Highlights
-
•
The combination of digestive enzymes (amylase, protease, cellulase, xylanase and beta glucanase) in the bovine diet improves growth performance.
-
•
The consumption of exogenous enzymes increased the concentration of short chain fatty acids in the ruminal fluid.
-
•
Blend enzymes intake per animals increasing the amount of unsaturated fatty acids and reducing the amount of saturated fatty acids in the meat.
-
•
The intake of exogenous enzymes has positive effect on oxidative stability in meat.
Keywords: Amylase, Protease, Cellulase, Xylanase, Beta glucanase
Abstract
The objective of this study was to evaluate if the inclusion of a blend composed of exogenous enzymes (amylase, protease, cellulase, xylanase and beta glucanase) in the individual and combined form in the feedlot steers diet has benefits on the physiology, rumen fermentation, digestibility and fatty acid profile in rumen and meat. The experiment used 24 animals, divided into 4 treatments, described as: T1-CON, T2-BLEND (0.5 g mixture of enzyme), T3-AMIL (0.5 g alpha-amylase), T4-BLEND+AMIL (0.5 g enzyme blend+ 0.5 g amylase). The concentration of mineral matter was higher in the meat of cattle of T4-BLEND+AMIL. A higher proportion of monounsaturated fatty acids was observed in the T3-AMIL group when compared to the others. The percentage of polyunsaturated fatty acids was higher in the T2-BLEND and T4-BLEND+AMIL compared to the T1-CON. The combination of exogenous enzymes in the diet positively modulate nutritional biomarkers, in addition to benefits in the lipid and oxidative profile meat.
1. Introduction
The demand for beef increases every year, which is why the need to intensify beef production systems has arisen. However, there is a huge challenge to this intensification since the 90 s, which is to produce in a profitable and sustainable way [1]. Still in the 1980s, there was the perception that a reduction in the production cycle, an increase in carcass yield, greater production per area, freeing up pasture areas for other categories and a faster return on investment are some of the advantages of the confined system [2]. However, the high cost of the diet provided to the animals was a limitation of this production system, due to the high added value of the ingredients, mainly cereals; nonetheless, the animal confinement system has grown worldwide.
Aiming to maximize the diet, the use of enzymatic additives is a good alternative to increase the performance of the animals through the mutual action of the enzymes provided and produced by the ruminal microbiota [3], which aims to increase digestion and seeks greater use of feed, and consequently greater weight gain. In order to maximize the use of the diet, the process of protein degradation in the rumen undergoes the action of the protease enzyme, involved in the breakdown of molecules to generate free amino acids through hydrolysis caused by the action of water and the action of enzymes [4]. In this context, in addition to the new formation of protein chains, the remaining amino acids serve as a substrate for microorganisms that will be sources of microbial protein, which is characterized as the main metabolizable source in ruminants, which leads to the understanding that the multiplication of microorganisms promotes improvements both in performance and in the health of these animals. The use of fibrolytic enzymes such as cellulase and xylanase has shown good results in the digestibility of dry matter and fiber, since the forage digestion process is often considered incomplete [5], which justifies the use of exogenous fibrolytic enzymes to act in conjunction with enzymes produced by microorganisms in the ruminal environment, thus enhancing fiber degradation [[6], [7], [8]].
Starchy grains are the main source of energy in confined ruminant diets, mainly corn [9]. With the processing it is possible to change the physical and chemical characteristics of the starch [10]. Knowing this, research with the inclusion of alpha amylase enzymes aims to optimize the digestion of starch, which can pass intact through the rumen. Due to the consistency of the endosperm, hard or farinaceous and the protein matrix that covers the starch, because it works as a physical and chemical barrier that prevents the hydrolysis of the starch molecule [[11], [12]]. However, this is extremely necessary in the digestion process to generate a maltose molecule that is equivalent to two glucose molecules, necessary to increase feed efficiency.
There are studies on the addition of exogenous enzymes in the diet of production animals, but it is considered insufficient when the experimental condition is in enzymatic association in order to evaluate digestibility, rumen environment and their effects on performance [[13], [14]]. We also agree that further studies are needed in order to seek to improve production in confinement, where a detail can reflect on profitability and consequently sustainability. Therefore, our hypothesis is that the combination of protease and amylase as an additive in the bovine diet will improve the digestibility. For this reason, this study was to evaluate if the inclusion of a blend composed of exogenous enzymes (amylase, protease, cellulase, xylanase and beta glucanase) in the individual and combined form in the feedlot steers diet has benefits on the physiology, rumen fermentation, digestibility and fatty acid profile in rumen and meat.
2. Material and methods
2.1. Facilities and animals
The study was carried out in the ruminant sector at the experimental farm of the Universidade do Estado de Santa Catarina (FECEO/UDESC) in the municipality of Guatambú (Latitude: 27° 8′ 5′' South, Longitude: 52° 47′ 15′' West), located in the western region of Santa Catarina. The animals were housed in appropriate confinement, allocated in individual pens measuring 1.5 × 7.0 m with a concrete floor, equipped with automatic drinkers and a freely accessible feeder. The animals' feeding area had cover, which allowed the animals to shelter from the weather conditions. The shed had north-south solar orientation, which allowed contact of the animals with sunlight. Twenty-four castrated male Holstein steers with an average weight of 336±4.68 kg and an average age of 12 months were used as an experimental model.
2.2. Test products
Tecmax Pro-Ruminantes® (Toledo – PR, Brazil) is a fermented product of Bacillus subtilis, algaroba bran, inactivated and dehydrated sugar cane yeast, fermentation product of Aspergillus Niger; which presents the following guarantee levels in its technical sheet: Protease (min.) 7500 U/g, cellulase (min.) 2700 U/g, xylanase (min.) 1200 U/g, beta glucanase (min.) 300 U/g. The alpha-amylase (corn starch and amylase) have guaranteed levels of amylase (min.) 1000 U/g. Therefore, the exogenous enzymes used were amylase and an enzyme mixture containing mainly protease in addition to cellulase, xylanase and beta glucanase; as well as they combined.
