Abstract
Sugarcane juice is a nutritious and energetic drink. For its processing, the use of supercritical carbon dioxide (SC-CO2) technology as an intervention potentially capable of rendering a high quality product can be considered. This study evaluated the combined effect of SC-CO2 and mild temperatures, primarily aiming for the reduction of endogenous microorganisms and enzymes in sugarcane juice (pH~5.5). Pressures (P) ranging from 74 to 351 bar, temperatures (T) between 33 and 67 °C, and holding times (t) between 20 and 70 min were tested in a central composite rotational design. Seventeen trials were performed, comprising three replicates at the central points. Counts of aerobic mesophiles, molds and yeasts, lactic acid bacteria and coliforms at 45 °C, determination of polyphenol oxidase (PPO) and peroxidase (POD) activities, and measurement of color parameters in freshly extracted and processed juice’s samples were carried out. The pH of fresh and processed juice varied between 4.6 and 6.0, and between 4.6 and 6.3, respectively. The number of decimal reductions achieved in mesophiles, molds and yeasts, lactic acid bacteria and coliforms varied between 0.1 and 3.9, 2.1 and 4.1, 0.0 and 2.1, and 0.3 to 2.5, respectively. The percentages of PPO reduction ranged from 3.51% to 64.18%. Regarding the POD, reductions between 0.27% and 41.42% were obtained. Color variations between fresh and processed samples varied between 2.0 and 12.3. As for mesophiles, molds and yeasts reduction, and soluble solids variation, none of the variables or their interactions were significant. In terms of polyphenol oxidase (PPO) reduction, only t was significant; however, T, t, and the interaction between them significantly affected the peroxidase (POD) reduction. In regards to pH variation, P, and the interaction between T and t were significant. P, T, t, and the interaction between T and t played a significant effect on color. The combination of mild temperatures and SC-CO2 can be potentially used for cane juice preservation.
Subject terms: Technology, Applied microbiology
Introduction
Sugarcane juice is a nutritious and energetic green brownish beverage with low acidity (pH 4.8-5.5) and high water activity (Aw~0.99). The juice’s composition depends on the variety, cultivar, maturation stage, soil, climatic and agricultural conditions. Cane juice has a limited shelf life due to its rapid microbiological and enzymic deterioration1–3, and when processed in industrial plants is subjected to heat treatment in order to inactivate enzymes, spoilage and potentially pathogenic microorganisms. Thermal processing, depending on its intensity, may however damage the sensory, functional and nutritional juice’s quality4. During extraction, the juice is exposed to oxygen, a reactant for enzymic browning, catalyzed by polyphenol oxidase (PPO) and peroxidase (POD), so the enzymic inactivation guarantees a higher quality product1. Additionally, it is necessary to eliminate spoilage microorganisms, such as Leuconostoc mesenteroides, molds and yeasts. L. mesenteroids produces lactic acid and changes the juice’s viscosity during storage1.
The industrialization of cane juice is rapidly growing in Brazil, and over the last few years more than 10 brands of processed cane juice have been launched. The preservation technologies frequently combine acidification, chemical preservatives, heat treatment and refrigeration, and vary among brands. Nevertheless, the sensory quality of the products available on the market is questionable. As an alternative to heat treatment, the technology that employs supercritical carbon dioxide (SC-CO2) is a promising intervention to inactivate pathogens and spoilage enzymes and microorganisms. This non-thermal technique consists of exposing food or beverages to high pressure (beyond 74 bar), and the main advantage is the preservation of the food sensory attributes1. In contrast, conventional heat treatments may trigger the onset of nutritional and sensory losses. A supercritical fluid is defined as any substance maintained above its critical temperature and pressure. The critical temperature is the highest temperature at which a gas can be converted into a liquid by increasing pressure. Critical pressure is the highest pressure at which a liquid can be converted into a gas by increasing the temperature of the liquid5. In SC-CO2 treatment, the food is exposed to pressurized CO2 for a certain period of time. The supercritical fluid diffuses through the food, showing a microbicidal effect, whose intensity depends on the pressure and holding time6–8. The temperature and pressure that characterize the critical state/point of carbon dioxide are 31.1 °C and 73.8 bar4.
Gómez-López et al.9 reported the specific energy required by the pressure change technology (PCT) application. The energy consumption per unit mass of treated product has been estimated and compared to that required by conventional indirect thermal technology. The estimated specific energy consumption was respectively 162.6 kJ/kg for indirect thermal and 26.3 kJ/kg for PCT. On the other hand, completely non-thermal processes such as PCT do not involve any energy costs for product and equipment cooling. Therefore, the energy consumption involved in this technology is only due to product and inert gas pumping and compression. Vignali et al.10 stated a comparison of typical specific working energy costs for thermal and non-thermal treatments in terms of KJ per kilograms of processed product. Non-thermal approaches seem to offer the most effective alternative in terms of nutrients and fresh-like characteristics preservation as well as working energy costs saving. Nevertheless, the SC-CO2 treatment has not been considered in that study because the literature is scarce and the data available for microbial inactivation are very low in comparison to the other technologies. According to Bocker and Silva11, CO2 stands out as the best cost-benefit among supercritical fluids since it has a low cost and is non-toxic. The critical conditions of CO2 (73,8 Bar and 31 °C) are moderate compared to those of other fluids used in the supercritical state. These moderate conditions reduce the process energy expenditure and promote less damage to the nutritional properties of the food matrices.
Many studies address the direct injection application of SC-CO2 in the inactivation of enzymes and microorganisms in fruit juices1,12–14. The SC-CO2 proved to be efficient in the inactivation of microorganisms and enzymes. Additionally, the low toxicity and cost are important advantages of CO2 since it is naturally found in the atmosphere. The use of SC-CO2 under mild conditions is a technique that, when used in juices, allows greater preservation of thermally unstable constituents, such as phenolic compounds, flavonoids and anthocyanins15. Nevertheless, no work targeting the stabilization of SC-CO2-treated cane juice has been found. This study was primarily conducted to evaluate the combined effect of mild temperatures and SC-CO2 on microorganisms and enzymes in cane juice.
Results and discussion
Physicochemical tests
Table 1 exhibits the pH and soluble solids values determined in raw and processed cane juice.
Table 1.
Physicochemical parameters of raw and SC-CO2-treated cane juice.
