Abstract
Background
Acute kidney injury (AKI) is common in patients undergoing cardiac surgery among whom it is associated with poor outcomes, prolonged hospital stays and increased mortality. Statin drugs can produce more than one effect independent of their lipid lowering effect, and may improve kidney injury through inhibition of postoperative inflammatory responses.
Objectives
This review aimed to look at the evidence supporting the benefits of perioperative statins for AKI prevention in hospitalised adults after surgery who require cardiac bypass. The main objectives were to 1) determine whether use of statins was associated with preventing AKI development; 2) determine whether use of statins was associated with reductions in in‐hospital mortality; 3) determine whether use of statins was associated with reduced need for RRT; and 4) determine any adverse effects associated with the use of statins.
Search methods
We searched the Cochrane Renal Group's Specialised Register to 13 January 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) that compared administration of statin therapy with placebo or standard clinical care in adult patients undergoing surgery requiring cardiopulmonary bypass and reporting AKI, serum creatinine (SCr) or need for renal replacement therapy (RRT) as an outcome were eligible for inclusion. All forms and dosages of statins in conjunction with any duration of pre‐operative therapy were considered for inclusion in this review.
Data collection and analysis
All authors extracted data independently and assessments were cross‐checked by a second author. Likewise, assessment of study risk of bias was initially conducted by one author and then by a second author to ensure accuracy. Disagreements were arbitrated among authors until consensus was reached. Authors from two of the included studies provided additional data surrounding post‐operative SCr as well as need for RRT. Meta‐analyses were used to assess the outcomes of AKI, SCr and mortality rate. Data for the outcomes of RRT and adverse effects were not pooled. Adverse effects taken into account were those reported by the authors of included studies.
Main results
We included seven studies (662 participants) in this review. All except one study was assessed as being at high risk of bias. Three studies assessed atorvastatin, three assessed simvastatin and one investigated rosuvastatin. All studies collected data during the immediate perioperative period only; data collection to hospital discharge and postoperative biochemical data collection ranged from 24 hours to 7 days. Overall, pre‐operative statin treatment was not associated with a reduction in postoperative AKI, need for RRT, or mortality. Only two studies (195 participants) reported postoperative SCr level. In those studies, patients allocated to receive statins had lower postoperative SCr concentrations compared with those allocated to no drug treatment/placebo (MD 21.2 µmol/L, 95% CI ‐31.1 to ‐11.1). Adverse effects were adequately reported in only one study; no difference was found between the statin group compared to placebo.
Authors' conclusions
Analysis of currently available data did not suggest that preoperative statin use is associated with decreased incidence of AKI in adults after surgery who required cardiac bypass. Although a significant reduction in SCr was seen postoperatively in people treated with statins, this result was driven by results from a single study, where SCr was considered as a secondary outcome. The results of the meta‐analysis should be interpreted with caution; few studies were included in subgroup analyses, and significant differences in methodology exist among the included studies. Large high quality RCTs are required to establish the safety and efficacy of statins to prevent AKI after cardiac surgery.
Keywords: Adult, Humans, Acute Kidney Injury, Acute Kidney Injury/prevention & control, Acute Kidney Injury/therapy, Atorvastatin, Cardiac Surgical Procedures, Cardiopulmonary Bypass, Cardiopulmonary Bypass/adverse effects, Coronary Artery Bypass, Coronary Artery Bypass/adverse effects, Creatinine, Creatinine/blood, Fluorobenzenes, Fluorobenzenes/therapeutic use, Heptanoic Acids, Heptanoic Acids/therapeutic use, Hydroxymethylglutaryl‐CoA Reductase Inhibitors, Hydroxymethylglutaryl‐CoA Reductase Inhibitors/therapeutic use, Length of Stay, Pyrimidines, Pyrimidines/therapeutic use, Pyrroles, Pyrroles/therapeutic use, Randomized Controlled Trials as Topic, Renal Replacement Therapy, Rosuvastatin Calcium, Simvastatin, Simvastatin/therapeutic use, Sulfonamides, Sulfonamides/therapeutic use
Plain language summary
Do statins prevent kidney failure in adults after surgery where cardiac bypass is used?
Heart surgeries are performed routinely in developed countries and their safety has increased significantly. However, heart surgery is still associated with complications. Of those, kidney failure is of great importance; and patients whose kidneys fail after a heart operation are more likely to have other health problems. Preventing kidney failure after heart surgery is therefore a major health issue.
Cardiac bypass, which replaces heart and lung functions by diverting blood from major vessels to a machine during surgery, is required for most major heart operations. Cardiac bypass is known to release molecules that cause inflammation into the blood. In susceptible people, these molecules could cause kidney failure. Statins are drugs used to decrease fatty acid (lipid) levels in the blood and help prevent cardiac and vascular disease. Statins also seem to decrease the general level of inflammation in the blood. Although animal studies have suggested that statins could help to prevent kidney failure after heart surgery, they can also cause adverse effects. More evidence is required before statins can be routinely used.
This review aims to evaluate evidence on the use of statins at the time of heart surgery to investigate if use can help to prevent kidney failure and how well statins are tolerated among patients. We searched the literature to January 2015 and included seven studies that involved a total of 662 participants to inform our assessment. In these studies, patients planned for heart surgery received statins or placebo (or no treatment at all). Five studies (467 participants) reported rates of kidney failure. We found that there was a high risk of bias in six of the seven included studies.
We found no difference in the rate of kidney failure between patients who received statins and those who did not. Two studies (195 patients) reported serum creatinine (a marker of kidney function) after the operation. We found that serum creatinine was lower in patients in the statin group (indicating better kidney function). Other conclusions were limited by the small number of studies. However, patients who received statins did not seem to need less dialysis. They did not have a higher rate of death in hospital and did not have an increased rate of adverse events.
Summary of findings
for the main comparison.
| Statin therapy compared with placebo for reduction in AKI after cardiac surgery with bypass | ||||||
|
Patient or population: adults undergoing surgery requiring cardiac bypass Settings: hospital inpatients Intervention: statin treatment prior to cardiac bypass surgery Comparison: placebo or no drug therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no drug therapy | Statins | |||||
|
Acute kidney injury Follow‐up: ICU stay |
27/232 (11.6%) | 21/235 (8.9%) | RR 0.76 (0.46 to 1.28) | 467 (5) | ⊕⊕⊝⊝ low | No significant difference. High risk of bias in most studies |
|
Serum creatinine Follow‐up: first 72 h post‐operative |
The mean serum creatinine ranged across control groups from 77.6 to 106 µmol/L | The mean serum creatinine in intervention groups was on average 21 µmol/L lower (95% CI ‐31.09 to ‐11.41) |
195 (2) | ⊕⊕⊝⊝ low | Significant difference, both studies have high risk of bias | |
|
Renal replacement therapy Follow‐up: ICU stay |
5/124 (4.0%) | 4/127 (3.1%) | RR 0.8 (0.23 to 2.81) | 251 (2) | ⊕⊝⊝⊝ very low | No significant difference. Only one study reported the need for renal replacement therapy |
|
Mortality Follow‐up: hospital stay |
0/189 (0%) | 3/192 (1.6%) | RR 3.86 (0.43 to 34.4) | 381 (5) | ⊕⊝⊝⊝ very low | No significant difference. Very low event rate. High risk of bias amongst studies |
|
Adverse events Follow‐up: treatment duration |
8/70 (11.4%) | 9/70 (12.9%) | RR 1.13 (1.47 to 2.68) | 140 (1) | ⊕⊝⊝⊝ very low | No significant difference. Only one study adequately reported adverse events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
ICU ‐ intensive care unit
Background
Description of the condition
AKI is a common complication of cardiac surgery; incidence ranges from 1% to 30% according to the definition used (Conlon 1999; Mariscalco 2011; Ostermann 2000; Thakar 2003). Cardiac surgery‐associated AKI (csa‐AKI) has been shown to be independently associated with increased morbidity and mortality (Chertow 1997) and a more complicated course of hospital admission. AKI is associated with longer intensive care unit (ICU) stays and increased costs, especially when renal replacement therapy (RRT) is required (Coca 2009; Srisawat 2010; Swaminathan 2007).
Cardiopulmonary bypass use has been identified as an important risk factor for occurrence of csa‐AKI (Bove 2004; Chawla 2012; Lamy 2012), and morbidity and mortality has not changed over the last decade despite significant advances in bypass technology (Swaminathan 2007). Other risk factors for development of AKI post cardiopulmonary bypass include pre‐existing chronic kidney disease (CKD) (Chertow 1997), older age (Mangano 1998), female gender (Asimakopoulos 2005), reduced left ventricular function (Mistiaen 2009), congestive heart failure (Mangano 1998), diabetes mellitus (Chukwuemeka 2005), peripheral vascular disease (Chukwuemeka 2005), pre‐operative use of an intra‐aortic balloon pump (Bove 2004), need for emergent surgery (Bove 2004), pre‐existing anaemia (Karkouti 2009), cardiopulmonary bypass time (Bove 2004), duration of cross clamp time (Mistiaen 2009) and requirement for vasopressor support (Arora 2008).
Although an overall decrease in renal blood flow has been shown to significantly contribute to the diminished glomerular filtration rate (GFR) observed in ischaemic kidney injury, decreased renal blood flow alone cannot account for the total reduction in GFR. Renal toxicity is also thought to be mediated by cardiopulmonary bypass triggered activation of bone marrow derived cells, endothelial cells and renal epithelial cells resulting in reactive oxygen species generation and release of inflammatory mediators (Boyle 1997). The adhesion of inflammatory cells to activated endothelium in peritubular capillaries of the outer medulla leads to medullary congestion and hypoxic injury to the proximal tubule. Pro‐inflammatory cytokines secreted by infiltrating and resident cells contribute to further tissue injury until inflammatory resolution and tubular epithelial proliferation occurs bringing a return to normal tissue function (Devarajan 2006).
Description of the intervention
Statins competitively inhibit the enzyme 3‐hydroxy 3‐methylglutaryl CoA (HMG CoA) reductase which catalyses the rate limiting step in cholesterol synthesis (Endo 1976). Since approval for clinical use in 1987 statins have been shown to reduce the progression of atherosclerosis, improve survival and reduce the risks of vascular death, non‐fatal myocardial infarction, stroke, and the need for coronary revascularisation across a wide range of cholesterol levels (Baigent 2005).
How the intervention might work
In addition to their lipid lowering actions, statins have been shown to exert anti‐inflammatory and pleiotropic effects (Haslinger‐Löffler 2008). In experimental models of AKI (Gueler 2002; Inman 2005; Yokota 2003), statins have been shown to preserve kidney function. Similarly, Mariscalco 2011 suggested that statins could reduce postoperative leukocyte and endothelial activation and result in significantly lower postoperative pro‐inflammatory serum cytokine levels.
There is some evidence that statin administration before percutaneous coronary interventions reduces periprocedural cardiovascular events. However, results are inconsistent when AKI (contrast‐induced nephropathy) was considered as an outcome (Jo 2008; Ozan 2010; Patti 2008). A recent meta‐analysis (Li 2012) supported the use of statins to prevent contrast‐induced nephropathy but concluded that their use must be considered in the context of variable patient demographics. Although the pathophysiology of contrast‐induced nephropathy is not fully understood, inflammation and oxidative stress may play an important role similar to a postulated mechanism of kidney injury after cardiac bypass surgery. Only small RCTs have been published to date, and results among patients undergoing cardiac surgery are conflicting.
Why it is important to do this review
Current evidence consists of numerous observational studies and a few small RCTs. Large observational studies have generated conflicting results: some suggest that statin administration could be associated with lower csa‐AKI incidence (Billings 2010; Clark 2006; Tabata 2007) but others have suggested no effect (Ali 2005; Pan 2004). Given their retrospective nature, such studies are limited by selection bias and their conclusions must be considered with care. Several systematic reviews have evaluated the overall benefits of perioperative statins in cardiac surgery (Liakopoulos 2008; Liakopoulos 2012); however, none have focused on AKI as a primary outcome.
There is a considerable need for effective therapies that prevent AKI in this setting because kidney dysfunction after surgery results in significantly increased morbidity compared with patients who maintain normal kidney function (Karkouti 2009). In general, prevention of kidney reperfusion injury after cardiac bypass surgery involves correction of dehydration and minimising nephrotoxins. Therefore, evidence indicating that statins help to prevent kidney injury in this setting will help to address this therapeutic gap. Recent evidence (including among patients not requiring RRT following AKI) suggests that kidney injury is associated with increased long‐term mortality risk independent of residual kidney function, with risk proportionate to the severity of the kidney injury (Lafrance 2010).
