Abstract
The effects of heterologous gene dosage as well as Salmonella typhimurium strain variability on immune response toward both the heterologous antigen, the nontoxic mutant of the Escherichia coli heat-labile enterotoxin LTK63, and the carrier Salmonella strain have been analyzed. Effects of a single integration into the host DNA and different-copy-number episomal vectors were compared in S. typhimurium Δcya Δcrp Δasd strains of two different serotypes, UK-1 and SR-11. Expression of the enterotoxin in the different Salmonella isolates in vitro was found to vary considerably and, for the episomal vectors, to correlate with the plasmid copy number. LTK63-specific serum immunoglobulin G (IgG) and mucosal immunoglobulin A (IgA) antibodies were highest in mice immunized with the high-level-expression strain. High anti-LTK63 IgG and IgA titers were found to correspond to higher anti-Salmonella immunity, suggesting that LTK63 exerts an adjuvant effect on response to the carrier. Statistically significant differences in anti-LTK63 immune response were observed between groups of mice immunized with the attenuated Δcya Δcrp UK-1 and SR-11 derivatives producing the antigen at the same rate. These data indicate that the same attenuation in S. typhimurium strains of different genetic backgrounds can influence significantly the immune response toward the heterologous antigen. Moreover, delivery of the LTK63 enterotoxin to the immune system by attenuated S. typhimurium strains is effective only when synthesis of the antigen is very high during the initial phase of invasion, while persistence of the S. typhimurium strain in deep tissues has only marginal influence.
Enterotoxigenic Escherichia coli strains produce a plasmid-encoded heat-labile enterotoxin (LT) (15, 34) related to cholera toxin (CT) (9, 35). LT is composed of two subunits, A and B, which are exported to the periplasmic space, where they assemble into an AB5 multimeric complex (16). Several mutants of LT-A have been constructed, and in particular, a nontoxic mutant which contains a substitution of serine 63 with lysine (LTK63) has been shown to maintain the structural and immunogenic properties of wild-type LT (21, 27, 28). LTK63 has also been found to display the strong mucosal adjuvant activity pertaining to wild-type LT. Efficient induction of mucosal immune response, specifically in the mouse vagina, has been achieved via the intranasal route of immunization (10). For the development of oral vaccines, however, it would be desirable to exploit the properties of LTK63 for enhancing antigen-specific immune response in the intestinal mucosa by means of oral delivery of the potent mucosal adjuvant.
Oral delivery of antigens by live vaccines is known to lead to a more effective production of antigen-specific antibodies in mucosal secretions than oral administration of the soluble antigen (36, 39). Several antigen delivery systems which use as carriers mutant intracellular pathogens that have lost the ability to persist and produce the disease while retaining limited growth in vivo have been developed. In particular, attenuated Salmonella mutants are suitable immunological carriers for virulence determinants from other enteric bacteria in that they can induce humoral immune response selectively at the site of colonization, the gut mucosa. Vaccine strains of Salmonella have been successfully attenuated by introducing different types of mutations (5, 8, 23, 26). Notably, Salmonella strains with a galactose epimerase (galE) mutation (18) or deletions in genes for the biosynthesis of aromatic compounds (aro mutants) (11, 12, 17, 19) or in the adenylate cyclase (cya) and cyclic AMP receptor protein (crp) genes (6) are the most extensively characterized.
Delivery of the B subunit of the E. coli enterotoxin (LT-B) by a galE mutant of Salmonella typhimurium has been shown to elicit low levels of anti-LT-B serum and mucosal antibodies. Since the vector used for expression of LT-B was rapidly lost in vivo, i.e., in the absence of the antibiotic required for selection of the plasmid, the level of immune response could be correlated only with the amount of antigen expressed during the initial phase of invasion (3).
Recently, direct comparison between the aroA aroD/pnirB and the Δcya Δcrp Δasd/asd+ delivery systems for the ability to induce humoral and cellular immunity after a single immunization showed that the former vaccine strain had greater potential as a carrier for antigen delivery (20). However, the balanced lethal asd system for in vivo selection of plasmids expressing heterologous antigens in the attenuated Δcya Δcrp Δasd strains is still very attractive in that asd+ plasmids do not require antibiotic resistance markers for selection while stably maintained in vivo (24). In addition, the Δcya Δcrp Δasd/asd+ delivery system has been reported to induce protective immunity against several pathogens (25, 29, 40). Most of these studies have restricted analysis of the immune response to antigens expressed from the same asd+ plasmid carried by Δcya Δcrp Δasd mutants usually of the same S. typhimurium serotype. In this work, we have analyzed the influence of heterologous gene dosage, and thus level of expression, as well as S. typhimurium strain variability on immune response toward both the heterologous antigen, a nontoxic mutant of E. coli LT, and the carrier Salmonella strain. Effects of a single integration into the host DNA and episomal vectors at different copy numbers were compared in S. typhimurium strains of two different Δcya Δcrp Δasd serotypes, UK-1 and SR-11.
MATERIALS AND METHODS
Strains, plasmids, and media.