| Trial | Treatment | pH | Soluble solids (°Brix) |
|---|---|---|---|
| 1 | raw | 5.4 ± 0.1 | 25.3 ± 0.0 |
| 213 bar/50 °C/45 min | 5.3 ± 0.0 | 24.9 ± 0.0 | |
| ∆ | −0.1 | −0.4 | |
| 2 | raw | 5.2 ± 0.1 | 24.2 ± 0.1 |
| 130 bar/40 °C/30 min | 5.0 ± 0.1 | 24.0 ± 0.1 | |
| ∆ | −0.2 | −0.2 | |
| 3 | raw | 4.6 ± 0.0 | 22.1 ± 0.1 |
| 295 bar/40 °C/30 min | 4.6 ± 0.1 | 21.8 ± 0.1 | |
| ∆ | 0.0 | −0.3 | |
| 4 | raw | 5.4 ± 0.1 | 25.2 ± 0.1 |
| 130 bar/60 °C/30 min | 5.1 ± 0.1 | 25.0 ± 0.1 | |
| ∆ | −0.3 | −0.2 | |
| 5 | raw | 5.4 ± 0.1 | 24.3 ± 0.0 |
| 295 bar/60 °C/30 min | 5.2 ± 0.1 | 24.0 ± 0.1 | |
| ∆ | −0.2 | −0.3 | |
| 6 | raw | 5.4 ± 0.0 | 23.5 ± 0.1 |
| 213 bar/50 °C/45 min | 5.4 ± 0.0 | 23.2 ± 0.1 | |
| ∆ | 0.0 | −0.3 | |
| 7 | raw | 5.4 ± 0.1 | 23.6 ± 0.1 |
| 130 bar/40 °C/60 min | 5.0 ± 0.1 | 23.2 ± 0.1 | |
| ∆ | −0.4 | −0.4 | |
| 8 | raw | 5.3 ± 0.1 | 23.7 ± |
| 295 bar/40 °C/60 min | 5.1 ± | 23.6 ± | |
| ∆ | −0.2 | -0.1 | |
| 9 | raw | 4.6 ± 0.1 | 21.4 ± 0.1 |
| 130 bar/60 °C/60 min | 4.4 ± 0.0 | 21.2 ± 0.1 | |
| ∆ | −0.2 | −0.2 | |
| 10 | raw | 5.3 ± 0.1 | 18.7 ± 0.1 |
| 295 bar/60 °C/60 min | 5.5 ± 0.0 | 18.7 ± 0.1 | |
| ∆ | 0.2 | 0.0 | |
| 11 | raw | 5.5 ± 0.1 | 20.6 ± 0.1 |
| 213 bar/50 °C/45 min | 5.6 ± 0.1 | 20.4 ± 0.0 | |
| ∆ | 0.1 | −0.2 | |
| 12 | raw | 6.0 ± 0.1 | 19.0 ± 0.1 |
| 74 bar/ 50 °C/ 45 min | 6.3 ± 0.0 | 18.7 ± 0.1 | |
| ∆ | 0.3 | −0.3 | |
| 13 | raw | 5.6 ± 0.1 | 20.9 ± 0.1 |
| 351 bar/50 °C/45 min | 5.4 ± 0.0 | 20.8 ± 0.1 | |
| ∆ | −0.2 | −0.1 | |
| 14 | raw | 5.4 ± 0.1 | 20.4 ± 0.1 |
| 213 bar/33 °C/45 min | 5.2 ± 0.0 | 20.1 ± 0.1 | |
| ∆ | −0.2 | −0.3 | |
| 15 | raw | 5.6 ± 0.1 | 19.9 ± 0.1 |
| 213 bar/67 °C/45 min | 5.5 ± 0.1 | 19.9 ± 0.0 | |
| ∆ | −0.1 | 0.0 | |
| 16 | raw | 5.6 ± 0.1 | 18.6 ± 0.1 |
| 213 bar/50 °C/20 min | 5.6 ± 0.0 | 18.6 ± 0.1 | |
| ∆ | 0.0 | 0.0 | |
| 17 | raw | 5.3 ± 0.1 | 18.5 ± 0.1 |
| 213 bar/50 °C/70 min | 5.4 ± 0.0 | 18.2 ± 0.1 | |
| ∆ | 0.1 | −0.3 |
Mean values of 3 replicates ± standard deviation.
Δ - variation.
The pH values ranged from 4.6 to 6.0 in the raw juice and between 4.4 and 6.3 for the processed one. The treatments reduced up to 0.4 units in the pH; however, the pH from trials 3 (295 bar/40 °C/30 min), 6 (213 bar/50 °C/45 min) and 16 (213 bar/50 °C/20 min) remained unchanged. The variety of cane, type of soil, fertilization, climatic conditions, degree of maturity, harvesting and extraction methods are important factors to be considered in the variation of juice’s pH. Bomdespacho et al.16 evaluated different cultivars of raw cane juice and reported an average pH equivalent to 5.05. This data is close to the values found in most treatments performed in the present study, with the exception of trials 3, 9 and 12.
Regarding the soluble solids content, variations between 18.5 and 25.3 °Brix were determined in the raw juice, and between 18.2 and 25.0 for the processed beverage. The variations (Δ) in this parameter caused by the treatment ranged between 0.0 and 0.4. With the exception of trials 15 and 16, there was a reduction in this parameter. These phenomena may be related to the variation of the treatments submitted, as well as to the batch used on the day of the respective trials. Bomdespacho et al.16 reported an average of 21.2 °Brix in fresh juice extracted from different cultivars. This result is in the range obtained in this study. In all 17 trials, no meaningful variations (Δ ≤ 0.4) were observed between processed and raw juice. These findings are positive as they lead to the hypothesis that there was no significant difference between the pH values and soluble solids after the treatments applied.
Microbiological assays
Table 2 reports the microbial counts in raw and SC-CO2-treated cane juice as well as the log reduction achieved in each trial.
Table 2.
Microbial counts (logCFU/mL) in raw and SC-CO2-treated cane juice.
| Trial | Treatment | Coliforms (45 °C) | Mesophiles | Lactic bacteria | Molds and yeasts |
|---|---|---|---|---|---|
| raw | 1.5 ± 0.2 | 3.6 ± 0.1 | 1.0 ± 0.0 | 3.5 ± 0.3 | |
| 1 | 213 bar/50 °C/45 min | <1est | 1.8 ± 0.1 | <1est | 1.1 ± 0.1 |
| log red | >0.5 est | 1.8 | - | 2.4 | |
| raw | <1est | 3.1 ± 0.4 | 2.6 ± 0.3 | 3.5 ± 0.3 | |
| 2 | 130 bar/40 °C/30 min | <1est | 2.7 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.2 |
| log red | - | 0.4 | 1.4 | 2.1 | |
| raw | <1est | 4.6 ± 0.3 | 1.3 ± 0.1 | 2.4 ± 0.2 | |
| 3 | 295 bar/40 °C/30 min | <1est | 2.6 ± 0.3 | < 1est | 0.3 ± 01 |
| log red | - | 2.0 | > 0.3 est | 2.1 | |
| raw | 2.5 ± 0.1 | 4.6 ± 0.2 | 3.0 ± 0.2 | 4.2 ± 0.4 | |
| 4 | 130 bar/60 °C/30 min | 0.7 ± 0.2 | 1.6 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| log red | 1.8 | 3.0 | 2.0 | 3.2 | |
| raw | 1.3 ± 0.3 | 3.7 ± 0.1 | 3.1 ± 0.2 | 3.8 ± 0.5 | |
| 5 | 295 bar/60 °C/30 min | <1est | 1.5 ± 0.1 | 1.5 ± 0.2 | 1.6 ± 0.3 |
| log red | >0.3 | 2.2 | 1.6 | 2.2 | |
| raw | <1est | 4.9 ± 0.3 | 3.0 ± 0.3 | 4.1 ± 0.3 | |
| 6 | 213 bar/50 °C/45 min | <1est | 1.7 ± 0.2 | 1.6 ± 0.3 | 0.9 ± 0.2 |
| log red | - | 3.2 | 1.4 | 3.2 | |
| raw | 2.3 ± 0.1 | 2.7 ± 0.2 | 2.2 ± 0.2 | 4.9 ± 0.3 | |
| 7 | 130 bar/40 °C/60 min | <1est | <1est | 2.1 ± 0.2 | 1.7 ± 0.2 |
| log red | >1.3est | >1.7est | 0.1 | 3.2 | |
| raw | <1est | 6.0 ± 0.3 | 3.5 ± 0.2 | 4.1 ± 0.3 | |
| 8 | 295 bar/40 °C/60 min | <1est | 2.1 ± 0.2 | 1.4 ± 0.2 | 1.8 ± 0.2 |
| log red | - | 3.9 | 2.1 | 2.3 | |
| raw | 3.5 ± 0.3 | 4.7 ± 0.3 | < 1est | 5.4 ± 0.2 | |
