was recorded. (D) The force threshold to induce AP firing of individual neuronal units was reduced in rats with SNI, which was reversed in rats with acute intra-mPFC WIN-55,212–2 injection. Effects of treatments on SNI and sham rats on AP firing in response to 8 g (E), 16 g (F), 29 g (G), and noxious or mvF with tungsten tip (H) punctate stimulation. (I) Representative traces show AP firing response to 10s noxious punctate stimulation in SNI+vehicle/mvF (top) and SNI+ WIN-55,212–2/mvF (bottom). Each dot represents an individual neuron. Four rats were used for each of the Sham+Vehicle and Sham+WIN groups. Six and seven rats were used for SNI+Vehicle and SNI+WIN, respectively. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001 with two-way repeated measure ANOVA followed by Tukey post hoc analysis.
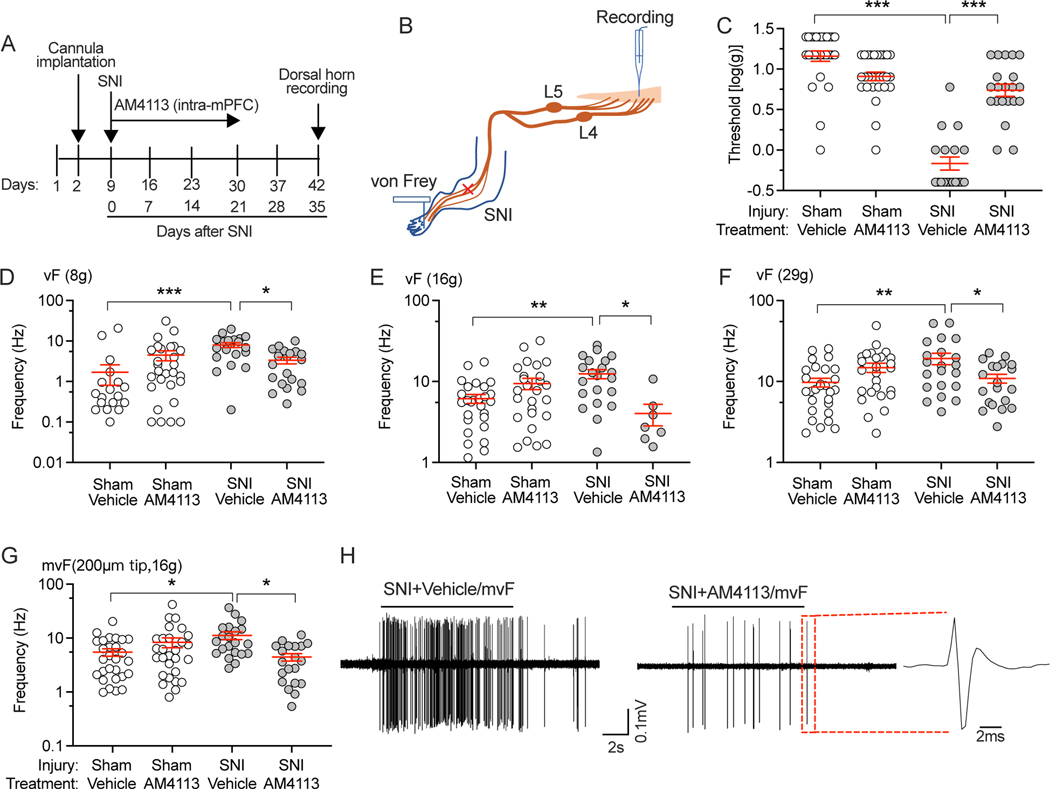
The development of pain-like behaviors is alleviated by the blockade of CB1Rs in the mPFC in the early phase of neuropathic pain.