The bacterial strains and plasmids used are listed in Table 1. The Δcya Δcrp Δasd S. typhimurium strains were kindly provided by Roy Curtiss III, Washington University, St. Louis, Mo.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| E. coli χ6212 | Φ80d lacZ ΔM15 deoR Δ(lacZYA-argF)U169 supE44 λ− gyrA recA1 relA1 endA1 ΔasdA4 Δ[zhf-2::Tn10] hsdR17 (r− m+) | R. Curtiss III |
| S. typhimurium | ||
| χ4072 SR-11 | pStSR100−gyrA1816 Δcya-1 Δcrp-1 ΔasdA1 Δ(zhf-4::Tn10) | 24 |
| χ4217 SR-11 | pStSR101+gyrA1816 Δcya-1 Δcrp-1 ΔasdA1 Δ(zhf-4::Tn10) | 33 |
| χ3985 UK-1 | pStV1+ Δcya-12 Δ(zid-62::Tn10) Δcrp-11 Δ(zhb::Tn10) | 7 |
| χ3987 UK-1 | pStV1+ Δcya-12 Δ(zid-62::Tn10) Δcrp-11 Δ(zhc-1431::Tn10) ΔasdA1 Δ(zhf-4::Tn10) | 14 |
| χ4217-10E | χ4217 with insertion of mini-Tn5 asd elt-K63 | 2 |
| χ3987-18A | χ3987 with insertion of mini-Tn5 asd elt-K63 | 2 |
| Plasmids | ||
| pBluescript KS-LT | E. coli vector with LT gene inserted into the SmaI-HindIII sites | 28 |
| pBluescript KS-LTK63 | E. coli vector with mutant LTK63 gene inserted into the SmaI-HindIII sites | 28 |
| pYA3074 | Low-copy-number Asd+ vector with pSC101 replicon | R. Curtiss III |
| pYA3149 | Medium-high-copy-number Asd+ vector with pBR322 replicon | R. Curtiss III |
| pYA3137 | High-copy-number Asd+ vector with pUC replicon | R. Curtiss III |
The complex medium used for routine bacterial growth was Luria broth (LB) medium. The minimal medium used was that described by Curtiss and Kelly (6). When required, the antibiotics ampicillin, chloramphenicol, tetracycline, and nalidixic acid were added to culture media at concentrations of 100, 50, 30, 12.5, and 40 μg per ml, respectively. For growth of the Δasd Salmonella strains, the amino acids methionine (20 μg/ml), threonine (8 μg/ml), isoleucine (20 μg/ml), and/or diaminopimelic acid (50 μg/ml) were added to the culture media.
For immunization experiments, bacteria from static overnight cultures were diluted 1:20 in prewarmed LB and grown at 37°C under aeration to give 109 CFU/ml. CFU were determined by plating serial dilutions of cultures of LB agar plates or Difco MacConkey base supplemented with 1% maltose and nalidixic acid for strains χ4072 and χ4217.
DNA manipulations.
Extraction and purification of plasmid DNA were carried out as described by Sambrook et al. (31). Genomic DNA extraction was performed as described by Rossolini et al. (30). Southern blot experiments were performed by bidirectional transfer of DNA from agarose gels to nylon membranes. Specific DNA probes were obtained amplifying DNA sequences by PCR and labeled with [α-32P]dATP, using the random-primers method.
The pBluescript KS plasmids containing the wild-type elt or the eltK63 mutated gene (provided by M. Pizza) were described previously (28). The 2-kb BamHI-SalI fragment carrying elt or eltK63 was subcloned into the pYA3074, pYA3149, and pYA3137 asd vectors (provided by R. Curtiss III). The resulting plasmids were transformed and amplified in E. coli χ6212 and then purified and transformed into the attenuated S. typhimurium strains. Transformants were selected in LB medium. The fermentation pattern, auxotrophic requirements, and lipopolysaccharide content were routinely analyzed in all strains. The completely smooth lipopolysaccharide was examined by polyacrylamide gel electrophoresis (PAGE) and silver staining after periodic acid treatment (38).
The number of copies per cell of each construct was determined by Southern blot analysis of total DNA extracted from the different strains, digested with BamHI and SalI, and hybridized to an elt-specific probe and to a probe specific for the spvC gene of S. typhimurium. The relative intensity of the elt-specific band was calculated by using a densitometer (Ultroscan; LKB).
The nucleotide sequence of mini-Tn5 asd eltK63 insertions was determined from DNA fragments obtained by inverse PCR. Briefly, SalI-XhoI-digested genomic DNA fragments were incubated with T4 DNA ligase overnight at 15°C. Inverse PCR was performed by using an Expand 20 kbplus PCR kit (Boehringer) in a total volume of 50 μl with 6 ng of DNA (2 μl of the ligation mixture) as a template and 300 nM each of the following primers: 5′-ACAGACGTGAGCCTGAAAGGTTTGG-3′ and 5′-CTTCCACTACAGGGAGCTGTTATAGC-3′ (complementary to the promoter and to the 3′ end of the coding region of eltK63, respectively).
SDS-PAGE and immunoblot analysis.
Bacterial cultures were grown to the logarithmic or stationary phase in LB broth. Cells were collected and lysed by boiling (5 to 10 min) in LSB buffer (2% sodium dodecyl sulfate [SDS], 10% glycerol, 100 mM dithiothreitol, 62.5 mM Tris-HCl [pH 6.8]). Total proteins in cell extracts were quantified with a micro-bicinchoninic acid protein assay reagent kit (Pierce, Rockford, Ill.). Proteins were separated by SDS-PAGE (15% polyacrylamide gel) and transferred to nitrocellulose membranes. Mouse polyclonal anti-LT antiserum was used as the primary antibody. Bands were visualized by enhanced chemiluminescence (Amersham Life Science) or with 4-chloro-1-naphthol (Sigma) as the substrate. Evaluation of the amount of LT produced by the different strains was obtained by densitometric analysis of bands, using purified enterotoxin or CT as a reference.
Immunization and sampling.
Groups of five or seven 6- to 8-week-old female BALB/c mice (Charles River, Calco, Como, Italy) were immunized intragastrically with two or four doses containing ca. 8 × 108 CFU of each Salmonella strain. Mice were deprived of water and food for 12 h and fed with 30 μl of 0.2 M sodium bicarbonate to neutralize stomach acidity 30 min prior to immunization. Groups of mice were immunized on days 0 and 10 to 14 or on days 0, 10, 17, and 24.
