Abstract
Purpose
Adolescent and young adult cancer survivors (AYAs) are at increased risk of long-term and late effects, and experience unmet needs, impacting their health-related quality of life (HRQoL). In order to provide and optimize supportive care and targeted interventions for this unique population, it is important to study HRQoL factors’ interconnectedness on a population level. Therefore, this network analysis was performed with the aim to explore the interconnectedness between HRQoL factors, in the analysis described as nodes, among long-term AYAs.
Methods
This population-based cohort study used cross-sectional survey data of long-term AYAs, who were identified by the Netherlands Cancer Registry (NCR). Participants completed a one-time survey (SURVAYA study), including the EORTC survivorship questionnaire (QLQ-SURV111) to assess their long-term HRQoL outcomes and sociodemographic characteristics. The NCR provided the clinical data. Descriptive statistics and a network analysis, including network clustering, were performed.
Results
In total, 3596 AYAs (on average 12.4 years post diagnosis) were included in our network analysis. The network was proven stable and reliable and, in total, four clusters were identified, including a worriment, daily functioning, psychological, and sexual cluster. Negative health outlook, part of the worriment cluster, was the node with the highest strength and its partial correlation with health distress was significantly different from all other partial correlations.
Conclusion
This study shows the results of a stable and reliable network analysis based on HRQoL data of long-term AYAs, and identified nodes, correlations, and clusters that could be intervened on to improve the HRQoL outcomes of AYAs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-023-08295-0.
Keywords: Adolescents and young adults, Cancer, Survivorship, Network analysis, HRQoL, Questionnaire study
Introduction
Adolescent and young adult cancer survivors (AYAs), those aged 15–39 years at initial cancer diagnosis, are considered a unique group with age-specific challenges, from cancer diagnosis until end of life [1, 2]. This age range is, however, flexibly applied depending on the research question of interest, country, and health care system [3]. In the Netherlands, pediatric oncology is centralized and includes patients until 18 years of age at diagnosis. The Dutch AYA definition applies to all cancer patients initially diagnosed between 18 and 39 years. As the overall cancer incidence of AYAs has increased over the last decades and the 5-year relative survival is now exceeding 80%, a large part of this growing population will eventually become long-term survivors [4]. As a result of the cancer diagnosis and treatment, these AYAs are at increased risk of long-term (e.g., infertility) and late effects (e.g., secondary malignancies), and experience unmet (age-specific) needs related to finances and mental health for example [5–7]. AYAs are in a particularly exposed position for these risk factors due to their often invasive and long-lasting treatments [8], and being diagnosed during a complex phase of life, including many physical, emotional, and social transitions [9].
AYAs can have a long life ahead in which suffering from these long-term and late effects can have a significant impact on their health-related quality of life (HRQoL). HRQoL is defined by the survivor’s own perception of one’s health or well-being, including physical, mental, and social aspects [10]. Several studies have focused on the HRQoL issues among AYAs: literature shows that AYAs are at increased risk of fatigue [11], cognitive impairment [12], work and financial problems [12, 13], psychological distress [14], and body image issues [15], which can result in a diminished HRQoL. In addition, studies described impacted physical health and functioning [16–19], and lower mental health [17–19] in AYAs compared to the general population/older cancer survivors [19]. In line, the systematic review of Quinn showed that AYAs are more likely to have impaired HRQoL compared to the general population, although QoL was difficult to measure due to their age-specific needs [20].
Many factors can independently impact HRQoL, yet they often co-occur in cancer patients [21–25]. To study the interconnectedness between these factors, a network analysis can be performed. It provides insight into the relationships among symptoms, risk factors, and protective factors. Network approaches involve the identification of symptoms and factors (network nodes) and the relations among them (positive or negative associations between nodes) [26]. Taking into account the dependence of factors, this type of analysis is more likely reflecting reality compared to focusing on these factors independently [27].
Although network analyses have been performed previously among cancer survivors in general [27–29], they are lacking among AYAs, especially when focusing on HRQoL outcomes. Gaining these insights is of importance to optimize supportive care and provide targeted interventions for this unique long-term surviving population. Within a large population-based sample of long-term AYAs, using an exploratory approach, we want to (1) assess HRQoL in long-term AYAs, (2) identify the most central nodes in a HRQoL network, and (3) determine how these nodes are linked to one another ((strengths of) interconnectedness).
Methods
Study population and data collection
Data of the population-based, cross-sectional SURVAYA study was used, which was approved by the Institutional Review Board (IRBd18122) and registered within clinical trial registration (NCT05379387). The study population is extensively described previously [30]. In short, the SURVAYA study was performed among AYAs (18–39 years old at time of initial cancer diagnosis) diagnosed with cancer between 1999 and 2015 (5–20 years post diagnosis at study invitation), treated at the Netherlands Cancer Institute or one of the University Medical Centers in the Netherlands, and registered within the Netherlands Cancer Registry (NCR). Survivors were invited to complete a one-time questionnaire within PROFILES (Patient Reported Outcomes Following Initial treatment and Long-term Evaluation of Survivorship) [31].
Measures
Sociodemographic characteristics were obtained through a one-time questionnaire including age at time of questionnaire, sex at birth, marital status, and educational level. Clinical characteristics, obtained by the NCR, include tumor type, stage, primary treatment received, and time since diagnosis. Tumor type was classified according to the third International Classification of Diseases for Oncology (ICDO-3) [32]. Cancer stage was classified according to TNM or Ann Arbor Code (Hodgkin lymphoma and Non-Hodgkin lymphoma) [33].