This review focused on AKI and procedures requiring cardiac bypass, except cardiac transplantation surgery and correction of congenital cardiac defects. All levels of kidney injury were included.
Objectives
This review aimed to look at the evidence supporting the benefits of perioperative statins for AKI prevention in hospitalised adults after surgery who require cardiac bypass. The main objectives were:
To determine whether use of statins was associated with preventing AKI development
To determine whether use of statins was associated with reductions in in‐hospital mortality
To determine whether use of statins was associated with reduced need for RRT
To determine any adverse effects associated with the use of statins.
Methods
Criteria for considering studies for this review
Types of studies
All published RCTs that compared statin use with placebo or standard treatment given preoperatively to patients undergoing surgery who required cardiac bypass were eligible for inclusion. The dose, type or duration of statins used was not restricted. Case reports and non‐randomised studies were not eligible for inclusion.
Types of participants
Inclusion criteria
All adult patients (aged > 18 years) undergoing surgery requiring cardiopulmonary bypass were included. Studies including children or mixed child and adult populations were excluded unless data were presented separately. Studies that included patients undergoing cardiac surgery who did not require cardiopulmonary bypass were excluded.
Exclusion criteria
Patients on extracorporeal RRT before the initiation of the study
Patients undergoing cardiac transplantation or corrective surgery for congenital heart disease
Kidney transplant recipients.
Types of interventions
We considered studies that investigated the use of statin therapy for any given duration and dose prior to surgery in people requiring cardiac bypass compared with placebo, no drug therapy or standard clinical care. All statins were considered for inclusion, with a priori subgroup analysis defined.
Types of outcome measures
Primary outcomes
The primary outcome is the incidence of AKI after cardiac surgery.
AKI is defined using the AKIN or RIFLE classification wherever provided. The Acute Kidney Injury Network (AKIN) (Mehta 2007) defines three stages of injury (1, 2 and 3) based on increasingly severe reductions of kidney function (Stage 1: increase in serum creatinine (SCr) ≥ 0.3 mg/dL, or 1.5‐ to 2‐fold increase from baseline; Stage 2: increase in SCr ≥ 150% to 200% from baseline; Stage 3: increase in SCr > 3‐fold from baseline, > 300% from baseline, ≥ 4.0 mg/dL, or initiation of RRT regardless of stage). The RIFLE criteria, proposed by the Acute Dialysis Quality Initiative group (Bellomo 2004) defines five levels of AKI (Risk, Injury, Failure, Loss and End‐stage kidney disease) based on incremental reductions in kidney function.
If AKI was not reported in the context of these criteria, efforts were made to classify patients.
Secondary outcomes
SCr changes during the same hospitalisation
In‐hospital mortality
Need for RRT during the same hospitalisation
Adverse effects attributed to the intervention (elevated liver enzymes, creatinine kinase levels, rhabdomyolysis or drug withdrawal).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register to 13 January 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Further potential studies were assessed through handsearching reference lists of nephrology textbooks, review articles and relevant studies. Letters were submitted seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
Literature search was performed using the predefined search strategy (Appendix 1) to identify studies eligible for inclusion. The titles and abstracts were screened initially. Studies that potentially contained information relevant to the review were retained, whereas studies that did not meet the pre‐specified criteria were excluded. All potentially relevant studies were then uploaded to a reference management database. Each author independently reviewed the abstract or full text (where required) of the retained articles to assess suitability for inclusion in this review. Reasons for exclusion were noted. Cross checking was performed by at least one further author to determine the final eligibility for inclusion, and disagreements were arbitrated amongst all three authors until a consensus was reached.
Data extraction and management
Data were extracted independently by all three authors using a standardised data extraction form, which was designed by AS and initially piloted by all three authors. The information obtained from each included study was cross‐checked by at least one additional author. Disagreements were arbitrated between all three authors until a consensus was reached. Where more than one publication of a study existed, reports were grouped together and the most complete data set was included. Any discrepancy between published versions has been highlighted. Unclear data were clarified through correspondence with the study authors and any data obtained in this way were included in the review and analysis. Data extracted included study design, inclusion and exclusion criteria, patient numbers and characteristics. Treatment regimen and duration as well as duration of follow‐up were included. For outcomes of interest (AKI, SCr, mortality, need for RRT, adverse events), the raw data were extracted using mean, median and standard deviations for continuous outcomes, and event rate for dichotomous outcomes. Where data were collected at more than one time‐point, we extracted all data and analysed different time‐points for comparison.
Assessment of risk of bias in included studies
All three authors independently performed the risk of bias assessment for between two to three of the included studies, with each assessment cross‐checked by a second author. Any disagreements were arbitrated between all three authors until a consensus was reached. Risk of bias was assessed using a risk of bias assessment tool (see Appendix 2), and according to the standards of the Cochrane Collaboration (Higgins 2011). The following items were assessed.
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
In each domain, studies were labelled as low, high or unclear risk of bias with consideration given to the presence or absence of sufficient information to make a determination. Reasons for assessment were documented (See Characteristics of included studies), and a risk of bias summary is presented.
Measures of treatment effect
For dichotomous outcomes (e.g. proportion of AKI, need for RRT, mortality rates and number of adverse events), results are expressed as risk ratios (RR) with 95% confidence intervals (CI), calculated using the random effects model. A RR < 1 favours statin treatment over control. As postoperative SCr is a continuous outcome it was first converted to standardised units (µmol/L) and the mean difference (MD) with 95% CI was subsequently used for comparisons.
Unit of analysis issues
Studies with non‐standard designs, such as cross‐over studies and cluster‐randomised studies were not included in this review.
Dealing with missing data
Any missing outcome data were requested from the corresponding author of the study. All the relevant information obtained via this method was included in this review. In one included study (Almansob 2012), there was lack of information surrounding SCr levels which were graphically presented. After written correspondence, the authors of this study provided mean and standard deviation of the SCr level and blood urea nitrogen (BUN) preoperatively, and at 24 hours, 48 hours and 72 hours postoperatively. Data surrounding need for RRT and mortality data were also provided. An author from Prowle 2012 was contacted for perioperative SCr data, which were also provided. No further missing outcome data were imputed for the purposes of this review. Given the universally short follow‐up periods of the included studies, loss to follow‐up was minimal. In one study that reported 15 patients dropped out (Prowle 2012), data within this study were analysed on intention‐to‐treat principle, followed by reanalysis on per‐protocol basis, in which no outcomes were materially affected. The intention‐to‐treat data from this study were used for the purposes of this review. In the only other study reporting significant drop outs (Almansob 2012), only those completing the study were included in the analysis, and study data were used as reported.
Assessment of heterogeneity
We assessed the heterogeneity between included studies using the Chi2 test on N‐1 degrees of freedom with an alpha of 0.05 used for statistical significance and with the I2 statistic (Higgins 2003). We considered statistical significance in heterogeneity surrounding calculation of the I2, although Chi2 with alpha < 0.05 was also considered. Based on the Cochrane Handbook (Higgins 2011), I2 of 0% to 40% may not be important, I2 of 30% to 60% may represent moderate heterogeneity, I2 of 50% to 90% may represent substantial heterogeneity and I2 of 75% to 100% may represent considerable heterogeneity. Visual inspection of forest plots provided added evidence of heterogeneity.
Assessment of reporting biases
In order to detect any publication bias, electronic searching of web sites with current clinical study protocols was undertaken. Funnel plot analysis (Sterne 2001) was planned to further investigate, however there were insufficient number of included studies and this analysis was not feasible. We also assessed selective reporting of outcomes in the included studies. Language bias was minimized by not applying any language restrictions during the search strategy.
Data synthesis
The data from the available studies were pooled using the random effects model (DerSimonian 1986), however the fixed effects model (Egger 1997) was also undertaken for comparison. The random effects model was used for the primary analysis as there is variability in the form, dose and duration of statin therapy used amongst studies of this nature, and some heterogeneity was expected. Before a pooled data analysis was performed for each outcome of interest, we assessed all the relevant studies to determine the eligibility of data comparison. We excluded pooled data analysis and only presented the result with narrative description when there were not sufficient comparable data available for a specific outcome.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity such as those amongst participants, treatments and study quality. Heterogeneity in patients could be related to age, preoperative renal pathology and other risk factors for renal reperfusion injury. Heterogeneity in treatments could be related to prior agent(s) used and the dose, duration and type of statin used. Despite small numbers of included studies, we investigated the potential differences through subgroup analysis according to type of statin used.
Sensitivity analysis
We planned to perform sensitivity analyses if there were sufficient studies identified, in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified above
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Too few studies were identified that met the inclusion criteria for this review, therefore sensitivity analyses were not undertaken.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies
Results of the search
We identified 89 records from the Cochrane Renal Group's Specialised Register (Figure 1); no studies were identified from searches of the reference lists of relevant review articles (Liakopoulos 2008; Morgan 2009). After screening titles, abstracts and the full text, 53 studies (71 records) were excluded and two ongoing studies were identified (NCT01547455; STATIN AKI 2011). Seven studies met our inclusion criteria (Almansob 2012; Chello 2006; Christenson 1999; Mannacio 2008; Prowle 2012; Tamayo 2009; Spadaccio 2010).
1.
Study flow diagram.
Prior to publication of this review a final search of the Specialised Register identified five new potential studies and these will be assessed for inclusion in a future update of this review (de Oliveira 2012; Han 2013; Liu 2013; NAPLES II Study 2009; ROSA‐cIN Trial 2013).
Included studies
The seven studies included in our analyses were all RCTs and included a treatment arm of statin and comparator arm of no drug treatment or placebo. The studies included had variable primary outcomes, however all included at least one of the pre‐specified outcomes of interest for this review. Authors were contacted to provide data for SCr and urine output and additional information for SCr was gained from two of the included studies.
See Characteristics of included studies
Design
After evaluation, seven RCTs published between 1999 and 2012 were included in this review. All were published in English.
Sample size
Sample size varied from 40 (Christenson 1999) to 200 participants (Mannacio 2008) to provide data on a total of 662 adult patients undergoing cardiac surgery, of whom 334 received statin therapy and 328 received placebo or no drug therapy.
Setting
Five of the seven studies were undertaken in European centres, and one each in China and Australia. All were undertaken in inpatient hospital settings with follow‐up limited to inpatient hospital stays.
Participants
All studies evaluated adult inpatients undergoing cardiac bypass. One study exclusively enrolled patients undergoing non‐coronary artery surgery (Almansob 2012) compared to the other included studies that predominantly evaluated those undergoing coronary artery bypass surgery. Most studies excluded those with baseline kidney impairment (Chello 2006; Mannacio 2008; Prowle 2012; Spadaccio 2010; Tamayo 2009) although of note, there was no consensus amongst these studies on levels of kidney impairment that led to exclusion.
Interventions
Three studies evaluated atorvastatin (Prowle 2012; Spadaccio 2010; Chello 2006), three evaluated simvastatin (Almansob 2012; Christenson 1999; Tamayo 2009) and one study evaluated rosuvastatin (Mannacio 2008). All studies compared pre‐operative statin treatment to placebo or no drug treatment. Use of statins prior to enrolment was variable, with three studies (Almansob 2012;Christenson 1999Tamayo 2009) not reporting prior use. Two studies (Chello 2006; Spadaccio 2010) had a mandated statin‐free period of more than one year prior to enrolment, and two studies had much shorter statin‐free periods of a minimum 30 days (Mannacio 2008) and 24 hours (Prowle 2012) prior to enrolment.
Doses of statin used varied; atorvastatin ranged from 20 mg/d (Chello 2006; Spadaccio 2010) to 40 mg/d (Prowle 2012), simvastatin was 20 mg/d (Almansob 2012; Christenson 1999; Tamayo 2009) and rosuvastatin was 20 mg/d (Mannacio 2008). Of note, only Christenson 1999 titrated statin dose to lipid levels, with simvastatin increased to 40 mg two weeks prior to surgery if adequate lipid lowering had not been achieved.