2.3. Experimental design, diets and feeding
The experiment was a randomized controlled design to standardize the initial body weight, with the animals divided into 4 treatments with 6 repetitions each: T1-CON (traditional confinement diet, T2-BLEND (0.5 g mixture of protease enzymes, cellulase, xylanase and beta glucanase per kg of DM diet), T3-AMIL (0.5 g alpha-amylase per kg of DM diet), T4-BLEND+AMIL (0.5 g enzyme blend + 0.5 g amylase per kg of DM).
Previously, the animals went through a period of adaptation to the diet (15 days), where the protocol of gradual inclusion of concentrate was used (40:60, 60:40, 70:30–concentrate ratio: roughage) in the periods 1–5, 5–10, 10–15 days of experiment, respectively. This gradual adaptation of the animals occurred because previously the animals were on winter pasture (oats and ryegrass), receiving protein in the feeder once a day. The diets were calculated according to the nutritional requirements of the animal category for an estimated average daily gain (ADG) of 1.5 kg of body weight (BR-CORTE, 2016). The daily diet was divided into two similar meals (08 AM and 16 PM), being supplied as a Total Mixed Ration (TMR: concentrate + silage). Enzymes were added to the TMR in the animal feeder, a top-dressed mixture.
2.4. Sample and data collection
2.4.1. Growth performance
Zootechnical performance was evaluated as complementary information for this research. Steers were weighed individually at 9 times (days 1, 15, 30, 45, 60, 75, 90, 105 and 120 of the experiment). All weighings were performed in the morning, with the animals fasting, with the aid of a digital electronic scale. With the body weight data, it was possible to calculate weight gain (WG) (WG = initial BW – final BW) and average daily gain (ADG) [(final BW–initial BW) / number of days)]. This body weight information was used to individually calculate the animals' diet for the subsequent 15 days; thus, each animal received the volume of feed proportional to its body weight. Feed leftovers were weighed and recorded daily during the morning; important information to calculate feed efficiency.
2.4.2. Sample collection
Blood collections were performed on days 1, 15, 60 and 120, through the caudal vein, with the aid of needles and vacuolated tubes, without anticoagulants to obtain serum for biochemical analysis and levels of oxidants and antioxidants. Vacuolated tubes with anticoagulants (EDTA) were also used for hematological analyses. The tubes were kept refrigerated at 10 °C, in an isothermal box until arrival at the laboratory. For serum separation, tubes were centrifuged without anticoagulant (7500 RPM for 10 min). The serum was transferred to microtubes, identified and stored at -20 °C until laboratory analysis. Using an esophageal probe with a vacuum system, the collection of ruminal fluid from bovines was carried out. This material had pH analyzed instantly and the rest of the material was frozen for analysis of volatile fatty acids.
On day 121 of the experiment, the animals were slaughtered in a specialized slaughterhouse for cattle. The slaughters followed current legislation, and were under veterinary inspection. A fragment of liver and longissimus dorsi muscle were collected for analysis of fatty acid profiles, stored at −20 °C until analysis.
2.5. Laboratory analysis
2.5.1. Chemical composition of diet and feces
Feed and feces samples were dried in a forced-air oven at 56 °C for at least 72 h and ground in a 1 mm sieve for analysis. Total DM contents in feed and feces samples were determined by oven drying at 110 °C for 24 h. Ash was determined by combustion at 600 °C for 3 h, and organic matter was determined by mass difference. Total nitrogen was assayed using the Kjeldahl method (Method 984.13; AOAC 1997). The neutral detergent fiber (NDF) analyses included ash and were based on the procedures described by Mertens (2002). The chemical composition of feeds is shown in Table 1.
Table 1.
Ingredients and chemical composition of the diet.
| Feed | Dry matter (kg/animal/day) | |
|---|---|---|
| Corn silage | 2.29 | |
| Concentrate1 | 7.04 | |
| Total | 9.33 | |
| Chemical composition% | Corn silage | Concentrate |
|---|---|---|
| Dry matter | 35.33 | 89.07 |
| Crude protein | 5.97 | 17.70 |
| NDF | 43.02 | 31.24 |
| ADF | 21.76 | 5.93 |
| Ethereal extract | 1.16 | 4.10 |
| Ashes | 4.56 | 6.59 |
| Starch | 22.6 | 37.35 |
2.5.2. Apparent digestibility coeficiente
Indigestible neutral detergent fiber (iNDF) was used as an internal marker to calculate apparent feed digestibility (Cochran et al., 1986; Huhtanen et al., 1994). The feed and feces samples were weighed in bags with 16-µm porosity and incubated in the rumen of cattle for 288 h. Then they were washed with tap water, treated with neutral detergent in an autoclave (SENGER et al., 2008), and dried in a forced-air ventilation oven at 55 °C. Digestibility was calculated as 1 – (iNDF in feed (% of DM)/iNDF in feces (% of DM).
2.5.3. Hematology
Hemoglobin concentration, total erythrocyte and leukocyte count and percentage of hematocrit were performed immediately upon arrival at the laboratory, with the aid of the Sysmex electronic device (model KX-21 N). The leukocyte differential followed the technique described by [15], using blood smear and light microscope identification.
2.5.4. Serum biochemistry
Levels of total proteins, glucose, albumin, cholesterol and urea were made using the Bio-2000 (BioPlus®) semi-automatic analyzer and commercial kits Analisa®, and their respective methodologies. The levels of globulins were obtained with mathematical calculation (total proteins - albumin).
2.5.5. Tissue oxidative status
Liver and meat fragments were homogenized (1v/9v) in saline solution, centrifuged for 10 min at 5600 g [16]. Then, the supernatant was collected, stored in microtubes under freezing (-20 °C) until analysis.
The levels of non-enzymatic antioxidants in meat and liver (protein thiols) were evaluated, following the methodology described by [17] and the results were expressed in nmol SH/mg protein. Glutathione S-transferase (GST) activity in the homogenate was measured based on the method described by researchers [[18], [19]] and the result was expressed as µmol CDNB/min/mg protein. Superoxide dismutase (SOD) activity was performed according to the methodology described by [20], with the results expressed U SOD/mg protein. The catalase activity (CAT) in the homogenized meat and liver followed the technique described by [21], and the data were expressed U CAT/mg protein.