| 9 | 130 bar/60 °C/60 min | 2.2 ± 0.1 | 1.7 ± 0.1 | < 1est | 2.6 ± 0.1 |
| log red | 1.3 | 3.0 | - | 2.8 | |
| raw | 5.2 ± 0.1 | 3.0 ± 0.1 | 4.0 ± 0.1 | 4.9 ± 0.6 | |
| 10 | 295 bar/60 °C/60 min | 2.9 ± 0.3 | 2.0 ± 0.3 | 2.2 ± 0.1 | 1.5 ± 0.1 |
| log red | 2.3 | 1.0 | 1.8 | 3.4 | |
| raw | 3.9 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.2 | 4.9 ± 0.4 | |
| 11 | 213 bar/50 °C/45 min | 1.4 ± 0.3 | 1.9 ± 0.1 | 0.7 ± 0.1 | 2.7 ± 0.2 |
| log red | 2.5 | 0.1 | 1.4 | 2.2 | |
| raw | 3.3 ± 0.1 | 5.5 ± 0.6 | 0.7 ± 0.2 | 4.8 ± 0.4 | |
| 12 | 74 bar/ 50 °C/ 45 min | 2.3 ± 0.4 | 3.2 ± 0.2 | 0.4 ± 0.2 | 1.9 ± 0.1 |
| log red | 1.0 | 2.3 | 0.3 | 2.9 | |
| raw | 3.4 ± 0.2 | 5.6 ± 0.1 | 3.9 ± 0.2 | 4.2 ± 0.4 | |
| 13 | 351 bar/50 °C/45 min | 2.5 ± 0.2 | 3.6 ± 0.3 | 2.8 ± 0.2 | 1.8 ± 0.3 |
| log red | 0.9 | 2.0 | 1.1 | 2.4 | |
| raw | 3.3 ± 0.1 | 5.6 ± 0.3 | 1.2 ± 0.1 | 5.1 ± 0.5 | |
| 14 | 213 bar/33 °C/45 min | 2.8 ± 0.2 | 2.8 ± 0.1 | < 1est | 2.6 ± 0.3 |
| log red | 0.5 | 2.8 | > 0.2est | 2.5 | |
| raw | 1.8 ± 0.2 | 5.9 ± 0.5 | 2.5 ± 0.4 | 4.7 ± 0.1 | |
| 15 | 213 bar/67 °C/45 min | <1est | 4.1 | < 1est | 0.6 ± |
| log red | >0.8est | 1.8 | 0.0 | 4.1 | |
| raw | 2.8 ± 0.3 | 5.3 ± 0.5 | 3.7 ± 0.1 | 5.3 ± 0.2 | |
| 16 | 213 bar/50 °C/20 min | 0.8 ± 0.1 | 3.2 ± 0.2 | 3.5 ± 0.3 | 1.8 ± 0.2 |
| log red | 2.0 | 2.1 | 0.2 | 3.5 | |
| raw | 2.9 ± 0.2 | 4.8 ± 0.1 | 3.6 ± 0.3 | 4.3 ± 0.2 | |
| 17 | 213 bar/50 °C/70 min | 0.8 ± 0.1 | 2.8 ± 0.2 | 2.8 ± 0.1 | 1.6 ± 0.3 |
| log red | 2.1 | 2.0 | 0.8 | 2.7 |
Mean values of 3 replicates ± standard deviation.
est – estimate count (under detection limit).
The results exhibited in Table 2 show the potential of SC-CO2 in the reduction of contaminants in raw cane juice. The reductions achieved by the different trinomials were 2.5 log for coliforms, 3.9 log for aerobic mesophiles, 2.1 log for lactic acid bacteria, and 4.1 log for molds and yeasts.
The lactic bacteria counts in raw juice varied between 1.0 and 4.0 logCFU/mL. For the processed sample, counts ranged from <1.0est to 3.5 logCFU/mL; comparison with data from other studies was not possible, once counts were carried out after cane fermentation, as reported by Silva et al.17, who performed counts after 3, 11 and 24 h of fermentation. The lactic bacteria contamination, such as Leuconostoc mesenteroides and some species of the Lactobacillus, can trigger the synthesis of dextrans (polysaccharides formed by glucose units) (Koblitz)18, forming gums in the juice, leading to its rejection. The discrepancy among counts within the same group of microorganisms in raw juice may be attributed to failures in the hygiene procedures of the raw material, utensils and/or equipment used in the extraction. This event is usual when it comes to street vending.
According to Prati, Moretti and Cardello19, mesophilic counts above 6.0 logCFU/mL may be related to hygienic-sanitary deficiencies in the extraction and/or storage of cane juice. In this study both the raw and processed juice exhibited counts within the range 1.6–6.0 logCFU/mL. For molds and yeasts, counts were between 2.4 and 5.4 logCFU/mL; Jay20 holds that values above 3.0 logCFU/mL can cause undesirable changes.
The efficiency of SC-CO2 treatment on microbial inactivation is associated with the modification of intracellular and extracellular pH, and also the length of time CO2 diffuses into the cells. Therefore, the holding time of treatment greatly impacts the microbial inactivation rate4.
Dhansu et al.21 pasteurized cane juice at 65 °C/25 min, and stored it under refrigeration, achieving a shelf life of 60 days. Oliveira et al.1 pasteurized the juice at 70 °C/25 min; the lactic acid bacteria counts in raw and processed cane juice were (5.9 and 1.3) logCFU/mL respectively, reaching 4.6 log reduction. The molds and yeasts’ counts in raw and processed juice were (6.1 and 1.7) logCFU/mL respectively. Gomes et al.22 optimized the time x temperature binomial used in the pasteurization of whole cane juice; temperatures and holding times ranging between 78 and 92 °C, and from 16 to 44 s, were tested. Regarding the reduction of microorganisms, the treatment at 90 °C/40 s was the most efficient, achieving 4.6 log reductions for mesophiles. For molds and yeasts, 3.2 log reductions were reached.
Hart et al.23 reported the application of SC-CO2 in the inactivation of spores in foods, highlighting how this technique can be more efficient in preserving nutritional and sensory characteristics as compared to high hydrostatic pressure techniques and thermal methods at high temperatures. The action of SC-CO2 occurs through disruption of the cell wall, coating, cortex and membranes, and degradation of proteins. More in-depth studies on larger scales are needed to disseminate this technology in the processing of fruits and juices.
Eggleston24 reported the microbiological, enzymic and chemical deterioration (acid degradation) of sucrose in cane juice. The findings indicated that the growth of microorganisms is relevant for sucrose degradation. After 14 h, the largest contribution was microbiological, accounting for 93% of losses, while enzymatic degradation contributed with 5.7% of losses and chemical degradation with 1.3%.
Enzymic tests
The endogenous enzymes activity (polyphenol oxidase and peroxidase) as well as the percentages of reduction achieved by different treatments are shown in Table 3.
Table 3.
