Abstract
Meningococcal sodC encodes periplasmic copper- and zinc-cofactored superoxide dismutase (Cu,Zn SOD) which catalyzes the conversion of the superoxide radical anion to hydrogen peroxide, preventing a sequence of reactions leading to production of toxic hydroxyl free radicals. From its periplasmic location, Cu,Zn SOD was inferred to acquire its substrate from outside the bacterial cell and was speculated to play a role in preserving meningococci from the action of microbicidal oxygen free radicals produced in the context of host defense. A sodC mutant was constructed by allelic exchange and was used to investigate the role of Cu,Zn SOD in pathogenicity. Wild-type and mutant meningococci grew at comparable rates and survived equally long in aerobic liquid culture. The mutant showed no increased sensitivity to paraquat, which generates superoxide within the cytosol, but was approximately 1,000-fold more sensitive to the toxicity of superoxide generated in solution by the xanthine/xanthine oxidase system. These data support a role for meningococcal Cu,Zn SOD in protection against exogenous superoxide. In experiments to translate this into a role in pathogenicity, wild-type and mutant organisms were used in an intraperitoneal mouse infection model. The sodC mutant was significantly less virulent. We conclude that periplasmic Cu,Zn SOD contributes to the virulence of Neisseria meningitidis, most likely by reducing the effectiveness of toxic oxygen host defenses.
Neisseria meningitidis is a major cause of life-threatening bacterial infection throughout the world, causing a range of conditions from meningitis to fulminant meningococcal septicemia, with a mortality rate as high as 60% despite treatment with potent antibiotics and all the resources of modern intensive care (26). Much attention is accordingly focused on possibilities for prevention of disease and therefore on understanding the mechanisms employed by the meningococcus to facilitate its survival in the course of invasive infection. During meningococcal disease, organisms continue to proliferate despite exposure to the microbicidal actions of proteins such as the components of the complement system and toxic small molecules, including oxygen free radicals generated by phagocytic cells (for recent reviews, see reference 12).
Superoxide dismutase (SOD) catalyzes the dismutation of the highly reactive superoxide radical anion to hydrogen peroxide and molecular oxygen (37). The removal of superoxide effectively blocks secondary reactions that otherwise would lead to formation of the promiscuously reactive hydroxyl radical, which is highly damaging to all classes of biological macromolecules. Two main classes of SOD have been identified in bacteria. Metalloenzymes containing manganese or iron (Mn SOD and Fe SOD, respectively) exhibit close primary sequence similarity to each other and are found in the bacterial cytosol. Bacterial copper- and zinc-cofactored SOD (Cu,Zn SOD) is an entirely distinct enzyme recently described in a wide range of gram-negative pathogens, where it is found in the periplasm (3, 5, 19, 30–33, 47). A role for periplasmic SOD in the virulence of bacterial pathogens has been proposed in light of the theoretical capacity of such an enzyme to dismutate superoxide generated outside the bacterial cell, for example, in the course of the microbicidal respiratory burst of phagocytic cells. Evidence in support of such a role has been conflicting in the case of Brucella abortus (34, 49), but clear evidence has recently been obtained for a role for Cu,Zn SOD in the virulence of Salmonella typhimurium (16, 19). Here we report that in N. meningitidis, as in Salmonella, the periplasmic Cu,Zn SOD protects organisms from the toxic effects of superoxide generated outside the cell in vitro and that a Cu,Zn SOD mutant shows attenuated virulence in a mouse model of meningococcal infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
N. meningitidis MC12, MC14, MC19, MC50, MC54, C311, and MC58 (50) were generously provided by M. Virji, University of Reading, Reading, United Kingdom. Neisseria gonorrhoeae MS11 was obtained from B. Robertson, Imperial College School of Medicine at St. Mary’s Hospital, London, United Kingdom. Additional gonococcal isolates and commensal neisseriae were from the collection of C. Ison, Imperial College School of Medicine at St. Mary’s Hospital. Escherichia coli QC779, a sodA sodB mutant (39), was kindly provided by D. Touati, Jacques Monod Institut, University of Paris, Paris, France. E. coli SURE (Stratagene) was used as a host strain for cloning. E. coli DH5α (23) was used as a positive control for SOD expression.
Antibiotics were used at the following concentrations: for the culture of E. coli, 100 μg of ampicillin/ml, 50 μg of kanamycin/ml, and 20 μg of chloramphenicol/ml; for N. meningitidis, 100 μg of kanamycin/ml.
