Abstract
Rabbit oncology is gaining more attention as more pet rabbits are surviving beyond their normal lifespans. Due to the limited epidemiological information on pet rabbits’ tumors in Thailand, this study aimed to report the prevalence and the potential risk factors associated with tumors in pet rabbits in Thailand. From 2018 to 2022, 93 tissue biopsies from tumor-suspected lesions on pet rabbits were gathered from animal hospitals in Bangkok and Chonburi provinces, Thailand. According to histopathology confirmation, tumors and tumor-like lesions were diagnosed. In this study, the overall tumors were 67.74% (n=63) out of the submitted cases (n=93). The most commonly affected organ systems were reproduction (65.08%) and integumentary (22.22%). Rabbits older than 5 years were 3.85 times more likely to have reproductive tumors than younger rabbits (95% confidence interval (CI): 1.45–10.27, P≤0.01), and the most frequently occurring tumor type was uterine adenocarcinoma. Furthermore, male rabbits had a 17.02 times higher probability of developing cutaneous tumors than female rabbits (95% CI: 4.19–69.11, P≤0.001), and the most frequently occurring tumor type was soft tissue sarcoma. The results of this study thus suggested that the age and sex of the rabbits were potential risk factors for tumor development in Thailand. The knowledge gained from our study also provided the recommendation for owners to monitor their rabbits’ health annually, particularly after late middle age, and rendered guidance for tumor detection in practical clinics.
Keywords: occurrence, pet rabbit, risk factor, tumor, Thailand
Pet rabbits are among the small animals becoming more popular in Thailand because of their small space requirements and less time-consuming management. The provinces of Bangkok and Chonburi, which are located in the middle of the country, are among the most outstanding urbanized cities, with a significant number of specialists in exotic pets and the largest pet rabbit market. The Holland Lop, Netherland Dwarf, and Lion Head are among the breeds most frequently seen in Thailand. The majority of owners raised them indoors and fed them commercially fortified food. The exact number of pet rabbits raised in Thailand is unclear. But during the past few years, they have become more common as clinic clients.
The advancement of rabbit medicine has led to healthier and longer lives for rabbits [15]. According to various literary works, pet rabbits often live for 5 to 10 years. However, in recent years, the lifespan of rabbits has increased, and it is more typical to see animals older than 10 years, especially in the case of medium-sized rabbits [8, 19]. While dwarf and giant breeds have a lower life expectancy and geriatric disorders may be present in these breeds as early as 4 to 5 years of age, medium-sized rabbits may be termed geriatric at 7 years of age [8]. The increase in life expectancy of pet rabbits results in the development of degenerative diseases such as intervertebral disc degeneration [20], chronic renal disease [28], disorders of the vertebral column including spondylosis, calcified discs, narrowed discs, joint arthritis [22], and tumor diseases [32]. However, the knowledge of tumor diseases in this animal is still limited and needs the development of skills for approaching, diagnosing, and treating them [34].
There could be two original mechanisms that caused the tumor in pet rabbits: virus-induced and non-virus-induced causes. Shope fibroma and papilloma are examples of virus-induced tumors caused by the Rabbit Fibroma Virus, a Leporipoxvirus spread by fleas and mosquitoes. The tumor spread in immunocompromised, young-adult, or newborn rabbits, resulting in their death [16, 26, 27, 34]. The non-viral cutaneous tumors were reported as case series or case reports that originated from epithelial, mesenchymal, or hematopoietic cell origins. The common epithelial cell cutaneous tumor was reported as basal cell tumor [7, 21], while a single case report presented trichoepithelioma [1], tricholemmoma [24], and sebaceous gland adenocarcinoma [31]. A variety of mesenchymal cell tumor origins were reported, including lipoma, malignant fibrous histiocytoma, and hemangiosarcoma [34]. The most common hematopoietic tumor seen in young rabbits was lymphoma with or without cutaneous involvement [6, 13, 36]. The previous study found that pet rabbits had a tumor prevalence of 14.4%, the majority of which were morphologically malignant. When compared to age, prevalence increased to as high as 47.2% in rabbits older than 6 years. The most common type of tumor was uterine adenocarcinoma, which caused female rabbits to be more frequently affected by neoplasia [6].
A number of epidemiological factors, such as environment, host, agent, and time, were related to animal cancer. Age, breed, and sex of the host were associated with the risk of developing cancer in animals, while the variation in the geographic distribution of different types of animal cancer was an illustration of some environmental impacts [11]. In humans, the diverse environments and situations in the different regions (Asia vs. the West) generated different lifestyles and cultures, which in turn led to different cancer patterns based on gender and age [9, 23]. Studies on the occurrence and prevalence of tumors in pet rabbits were conducted in various regions of the world [3, 4, 6, 34], with Asia having the fewest reports [29].