Serum lipid peroxidation was measured as the amount of thiobarbituric acid reactive substances (TBARS) according to researchers [[22], [23]]. The reaction was read in a spectrophotometer at 535 nm. The result will be expressed in nmoles of malondialdehyde/ml of homogenate. The determination of reactive oxygen species (ROS) was based on the technique described by researchers [[24], [25], [26]] and the results were expressed in U DCFH/mg protein.
2.5.6. Determination of short chain fatty acids in ruminal liquid
The rumen fluid samples were thawed to 5 °C and agitated manually in order to homogenize them. 1 mL aliquots of the supernatant from rumen fluid samples were collected in polypropylene microtubes (2 mL) and then centrifuged for 5 min (12,300 × g). Then 250 μL of the supernatant was removed and transferred to a new microtube containing 250 μL of formic acid. The mixture was manually shaken and centrifuged for 3 min. After centrifugation, 250 μL of the supernatant of the mixture was collected into an injection vial. 500 μL of 3-octanol solution (665 μg mL−1 in methanol) was added, used as an internal standard, and homogenized. The samples were injected into a gas chromatograph equipped with a flame ionization detector (GC-FID; Varian Star 3400, Palo Alto, USA) and an autosampler (Varian 8200CX, Palo Alto, USA). 1 μL of the extract was injected in split mode at 1:10. The carrier gas used was hydrogen at a constant pressure of 20 psi. The analytes (acetic, propionic, butyric, valeric, and isovaleric acids) were separated by a CP WAX 52CB capillary column (60 m x 0.25 mm; 0.25 μm stationary phase thickness). The initial column temperature was set at 80 °C for 1 min and increasing to 120 °C at a rate of 8 °C min–1, than up to 230 °C by 20 °C min–1, where it remained for 1 min. Injector and detector temperatures were set at 250 °C. The validation of the method comprised the following parameters: selectivity, linearity, linear range, repeatability, precision, limit of detection (LOD) and limit of quantification (LOQ) for acetic, propionic, butyric, valeric and isovaleric acids. Analytical parameters are shown in Table Supplementary 1. Linearity was evaluated by calculating a regression equation using the least squares method. LOD and LOQ values were achieved by sequential dilutions up to signal-to-noise ratios of 3:1 and 6:1, respectively. Precision was assessed by analyzing the repeatability of six replicate samples. Accuracy was determined by recovering known amounts of standard substances added to a diluted sample. The results were expressed in mmol L − 1 of each SCFA in rumen fluid.
2.5.7. Profile of fatty acids in meat and feed
The extraction was performed by the Bligh and Dyer method [27] with some modifications. 1.5 g of bovine muscle samples, 0.5 mL of water, 5 mL of methanol and 2.5 mL of chloroform were added to a 15 mL polypropylene tube and mechanical stirring was performed for 60 min. Subsequently, 2.5 mL of chloroform and 1.5 % Na2SO4 solution were added to promote a biphasic system [28]. This mixture was shaken for 2 min, and then centrifuged for 15 min at 2000 rpm. Lipids obtained from the chloroform phase were subjected to fatty acid analysis.
Methylation was performed by a transesterification method proposed by [29]. We added to the extracted lipids 1 mL of 0.4 M koh methanolic solution in a test tube and vortexed for 1 min. The samples were kept in a water bath for 10 min at the boiling point. Subsequently, they were cooled to room temperature and 3 mL of 1 M H2SO4 solution was added and vortexed and kept in a water bath for 10 min. After cooling, 2 mL of hexane was added and centrifuged at 2000 rpm for 10 min. Finally, hexane with fatty acid methyl esters (FAME) was subjected to chromatography analysis.
For FAME determination, a TRACE 1310 gas chromatograph model equipped with a flame ionization detector (Thermo Scientific) was used. One microliter of samples was injected into a split/splitless injector operated in the 1:20 split ratio mode at 250 °C. Hydrogen was used as carrier gas at a constant flow rate of 1.5 mL/min. Separation of FAMEs was performed using an RT 2560 chromatography column (100 m × 0.25 mm × 0.20 μm thick film, Restek, USA). The initial oven temperature was programmed at 130 °C for 5 min and increased to 180 °C at a rate of 8 °C/min. Then, increasing to 210 °C, at a rate of 4 °C/min, and finally to 250 °C, increasing 20 °C/min, and maintained for 7 min in isotherm. The detector temperature was held constant at 250 °C. FAME compounds were identified by comparing the experimental retention time with the authentic standard (FAME Mix-37, Sigma Aldrich, St. Louis, MO). The results were presented as a percentage of each FA identified in the lipid fraction, considering the equivalent factor of PPI chain size for FID and ester conversion factor for the respective acid, according to Visentainer [[30], [31]]. Results of the fatty acid profile of feeds were presented in Supplementary Material 1.
2.5.8. Statistical analysis
All data were analyzed using the SAS MIXED procedure (SAS Inst. Inc., Cary, NC, USA; version 9.4), with Satterthwaite approximation to determine denominator degrees of freedom for the fixed effects test. Growth performance data (exception for body weight -BW), carcass traits were tested for fixed treatment effect using animal (treatment) as random effect. BW, blood count, serum biochemistry, and ruminal variables were analyzed as repeated measures and tested for fixed effects of treatment, day, and treatment × day, using animal (treatment) as the random effect. The d 1 results were included as an independent covariate. Also, for these variables, to generate the average per treatment, the d 1 results were removed from the dataset, but were kept as a covariate. The first-order autoregressive covariance structure was selected according to the lowest Akaike information criterion. Means were separated using the PDIFF method (Tukey test) and all results were reported as LSMEANS followed by SEM. Significance was defined when P ≤ 0.05 and trend when P > 0.05 and ≤ 0.10.