Serum samples and intestinal mucus were collected on days 14, 17, 24, 36, 50, and 66. Blood samples were collected from the retro-orbital sinus of each anesthetized mouse. To collect gut secretions, mice were sacrificed, the small intestine was excised, and the mucus was scraped from the luminal surface. Mucus samples were collected in centrifuge tubes containing 5 μl each of 0.1 M phenylmethylsulfonyl fluoride in ethanol, 1% bovine serum albumin, and 1% sodium azide. After centrifugation at 12,000 × g for 10 min at 4°C, supernatant fluids were collected, 5 μl of a solution containing 0.1 M phenylmethylsulfonyl fluoride and 1% sodium azide was added, and samples were stored at −20°C.
Spleens and Peyer’s patches were removed from sacrificed animals and processed as described by Curtiss and Kelly (6). After disruption in a Dounce tissue homogenizer, suspensions were diluted in phosphate-buffered saline (PBS) and plated on MacConkey agar containing 1% lactose. The expected phenotype of S. typhimurium strains recovered from immunized animals was verified on MacConkey agar supplemented with 1% maltose.
ELISA for LT and Salmonella.
Individual mouse serum and mucus samples were tested for immunoglobulin A (IgA) and IgG antibodies against LT or whole Salmonella cells by enzyme-linked immunosorbent assay (ELISA). A PBS solution of purified LT (a gift from M. Pizza) was absorbed to 96-well ELISA plates (Greiner-GmbH, Kremsmunster, Austria) at 0.2 μg per well overnight at 4°C. For anti-Salmonella antibody titration, bacteria were grown overnight, harvested by centrifugation, and resuspended in PBS at 3 × 1011 CFU/ml. Bacteria were heat killed for 10 min at 80°C and stored at −20°C. Each sample well was coated with 0.1 ml of this suspension diluted 100-fold in 0.1 M carbonate buffer (pH 9.6). Plates were blocked with PBS containing 0.05% Tween 20 and 1% bovine serum albumin for 2 h at 37°C. After washing with PBS, an anti-isotype secondary antibody (Sigma) was added and plates were incubated for 2 h at 37°C. Serum samples were incubated with alkaline phosphatase-conjugated goat anti-mouse IgG (γ-chain specific) antibodies, mucus samples were incubated with biotin-conjugated goat anti-mouse IgA (α-chain specific) antibodies. Plates were washed with PBS, developed with a solution of p-nitrophenyl phosphate (1 mg/ml) in 1 M diethanolamine buffer (pH 9.8), and read in an ELISA reader (Titertek Multiscan) at 405 nm. Titers were expressed as the last dilution that gave an optical density at 405 nm of 0.1. Under these experimental conditions, preimmune samples always gave an optical density at 405 nm of <0.1 from the first dilution.
Nucleotide sequence accession number.
The nucleotide sequence of the mini-Tn5 insertion in the virulence plasmid described in this work has been assigned GenBank accession no. AF025956.
RESULTS
Influence of copy number on expression level of LT-K63 in vitro.
Expression of LTK63 by S. typhimurium attenuated strains carrying the eltK63 gene cloned into the pYA plasmids listed in Table 1 was examined. Western blot analysis of total-cell extracts and periplasmic fractions from logarithmic- and stationary-growth-phase cultures of the different S. typhimurium strains showed, as expected, a good correlation between the amount of LTK63 produced by each strain and the copy number of the recombinant plasmid carried by the strain. Expression of the enterotoxin gene, which is under the control of its own promoter in these constructs, was constitutive in all strains analyzed and in all conditions tested.
Representative results are shown in Fig. 1a. A fivefold difference was observed between the amount of toxin synthesized by strain χ3987:pYA3074-LTK63 (Fig. 1a, lane 3) and that produced by χ3987:pYA3137-LTK63 (Fig. 1a, lane 5). The medium-high-copy-number plasmid pYA3149, in turn, could direct synthesis of LTK63 at a level slightly lower than that found for the high-copy-number plasmid pYA3137 in the same strain (Fig. 1a; compare lanes 4 and 5). However, some differences were found between the strains in that χ4072:pYA3137-LTK63 produced the same amount of enterotoxin as χ4072:pYA3149-LTK63 but showed an unusual growth curve with a 3-h lag phase followed by an exponential growth phase with the same doubling time as the other strains. Furthermore, it is noteworthy that strain χ4072:pYA3137-LTK63 lysed during static growth.
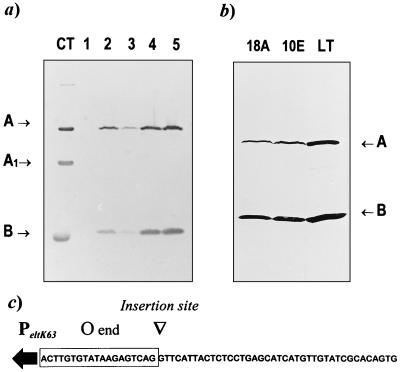
FIG. 1.
Western blot analysis of expression of LTK63 in different S. typhimurium strains, using an anti-LT antibody. (a) Total-cell extracts of stationary-growth-phase cultures of strains χ3987 (lane 1), χ3987-18A (lane 2), χ3987:pYA3074-LTK63 (lane 3), χ3987:pYA3149-LTK63 (lane 4), and χ3987:pYA3137-LTK63 (lane 5); (b) total-cell extracts of late-logarithmic-growth-phase cultures of eltK63 single-copy integrants. Total proteins in cell extracts were quantified, and 30 μg of each sample was used for the immunoblot. Purified CT (3 μg) and LT (3 μg) were used as a control and as a molecular mass marker for the A, A1, and B subunits of the toxins. (c) Nucleotide sequence of the integration site of the mini-Tn5 asd eltK63 into the virulence plasmid of strains χ3987-18A and χ4217-10E. The sequence of the mini-Tn5 19-bp O end flanking the eltK63 promoter (PeltK63; arrow) is boxed.