Neisserial strains were cultured on GC agar (Difco) supplemented with 2% Vitox (Oxoid), GC1 agar (1), or brain heart infusion agar (Oxoid) containing 1% horse serum in 5% CO2 at 37°C.
Recombinant DNA methods.
Standard methods were used for genomic and plasmid DNA preparation, restriction enzyme analysis, Southern blotting, and cloning (40). Southern hybridizations were carried out under conditions of 80% stringency. Routine cloning was carried out with the cloning vectors pBluescript (Stratagene) and pACYC184 (13). DNA sequencing was carried out by the dideoxy chain termination method (41), with Sequenase version 2.0 (Amersham).
Construction of sodC::bla fusion.
To investigate the export of SodC beyond the cytoplasmic membrane, the sodC-containing insert of pJSK205 was recloned into pACYC184. After linearization with BamHI and brief exonuclease digestion, the promotorless, leader peptide-lacking bla gene from pJBS633 (10) was ligated into the digestion product. The bla gene was shown to be in frame with the 5′ sequence of sodC.
Construction of the sodC mutant of N. meningitidis.
A kanamycin resistance (Kmr) cassette excised from pUC4Kan (Pharmacia) was inserted into the BamHI site of the cloned sodC gene (pJSK207). MC58 was transformed with linearized plasmid as described by Nassif et al. (38). Allelic replacement of the wild-type gene was confirmed by paired Southern hybridization of ClaI-digested chromosomal DNA from kanamycin-resistant transformants, probed with a digoxigenin (DIG)-labelled HindIII/EcoRI fragment from pJSK204 and a DIG-labelled Kmr cassette from pUC4Kan (data not shown).
Preparation of meningococci for studies in liquid culture.
Plate-grown N. meningitidis organisms were harvested into phosphate-buffered saline (PBS) and centrifuged at 70 × g for 1 min in order to remove large aggregates. The supernatant suspension was adjusted to 107 CFU/ml with culture medium. Aliquots of 10 ml were placed in 50-ml tubes sealed with a vented cap containing a 0.2-μm-pore-size filter (Becton Dickinson) to ensure that aerobic conditions were maintained. Liquid cultures were incubated at 37°C, with shaking at 180 rpm.
Extraction of bacterial proteins, gel electrophoresis, and detection of SOD activity.
In order to detect Cu,Zn SOD activity, meningococci grown overnight on supplemented GC agar or cell pellets from aerobically grown E. coli were suspended in a solution of 50 mM Tris (pH 7.8) (BDH)–1 mM CuSO4 (Tris-CuSO4) and then lysed by three freeze-thaw cycles. Whole-cell extracts (WCE) were separated by nondenaturing polyacrylamide gel electrophoresis (PAGE) under the following conditions: 4.5% (wt/vol) stacking gel (pH 8.3), 10% (wt/vol) separating gel (pH 8.9), and a buffering system essentially the same as that of Davies (15), but with modification of the upper buffer to pH 8.9 with 10 M NaOH. SOD activity was visualized on these gels by the method of Beauchamp and Fridovich (2) as modified by Steinman (45). Cu,Zn SOD activity was inhibited by soaking the gel in 2 mM diethyldithiocarbamate (DEDC) for 30 min prior to activity staining (5). Fe SOD was inhibited by the addition of hydrogen peroxide to a final concentration of 5 mM at the time of incubation with riboflavin, N,N,N′,N′-tetramethylethylenediamine and nitroblue tetrazolium (14, 18).
To investigate the expression of meningococcal Cu,Zn SOD during different phases of growth, liquid cultures of MC58 were prepared in Mueller-Hinton broth at an initial density of 107 CFU/ml and incubated at 37°C, with shaking at 180 rpm. A 4-ml sample extracted at 4 h, and 2-ml samples extracted at 8, 12, and 20 h were centrifuged (13,000 × g, 10 min). The pellets were washed with PBS and resuspended in 200 μl of Tris-CuSO4. The cells were lysed by four freeze-thaw cycles, then centrifuged to remove cell debris (13,000 × g, 10 min). Supernatants were assayed for protein by the method of Bradford (8) and were adjusted to equivalent protein concentrations prior to assay for SOD activity.
Quantification of growth of N. meningitidis.
Meningococcal liquid cultures were studied in triplicate in Mueller-Hinton broth (Difco) supplemented with 2% Vitox, from a starting density of 107 CFU/ml. Over a 48-h period, 20-μl samples were removed from each culture every 2 h, diluted 10-fold serially, and plated on supplemented GC agar for determination of viable CFU. This experiment was performed on two occasions.
Paraquat sensitivity.