The EORTC QLQ-SURV111 [34], a cancer core survivorship questionnaire that is currently being developed by the European Organization for Research and Treatment of Cancer (EORTC), was used for our network analysis. This questionnaire assesses long-term HRQoL outcomes, including physical, mental, and social HRQoL issues specifically relevant to cancer survivors. We selected 8 functioning scales (physical functioning, cognitive functioning, emotional functioning, role functioning, body image, symptom awareness, sexual functioning, and overall quality of life), 9 symptoms scales (fatigue, sleep problems, pain, social interference, health distress, negative health outlook, social isolation, symptom checklist, and sexual problems), and 3 single items (financial difficulties, worry cancer risk family, and treated differently). Scales and items measuring positive factors of HRQoL and items that were not applicable for all participants were excluded from the analysis. The rationale for this selection is that including these positive scales/items and optional items would result in difficulties with respectively interpreting network associations to intervene on and the development of a network structure. Participants scored the items on a 4-point Likert scale from 1 (not at all) to 4 (very much). Overall quality of life scores ranged from 1 (very poor) to 7 (excellent). All scales and single items were linearly transformed to a “0–100” scale [35]. A higher score on the functioning scales indicates better HRQoL/functioning, while a higher score for symptoms indicates more complaints. For our analysis, we transformed the scores of the symptom scales once again, so a higher score on the symptom scale indicates fewer complaints. Now, a higher score on all scales corresponds with better functioning, fewer complaints, and a better overall quality of life.
Data analysis
Data were analyzed using SPSS Statistics (IBM Corporation, version 26.0, Armonk, NY, USA) and R version 4.2.1 packages MVN, huge, qgraph, and bootnet. Descriptive statistics were calculated and presented as frequencies, percentages, means, and standard deviations.
We used listwise deletion to exclude participants with missing items on the EORTC QLQ-SURV111 questionnaire, except for the items related to sexuality. This is because sexual functioning and problems can be an important aspect of HRQoL, and missing responses to sensitive questions like sexuality are common [36]. If half of the items from the two sexuality scales were answered, we assumed that the missing item had a value equal to the item that was present for that respondent according to the QLQ-SURV111 scoring manual. In case both items for the scale were missing, we used a copy mean imputation of the study population.
We assessed the assumption of multivariate normality with Mardia’s test [37], which needs to be fulfilled prior to estimating the network [38]. Mardia’s multivariate skewness and kurtosis coefficients of the numeric scales were calculated [37]. In the case of multivariate normality, both p-values of skewness and kurtosis should be greater than 0.05 [37]. As the data were not multivariate normally distributed according to Mardia’s test (p<0.05), a nonparanormal transformation was applied to relax the normality assumption [39].
In our network model, HRQoL is conceptualized as a network of mutually interrelated factors. Because data are continuous scales or items, we used Guassian Graphical model (GGM) [26, 40]. Nodes represent the selected HRQoL scales and items, and edges (links connecting two nodes) represent the regularized partial correlation coefficients after controlling for all other nodes. The thickness of the edge visualizes the strength, and the color a positive (red) or negative (blue) partial correlation. The partial correlation is indicated as very small (r<0.1), small (0.1≤r<0.3), moderate (0.3≤r<0.5), and large (r>0.5) [41].
We applied graphical lasso tuned with the Extended Bayesian Information Criterion (EBIC) [38, 42]. The EBIC hyperparameter, used to set the preferred simplicity of the model, was set to 0.5 to minimize spurious connections [38]. Graphical lasso is a form of lasso regularization to prevent that edges between two nodes are spurious because of other nodes (i.e., conditional independence association) and small edges were shrinked to zero by dropping them from the model [42]. In this way, the estimated network is not over fitted and interpretable.
We estimated node strength (i.e., number and strength of edges between nodes), betweenness (i.e., how often a node lies in shortest path between any combination of two nodes), and closeness (i.e., average distance from one node to all other nodes, which indicates how fast a node can be reached), which are indices of node centrality [38, 43].
Bootstrapping was performed to explore the accuracy and stability of the network [38]. To estimate the accuracy of edge weights, 95% bootstrapped confidence intervals (CIs) around each edge in the network were calculated. Non-parametric bootstrapping (1000 bootstrap samples) was used to construct CIs. To estimate the stability of node centrality, we applied case-dropping bootstrap (1000 bootstrap samples) to calculate the correlational stability coefficient (CS coefficient) [38]. This coefficient represents the maximum proportion of participants that can be dropped from the analysis with the correlation between the original centrality indices and the subset centrality indices of at least 0.7 with 95% probability [44]. The CS coefficients of at least above 0.25, but preferable above 0.5, are considered stable [44]. Additionally, the bootstrapped values were used to test the significance of edge weights and node strength [38]. These bootstrapped difference tests indicate the difference between two different edge weights or node strengths. A bootstrapped CI around these difference scores was calculated [38].
Detection of communities was performed using the Louvain clustering method, a hierarchical clustering method based on multi-level modularity optimization algorithm [45].
Results
Characteristics AYA cancer survivors
In total, 11296 AYAs were invited to participate in the study, of whom 4010 (36%) responded. After excluding 414 records with missing data, we included 3596 AYAs in our final analysis; their sociodemographic and clinical characteristics are described in Table 1. AYAs were on average 31.5 years old at diagnosis and mostly female (61%). The average time since diagnosis was 12.4 years and the most common cancer types were breast cancer (24%), germ cell tumors (18%), lymphoid hematological malignancies (15%), and tumors of female genitalia (11%).
Table 1.