Polyphenol oxidase (PPO) e peroxidase (POD) activities (U) in raw and SC-CO2-treated cane juice.
| Trial | Treatment | PPO | POD |
|---|---|---|---|
| raw | 11.4 ± 0.0 | 226.7 ± 0.1 | |
| 1 | 213 bar/50 °C/45 min | 7.7 ± 0.2 | 198.0 ± 0.5 |
| red (%) | 32.5 | 12.7 | |
| raw | 11.0 ± 0.5 | 245.1 ± 0.5 | |
| 2 | 130 bar/40 °C/30 min | 5.4 ± 0.5 | 202.2 ± 2 |
| red (%) | 50.9 | 17.5 | |
| raw | 11.0 ± 0.1 | 238.4 ± 1 | |
| 3 | 295 bar/40 °C/30 min | 6.6 ± 0.1 | 202.2 ± 2 |
| red (%) | 40.0 | 15.2 | |
| raw | 11.0 ± 0.1 | 231.6 ± 7 | |
| 4 | 130 bar/60 °C/30 min | 6.1 ± 0.3 | 202.2 ± 2 |
| red (%) | 44.5 | 12.7 | |
| raw | 11.0 ± 0.1 | 202.2 ± 2 | |
| 5 | 295 bar/60 °C/30 min | 3.9 ± 0.0 | 192.9 ± 0.3 |
| red (%) | 64.5 | 4.6 | |
| raw | 11.4 ± 0.0 | 235.6 ± 2 | |
| 6 | 213 bar/50 °C/45 min | 7.7 ± 0.3 | 190.7 ± 0.0 |
| red (%) | 32.5 | 19.1 | |
| raw | 11.4 ± 0.0 | 232.2 ± 6 | |
| 7 | 130 bar/40 °C/60 min | 9.4 ± 0.3 | 183.8 ± 4.1 |
| red (%) | 17.5 | 20.8 | |
| raw | 11.4 ± 0.0 | 231.1 ± 8 | |
| 8 | 295 bar/40 °C/60 min | 8.0 ± 0.2 | 215.1 ± 3 |
| red (%) | 29.8 | 6.9 | |
| raw | 10.0 ± 0.2 | 56.1 ± 4 | |
| 9 | 130 bar/60 °C/60 min | 9.3 ± 2.0 | 34.0 ± 7 |
| red (%) | 7.0 | 39.4 | |
| raw | 3.4 ± 0.6 | 56.1 ± 4 | |
| 10 | 295 bar/60 °C/60 min | 2.5 ± 0.4 | 34.0 ± 7 |
| red (%) | 26.5 | 39.4 | |
| raw | 2.3 ± 0.6 | 8.4 ± 2 | |
| 11 | 213 bar/50 °C/45 min | 0.8 ± 0.4 | 7.2 ± 2 |
| red (%) | 63.2 | 14.3 | |
| raw | 1.8 ± 0.6 | 8.1 ± 2 | |
| 12 | 74 bar/ 50 °C/ 45 min | 0.7 ± 0.4 | 6.7 ± 2 |
| red (%) | 61.1 | 17.3 | |
| raw | 2.4 ± 0.5 | 8.1 ± 0.2 | |
| 13 | 351 bar/50 °C/45 min | 0.9 ± 0.5 | 5.6 ± 2 |
| red (%) | 62.5 | 30.9 | |
| raw | 18.0 ± 0.7 | 7.3 ± 2 | |
| 14 | 213 bar/33 °C/45 min | 17.4 ± 0.5 | 7.2 ± 2 |
| red (%) | 3.3 | 1.4 | |
| raw | 21.9 ± 0.7 | 8.8 ± 2 | |
| 15 | 213 bar/67 °C/45 min | 20.5 ± 0.3 | 5.2 ± 0.1 |
| red (%) | 6.4 | 40.9 | |
| raw | 2.7 ± 0.2 | 7.1 ± 2 | |
| 16 | 213 bar/50 °C/20 min | 1.2 ± 0.0 | 7.1 ± 2 |
| red (%) | 55.6 | 0.0 | |
| raw | 3.4 ± 0.6 | 5.1 ± 0.1 | |
| 17 | 213 bar/50 °C/70 min | 2.1 ± 0.4 | 5.0 ± 0.4 |
| red (%) | 38.2 | 2.0 |
Mean values of 3 replicates ± standard deviation.
Red reduction.
The results exhibited in Table 3 suggest the potential of SC-CO2 combined with mild temperatures to inactivate the endogenous enzymes that are responsible for the degradation of the color, flavor and the nutritional values of cane juice. The percentages of PPO (3.3–64.5%) and POD (0.0–40.9%) reduction varied widely. The trinomial applied in trial 5 (295 bar/60 °C/30 min) reached the greatest PPO inactivation (64.5%), suggesting that temperature had a more significant effect on the percentage of reduction; however, this hypothesis will be confirmed in the light of the statistical analysis of the effects of the variables studied. POD exhibited greater resistance to the treatments in most trials. The trinomial applied in trial 15 (213 bar/67 °C/45 min) reached the highest percentage of inactivation (40.9%). Marszałek et al.12 studied the effect of supercritical carbon dioxide on PPO and POD in mushroom and radish; surprisingly, PPO was more resistant to temperature and pressure than POD. In this study, similar result was observed in trials 7, 9, 10 and 15, i.e., PPO was more resistant to SC-CO2. In most trials, however, the percentage of POD reduction in the juice was lower than PPO. This finding suggests that the type of food matrix also influences the impact of the technology that is applied. The food matrix can interfere with the intermolecular bonds of the two enzymes depending on the amount of water in the medium, since the impact of pressure on intra and intermolecular interactions can also be correlated with the ability of the functional groups of the enzymes to interact with water (Marszałek et al.)25. The deactivation of enzymes exposed to high temperatures and prolonged times is explained by changes in the tertiary and secondary structures of the protein. The thermal stability of enzymes depends on a number of factors such as source, species, nature of the food matrix (Iqbal et al.)26.
Color parameters
The color parameters instrumentally measured in cane juice are presented in Table 4.
Table 4.
Color parameters determined in raw and SC-CO2-treated cane juice.
| Trial | Treatment | L* | a* | b* | chroma | °hue |
|---|---|---|---|---|---|---|
| 1 | raw | 33.67 ± 0.02 | 8.71 ± 0.01 | 29.95 ± 0.02 | 31.2 | 73.8 |
| 213 bar/50 °C/45 min | 37.91 ± 0.01 | 6.25 ± 0.02 | 27.92 ± 0.02 | 28.6 | 77.4 | |
| 2 | raw | 34.17 ± 0.02 | 10.5 ± 0.01 | 32.55 ± 0.01 | 34.2 | 72.1 |
| 130 bar/40 °C/30 min | 35.75 ± 0.03 | 9.37 ± 0.02 | 30.02 ± 0.01 | 31.4 | 72.7 | |
| 3 | raw | 32.26 ± 0.02 | 10.5 ± 0.02 | 26.03 ± 0.01 | 28.1 | 68.1 |
| 295 bar/40 °C/30 min | 34.92 ± 0.01 | 9.02 ± 0.01 | 26.40 ± 0.01 | 27.9 | 71.1 | |
| 4 | raw | 36.75 ± 0.01 | 6.19 ± 0.02 | 36.71 ± 0.03 | 37.2 | 80.4 |
| 130 bar/60 °C/30 min | 37.77 ± 0.02 | 6.27 ± 0.02 | 34.55 ± 0.02 | 35.1 | 79.7 | |
| 5 | raw | 38.93 ± 0.03 | 4.61 ± 0.01 | 35.44 ± 0.05 | 35.7 | 82.6 |
| 295 bar/60 °C/30 min | 44.14 ± 0.02 | 3.96 ± 0.05 | 37.62 ± 0.03 | 37.6 | 84.0 | |