Due to the limited information about rabbit tumor diseases occurring in Thailand, our study aimed to provide epidemiological data on pet rabbit tumors in terms of prevalence and risk factors for a naturally occurring tumor in pet rabbits in Bangkok and Chonburi provinces, Thailand. The findings of this study could be used as guidelines for further research, as well as providing information on potential types of tumors and predisposing factors for exotic pet veterinarians in tumor diagnosis and treatment during the gap periods of histopathological examination or in some cases that denied confirmation tests.
MATERIALS AND METHODS
Study population
The data used in this study came from hospital records of rabbits that underwent routine ovarian hysterectomy or had clinical signs of depression, anorexia, poor general health, and abnormal palpable masses. To confirm the suspected lesion, radiography and ultrasonography were employed. From July 2018 to June 2022, tissue biopsies from 93 pet rabbits (1–3 samples per month) were distributed across the years from four animal hospitals—two hospitals in Bangkok and two hospitals in Chonburi provinces, Thailand. Based on histological findings, the samples were diagnosed as tumors and tumor-like lesions by a certified pathologist from the Department of Pathology, Faculty of Veterinary Medicine, Chulalongkorn University, Bangkok. Case descriptions including age, sex, breed, body weight, and tumor location were also recorded. The rabbits’ age ranges were classified into four groups based on their life stages: young adult (1–3 years), middle age (>3–5 years), late middle age (>5–6 years), and old age (>6–10 years) [12].
Descriptive and statistical analysis
The percentage of occurrence (total cases, tumors, and tumor-like lesions), as well as the relative frequency (RF) categorized by organ systems (reproductive, integumentary, mammary gland, urinary, respiratory, and gastrointestinal), age, sex, tumor and tumor-like lesions, and affected area, were calculated and analyzed. The descriptive and analytic statistics were computed for prevalence and the most significant variables. To assess the influence of age, sex, breed, and body weight on the development of reproductive and integument tumors, the analysis with likelihood ratio test (odds ratios; OR) and Pearson’s χ2 test (or Fisher’s exact test), using Epitools Epidemiological Calculators [30] was applied. Potential risk factors were defined as age (classified into two groups: rabbits aged 0–5.0 years and >5.0 years), sex (male, female), breed (Holland Lop, Lionhead, Netherland dwarf, Teddy bear, Woody Toy, and mixed breeds), and body weight (classified into 3 groups: <1.0, 1.0–2.0, and >2.0 kg). Results were reported as odds ratios (OR) with their associated 95% confidence intervals (CI), and the significant level was considered at P value less than 0.05.
RESULTS
Tumor in different organ systems of pet rabbit
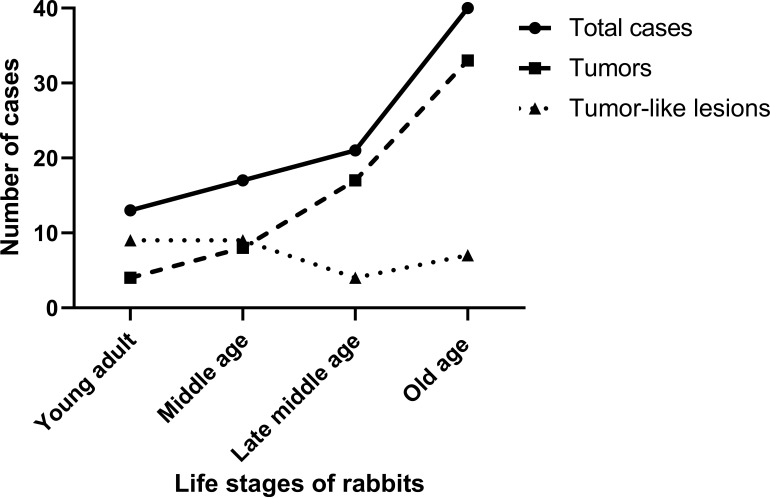
A total of 93 cases were suspected of being tumors by the animal hospitals during 2018–2022. According to the organ system classification, the reproductive (74.19%) and integumentary (16.13%) systems had the highest reported case numbers. However, only 63 cases (67.74%) of the total 93 cases were identified as tumors, with the highest number of tumors occurring in the reproductive system (65.08%), followed by the integumentary system (22.22%) and mammary gland (4.76%), respectively. Other tumors in different organ systems, such as the urinary system (perirenal lipoma and nephroblastoma), respiratory system (fibrosarcoma and mediastinal lymphoma), and gastrointestinal system (oral fibroma), were found sporadically. According to sex categorization, the prevalence of tumors in pet rabbits was found mostly in females (Table 1). The median age of pet rabbits diagnosed with tumors was 6.7 years (interquartile range (IQR): 1–11 years), while that of rabbits diagnosed with tumor-like lesions was 4.1 years (IQR: 1–11 years) (Table 2). Based on life stage distribution, the number of tumor cases gradually increased as the rabbit aged, while the number of tumor-like cases did not (Fig. 1).