Sociodemographic and clinical characteristics of the study cohort
| Respondents N = 3596 | |||
|---|---|---|---|
| n | % | ||
| Gender | Male | 1420 | 39 |
| Female | 2176 | 61 | |
| Age at diagnosis mean (SD) | 31.5 (5.9) | ||
| 18–24 years | 569 | 16 | |
| 25–34 years | 1584 | 44 | |
| 35–39 years | 1443 | 40 | |
| Age at completing questionnaire mean (SD) | 44.5 (7.5) | ||
| Time since diagnosis | 12.4 (4.5) | ||
| 5–10 years | 1452 | 40 | |
| 11–15 years | 1247 | 35 | |
| 16–20 years | 897 | 25 | |
| Type of cancer | Breast cancer | 846 | 23.5 |
| Germ cell tumors | 637 | 17.7 | |
| Lymphoid hematological malignancies | 540 | 15.0 | |
| Female genitalia tumors | 383 | 10.7 | |
| Melanoma | 252 | 7.0 | |
| Thyroid cancer | 224 | 6.2 | |
| Bone or soft tissue sarcoma | 161 | 4.5 | |
| Myeloid hematological malignancies | 136 | 3.8 | |
| Central nervous system tumors | 131 | 3.6 | |
| Head and neck cancer | 107 | 3.0 | |
| Digestive tract tumors | 104 | 2.9 | |
| Other* | 75 | 2.1 | |
| Tumor stage | I | 1537 | 42.7 |
| II | 957 | 26.6 | |
| III | 515 | 14.3 | |
| IV | 165 | 4.6 | |
| Missing | 422 | 11.7 | |
| Primary treatment modality | Surgery | 2799 | 77.8 |
| Chemotherapy | 2030 | 56.5 | |
| Radiotherapy | 1716 | 47.7 | |
| Hormonal therapy | 435 | 12.1 | |
| Targeted therapy | 282 | 7.8 | |
| Stem cell therapy | 130 | 3.6 | |
| Marital status (at time of questionnaire) | In a relation | 3001 | 83.5 |
| Educational level | Primary school or equivalent | 16 | 0.4 |
| Secondary school or equivalent | 1514 | 42.1 | |
| College/university or equivalent | 2060 | 57.3 | |
| Missing | 6 | 0.2 | |
*Other includes urinary tract, respiratory tract, male genitalia, neuroblastoma, adrenal, paraganglioma, and eyes
The mean scores of the functioning and symptom scales of the QLQ-SURV111 are shown in Table 2. The overall global quality of life score of AYAs was on average 77.3 (SD 18.7). The functioning scale with the highest score was physical functioning (mean 91.7; SD 13.9), whereas sexual functioning was the scale with the lowest score (mean 43.7; SD 25.5). AYAs scored the lowest on the symptom scales social isolation (mean 69.1; SD 30.7), fatigue (mean 70.3; SD 26.0), and negative health outlook (mean 74.2; SD 20.0).
Table 2.
The long-term HRQoL outcomes from the cancer survivorship core questionnaire (QLQ-SURV111) of the AYA cancer survivors, arranged by scale and total score mean
| Scales | Short names | Functional/symptom | Number of items (n) | Item numbers | Raw scores mean (SD) |
Total scores mean (SD) |
|---|---|---|---|---|---|---|
| Physical functioning | PF | Functional | 5 | 1–5 | 1.2 (0.4) | 91.7 (13.9) |
| Role functioning | RF | Functional | 3 | 69–71 | 1.5 (0.7) | 83.7 (24.5) |
| Emotional functioning | EF | Functional | 7 | 52–58 | 1.6 (0.6) | 81.0 (19.9) |
| Cognitive functioning | CF | Functional | 4 | 47, 48, 50, 51 | 1.6 (0.7) | 79.6 (22.6) |
| Body image | BI | Functional | 2 | 40, 41 | 1.7 (0.7) | 77.8 (24.6) |
| Overall quality of life | QL | Functional | 1 | 121 | 5.6 (1.1) | 77.3 (18.7) |
| Symptom awareness | SA | Functional | 2 | 76, 77 | 2.2 (0.8) | 60.9 (25.4) |
| Sexual functioning | SF | Functional | 2 | 111, 112 | 2.3 (0.8) | 43.7 (25.5) |
| Financial difficulties | FD | Symptom | 1 | 68 | 1.3 (0.7) | 89.4 (23.8) |
| Social interference | Sif | Symptom | 2 | 73, 74 | 1.4 (0.7) | 88.1 (22.0) |
| Worry cancer risk family | WF | Symptom | 1 | 61 | 1.4 (0.7) | 86.6 (23.1) |
| Symptom checklist | SC | Symptom | 17 | 20, 21, 23–26, 28, 30–38, 75 | 1.4 (0.4) | 85.8 (13.5) |
| Treated differently | TD | Symptom | 1 | 107 | 1.4 (0.7) | 85.2 (22.6) |
| Pain | PA | Symptom | 2 | 22, 72 | 1.5 (0.7) | 84.2 (23.0) |
| Sexual problems | SP | Symptom | 2 | 113, 117 | 1.5 (0.8) | 82.8 (26.3) |
| Health distress | HD | Symptom | 3 | 63–65 | 1.6 (0.7) | 79.0 (22.3) |
| Sleep problems | SL | Symptom | 4 | 16–19 | 1.8 (0.7) | 74.5 (23.6) |
| Negative health outlook | NHO | Symptom | 7 | 60, 62, 64, 81, 82, 98, 99 | 1.8 (0.6) | 74.2 (20.0) |
| Fatigue | FA | Symptom | 4 | 6–9 | 1.9 (0.8) | 70.3 (26.0) |
| Social isolation | SI | Symptom | 2 | 89, 90 | 1.9 (0.9) | 69.1 (30.7) |
Network analysis
Overall network
The partial correlation network model is shown in Fig. 1. In our sample, health distress had a strong partial correlation with negative health outlook (r = 0.71) and moderate partial correlation with worries about family getting cancer (r =0.48). Symptom checklist had a strong partial correlation with pain (r =0.67). Role functioning was strongly partially correlated to physical functioning (r =0.70) and to social interference (r =0.72), and there was a moderate partial correlation between sexual functioning and sexual problems (r =0.43).
Fig. 1.