Paraquat is a redox-cycling agent which readily penetrates cells, including capsulate bacteria, and which, under aerobic conditions, generates a bactericidal flux of superoxide in the cytosol (17, 24). Suspensions of wild-type and sodC mutant N. meningitidis were prepared in PBS, and approximately 300 CFU was plated onto supplemented GC agar containing various concentrations of paraquat. Plates were incubated overnight at 37°C with 5% CO2, and CFU were counted in order to determine survival relative to that on control plates lacking paraquat.
Exposure to X/XO.
Meningococcal cultures were studied in GC1 medium (1) containing 100 μM xanthine (X) (Sigma), at an initial density of 107 CFU/ml. In order to generate a superoxide flux, xanthine oxidase (XO) (Sigma) was added at a concentration of 7 mU/ml (sufficient to catalyze reduction of 15 nM ferricytochrome c at a rate of 46 pmol/min) and cultures were incubated at 37°C, with shaking at 180 rpm. Samples (20 μl) were removed at intervals in order to assess viability by serial dilution and plating. Bovine SOD (0.01 U/ml) or bovine catalase (40 U/ml) (both from Sigma) was added to some cultures.
Infection of mice with N. meningitidis.
Fifty 6-week-old NIH mice (Harlan) were infected by using the intraperitoneal (i.p.) infection model (28, 35). Bacteria were grown in Mueller-Hinton broth for 4 h, adjusted to the required density with the same medium, and mixed with an equal volume of sterile iron dextran (100 mg of iron/ml; Sigma). Mice received the appropriate challenge dose i.p. in a 0.5-ml suspension, and 24 h later a second i.p. injection, of 0.25 ml of saline containing iron dextran (8 mg of iron/ml), was administered. Deaths were monitored for 4 days after infection. Meningococci of appropriate phenotypes (wild type, kanamycin sensitive; sodC mutant, kanamycin resistant) were recovered from blood, livers, and spleens of dead animals to confirm the cause of death. Results were analyzed by the chi-square statistical test with 1 degree of freedom and continuity correction.
Nucleotide sequence accession number.
The sequence of the entire N. meningitidis sodC gene has been deposited in the EMBL database under accession no. AJ001313.
RESULTS AND DISCUSSION
Distribution, nucleotide sequence, and localization of sodC.
We have previously cloned a 310-bp PCR product (pJSK204) from within the 3′ region of sodC, a gene encoding Cu,Zn SOD in the N. meningitidis serogroup B strain MC58 (32). With this DNA as a probe in Southern hybridization, sodC was found to be present in all strains of a collection of four serogroup B, one serogroup C, and two serogroup A N. meningitidis strains (Fig. 1); these serogroups cause more than 90% of meningococcal disease (20). Eight clinical isolates of N. gonorrhoeae were examined, and no hybridization to pJSK204 was detected. As shared meningococcal and gonococcal genes are predicted to be nearly 90% identical (21, 27), this suggests that sodC is absent from N. gonorrhoeae. In confirmation, no Cu,Zn SOD activity was detectable in total-protein extracts of N. gonorrhoeae MS11 (data not shown). In addition, no hybridization was found to chromosomal DNA from the commensal neisserial species N. lactamica, N. cinerea, N. polysaccharea, and N. mucosa, suggesting that, among neisseriae, sodC is found only in the meningococcus.
FIG. 1.
Southern blot of seven meningococcal strains and one gonococcal strain. Genomic DNA (lane 1, MS11 [gonococcal]; lane 2, MC19 [serogroup B]; lane 3, MC54 [serogroup B]; lane 4, MC12 [serogroup A]; lane 5, MC14 [serogroup A]; lane 6, MC58 [serogroup B]; lane 7, C311 [serogroup B]; lane 8, MC50 [serogroup C]) was digested with ClaI and hybridized with a sodC-containing, DIG-labelled HindIII/EcoRI fragment from pJSK204.
To isolate the entire gene for further study, a partial ClaI library prepared from MC58 DNA was probed with pJSK204, and a positive clone (pJSK205) was isolated. Oligonucleotides complementary to known meningococcal sodC were used to sequence the entire open reading frame and its flanking DNA. The translated sequence displayed a high level of similarity to Cu,Zn SODs from other bacteria; the deduced meningococcal sequence contained all the residues recognized to have key structural or functional significance (6, 7, 48). Typical of bacterial Cu,Zn SODs, the translated sequence contains a 22-amino-acid N-terminal domain characteristic of a leader peptide, suggesting that SodC is localized outside the cytoplasm. Single colonies of E. coli transformed with a construct containing a meningococcal sodC leader peptide-bla fusion showed high-level ampicillin resistance, confirming that the cloned meningococcal sodC leader peptide, when expressed in E. coli, directs the protein product beyond the cytoplasmic membrane (10). In all cases where the subcellular localization of native bacterial Cu,Zn SOD has been determined, it has been found to be periplasmic (4, 44, 46). In this light, our data strongly suggest a periplasmic location for the enzyme in N. meningitidis. As superoxide can cross the bacterial outer membrane but not the cytoplasmic membrane (24), this suggests that Cu,Zn SOD may play a role in protection of meningococci against superoxide radicals produced outside the cell.