To assess if the phenotype conferred to strain χ4072 by plasmid pYA3137-LTK63 was due to the presence of the high-copy-number plasmid or to toxicity of LTK63, a preliminary analysis of the attenuated strains carrying plasmid pYA3137 with and without the eltK63 insert was carried out by plating cells from static growth onto selective medium. Lysis was observed only for strain χ4072 containing the eltK63 insert in plasmid pYA3137. The plasmid without the insert did not cause lysis in any of the strains analyzed (data not shown). Likely, expression of eltK63 from the high-copy-number plasmid is toxic for strain χ4072 during static growth.
Two S. typhimurium strains, χ3987-18A and χ4217-10E, carrying a single-copy integration of the eltK63 gene into the genome and producing equally high amounts of LTK63, were also analyzed for expression of the enterotoxin. The two strains were obtained by inserting a single copy of a mini-Tn5 asd eltK63 minitransposon into the genomes of S. typhimurium χ3987 and χ4217. It should be noted that strain χ4217 is an SR-11 derivative (33), while strain χ3987 is a derivative of the S. typhimurium UK-1 strain χ3985 (14). Insertion of the mini-Tn5 asd eltK63 was found to have occurred in the same locus of the virulence plasmid in both strains χ3987-18A and χ4217-10E. The nucleotide sequence of this locus at the junction of the O end of the minitransposon was determined from DNA fragments obtained by inverse PCR. The locus does not show any homology to known S. typhimurium sequences (Fig. 1c).
In Fig. 1a, lane 2, the level of LTK63 production of strain χ3987-18A is compared with those of the episomal systems used for expression in the same strain. The amount of LTK63 found in the single-copy integrant was threefold greater than that observed for the low-copy-number plasmid pYA3074. Figure 1b shows a direct comparison of the level of LTK63 expression in late-logarithmic-growth-phase cultures of the eltK63 single-copy integrants.
It was also found that in all strains analyzed, the LT was secreted to the periplasmic space (data not shown), where presumably it is assembled into its heterohexameric form, as it has been shown that assembly of LT takes place in the periplasm in E. coli (16).
Serum antibody response induced by S. typhimurium χ3987 expressing different levels of LTK63.
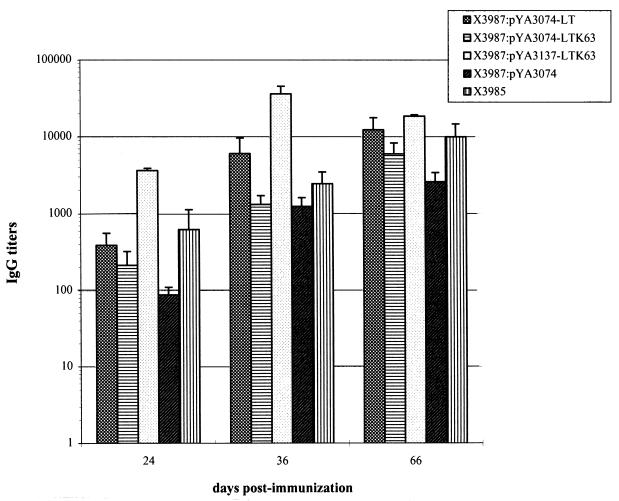
Groups of five BALB/c mice were immunized orally with strain χ3987 containing the low-copy-number plasmids pYA3074-LT and pYA3074-LTK63 or the high-copy-number plasmid pYA3137-LTK63. The same strain carrying the pYA3074 vector without the LTK63 insert and the parental attenuated asd+ strain χ3985 were used as control strains. A single oral booster dose of 8 × 108 S. typhimurium cells was administered 10 days after the first inoculation. The Salmonella-specific IgG titers in serum samples from immunized animals 24, 36, and 66 days after the first dosing were found to differ significantly particularly at day 24, in that strain χ3987:pYA3137-LTK63 induced an average 10-fold-higher response. A diminished, but still significant, difference was observed at day 66 (Fig. 2). A possible explanation for the less pronounced difference at day 66 in mice receiving the high-copy-number plasmid strain could be that the higher anti-Salmonella titers elicited by this strain after the first immunization result in a reduced number of organisms able to colonize after the second inoculum.
FIG. 2.
Influence of amounts of LT or LTK63 synthesized by S. typhimurium on anti-Salmonella immune response. Serum IgG activity to S. typhimurium in mice immunized with two doses of strain χ3987 containing the low-copy-number plasmids pYA3074-LT and pYA3074-LTK63, the high-copy-number plasmid pYA3137-LTK63, or the control plasmid pYA3074 without the enterotoxin gene insert was determined. The two doses were given to groups of five mice on days 0 and 10. IgG responses were detected by ELISA 24, 36, and 66 days after primary immunization. The standard error of the mean was calculated for all data, and mean values were compared by Student’s t test. P values of ≤0.0001 were considered statistically significant.
No significant difference was observed between the antibody responses induced by strains χ3987:pYA3074-LT and χ3987:pYA3074-LTK63. When the anti-Salmonella IgG titers induced by the two control strains are compared, it should be noted that the asd mutation present in strain χ3987 further attenuates S. typhimurium. Thus, the higher anti-Salmonella IgG titers elicited by the asd+ strain χ3985 are presumably due to a higher persistence of this strain in deep organs (Table 2).
TABLE 2.