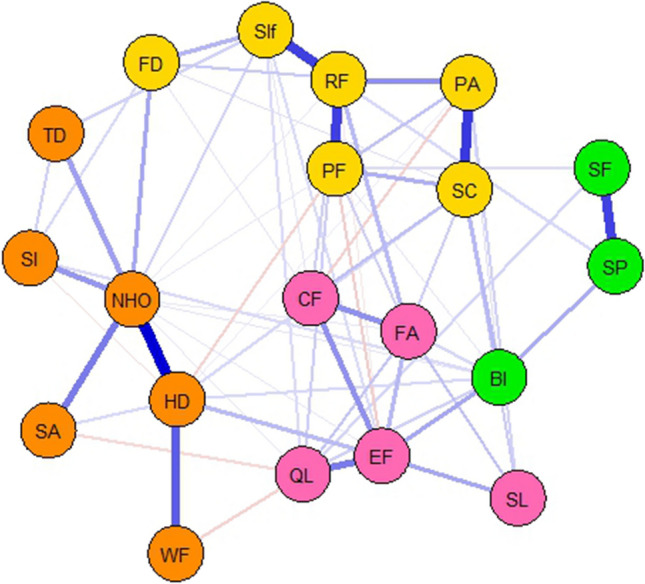
The (cluster) network of HRQoL outcomes of long-term AYA cancer survivors. In this partial correlation network model, the nodes (PF: physical functioning, CF: cognitive functioning, EF: emotional functioning, RF: role functioning, BI: body image, SA: symptom awareness, SF: sexual functioning, FA: fatigue, SL: sleep problems, PA: pain, Sif: social interference, HD: health distress, NHO: negative health outlook, SI: social isolation, SC: symptom checklist, SP: sexual problems, FD: financial difficulties, WF: worried about family getting cancer, TD: people treating you differently, QL: overall quality of life) represent all the HRQoL scales of the QLQ-SURV111 and the edges (links connecting two nodes) represent the regularized partial correlation coefficients after controlling for all other nodes. The blue color indicates a positive partial correlation and a red color a negative partial correlation between two nodes. The thickness of the edge visualizes the strength of the partial correlation between two nodes. The four clusters that we identified within our network model include in orange the worriment cluster, in yellow the daily functioning cluster, in pink the psychological cluster, and in green the sexual cluster
The bootstrapped confidence intervals of estimated edge weights (Fig. 2) show that the previously described strong/moderate correlations in our network are robust. The negative correlations in our network, on the other hand, are not reliable. To test if a correlation between two nodes was significantly different from other correlations, we used the edge difference test (Supplementary material Figure S1). This plot showed that the correlation between health distress and negative health outlook was significantly different from all other correlations.
Fig. 2.
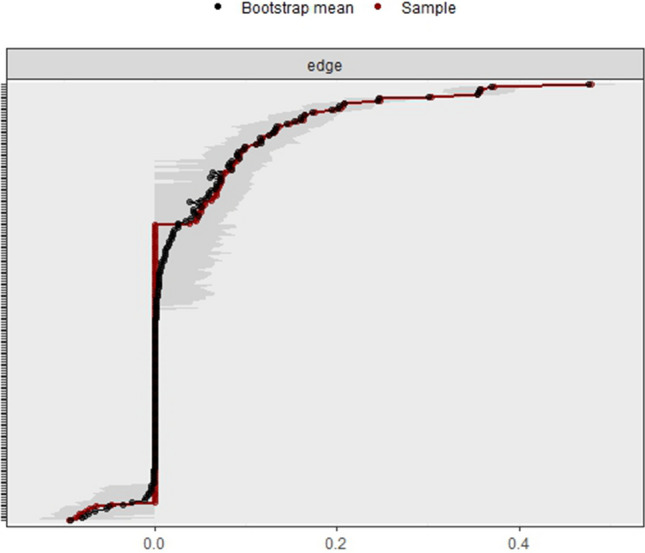
Bootstrapped confidence intervals of estimated edge weights. Each horizontal line represents one edge of the network, ordered from the edge with the highest edge weight to the edge with the lowest edge weight. The red line indicates the sample values and the gray lines are the bootstrapped CIs. The larger the gray line the less certain the edge value is
Cluster analysis
Within our network model, we identified four clusters (Fig. 1); (1) the worriment cluster (orange), (2) the daily functioning cluster (yellow), (3) the psychological cluster (pink), and (4) sexual cluster (green). The worriment cluster consists of the nodes: health distress, negative health outlook, symptom awareness, social isolation, worried about family getting cancer, and people treating you differently. Role functioning, physical functioning, pain, symptom checklist, social interference, and financial difficulties were all part of the daily functioning cluster. Emotional functioning, fatigue, sleep problems, cognitive functioning, and overall quality of life formed the psychological cluster. The sexual cluster consists of sexual functioning, sexual problems, and body image.
Network stability and centrality
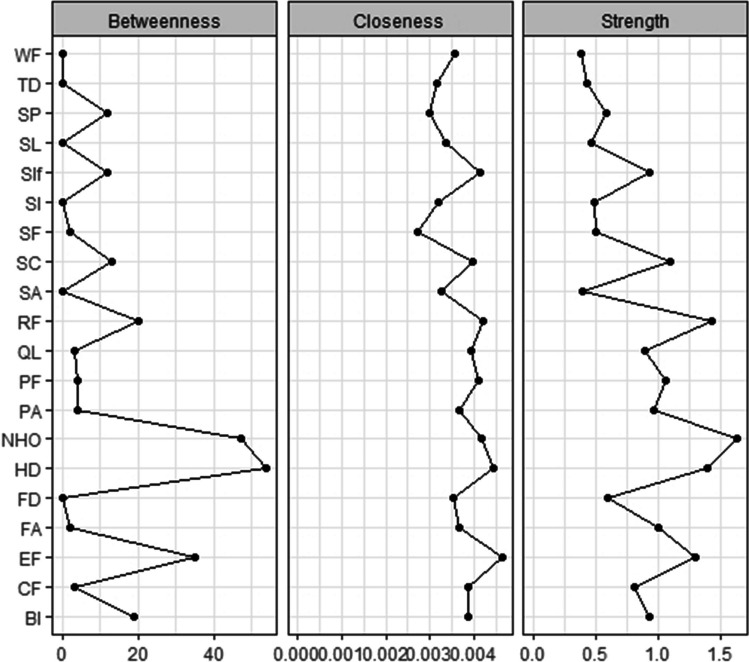
The CS coefficient for strength, closeness, and betweenness were 0.75, 0.75, and 0.75 respectively, indicating a stable and reliable network (Fig. 3). Regarding centrality, the nodes with the highest strength are the most central, and therefore the most important nodes of the network model. In our model, nodes with the highest strength were negative health outlook (standardized centrality estimates (SCE) = 1.60), role functioning (SCE = 1.40), health distress (SCE = 1.40), and emotional functioning (SCE = 1.30). In addition, health distress and negative health outlook had the highest betweenness, and emotional functioning and health distress had the highest closeness (Fig. 4). The centrality difference plot (Supplementary material Figure S2) demonstrates that the strength of the node negative health outlook significantly differed from the other nodes in the network model.