Expression of meningococcal Cu,Zn SOD.
In order to establish that the cloned meningococcal sodC encodes an active Cu,Zn SOD (the gene in Haemophilus influenzae, for example, does not [31]), pJSK205 was transferred into E. coli QC779, a sodA sodB mutant which does not express any detectable SOD in our experimental system during exponential growth. WCE were separated by nondenaturing PAGE and stained to visualize SOD activity (Fig. 2). No SOD activity was detected in exponential-phase cultures of QC779 or of QC779 containing pBluescript. MC58 produced several bands, while QC779 harboring pJSK205 produced a single band of activity, of the same electrophoretic mobility as the uppermost band in wild-type N. meningitidis. In each case this activity was inhibited by 2 mM DEDC, characteristic of Cu,Zn SOD (5). That the remaining bands present in the wild-type meningococcal extract were attributable to isoforms of Fe SOD was confirmed by H2O2 treatment, which selectively abolishes this activity (14).
FIG. 2.
SOD activity of WCE, uninhibited (A) or inhibited with DEDC (B) or H2O2 (C), visualized in a 10% nondenaturing gel. Lanes 1, MC58; lanes 2, E. coli DH5α (expressing SodA and SodB); lanes 3, QC779 containing pJSK205. The arrow highlights the position of the cloned meningococcal Cu,Zn SOD in QC779, which has the same electrophoretic mobility as the wild-type enzyme.
To investigate the phase of bacterial growth during which sodC is expressed, samples were withdrawn from aerobic liquid culture at various times and assayed for Cu,Zn SOD. SodC activity, measured in samples containing 10 μg of total protein, was readily detectable in lysates prepared from exponentially growing N. meningitidis and continued to accumulate to modestly higher levels in stationary phase (data not shown). In this respect sodC expression in the meningococcus differs markedly from that in E. coli, where the gene is expressed significantly only during stationary phase (30), but resembles that in Legionella pneumophila (47) and Caulobacter crescentus (42).
Attenuation of the N. meningitidis sodC mutant.
A sodC mutant was constructed by interruption of the coding sequence with a kanamycin resistance cassette. Loss of Cu,Zn SOD activity was confirmed by nondenaturing PAGE (Fig. 3), and the mutant was used to investigate the role of Cu,Zn SOD in meningococcal biology.
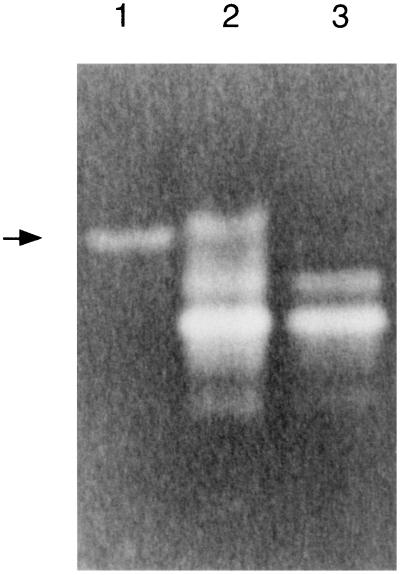
FIG. 3.
Nondenaturing gel stained to visualize SOD activity. Lane 1, QC779 containing pJSK205; lane 2, MC58 WCE; lane 3, MC58 sodC mutant WCE. The arrow indicates the position of meningococcal Cu,Zn SOD activity.
(i) In vitro.