Table 1. The overall case numbers of pet rabbits diagnosed with tumors and tumor-like lesions are categorized by organ systems and sex.
| Organ systems | Case numbers | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumors | Tumor-like lesions | Total cases | |||||||
| Female | Male | Total (% of all tumors) | Female | Male | Total (% of all tumor-like lesions) | Female | Male | Total (% of all cases) | |
| Reproductive system | 32 | 9 | 41 (65.08) | 27 | 1 | 28 (93.33) | 59 | 10 | 69 (74.19) |
| Integumentary system | 3 | 11 | 14 (22.22) | 0 | 1 | 1 (0.33) | 3 | 12 | 15 (16.13) |
| Mammary gland | 3 | 0 | 3 (4.76) | 0 | 0 | 0 (0) | 3 | 0 | 3 (3.23) |
| Urinary system | 0 | 1 | 2 (3.17) | 1 | 0 | 0 (0) | 1 | 1 | 2 (2.15) |
| Respiratory system | 1 | 1 | 2 (3.17) | 0 | 0 | 0 (0) | 1 | 1 | 2 (2.15) |
| Gastrointestinal system | 1 | 1 | 1 (1.59) | 0 | 0 | 1 (0.33) | 1 | 1 | 2 (2.15) |
| Total | 40 | 23 | 63 | 28 | 2 | 30 | 68 | 25 | 93 |
Table 2. The median and mean ages (years) of the cases diagnosed with tumors and tumor-like lesions are categorized by organ systems.
| Organ systems | Median* / Mean† age in years (range) | ||
|---|---|---|---|
| Tumors | Tumor-like lesions | Total cases | |
| Reproductive system | 6.15/6.55 (1–10) | 4/4.29 (1–9) | 6/5.64 (1–10) |
| Integumentary system | 8/7.51 (3–10.1) | 11 | 9/7.74 (3–11) |
| Mammary gland | 8/7.97 (5.9–10) | - | 8/7.97 (5.9–10) |
| Gastrointestinal system | 5.9 | 8.7 | 7.3 (5.9–8.7) |
| Urinary system | 7.5 (4–11) | - | 7.5 (4–11) |
| Respiratory system | 5.1 (4.2–6) | - | 5.1 |
| Total | 6.7/6.81 (1–11) | 4.1/4.67 (1–11) | 6/6.13 (1–11) |
*Median age=middle age that separates the greater and lesser halves of the data set (second quartile). †Mean age=arithmetic average of the age. If ≤2 cases with known age, only one value was indicated.
Fig. 1.
The number of cases and their distribution by rabbit life stage. Young adults (1–3 years), middle age (>3–5 years), late middle age (>5–6 years), and old age (>6–10 years) were the four categories used to categorize different life phases.
Tumor of reproductive systems in pet rabbits
Only 41 (59.42%) out of the 69 reproductive cases had tumor diagnoses (Table 1). The remaining 28 cases (40.58%) represented inflammatory, hyperplasia, cystic, and degenerative conditions. In addition, the prevalence of tumor cases in females (n=32) was higher than in males (n=9). Female rabbits had 1.58 times the odds of a reproductive tumor diagnosis compared to male rabbits (95% CI=0.61–4.07 and P=0.47) (Table 3). The median age of rabbits diagnosed with reproductive tumors was 6.15 years (IQR: 1–10 years) (Table 2). Moreover, rabbits over 5 years old had 3.85 times the odds of reproductive tumors compared to rabbits under or equal to 5 years old (95% CI=1.45–10.27 and P=0.01) (Table 3). Uterine adenocarcinoma was the most common type of female reproductive tumor in pet rabbits (43.90%) with a median age of 7 years (IQR: 4.1–10 years), while cystic endometrial hyperplasia was the most common type of female reproductive tumor-like lesion (75%), with a median age of 4.05 years (IQR: 1–8.7 years) (Table 4). The most frequent male reproductive tumor in pet rabbits was an interstitial cell tumor (17.07%) with a median age of 8 years (IQR: 6–9 years) (Table 4).