3. Results
3.1. Performance of steers supplemented with exogenous enzymes
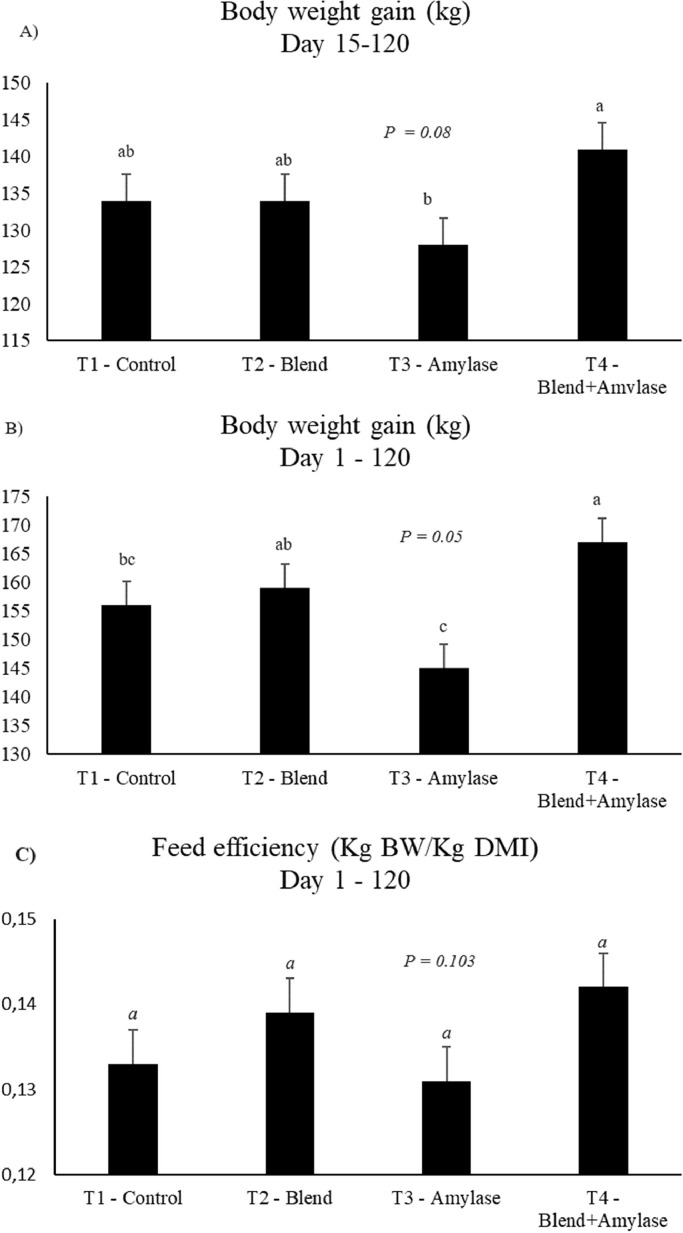
The zootechnical results are shown in Fig. 1 and Supplementary material 3. The body weight gain tended to be greater with the treatment in the T4-BLEND+AMIL in the period between days 15 and 120, (P = 0.08–Fig. 1), when compared to the T3-AMIL treatment, as well as verified, T4-BLEND+AMIL had greater body weight gain throughout the entire experimental period, from days 1 to 120 (P = 0.05–Fig. 1) when compared to T3-AMIL. Mean daily gain tended to be higher on treatment at T4, on days 16 to 120 (P = 0.09), when compared to T3-AMIL. Total dry matter intake, feed conversion and feed efficiency did not differ between treatments.
Fig. 1.
Mean and standard error (SEM) of body weight gain and feed efficiency of steers (n = 6 per group) fed with exogenous enzymes during the finishing period in a confinement system. P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters on the bar (a, b, c), which reports each group.
3.1.1. Blood count of steers
The results of the hemograms are shown in Table 2. Higher erythrocyte counts showed a trend of interaction between treatment x day, being higher (P = 0.08) in the T3-AMIL animals compared to the other treatments on day 15, on day 120 the treatment T4-BLEND+AMIL presented higher counts when compared to T2-BLEND. The percentage of hematocrit showed a trend towards treatment effect, that is, lower (P = 0.10) in T3-AMIL at 15 days. There was also an interaction of days versus treatment for hematocrit, with T2-BLEND being lower in the blood of cattle (P = 0.05) in the 120 days of the experiment. Hemoglobin levels showed a trend of interaction between days and treatment (P = 0.08) on days 15 and 120 of the experiment, following a similar behavior for erythrocyte count and hematocrit. The numbers of leukocytes, lymphocytes, monocytes, granulocytes, distribution of red cells and number of platelets did not differ between treatments.
Table 2.
Mean and standard error (SEM) of blood count of steers supplemented with amylase and blend enzymes.