Fig. 3.
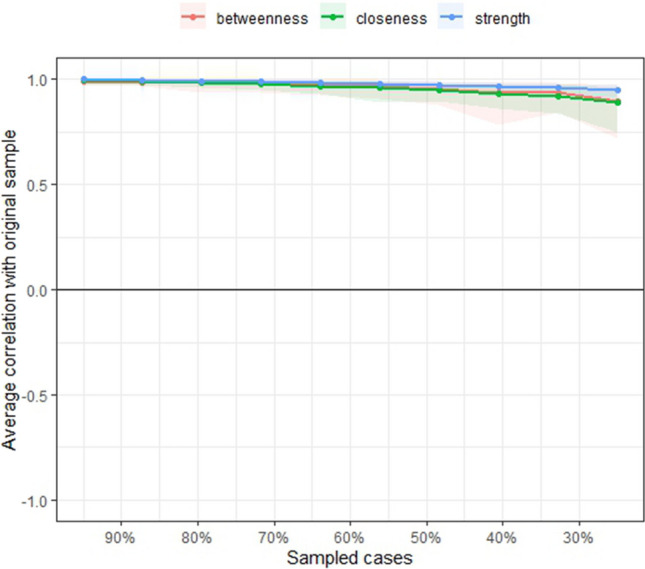
Correlational stability plot of centrality indices by case-dropping subset bootstrap. Correlations between centrality indices of network sampled with persons dropped and the original sample. Lines indicate the means and areas indicate the range from the 2.5th quantile to the 97.5th quantile
Fig. 4.
Central indices of the network. Centrality indices are shown as standardized z-scores. PF: physical functioning, CF: cognitive functioning, EF: emotional functioning, RF: role functioning, BI: body image, SA: symptom awareness, SF: sexual functioning, FA: fatigue, SL: sleep problems, PA: pain, Sif: social interference, HD: health distress, NHO: negative health outlook, SI: social isolation, SC: symptom checklist, SP: sexual problems, FD: financial difficulties, WF: worried about family getting cancer, TD: people treating you differently, QL: overall quality of life
Discussion
This study shows the results of a stable and reliable network analysis based on HRQoL data of 3596 long-term AYAs, as the first to our knowledge. Although the overall global quality of life score was 77.3 on average, the lowest functioning scale score was sexual functioning and the lowest symptom scale scores were social isolation, fatigue, and negative health outlook. This network showed several strong/moderate partial correlations, with the partial correlation between health distress and negative health outlook being the only one significantly different from all other. Also, the strength of the node negative health outlook was significantly different from all other nodes. In total, four clusters of negative symptom and functioning HRQoL scales were identified, including a worriment cluster, daily functioning cluster, psychological cluster, and sexual cluster.
As this study is the first to apply a network analysis based on a wide range of HRQoL data of AYAs using the relatively new QLQ-SURV111 questionnaire, findings are difficult to compare with other studies. Although previous studies had similar aims, they mostly used the EORTC QLQ-C30 to assess HRQoL and studied adult cancer survivors [28, 46]. This was also the case in the network analysis conducted by Rooij et al. in a heterogeneous sample of adult cancer survivors where the EORTC QLQ-C30 was used [28]. In their analysis, fatigue was consistently central and had moderate direct relationships with emotional symptoms, cognitive symptoms, appetite loss, dyspnea, and pain. Fatigue being the most central symptom was in contrast with our results, which might be explained by the much younger population in our study (31.5 years vs 61 years) and the difference in time since diagnosis, which was considerably longer in our study (12.4 years vs 4.2 years). In our network, negative health outlook was the node with the highest strength, which had a strong correlation with health distress within the worriment cluster. This suggests that psychological and emotional issues remain more of relevance to AYA cancer survivors also after long-term follow-up, and are better picked up by the QLQ-SURV111 questionnaire that covers a more complete range of relevant survivorship issues.
Other network studies focused specifically on a construct (e.g., fear of recurrence) or predefined clusters of symptoms, for example, the network analysis on fear of cancer recurrence, anxiety, and depression in breast cancer patients of Yang et al. [46]. In their network, “having trouble relaxing” was the most central node, anxiety and depression were well-connected, and fear of cancer recurrence formed a distinct cluster. The use of the broad range of survivorship issues of the QLQ-SURV111, including psychological, social, physical symptoms and functioning, allowed us to explore clusters within our network as well. An interesting cluster that emerged was the worriment cluster, which consisted of negative health outlook, health distress, symptom awareness, social isolation, worried about family getting cancer, and people treating you differently. Although our questions regarding worries were different, one other network analysis on AYAs focused on the construct of fear of cancer recurrence [47]. Here, the researchers found fear of serious medical interventions as the most central symptom in their network, with the highest node strengths for fear of pain, fear of relying on strangers for activities of daily living, and fear of severe medical treatments. Based on their results, which emphasize the centrality of emotional issues among AYA patients, they stress the importance of prioritizing these symptoms for interventions. However, as stated previously, comparing the results of these studies with our results should be done cautiously as study populations, study designs, aims, and used questionnaires differ. The lack of studies among AYAs to compare our findings with stresses the need for more AYA-specialized research.