| 6 | raw | 69.03 ± 0.10 | 0.96 ± 0.0 | 36.60 ± 0.02 | 36.6 | 88.5 |
| 213 bar/50 °C/45 min | 63.14 ± 0.02 | 4.62 ± 0.01 | 43.95 ± 0.01 | 44.2 | 84.0 | |
| 7 | raw | 40.67 ± 0.01 | 4.89 ± 0.01 | 38.58 ± 0.02 | 38.9 | 82.8 |
| 130 bar/ 40 °C/ 60 min | 42.55 ± 0.02 | 4.89 ± 0.01 | 37.84 ± 0.01 | 38.2 | 82.6 | |
| 8 | raw | 72.92 ± 0.01 | 1.22 ± 0.02 | 36.71 ± 0.02 | 36.7 | 88.1 |
| 295 bar/40 °C/60 min | 73.06 ± 0.01 | 4.04 ± 0.01 | 42.28 ± 0.02 | 42.5 | 84.5 | |
| 9 | raw | 71.09 ± 0.02 | 2.64 ± 0.04 | 35.90 ± 0.03 | 36.0 | 85.8 |
| 130 bar/60 °C/60 min | 60.91 ± 0.02 | 3.97 ± 0.02 | 39.33 ± 0.02 | 39.5 | 84.2 | |
| 10 | raw | 37.02 ± 0.01 | 16.09 ± 0.0 | 45.84 ± 0.01 | 40.6 | 70.7 |
| 295 bar/60 °C/60 min | 50.14 ± 0.02 | 14.74 ± 0.0 | 50.67 ± 0.02 | 52.8 | 73.8 | |
| 11 | raw | 31.48 ± 0.02 | 4.60 ± 0.03 | 30.29 ± 0.03 | 30.6 | 81.4 |
| 213 bar/50 °C/45 min | 32.53 ± 0.01 | 5.80 ± 0.03 | 24.75 ± 0.02 | 25.4 | 76.8 | |
| 12 | raw | 55.16 ± 0.02 | 5.14 ± 0.02 | 41.59 ± 0.02 | 41.9 | 83.0 |
| 74 bar/ 50 °C/ 45 min | 49.73 ± 0.02 | 6.78 ± 0.02 | 34.34 ± 0.01 | 35.0 | 78.8 | |
| 13 | raw | 49.15 ± 0.01 | 5.65 ± 0.01 | 44.44 ± 0.01 | 44.8 | 82.8 |
| 351 bar/50 °C/45 min | 51.37 ± 0.01 | 4.33 ± 0.02 | 37.39 ± 0.05 | 37.6 | 83.4 | |
| 14 | raw | 60.81 ± 0.02 | 4.94 ± 0.01 | 44.73 ± 0.01 | 44.9 | 83.7 |
| 213 bar/33 °C/45 min | 66.36 ± 0.02 | 4.05 ± 0.04 | 38.75 ± 0.02 | 39.0 | 84.0 | |
| 15 | raw | 58.49 ± 0.01 | 3.05 ± 0.05 | 40.05 ± 0.02 | 40.2 | 85.6 |
| 213 bar/67 °C/45 min | 52.32 ± 0.02 | 4.25 ± 0.03 | 36.51 ± 0.01 | 36.8 | 83.4 | |
| 16 | raw | 52.49 ± 0.03 | 6.53 ± 0.05 | 45.88 ± 0.02 | 46.3 | 81.9 |
| 213 bar/50 °C/20 min | 52.82 ± 0.02 | 6.63 ± 0.04 | 41.09 ± 0.02 | 41.6 | 80.8 | |
| 17 | raw | 63.63 ± 0.01 | 4.84 ± 0.03 | 46.17 ± 0.05 | 46.4 | 84.0 |
| 213 bar/50 °C/70 min | 65.70 ± 0.04 | 4.58 ± 0.02 | 42.14 ± 0.02 | 42.4 | 83.8 |
L* (lightness) = 0 (black). 100 (white). +a* = red. −a* = green. +b* = yellow. −b* = blue.
Mean values of 3 replicates ± standard deviation.
.
The parameter L*, which represents lightness, varied widely for raw (32.3–72.9) and processed (32.5–73.1) juice. Most treatments positively influenced the lightness of the juice samples, which is most likely related to enzymic inactivation (Table 3). This result could favor the juice’s sensory acceptance, assuming the consumers prefer a lighter drink. Similarly, there was a great variation in the a* parameter for fresh (1–16.1) and processed (4.0–14.7) juice. The b* parameter also varied considerably for raw (26.0–46.2) and processed (24.8–50.7) samples. Regarding the chroma parameter (C*), significant variations were also observed for raw (28.1–46.4) and processed (25.4–52.8) juice. Chroma correlates to saturation, characterizing the sample’s color as “vivid” or opaque (dull). This attribute is independent of lightness and °hue. Saturation ranges from purple-red to green, and increases from the center (0) to the edge of the color wheel. Oliveira et al.1 stated that low C* values represent gray, and values close to 60 represent vivid colors; they found C* values close to 9 (more neutral color) for raw cane juice, in contrast to the present study. Meerod27 studied different cultivars of raw material, which showed divergent colors at various levels. Following the same behavior as the previous parameters, the hue angle also showed great variation for raw (68.1–88.5) and processed (71.1–84.5°) juices. Hue, measured in degrees, classifies color (green, yellow, blue, etc.). The ranges determined in this study are positioned in the first quadrant of the color circle, and can be classified between yellow-red and yellow. The wide variation ranges in the color parameters can be explained by the variability inherent to the raw material; juice samples extracted on different days, from different stalks, were used during the course of this research.
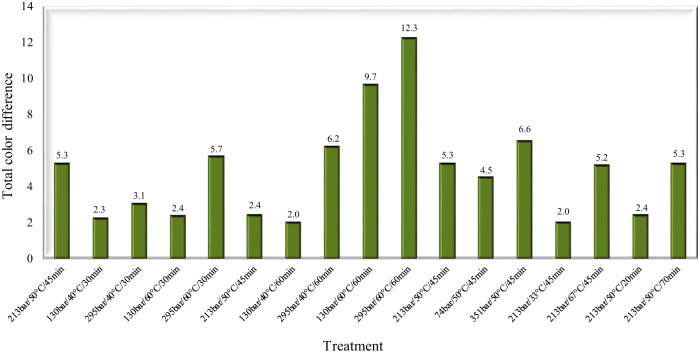
Figure 1 illustrates the total color difference (TCD) between raw and processed cane juice.
Fig. 1.
Total color difference (TCD) between raw and SC-CO2-processed cane juice.
The highest (12.3) and lowest (2.0) values of total color difference (∆E*) were determined for juice treated at 295 bar/ 60 °C/ 60 min and (130 bar/ 40 °C/ 60 min and 213 bar/ 33 °C/45 min), respectively. Bernard et al.28 states that ∆E* values less than 3 cannot be easily detected by the human eye, and values greater than 12 represent different color “spaces”. Therefore, of the 17 tests carried out, only six preserved the original color of the juice, in terms of its sensory perception.
Statistical analysis
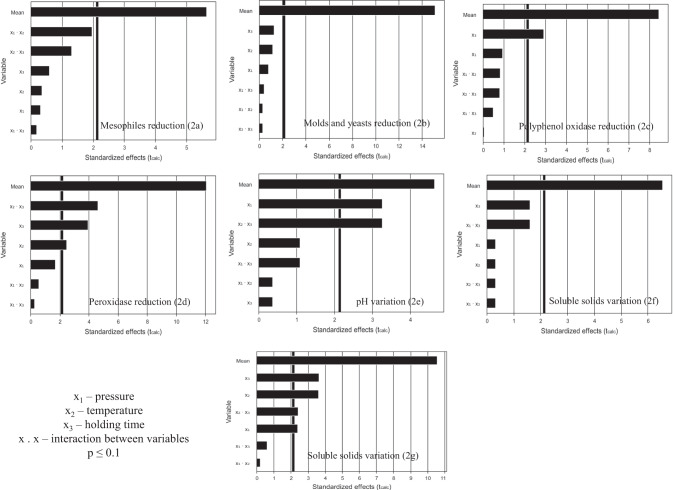
Because the log reduction in coliforms and lactic bacteria could not be calculated in some trials (Table 3), these responses were not subjected to the statistical analysis. Figure 2 demonstrates the Pareto diagrams, built to investigate which parameters/variables (pressure/P/x1, temperature/T/x2, holding time/t/x3) were significant (p ≤ 0.1) in the studied responses. The terms that were not statistically significant were incorporated into the lack-of-fit to calculate the coefficient of determination (R2).