Persistence of S. typhimurium strains in deep organsa
| Strain | No. of bacteria
|
|||
|---|---|---|---|---|
| Peyer’s patches
|
Spleen
|
|||
| 24 dpi | 50 dpi | 24 dpi | 50 dpi | |
| χ3985 | 1.1 × 104 | 0.8 × 101 | 1.7 × 104 | 2.4 × 103 |
| χ3987-18A | 5.2 × 103 | 0 | 6.3 × 103 | 7.5 × 101 |
| χ4217-10E | 4.6 × 103 | 1.2 × 102 | 9.9 × 103 | 9.5 × 102 |
| χ3987:pYA3137/LT-K63 | 1.1 × 103 | 1.0 × 101b | 2.7 × 103 | 1.3 × 102b |
| χ3987:pYA3149/LT-K63 | 1.8 × 103 | 0b | 1.4 × 103 | 0b |
| χ4072:pYA3149/LT-K63 | 2.9 × 103 | 0.3 × 101b | 1.7 × 102 | 0.2 × 101b |
Groups of seven BALB/c mice were inoculated intragastrically with two doses of 8 × 108 S. typhimurium organisms. Peyer’s patches and spleens were removed at specified days postimmunization (dpi), homogenized in PBS, and plated on MacConkey agar containing 1% lactose.
Recovery of S. typhimurium from Peyer’s patches and spleens of mice immunized with four doses of 8 × 108 S. typhimurium organisms.
Analysis of LT-specific IgG titers in the same serum samples was carried out only for the three χ3987 strains containing the LT or LTK63 construct. IgG titers were found to be negligible with both low-copy-number constructs, while samples from mice immunized with the high-copy-number plasmid-bearing strain χ3987:pYA3137-LTK63 showed high anti-LT IgG titers at days 24 (1:10,310), 36 (1:31,310), and 66 (1:20,970).
Influence of Salmonella genetic background on the induction of serum antibody response.
A similar immunization scheme was extended to include oral inoculation of groups of seven BALB/c mice with 8 × 108 cells of the three S. typhimurium strains carrying a single-copy insertion of the mini-Tn5 asd eltK63 minitransposon into the genomes of S. typhimurium χ3987 and χ4217 (Table 1).
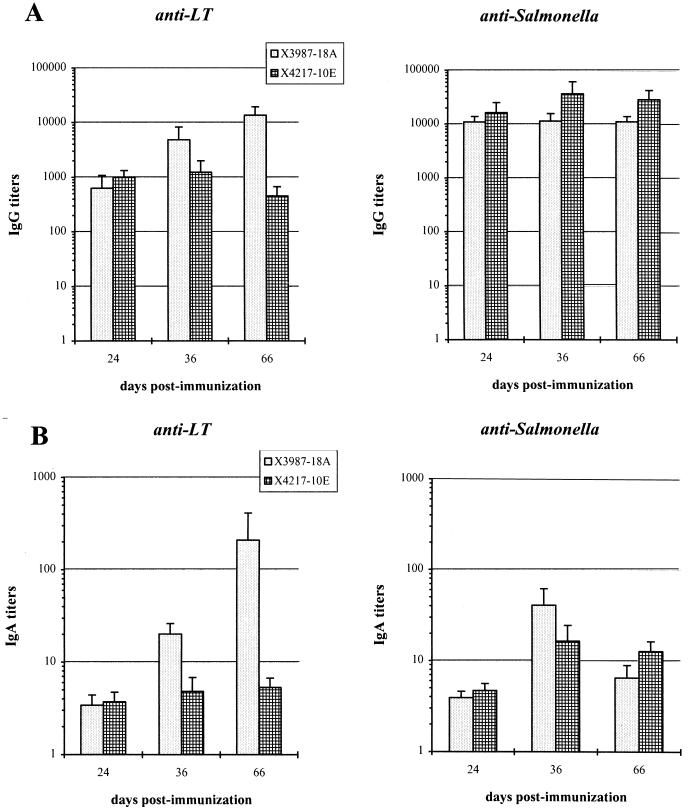
The LT-specific and Salmonella-specific IgG titers determined in serum samples from mice immunized with these strains are shown in Fig. 3A. While the anti-Salmonella serum responses were similar for the two strains, a significant difference was observed in the anti-LT titers, particularly at day 66 from immunization. Sera from mice immunized with strain χ3987-18A showed LT-specific IgG titers 10- to 100-fold higher than those in samples from the other group of mice. It is interesting that this occurs in spite of the fact that persistence of this S. typhimurium strain in deep organs 50 days after immunization was found to be over 10-fold lower than that of strain χ4217-10E (Table 2) and that the levels of LTK63 produced in vitro were equally high for the two strains (Fig. 1b). Possibly, this may be attributed to a difference in the level of expression of LTK63 in vivo during the initial steps of invasion.
FIG. 3.
ELISA analysis of immune response induced by two different genetic backgrounds carrying LTK63 single-copy integrations. Groups of seven mice were inoculated with the two S. typhimurium strains on days 0 and 14. Samples were collected at 24, 36, and 66 days after primary immunization. (A) Serum anti-LTK63 and anti-Salmonella IgG antibody response; (B) mucosal anti-LTK63 and anti-Salmonella IgA titers. Mean values of each group were compared by Student’s t test. P values of ≤0.05 were considered statistically significant.
Differential induction of serum antibody response by high LTK63 expression.