We have previously found that mPFC eCB/CB1R signaling is activated in the early phase of neuropathic pain [37], which could trigger subsequent suppression of CB1R signaling in the chronic phase. This suggests that blocking CB1R in the early phase of neuropathic pain could prevent later chronic pain, thereby supporting the role of mPFC eCB/CB1R signaling in antinociception. To examine this, we first deactivated CB1Rs in the mPFC by injecting CB1R antagonist AM4113 (1 μg/0.5μl/side, given daily) into the mPFC for 21d following SNI, while continuously assessing the sensory behavior responses up to 35d after SNI (Fig. 4A). We found that sham rats with and without AM4113 administration (Sham+vehicle and Sham+AM4113) didn’t show hypersensitivity to mechanical stimuli (Fig. 4B–I, Supple. Table. 1E and F), whereas rats subjected to SNI without intra-mPFC AM4113 injection (SNI+vehicle) developed a lower threshold for withdrawal from mechanical stimuli (von Frey), increased frequency of hyperalgesic responses to noxious stimuli (pin), and an elevated frequency of withdrawal from cold and acetone (Fig. 4B–I), which characterize a neuropathic pain state. These injury-induced changes were prevented by intra-mPFC injection of CB1R antagonist with AM4113 (SNI+AM4113) during the early phase after SNI (Fig. 4B–I). This alleviation of pain-like behaviors persisted during the 14 days after the termination of AM4113 administration, indicating that blocking mPFC CB1Rs in the early phase of neuropathic pain can prevent the development of pain chronification. The administration of CB1R antagonists has been linked to depression and increased risk of suicide [18; 45]. Moreover, mPFC activity has been shown to impact locomotor activity and aggressive behavior [51]. Hence, there may be potential confounding effects of AM4113 on pain-like behaviors. However, our results show that 21 days of intra-mPFC injection of AM4113 did not affect the locomotor activity or anxiety levels of rats (Supple. Fig. 4A–E).
Figure 4.
Effects of intra-mPFC injection of CB1R antagonist AM4113 in the early phase on evoked pain-like behaviors. (A) Timeline for intra-mPFC injection of AM4113 (1 μg/μl/side) and behavior tests in rats with SNI. Behavioral tests on 7, 14, and 21 days after SNI were performed before the intra-mPFC injection on that day. (A-I) The time course of sensory behavior tests are shown in the left panels, and AUC analysis in the right panels of intra-mPFC injection of CB1R antagonist, AM4113 blocked the development of threshold mechanical stimuli (von Frey, B, C), noxious mechanical stimuli (pin, D, E), brush (F, G), and cold (acetone, H, I) after SNI (n=11 rats/group). Each dot represents one rat. *p<0.05, **p<0.01, ***p<0.001 compared to baseline at 0 minute with two-way repeated measure ANOVA followed by Dunnett post hoc analysis. ##p<0.01, ###p<0.001 with two-way repeated measure ANOVA followed by Tukey post hoc analysis.
As activation of CB1Rs in the late phase of neuropathic pain by SNI can alleviate pain behaviors (Fig. 1), we tested whether agonists of CB1Rs can alleviate pain behaviors in the early phase of SNI when the activity eCB signaling is elevated. We found that intra-mPFC injection WIN didn’t show any effects in the early phase of neuropathic pain by SNI (Supple. Fig. 5A–H).
The incidence of SA in WDR neurons in the spinal cord DH is diminished by intra-mPFC injection of AM4113 in the early phase of neuropathic pain.
To directly examine whether interrupting mPFC eCB/CB1R signaling in the early phase after SNI can preserve normal mPFC eCB regulation of descending pain modulation to the spinal cord, we suppressed CB1R function by daily mPFC injections of AM4113. Then we recorded SA of DH WDR neurons in the chronic phase of pain, after a 2-week washout interval of no injections (Fig. 4A). Recording SA of DH WDR neurons in SNI or sham rats with treatment by either vehicle (saline+DMSO) or AM4113 in the first 3 weeks following SNI or sham surgery (Fig. 5A–B) showed that SNI increased the incidence of SA, but this was prevented by early treatment with mPFC AM4113 (Fig. 5C, Supple. Table. 1G). We observed no difference in SA firing rates across all groups (Fig. 5D). These findings suggest that in the early phase of neuropathic pain, suppression of CB1R activation in the mPFC can preserve descending regulation of pain signaling in the spinal cord, with a persisting effect. The number of recorded WDR neurons with RF in the hind paw was shown in Supple. Fig. 3B. The rats used in this study underwent the behavioral tests shown in Figure 4. Two rats died during the laminectomy surgeries.