Table 3. Univariate analysis of risk factors associated with pet rabbit tumors from animal hospitals in Central Thailand during 2018–2022.
| Risk factors | Categories | Tumor cases (%RFa) | OR | 95% CI (OR) | P valueb |
|---|---|---|---|---|---|
| Reproductive system (n=41) | |||||
| Age | >5 years | 34 (53.97) | 3.85 | 1.45–10.27 | 0.01* |
| 0–5 years | 7 (23.33) | ||||
| Sex | Female | 32 (47.06) | 1.58 | 0.61–4.07 | 0.47 |
| Male | 9 (36) | ||||
| Breed | Holland Lop | 8 (40) | 0.81 | 0.3–2.21 | 0.87 |
| Lionhead | 1 (50) | 1.27 | 0.08–21.02 | 1 | |
| Netherland dwarf | 9 (42.86) | 0.94 | 0.35–2.80 | 1 | |
| Teddy bear | 2 (40) | 0.84 | 0.13–5.26 | 1 | |
| Woody toy | 1 (100) | 3.89 | 0.15–97.99 | 0.44 | |
| Mixed | 20 (48.78) | 1.41 | 0.62–3.21 | 0.55 | |
| Body weight | <1 kg | 4 (36.36) | 0.69 | 0.19–2.56 | 0.821 |
| 1–2 kg | 27 (47.37) | 1.41 | 0.61–3.30 | 0.56 | |
| >2 kg | 10 (43.48) | 0.97 | 0.37–2.5 | 1 | |
| Integument system (n=14) | |||||
| Age | >5 years | 11 (17.46) | 1.9 | 0.49–7.41 | 0.529 |
| 0–5 years | 3 (10) | ||||
| Sex | Male | 11 (44) | 17.02 | 4.19–69.11 | <0.0001* |
| Female | 3 (4.41) | ||||
| Breed | Holland Lop | 3 (15) | 0.99 | 0.25–3.97 | 1 |
| Netherland dwarf | 2 (9.52) | 0.53 | 0.11–2.56 | 0.73 | |
| Mixed | 9 (64.29) | 2.64 | 0.81−8.62 | 0.174 | |
| Body weight | <1 kg | 1 (9.09) | 0.53 | 0.06–4.51 | 1 |
| 1–2 kg | 6 (10.34) | 0.39 | 0.12–1.24 | 0.182 | |
| >2 kg | 6 (26.09) | 2.74 | 0.83–8.96 | 0.1 | |
a Relative frequency (RF) is tumors cases given all total cases both tumors and tumor-like lesions. b χ2 test or Fisher’s exact test.* Significant level of P<0.05.
Table 4. Reproductive tumor and tumor-like lesions in pet rabbits.
| Type of lesions | Number of cases (% of total) | Median* / Mean† age in years (range) | Affected areas |
|---|---|---|---|
| Tumor | |||
| Uterine adenocarcinoma | 18 (43.90) | 7/6.91 (4.1–10) | Uterus |
| Luteoma | 3 (7.32) | 5.9/4.3 (1–6) | Ovary |
| Interstitial cell tumor (Leydig cell tumor) | 7 (17.07) | 8/7.6 (6–9) | Testis |
| Leiomyosarcoma | 4 (9.76) | 7/6.75 (4–9) | Uterus |
| Leiomyoma | 2 (4.88) | 3.5 (3–4) | Uterus |
| Uterine adenoma | 1 (2.44) | 5.9 | Uterus |
| Papillary adenoma | 2 (4.88) | 6 (6–6) | Ovary, oviduct, uterus |
| Soft tissue sarcoma | 2 (4.88) | 6.7 (6.7–6.7) | Ovary, uterus |
| Myxosarcoma | |||
| Fibrosarcoma | |||
| Seminoma | 1 (2.44) | 3 | Testis |
| Sertoli cell tumor | 1 (2.44) | 9 | Testis |
| Total | 41 | 6.15/6.55 (1–10) | Ovary, oviduct, uterus, testis |
| Tumor-like | |||
| Cystic endometrial hyperplasia | 21 (75) | 4.05/4.42 (1–8.7) | Uterus |
| Follicular cyst | 2 (7.14) | 1.65 (1.3–2) | Ovary |
| Uterine papillary hyperplasia | 1 (3.57) | 2.6 | Uterus |
| Persistent corpus luteum | 1 (3.57) | 1.3 | Ovary |
| Severe necrotic suppurative endometritis | 1 (3.57) | 5 | Uterus |
| Testicular atrophy | 1 (3.57) | 6 | Testis |
| Calcification | 1 (3.57) | 9 | Ovary |
| Total | 28 | 4/4.29 (1–9) | Ovary, uterus, testis |
*Median age=middle age that separates the greater and lesser halves of the data set (second quartile). †Mean age=arithmetic average of the age. If ≤2 cases with known age, only one value was indicated.