In order to interpret any difference between the pathogenic behaviors of the sodC mutant and the wild type, it was first necessary to demonstrate that Cu,Zn SOD was not essential for normal bacterial growth. During aerobic growth, low levels of superoxide radicals are steadily released into the cytosol in the course of electron transfer to oxygen. The action of cytosolic SOD, in concert with catalase, is accordingly essential for sustained viability under aerobic conditions (11); cytosolic-SOD mutants are clearly defective (17). However, the extracytoplasmic location of Cu,Zn SOD isolates the enzyme from free-radical flux generated in the cytosol, so we anticipated that the sodC mutant phenotype would not differ significantly from the wild-type phenotype in growth and survival in aerobic liquid culture. In a series of experiments, the viability of organisms grown in supplemented Mueller-Hinton broth under aerobic conditions was monitored for periods of up to 48 h. Wild-type and Cu,Zn SOD mutant phenotypes grew at comparable rates (doubling time of approximately 29 min in exponential phase) and reached similar densities (2 × 109 to 5 × 109 CFU/ml) under these conditions. They maintained these densities equally well in stationary phase, up to 38 h. Thereafter, the viable-cell densities dropped rapidly, so that live organisms could no longer be recovered after 48 h. Thus, meningococcal sodC mutants do not appear to be disabled under standard aerobic culture conditions in vitro. In agreement with these observations, Farrant et al. (19) recently demonstrated that wild-type and sodC mutant S. typhimurium grew at comparable rates and survived equally long in aerobic liquid culture. To stress the system further and subject N. meningitidis to an enhanced cytosolic-superoxide flux, organisms were grown in the presence of paraquat, a redox cycling reagent which penetrates the cytosol, greatly increasing superoxide production there, and which has been shown to penetrate and kill other neisserial organisms (25). The sensitivities of wild-type and sodC mutant meningococci at concentrations up to 200 μM paraquat were similar (data not shown). In conclusion, periplasmic Cu,Zn SOD in N. meningitidis does not appear to confer any additional protection over that afforded by the cytosolic enzyme against the superoxide flux generated in the cytoplasm as a result of normal aerobic respiration, or under conditions of enhanced superoxide flux in the cytosol.
To challenge meningococci with an extracellular source of superoxide, experiments using the X/XO superoxide-generating system were carried out. In a reaction catalyzed by XO, X is converted to urate, generating superoxide in the process. This system also generates H2O2, both directly by two-electron transfer to oxygen and indirectly through the dismutation (spontaneous and iron catalyzed) of superoxide. Both of these species, and products of the reaction between them, are microbicidal.
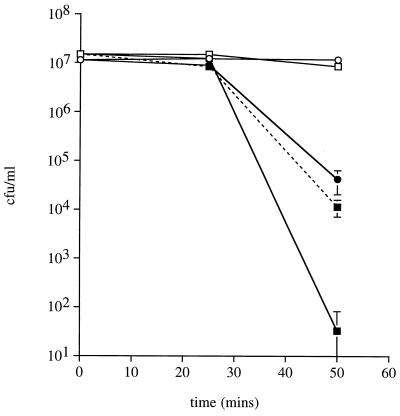
In GC1 broth containing 100 μM X, both wild-type and sodC mutant organisms remained fully viable over the 1-h course of the experiments. However, the toxic oxygen species generated by the addition of 7 mU of XO/ml reduced the viability of both wild-type and sodC mutant phenotypes, the latter much more than the former (approximately a 1,000-fold difference in survival [P < 0.03]) (Fig. 4). These results are consistent with the findings of Schnell and Steinman (42), who, working with C. crescentus, showed that periplasmic Cu,Zn SOD was protective in this system. They observed a difference in survival of as much as 20-fold between wild-type and sodC mutant phenotypes on exposure to X/XO.
FIG. 4.
Survival of wild-type and sodC mutant N. meningitidis MC58 exposed to 0.7 mU of XO/ml in the presence of 100 μM X. Circles, wild type; squares, sodC mutant; open symbols, control cultures; solid symbols, XO present; dashed line, sodC mutant in the presence of XO plus added exogenous SOD (0.01 U/ml).
To further investigate the partial protective effect that meningococcal Cu,Zn SOD activity provided in this experimental system, purified bovine Cu,Zn SOD or bovine catalase was added to the medium. Exogenous SOD (0.01 U/ml) caused a significant (P < 0.03) decrease in the sensitivity of the sodC mutant to the X/XO system, restoring survival to a level comparable to that of the wild type (Fig. 4). No comparable effect was seen with the addition of SOD to wild-type cultures (data not shown). We suspected that H2O2 might be in part responsible for the residual toxicity of X/XO seen in experiments with the wild type. The addition of 40 U of catalase/ml completely protected both phenotypes from killing (data not shown); in the case of the sodC mutant, this effect was obtained even if exogenous SOD was not added. These results are similar to those obtained with wild-type N. meningitidis by Archibald and Duong (1).