Figure 5.
Effects of intra-mPFC injection of CB1R antagonist AM4113 on the incidence of SA and SA firing rates of WDR neurons in the spinal cord DH in the early phase of neuropathic pain. (A) Timeline for intra-mPFC injection of AM4113 and dorsal horn recordings. (B) In SNI or sham SNI rats, vehicle (saline+DMSO) or AM4113 was injected via intra-mPFC, and DH WDR neurons’ activity was recorded. (C) The incidence of SA in recorded WDR neurons was categorized by injury (SNI vs. sham without treatment or with AM4113). Each dot represents the number of animals. (D) SA firing rates were recorded in different injury conditions with and without AM4113. Each dot represents an individual neuron in C. *p<0.05, **p<0.01, ***p<0.001 with two-way repeated measure ANOVA followed by Tukey post hoc analysis.
AP firing of DH WDR neurons evoked by plantar mechanical stimulation is diminished by intra-mPFC injection of AM4113 in the early phase of neuropathic pain.
We next examined the effects of intra-mPFC injection of AM4113 on regulating the threshold of mechanical force necessary to evoke AP firing by DH WDR neurons and their firing rates during suprathreshold mechanical and fully noxious stimulation of receptive fields in the glabrous plantar skin (Fig. 6A, B). The firing threshold in SNI and sham SNI rats with and without AM4113 treatment was determined by the application of graded von Frey fibers for each DH WDR neuron. This threshold was reduced in SNI rats, but this injury-induced change was prevented by intra-mPFC AM4113 administration during the early phase of pain (Fig. 6C, Supple. Table. 1H). Additionally, the AP firing rates of DH neurons in response to 8 g, 16 g, and 29 g standard von Frey fibers and 16 g modified von Frey fiber were increased in SNI rats, which was also reversed by intra-mPFC injection of AM4113 (Fig. 6D–H, Supple. Table. 1H). These findings show that blockade of CB1R in the mPFC in the early phase of neuropathic pain can preserve descending control of the dorsal horn. The rats in this study were the same group used for experiments in Figure 5, and only neurons without spontaneous activity were used for this purpose.
Figure 6.
Effects of intra-mPFC injection of CB1R antagonist AM4113 on the firing of DH WDR neurons evoked by plantar mechanical stimulation. (A) Timeline for intra-mPFC injection of AM4113 and dorsal horn recordings. (B) In SNI and sham rats treated with vehicle (saline+DMSO) or AM3113, AP firing was evoked by plantar von Frey application, and the activity of WDR neurons was recorded. (C) SNI reduced the force that induces single unit AP firing, which was reversed by mPFC AM4113 administration in the early phase of pain. Effects of treatments on SNI and sham SNI rats on AP firing in response to 8 g (D), 16 g (E), 29 g (F), and noxious or mvF with tungsten tip (G) punctate stimulation. (H) Representative traces show AP firing response to 10s noxious punctate stimulation in SNI+vehicle/mvF (left) and SNI+AM4113/mvF (right). Each dot represents an individual neuron. Four rats were used for the Sham+Vehicle group. Six rats were used for each of the other groups. Data represent means ± SEM. *p<0.05, **p<0.01, ***p<0.001 with two-way repeated measure ANOVA followed by Tukey post hoc analysis.
SA in peripheral sensory neurons is reduced after intra-mPFC AM4113 treatment in the early phase of neuropathic pain.