Duration of preoperative statin therapy was also variable between the studies, with periods ranging from commencement on the day of operation (Prowle 2012) through to four weeks preoperatively (Christenson 1999). Three of the studies commenced therapy three weeks prior to surgery (Chello 2006; Spadaccio 2010; Tamayo 2009) and two studies commenced therapy between five to seven days preoperatively (Almansob 2012; Mannacio 2008). The period of postoperative statin therapy was not clarified in 5/7 studies (Chello 2006; Christenson 1999; Mannacio 2008; Spadaccio 2010; Tamayo 2009). In two studies the statin was continued for three days postoperatively (Prowle 2012) and recommenced on day two postoperatively for ongoing therapy (Almansob 2012).
Outcomes
With the exception of Prowle 2012 none of the studies listed AKI as a primary outcome. For our pre‐specified outcomes of interest, 5/7 studies reported AKI (Chello 2006; Prowle 2012; Christenson 1999; Spadaccio 2010; Mannacio 2008), 3/7 studies reported SCr (Almansob 2012; Prowle 2012; Tamayo 2009), 2/7 studies reported need for RRT (Almansob 2012; Prowle 2012), 5/7 reported mortality (Almansob 2012; Chello 2006;Prowle 2012; Spadaccio 2010; Tamayo 2009), and 2/7 studies reported adverse events (Chello 2006; Prowle 2012). Standardisation of outcome reporting for the outcomes of AKI, SCr and adverse events was a significant issue. The definitions of AKI ranged from using the validated RIFLE criteria (Prowle 2012), to using a single SCr cut‐off (Christenson 1999) or increase in SCr (Mannacio 2008) to providing no clarifying definition (Chello 2006; Spadaccio 2010). No conversion to AKIN criteria was able to be achieved. Units of measure of SCr were able to be freely converted to µmol/L, however time point reporting varied widely amongst studies. Almansob 2012 reported SCr data at 24, 48 and 72 hour time points, while for Tamayo 2009 the time‐point of SCr was unable to be clarified, although data collection was ceased at 48 hours. Prowle 2012 reported only peak SCr values, which were taken one to five days postoperatively. Finally, adverse events were defined clearly in one study (Prowle 2012) as alanine transferase (ALT) > 3 times the upper limit or normal (ULN), creatine kinase (CK) > 5000 IU/L, or study drug being stopped by the treating clinician. Chello 2006 reported only that no adverse events were experienced, without qualification.
Excluded studies
Overall, 53 studies were excluded after critical appraisal of title, abstract or full text (See Characteristics of excluded studies). One study did not fulfil the criteria for a RCT; 24 contained no outcome of interest, 25 were excluded as they did not use cardiac bypass in the procedure and three did not use statins as a comparator arm, or used a statin in both arms.
Risk of bias in included studies
2.
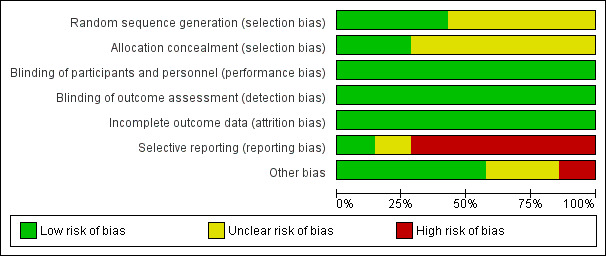
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
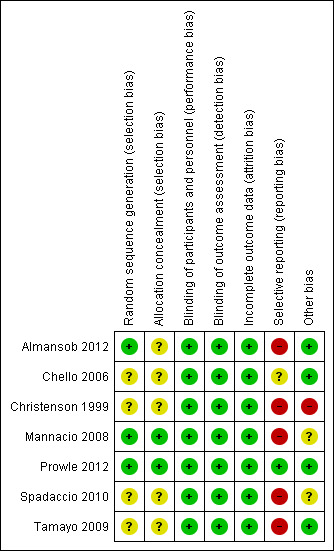
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall, the study quality was variable, with high risk of bias assessed in at least one domain for 5/7 studies (Almansob 2012; Mannacio 2008; Christenson 1999; Spadaccio 2010; Tamayo 2009). Only Prowle 2012 was considered truly to be at low risk of bias, with Chello 2006 assessed as unclear risk of bias based on identification of potential selection bias.
Allocation
Only three of the studies were assessed as low risk, with adequate description of randomisation methods including the use of computer generated algorithms (Almansob 2012; Mannacio 2008; Prowle 2012). The other four studies (Chello 2006; Christenson 1999; Spadaccio 2010; Tamayo 2009) made variable mention of randomisation, however they did not provide enough detailed information regarding actual randomisation procedures undertaken to determine risk of bias, and were therefore deemed to be unclear.
Blinding
Whilst the mention of 'blinding' was made in the majority of studies, the methods by which this was achieved were not clearly described. Only two of the studies made mention of use of placebo and the methods undertaken to administer this (Mannacio 2008; Prowle 2012). Despite this, the outcome measures of interest for this review (AKI, need for RRT, SCr, mortality and adverse events) are objective, and thus it was felt that whilst this represented poor reporting practices for the majority of the studies it did in fact constitute a low risk of bias for these outcome measures.
Incomplete outcome data
Most studies reported no loss to follow‐up or incomplete outcome data. This is likely secondary to the short follow‐up period, with no long‐term follow‐up undertaken in any study. Two studies reported patient drop out, with incomplete data collection. One study (Prowle 2012) reported a number of patient drop‐outs (15), however study results were not affected when reanalysed on a per protocol basis, with the primary analysis remaining as intention to treat. The missing data were evenly spread across groups (statin (8); control (7)) and dropout was for comparable reasons. Given this transparency, it was felt that this represented low risk of bias. The second study (Almansob 2012) reported that 19 patients were excluded from the analysis (statin (9), placebo (10)). The drop out was for comparable reasons, including liver dysfunction (statin (2), placebo (3)), and non‐completion not further defined (statin (4), placebo (6)). A further two patients in the statin group underwent surgery without cardiac bypass. Whilst these patients were excluded from the analysis, the dropout is even across groups and for comparable reasons, so again, was felt to represent low risk of bias.
Selective reporting
All the studies reported the outcomes that were prespecified in their methods. However, 5/7 studies were felt to have high risk of reporting bias (Almansob 2012; Christenson 1999; Mannacio 2008; Spadaccio 2010; Tamayo 2009) as drug studies would be expected to report adverse events as outcome data and these studies failed to do so. Prowle 2012 very clearly reported definitions of adverse events and event rate. Chello 2006 did report that no adverse drug related events were seen, however failed to define what constituted an event. As such, this was determined to be unclear risk of bias as judgement was unable to be made.
Other potential sources of bias
Christenson 1999 was industry funded, however made no mention of the role that this funding body played in the design and execution of the study or analysis of results, therefore risk of bias was deemed high. Two studies did not reporting funding and were deemed unclear (Mannacio 2008; Spadaccio 2010). No other potential sources of bias were identified.
Effects of interventions
See: Table 1
See Table 1. Seven studies enrolling 662 participants were evaluated. Both fixed and random effects models were used in the initial analysis. No significant differences were found, therefore the summary estimates reported here were from the random effects models. Given the limited number of included studies, no sensitivity analysis was undertaken.
Postoperative AKI
Five studies (467 participants) reported postoperative AKI (Chello 2006; Prowle 2012; Christenson 1999; Spadaccio 2010; Mannacio 2008). There was no significant difference between groups in preventing postoperative AKI (Analysis 1.1 (5 studies, 467 participants): RR 0.76, 95% CI 0.46 to 1.28). There was no significant heterogeneity demonstrated (I2 = 0%). Despite the limited number of studies, a subgroup analysis by statin class was performed. For those studies using atorvastatin no statistically significant difference was seen in postoperative AKI between groups (Analysis 1.1.1 (3 studies, 190 participants): RR 0.83, 95% CI 0.46 to 1.49). For the single studies assessing simvastatin and rosuvastatin, no differences were seen between the groups (Analysis 1.1.2; Analysis 1.1.3). Subclass differences were assessed, with a calculated I2 of 34%, indicating a potential low level of variability amongst studies when considering result by statin subgroup. Whilst this may indicate different effects by statin (i.e. trends to benefit versus harm by class used), the low event rates overall and wide confidence intervals seen in conjunction with the low number of studies in each subgroup make it difficult to draw firm conclusions.
1.1. Analysis.
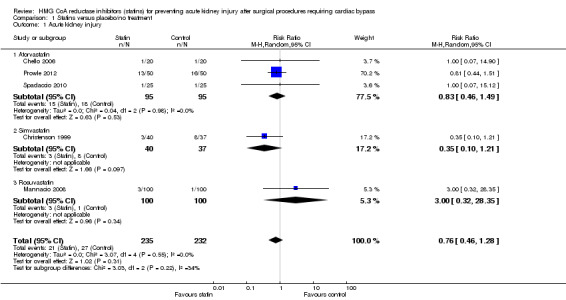
Comparison 1 Statins versus placebo/no treatment, Outcome 1 Acute kidney injury.
Postoperative serum creatinine
Three studies (195 participants) reported postoperative SCr (Almansob 2012; Prowle 2012; Tamayo 2009). Given differences in outcome reporting previously described, pooled analysis was only possible for two of these studies (Almansob 2012; Tamayo 2009 (195 participants). Statin treatment significantly decreased 24 hour postoperative SCr (Analysis 1.2 (2 studies, 195 participants): MD ‐21.25 µmol/L, 95% CI ‐31.09 to ‐11.41; I2 = 0%) with the result driven by differences seen in one study (Almansob 2012) which received 85.7% of the weighting. As data were available at two time points for this study, analysis of the 48 hour SCr was also significantly decreased in the statin group (Analysis 1.3 (2 studies, 195 participants): MD ‐20.97µmol/L, 95% CI ‐34.58 to ‐7.35; I2 = 12%). Given that Tamayo 2009) collected data to a maximum of 48 hours, the 72 hour time point was not undertaken.
1.2. Analysis.
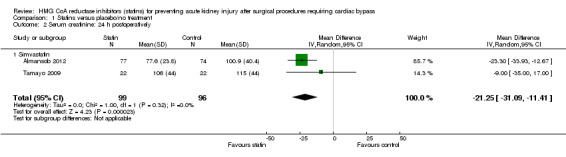
Comparison 1 Statins versus placebo/no treatment, Outcome 2 Serum creatinine: 24 h postoperatively.
1.3. Analysis.
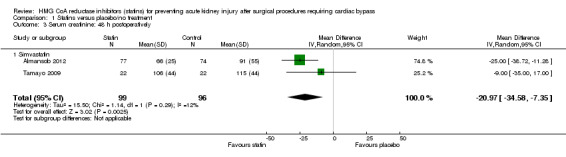
Comparison 1 Statins versus placebo/no treatment, Outcome 3 Serum creatinine: 48 h postoperatively.
Perioperative mortality
Mortality outcomes were reported in six studies (458 participants) (Almansob 2012; Chello 2006; Christenson 1999; Prowle 2012; Spadaccio 2010; Tamayo 2009). Given the universally short periods of follow‐up, low numbers of deaths were reported, all within the statin treatment arm of two studies (Almansob 2012; Tamayo 2009). Despite the individual studies showing trends to favour placebo for this outcome, there was no statistically significant difference in mortality seen between groups in the pooled analysis (Analysis 1.4 (6 studies, 458 participants): RR 3.86, 95% CI 0.43 to 34.40). There was no significant heterogeneity (I2 = 0%) seen. With low event rates demonstrated confidence intervals were extremely wide, again making any interpretation of these results difficult.
1.4. Analysis.
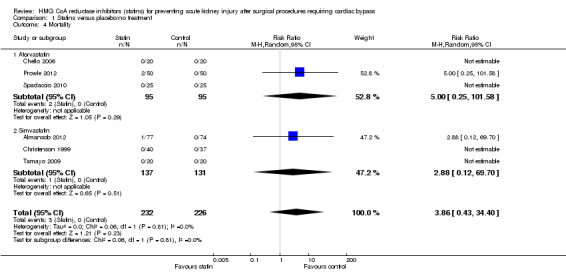
Comparison 1 Statins versus placebo/no treatment, Outcome 4 Mortality.