The complexity of the mixture of toxic oxygen species generated by X/XO makes the results of protection experiments like these susceptible to various interpretations. We propose the following. Superoxide has a modest toxicity in its own right but can dismutate spontaneously, or much more rapidly in a catalyzed reaction, to produce H2O2, which is more toxic, and the two can react to form highly toxic hydroxyl radicals (22). In the presence of iron, which is an essential component of GC1 medium, this reaction (now referred to as the iron-catalyzed Haber-Weiss reaction) will occur particularly rapidly. The final balance of toxic species will be critically dependent on the relative extent to which these reactions proceed. We suggest that by studying the sodC mutant in the presence of exogenous SOD, we have mimicked the wild-type phenotype with its periplasmic Cu,Zn SOD, allowing superoxide to be eliminated and with it the capacity to form hydroxyl radicals by reaction with H2O2. H2O2 is still present, of course, and is likely to be responsible for the residual toxicity seen. When catalase is added to either the sodC mutant or the wild type, the only residual toxicity left in the system is that of superoxide, which is not detectable in our assay.
(ii) In vivo.
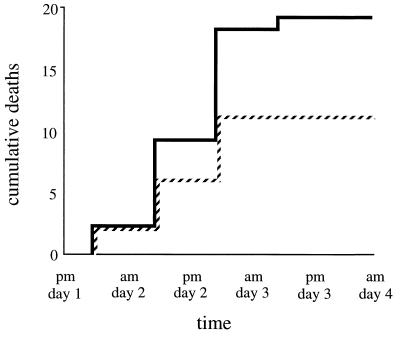
To investigate whether the protective role of Cu,Zn SOD against environmental superoxide is important in the context of infection, where meningococci are subject to host defenses involving oxygen free radicals, a mouse model was employed (28, 35). Although there is no satisfactory model that reproduces natural meningococcal disease, this system has been of use in distinguishing between meningococci of high and low virulence (9, 29, 36, 43). Mice were inoculated i.p. with bacteria and iron dextran, and survival was monitored for 4 days. When an infecting dose of 2 × 106 CFU was used, 25 of 25 animals given the wild type and 24 of 25 given the sodC mutant died, suggesting that they were overwhelmed by this number of bacteria. However, a significant difference in survival (χ2 = 4.08; P < 0.05) was observed between animals given 5 × 105 CFU of wild-type versus sodC mutant meningococci; the mutant exhibited attenuated virulence. In each case the whole of the lethal effect was seen by the end of 48 to 60 h (Fig. 5). Only 6 of 25 animals infected with wild-type meningococci survived, compared to 14 of 25 infected with the sodC mutant. Twenty-four hours after infection, at the time of the second iron dextran injection, the majority of animals given wild-type MC58 looked ill (immobile, with ruffled fur), whereas animals infected with the sodC mutant were mainly recovering. Thus, expression of Cu,Zn SOD is associated with enhanced early virulence in this model.
FIG. 5.
Cumulative deaths of i.p.-infected mice. Fifty mice were infected with 5 × 105 wild-type (continuous line) or sodC mutant (dashed line) meningococci and monitored for 4 days.
These studies using a sodC knockout mutant have defined a role for Cu,Zn SOD in meningococcal biology, protecting organisms against exogenous superoxide challenge. This is reflected in reduced virulence of the sodC mutant in a mouse model of infection, suggesting that protection against superoxide produced during host defense reactions may be an important strategy enabling meningococci to survive in the course of systemic human infection. It will be interesting to evaluate the comparative virulence of wild-type and sodC mutant meningococci in other, better models of infection as these are developed, but in the meantime these data have suggested a series of experiments, now under way, to assess the extent to which Cu,Zn SOD protects meningococci from uptake and killing in a range of different phagocytic cells.
ACKNOWLEDGMENTS
This work was funded by grants to J.S.K. from the Meningitis Research Foundation and the World Health Organization.
K.E.W. and K.L.R.D. contributed equally to this study.