As elevated excitability and SA of peripheral sensory neurons is a key element contributing to the maintenance of chronic pain [11; 38], we asked whether SA of DRG neurons was also changed after the AM4113 treatment in the early phase after SNI. Indeed, we found reduced SA of DRG neurons recorded with teased fiber single unit dorsal root recording in SNI rats treated AM4113 (Fig. 7A, B). Since efferent (retrograde) impulses originating in the DH and traveling to DRG (so-called dorsal root reflex activity) can contribute to pain genesis and inflammatory tissue changes [12; 32; 35; 47; 52], we measured this by recording dorsal root efferent activity. First, dorsal root teased fiber recordings showed increased retrograde SA after SNI, as we have previously observed in a monosodium iodoacetate-induced osteoarthritis pain rat model [12]. This was eliminated by dorsal root entry zone application of either AMPAR blocker (CNQX) or sodium channel blockers (lidocaine, Fig. 7C–G), confirming it as dorsal root reflex activity. Furthermore, mPFC AM4113 in the early phase after SNI reduces the incidence of fibers with this activity (Fig. 7D, Supple. Table. 1I). These findings suggest that blockade of CB1R in the mPFC in the early phase of neuropathic pain can modulate the activity of DRG neurons possibly by descending control of central terminals of DRG neurons to reduce dorsal root reflex activity. Two rats in both the SNI+vehicle and SNI+AM4113 groups were from those tested in the behavioral experiments depicted in Figure 4. The remaining rats were sourced from separate rat cohorts.
Figure 7.
Effects of intra-mPFC injection of CB1R antagonist AM4113 on the firing of DRG neurons. (A) Timeline for intra-mPFC injection of AM4113 and teased dorsal root recordings in rats with SNI. (B) Preparation design and sample trace (bottom) of recorded afferent activity from the L4 dorsal root. (C) Incidence of SA in L4 dorsal root bundles teased from rats with ShamSNI and SNI, with and without AM4113 treatment. ***p<0.01 by two-way ANOVA with Tucky post hoc comparison. (D) Preparation design for recording efferent SA from the L4 dorsal root. (E) Incidence of SA in L4 dorsal root bundles teased from rats with ShamSNI and SNI, with and without AM4113 treatment. Sample traces show spontaneous retrograde firing, which is blocked by lidocaine (F) and CNQX (G) at the dorsal root entry zone. **p<0.01 by two-way ANOVA with Tucky post hoc comparison. Each dot represents one rat in C and E.
In summary, our data support the mechanism outlined in Figure 8: at baseline, ongoing mPFC neuronal activity causes the generation of eCBs that suppress GABA inhibitory input onto pyramidal neurons from local interneurons (Fig. 8A), thus supporting mPFC outflow which maintains a normal descending control (Fig. 8B). When pain begins, harmful stimuli activate the mPFC, leading to an eCB-dependant decrease in GABA release and increased activity of mPFC pyramidal neurons. This increased activity may potentially increase the strength of descending inhibition to suppress/compensate nerve injury-related activation (Fig. 8C). The application of CB1R antagonist to counteract this elevated eCB signaling takes long-lasting analgesic effects in the chronic phase of pain (Fig. 8C). However, strong and consistent activation of CB1R signaling can also lead to the increased inhibitory synaptic transmission through a loss of eCB signaling function. This occurs during the persistent phase of pain, which suppresses mPFC function and reduces descending control (Fig. 8D). In this phase, the agonist of CB1R takes analgesic effects by reducing GABA release, which subsequently increases the activity of mPFC neurons (Fig. 8D).
Figure 8.