Need for postoperative renal replacement therapy
Need for postoperative RRT was reported in two studies (Almansob 2012; Prowle 2012). Almansob 2012 reported no need for RRT in either group and there were near equal numbers needing RRT reported in Prowle 2012. There was no statistically significant difference between statin treatment and placebo (Analysis 1.5 (2 studies, 251 participants): RR 0.80, 95% CI 0.23 to 2.81). There was no significant heterogeneity demonstrated (I2 = 0%)
1.5. Analysis.
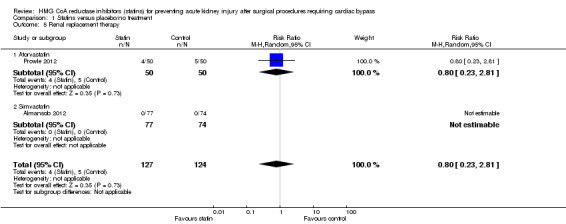
Comparison 1 Statins versus placebo/no treatment, Outcome 5 Renal replacement therapy.
Adverse events
Two studies (140 participants) reported adverse events however one study (Chello 2006) did not report adverse events related to statin use therefore no pooled analysis was undertaken. Prowle 2012 reported rise in ALT x 3 upper limit of normal, creatine kinase rise > 5000 IU/L and study drug cessation, the compared event rate between the groups did not reach statistical significance (P = 0.36, 0.36 and 1.0 respectively).
Prowle 2012 reported adverse events related to statin use. There was no significant difference in rise in ALT x 3 upper limit of normal (Analysis 1.6.1 (100 participants): RR 0.25, 95% CI 0.03 to 2.16), rise in creatine kinase > 5000 IU/L (Analysis 1.6.2 (100 participants): RR 4.00, 95% CI 0.46 to 34.54) or study drug cessation (Analysis 1.6.3 (100 participants): RR 1.33, 95% CI 0.31 to 5.65) between atorvastatin and placebo.
1.6. Analysis.
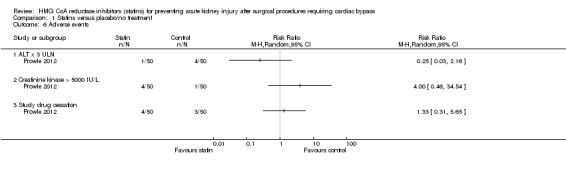
Comparison 1 Statins versus placebo/no treatment, Outcome 6 Adverse events.
Discussion
Summary of main results
After a search of the literature, seven RCTs with a total of 662 participants that addressed the use of statin intervention prior to cardiac bypass surgery and its effects on perioperative kidney injury were identified. All the included studies assessed our prespecified primary outcome of AKI, or one of our secondary outcomes of SCr, need for RRT or adverse events, although with the exception of one (Prowle 2012), all kidney outcomes were considered as secondary within the original studies. Three studies assessed atorvastatin (Chello 2006; Prowle 2012; Spadaccio 2010; 190 participants), three studies assessed simvastatin (Almansob 2012; Christenson 1999; Tamayo 2009; 272 participants) and one study used rosuvastatin (Mannacio 2008; 200 participants), with high variability in drug washout period (24 hours (Prowle 2012) to more than one year (Chello 2006; Spadaccio 2010)). Duration of pre‐operative drug therapy ranged from treatment commencing on the operative day (Prowle 2012) to four weeks preoperatively (Christenson 1999). All studies were assessed as having a high risk of bias, with the exception of Prowle 2012 which was assessed as having a low risk of bias in all five assessed domains. Study follow‐up was uniformly short, with all the studies collecting data to hospital discharge, and biochemical data collection ranging from 24 hours postoperatively (Spadaccio 2010) up to seven days (Almansob 2012; Christenson 1999).
Overall for the outcome of AKI, the studies were variable in their results, however no individual study showed significant differences between statin treated and non‐drug treated groups. Our resultant meta‐analysis therefore showed no significant difference between the statin and comparator arms for incidence of AKI postoperatively (RR 0.76, 95% CI 0.46 to 1.28) (See Table 1). The only finding of significance from all outcomes assessed was the difference in postoperative SCr between statin and placebo groups, an outcome which was measured in three studies (Almansob 2012; Prowle 2012; Tamayo 2009). Of these, significant variability in time‐point measurements allowed only two of the studies to be pooled for analysis. The third study (Prowle 2012) showed no significant difference in maximal SCr reached between postoperative days 1 to 5 between the statin and placebo arms (rise of 28 µmol/L (16 to 55) versus 29 (16 to 54), P = 0.62). In the meta‐analysis (Almansob 2012; Tamayo 2009), the group treated with statin showed a mean postoperative SCr of 21.25 µmol/L lower than the non‐drug treated group (95% CI ‐31.09 to ‐11.41, P < 0.0001). This result was largely driven through the strongly positive results in one study (Almansob 2012), where SCr data at 24, 48 and 72 hour time‐points showed a significant benefit of statin therapy over no drug treatment (24 hour (MD ‐23.30 µmol/L, 95% CI ‐33.93 to ‐12.67, P < 0.0001). For all other assessed outcomes (need for RRT, mortality, adverse events) no statistically or clinically significant difference was found between statin therapy and placebo or no therapy. Reliable conclusions were not able to be drawn from these analyses given the low numbers of studies reporting these outcomes, and concomitant low event rates.
Overall completeness and applicability of evidence
There are significant limitations to this review. Given the lack of large, high‐quality RCTs, our meta‐analysis was limited to small heterogeneous studies. Studies were conducted in a range of settings, from Europe to China and Australia, with potentially important socio‐demographic and procedural differences between the centres. Similarly, despite recruiting populations of adult patients undergoing cardiac bypass, the included studies had different protocols and preoperative treatment duration, with inclusions ranging from exclusively statin naive patients (Christenson 1999) to accepting different periods of drug washout, a setting unlikely to be seen in current clinical practice. For all studies but one (Prowle 2012), the statin was administered before the operative day, but the pre‐operative treatment duration ranged from seven days (Almansob 2012) to four weeks (Christenson 1999). In addition, different statins were used as part of these protocols. Three studies used atorvastatin (Chello 2006; Prowle 2012; Spadaccio 2010), three used simvastatin (Almansob 2012, Christenson 1999; Tamayo 2009) and Mannacio 2008 used rosuvastatin. To take into account this potential for different effects across drug formulation, subgroup analyses were performed. However given the small number of patients, the power to detect a difference is very limited. The outcomes of interest for this review were largely secondary outcomes within the included studies, and therefore lack of consensus definition for AKI was an issue. Two studies (Christenson 1999; Mannacio 2008) used SCr cut‐offs to dichotomise patients into AKI or no AKI (125 µmol/L for the first and 221µmol/L for the latter), while Prowle 2012 used the currently recommended RIFLE criteria (Bellomo 2004), which are based both on a ratio of peak over baseline SCr level and urinary outputs. Finally, two studies (Chello 2006; Spadaccio 2010) did not report their definition for AKI.
Overall, only minimal information about post‐operative kidney function was available in these reports as AKI was only a secondary outcome in all but one study (Prowle 2012). The event numbers were low for many outcomes, and therefore power to detect differences between the groups may be limited. The safety of statin use in surgery requiring cardiac bypass was unable to be assessed as a secondary outcome due to a lack of reported data in the majority of included studies.
Quality of the evidence
With the exception of Prowle 2012, all of the studies were assessed as having high risk of bias in at least one domain. Drug studies would be expected to report clearly on the presence or absence of adverse events related to drug administration, however only Prowle 2012 adequately assessed and reported these events. In addition, most studies stated that randomisation was undertaken, without describing the methods of randomisation, allocation concealment and blinding of study participants. In cases where blinding was stated to have occurred, several studies did not describe use of a placebo, raising significant questions as to how blinding could possibly have been adequate. Despite this, outcome measures in this case were objective and therefore most studies were assessed as low risk of bias in this domain. Authors of the included studies were therefore not contacted to further clarify randomisation strategies. Compliance to newly prescribed medication may well be poor, which would potentially significantly affect the outcomes of the included studies. None of the included studies measured or addressed the issue of compliance directly, although one (Christenson 1999) did measure serial lipid profiles to assess preoperative change in the setting of statin use. Overall, given that 6/7 included studies had identified high risk of bias in at least one domain in addition to significant differences in methodology and outcome reporting practices, the quality of the evidence was assessed as low or very low across all outcome measures (See Table 1).
Potential biases in the review process
We believe that all relevant studies were identified. A thorough search strategy was devised and all major databases were searched for relevant studies, with no language restrictions applied. All three authors assessed the studies for inclusion in the review and the risk of bias, with discrepancy discussed between the three authors. The biggest limitation of the review process was that not all studies reported the outcome of interest or reported in a different fashion. This is related to the lack of thoroughly accepted definition for AKI. Despite attempting to obtain additional data, we were not able to obtain sufficient data to adequately classify all patients according to the RIFLE (Bellomo 2004) or AKIN (Mehta 2007) classification, and therefore our primary outcome (AKI) was considered as a dichotomous outcome rather than a stratified outcome based on reporting in the included studies. We did not include the results of unpublished studies. Studies with both positive and negative results were identified, making the possibility of publication bias less likely. No sensitivity analysis was able to be undertaken given the small number of studies eligible for inclusion in this review.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first completed review that assesses the impact of preoperative statin use for surgery requiring cardiac bypass on the incidence of postoperative AKI. However, there have been systematic reviews that examined multiple clinical outcomes with the use of preoperative statin therapy after cardiac surgery. A systematic review with meta‐analysis (Liakopoulos 2008) included more than 30,000 participants. It was shown that statin therapy had no effect on postoperative kidney failure (OR 0.78, 95% CI 0.46 to 1.31). This was in agreement with our findings. However, it reported an incidence of 4.1% kidney failure in a total of 6408 patients. This was much lower than our incidence of 10.3% AKI out of total of 467 patients. The discrepancy could be due to the fact that the majority of studies included in Liakopoulos 2008 were retrospective observational studies (only two small RCTs out of five studies), whereas this review included five RCTs for this particular analysis. There was also significant heterogeneity observed in Liakopoulos 2008 (P = 0.05; I2 = 58.3%).
In a more recent Cochrane review performed by the same author (Liakopoulos 2012), four studies reported the incidence of kidney failure in a total of 367 participants. The incidence of kidney failure was 3.2% in the statin group compared to 7.1% in the control group. This Cochrane analysis showed that statin treatment did not lead to a significant reduction of the odds of kidney failure (OR 0.41, 95% CI 0.15 to 1.12; P = 0,08). Our analysis also did not show any statistically significant difference between the two groups (RR 0.76; 95% CI: 0.46 to 1.28). A significant difference between our analysis and the previous Cochrane review related to inclusion in this review of a recent RCT reporting the outcome of AKI (Prowle 2012). It was a well‐designed study with low risk of bias and it carried an important weight in the pooled analysis. In Prowle 2012, there were 13/50 patients with AKI in the statin group compared to 16/50 in the placebo group, (RR 0.81, 95% CI 0.44 to 1.51). This result would have likely attenuated the statistical trend observed in Liakopoulos 2008.
Authors' conclusions
Implications for practice.
When considering the five RCTs (467 participants) that reported the primary outcome of AKI, our analysis demonstrated no reduction in AKI incidence in the setting of preoperative statin therapy. Whilst postoperative SCr was significantly lower in those treated with statins preoperatively (compared to placebo), only two studies (195 participants) adequately reported this outcome and results were driven by the results of a single study. As such, there is significant uncertainty regarding the strength of this result, and we are unable to draw a definitive conclusion. Overall, no reduction in mortality or need for RRT was seen with statin treatment. Adverse events were poorly reported in most studies, and no significant differences seen in the only study that reported this outcome adequately. Overall, there was no evidence of improved kidney health outcomes or mortality associated with empirical statin therapy for adults undergoing surgery requiring cardiac bypass.
Implications for research.
Although our analysis did not show any benefit of preoperative statin therapy in the reduction of AKI for cardiopulmonary bypass surgery, our conclusion was drawn from only small number of studies with limited number of patients in total. Most of the studies were designed to investigate the incidence of AKI as a secondary outcome. Moreover, the outcome definition varied among different studies. Therefore further larger studies with their main focus on perioperative kidney function are required in order to investigate any potential effect of preoperative statin therapy. It is also necessary for future studies to have a standardised definition of AKI, such as the AKIN or RIFLE consensus classification which would allow more meaningful comparisons to be made.
Further research is also required to assess any potential benefit of different types of statin therapy and different dose regimens. As there are newer statin agents emerging in the market, more studies are required to explore any potential renoprotective benefit of such agents. Upcoming studies should also collect detailed data regarding adverse events associated with statin use, as this was lacking in most of the RCTs included in this review.