REFERENCES
- 1.Archibald F S, Duong M-N. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun. 1986;51:631–641. doi: 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchamp C O, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 3.Beck B L, Tabatabai L B, Mayfield J E. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry. 1990;29:372–376. doi: 10.1021/bi00454a010. [DOI] [PubMed] [Google Scholar]
- 4.Benov L, Chang L Y, Day B, Fridovich I. Copper, zinc superoxide dismutase in Escherichia coli: periplasmic localization. Arch Biochem Biophys. 1995;319:508–511. doi: 10.1006/abbi.1995.1324. [DOI] [PubMed] [Google Scholar]
- 5.Benov L T, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269:25310–25314. [PubMed] [Google Scholar]
- 6.Bordo D, Djinovic K, Bolognesi M. Conserved patterns in the Cu,Zn superoxide dismutase family. J Mol Biol. 1994;238:366–386. doi: 10.1006/jmbi.1994.1298. [DOI] [PubMed] [Google Scholar]
- 7.Bourne Y, Redford S M, Steinman H M, Lepock J R, Tainer J A, Getzoff E D. Novel dimeric interface and electrostatic recognition in bacterial Cu,Zn superoxide dismutase. Proc Natl Acad Sci USA. 1996;93:12774–12779. doi: 10.1073/pnas.93.23.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Brener D, DeVoe I W, Holbein B E. Increased virulence of Neisseria meningitidis after in vitro iron-limited growth at low pH. Infect Immun. 1981;33:59–66. doi: 10.1128/iai.33.1.59-66.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broome-Smith J, Spratt B. A vector for the construction of translational fusions to TEM β-lactamase and the analysis of protein export signals and membrane protein technology. Gene. 1986;49:341–349. doi: 10.1016/0378-1119(86)90370-7. [DOI] [PubMed] [Google Scholar]
- 11.Carlioz A, Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase essential for aerobic life? EMBO J. 1986;5:623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright K. Meningococcal disease. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1995. [Google Scholar]
- 13.Chang L E, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crapo J D, McCord J M, Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- 15.Davies B J. Disc electrophoresis. II. Method and application to human serum proteins. Ann N Y Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 16.De Groote M A, Ochsner U A, Shiloh M, Nathan C, McCord J, Dinauer M C, Fang F C. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. The role of Cu,Zn-superoxide dismutase (SodC) in Salmonella typhimurium pathogenesis, abstr. B-279; p. 77. [Google Scholar]
- 17.D’mello R A, Langford P R, Kroll J S. Role of bacterial Mn-cofactored superoxide dismutase in oxidative stress responses, nasopharyngeal colonization, and sustained bacteremia caused by Haemophilus influenzae type b. Infect Immun. 1997;65:2700–2706. doi: 10.1128/iai.65.7.2700-2706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunlap P V, Steinman H M. Strain variation in bacteriocuprein superoxide dismutase from symbiotic Photobacterium leiognathi. J Bacteriol. 1986;165:393–398. doi: 10.1128/jb.165.2.393-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrant J L, Sansone A, Canvin J R, Pallen M J, Langford P R, Wallis T S, Dougan G, Kroll J S. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- 20.Gotschlich E C. Unsolved problems in the prevention of meningococcal meningitis. In: Beers R F Jr, Bassett E G, editors. The role of immunological factors in infections: allergic and autoimmune process. New York, N.Y: Raven Press; 1976. pp. 91–102. [Google Scholar]
- 21.Guibourdenche M, Popoff M Y, Riou J Y. Deoxyribonucleic acid relatedness among Neisseria gonorrhoeae, N. meningitidis, N. lactamica, N. cinerea and “Neisseria polysaccharea.”. Ann Inst Pasteur Microbiol. 1986;137B(2):177–185. doi: 10.1016/s0769-2609(86)80106-5. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B, Gutteridge J M C. Free radicals in biology and medicine. Oxford, United Kingdom: Oxford University Press; 1985. pp. 118–119. [Google Scholar]
- 23.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 24.Hassan H M, Fridovich I. Paraquat and Escherichia coli. J Biol Chem. 1979;254:10846–10852. [PubMed] [Google Scholar]
- 25.Hassett D J, Britigan B E, Svendsen T, Rosen G M, Cohen M S. Bacteria form intracellular free radicals in response to paraquat and streptonigrin. Demonstration of the potency of hydroxyl radical. J Biol Chem. 1987;262:13404–13408. [PubMed] [Google Scholar]
- 26.Heyderman R S, Klein N J, Levin M. Pathophysiology and management of meningococcal septicaemia. Recent Adv Paediatr. 1992;11:1–18. [Google Scholar]
- 27.Hoke C, Vedros N A. Taxonomy of the neisseriae: deoxyribonucleic acid base composition, interspecific transformation, and deoxyribonucleic acid hybridization. Int J Syst Bacteriol. 1982;32:57–66. [Google Scholar]