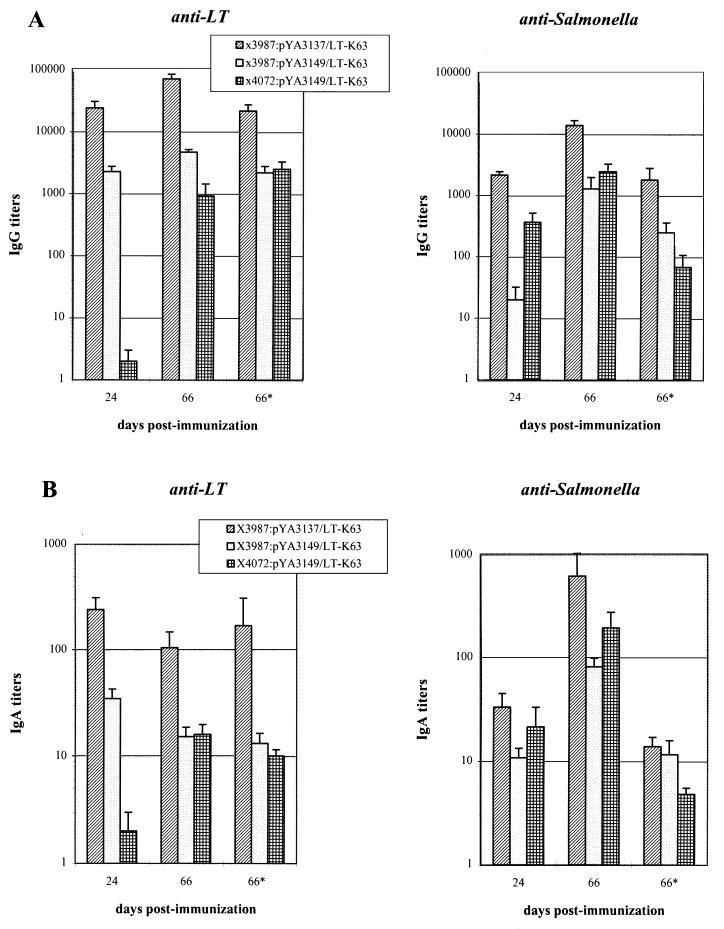
Since the amount of enterotoxin produced by S. typhimurium seemed the most crucial parameter in determining a good immune response, an immunization experiment was carried out with four doses of the S. typhimurium strains containing the pYA3149-LTK63 and pYA3137-LTK63 constructs. The strains carrying the medium-high-copy-number plasmid were included in this experiment since strain χ4072:pYA3137-LTK63 lysed after growth in static culture. In addition, in order to compare this immunization scheme with the previous ones, groups of seven mice were inoculated with only two doses of the same plasmid-bearing S. typhimurium strains used for immunizing mice with four doses. The immune response in these animals was evaluated only at day 66 from the first inoculum. The results presented in Fig. 4A show that both the anti-LTK63 and the anti-Salmonella IgG titers were highest for the group of mice immunized with strain χ3987:pYA3137-LTK63.
FIG. 4.
ELISA analysis of immune response induced by UK-1 (χ3987) and SR-11 (χ4072) strains expressing high levels of LTK63. Groups of seven mice were immunized with two (on days 0 and 10) or four doses (on days 0, 10, 17, and 24) of strains χ3987:pYA3137-LTK63, χ3987:pYA3149-LTK63, and χ4072:pYA3149-LTK63. Samples were collected at day 66 after primary immunization from mice immunized with two doses (66*) and at 24 and 66 days from mice immunized with four doses. (A) Serum anti-LTK63 or anti-Salmonella total IgG titers; (B) mucosal anti-LTK63 or anti-Salmonella total IgA titers. Mean values of each group were compared by Student’s t test. P values of ≤0.001 (A) and ≤0.01 (B) were considered statistically significant.
Analysis of mucosal antibody response.
Intestinal mucus samples from mice immunized with the S. typhimurium strains reported in Table 2 were analyzed for LTK63-specific and Salmonella-specific IgA responses. The mean IgA titers of each group of seven mice are shown in Fig. 3B and 4B. The anti-LTK63 secretory response was clearly higher in mice inoculated with the medium-high- and high-episomal-expression constructs (Fig. 4B) than in mice immunized with the eltK63 integrants (Fig. 3B), with the exception of the group immunized with strain χ3987-18A. The high anti-LTK63 IgA titers in the latter group of mice correlate well with the anti-LTK63 serum IgG titers found in the same group (Fig. 3A), although the variation of titers in individual mice was greater for the IgA samples than for the IgG samples.
A correlation was observed also between the anti-Salmonella IgG (Fig. 4A) and IgA titers (Fig. 4B) of mice immunized with four doses of the strains carrying plasmid pYA3149-LTK63 or pYA3137-LTK63. Animals inoculated with strain χ3987:pYA3137-LTK63 always showed higher systemic and local immune responses than the other two groups of mice. Moreover, after the fourth immunization, higher IgG titers were paralleled by higher IgA titers in all groups (Fig. 4, day 66).
The stability in vivo of the eltK63 constructs was assessed by Southern blot analysis of genomic or plasmid DNA extracted from bacteria isolated from Peyer’s patches and spleens of immunized animals. All strains were found to have maintained the eltK63 construct. Western blot analysis of cell extracts from the same S. typhimurium isolates also confirmed that the level of LTK63 synthesis was unchanged after the passage in vivo (data not shown).
DISCUSSION
As most studies on attenuated Salmonella carriers have analyzed differences in the induction of immunity between levels of antigen expression (3, 4), selection for vector maintenance in vivo, or attenuation of the delivery system (20, 37), we have compared the immune responses induced by the same antigen when delivered by different strain-plasmid combinations. In particular, we have analyzed a single integration into the host DNA and multicopy episomal vectors in three Δcya Δcrp Δasd strains of different genetic backgrounds expressing a nontoxic mutant of the E. coli LT.
A first comparison of variation in antigen delivery by the same S. typhimurium strain in relation to different expression levels of the antigen was made by analyzing the systemic response in mice immunized with strain χ3987 containing the low- and high-expression constructs. The lack of an LTK63-specific immune response in mice immunized with the low-expression construct, in contrast to high levels of IgG antibodies elicited by the high-expression vector, correlates with a fivefold difference in amount of LTK63 synthesized in vitro by the two constructs. This finding suggests that for this S. typhimurium strain, the threshold for induction of anti-LTK63 systemic response is high (see below). Alternatively, expression in vivo of LTK63 from the two plasmids may not correlate with what is seen in vitro, although expression in both constructs is driven by the same promoter.
An interesting finding of this experiment is that strain χ3987:pYA3137-LTK63 induced anti-Salmonella IgG titers significantly higher, during the first 3 to 5 weeks postimmunization, than those induced by control strains or by low LTK63 producers. LTK63 has been previously shown to exert an adjuvant effect on immune response to different antigens administered to mice by different routes (10).