The results of a network analysis can provide more insight in the HRQoL of AYAs, in which symptoms and functioning can influence each other, instead of perceiving them as individual factors [48]. Identifying the most important factors in a network can help to address these problems with targeted interventions and healthcare, and lead to novel research ideas. First, we recommend to replicate network analysis studies in other groups of AYAs to be able to make comparisons between studies with similar study populations and draw conclusions with more certainty—changing the exploratory approach into a confirmative approach. Ideally, longitudinal data needs to be collected to draw conclusions over time and adapt healthcare to these time-related changes where needed. This could even be specific to longitudinal ecological momentary assessment (EMA) in which participants are asked to complete (parts of) the expected momentary dynamic items of the HRQoL questionnaire multiple times a day during a study period. In this way, variations over time can be taken into account to a more detailed and individual level. Inter- and intra-individual differences over time might result in changes in prevalence (scores of the scales or items), partial correlations of nodes, centrality, and clusters formed.
In addition, subgroup analysis should be performed to make healthcare interventions even more tailored. AYAs subgroup analysis could focus on age, gender, stage, treatment, and type of cancer. In order to establish tailored care, it is important to make the subgroups as specific as possible while remaining a stable and reliable network. However, it should also be noted that these results represent means and thus differences may exist on an individual level in clinical practice.
Future research can lead to and optimize supportive care and targeted interventions, like psycho-oncological care and psychosocial, behavioral, and supportive interventions [49]. For example, the strong significant correlation between health distress and negative health outlook, which is part of the worriment cluster in this study, might be intervened on by distress screening and renewed/tailored, age-specific psycho-oncological aftercare [50]. For healthcare providers (HCPs) involved in AYA healthcare, and in general, visualization of the nodes, correlations, and clusters may help to understand the cohesion between different factors/symptoms and the influence they might have on each other, and more important, how a targeted intervention can influence several HRQoL outcomes simultaneously. This advocates for holistic and age-specific psycho-oncological aftercare in which multiple factors are targeted simultaneously by a multidisciplinary team of HCPs, to be as efficient and effective as possible.
Strengths and limitations
This explorative study represents the very first network analysis using data on a range of HRQoL outcomes of long-term AYAs to our knowledge. Strengths include the large sample size, the establishment of a stable and reliable network, and the inclusion of a wide range of survivorship issues. However, the results should be interpreted with caution as the EORTC QLQ-SURV111 is not yet finalized and validated. In addition, our study included mostly females and over 40% was diagnosed with a stage I tumor. With a response rate of 36%, there are several subgroups (males, AYAs with a more aggressive disease, and AYAs diagnosed at the age of 18–24) underrepresented in this analysis who might have different HRQoL outcomes [30]. Results may therefore not be generalizable to the total AYAs population. Also, we have not taken a closer look at the outcomes of specific subgroups (between groups), as the study group as a whole was analyzed. As mentioned previously, the subgroup analyses should be part of future research to tailor interventions with a risk-based approach. In line with this, due to the methodology of this study, i.e., a cross-sectional questionnaire study, no causal pathways or changes in HRQoL factors over time can be assessed. In the future, this might be tackled by using longitudinal data instead of cross-sectional data.
Conclusions
This innovative network analysis provides insight in the nodes, correlations, and clusters that could be targeted to improve the HRQoL outcomes of AYAs. Future studies with longitudinal data and subgroup analyses can tailor the interventions and provided healthcare even more, specifically for those at risk of poor HRQoL outcomes. With these insights, more targeted interventions and healthcare can be provided and developed.
Supplementary information
(DOCX 98 kb)
Acknowledgements
The authors wish to thank all the patients for their participation in the study and the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry.
Author contribution
Conceptualization: C.V., S.H.M.J., T.I.B., D.W., D.C.R., C.D., and O.H.; data curation: C.V. and T.I.B.; formal analysis: T.I.B., D.W., and C.D.; funding acquisition: W.T.A.G. and O.H.; investigation: T.I.B., D.W., C.V., D.C.R., C.D., and S.H.M.J.; methodology: T.I.B., D.W., C.V., D.C.R., C.D., and S.H.M.J.; project administration: C.V.; resources: T.I.B., D.W., C.V., D.C.R., C.D., and S.H.M.J.; software: T.I.B., D.W., C.V., D.C.R., C.D., and S.H.M.J.; supervision: W.T.A.G. and O.H.; validation: T.I.B., D.W., C.V., D.C.R., C.D., and S.H.M.J.; visualization: T.I.B., D.W., C.V., D.C.R., C.D., and S.H.M.J.; writing—original draft preparation: C.V., D.C.R., and S.H.M.J.; writing—review and editing: T.I.B., D.W., C.V., D.C.R., C.D., R.T., R.B., S.E.J.K., J.M.K., J.M.T., M.E.M.M.B., T.H., R.I.L., J.N., M.C.M.K., W.T.A.G., S.H.M.J., and O.H.; all authors have read and approved the final manuscript.
Funding
This study was funded by a VIDI grant (VIDI198.007) and an investment grant (#480-08-009) from the Netherlands Organization for Scientific Research. This research was also supported by an institutional grant of the Dutch Cancer Society and of the Dutch Ministry of Health, Welfare and Sport.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.
Declarations
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Netherlands Cancer Institute Institutional Review Board (IRBd18122) on February 6, 2019.