- 28.Holbein B E. Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun. 1981;34:120–125. doi: 10.1128/iai.34.1.120-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holbein B E. Differences in virulence for mice between disease and carrier strains of Neisseria meningitidis. Can J Microbiol. 1981;27:738–741. doi: 10.1139/m81-113. [DOI] [PubMed] [Google Scholar]
- 30.Imlay K R C, Imlay J A. Cloning and analysis of sodC, encoding the copper-zinc superoxide dismutase of Escherichia coli. J Bacteriol. 1996;178:2564–2571. doi: 10.1128/jb.178.9.2564-2571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroll J S, Langford P R, Loynds B M. Copper-zinc superoxide dismutase of Haemophilus influenzae and H. parainfluenzae. J Bacteriol. 1991;173:7449–7457. doi: 10.1128/jb.173.23.7449-7457.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroll J S, Langford P R, Wilks K E, Keil A D. Bacterial [Cu,Zn]-superoxide dismutase: phylogenetically distinct from the eukaryotic enzyme and not so rare after all! Microbiology. 1995;141:2271–2279. doi: 10.1099/13500872-141-9-2271. [DOI] [PubMed] [Google Scholar]
- 33.Langford P R, Loynds B M, Kroll J S. Copper-zinc superoxide dismutase in Haemophilus species. J Gen Microbiol. 1992;138:517–522. doi: 10.1099/00221287-138-3-517. [DOI] [PubMed] [Google Scholar]
- 34.Latimer E, Simmers J, Sriranganathan N, Roop II R M, Schurig G G, Boyle S M. Brucella abortus deficient in copper/zinc superoxide dismutase is virulent in BALBc mice. Microb Pathog. 1992;12:105–113. doi: 10.1016/0882-4010(92)90113-3. [DOI] [PubMed] [Google Scholar]
- 35.Mackinnon F G, Gorringe A R, Funnell S G P, Robinson A. Intranasal infection of infant mice with Neisseria meningitidis. Microb Pathog. 1992;12:415–420. doi: 10.1016/0882-4010(92)90004-8. [DOI] [PubMed] [Google Scholar]
- 36.Masson L, Holbein B E. Influence of nutrient limitation and low pH on serogroup B Neisseria meningitidis capsular polysaccharide levels: correlation with virulence for mice. Infect Immun. 1985;47:465–471. doi: 10.1128/iai.47.2.465-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCord J M, Fridovich I. Superoxide dismutase. An enzymatic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 38.Nassif X, Puaoi D, So M. Transposition of Tn1545-Δ3 in the pathogenic neisseriae: a genetic tool for mutagenesis. J Bacteriol. 1991;173:2147–2154. doi: 10.1128/jb.173.7.2147-2154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natvig D O, Imlay K, Touati D, Hallewell R A. Human copper-zinc superoxide dismutase-deficient Escherichia coli mutants. J Biol Chem. 1987;262:14697–14701. [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnell S, Steinman H M. Function and stationary-phase induction of periplasmic copper-zinc superoxide dismutase and catalase/peroxidase in Caulobacter crescentus. J Bacteriol. 1995;177:5924–5929. doi: 10.1128/jb.177.20.5924-5929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schryvers A B, Gonzalez G C. Comparison of the abilities of different protein sources of iron to enhance Neisseria meningitidis infection in mice. Infect Immun. 1989;57:2425–2429. doi: 10.1128/iai.57.8.2425-2429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stabel T J, Sha Z, Mayfield J E. Periplasmic location of Brucella abortus Cu/Zn superoxide dismutase. Vet Microbiol. 1994;38:307–314. doi: 10.1016/0378-1135(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 45.Steinman H M. Bacteriocuprein superoxide dismutases in pseudomonads. J Bacteriol. 1985;162:1255–1260. doi: 10.1128/jb.162.3.1255-1260.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinman H M, Ely B. Copper-zinc superoxide dismutase of Caulobacter crescentus: cloning, sequencing, and mapping of the gene and periplasmic location of the enzyme. J Bacteriol. 1990;172:2901–2910. doi: 10.1128/jb.172.6.2901-2910.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St. John G, Steinman H M. Periplasmic copper-zinc superoxide dismutase of Legionella pneumophila: role in stationary-phase survival. J Bacteriol. 1996;178:1578–1584. doi: 10.1128/jb.178.6.1578-1584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tainer J A, Getzoff E D, Beem K M, Richardson J S, Richardson D C. Determination and analysis of the 2Å structure of bovine copper, zinc superoxide dismutase. J Mol Biol. 1982;160:181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- 49.Tatum F M, Detilleux P G, Sacks J M, Halling S M. Construction of Cu-Zn superoxide dismutase deletion mutants of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infect Immun. 1992;60:2863–2869. doi: 10.1128/iai.60.7.2863-2869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Virji M, Kayhty H, Ferguson D J P, Alexandrescu C, Heckels J E, Moxon E R. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5:1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]