| Items | Treatments1 | SEM | P – values | ||||
|---|---|---|---|---|---|---|---|
| T1 (n = 6) Control | T2 (n = 6) Blend | T3 (n = 6) Amylase | T4 (n = 6) Blend+Amylase | Treat | Treat × Day | ||
| Erythrocytes (x106 µL) | 0.31 | 0.08 | |||||
| d 1 | 7.92 | 7.03 | 6.73 | 7.69 | 0.44 | ||
| d 15 | 8.21a | 7.58a | 6.47b | 8.03a | 0.42 | ||
| d 60 | 6.82 | 6.69 | 6.64 | 7.17 | 0.44 | ||
| d 120 | 7.09ab | 6.52b | 7.29ab | 7.89a | 0.42 | ||
| Average2 | 7.40 | 6.93 | 6.80 | 7.70 | 0.34 | ||
| Hematocrit (%) | 0.10 | 0.05 | |||||
| d 1 | 32.3 | 30.1 | 30.1 | 31.9 | 1.44 | ||
| d 15 | 32.9a | 32.0ab | 29.3b | 32.6a | 1.41 | ||
| d 60 | 29.9 | 29.8 | 31.5 | 30.6 | 1.62 | ||
| d 120 | 32.0bc | 28.8c | 34.1ab | 36.2a | 1.41 | ||
| Average2 | 31.6ab | 30.2b | 31.7ab | 33.1a | 0.66 | ||
| Hemoglobin (g/dL) | 0.26 | 0.08 | |||||
| d 1 | 11.0 | 10.4 | 10.2 | 11.0 | 0.55 | ||
| d 15 | 11.3a | 10.9ab | 9.75b | 11.7a | 0.55 | ||
| d 60 | 10.7 | 10.8 | 11.0 | 11.3 | 0.63 | ||
| d 120 | 11.5ab | 10.4b | 12.3a | 12.9a | 0.55 | ||
| Average2 | 11.2 | 10.6 | 11.0 | 12.0 | 0.47 | ||
| Leukocytes (x103 µL) | 0.93 | 0.88 | |||||
| d 1 | 3.84 | 3.79 | 3.32 | 2.87 | 0.71 | ||
| d 15 | 4.79 | 4.57 | 4.71 | 4.39 | 0.71 | ||
| d 60 | 5.51 | 6.30 | 6.50 | 6.22 | 0.81 | ||
| d 120 | 4.56 | 5.41 | 5.74 | 6.21 | 0.71 | ||
| Average2 | 5.09 | 5.55 | 5.61 | 5.39 | 0.58 | ||
| Lymphocytes (x103 µL) | 0.49 | 0.88 | |||||
| d 1 | 2.63 | 2.69 | 2.43 | 2.39 | 0.49 | ||
| d 15 | 3.50 | 3.14 | 3.53 | 2.61 | 0.49 | ||
| d 60 | 3.65 | 3.55 | 4.38 | 3.98 | 0.56 | ||
| d 120 | 3.25 | 3.25 | 4.11 | 3.74 | 0.49 | ||
| Average2 | 3.50 | 3.36 | 3.98 | 3.39 | 0.30 | ||
| Monocytes (x103 µL) | |||||||
| d 1 | 0.28 | 0.27 | 0.28 | 0.24 | 0.09 | 0.19 | 0.29 |
| d 15 | 0.31 | 0.27 | 0.32 | 0.28 | 0.10 | ||
| d 60 | 0.85 | 1.21 | 0.95 | 0.81 | 0.12 | ||
| d 120 | 0.63 | 0.88 | 0.74 | 0.77 | 0.10 | ||
| Average2 | 0.60 | 0.79 | 0.67 | 0.62 | 0.07 | ||
| Granulocytes (x103 µL) | 0.12 | 0.59 | |||||
| d 1 | 1.15 | 0.95 | 0.69 | 0.89 | 0.20 | ||
| d 15 | 1.30 | 1.30 | 0.87 | 1.09 | 0.21 | ||
| d 60 | 1.33 | 1.69 | 1.16 | 0.99 | 0.24 | ||
| d 120 | 0.99 | 1.42 | 0.87 | 1.29 | 0.21 | ||
| Average2 | 1.20 | 1.47 | 0.97 | 1.13 | 0.18 | ||
| RDW-CV | 0.88 | 0.50 | |||||
| d 1 | 21.2 | 22.3 | 21.8 | 21.3 | 1.30 | ||
| d 15 | 21.2 | 23.6 | 22.4 | 21.6 | 1.39 | ||
| d 60 | 26.2 | 24.7 | 25.9 | 25.3 | 1.67 | ||
| d 120 | 22.9 | 21.0 | 23.2 | 22.2 | 1.39 | ||
| Average2 | 23.4 | 23.1 | 23.8 | 23.0 | 1.00 | ||
| Platelets (x103 µL) | 0.89 | 0.16 | |||||
| d 1 | 395 | 345 | 329 | 372 | 55.7 | ||
| d 15 | 380 | 289 | 305 | 423 | 62.0 | ||
| d 60 | 235 | 119 | 145 | 161 | 70.7 | ||
| d 120 | 130 | 283 | 223 | 229 | 62.0 | ||
| Average2 | 248 | 230 | 224 | 271 | 45.9 | ||
Treatments were: T1 Control- Control treatment, T2 blend– Treatment with 0.5 g of blend per kg of DM in the diet, T3 Amylase- Treatment with 0.5 g of amylase per kg of DM in the diet, T4 blend + Amylase- Treatment with 0.5 g enzyme blend+ 0 0.5 g amylase per kg of DM in the diet.
The d 1 results were removed from the data set to generate the average per treatment in the statistical analysis.
a,b,c Note: P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters (a,b,c) on the same line.
3.1.2. Serum biochemistry
The biochemistry results are shown in Table 3. There was a tendency for treatment to influence glucose levels, the T1-CON group was superior to T2-BLEND and T3-AMIL, while T4-BLEND+AMIL was superior to T2-BLEND and equal to T3-AMIL (P = 0.005). Albumin, globulin, total protein, urea and cholesterol levels did not differ between treatments.
Table 3.
Mean and standard error (SEM) of serum biochemistry of steers supplemented with amylase and blend enzymes.