Fig. 2. Pareto diagrams for cane juice treated with SC-CO2.
Pareto diagrams for mesophiles reduction (2a), molds and yeasts reduction (2b), polyphenol oxidase reduction (2c), peroxidase reduction (2d), pH variation (2e), soluble solids variation (2 f) and total color difference (2 g).
As for mesophiles, molds and yeasts reduction, and soluble solids variation, none of variables or their interactions were significant. In terms of polyphenol oxidase (PPO) reduction, only t (x3) was significant; however, the parameters T (x2), t (x3), and the interaction between them (x2.x3) played a significant effect on the peroxidase (POD) reduction. In regards to pH variation, P (x1) and the interaction between T and t (x2.x3) were significant. Finally, P, T, t, and the interaction between T and t were significant in the total color difference.
Only the significant variables were encompassed into the mathematical model, whose statistical significance was evaluated through analysis of variance (ANOVA). Table 5 exhibits the ANOVA carried out for the models (1st and 2nd orders) generated for the responses POD reduction and total color difference (TDC); the models for other responses were not statistically significant (p > 0.1). The coded predicted models obtained for the aforementioned responses are represented by Eqs. 1 and 2.
| 1 |
Table 5.
Analysis of variance (p ≤ 0.1) for peroxidase reduction and total color difference in cane juice.
| Response | Variation source | Sum of squares | Degrees of freedom | Mean square | F value | |
|---|---|---|---|---|---|---|
| Fcalc | Ftab | |||||
| Peroxidase reduction 1st order | Regression | 1112.0 | 3 | 370.7 | 13.8 | 3.1 |
| Residual | 188.4 | 7 | 26.9 | |||
| Total | 1300.4 | |||||
| R2 | 0.86 | |||||
| Peroxidase reduction 2nd order | Regression | 1356.7 | 2 | 678.3 | 6.2 | 2.73 |
| Residual | 1536.4 | 14 | 109.7 | |||
| Total | 2893.1 | |||||
| R2 | 0.47 | |||||
| Total color difference 1st order | Regression | 99.1 | 4 | 24.8 | 13.1 | 3.2 |
| Residual | 11.5 | 6 | 1.9 | |||
| Total | 110.6 | |||||
| R2 | 0.90 | |||||
| Total color difference 2nd order | Regression | 99.8 | 4 | 25.0 | 10.0 | 2.5 |
| Residual | 29.5 | 12 | 2.5 | |||
| Total | 129.3 | |||||
| R2 | 0.77 |
Y1 – POD reduction (%)
x2 – Temperature (T)
x3 – holding time (t)
| 2 |
Y2 – Total color difference
x1 – Pressure (P)
x2 – Temperature (T)
x3 – holding time (t)
For practical purposes, it is desirable that the fitted model be as simple as possible and contain the smallest possible number of parameters without giving up the quality assured in the careful selection of the experimental design. The models herein presented were re-parameterized/reduced because the parameters with little or no influence on the outcome of the final fit were excluded.
Regarding the response POD reduction, Table 5 shows that the 1st order model (R2 = 0.86) better fitted to experimental data than the 2nd order model (R2 = 0.47). For both orders, Fcalc was greater than Ftab. Similarly, as for the total color difference (TCD), the 1st order mathematical model (R2 = 0.90) best fitted to experimental data. The coded Eqs. 1 and 2 can be used to predict the percentage of POD reduction and the TCD that can be achieved in cane juice processed under the same conditions of this study. The coded model is that whose regression coefficients are obtained from the matrix of coded variables (−α, −1, 0, +1, +α). Given this, to obtain a predicted value from the model one must replace the values in the coded equation. In contrast, if using real values for the variables in the model, the predicted value may be incorrect and even absurd. Of particular relevance is the claim that the first order mathematical models hereby presented (Eqs. 1 and 2) are only valid in a range of pressure from 130 to 295 bar, temperature from 40 to 60 min, and holding time between 30 and 60 min (Table 6).
Table 6.
Actual and coded levels tested in the central composite rotational design (CCRD) for cane juice processing with SC-CO2.
| Variable | code | −1.68 (-α) | −1 | 0 | +1 | +1.68 ( + α) |
|---|---|---|---|---|---|---|
| Pressure (bar) | x1 | 74 | 130 | 213 | 295 | 351 |
| Temperature (°C) | x2 | 33 | 40 | 50 | 60 | 67 |
| Time (min) | x3 | 20 | 30 | 45 | 60 | 70 |
(−1.68) lower axial point, (−1) lower level, (0) central point, (+1) upper level, (+1.68) upper axial point. α = (2n)1/4 = 1.68. n = number of variables (3).
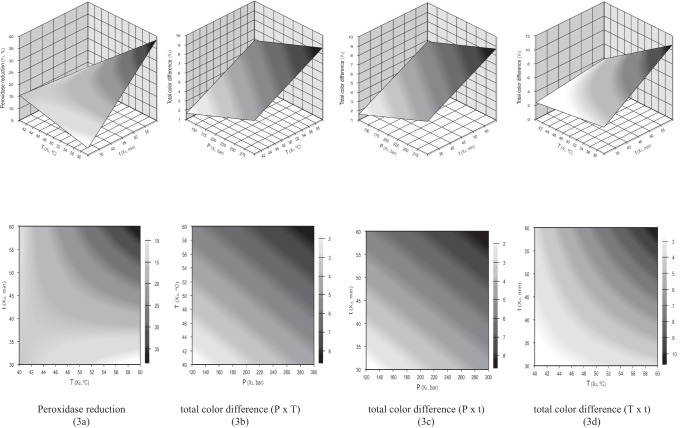
Figure 3 depicts the response surfaces and contour curves that represent Eqs. 1 and 2. By analyzing the surface for POD reduction, one can identify the existence of an optimal range for the temperature (57–60 °C) and holding time (56–60 min), regardless the pressure (in the range 130–295 bar). As for TDC, the ranges 130–150 bar, 40–43 °C and 30–35 min, within which the color difference between raw and processed juice is minimal, represent the optimal conditions in this experiment. This is of much greater interest than a simple point value, because it provides information about the “robustness” of the process, and most notably, it is the variation in pressure, temperature and holding time that may be permitted around optimal values which still maintains the process under optimized conditions. This finding is fundamental for the control engineer to define and maintain the pressure, temperature and time sensors and controller levels. This directly affects viability and process implementation (Rodrigues and Iemma)29.
Fig. 3. Response surfaces and contour curves for peroxidase reduction (3a), and total difference color (3b, 3c, 3d) in cane juice treated with SC-CO2.
Response surfaces.
A fact worth highlighting is that studies addressing the use of SC-CO2 in cane juice processing have not been found. In this way, data comparison could not be made. The combination of supercritical carbon dioxide and mild temperatures exhibited a meaningful effect on microorganism’s reduction in sugarcane juice, under the conditions of this study. Endogenous enzymes that deteriorate the juice’s quality were partially inactivated. None of variables (pressure/P, temperature/T, holding time/t) or their interactions were significant in mesophiles, molds and yeasts reduction, or soluble solids variation. In terms of polyphenol oxidase (PPO) reduction, only t was significant; however, T, t and the interaction between them played a significant effect on the peroxidase (POD) reduction. In regards to pH variation, P and the interaction between T and t were significant. Finally, P, T, t, and the interaction between T and t were significant in the total color difference. The optimal parameters (P, T and t) determined in this study varied for different responses. The combination of mild temperatures and SC-CO2 can be potentially used for cane juice preservation.