Acknowledgements
We are grateful to the Cochrane Renal Group for their assistance, particularly Narelle Willis who helped us establish the protocol and Gail Higgins who undertook the searches for this review.
We also wish to thank the team in the Department of Epidemiology and Preventative Medicine at Monash University, particularly Renea Johnston and Rachelle Buchbinder for their help in preparation of the initial manuscript
We wish to thank Associate Professor Kevan Polkinghorne from the Department of Nephrology at Monash Medical Centre for his valuable assistance in preparing the final manuscript
Dr Michelle Lewicki is supported through a Jacquot Research Entry Scholarship, RACP Foundation Australia
Dr Antoine Schneider is supported through an MGS‐MIPS scholarship, Monash University, Australia
Finally, we would like to thank Dr Jingsong Ou, Dr John Prowle and Rinaldo Bellomo for providing additional data related to their studies at our request.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE | 1. exp Coronary Artery Bypass/ 2. bypass.tw. 3. coronary artery bypass$.tw. 4. coronary bypass grafting surgery.tw. 5. ((valve$ or cardiac) and surgery).tw. 6. CABG.tw. 7. Thoracic Surgery/ 8. cardiac surgery.tw. 9. valve replacement$.tw. 10. or/1‐9 11. exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ 12. statin$.tw. 13. "HMG‐CoA".tw. 14. hydroxymethylglutaryl‐coa reductase inhibitor$.tw. 15. simvastatin.tw. 16. fluvastatin.tw. 17. cervistatin.tw. 18. lovastatin.tw. 19. pravastatin.tw. 20. atorvastatin.tw. 21. rosuvastatin.tw. 22. Lipitor.tw. 23. Baycol.tw. 24. Lescol.tw. 25. Mevcor.tw. 26. Altocar.tw. 27. Pravacol.tw. 28. Lipostat.tw. 29. Zocor.tw. 30. Crestor.tw. 31. or/11‐30 32. exp Acute Kidney Injury/ 33. (acute kidney failure or acute renal failure).tw. 34. (acute kidney injur$ or acute renal injur$).tw. 35. (acute kidney insufficie$ or acute renal insufficie$).tw. 36. acute tubular necrosis.tw. 37. (ARF or AKF or ATN).tw. 38. or/32‐37 39. and/10,31 40. and/10,38 41. and/31,38 42. or/39‐41 |
| EMBASE | 1. exp coronary artery surgery/ 2. thorax surgery/ 3. bypass.tw. 4. coronary artery bypass$.tw. 5. coronary bypass grafting surgery.tw. 6. ((valve$ or cardiac) and surgery).tw. 7. CABG.tw. 8. cardiac surgery.tw. 9. valve replacement$.tw. 10. or/1‐9 11. exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ 12. statin$.tw. 13. "HMG‐CoA".tw. 14. hydroxymethylglutaryl‐coa reductase inhibitor$.tw. 15. (simvastatin or fluvastatin or cervistatin or lovastatin or pravastatin or atorvastatin or rosuvastatin).tw. 16. (Lipitor or Baycol or Lescol or Mevcor or Altocar or Pravacol or Lipostat or Zocor or Crestor).tw. 17. or/11‐16 18. exp acute kidney failure/ 19. (acute kidney failure or acute renal failure).tw. 20. (acute kidney injur$ or acute renal injur$).tw. 21. (acute kidney insufficie$ or acute renal insufficie$).tw. 22. acute tubular necrosis.tw. 23. (ARF or AKF or ATN).tw. 24. or/18‐23 25. and/10,17 26. and/10,24 27. and/17,24 28. or/25‐27 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Statins versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute kidney injury | 5 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.28] |
| 1.1 Atorvastatin | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.49] |
| 1.2 Simvastatin | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.21] |
| 1.3 Rosuvastatin | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 28.35] |
| 2 Serum creatinine: 24 h postoperatively | 2 | 195 | Mean Difference (IV, Random, 95% CI) | ‐21.25 [‐31.09, ‐11.41] |
| 2.1 Simvastatin | 2 | 195 | Mean Difference (IV, Random, 95% CI) | ‐21.25 [‐31.09, ‐11.41] |
| 3 Serum creatinine: 48 h postoperatively | 2 | 195 | Mean Difference (IV, Random, 95% CI) | ‐20.97 [‐34.58, ‐7.35] |
| 3.1 Simvastatin | 2 | 195 | Mean Difference (IV, Random, 95% CI) | ‐20.97 [‐34.58, ‐7.35] |
| 4 Mortality | 6 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.43, 34.40] |
| 4.1 Atorvastatin | 3 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 101.58] |
| 4.2 Simvastatin | 3 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [0.12, 69.70] |
| 5 Renal replacement therapy | 2 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.23, 2.81] |
| 5.1 Atorvastatin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.23, 2.81] |
| 5.2 Simvastatin | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 ALT x 3 ULN | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Creatinine kinase > 5000 IU/L | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Study drug cessation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almansob 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation used |
| Allocation concealment (selection bias) | Unclear risk | Information not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "... patients, surgeons, anaesthetists, perfusionists, ultrasound physicians, individuals collecting the samples... were all blinded". Blinding methods for the control group not reported. However, this was unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...people performing the data analysis were all blinded" No comment on how this was achieved, but given objective outcome measures, considered low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram: 9 patients from statin arm and 10 from placebo arm were excluded from the analysis: 4 statin and 6 placebo arm participants did not complete the study, 2 statin and 3 placebo arm participants had liver dysfunction postoperatively, 2 statin arm participants did not undergo bypass. 68 participants in statin arm and 64 in placebo arm underwent analysis. Despite there being evidence of missing data, this was assessed as low risk of bias, because missing data were balanced across groups for similar reasons and therefore was unlikely to have a clinically relevant impact |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes were reported. Comment: We would expect a drug study to clearly describe the outcome of drug and non‐drug related adverse events which did not occur here, thus this is deemed high risk. |
| Other bias | Low risk | No other risk of bias was identified |
Chello 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects fulfilling inclusion criteria were randomised". Randomisation method details not specified |
| Allocation concealment (selection bias) | Unclear risk | Information not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Both patients and physicians were blinded to the drug assignment group”. Placebo was not described so it is unclear how this was possible. However, the outcomes of interest were objective and therefore unlikely to be affected |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Information not provided. This was assessed as low risk because the outcomes of interest were objective and therefore unlikely to be affected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence or reporting of dropouts |
| Selective reporting (reporting bias) | Unclear risk | "All patients randomised to atorvastatin treatment did not experience any side effect related to the drug" The definition of adverse events was not given, nor was this a prespecified secondary outcome, therefore we deem this unclear risk of bias as clear judgement is unable to be made |
| Other bias | Low risk | No other risk of bias was identified for this study |
Christenson 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided |
| Allocation concealment (selection bias) | Unclear risk | Information not provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "two independent surgeons who were both unaware of the patients group identity, performed all operations" Comment: no other comment is made regarding blinding of participants, use of placebo or methods of blinding the surgeons. Despite this, outcome measures were objective, and therefore low risk of bias is assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information was provided Comment: Low risk of bias is assigned secondary to objective outcome measures unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patient dropouts described |
| Selective reporting (reporting bias) | High risk | No information provided Comment: No mention of adverse events which would be expected in such a drug study. As this is an expected outcome, this is assigned high risk of bias |
| Other bias | High risk | Pharmaceutical company‐sponsored study |
Mannacio 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "selection of patients for placebo or RSV treatment was obtained on admission by means of a computer generated algorithm" |
| Allocation concealment (selection bias) | Low risk | "randomisation was fully blinded without any account of clinical or demographic features" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo for participant blinding, personnel blinding and other methods of concealment not described. However, this was deemed low risk because it was unlikely to influence the objective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No explicit description of blinding, but these were objective, and therefore deemed to be low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of patient dropout; flow diagram not provided. However, given the short follow‐up period, there were potentially no dropouts |
| Selective reporting (reporting bias) | High risk | Mortality was inadequately reported and there was no mention of adverse events |
| Other bias | Unclear risk | Funding not reported; no other bias was noted in this study |
Prowle 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The hospital pharmacy clinical trials coordinator used a Microsoft Excel‐based random number generator to create the randomisation list using a permuted block strategy with blocks of 10" |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment to patients, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators was ensured by central randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Atorvastatin or placebo medication was prepared in capsules of identical appearance"; "the study drug or placebo was administered orally in the pre‐operative area by a study investigator" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Treatment allocation was only revealed after data analysis had been performed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram: 8 cases (2 emergency, 1 withdrew consent, 1 study drug stopped, 1 no CPB), 7 controls (3 emergency, 3 study drug stopped, 1 no CPB). Given that the spread of patient loss was even across both groups this was felt to represent low risk of bias. Furthermore, data were analysed on ITT basis, followed by PP basis. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | No other bias was identified in this study |
Spadaccio 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that recruited patients were "randomised" but no details of randomisation method are provided |
| Allocation concealment (selection bias) | Unclear risk | No methods of allocation concealment mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both patients and physicians were blinded to the assignment group." Blinding methods not described; use of placebo not described. However, this was unlikely to influence outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although reporting was suboptimal, it was unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data |
| Selective reporting (reporting bias) | High risk | No mention of adverse events which would be expected in such a drug study |
| Other bias | Unclear risk | Funding not reported; no other bias was noted in this study |
Tamayo 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “except for perfusionists, clinical team was blinded to the randomisation”. Comment: it is unclear why the perfusionists were unblinded, and how the blinding of other members of the team occurred as no placebo is mentioned. However this is unlikely to influence the objective outcomes, therefore is deemed low risk |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information is provided Comment: despite lack of information, this is deemed low risk as it is unlikely to influence the objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs and no missing data were reported for this study |
| Selective reporting (reporting bias) | High risk | No information provided Comment: There is no mention of adverse events which would be expected in such a drug study, therefore this is deemed high risk |
| Other bias | Low risk | Funding not reported; no other bias was noted in this study |
ACEi ‐ angiotensin‐converting enzyme inhibitors; AKI ‐ acute kidney injury; CABG ‐ coronary artery bypass grafting; CHF ‐ chronic heart failure; CKD ‐ chronic kidney disease; CPB ‐ cardiopulmonary bypass; CrCl ‐ creatinine clearance; DM ‐ diabetes mellitus; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; ITT ‐ intention to treat; LVEF ‐ left ventricular ejection fraction; MI ‐ myocardial infarction; NSAID ‐ nonsteroidal anti‐inflammatory drug; PP ‐ per protocol; RRT ‐ renal replacement therapy; SCr ‐ serum creatinine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acikel 2010 | No procedure of interest |
| Almeida 2010 | No procedure of interest |
| Antoniades 2010 | No outcome of interest |
| Antoniades 2010a | No outcome of interest |
| ARMYDA‐CIN 2011 | No procedure of interest |
| Barbir 1994 | No outcome of interest |
| Berkan 2009 | No outcome of interest |
| Caorsi 2008 | No outcome of interest |
| Chello 2003 | No outcome of interest |
| Chello 2005 | No outcome of interest |
| Chello 2007 | No outcome of interest |
| Chen 2009a | No procedure of interest |
| Cobbaert 1992 | No outcome of interest |
| Coccia 2007 | No outcome of interest |
| Dotani 2003 | No outcome of interest |
| Dunkelgrun 2009 | No procedure of interest |
| Durazzo 2004 | No procedure of interest |
| Faulkner 2000 | No procedure of interest |
| Flaker 1999 | No procedure of interest |
| FLARE Study 1999 | No procedure of interest |
| Florens 2001 | No outcome of interest |
| Foley 1994 | No procedure of interest |
| Han 2014 | No procedure of interest |
| Ji 2009 | No outcome of interest |
| Kesteloot 1992 | No outcome of interest |
| Kourliouros 2011 | No outcome of interest |
| Krivoy 2008 | No outcome of interest |
| Li 2012a | No procedure of interest |
| LIPS Study 2002 | No procedure of interest |
| Lotfi 2008 | No procedure of interest |
| Ludman 2010 | No placebo arm |
| Morawietz 2006 | No outcome of interest |
| Nakamura 2006b | No outcome of interest |
| Ozhan 2010 | No procedure of interest |
| Post‐CABG Trial 1997 | Statin treatment used in both arms |
| PRATO‐ACS Study 2014 | No procedure of interest |
| PROMISS Study 2008 | No procedure of interest |
| Qian 2008 | No outcome of interest |
| Radaelli 2007 | Dual agents used (ACEi + statin), no placebo arm |
| RAP Study 2010 | Data extracted from RCT, not randomised to statin versus placebo |
| Riahi 2006 | No outcome of interest |
| Sasmazel 2010 | No outcome of interest |
| Schanzer 2008 | Not procedure of interest |
| Schouten 2009 | Not procedure of interest |
| Song 2008 | No outcome of interest |
| STATIN‐STEMI Study 2010 | No procedure of interest |
| Su 2011 | No procedure of interest |
| Sun 2009a | No outcome of interest |
| Tetik 2010 | Statin treatment given in both arms of study |
| Toso 2010 | No procedure of interest |
| Xinwei 2009 | No procedure of interest |
| Zhou 2009 | No procedure of interest |
| Zhou 2010 | No outcome of interest |
ACEi ‐ angiotensin‐converting enzyme inhibitor; RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01547455.