Consent to participate
Informed consent was obtained from all subjects (responders) involved in the study.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Tom I. Bootsma and Deborah van de Wal share first authorship.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meeneghan MR, Wood WA. Challenges for cancer care delivery to adolescents and young adults: present and future. Acta Haematol. 2014;132(3-4):414–422. doi: 10.1159/000360241. [DOI] [PubMed] [Google Scholar]
- 2.Adolescent and Young Adult Oncology Progress Review Group, "Closing the gap: research and care imperatives for adolescents and young adults with cancer," 2006.
- 3.Smith AW, et al. Next steps for adolescent and young adult oncology workshop: an update on progress and recommendations for the future. Cancer. 2016;122(7):988–999. doi: 10.1002/cncr.29870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Meer DJ, et al. Incidence, survival, and mortality trends of cancers diagnosed in adolescents and young adults (15–39 years): a population-based study in The Netherlands 1990–2016. Cancers. 2020;12(11):3421. doi: 10.3390/cancers12113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen SH, van der Graaf WT, van der Meer DJ, Manten-Horst E, Husson O. Adolescent and young adult (AYA) cancer survivorship practices: an overview. Cancers. 2021;13(19):4847. doi: 10.3390/cancers13194847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keegan TH, et al. Unmet adolescent and young adult cancer survivors information and service needs: a population-based cancer registry study. J Cancer Surviv. 2012;6:239–250. doi: 10.1007/s11764-012-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen SHM, Vlooswijk C, Manten-Horst E, Sleeman SHE, Bijlsma RM, Kaal SEJ, Kerst JM, Tromp JM, Bos MEMM, van der Hulle T, Lalisang RI, Nuver J, Kouwenhoven MCM, van der Graaf WTA, Husson O (2023) Learning from long-term adolescent and young adult (AYA) cancer survivors regarding their age-specific care needs to improve current AYA care programs. Cancer Med 12(12):13712–13731. 10.1002/cam4.6001 [DOI] [PMC free article] [PubMed]
- 8.Coccia PF, et al. Adolescent and young adult oncology, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(1):66–97. doi: 10.6004/jnccn.2018.0001. [DOI] [PubMed] [Google Scholar]
- 9.Bleyer A. Adolescent and young adult (AYA) oncology: the first A. Pediatr Hematol Oncol. 2007;24(5):325–336. doi: 10.1080/08880010701316850. [DOI] [PubMed] [Google Scholar]
- 10.Evans DR. Enhancing quality of life in the population at large. Soc Indic Res. 1994;33:47–88. doi: 10.1007/BF01078958. [DOI] [Google Scholar]
- 11.Nowe E, Stöbel-Richter Y, Sender A, Leuteritz K, Friedrich M, Geue K. Cancer-related fatigue in adolescents and young adults: a systematic review of the literature. Crit Rev Oncol Hematol. 2017;118:63–69. doi: 10.1016/j.critrevonc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Brock H, et al. Work ability and cognitive impairments in young adult cancer patients: associated factors and changes over time—results from the AYA-Leipzig study. J Cancer Surviv. 2022;16(4):771–780. doi: 10.1007/s11764-021-01071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen S, van der Meer D, van Eenbergen M, Manten-Horst E, van der Graaf W, Husson O. Short-and long-term impact of cancer on employment and financial outcomes of adolescents and young adults (AYAs): a large population-based case-control registry study in the Netherlands. ESMO open. 2022;7(4):100521. doi: 10.1016/j.esmoop.2022.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michel G, François C, Harju E, Dehler S, Roser K. The long-term impact of cancer: evaluating psychological distress in adolescent and young adult cancer survivors in Switzerland. Psycho-oncology. 2019;28(3):577–585. doi: 10.1002/pon.4981. [DOI] [PubMed] [Google Scholar]
- 15.Moore JB, et al. A qualitative assessment of body image in adolescents and young adults (AYAs) with cancer. Psycho-Oncology. 2021;30(4):614–622. doi: 10.1002/pon.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen NH, et al. Differences in functioning between young adults with cancer and older age groups: a cross-sectional study. Eur J Cancer Care. 2022;31(6):e13660. doi: 10.1111/ecc.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulte FS, Chalifour K, Eaton G, Garland SN. Quality of life among survivors of adolescent and young adult cancer in Canada: a Young Adults With Cancer in Their Prime (YACPRIME) study. Cancer. 2021;127(8):1325–1333. doi: 10.1002/cncr.33372. [DOI] [PubMed] [Google Scholar]
- 18.Husson O, et al. Health-related quality of life in adolescent and young adult patients with cancer: a longitudinal study. J Clin Oncol. 2017;35(6):652–659. doi: 10.1200/JCO.2016.69.7946. [DOI] [PubMed] [Google Scholar]
- 19.Harju E, Roser K, Dehler S, Michel G. Health-related quality of life in adolescent and young adult cancer survivors. Support Care Cancer. 2018;26:3099–3110. doi: 10.1007/s00520-018-4151-z. [DOI] [PubMed] [Google Scholar]
- 20.Quinn GP, Gonçalves V, Sehovic I, Bowman ML, Reed DR (2015) Quality of life in adolescent and young adult cancer patients: a systematic review of the literature. Patient Related Outcome Measures:19–51. 10.2147/PROM.S51658 [DOI] [PMC free article] [PubMed]
- 21.Gold M, et al. Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur J Oncol Nurs. 2016;20:97–105. doi: 10.1016/j.ejon.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng KK, Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. 2011;78(2):127–137. doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Lin S, Chen Y, Yang L, Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs. 2013;22(9-10):1281–1290. doi: 10.1111/jocn.12228. [DOI] [PubMed] [Google Scholar]