| Items | Treatments1 | SEM | P – values | ||||
|---|---|---|---|---|---|---|---|
| T1 (n = 6) Control | T2 (n = 6) Blend | T3 (n = 6) Amylase | T4 (n = 6) Blend+Amylase | Treat | Treat × Day | ||
| Albumin (g/dL) | 0.88 | 0.45 | |||||
| d 1 | 6.36 | 5.86 | 6.07 | 5.58 | 0.29 | ||
| d 15 | 2.50 | 2.63 | 2.64 | 2.78 | 0.29 | ||
| d 60 | 2.76 | 2.83 | 2.52 | 3.40 | 0.29 | ||
| d 120 | 3.05 | 3.01 | 2.89 | 2.90 | 0.29 | ||
| Average2 | 2.89 | 2.79 | 2.72 | 2.91 | 0.14 | ||
| Globulin (g/dL) | 0.48 | 0.20 | |||||
| d 1 | 3.30 | 2.91 | 3.51 | 3.21 | 0.79 | ||
| d 15 | 6.02 | 5.81 | 5.99 | 5.71 | 0.79 | ||
| d 60 | 9.12 | 11.8 | 12.3 | 9.99 | 0.79 | ||
| d 120 | 4.15 | 4.99 | 3.9 | 5.14 | 0.79 | ||
| Average2 | 6.45 | 7.43 | 7.48 | 6.94 | 0.53 | ||
| Total protein (g/dL) | 0.73 | 0.36 | |||||
| d 1 | 9.68 | 8.74 | 9.61 | 8.78 | 0.83 | ||
| d 15 | 8.53 | 8.41 | 8.65 | 8.48 | 0.83 | ||
| d 60 | 11.9 | 14.6 | 14.8 | 13.4 | 0.83 | ||
| d 120 | 7.21 | 7.98 | 6.8 | 8.03 | 0.83 | ||
| Average2 | 9.38 | 10.2 | 10.3 | 9.81 | 0.57 | ||
| Glucose (mg/dL) | 0.06 | 0.86 | |||||
| d 1 | 97.7 | 98.6 | 97.0 | 93.9 | 6.37 | ||
| d 15 | 84.8 | 77.1 | 82.4 | 87.7 | 6.37 | ||
| d 60 | 105 | 86.6 | 89.4 | 106 | 6.37 | ||
| d 120 | 96.4 | 72.9 | 85.4 | 84.9 | 6.37 | ||
| Average2 | 95.5a | 78.9c | 85.7bc | 92.6ab | 3.24 | ||
| Urea (mg/dL) | 0.98 | 0.18 | |||||
| d 1 | 23.6 | 20.8 | 22.6 | 21.6 | 1.87 | ||
| d 15 | 26.2 | 23.3 | 22.9 | 23.3 | 1.87 | ||
| d 60 | 26.6 | 25.3 | 25.6 | 28.1 | 1.87 | ||
| d 120 | 27.1 | 31.7 | 32.1 | 28.4 | 1.87 | ||
| Average2 | 27.1 | 26.3 | 27.1 | 26.4 | 1.51 | ||
| Cholesterol (mg/dL) | 0.75 | 0.51 | |||||
| d 1 | 108 | 97.6 | 86.8 | 97.2 | 11.7 | ||
| d 15 | 103 | 87.1 | 90.4 | 117 | 11.7 | ||
| d 60 | 98.6 | 106 | 97.1 | 103 | 11.7 | ||
| d 120 | 116 | 113 | 138 | 125 | 11.7 | ||
| Average2 | 109 | 102 | 105 | 115 | 9.40 | ||
Treatments were: T Control- Control treatment, T2 blend– Treatment with 0.5 g of blend per kg of DM in the diet, T3 Amylase- Treatment with 0.5 g of amylase per kg of DM in the diet, T4 blend + Amylase- Treatment with 0.5 g enzyme blend + 0 0.5 g amylase per kg of DM in the diet.
The d 1 results were removed from the data set to generate the average per treatment in the statistical analysis.
a,b,c Note: P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters (a,b,c) on the same line.
3.1.3. Apparent digestibility coeficiente
Apparent digestibility coefficients are presented in Table 4. There was a treatment effect for digestibility of ether extract (EE) and starch. The T3-AMIL and T4-BLEND+AMIL treatments showed higher EE digestibility compared to the others (P = 0.01), which did not differ from each other. Starch digestibility was higher in groups T3-AMIL and T4-BLEND+AMIL compared to T1-CON; T2-BLEND did not differ (P = 0.05). The crude protein digestibility coefficient tended to be higher in groups T2-BLEND and T3-AMIL compared to T1-CON (P = 0.07). The coefficients of dry matter, organic matter, insoluble fiber in neutral detergent, insoluble fiber in acid detergent did not differ between treatments.
Table 4.
Mean and standard error (SEM) of apparent digestibility coeficiente (ADC) of cattle fed exogenous enzymes.
| Variables (%) | T1 (n = 6) Control | T2 (n = 6) Blend | T3 (n = 6) Amylase | T4 (n = 6) Blend+Amylase | SEM | P- trat |
|---|---|---|---|---|---|---|
| Dry Matter | 61.7 | 65.4 | 67.6 | 66.4 | 2.41 | 0.39 |
| Organic Matter | 65.4 | 68.8 | 71.2 | 70.3 | 2.93 | 0.26 |
| Crude protein | 54.2b | 58.1a | 61.3a | 57.1ab | 1.08 | 0.07 |
| NDF | 59.6 | 62.9 | 61.5 | 64.5 | 2.58 | 0.46 |
| ADF | 46.1 | 46.6 | 51.0 | 49.5 | 2.36 | 0.37 |
| Ether extract | 36.6b | 42.8b | 53.0a | 55.2a | 2.79 | 0.01 |
| Starch | 73.0b | 76.9ab | 81.3a | 81.8a | 2.01 | 0.05 |
1Treatments were: T1 Control- Control treatment, T2 blend– Treatment with 0.5 g of blend per kg of DM in the diet, T3 Amylase- Treatment with 0.5 g of amylase per kg of DM in the diet, T4 blend + Amylase- Treatment with 0.5 g enzyme blend+ 0 0.5 g amylase per kg of DM in the diet.
a,b,c Note: P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters (a,b,c) on the same line.
3.1.4. Profile of short-chain fatty acids in ruminal fluid
The results of the profile of short-chain fatty acids in the rumen are shown in Table 5. The T4-BLEND+AMIL group had a higher concentration of acetic acid than the other groups that were equal to each other (P = 0.05). T3-AMIL and T4-BLEND+AMIL had a higher concentration of propionic acid (P = 0.01) than T1-CON, with T2-BLEND being similar for all groups. Butyric acid concentration had a strong tendency to be higher in T4-BLEND+AMIL (P = 0.06) compared to T1-CON. There was no treatment effect for isovaleric and valeric acid concentration between groups.
Table 5.