Model of mPFC regulation of descending pathway by eCB after pain. (A) The synaptic model shows the physiological functions of eCB signaling. Excitatory axonal terminals release glutamate, which activates metabotropic glutamate receptors (mGluRs), triggering a cascade that activates phospholipase C (PLC) through G-protein. Phospholipase C cleaves phosphatidylinositol 1,4,5-bisphosphate into diacylglycerol and inositol 1,4,5-trisphosphate (IP3). Diacylglycerol is converted into 2-AG by DAGL, which then crosses the synapse and activates CB1 receptors on the inhibitory axon terminal, leading to a decrease in GABA release. (B) At baseline, projections from the mPFC maintain a normal descending control pathway. Ongoing mPFC neuron activity causes the generation of eCBs that suppress GABA inhibitory input from local neurons, thus supporting mPFC activity. (C) Painful injury initially activates the mPFC, causing greater eCB production that further impedes GABAergic inhibition, but also leads to loss-of-function of CB1Rs. The usage of CB1R antagonists is supposed to alleviate this process in this phase. (D) During chronic pain, loss-of-function of CB1Rs breaks the feedback loop, leading to unopposed inhibitory input, suppresses mPFC function, and a shift to depression. The usage of CB1R agonists is supposed to alleviate this process.
Discussion:
In this study, we showed that activation of CB1Rs in the chronic phase after SNI in the mPFC palliatively reversed pain-like behaviors via descending mechanisms. Blocking CB1Rs in the early phase after SNI was found to mitigate pain chronification and supports the idea that the dynamic change of eCB-dependent mPFC activity after SNI plays a role in pain chronification.
New observations suggest the involvement of cortical sites in regulating pain at the dorsal horn level. In the rat tibial nerve transection neuropathic pain model, chemo-inhibition of the preliminary subdivision of mPFC projections to PAG neurons exacerbated pain-like behaviors only in the acute phase (3 days and 7 days post-injury) but not in the chronic phase (after 14 days post-injury) [23]. On the contrary, Opto-activation of the pyramidal neurons in the prelimbic region of mPFC on day 14 post-injury produced antinociception and reduced spinal dorsal horn neuronal activity evoked by plantar mechanical stimulation in neuropathic rats [23]. These results align with our current behaviors data (Fig. 1&4) and in vivo electrophysiological recordings (Fig. 2, 3, 5&6), which support the notion that mPFC’s functional changes of mPFC can modulate descending control of pain. However, our present study further demonstrated the critical role of the eCB signaling system in the mPFC in descending pain modulation.
In the chronic phase of neuropathic pain, the loss of CB1R-dependent disinhibition of the mPFC results in mPFC hypoactivity and the manifestation of chronic pain [37]. This suggests that eCB signaling-mediated mPFC hypoactivity may be a contributing factor to the loss of descending control inhibition and can be remedied by activating CB1Rs. Our study demonstrates that activating CB1Rs in the mPFC during the chronic phase of neuropathic pain can quickly alleviate hypersensitivity to mechanical stimuli (Fig. 1), and spinal cord DH sensitization through descending control mechanisms (Fig. 2&3). These findings suggest that the dynamic change of eCB-dependent mPFC activity following SNI plays a role in pain chronification and that targeting the eCB signaling can treat pain at different states of pain development, albeit with different strategies in each state.
The decline in mPFC activity during chronic pain leads to a loss of descending inhibition in both patients and preclinical pain models [2; 37; 39; 49], while increased PFC activation reduces chronic pain in patients [7; 56] and in rats [23]. There is strong evidence that the mPFC undergoes changes during the transition from acute to chronic pain. Our previous study revealed that these changes in mPFC synaptic function and neuronal activity are regulated by the endocannabinoid (eCB) signaling system [37]. The mPFC is temporarily hyperactive after injury, but then becomes hypoactive during sustained neuropathic pain. Our previous study found that blocking long-term activation of CB1Rs after the injury can prevent mPFC hypoactivity in the chronic phase of the neuropathic pain [37]. Our current study showed that preventing CB1Rs from being overactivated with a blocker in the early phase of neuropathic pain reduced the development of pain-like behaviors in the chronic phase (Fig. 4) through its effect on the activity of DH and DRG neurons (Fig. 5–7). We speculate that the activity and descending control of the mPFC can be maintained by preventing CB1 receptors from being overactivated during the initial stages of neuropathic pain, potentially reducing the progression of chronic pain.