- 24.Bjerkeset E, Röhrl K, Schou-Bredal I. Symptom cluster of pain, fatigue, and psychological distress in breast cancer survivors: prevalence and characteristics. Breast Cancer Res Treat. 2020;180:63–71. doi: 10.1007/s10549-020-05522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang K-H, Cho O-H, Yoo Y-S. Symptom clusters of ovarian cancer patients undergoing chemotherapy, and their emotional status and quality of life. Eur J Oncol Nurs. 2016;21:215–222. doi: 10.1016/j.ejon.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Borsboom D, et al. Network analysis of multivariate data in psychological science. Nat Rev Methods Prim. 2021;1(1):58. doi: 10.1038/s43586-021-00055-w. [DOI] [Google Scholar]
- 27.Schellekens MP, Wolvers MD, Schroevers MJ, Bootsma TI, Cramer AO, van der Lee ML. Exploring the interconnectedness of fatigue, depression, anxiety and potential risk and protective factors in cancer patients: a network approach. J Behav Med. 2020;43:553–563. doi: 10.1007/s10865-019-00084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Rooij BH, et al. Symptom clusters in 1330 survivors of 7 cancer types from the PROFILES registry: a network analysis. Cancer. 2021;127(24):4665–4674. doi: 10.1002/cncr.33852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kossakowski JJ, Epskamp S, Kieffer JM, van Borkulo CD, Rhemtulla M, Borsboom D. The application of a network approach to Health-Related Quality of Life (HRQoL): introducing a new method for assessing HRQoL in healthy adults and cancer patients. Qual Life Res. 2016;25:781–792. doi: 10.1007/s11136-015-1127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlooswijk C, et al. Recruiting adolescent and young adult cancer survivors for patient-reported outcome research: experiences and sample characteristics of the SURVAYA study. Curr Oncol. 2022;29(8):5407–5425. doi: 10.3390/curroncol29080428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Poll-Franse LV, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14):2188–2194. doi: 10.1016/j.ejca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, et al. International classification of diseases for oncology. 3. World Health Organization; 2000. [Google Scholar]
- 33.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. John Wiley & Sons; 2011. [Google Scholar]
- 34.van Leeuwen M, Kieffer JM, Young TE, Annunziata MA, Arndt V, Arraras JI, Autran D, Hani HB, Chakrabarti M, Chinot O, Cho J, da Costa Vieira RA, Darlington AS, Debruyne PR, Dirven L, Doege D, Eller Y, Eichler M, Fridriksdottir N, Gioulbasanis I, Hammerlid E, van Hemelrijck M, Hermann S, Husson O, Jefford M, Johansen C, Kjaer TK, Kontogianni M, Lagergren P, Lidington E, Lisy K, Morag O, Nordin A, Al Omari ASH, Pace A, De Padova S, Petranovia D, Pinto M, Ramage J, Rammant E, Reijneveld J, Serpentini S, Sodergren S, Vassiliou V, Leeuw IV, Vistad I, Young T, Aaronson NK, van de Poll-Franse LV, EORTC QLG (2023) Phase III study of the European Organisation for Research and Treatment of Cancer Quality of Life cancer survivorship core questionnaire. J Cancer Surviv 17(4):1111–1130. 10.1007/s11764-021-01160-1 [DOI] [PubMed]
- 35.PM ANF, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group . The EORTC QLQ-C30 Scoring Manual. 3. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 36.van de Poll-Franse LV, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667–675. doi: 10.1016/j.ejca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Korkmaz S, Göksülük D, Zararsiz G (2014) MVN: an R package for assessing multivariate normality. The R Journal 6:151–162. 10.32614/RJ-2014-031
- 38.Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods. 2018;50:195–212. doi: 10.3758/s13428-017-0862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Lafferty J, Wasserman L (2009) The nonparanormal: semiparametric estimation of high dimensional undirected graphs. J Mach Learn Res 10. 10.1145/1577069.1755863
- 40.Epskamp S, Fried EI. A tutorial on regularized partial correlation networks. Psychol Methods. 2018;23(4):617. doi: 10.1037/met0000167. [DOI] [PubMed] [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioral sciences. Academic press; 2013. [Google Scholar]
- 42.Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Stat Soc : Series B (Methodological) 1996;58(1):267–288. [Google Scholar]
- 43.Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: generalizing degree and shortest paths. Soc Networks. 2010;32(3):245–251. doi: 10.1016/j.socnet.2010.03.006. [DOI] [Google Scholar]
- 44.Costenbader E, Valente TW. The stability of centrality measures when networks are sampled. Soc Networks. 2003;25(4):283–307. doi: 10.1016/S0378-8733(03)00012-1. [DOI] [Google Scholar]
- 45.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech : theory Exp. 2008;2008(10):P10008. doi: 10.1088/1742-5468/2008/10/P10008. [DOI] [Google Scholar]
- 46.Yang Y, et al. Network connectivity between fear of cancer recurrence, anxiety, and depression in breast cancer patients. J Affect Disord. 2022;309:358–367. doi: 10.1016/j.jad.2022.04.119. [DOI] [PubMed] [Google Scholar]
- 47.Richter D, Clever K, Mehnert-Theuerkauf A, Schönfelder A. Fear of recurrence in young adult cancer patients—a network analysis. Cancers. 2022;14(9):2092. doi: 10.3390/cancers14092092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miaskowski C, Barsevick A, Berger A, Casagrande R, Grady PA, Jacobsen P, Kutner J, Patrick D, Zimmerman L, Xiao C, Matocha M, Marden S (2017) Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst 109(4):djw253. 10.1093/jnci/djw253 [DOI] [PMC free article] [PubMed]
- 49.Zhang A, Wang K, Zebrack B, Tan CY, Walling E, Chugh R. Psychosocial, behavioral, and supportive interventions for pediatric, adolescent, and young adult cancer survivors: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;160:103291. doi: 10.1016/j.critrevonc.2021.103291. [DOI] [PubMed] [Google Scholar]
- 50.Patterson P, et al. The clinical utility of the Adolescent and Young Adult Psycho-Oncology Screening Tool (AYA-POST): perspectives of AYA cancer patients and healthcare professionals. Front Psychol. 2022;13:872830. doi: 10.3389/fpsyg.2022.872830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 98 kb)
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.