Tumor of integumentary systems in pet rabbits
According to Table 1, 14 (93.33%) out of the 15 integumentary cases had tumor diagnoses. In contrast to the reproductive systems, the presence of tumor cases in males (n=11) was significantly higher than in females (n=3) (OR=17.02, 95% CI=4.19–69.11, and P<0.001) (Table 3). The median age of rabbits diagnosed with cutaneous tumors was 8 years (IQR: 3–10.1 years) (Table 2). The rabbits weighing over 2 kg had 2.74 times the odds of having skin tumors (95% CI=0.83–8.96 and P=0.1). In addition, the mixed breed rabbits tended to have the tumor 2.64 times (95% CI=0.81–8.62 and P=0.174) compared to other pure breeds (Table 3). Soft tissue sarcoma was the most common type of cutaneous tumor in pet rabbits (Table 5). The anatomical localization of cutaneous tumors was shown in Table 5.
Table 5. Cutaneous tumor and tumor-like lesions in pet rabbits.
| Type of lesions | Number of cases (% of total) | Median* / Mean† age in years (range) | Locations |
|---|---|---|---|
| Tumor | |||
| Soft tissue sarcoma | 6 (42.86) | 8/7.83 (5–10.1) | Left hindlimb, left flank, lumbar, ventral abdomen, forelimb |
| -Malignant fibrous histiocytoma | |||
| -Neurofibrosarcoma or hemangiopericytoma | |||
| -Hemangiopericytoma or fibrosarcoma | |||
| -Myosarcoma or fibrosarcoma | |||
| -Giant cell sarcoma | |||
| -Fibrosarcoma | |||
| Basal cell tumor (Trichoblastoma) | 3 (21.43) | 7/6.33 (3–9) | Shoulder |
| Squamous cell carcinoma | 2 (14.29) | 9.5 (9–10) | Upper lip |
| Apocrine cystic papillary adenoma | 1 (7.14) | 9 | Face |
| Lipoma | 1 (7.14) | 4.1 | Neck |
| Unidentified carcinoma | 1 (7.14) | 7 | Dorsal back |
| Total | 14 | 8/7.51 (3–10.1) | Limb, flank, lumbar, ventral abdomen, shoulder, lip, face, neck |
| Tumor-like | |||
| Third eyelid glandular hyperplasia | 1 (100) | 11 | Eye |
| Total | 1 | 11 | Eye |
*Median age=middle age that separates the greater and lesser halves of the data set (second quartile). †Mean age=arithmetic average of the age. If ≤2 cases with known age, only one value was indicated.
DISCUSSION
In this study, reproductive tumors were the most common, followed by cutaneous tumors in pet rabbits. This was compatible with some studies [6], but it was contrary to some earlier claims suggesting cutaneous tumors were more common [3]. Animal sex and age were the potential risk factors for the occurrence of the tumor. Our study indicated that tumors tend to affect females more frequently, which was similar to the previous report [6]. When comparing the prevalence with the life stage of the rabbits, tumor prevalence gradually increased in aged rabbits, whereas that of tumor-like lesions did not (Fig. 1). The common life stage of rabbits with tumors was old age (median: 6.7 years of life; IQR: 1–11 years), while previous study in European country reported that the life stage of rabbits with neoplasms occurred earlier at late middle age (median: 6 years of life [6] and mean: 5.7 years of age [3]). This different result implied that Asian environmental factors, in addition to host factors, might influence the age-related tumor’s occurrence in various parts of the world.
Similar to other previous reports, the majority of tumor cases in this study were reproductive tumors, which were more common in females than males [6, 14, 32]. The affected life stage of the rabbits with the tumor was old age (median: 6.15 years of life; IQR: 1–10 years) compared to that of rabbits with a tumor-like lesion was middle age (median: 4 years of life; IQR: 1–9 years). Interestingly, the probabilities of reproductive tumors were 3.85 times significantly higher in rabbits over 5 years of age compared to rabbits under or equal to 5 years of age. This finding was consistent with earlier studies, which demonstrated that tumor-like lesions were more common in younger animals, while the incidence of reproductive tumors increased with age [5]. The most common female reproductive tumor, similar to previous studies [3, 5, 6], was uterine adenocarcinoma, while the most common reproductive tumor-like lesion was cystic endometrial hyperplasia [5, 18, 29, 35]. The high prevalence of cystic endometrial hyperplasia and uterine adenocarcinoma might be related each other. Age-related increases in the prevalence of uterine adenocarcinomas might be due to hormonal stimulation, specifically progesterone and estrogen, which play a crucial role in tumor growth [2, 33]. Although cystic endometrial hyperplasia (CEH) was more frequently observed in middle-aged to older rabbits [5], its prevalence also rose with age. It was thought to develop from estrogen or progesterone stimulation and was proposed as a precursor to uterine adenocarcinoma in later life stages [2]. As in a previous study, interstitial cell tumor was the most common male reproductive tumor, followed by seminoma and Sertoli cell tumor [3].