| Trial name or title | Anti‐inflammatory and renoprotective effect of pretreatment loading dose atorvastatin in CABG |
| Methods | Randomised double blind parallel study |
| Participants | Adult patients >18 y old having elective CABG with cardiopulmonary bypass |
| Interventions |
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | March 2012 |
| Contact information | Shan Zhou MD, +86 15201506389, zhoushan.whu@live.cn |
| Notes |
STATIN AKI 2011.
| Trial name or title | Short‐term atorvastatin's effect on acute kidney injury following cardiac surgery |
| Methods | RCT |
| Participants | Adults > 18 y undergoing cardiac surgery |
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | July 2009 |
| Contact information | Frederic T. Billings, Vanderbilt University Medical Centre: frederic.t.billings@vanderbilt.edu |
| Notes | Estimated completion July 2015 |
ACEi ‐ angiotensin‐converting enzyme inhibitor; AKI ‐ acute kidney injury; CABG ‐ coronary artery bypass grafting; CrCl ‐ creatinine clearance; ICU ‐ intensive care unit; RCT ‐ randomised controlled trial; RRT ‐ renal replacement therapy; SCr ‐ serum creatinine
Differences between protocol and review
Although our published protocol specified conversion of raw SCr data to classify patients according to AKIN criteria, this was not possible secondary to insufficient data being available from included studies. As such, patients were assessed as having developed AKI as specified by the study authors.
Contributions of authors
Draft the protocol: ML, AS, IN
Study selection: ML, AS, IN
Extract data from studies: ML, AS, IN
Enter data into RevMan: AS, ML
Carry out the analysis: ML, AS, IN
Interpret the analysis: ML
Draft the final review: ML, AS, IN
Disagreement resolution: ML, AS, IN
Update the review: ML
Sources of support
Internal sources
No sources of support supplied
External sources
-
Dr M Lewicki, Australia.
Supported through a Jacquot Research Entry Scholarship, RACP Foundation Australia
-
Dr A Schneider, Australia.
Supported through a MGS‐MIPRS Research Scholarship, Monash University Australia
Declarations of interest
Michelle Lewicki: None known
Irene Ng: None known
Antoine G Schneider: None known
New
References
References to studies included in this review
Almansob 2012 {published data only}
- Almansob MA, Xu B, Zhou L, Hu XX, Chen W, Chang FJ, et al. Simvastatin reduces myocardial injury undergoing noncoronary artery cardiac surgery: A randomized controlled trial. Arteriosclerosis Thrombosis & Vascular Biology 2012;32(9):2304‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chello 2006 {published data only}
- Chello M, Patti G, Candura D, Mastrobuoni S, Sciascio G, Agro F, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Critical Care Medicine 2006;34(3):660‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Christenson 1999 {published data only}
- Christenson JT. Preoperative lipid control with simvastatin protects coronary artery bypass grafts from obstructive graft disease. American Journal of Cardiology 2001;88(8):896‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Christenson JT. Preoperative lipid control with simvastatin reduces the risk for graft failure already 1 year after myocardial revascularization. Cardiovascular Surgery 2001;9(1):33‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Christenson JT. Preoperative lipid‐control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. European Journal of Cardio‐Thoracic Surgery 1999;15(4):394‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mannacio 2008 {published data only}
- Mannacio VA, Iorio D, Amicis V, Lello F, Musumeci F. Effect of rosuvastatin pretreatment on myocardial damage after coronary surgery: a randomized trial. Journal of Thoracic & Cardiovascular Surgery 2008;136(6):1541‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prowle 2012 {published data only}
- Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Haase M, et al. Pilot double‐blind, randomized controlled trial of short‐term atorvastatin for prevention of acute kidney injury after cardiac surgery. Nephrology 2012;17(3):215‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Prowle JR, Licari E, Calzavacca P, Ligabo EV, Echeverri JE, Hasse M, et al. Peri‐operative statin therapy does not alter the incidence of acute kidney injury after cardiopulmonary bypass: CREAT, the cardiopulmonary bypass, renal injury and atorvastatin trial. clinicaltrials.gov NCT00910221 [abstract]. Intensive Care Medicine 2010;36:S88. [EMBASE: 70290230] [Google Scholar]
Spadaccio 2010 {published data only}
- Spadaccio C, Pollari F, Casacalenda A, Alfano G, Genovese J, Covino E, et al. Atorvastatin increases the number of endothelial progenitor cells after cardiac surgery: a randomized control study. Journal of Cardiovascular Pharmacology 2010;55(1):30‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tamayo 2009 {published data only}
- Tamayo E, Alonso O, Alvarez FJ, Castrodeza J, Florez S, Stefano S. Effects of simvastatin on acute‐phase protein levels after cardiac surgery [Efecto de la simvastatina en la concentracion de las proteinas de fase aguda despues de la cirugia cardiaca]. Medicina Clinica 2008;130(20):773‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tamayo E, Alvarez FJ, Alonso O, Bustamente R, Castrodeza J, Soria S, et al. Effects of simvastatin on systemic inflammatory responses after cardiopulmonary bypass. Journal of Cardiovascular Surgery 2009;50(5):687‐94. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Acikel 2010 {published data only}
- Acikel S, Muderrisoglu H, Yildirir A, Aydinalp A, Sade E, Bayraktar N, et al. Prevention of contrast‐induced impairment of renal function by short‐term or long‐term statin therapy in patients undergoing elective coronary angiography. Blood Coagulation & Fibrinolysis 2010;21(8):750‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Almeida 2010 {published data only}
- Almeida R, Coelho OR, Meneses FH, Oliveira G, Molinari G, Franca R, et al. The APVS (atorvastatin in the perioperative of vascular surgery) study [abstract]. Circulation 2010;122(2):e345. [EMBASE: 70233157] [Google Scholar]
Antoniades 2010 {published data only}
- Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, et al. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH‐oxidase activity. Circulation 2010;122(11 Suppl):S66‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Antoniades C, Bakogiannis C, Tousoulis D, Zhang MH, Demosthenous M, Antonopoulos A, et al. Preoperative atorvastatin treatment rapidly improves vein graft redox state by inhibiting rac1 and NADPH oxidase activity in patients undergoing elective CABG [abstract]. European Heart Journal 2010;31(Suppl 1):310. [EMBASE: 70280690] [DOI] [PubMed] [Google Scholar]
- Antoniades C, Demosthenous M, Margaritis M, Tousoulis D, Antonopoulos A, Paschalis A, et al. Short‐term preoperative treatment with atorvastatin improves myocardial redox state in patients undergoing elective CABG by affecting NADPH oxidase activity and NOS coupling [abstract]. European Heart Journal 2010;31(Suppl 1):60. [EMBASE: 70279719] [Google Scholar]
Antoniades 2010a {published data only}
- Antoniades CA, Paschalis A, Tousoulis D, Bakogiannis C, Dimosthenous M, Antonopoulos AS, et al. Atorvastatin rapidly modifies vascular redox and increases nitric oxide bioavailability in human arteries [abstract]. Journal of the American College of Cardiology 2010;55(10 Suppl 1):A123. [EMBASE: 70096347] [Google Scholar]
- Demosthenous M, Antoniades C, Paschalis A, Tousoulis D, Bakogiannis C, Antonopoulos AS, et al. Atorvastatin rapidly modifies vascular redox state and improves nitric oxide bioavailability in human vessels obtained from patients with advanced atherosclerosis [abstract]. European Heart Journal 2010;31(Suppl 1):1023. [EMBASE: 70283432] [Google Scholar]
ARMYDA‐CIN 2011 {published data only}
- Patti G, Ricottini E, Nusca A, Colonna G, Pasceri V, D'Ambrosio A, et al. Short‐term, high‐dose Atorvastatin pretreatment to prevent contrast‐induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA‐CIN [atorvastatin for reduction of myocardial damage during angioplasty‐‐contrast‐induced nephropathy] trial. American Journal of Cardiology 2011;108(1):1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barbir 1994 {published data only}
- Barbir M, Hunt BJ, Galloway D, Taylor A, Ilsley C, Mitchell A, et al. A randomized pilot trial of low‐dose combination lipid‐lowering therapy following coronary artery bypass grafting. Clinical Cardiology 1994;17(2):59‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berkan 2009 {published data only}
- Berkan O, Katrancioglu N, Ozker E, Ozerdem G, Bakici Z, Yilmaz MB. Reduced P‐selectin in hearts pretreated with fluvastatin: a novel benefit for patients undergoing open heart surgery. Thoracic & Cardiovascular Surgeon 2009;57(2):91‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Caorsi 2008 {published data only}
- Caorsi C, Pineda F, Munoz C. Pravastatin immunomodulates IL‐6 and C‐reactive protein, but not IL‐1 and TNF‐alpha, in cardio‐pulmonary bypass. European Cytokine Network 2008;19(2):99‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chello 2003 {published data only}
- Chello M, Mastroroberto P, Patti G, D'Ambrosio A, Morichetti MC, Sciascio G, et al. Simvastatin attenuates leucocyte‐endothelial interactions after coronary revascularisation with cardiopulmonary bypass. Heart 2003;89(5):538‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chello 2005 {published data only}
- Chello M, Goffredo C, Patti G, Candura D, Melfi R, Mastrobuoni S, et al. Effects of atorvastatin on arterial endothelial function in coronary bypass surgery. European Journal of Cardio‐Thoracic Surgery 2005;28(6):805‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chello 2007 {published data only}
- Chello M, Anselmi A, Spadaccio C, Patti G, Goffredo C, Sciascio G, et al. Simvastatin increases neutrophil apoptosis and reduces inflammatory reaction after coronary surgery. Annals of Thoracic Surgery 2007;83(4):1374‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2009a {published data only}
- Chen GL, Su JZ. Atorvastatin attenuated contrast induced renal function damage. Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 2009;37(5):389‐93. [MEDLINE: ] [PubMed] [Google Scholar]
Cobbaert 1992 {published data only}
- Cobbaert C, Sergeant P, Meyns B, Szecsi J, Kesteloot H. Time course of serum Lp(a) in men after coronary artery bypass grafting. Acta Cardiologica 1992;47(6):529‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Coccia 2007 {published data only}
- Coccia R, Spadaccio C, Foppoli C, Perluigi M, Covino E, Lusini M, et al. The effect of simvastatin on erythrocyte membrane fluidity during oxidative stress induced by cardiopulmonary bypass: a randomized controlled study. Clinical Therapeutics 2007;29(8):1706‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dotani 2003 {published data only}
- Dotani MI, Morise AP, Haque R, Jain AC, Gupta N, Gibson CM. Association between short‐term simvastatin therapy before coronary artery bypass grafting and postoperative myocardial blood flow as assessed by positron emission tomography. American Journal of Cardiology 2003;91(9):1107‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dunkelgrun 2009 {published data only}
- Dunkelgrun M, Boersma E, Schouten O, Koopman‐van Gemert AW, Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Annals of Surgery 2009;249(6):921‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Durazzo 2004 {published data only}
- Caramelli B, Durazzo A, Ikeoka D, Bernoche C, Monachini M, Puech Leao P, et al. Short‐term treatment with atorvastatin for prevention of cardiac complications after vascular surgery [abstract no: 086]. Atherosclerosis Supplements 2002;3(2):83. [CENTRAL: CN‐00831428] [Google Scholar]
- Durazzo AE, Machado FS, Ikeoka DT, Bernoche C, Monachini MC, Puech‐Leao P, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. Journal of Vascular Surgery 2004;39(5):967‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Faulkner 2000 {published data only}
- Faulkner MA, Wadibia EC, Lucas BD, Hilleman DE. Impact of pharmacy counseling on compliance and effectiveness of combination lipid‐lowering therapy in patients undergoing coronary artery revascularization: a randomized, controlled trial. Pharmacotherapy: The Journal of Human Pharmacology & Drug Therapy 2000;20(4):410‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flaker 1999 {published data only}
- Flaker GC, Warnica JW, Sacks FM, Moye LA, Davis BR, Rouleau JL, et al. Pravastatin prevents clinical events in revascularized patients with average cholesterol concentrations. Cholesterol and Recurrent Events CARE Investigators. Journal of the American College of Cardiology 1999;34(1):106‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
FLARE Study 1999 {published data only}
- Lloyd GW, Jackson G, Foley DP, Boersma E, Shepherd J, Serruys PW. The influence of plasma lipoprotein (a) on angiographic restenosis and coronary events in patients undergoing planned coronary balloon angioplasty. Ancillary analysis of the Fluvastatin Angioplasty Restenosis (FLARE) trial. Atherosclerosis 2001;158(2):445‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serruys PW, Foley DP, Jackson G, Bonnier H, Macaya C, Vrolix M, et al. A randomized placebo‐controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. European Heart Journal 1999;20(1):58‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Florens 2001 {published data only}