Methods
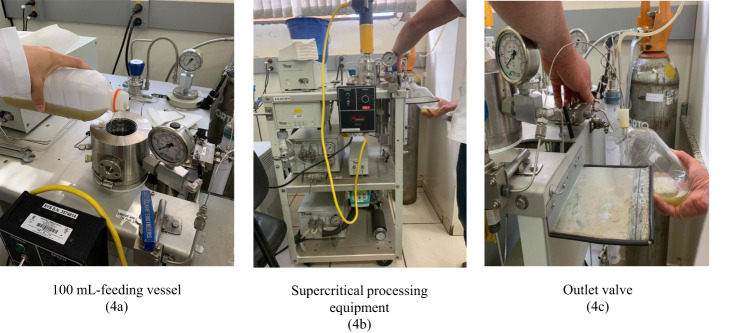
The cane juice was procured from a local vendor, in the city of Pirassununga/SP-Brazil. The freshly extracted juice was collected in a plastic bottle by the vendor, kept on ice in an isothermal container, and rapidly transported to the laboratory in the Food Engineering Department at the University of Sao Paulo. The juice’s sample was divided into two parts and collected in previously sterilized glass bottles with screw caps. One fraction was used as a control (unprocessed juice) and the other one was treated with SC-CO2. Figure 4 illustrates the juice processing.
Fig. 4. Cane juice processing (4a. 100 mL-feeding vessel, 4b. supercritical processing equipment, 4c. outlet valve).
Juice processing.
The treatment of cane juice with direct injection of SC-CO2 was carried out in a 100 mL-reactor of a supercritical fluid system (Thar Technologies SFE-500, Pittsburgh/USA) available at the Laboratory of High Pressure Technology and Natural Products. The juice sample (100 mL) was transferred to the reactor and kept under the pre-set conditions (subsequently described). At the end of the treatment the sample was moved out through rapid depressurization to a sterilized glass flask.
Table 6 points out the independent variables and their actual and coded levels tested according to Rodrigues and Iemma29. Pressures (P) in the range of 74 to 351 bar, temperatures (T) between 33 and 67 °C, and holding times (t) varying from 20 to 70 min were tested in a central composite rotatable design. Seventeen trials were performed. This study aimed at exploring a wide range of CO2 pressure (above the critical one – 73.8 bar). Also the operational limits of the equipment available to conduct this study were considered in the range of the investigated parameters set. Because no study carried out with cane juice was found, no reference is herein mentioned. As for the temperature, mild values were targeted to preserve the original quality of the juice.
To get an approximate statistical inference, three trials were conducted at the central point of the experimental space; they can provide valuable information on the behavior of the responses between the levels attributed to the factors, and demonstrate the repeatability of the process (Rodrigues and Iemma)29.
The physicochemical, enzymic, microbiological analysis and instrumental determination of color parameters were carried out on raw and processed samples to evaluate the performance of multiple combinations of the processing’s parameters (pressure, temperature and holding time) and are as follows. All assays were performed in triplicate as described in Petrus and Simões30.
The physicochemical tests were performed according to the Association of Official Analytical Chemists (AOAC, 2010)31. An Analyzer model 300 M was used to determine the pH. The soluble solids content (expressed in °Brix) was determined in a Reichert model AR 200 portable digital refractometer.
Counts of mesophiles, molds and yeasts, lactic bacteria, and coliforms (at 45 °C) were conducted following the protocol described in the Compendium of Methods for the Microbiological Examination of Foods (Salfinger and Tortorello)32.
The protocols adapted from ref. 33 were used to determine the polyphenol oxidase (PPO) and peroxidase (POD) activities.
Five and half milliliters of 0.2 M phosphate buffer solution (pH 6.0) and 1.5 mL of 0.2 M catechol were added into a test tube and maintained at 25 °C for 10 min. Then 1.0 mL of the diluted sample in deionized water (1:10) was added. The tube was stirred for 15 s and returned to the water bath at 25 °C for 30 min. The absorbance was read in a spectrophotometer at 425 nm. The blank was prepared by diluting the sample in deionized water.
Seven milliliters of 0.2 M phosphate buffer solution (pH 5.5) and 1.0 mL of the diluted sample (juice) in deionized water (1:10) were added to a test tube and maintained in a heat bath at 35 °C for 10 min. Then 1.5 mL of 0.05% guaiacol and 0.5 mL of 0.1% hydrogen peroxide were added. The tube was magnetically stirred for 15 s and returned to the bath at 35 °C for 15 min. Finally, the absorbance was read in a spectrophotometer at 470 nm. One (1) unit of enzyme activity (U) was defined as the amount of enzymic extract capable of increasing absorbance at 425 and 470 nm for PPO and POD, respectively, at rates of 0.001 units per minute.
The color parameters (L*, a* and b*) of unprocessed and treated juice’s samples were measured in a Hunterlab Ultra-Scan colorimeter (Hunter Associates Laboratory, Model SN7877 Reston, VA/USA). The iluminant D65 and observation angle at 10° were set up. The parameters a* and b* were used to determine chroma (C) and hue angle (°hue) (Eq. 3 and 4). To compare the raw juice to the processed one, total color difference (TCD) was calculated by Eq. 5. Also L*, a* and b* were inserted in the EasyRGB to convert them into color image (EasyRGB)34.
| 3 |
| 4 |
| 5 |
L*: lightness (0 a 100).
a*: coordinate red (+60) / green (−60).
B: coordinate yellow (+60) / blue (−60).
ΔL*: lightness variation
Δa*: red/green variation
Δb*: yellow/blue variation
Data from the central composite rotatable design were first subjected to the analysis of effects to identify the variable(s) (P, T and t) that had significant effect on the responses (PPO, POD, coliforms, mesophiles, molds and yeasts, and lactic bacteria reduction, total color difference, pH and soluble solids variation), at 10% of significance. Due to the high variability of processes involving microorganisms and enzymes, p-values below 10 percent (p ⩽ 0.1) are considered significant parameters, as stated by Rodrigues and Iemma (2015). The analysis of regression was performed for both 1st and 2nd (responses including axial points) orders. Then the mathematical model was re-parameterized considering only the statistically significant coefficients. The analysis of variance (ANOVA) was undertaken to evaluate if the model was statistically significant. If so, the response surface was generated. Statistical tests were performed using the software Protimiza Experimental Design (http://experimental-design.protimiza.com.br).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Grateful acknowledgment is made for the grant (2020/08011-5) provided by the Sao Paulo Research Foundation/Brazil (Fundação de Amparo à Pesquisa do Estado de São Paulo), and support offered by the Laboratory of High Pressure Technology and Natural Products.
Author contributions
F.C.P. performed the assays and reviewed the manuscript. T.C.K.M. performed the assays and reviewed the manuscript. G.C.D. reviewed the manuscript. A.L.dO. helped to design the study and reviewed the manuscript. R.P. designed the study and wrote the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41538-023-00242-x.
References
- 1.Oliveira ACG, Spoto MHF, Canniatti-Brazaca SG, Souza CWO, Sousa CP. Percepção dos consumidores sobre o comércio de alimentos de rua e avaliação do teste de mercado do caldo de cana processado e embalado em seis municípios do estado de São Paulo. Rev. Aliment. Nutr. 2007;18:397–403. [Google Scholar]
- 2.Prado SPT, Bergamini AMM. Avaliação do perfil microbiológico e microscópico do caldo de cana in natura comercializado por ambulantes. Rev. Inst. Adolfo Lutz. 2010;69:55–61. doi: 10.53393/rial.2010.v69.32675. [DOI] [Google Scholar]
- 3.Sankhla S, Chaturvedi A, Kuna A, Dhanlakshmi K. Preservation of sugarcane juice using hurdle technology. Sugar Tech. 2012;14:26–39. doi: 10.1007/s12355-011-0127-8. [DOI] [Google Scholar]
- 4.Silva EK, Meireles MAA, Saldana MDA. Supercritical carbon dioxide technology: A promising technique for the non-thermal processing of freshly fruit and vegetable juices. Trends Food Sci. Technol. 2020;97:381–390. doi: 10.1016/j.tifs.2020.01.025. [DOI] [Google Scholar]
- 5.Araújo, J. M. A. Química de Alimentos: teoria e prática 7th edn, Chapter 23 (Ed UFV, 2019).