Cutaneous tumors were the second most frequent tumors in this study. Similar to earlier studies [3, 6, 25], male rabbits were more likely than female rabbits to develop cutaneous tumors. According to this study, male rabbits had a 17.02 times higher probability of developing cutaneous tumors than female rabbits, and the risk was 2.74 times higher in rabbits weighing more than 2 kg, even though it was just a trend. The affected life stage of the rabbits with cutaneous tumors was old age (median: 8 years of age; IQR: 3–10.1 years), which was different from prior studies that reported the tumor occurred earlier in life at 5–5.51 years of age [3, 6]. Since such different age-related evidences were reported in European region, our result suggested the idea that other factors, in addition to the host factor but also environmental factors, might influence the occurrence of the cutaneous tumor in Thailand. Additionally, more than 80% of cutaneous cases were tumor, and the majority of the cutaneous tumors in this study were malignant [3, 25], in which soft tissue sarcoma, rather than trichoblastoma [17], was the most prevalent cutaneous tumor.
According to a previous report, most mammary gland tumors (>70%) were malignant, with predisposing factors including age (older rabbits with a mean age of 5.5 years; range, 2–14), breed (New Zealand White, English, and Belgian breeds), gender (intact female), and multiparity [4, 10, 32]. Estrogen and progesterone were suggested as the crucial factors influencing mammary cancer occurrence in pet rabbits [10]. In this study, mammary tumors were occasionally found, particularly in older female rabbits (median: 8 years of age; IQR: 5.9–10 years), most of which were malignant (adenocarcinoma and malignant myoepithelioma). There were other infrequent reports of mediastinal lymphoma, oral fibroma, fibrosarcoma, abdominal lipoma, and nephroblastoma, which were also found sporadically in this study.
The limitation of the present study was a retrospective study, in which some neoplastic masses were not identified or reported by the initial diagnostic service. Because of economic considerations, several domestic rabbit owners declined an antemortem diagnosis, resulting in limited case numbers of some neoplasia. Moreover, some internal masses might be unnoticed by the owner, and some owners preferred not to have a necropsy or confirm them histopathologically. It was, therefore, possible that the actual prevalence of neoplasia in pet rabbits may be higher than in the current study. Moreover, the history that might provide information on risk factors such as rabbit behavior, neutering, parity, and vaccine status was lacking in this study.
In conclusion, the reproduction and integumentary were the most prevalently affected systems by tumors, especially uterine adenocarcinomas and soft tissue sarcomas. The possible risk factors associated with the tumors were the rabbits’ sex and age. The older the rabbits got, the more reproductive tumors were observed, especially in female. Furthermore, male rabbits had a higher probability of developing cutaneous tumors than female ones. The knowledge from our study could be useful for practitioners for making a differential diagnosis of tumor in old rabbits and help suggesting the owners to check on their pet’s health annually, especially after age 5 or in late middle life. Furthermore, the findings of this study also suggested future investigations into the prevalence and other risk factors for the development of pet rabbit tumors in the Asian region.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank our colleagues and staff from Premier Pet Hospital, V Pet Hospital, and Pet Friends Animal Hospital for their data contributions. We especially appreciate our team: Wachirawich Nimitpan, Yanisa Tongsari, Suttichai Chompen, Phornkamol Maikonglay, and Anusara Chanthopha for their hard work and encouragement.