In addition to the well-established DH mechanisms of descending control, we also found that mPFC can control SA of DRG neurons possibly by reducing efferent (retrograde) impulse trains (Fig. 7). The dorsal root reflex processes by which retrograde impulse trains are generated in sensory neurons has been well-delineated [52]. Increased afferent input originating from peripheral sites of injury or inflammation cause dorsal horn release of excitatory neurotransmitters and depolarization of sensory neuron central terminals (i.e., primary afferent depolarization), which in turn generates impulse trains firing antidromically back to the periphery (i.e., dorsal root reflexes), not only in the active afferent but also in neighboring units [52]. This triggers the peripheral release of inflammatory peptides from sensory neurons’ peripheral terminals such as skin, joints, meninges, and viscera [35; 47; 52], and neuronal somata in the DRG [32], which incites inflammatory cascades. We found this efferent activity in the MIA-OA model [12]. Our study revealed that the descending control pathway could regulate efferent activity in sensory neurons, which aligns with prior findings that the PAG could control the dorsal root reflex [42].
The sprouting of postganglionic sympathetic fibers into the DRG after peripheral nerve injury also contributes to SA of sensory neurons, including recently described waves of simultaneously firing DRG neuron somata [58], and resulting spontaneous pain in animal models and in patients after nerve injury [15; 46; 54; 58]. Consistent with this concept, microsympathectomy (cutting segmental grey rami communicans) has analgesic effects in preclinical pain models, and local sympathetic blockade or lesion can treat pain conditions in patients [27; 55; 59]. Peripheral sympathetic neuronal activity is driven by preganglionic neurons of the intermediolateral nucleus, which are regulated by descending control from the rostral ventrolateral medulla and hypothalamus, which are in turn regulated by the mPFC directly or indirectly [36; 43; 48]. We therefore speculate that mPFC activation may be a source of elevated activity of the DRG sympathetic innervation, and thus cause sensory neuron hyperexcitability. Further investigation is necessary to uncover the mechanisms behind this descending control of DRG neuronal activity.
In summary, the present study demonstrates that altering CB1R activity in the mPFC, either by activating Cb1Rs in the chronic phase or blocking them in the early phase, can mitigate pain-like behaviors by modulating descending pain mechanism. This supports the new idea that changes in eCB-dependent mPFC activity play a role in the chronification of pain. Additionally, this study provides evidence that controlling mPFC eCB signaling can affect DRG activity through descending control pathways.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01NS112194 to BP. There are no conflicts of interest to declare.
Data availability statement:
Most of the data used to support the findings of this study are included in this study. Other data are available on request from the corresponding author.
References:
- [1].Alvarado S, Tajerian M, Millecamps M, Suderman M, Stone LS, Szyf M. Peripheral nerve injury is accompanied by chronic transcriptome-wide changes in the mouse prefrontal cortex. Mol Pain 2013;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15(8):1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull 2000;52(5):319–330. [DOI] [PubMed] [Google Scholar]
- [4].Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry 2011;69(12):1133–1139. [DOI] [PubMed] [Google Scholar]
- [5].Boada MD, Gutierrez S, Aschenbrenner CA, Houle TT, Hayashida K, Ririe DG, Eisenach JC. Nerve injury induces a new profile of tactile and mechanical nociceptor input from undamaged peripheral afferents. J Neurophysiol 2015;113(1):100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Borges G, Neto F, Mico JA, Berrocoso E. Reversal of monoarthritis-induced affective disorders by diclofenac in rats. Anesthesiology 2014;120(6):1476–1490. [DOI] [PubMed] [Google Scholar]
- [7].Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, Panetta M, Giglia G, Fierro B. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain 2011;12(2):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brodkin J, Frank D, Grippo R, Hausfater M, Gulinello M, Achterholt N, Gutzen C. Validation and implementation of a novel high-throughput behavioral phenotyping instrument for mice. J Neurosci Methods 2014;224:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 1995;363(4):615–641. [DOI] [PubMed] [Google Scholar]