By comparing S. typhimurium strains with different genetic backgrounds and expressing the same amount of LTK63 from a single integration site, the influence of strain variability could be inferred from the anti-LTK63 antibody response, while both systemic and local immune responses to Salmonella were not significantly different for the different strains. Only strain χ3987-18A could elicit high LTK63-specific IgG and IgA titers. Considering that strain χ4217-10E contains the eltK63 gene integrated in the same locus of the virulence plasmid as strain χ3987-18A and can persist longer in deep tissues, this result implies that very early events at the priming of the immune response are particularly critical in the induction of an anti-LT immune response (4). Furthermore, the χ3987-18A genetic background may be intrinsically more effective in priming anti-LTK63 immune responses. The observation that high expression of the antigen is crucial during the early phase of the induction of the immune response is consistent with the finding by others that oral administration of Salmonella strains expressing LT-B induces a dramatic increase in the level of mRNA of some cytokines (e.g., interleukin-12) at mucosal sites within hours following immunization (1).
One of the advantages of the Δcya Δcrp/asd system is the effectiveness of the asd selection for plasmid maintenance in vivo (7, 13, 24). As previously shown by other reports, the stability in vivo of the asd vectors used in this study for expression of LTK63 was found to be very high even for the high-copy-number plasmid pYA3137. It should be noted, however, that expression of LTK63 from the latter plasmid conferred an unusual phenotype to strain χ4072 in vitro: lysis after static growth. For this reason, the analysis of immune response had to be extended to strains containing the medium-high-copy-number plasmid pYA3149. Comparison between both systemic and local antibody responses induced by UK-1 and SR-11 strains expressing LTK63 from the high- or the medium-high-copy-number plasmids clearly showed that, in addition to any influence of the S. typhimurium strain delivering the antigen, the level of expression of the heterologous antigen is critical for a high immune response. A 2-fold difference in amount of LTK63 produced by strain χ3987:pYA3137-LTK63 was sufficient to enhance both IgG and IgA titers 10-fold. An adjuvant effect of LTK63 could be inferred also from these data, since higher Salmonella-specific titers correlated with higher expression of LTK63.
In conclusion, our data indicate that the same attenuation in different genetic background S. typhimurium strains, expressing the recombinant antigen at the same level, can produce significantly different immune responses. Moreover, delivery of the LTK63 antigen to the immune system by attenuated S. typhimurium strains is effective only when synthesis of the antigen is very high during the initial phase of invasion, while persistence of the S. typhimurium strain in deep tissues has only marginal influence.
ACKNOWLEDGMENTS
We are very grateful to Roy Curtiss III for providing plasmids and attenuated strains and for helpful advice. We thank Mariagrazia Pizza, Maria Teresa De Magistris, and Giuseppe Del Giudice for many valuable discussions and critical reading of the manuscript.
M.B. was supported by a CNPq/Rhae scholarship (260023/93.0) and by an UNIDO/ICGEB fellowship.
REFERENCES
- 1.Bost K L, Clements J D. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1995;63:1076–1083. doi: 10.1128/iai.63.3.1076-1083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brocchi, M., M. G. Covone, E. Palla, and C. L. Galeotti. Unpublished data.
- 3.Cárdenas L, Clements J D. Stability, immunogenicity and expression of foreign antigens in bacterial vaccine vectors. Vaccine. 1993;11:126–135. doi: 10.1016/0264-410x(93)90007-k. [DOI] [PubMed] [Google Scholar]
- 4.Cárdenas L, Dasgupta U, Clements J D. Influence of strain viability and antigen dose on the use of attenuated mutants of Salmonella as vaccine carriers. Vaccine. 1994;12:833–840. doi: 10.1016/0264-410x(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 5.Chatfield S N, Fairweather N, Charles I, Pickard D, Levine M, Hone D, Posada M, Strugnell R A, Dougan G. Construction of a genetically defined Salmonella typhi Ty2 aroA, aroC mutant for the engineering of a candidate oral typhoid-tetanus vaccine. Vaccine. 1992;10:53–60. doi: 10.1016/0264-410x(92)90420-o. [DOI] [PubMed] [Google Scholar]
- 6.Curtiss R, III, Kelly S M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtiss R, III, Kelly S M, Gulig P A, Nakayama K. Selective delivery of antigens by recombinant bacteria. Curr Top Microbiol Immunol. 1989;146:35–49. doi: 10.1007/978-3-642-74529-4_4. [DOI] [PubMed] [Google Scholar]
- 8.Curtiss R., III . Attenuated Salmonella strains as live vectors for the expression of foreign antigens. In: Woodrow G C, Levine M M, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1990. pp. 161–188. [Google Scholar]
- 9.Dallas W S, Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980;288:499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- 10.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougan G. The molecular basis for the virulence of bacterial pathogens: implications for oral vaccine development. Microbiology. 1994;140:215–224. doi: 10.1099/13500872-140-2-215. [DOI] [PubMed] [Google Scholar]
- 12.Dougan G, Chatfield S N, Pickard D, O’Callaghan D, Maskell D. Construction and characterization of Salmonella vaccine strains harbouring mutations in two different aro genes. J Infect Dis. 1988;158:1329–1335. doi: 10.1093/infdis/158.6.1329. [DOI] [PubMed] [Google Scholar]
- 13.Galan J E, Nakayama K, Curtiss R., III Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella typhimurium. Gene. 1990;94:29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 14.Gentry-Weeks C R, Hultsch A-L, Kelly S M, Keith J M, Curtiss R., III Cloning and sequencing of a gene encoding a 21-kilodalton outer membrane protein from Bordetella avium and expression of the gene in Salmonella typhimurium. J Bacteriol. 1992;174:7729–7742. doi: 10.1128/jb.174.23.7729-7742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyles C L, Barnum D A. A heat-labile enterotoxin from strains of E. coli enteropathogenic for pigs. J Infect Dis. 1969;120:419–426. doi: 10.1093/infdis/120.4.419. [DOI] [PubMed] [Google Scholar]