Mean and standard error (SEM) of total short-chain fatty acids (SCFA) in ruminal fluid and profile of volatile fatty acids in the rumen of cattle that consumed exogenous enzymes.
| Variables | T1 (n = 6) Control | T2 (n = 6) Blend | T3 (n = 6) Amylase | T4 (n = 6) Blend+Amylase | SEM | P- trat | P- trat x day |
|---|---|---|---|---|---|---|---|
| Acetic acid (mmol/L | 0.05 | 0.05 | |||||
| d60 | 64.7ab | 51.5b | 58.2ab | 69.0a | 1.98 | ||
| d120 | 68.4b | 77.2a | 75.1ab | 77.9a | 1.57 | ||
| Average | 66.5b | 64.3b | 66.6b | 73.4a | 1.45 | ||
| Propionic acid (mmol/L) | 0.01 | 0.01 | |||||
| d60 | 15.6bc | 13.7c | 17.0ab | 19.7a | 0.74 | ||
| d120 | 18.0b | 26.9a | 29.4a | 28.8a | 0.81 | ||
| Average | 16.8b | 20.3ab | 23.2a | 24.2a | 0.75 | ||
| Butiric acid (mmol/L) | 0.06 | 0.04 | |||||
| d60 | 10.9 | 10.81 | 10.6 | 12.6 | 0.65 | ||
| d120 | 13.3b | 15.5ab | 15.6ab | 17.1a | 0.66 | ||
| Average | 12.1b | 13.1ab | 13.1ab | 14.9a | 0.62 | ||
| Isovaleric acid (mmol/L) | 0.59 | 0.23 | |||||
| d60 | 1.82 | 1.60 | 1.49 | 1.70 | 0.11 | ||
| d120 | 1.72 | 2.23 | 1.74 | 2.05 | 0.09 | ||
| Average | 1.77 | 1.91 | 1.61 | 1.87 | 0.1 | ||
| Valeric acid (mmol/L) | 0.44 | 0.62 | |||||
| d60 | 1.77 | 1.51 | 1.59 | 1.85 | 0.21 | ||
| d120 | 2.01 | 2.50 | 2.35 | 2.61 | 0.36 | ||
| Average | 1.89 | 2.00 | 1.97 | 2.23 | 0.25 | ||
| SCFA (mmol/L) | 0.01 | 0.01 | |||||
| d60 | 94.7b | 79.1c | 88.8b | 104.8a | 2.04 | ||
| d120 | 103.4b | 124.3a | 124.2a | 128.4a | 1.96 | ||
| Average | 99.0b | 101.7b | 106.5b | 116.6a | 2.01 |
1Treatments were: T1 Control- Control treatment, T2 blend– Treatment with 0.5 g of blend per kg of DM in the diet, T3 Amylase- Treatment with 0.5 g of amylase per kg of DM in the diet, T4 blend + Amylase- Treatment with 0.5 g enzyme blend+ 0 0.5 g amylase per kg of DM in the diet.
2The d 1 results were removed from the data set to generate the average per treatment in the statistical analysis.
a,b,c Note: P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters (a,b,c) on the same line.
Total short-chain fatty acids (SCFA) in the ruminal fluid was higher in T4-BLEND+AMIL animals, referring to the interaction between treatment and day (day 60) compared to the other groups. Interaction treatment x day was also verified on day 120, with higher SCFA in all groups that consumed the enzymes compared to T1-CON. Treatment effect was verified in the SCFA concentration, higher in all treatments (T1, T2 and T3) compared to control (T1).
3.1.5. Meat composition and carcass weight/yield
The results of the centesimal composition of meat and carcass are shown in Table 6. There was no effect of treatment on weight and carcass yield. There was influence of the treatment on the percentage of fat in the meat (P = 0.007) in animals from T3-AMIL, higher levels when compared to T2-BLEND and T4-BLEND+AMIL. The concentration of mineral matter was higher in the meat of cattle that consumed the enzymatic combination T4-BLEND+AMIL when compared to T1-CON and T2-BLEND. As for the content of dry matter and protein there was no difference between treatments.
Table 6.
Mean and standard error (SEM) of meat and carcass composition of steers supplemented with amylase and protease enzymes.
| Items | Treatments1 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| T1 (n = 6) Control | T2 (n = 6) Blend | T3 (n = 6) Amylase | T4 (n = 6) Blend+Amylase | Trat | ||
| Carcass variables | ||||||
| Carcass weight (kg) | 251 | 244 | 239 | 246 | 9.79 | 0.86 |
| Carcass yield (%) | 50.5 | 49.2 | 49.5 | 48.9 | 0.71 | 0.43 |
| Meat variables | ||||||
| Dry matter (%) | 31.8 | 30.6 | 32.3 | 33.1 | 1.13 | 0.48 |
| Protein (%) | 25.1 | 25.7 | 23.8 | 25.7 | 0.78 | 0.30 |
| Fat (%) | 2.79b | 2.05b | 3.84a | 1.97b | 0.38 | 0.007 |
| Ash (%) | 3.91bc | 2.85c | 4.66ab | 5.43a | 0.40 | 0.001 |
Treatments were: T1 Control- Control treatment, T2 blend– Treatment with 0.5 g of blend per kg of DM in the diet, T3 Amylase- Treatment with 0.5 g of amylase per kg of DM in the diet, T4 blend + Amylase- Treatment with 0.5 g enzyme blend+ 0 0.5 g amylase per kg of DM in the diet.
a,b,cNote: P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters (a,b,c) on the same line.
3.1.6. Meat fatty acid profile
The results of the fatty acid profile in meat are shown in Table 7. We verified the influence of treatments for the presence of saturated, monounsaturated and polyunsaturated fatty acids in different treatments. We highlight the lower amount of saturated fatty acids in the meat of cattle that consumed the mixture of enzymes T4-BLEND+AMIL compared to T1-CON and T3-AMIL. A higher proportion of monounsaturated fatty acids was observed in the T3-AMIL group when compared to the others. The percentage of polyunsaturated fatty acids was higher in the T2-BLEND and T4-BLEND+AMIL groups when compared to the T1-CON control, and lower in the meat of the T3-AMIL cattle compared to the other groups.
Table 7.