- [10].Chao D, Mecca CM, Yu G, Segel I, Gold MS, Hogan QH, Pan B. Dorsal root ganglion stimulation of injured sensory neurons in rats rapidly eliminates their spontaneous activity and relieves spontaneous pain. Pain 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chao D, Mecca CM, Yu G, Segel I, Gold MS, Hogan QH, Pan B. Dorsal root ganglion stimulation of injured sensory neurons in rats rapidly eliminates their spontaneous activity and relieves spontaneous pain. Pain 2021;162(12):2917–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chao D, Tran H, Hogan QH, Pan B. Analgesic Dorsal Root Ganglion Field Stimulation Blocks Both Afferent and Efferent Spontaneous Activity in Sensory Neurons of Rats with Monosodium Iodoacetate-induced Osteoarthritis. Osteoarthritis Cartilage 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain 2020;161(12):2872–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [15].Chen SS, Zhang JM. Progress in Sympathetically Mediated Pathological Pain. J Anesth Perioper Med 2015;2(4):216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheriyan J, Sheets PL. Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. J Neurosci 2018;38(20):4829–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994;59(3):369–376. [DOI] [PubMed] [Google Scholar]
- [18].Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 2007;370(9600):1706–1713. [DOI] [PubMed] [Google Scholar]
- [19].Cross L, Brown MW, Aggleton JP, Warburton EC. The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn Mem 2012;20(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000;87(2):149–158. [DOI] [PubMed] [Google Scholar]
- [21].Dougherty PM, Chen J. Relationship of membrane properties, spike burst responses, laminar location, and functional class of dorsal horn neurons recorded in vitro. J Neurophysiol 2016;116(3):1137–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Drake RA, Hulse RP, Lumb BM, Donaldson LF. The degree of acute descending control of spinal nociception in an area of primary hyperalgesia is dependent on the peripheral domain of afferent input. J Physiol 2014;592(16):3611–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Drake RA, Steel KA, Apps R, Lumb BM, Pickering AE. Loss of cortical control over the descending pain modulatory system determines the development of the neuropathic pain state in rats. Elife 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feng Y, Li K, Roth E, Chao D, Mecca CM, Hogan QH, Pawela C, Kwok WM, Camara AKS, Pan B. Repetitive Mild Traumatic Brain Injury in Rats Impairs Cognition, Enhances Prefrontal Cortex Neuronal Activity, and Reduces Pre-synaptic Mitochondrial Function. Front Cell Neurosci 2021;15:689334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fischer G, Pan B, Vilceanu D, Hogan QH, Yu H. Sustained relief of neuropathic pain by AAV-targeted expression of CBD3 peptide in rat dorsal root ganglion. Gene Ther 2014;21(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J Neurosci 2006;26(16):4298–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, Barthel J, Costa B, Graciosa JR, Bruehl S, Reflex Sympathetic Dystrophy Syndrome A. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med 2013;14(2):180–229. [DOI] [PubMed] [Google Scholar]
- [28].Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, Karatsoreos IN, Mackie K, Viau V, Pickel VM, McEwen BS, Liu QS, Gorzalka BB, Hillard CJ. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 2011;31(29):10506–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology 2004;101(2):476–487. [DOI] [PubMed] [Google Scholar]
- [30].Hu P, McLachlan EM. Selective reactions of cutaneous and muscle afferent neurons to peripheral nerve transection in rats. J Neurosci 2003;23(33):10559–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang J, Gadotti VM, Chen L, Souza IA, Huang S, Wang D, Ramakrishnan C, Deisseroth K, Zhang Z, Zamponi GW. A neuronal circuit for activating descending modulation of neuropathic pain. Nat Neurosci 2019;22(10):1659–1668. [DOI] [PubMed] [Google Scholar]
- [32].Huang LY, Neher E. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron 1996;17(1):135–145. [DOI] [PubMed] [Google Scholar]