- 16.Hofstra H, Witholt B J. Kinetics of synthesis, processing and membrane transport of heat-labile enterotoxin, a periplasmic protein in E. coli. J Biol Chem. 1984;259:15182–15187. [PubMed] [Google Scholar]
- 17.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 18.Hone D, Morona R, Attridge S, Hackett J. Construction of a defined galE mutant of Salmonella for vaccine use. J Infect Dis. 1987;156:167–174. doi: 10.1093/infdis/156.1.167. [DOI] [PubMed] [Google Scholar]
- 19.Hone D, Harris A M, Chatfield S, Dougan G, Levine M M. Construction of genetically-defined double aro mutants of Salmonella typhi. Vaccine. 1991;9:810–816. doi: 10.1016/0264-410x(91)90218-u. [DOI] [PubMed] [Google Scholar]
- 20.Karem K L, Chatfield S, Kuklin N, Rouse B T. Differential induction of carrier antigen-specific immunity by Salmonella typhimurium live-vaccine strains after single mucosal or intravenous immunization of BALB/c mice. Infect Immun. 1995;63:4557–4563. doi: 10.1128/iai.63.12.4557-4563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magagnoli C, Manetti R, Fontana M R, Giannelli V, Giuliani M M, Rappuoli R, Pizza M. Mutations in the A subunit affect yield, stability, and protease sensitivity of nontoxic derivatives of heat-labile enterotoxin. Infect Immun. 1996;64:5434–5438. doi: 10.1128/iai.64.12.5434-5438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maskell D J, Sweeney K J, O’Callaghan D, Hormaeche C E, Liew F Y, Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin B subunit to the murine secretory and systemic immune system. Microb Pathog. 1987;2:211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- 23.Miller S I, Loomis W P, Alpuche-Aranda C, Behlau I, Hohmann E. The PhoP virulence regulon and live oral Salmonella vaccines. Vaccine. 1993;11:122–125. doi: 10.1016/0264-410x(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama K, Kelly S M, Curtiss R., III Construction of an ASD+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Bio/Technology. 1988;6:693–697. [Google Scholar]
- 25.Oyston P C F, Williamson E D, Leary S E C, Eley S M, Griffin K F, Titball R W. Immunization with live recombinant Salmonella typhimurium aroA producing F1 antigen protects against plague. Infect Immun. 1995;63:563–568. doi: 10.1128/iai.63.2.563-568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickard D, Li J, Roberts M, Maskell D, Hone D, Levine M M, Dougan G, Chatfield S. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun. 1994;62:3984–3993. doi: 10.1128/iai.62.9.3984-3993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizza M, Domenighini M, Hol W, Giannelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 28.Pizza M, Fontana M R, Giuliani M M, Domenighini M, Magagnoli C, Giannelli V, Nucci D, Hol W, Manetti R, Rappuoli R. A genetically detoxified derivative of heat-labile Escherichia coli enterotoxin induces neutralizing antibodies against the A subunit. J Exp Med. 1994;180:2147–2153. doi: 10.1084/jem.180.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redman T K, Harmon C C, Lallone R L, Michalek S M. Oral immunization with recombinant Salmonella typhimurium expressing surface protein antigen A of Streptococcus sobrinus: dose response and induction of protective humoral responses in rats. Infect Immun. 1995;63:2004–2011. doi: 10.1128/iai.63.5.2004-2011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossolini G M, Muscas P, Chiesurin A, Satta G. Molecular cloning and expression in Escherichia coli of the Salmonella typhi gene cluster coding for type 1 fimbriae. In: Cabello F, et al., editors. The biology of Salmonella. New York, N.Y: Plenum Publishing Co.; 1993. pp. 408–412. [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Sanderson K E, Hessel A, Rudd K E. Genetic map of Salmonella typhimurium, edition VIII. Microbiol Rev. 1995;59:241–303. doi: 10.1128/mr.59.2.241-303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schödel F, Kelly S M, Peterson D L, Milich D R, Curtiss R., III Hybrid hepatitis B virus core-pre-S proteins synthesized in avirulent Salmonella typhimurium and Salmonella typhi for oral vaccination. Infect Immun. 1994;62:1669–1676. doi: 10.1128/iai.62.5.1669-1676.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith H W, Halls S. Escherichia coli enterotoxin. J Pathol Bacteriol. 1967;93:531–543. doi: 10.1002/path.1700930212. [DOI] [PubMed] [Google Scholar]
- 35.Spicer E K, Kavanaugh W M, Dallas W S, Falkow S, Konigsberg W H, Shafer D. Sequence homologies between A subunits of E. coli and V. cholera enterotoxin. Proc Natl Acad Sci USA. 1981;78:50–54. doi: 10.1073/pnas.78.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staats H F, Jackson R J, Marinaro M, Takahashi I, Kiyono H, McGhee J R. Mucosal immunity to infection with implications for vaccine development. Curr Opin Immunol. 1994;6:572–583. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 37.Tacket C O, Hone D M, Curtiss III R, Kelly S M, Losonsky G, Guers L, Harris A M, Edelman R, Levine M M. Comparison of the safety and immunogenicity of ΔaroC ΔaroD and Δcya Δcrp Salmonella typhi strains in adult volunteers. Infect Immun. 1992;60:536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai C M, Frash C E. A sensitive stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1983;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 39.Walker R I. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Stanley S L. Oral immunization with an attenuated vaccine strain of Salmonella typhimurium expressing the serine-rich Entamoeba histolytica protein induces an antiamebic immune response and protects gerbils from amebic liver abscess. Infect Immun. 1996;64:1526–1531. doi: 10.1128/iai.64.5.1526-1531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]