REFERENCES
- 1.Altman NH, Demaray SY, Lamborn PB., Jr1978. Trichoepithelioma in a rabbit. Vet Pathol 15: 671–672. doi: 10.1177/030098587801500511 [DOI] [PubMed] [Google Scholar]
- 2.Asakawa MG, Goldschmidt MH, Une Y, Nomura Y. 2008. The immunohistochemical evaluation of estrogen receptor-alpha and progesterone receptors of normal, hyperplastic, and neoplastic endometrium in 88 pet rabbits. Vet Pathol 45: 217–225. doi: 10.1354/vp.45-2-217 [DOI] [PubMed] [Google Scholar]
- 3.Baum B. 2021. Not just uterine adenocarcinoma—neoplastic and non-neoplastic masses in domestic pet rabbits (Oryctolagus cuniculus): a review. Vet Pathol 58: 890–900. doi: 10.1177/03009858211002190 [DOI] [PubMed] [Google Scholar]
- 4.Baum B, Hewicker-Trautwein M. 2015. Classification and epidemiology of mammary tumours in pet rabbits (Oryctolagus cuniculus). J Comp Pathol 152: 291–298. doi: 10.1016/j.jcpa.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 5.Bertram CA, Müller K, Klopfleisch R. 2018. Genital tract pathology in female pet rabbits (Oryctolagus cuniculus): a retrospective study of 854 necropsy examinations and 152 biopsy samples. J Comp Pathol 164: 17–26. doi: 10.1016/j.jcpa.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 6.Bertram CA, Bertram B, Bartel A, Ewringmann A, Fragoso-Garcia MA, Erickson NA, Müller K, Klopfleisch R. 2021. Neoplasia and tumor-like lesions in pet rabbits (Oryctolagus cuniculus): a retrospective analysis of cases between 1995 and 2019. Vet Pathol 58: 901–911. doi: 10.1177/0300985820973460 [DOI] [PubMed] [Google Scholar]
- 7.Bunte RM, Page DG. 1997. Basal cell adenoma in a rabbit. Contemp Top Lab Anim Sci 36: 90. [PubMed] [Google Scholar]
- 8.Chitty J. 2014. Problems of the geriatric rabbit. pp. 277–283. In: BSAVA Manual of Rabbit Medicine, 1st ed. (Meredith A, Lord B eds.), British Small Animal Veterinary Association, Gloucester. [Google Scholar]
- 9.Crocetti E, De Angelis R, Buzzoni C, Mariotto A, Storm H, Colonna M, Zanetti R, Serraino D, Michiara M, Cirilli C, Iannelli A, Mazzoleni G, Sechi O, Sanoja Gonzalez ME, Guzzinati S, Capocaccia R, Dal Maso L, Zucchetto A, Caldarella A, Bovo E, Tagliabue G, Vercelli M, Falcini F, Randi G, Petrucci S, Ferretti S, Pannozzo F, Mangone L, Piffer S, Fusco M, Giacomin A, Tisano F, Zarcone M. AIRTUM Working group.2013. Cancer prevalence in United States, Nordic Countries, Italy, Australia, and France: an analysis of geographic variability. Br J Cancer 109: 219–228. doi: 10.1038/bjc.2013.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degner S, Schoon HA, Laik-Schandelmaier C, Aupperle-Lellbach H, Schöniger S. 2018. Estrogen receptor–α and progesterone receptor expression in mammary proliferative lesions of female pet rabbits. Vet Pathol 55: 838–848. doi: 10.1177/0300985818788611 [DOI] [PubMed] [Google Scholar]
- 11.Dorn CR. 1967. The epidemiology of cancer in animals. Calif Med 107: 481–489. [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta S, Sengupta P. 2018. Rabbits and men: relating their ages. J Basic Clin Physiol Pharmacol 29: 427–435. doi: 10.1515/jbcpp-2018-0002 [DOI] [PubMed] [Google Scholar]
- 13.Gómez L, Gázquez A, Roncero V, Sánchez C, Durán ME. 2002. Lymphoma in a rabbit: histopathological and immunohistochemical findings. J Small Anim Pract 43: 224–226. doi: 10.1111/j.1748-5827.2002.tb00063.x [DOI] [PubMed] [Google Scholar]
- 14.Harcourt-Brown FM. 2017. Disorders of the reproductive tract of rabbits. Vet Clin North Am Exot Anim Pract 20: 555–587. doi: 10.1016/j.cvex.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Heatley JJ, Smith AN. 2004. Spontaneous neoplasms of lagomorphs. Vet Clin North Am Exot Anim Pract 7: 561–577, v. doi: 10.1016/j.cvex.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 16.Hess L. 2004. Dermatologic diseases. pp. 194–202. In: Ferrets, Rabbits and Rodents, Clinical Medicine and Surgery, 2nd ed. (Quesenberry KE, Carpenter JW eds.), Elsevier, St. Louis. [Google Scholar]