- [33].Klein JC, Rushworth MF, Behrens TE, Mackay CE, de Crespigny AJ, D’Arceuil H, Johansen-Berg H. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage 2010;51(2):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kummer KK, Mitric M, Kalpachidou T, Kress M. The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. Int J Mol Sci 2020;21(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J Neurophysiol 1999;82(5):2602–2611. [DOI] [PubMed] [Google Scholar]
- [36].McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol 2015;27(6):446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mecca CM, Chao D, Yu G, Feng Y, Segel I, Zhang Z, Rodriguez-Garcia DM, Pawela CP, Hillard CJ, Hogan QH, Pan B. Dynamic Change of Endocannabinoid Signaling in the Medial Prefrontal Cortex Controls the Development of Depression After Neuropathic Pain. J Neurosci 2021;41(35):7492–7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mogil JS. The etiology and symptomatology of spontaneous pain. J Pain 2012;13(10):932–933; discussion 934–935. [DOI] [PubMed] [Google Scholar]
- [39].Ong WY, Stohler CS, Herr DR. Role of the Prefrontal Cortex in Pain Processing. Mol Neurobiol 2019;56(2):1137–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience 2009;161(3):671–679. [DOI] [PubMed] [Google Scholar]
- [41].Paxinos G WC. The rat brain in stereotaxic coordinates. London: Academic Press; 2014. [Google Scholar]
- [42].Peng YB, Wu J, Willis WD, Kenshalo DR. GABA(A) and 5-HT(3) receptors are involved in dorsal root reflexes: possible role in periaqueductal gray descending inhibition. J Neurophysiol 2001;86(1):49–58. [DOI] [PubMed] [Google Scholar]
- [43].Reppucci CJ, Petrovich GD. Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct 2016;221(6):2937–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Saab CY. Pain-related changes in the brain: diagnostic and therapeutic potentials. Trends Neurosci 2012;35(10):629–637. [DOI] [PubMed] [Google Scholar]
- [45].Santucci V, Storme JJ, Soubrie P, Le Fur G. Arousal-enhancing properties of the CB1 cannabinoid receptor antagonist SR 141716A in rats as assessed by electroencephalographic spectral and sleep-waking cycle analysis. Life Sci 1996;58(6):PL103–110. [DOI] [PubMed] [Google Scholar]
- [46].Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol 1999;28(9):743–761. [DOI] [PubMed] [Google Scholar]
- [47].Sorkin LS, Eddinger KA, Woller SA, Yaksh TL. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol 2018;40(3):237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol 2005;481(4):363–376. [DOI] [PubMed] [Google Scholar]
- [49].Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother 2012;12(5):577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev 2004;46(3):295–309. [DOI] [PubMed] [Google Scholar]
- [51].Wei J, Zhong P, Qin L, Tan T, Yan Z. Chemicogenetic Restoration of the Prefrontal Cortex to Amygdala Pathway Ameliorates Stress-Induced Deficits. Cereb Cortex 2018;28(6):1980–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Willis WD Jr., Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 1999;124(4):395–421. [DOI] [PubMed] [Google Scholar]
- [53].Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain 2010;11(3):280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xie W, Strong JA, Li H, Zhang JM. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol 2007;97(1):492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xie W, Strong JA, Zhang JM. Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain 2020;161(8):1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang S, Chang MC. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front Neurol 2020;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain 2022;163(2):e328–e332. [DOI] [PubMed] [Google Scholar]
- [58].Zheng Q, Xie W, Luckemeyer DD, Lay M, Wang XW, Dong X, Limjunyawong N, Ye Y, Zhou FQ, Strong JA, Zhang JM, Dong X. Synchronized cluster firing, a distinct form of sensory neuron activation, drives spontaneous pain. Neuron 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhu X, Xie W, Zhang J, Strong JA, Zhang JM. Sympathectomy decreases pain behaviors and nerve regeneration by downregulating monocyte chemokine CCL2 in dorsal root ganglia in the rat tibial nerve crush model. Pain 2022;163(1):e106–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Most of the data used to support the findings of this study are included in this study. Other data are available on request from the corresponding author.