- Florens E, Salvi S, Peynet J, Elbim C, Mallat Z, Bel A, et al. Can statins reduce the inflammatory response to cardiopulmonary bypass? A clinical study. Journal of Cardiac Surgery 2001;16(3):232‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foley 1994 {published data only}
- Foley DP, Bonnier H, Jackson G, Macaya C, Shepherd J, Vrolix M, et al. Prevention of restenosis after coronary balloon angioplasty: rationale and design of the Fluvastatin Angioplasty Restenosis (FLARE) Trial. The FLARE Study Group. American Journal of Cardiology 1994;73(14):50D‐61D. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Han 2014 {published data only}
- Han Y, Zhu G, Han L, Hou F, Huang W, Liu H, et al. Short‐term rosuvastatin therapy for prevention of contrast‐induced acute kidney injury in patients with diabetes and chronic kidney disease. Journal of the American College of Cardiology 2014;63(1):62‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ji 2009 {published data only}
- Ji Q, Mei Y, Wang X, Sun Y, Feng J, Cai J, et al. Effect of preoperative atorvastatin therapy on atrial fibrillation following off‐pump coronary artery bypass grafting. Circulation Journal 2009;73(12):2244‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kesteloot 1992 {published data only}
- Kesteloot H, Cobbaert C, Meyns B, Szecsi J, Lesaffre E, Sergeant P. Time course of serum lipid and lipoprotein levels after coronary bypass surgery: modification by pravastatin. Acta Cardiologica 1992;47(6):519‐28. [MEDLINE: ] [PubMed] [Google Scholar]
Kourliouros 2011 {published data only}
- Kourliouros A, Valencia O, Hosseini MT, Mayr M, Sarsam M, Camm J, et al. Preoperative high‐dose atorvastatin for prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. Journal of Thoracic & Cardiovascular Surgery 2011;141(1):244‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krivoy 2008 {published data only}
- Krivoy N, Adler Z, Saloma R, Hawadie A, Azzam ZS. Targeting C‐reactive protein levels using high‐dose atorvastatin before coronary artery bypass graft surgery. Experimental & Clinical Cardiology 2008;13(4):171‐4. [EMBASE: 2009154149] [PMC free article] [PubMed] [Google Scholar]
Li 2012a {published data only}
- Li W, Fu X, Wang Y, Li X, Yang Z, Wang X, et al. Beneficial effects of high‐dose atorvastatin pretreatment on renal function in patients with acute ST‐segment elevation myocardial infarction undergoing emergency percutaneous coronary intervention. Cardiology 2012;122(3):195‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LIPS Study 2002 {published data only}
- Arampatzis CA, Goedhart D, Serruys PW, Saia F, Lemos PA, Feyter P, et al. Fluvastatin reduces the impact of diabetes on long‐term outcome after coronary intervention‐‐a Lescol Intervention Prevention Study (LIPS) substudy. American Heart Journal 2005;149(2):329‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serruys PW, Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287(24):3215‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lotfi 2008 {published data only}
- Lotfi A, Schweiger MJ, Giugliano GR, Murphy SA, Cannon CP, TIMI 22 Investigators. High‐dose atorvastatin does not negatively influence clinical outcomes among clopidogrel treated acute coronary syndrome patients‐‐a Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis in Myocardial Infarction 22 (PROVE IT‐TIMI 22) analysis. American Heart Journal 2008;155(5):954‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ludman 2010 {published data only}
- Ludman AJ, Hausenloy DJ, Venugopal V, Babu G, Boston‐Griffiths E, Lawrence D, et al. The effect of high‐dose atorvastatin on a background of standard‐dose chronic statin therapy in patients undergoing cardiac surgery [abstract]. European Heart Journal 2010;31(Suppl 1):70. [EMBASE: 70279760] [Google Scholar]
Morawietz 2006 {published data only}
- Morawietz H, Erbs S, Holtz J, Schubert A, Krekler M, Goettsch W, et al. Endothelial Protection, AT1 blockade and Cholesterol‐Dependent Oxidative Stress: the EPAS trial. Circulation 2006;114(1 Suppl):1296‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakamura 2006b {published data only}
- Nakamura K, Masuda H, Kariyazono H, Arima J, Iguro Y, Yamada K, et al. Effects of atorvastatin and aspirin combined therapy on inflammatory responses in patients undergoing coronary artery bypass grafting. Cytokine 2006;36(5‐6):201‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozhan 2010 {published data only}
- Ozhan H, Erden I, Ordu S, Aydin M, Caglar O, Basar C, et al. Efficacy of short‐term high‐dose atorvastatin for prevention of contrast‐induced nephropathy in patients undergoing coronary angiography. Angiology 2010;61(7):711‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Post‐CABG Trial 1997 {published data only}
- Alaupovic P, Fesmire JD, Hunnighake D, Domanski M, Forman S, Knatterud GL, et al. The effect of aggressive and moderate lowering of LDL‐cholesterol and low dose anticoagulation on plasma lipids, apolipoproteins and lipoprotein families in post coronary artery bypass graft trial. Atherosclerosis 1999;146(2):369‐79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Campeau L, Lesperance J, Bilodeau L, Fortier A, Guertin MC, Knatterud GL. Effect of cholesterol lowering and cardiovascular risk factors on the progression of aortoiliac arteriosclerosis: a quantitative cineangiography study. Angiology 2005;56(2):191‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Canner PL, Thompson B, Knatterud GL, Geller N, Campeau L, Zucker D. An application of the Zucker‐Wittes modified ratio estimate statistic in the Post Coronary Artery Bypass Graft (CABG) clinical trial. Controlled Clinical trials 1997;18(4):318‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Domanski M, Tian X, Fleg J, Coady S, Gosen C, Kirby R, et al. Pleiotropic effect of lovastatin, with and without cholestyramine, in the post coronary artery bypass graft (Post CABG) trial. American Journal of Cardiology 2008;102(8):1023‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hunninghake DB. Therapeutic efficacy of the lipid‐lowering armamentarium: the clinical benefits of aggressive lipid‐lowering therapy. American Journal of Medicine 1998;104(2A):9S‐13S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Girotra S, Mittleman MA. Alcohol consumption, atherosclerotic progression, and prognosis among patients with coronary artery bypass grafts. American Heart Journal 2006;151(2):368‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Smith CC, Karlamangla AS, Moore AA. Moderate alcohol consumption and safety of lovastatin and warfarin among men: the post‐coronary artery bypass graft trial. American Journal of Medicine 2006;119(5):434‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Wellenius GA, Mittleman MA. Hematologic parameters, atherosclerotic progression, and prognosis in patients with previous coronary artery bypass grafting (from the Post CABG Trial). American Journal of Cardiology 2009;103(3):328‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low‐density lipoprotein cholesterol levels and low‐dose anticoagulation on obstructive changes in saphenous‐vein coronary‐artery bypass grafts [erratum appears in N Engl J Med 1997 Dec 18;337(25):1859]. New England Journal of Medicine 1997;336(3):153‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wee CC, Girotra S, Weinstein AR, Mittleman MA, Mukamal KJ. The relationship between obesity and atherosclerotic progression and prognosis among patients with coronary artery bypass grafts the effect of aggressive statin therapy. Journal of the American College of Cardiology 2008;52(8):620‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Mukamal KJ, Winkelmayer WC, Mittleman MA. Renal dysfunction increases the risk of saphenous vein graft occlusion: results from the Post‐CABG trial. Atherosclerosis 2007;193(2):414‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- White CW, Gobel FL, Campeau L, Knatterud GL, Forman SA, Forrester JS, et al. Effect of an aggressive lipid‐lowering strategy on progression of atherosclerosis in the left main coronary artery from patients in the post coronary artery bypass graft trial. Circulation 2001;104(22):2660‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PRATO‐ACS Study 2014 {published data only}
- Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high‐dose rosuvastatin for contrast‐induced nephropathy prevention in acute coronary syndrome: Results from the PRATO‐ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast‐induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome). Journal of the American College of Cardiology 2014;63(1):71‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PROMISS Study 2008 {published data only}
- Jo SH, Koo BK, Park JS, Kang HJ, Cho YS, Kim YJ, et al. Prevention of radiocontrast medium‐induced nephropathy using short‐term high‐dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial‐‐a randomized controlled study. American Heart Journal 2008;155(3):499. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Qian 2008 {published data only}
- Qian YJ, Xiao XJ, Yuan HS, Tang H, Shao HZ, Wei DM. Combination pharmacological cardioversion of permanent atrial fibrillation in post‐prosthetic mitral valve replacement outpatients: a novel approach for the treatment of atrial fibrillation. Journal of International Medical Research 2008;36(3):537‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Radaelli 2007 {published data only}
- Radaelli A, Loardi C, Cazzaniga M, Balestri G, DeCarlini C, Cerrito MG, et al. Inflammatory activation during coronary artery surgery and its dose‐dependent modulation by statin/ACE‐inhibitor combination. Arteriosclerosis, Thrombosis & Vascular Biology 2007;27(12):2750‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RAP Study 2010 {published data only}
- Billings FT 4th, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. Journal of Cardiothoracic & Vascular Anesthesia 2010;24(6):913‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riahi 2006 {published data only}
- Riahi S, Schmidt EB, Amanavicius N, Karmisholt J, Jensen HS, Christoffersen RP, et al. The effect of atorvastatin on heart rate variability and lipoproteins in patients treated with coronary bypass surgery. International Journal of Cardiology 2006;111(3):436‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sasmazel 2010 {published data only}
- Sasmazel A, Baysal A, Fedekar A, Buyukbayrak F, Bugra O, Erdem H, et al. The effect of statin therapy on stimulation of endothelium‐derived nitric oxide before and after coronary artery bypass surgery. Heart Surgery Forum 2010;13(4):E243‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schanzer 2008 {published data only}
- Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. Journal of Vascular Surgery 2008;47(4):774‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schouten 2009 {published data only}
- Schouten O, Boersma E, Hoeks SE, Benner R, Urk H, Sambeek MR, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. New England Journal of Medicine 2009;361(10):980‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2008 {published data only}
- Song YB, On YK, Kim JH, Shin DH, Kim JS, Sung J, et al. The effects of atorvastatin on the occurrence of postoperative atrial fibrillation after off‐pump coronary artery bypass grafting surgery. American Heart Journal 2008;156(2):373‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STATIN‐STEMI Study 2010 {published data only}
- Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG, et al. Efficacy of high‐dose atorvastatin loading before primary percutaneous coronary intervention in ST‐segment elevation myocardial infarction: the STATIN STEMI trial. [Erratum appears in JACC Cardiovasc Interv. 2012 Feb;5(2):248]. JACC: Cardiovascular Interventions 2010;3(3):332‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Su 2011 {published data only}
- Su JZ, Xue Y, Cai WQ, Huang QY, Chai DJ, Chen GL, et al. Association between high sensitivity C‐reactive protein and contrast induced acute kidney injury in patients with acute coronary syndrome undergoing percutaneous coronary intervention: impact of atorvastatin. Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 2011;39(9):807‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Sun 2009a {published data only}
- Sun YF, Mei YQ, Ji Q, Wang XS, Feng J, Cai JZ, et al. Effect of atorvastatin on postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2009;89(42):2988‐91. [MEDLINE: ] [PubMed] [Google Scholar]
Tetik 2010 {published data only}
- Tetik S, Ak K, Isbir S, Eksioglu‐Demiralp E, Arsan S, Iqbal O, et al. Clopidogrel provides significantly greater inhibition of platelet activity than aspirin when combined with atorvastatin after coronary artery bypass grafting: a prospective randomized study. Clinical & Applied Thrombosis/Hemostasis 2010;16(2):189‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toso 2010 {published data only}
- Toso A, Leoncini M, Maioli M, Gallopin M, Tedeschi D, Amato M, et al. Short‐term high‐dose atorvastatin for periprocedural myocardial infarction prevention in patients with renal dysfunction. Journal of Cardiovascular Medicine 2011;12(5):318‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Toso A, Maioli M, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Usefulness of atorvastatin (80 mg) in prevention of contrast‐induced nephropathy in patients with chronic renal disease. American Journal of Cardiology 2010;105(3):288‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xinwei 2009 {published data only}