- 6.Karaman H, Erkmen O. High carbon dioxide pressure inactivation kinetics of Escherichia coli in broth. Food Microbiol. 2001;18:11–16. doi: 10.1006/fmic.2000.0371. [DOI] [Google Scholar]
- 7.Ferrentino G, Balaban MO, Ferrari G, Poletto M. Food treatment with high pressure carbon dioxide: saccharomyces cerevisiae inactivation kinetics expressed as a function of CO2 solubility. J. Supercrit. Fluids. 2010;52:151–160. doi: 10.1016/j.supflu.2009.07.005. [DOI] [Google Scholar]
- 8.Zhang J, et al. Sterilization using high-pressure carbon dioxide. J. Supercrit. Fluids. 2006;38:354–372. doi: 10.1016/j.supflu.2005.05.005. [DOI] [Google Scholar]
- 9.Gómez-López VM, et al. Guidelines on reporting treatment conditions for emerging technologies in food processing. Crit. Rev. Food Sci. Nutr. 2022;62:5925–5949. doi: 10.1080/10408398.2021.1895058. [DOI] [PubMed] [Google Scholar]
- 10.Vignali G, et al. Non-conventional stabilization for fruit and vegetable juices: overview, technological constraints, and energy cost comparison. Food Bioprocess Technol. 2022;15:1729–1747. doi: 10.1007/s11947-022-02772-w. [DOI] [Google Scholar]
- 11.Bocker R, Silva EK. Innovative technologies for manufacturing plant-based non-dairy alternative milk and their impact on nutritional, sensory and safety aspects. Future Foods. 2022;5:1–12. doi: 10.1016/j.fufo.2021.100098. [DOI] [Google Scholar]
- 12.Marszalek K, et al. Enzymatic, physicochemical, nutritional and phytochemical profile changes of apple (Golden Delicious L.) juice under supercritical carbon dioxide and long-term cold storage. Food Chem. 2018;268:279–286. doi: 10.1016/j.foodchem.2018.06.109. [DOI] [PubMed] [Google Scholar]
- 13.Marszalek K, Kruszewski B, Wozniak L, Skapska S. The application of supercritical carbon dioxide for the stabilization of native and commercial polyphenol oxidases and peroxidases in cloudy apple juice (cv. Golden Delicious) Innov. Food Sci. Emerg. Technol. 2017;39:42–48. doi: 10.1016/j.ifset.2016.11.006. [DOI] [Google Scholar]
- 14.Marszalek K, Skapska S, Wozniak L, Sokolowska B. Application of supercritical carbon dioxide for the preservation of strawberry juice: Microbial and physicochemical quality, enzymatic activity and the degradation kinetics of anthocyanins during storage. Innov. Food Sci. Emerg. Technol. 2015;32:101–109. doi: 10.1016/j.ifset.2015.10.005. [DOI] [Google Scholar]
- 15.Jimenez-Sanchez C, Lozano-Sanchez J, Segura-Carretero A, Fernandez-Gutierrez A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017;57:501–523. doi: 10.1080/10408398.2013.867828. [DOI] [PubMed] [Google Scholar]
- 16.Bomdespacho LD, da Silva BTR, Lapa-Guimaraes J, Ditchfield C, Petrus RR. Cultivar affects the color change kinetics of sugarcane juice. Food Sci. Technol. 2018;38:96–102. doi: 10.1590/fst.15017. [DOI] [Google Scholar]
- 17.Silva DA, Martins LTS, Sorares LS. Microbiological evaluation of cane juice marketed by street vendors and formal establishments in Salvador, BA. Hig. Aliment. 2018;32:80–84. [Google Scholar]
- 18.Koblitz, M. G. B. Bioquímica dos Alimentos - Teoria e Aplicações Práticas, 2nd ed. (Guanabara Koogan, 2019).
- 19.Patricia P, Hermínio MR, Cardello BHMA. Elaboration of beverage composed by blends of clarified-stabilized sugar cane and juice’s acid fruits. Food Sci. Technol. 2005;25:147–152. [Google Scholar]
- 20.JAY, J. M. Do background microorganisms play a role in fresh food safety? Trends Food Sci. Technol.8. 10.1016/S0924-2244(97)01093-5 (1997).
- 21.Dhansu Pooja, et al. Different treatments for sugarcane juice preservation. Foods. 2023;2:311. doi: 10.3390/foods12020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Lima JP, et al. Sugarcane juice pasteurization: a search for the most effective parameters. Food Process. Preserv. Mag. 2020;44:e14842. [Google Scholar]
- 23.Hart A, et al. Application of supercritical fluid carbon dioxide in improving food shelf-life and safety by inactivating spores: a review. J. Food Sci. Technol. 2022;59:417–428. doi: 10.1007/s13197-021-05022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eggleston G. Deterioration of cane juice - sources and indicators. Food Chem. 2002;78:95–103. doi: 10.1016/S0308-8146(01)00390-9. [DOI] [Google Scholar]
- 25.Marszałek Krystian, et al. Comparative effect of supercritical carbon dioxide and high pressure processing on structural changes and loss of redox enzyme activity. J. CO2 Utilization. 2019;29:46–56. doi: 10.1016/j.jcou.2018.11.007. [DOI] [Google Scholar]
- 26.Iqbal A, et al. Activation and inactivation mechanisms of polyphenol oxidase during thermal and non-thermal methods of food processing. Food Bioproducts Process. 2019;117:2019. doi: 10.1016/j.fbp.2019.07.006. [DOI] [Google Scholar]
- 27.Meerod K, Weerawatanakorn M, Pansak W. Effect of liming process on physicochemical properties and phytochemical components of non-centrifugal sugar from different sugarcane cultivars. Agric. Res. 2020;9:35–45. doi: 10.1007/s40003-019-00409-7. [DOI] [Google Scholar]
- 28.Bernat N, et al. Hazelnut milk fermentation using probiotic Lactobacillus rhamnosus GG and inulin. Int. J. Food Sci. Technol. Oxford. 2014;49:2553–2562. doi: 10.1111/ijfs.12585. [DOI] [Google Scholar]
- 29.Rodrigues, M. I., Iemma, A. F. Experimental Design and Process Optimization (Taylor and Francis Group, 2015).
- 30.Petrus, R. R., Simões, B. S. (2021). Organic acid adaptations in sugarcane juice. Avanços em Ciência e Tecnologia de Alimentos Vol. 5. (Cientifica, 2021). 10.37885/210906208.
- 31.Association of Official Analytical Chemists - AOAC. Official methods of analysis of AOAC International (18th ed.) (AOAC International, 2010).
- 32.Salfinger, Y., Tortorello, M. L. (Ed). Compendium of methods for microbiological examination of foods (American Public Health Association, 2015).
- 33.Campos CF, Souza PEA, Coelho JV, Gloria MBA. Chemical composition, enzyme activity and effect of enzyme inactivation on flavor quality of green coconut water. J. Food Process. Preserv. 1996;20:487–500. doi: 10.1111/j.1745-4549.1996.tb00761.x. [DOI] [Google Scholar]
- 34.EASYRGB. Color calculator. Retrieved from http://easyrgb.com/index.php?X=CALC. Accessed June 6, (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.