- 17.Kanfer S, Reavill DR. 2013. Cutaneous neoplasia in ferrets, rabbits, and guinea pigs. Vet Clin North Am Exot Anim Pract 16: 579–598. doi: 10.1016/j.cvex.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 18.Künzel F, Grinninger P, Shibly S, Hassan J, Tichy A, Berghold P, Fuchs-Baumgartinger A. 2015. Uterine disorders in 50 pet rabbits. J Am Anim Hosp Assoc 51: 8–14. doi: 10.5326/JAAHA-MS-5812 [DOI] [PubMed] [Google Scholar]
- 19.Lennox AM. 2010. Care of the geriatric rabbit. Vet Clin North Am Exot Anim Pract 13: 123–133. doi: 10.1016/j.cvex.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung VYL, Hung SC, Li LC, Wu EX, Luk KDK, Chan D, Cheung KMC. 2008. Age-related degeneration of lumbar intervertebral discs in rabbits revealed by deuterium oxide-assisted MRI. Osteoarthritis Cartilage 16: 1312–1318. doi: 10.1016/j.joca.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Schlafer DH. 1992. A spontaneous skin basal cell tumor in a black French minilop rabbit. Lab Anim Sci 42: 94–95. [PubMed] [Google Scholar]
- 22.Mäkitaipale J, Harcourt-Brown FM, Laitinen-Vapaavuori O. 2015. Health survey of 167 pet rabbits (Oryctolagus cuniculus) in Finland. Vet Rec 177: 418. doi: 10.1136/vr.103213 [DOI] [PubMed] [Google Scholar]
- 23.Ng CJ, Teo CH, Abdullah N, Tan WP, Tan HM. 2015. Relationships between cancer pattern, country income and geographical region in Asia. BMC Cancer 15: 613. doi: 10.1186/s12885-015-1615-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira KD, França TN, González AP, Peixoto PV. 1999. Tricolemoma em coelho. Cienc Rural 29: 361–363. doi: 10.1590/S0103-84781999000200030 [DOI] [Google Scholar]
- 25.Otrocka-Domagała I, Paździor-Czapula K, Fiedorowicz J, Mikiewicz M, Piotrowska A, Gesek M. 2022. Cutaneous and subcutaneous tumours of small pet mammals—retrospective study of 256 cases (2014–2021). Animals (Basel) 12: 965. doi: 10.3390/ani12080965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prose PH, Friedman-Kien AE, Vilcek J. 1971. Morphogenesis of rabbit fibroma virus. Correlation with pathogenesis of the skin lesion. Am J Pathol 64: 467–478. [PMC free article] [PubMed] [Google Scholar]
- 27.Pulley LT, Shively JN. 1973. Naturally occurring infants fibroma in the domestic rabbit. Vet Pathol 10: 509–519. doi: 10.1177/030098587301000604 [DOI] [PubMed] [Google Scholar]
- 28.Reavill DR, Lennox AM. 2020. Disease overview of the urinary tract in exotic companion mammals and tips on clinical management. Vet Clin North Am Exot Anim Pract 23: 169–193. doi: 10.1016/j.cvex.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito K, Nakanishi M, Hasegawa A. 2002. Uterine disorders diagnosed by ventrotomy in 47 rabbits. J Vet Med Sci 64: 495–497. doi: 10.1292/jvms.64.495 [DOI] [PubMed] [Google Scholar]
- 30.Sergeant ESG. 2018. Epitools −Epidemiological Calculators: Ausvet. http://epitools.ausvet.com.au [accessed on October 15, 2022].
- 31.Suckow MA, Rebelatto MC, Schulman AA, HogenEsch H. 2002. Sebaceous adenocarcinoma of the external auditory canal in a New Zealand white rabbit. J Comp Pathol 127: 301–303. doi: 10.1053/jcpa.2002.0585 [DOI] [PubMed] [Google Scholar]
- 32.van Zeeland Y. 2017. Rabbit oncology: diseases, diagnostics, and therapeutics. Vet Clin North Am Exot Anim Pract 20: 135–182. doi: 10.1016/j.cvex.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 33.Vinci A, Bacci B, Benazzi C, Caldin M, Sarli G. 2010. Progesterone receptor expression and proliferative activity in uterine tumours of pet rabbits. J Comp Pathol 142: 323–327. doi: 10.1016/j.jcpa.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 34.von Bomhard W, Goldschmidt MH, Shofer FS, Perl L, Rosenthal KL, Mauldin EA. 2007. Cutaneous neoplasms in pet rabbits: a retrospective study. Vet Pathol 44: 579–588. doi: 10.1354/vp.44-5-579 [DOI] [PubMed] [Google Scholar]
- 35.Walter B, Poth T, Böhmer E, Braun J, Matis U. 2010. Uterine disorders in 59 rabbits. Vet Rec 166: 230–233. doi: 10.1136/vr.b4749 [DOI] [PubMed] [Google Scholar]
- 36.White SD, Campbell T, Logan A, Meredith A, Schultheiss P, Van Winkle T, Moore PF, Naydan DK, Mallon F. 2000. Lymphoma with cutaneous involvement in three domestic rabbits (Oryctolagus cuniculus). Vet Dermatol 11: 61–67. doi: 10.1046/j.1365-3164.2000.00159.x [DOI] [PubMed] [Google Scholar]