- Xinwei J, Xianghua F, Jing Z, Xinshun G, Ling X, Weize F, et al. Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrast‐induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. American Journal of Cardiology 2009;104(4):519‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhou 2009 {published data only}
- Zhou X, Jin YZ, Wang Q, Min R, Zhang XY. Efficacy of high dose atorvastatin on preventing contrast induced nephropathy in patients underwent coronary angiography. Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 2009;37(5):394‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Zhou 2010 {published data only}
- Zhou Q, Wang G, Gao C, Chen T. Effect of ulinastatin on perioperative inflammatory response to coronary artery bypass grafting with cardiopulmonary bypass. Zhong Nan da Xue Xue Bao. Yi Xue Ban [Journal of Central South University Medical Sciences] 2010;35(2):107‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
de Oliveira 2012 {published data only}
- Oliveira MS, Martins KB, Costa JR Jr, Abizaid A, Stadler J, Mattos LA, et al. Impact on renal function of rosuvastatin preload prior to elective percutaneous coronary intervention in chronic statin users [Impacto na funcao renal de uma dose de reforco de rosuvastatina previa a intervencao coronaria percutanea eletiva nos pacientes em uso cronico de estatina]. Revista Brasileira de Cardiologia Invasiva 2012;20(3):303‐8. [EMBASE: 2013021434] [Google Scholar]
Han 2013 {published data only}
- Han S, Li XM, Mohammed Ali LA, Fu NK, Jin DX, Cong HL. Effect of short‐term different statins loading dose on renal function and CI‐AKI incidence in patients undergoing invasive coronary procedures. International Journal of Cardiology 2013;168(5):5101‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu WJ, Zhang BC, Guo R, Wei YD, Li WM, Xu YW. Renoprotective effect of alprostadil in combination with statins in patients with mild to moderate renal failure undergoing coronary angiography. Chinese Medical Journal 2013;126(18):3475‐80. [MEDLINE: ] [PubMed] [Google Scholar]
NAPLES II Study 2009 {published data only}
- Briguori C, Visconti G, Focaccio A, Golia B, Chieffo A, Castelli A, et al. Novel approaches for preventing or limiting events (Naples) II trial: impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. Journal of the American College of Cardiology 2009;54(23):2157‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Quintavalle C, Fiore D, Micco F, Visconti G, Focaccio A, Golia B, et al. Impact of a high loading dose of atorvastatin on contrast‐induced acute kidney injury. Circulation 2012;126(25):3008‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ROSA‐cIN Trial 2013 {published data only}
- Kaya A, Kurt M, Tanboga IH, Isik T, Ekinci M, Aksakal E, et al. Rosuvastatin versus atorvastatin to prevent contrast induced nephropathy in patients undergoing primary percutaneous coronary intervention (ROSA‐cIN trial). Acta Cardiologica 2013;68(5):489‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01547455 {published data only}
- Zhou S. Anti‐inflammatory and renoprotective effect of pretreatment loading dose atorvastatin in CABG. www.clinicaltrials.gov/ct2/show/NCT01547455 (accessed 13 January 2015).
STATIN AKI 2011 {published data only}
- Billings FT 4th, Ball SK, Roberts LJ 2nd, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radical Biology & Medicine 2011;50(11):1480‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ali 2005
- Ali IS, Buth KJ. Preoperative statin use and in‐hospital outcomes following heart surgery in patients with unstable angina. European Journal of Cardio‐Thoracic Surgery 2005;27(6):1051‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arora 2008
- Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, et al. Preoperative use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(5):1266‐73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asimakopoulos 2005
- Asimakopoulos G, Karagounis AP, Valencia O, Alexander N, Howlader M, Sarsam MA, et al. Renal function after cardiac surgery off‐ versus on‐pump coronary artery bypass: analysis using the Cockroft‐Gault formula for estimating creatinine clearance. Annals of Thoracic Surgery 2005;79(6):2024‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baigent 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. [Erratum appears in Lancet. 2008 Jun 21;371(9630):2084], [Erratum appears in Lancet. 2005 Oct 15‐21;366(9494):1358]. Lancet 2005;366(9493):1267‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellomo 2004
- Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care 2004;8(4):R204‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Billings 2010
- Billings FT 4th, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. Journal of Cardiothoracic & Vascular Anesthesia 2010;24(6):913‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bove 2004
- Bove T, Calabrò MG, Landoni G, Aletti G, Marino G, Crescenzi G, et al. The incidence and risk of acute renal failure after cardiac surgery. Journal of Cardiothoracic & Vascular Anesthesia 2004;18(4):442‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boyle 1997
- Boyle EM Jr, Pohlman TH, Johnson MC, Verrier ED. Endothelial cell injury in cardiovascular surgery: the systemic inflammatory response. Annals of Thoracic Surgery 1997;63(1):277‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chawla 2012
- Chawla LS, Zhao Y, Lough FC, Schroeder E, Seneff MG, Brennan JM. Off‐pump versus on‐pump coronary artery bypass grafting outcomes stratified by preoperative renal function. Journal of the American Society of Nephrology 2012;23(8):1389‐97. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chertow 1997
- Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, et al. Preoperative renal risk stratification. Circulation 1997;95(4):878‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chukwuemeka 2005
- Chukwuemeka A, Weisel A, Maganti M, Nette AF, Wijeysundera DN, Beattie WS, et al. Renal dysfunction in high‐risk patients after on‐pump and off‐pump coronary artery bypass surgery: a propensity score analysis. Annals of Thoracic Surgery 2005;80(6):2148‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clark 2006
- Clark LL, Ikonomidis JS, Crawford FA Jr, Crumbley A 3rd, Kratz JM, Stroud MR, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: an 8‐year retrospective cohort study. Journal of Thoracic & Cardiovascular Surgery 2006;131(3):679‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coca 2009
- Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long‐term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta‐analysis. American Journal of Kidney Diseases 2009;53(6):961‐73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conlon 1999
- Conlon PJ, Stafford‐Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrology Dialysis Transplantation 1999;14(5):1158‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Devarajan 2006
- Devarajan P. Update on mechanisms of ischemic acute kidney injury. Journal of the American Society of Nephrology 2006;17(6):1503‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Phillips AN. Meta‐analysis: Principles and procedures. BMJ 1997;315(7121):1533‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endo 1976
- Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase by ML‐236A and ML‐236B fungal metabolites, having hypocholesterolemic activity. FEBS Letters 1976;72(2):323‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gueler 2002
- Gueler F, Rong S, Park JK, Fiebeler A, Menne J, Elger M, et al. Postischemic acute renal failure is reduced by short‐term statin treatment in a rat model. Journal of the American Society of Nephrology 2002;13(9):2288‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haslinger‐Löffler 2008
- Haslinger‐Löffler B. Multiple effects of HMG‐CoA reductase inhibitors (statins) besides their lipid‐lowering function. Kidney International 2008;74(5):553‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Inman 2005
- Inman SR, Davis NA, Mazzone ME, Olson KM, Lukaszek VA, Yoder KN. Simvastatin and L‐arginine preserve renal function after ischemia/reperfusion injury. American Journal of the Medical Sciences 2005;329(1):13‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jo 2008
- Jo SH, Koo BK, Park JS, Kang HJ, Cho YS, Kim YJ, et al. Prevention of radiocontrast medium‐induced nephropathy using short‐term high‐dose simvastatin in patients with renal insufficiency undergoing coronary angiography (PROMISS) trial‐‐a randomized controlled study. American Heart Journal 2008;155(3):499, e1‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Karkouti 2009
- Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 2009;119(4):495‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lafrance 2010
- Lafrance JP, Miller DR. Acute kidney injury associates with increased long‐term mortality. Journal of the American Society of Nephrology 2010;21(2):345‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamy 2012
- Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Off‐pump or on‐pump coronary‐artery bypass grafting at 30 days. New England Journal of Medicine 2012;366(16):1489‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2012
- Li Y, Liu Y, Fu L, Mei C, Dai B. Efficacy of short‐term high‐dose statin in preventing contrast‐induced nephropathy: a meta‐analysis of seven randomized controlled trials. PLoS One 2012;7(4):e34450. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liakopoulos 2008
- Liakopoulos OJ, Choi YH, Haldenwang PL, Strauch J, Wittwer T, Dörge H, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta‐analysis of over 30,000 patients. European Heart Journal 2008;29(12):1548‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liakopoulos 2012
- Liakopoulos OJ, Kuhn EW, Slottosch I, Wassmer G, Wahlers T. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008493.pub2] [DOI] [PubMed] [Google Scholar]
Mangano 1998
- Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Annals of Internal Medicine 1998;128(3):194‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mariscalco 2011
- Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: a relevant complication after cardiac surgery. Annals of Thoracic Surgery 2011;92(4):1539‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehta 2007
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care 2007;11(2):R31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mistiaen 2009
- Mistiaen W, Cauwelaert P, Muylaert P, Worm E. A thousand pericardial valves in aortic position: risk factors for postoperative acute renal function impairment in elderly. Journal of Cardiovascular Surgery 2009;50(2):233‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Morgan 2009
- Morgan C, Zappitelli M, Gill P. Statin prophylaxis and inflammatory mediators following cardiopulmonary bypass: a systematic review. Critical Care 2009;13(5):R165. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostermann 2000
- Ostermann ME, Taube D, Morgan CJ, Evans TW. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Medicine 2000;26(5):565‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ozan 2010
- Ozhan H, Erden I, Ordu S, Aydin M, Caglar O, Basar C, et al. Efficacy of short‐term high‐dose atorvastatin for prevention of contrast‐induced nephropathy in patients undergoing coronary angiography. Angiology 2010;61(7):711‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pan 2004
- Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation 2004;110(11 Suppl 1):II45‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patti 2008
- Patti G. Short‐term high‐dose atorvastatin for periprocedural myocardial infarction prevention in patients with renal dysfunction. Journal of Cardiovascular Medicine 2011;12(5):305‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Srisawat 2010
- Srisawat N, Lawsin L, Uchino S, Bellomo R, Kellum JA, BEST Kidney Investigators. Cost of acute renal replacement therapy in the intensive care unit: results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Critical Care 2010;14(2):R46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ 2001;327(7304):101‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swaminathan 2007
- Swaminathan M, Shaw AD, Phillips‐Bute BG, McGugan‐Clark PL, Archer LE, Talbert S, et al. Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Critical Care Medicine 2007;35(10):2286‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tabata 2007
- Tabata M, Khalpey Z, Pirundini PA, Byrne ML, Cohn LH, Rawn JD. Renoprotective effect of preoperative statins in coronary artery bypass grafting. American Journal of Cardiology 2007;100(3):442‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thakar 2003
- Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, et al. ARF after open‐heart surgery: Influence of gender and race. American Journal of Kidney Diseases 2003;41(4):742‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yokota 2003
- Yokota N, O'Donnell M, Daniels F, Burne‐Taney M, Keane W, Kasiske B, et al. Protective effect of HMG‐CoA reductase inhibitor on experimental renal ischemia‐reperfusion injury. American Journal of Nephrology 2003;23(1):13‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lewicki 2013
- Lewicki M, Ng I, Schneider AG. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010480] [DOI] [PMC free article] [PubMed] [Google Scholar]