Mean and standard error (SEM) of profile of fatty acids in the meat of steers supplemented with amylase and blend enzymes.
| Fatty acid (g/kg) | Treatments | SEM | P-value | |||
|---|---|---|---|---|---|---|
| T1 (n = 6) Control | T2 (n = 6) Blend | T3 (n = 6) Amylase | T4 (n = 6) Blend+Amylase | |||
| C8:0 (Caprylic) | 0.908ab | 1.605a | 0.579b | 1.744a | 0.121 | 0.010 |
| C12:0 (Lauric) | 0.472 | 0.650 | 0.532 | 0.521 | 0.048 | 0.654 |
| C14:0 (Myristic) | 20.12ab | 19.18ab | 23.598a | 17.861b | 0.823 | 0.010 |
| C14:1 (Myristoleic) | 5.232 | 4.776 | 5.616 | 4.497 | 0.337 | 0.102 |
| C15:0 (Pentadecanoic) | 2.279 | 2.401 | 2.498 | 2.204 | 0.083 | 0.856 |
| C16:0 (Palmitic) | 314.8a | 300.0b | 310.0ab | 300.9b | 2.397 | 0.071 |
| C16:1 (Palmitoleic) | 33.59 | 34.29 | 36.56 | 33.02 | 0.969 | 0.258 |
| C17:0 (Heptadecanoic) | 6.437 | 6.309 | 7.475 | 6.261 | 0.240 | 0.182 |
| C18:0 (Stearic) | 148.1a | 150.9a | 148.6a | 144.1b | 1.966 | 0.100 |
| C18:1n9c (Oleic) | 384.0b | 380.0b | 406.0a | 378.9b | 4.689 | 0.001 |
| C18:2n6c (Linoleic) | 53.33b | 67.96a | 37.33c | 73.74a | 4.202 | 0.001 |
| C20:0 (Arachidic) | 0.645b | 1.173a | 0.923a | 0.982a | 0.061 | 0.023 |
| C18:3n6 (?-Linolenic) | 0.558 | 0.626 | 0.420 | 0.589 | 0.047 | 0.362 |
| C20:1n9 (cis-11-Eicosenoic) | 2.382a | 1.000b | 1.304b | 1.347b | 0.054 | 0.060 |
| C18:3n3 (a-Linolenic) | 1.772b | 1.548b | 1.337b | 3.201a | 0.245 | 0.001 |
| C21:0 (Henicosanoic) | 2.014 | 1.864 | 2.534 | 1.867 | 0.105 | 0.201 |
| C20:2 (cis-11,14-Eicosadienoic) | 0.799 | 0.832 | 0.592 | 0.632 | 0.027 | 0.120 |
| C22:0 (Behenic) | 0.362b | 0.369b | 0.195c | 0.457a | 0.048 | 0.050 |
| C20:3n6 (cis-8,11,14-Eicosatrienoic) | 4.371a | 4.567a | 2.770b | 4.531a | 0.258 | 0.043 |
| C22:1n9 (Erucic) | 0.283 | 0.310 | 0.204 | 0.384 | 0.038 | 0.258 |
| C20:4n6 (Arachidonic) | 14.92cd | 16.21bc | 9.575d | 19.28a | 1.217 | 0.001 |
| C22:2 (cis-13,16-Docosadienoic) | 0.187 | 0.072 | 0.109 | 0.185 | 0.011 | 0.350 |
| C24:0 (Lignoceric) | 0.399bc | 0.551a | 0.266c | 0.461ab | 0.032 | 0.050 |
| C20:5n3 (cis-5,8,11,14,17-Eicosapentaenoic) | 0.692 | 0.680 | 0.374 | 0.792 | 0.051 | 0.135 |
| C24:1n9 (Nervonic) | 0.220 | 0.148 | 0.116 | 0.162 | 0.016 | 0.186 |
| C22:6n3 (cis-4,7,10,13,16,19-Docosahexaenoic) | 1.035 | 0.792 | 0.361 | 1.187 | 0.163 | 0.632 |
| AGS | 496.5a | 485.0ab | 497.2a | 477.4b | 2.053 | 0.038 |
| MUFA | 425.7b | 420.5b | 449.8a | 418.3b | 1.970 | 0.001 |
| PUFA | 77.67b | 93.29a | 52.87c | 104.14a | 2.381 | 0.001 |
1Treatments were: T1 Control- Control treatment, T2 blend– Treatment with 0.5 g of blend per kg of DM in the diet, T3 Amylase- Treatment with 0.5 g of amylase per kg of DM in the diet, T4 blend + Amylase- Treatment with 0.5 g enzyme blend+ 0 0.5 g amylase per kg of DM in the diet.
a,b,c Note: P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters (a,b,c) on the same line.
These results are related to individual changes in fatty acids, which when added together changed the fatty acid profile of the meat. We found that C8:0 levels were higher (P = 0.010) in T4-BLEND+AMIL when compared to T3-AMIL, the same was repeated for C14:0 (P = 0.010), but T4-BLEND+AMIL showed lower concentration than T3-AMIL, for C16:0 (P = 0.071) the T4-BLEND+AMIL treatment and T2-BLEND showed a lower concentration than the control treatment, for C18:0 (P = 0.100) the T2-BLEND and T3-AMIL were superior when compared to T4-BLEND+AMIL, for C:20 (P = 0.023) all treatments had a higher concentration than the control, for C22:0 (P = 0.050) T4-BLEND+AMIL was superior when compared to all other treatments, for C24:0 (P = 0.050) T2-BLEND was superior when compared to T3-AMIL.
The concentration of C18:1n9c (P = 0.001) had a treatment effect, with higher concentrations in T2-BREND when compared to control, T1-CON and T4-BLEND+AMIL; for C20:1n9 (P = 0.060) all treatments showed lower concentration compared to control (T1-CON).
The concentration of C18:3n3 (P = 0.001) in meat was higher in T4-BLEND+AMIL when compared to T3-AMIL, T2-BLEND, and T1-CON; for the levels of C20:3n6 (P = 0.043) we found that T4-BLEND+AMIL had a higher concentration when compared to T3-AMIL, but did not differ from the other treatments; the levels of C20:4n6 (0.001) in T4-BLEND+AMIL were higher when compared to T3-AMIL, T2-BLEND, and T1-CON.
3.1.7. Oxidative status in liver and meat
The results of oxidative status in liver and meat are shown in Table 8. In the liver, in general, we observed a lower concentration of TBARS in T2-BLEND animals (P = 0.050), when compared to T1-CON. The lowest production of ROS (P = 0.001) was verified in T3-CON, when compared to T2-BLEND and control, on the other hand SOD activity (P = 0.015) did not differ between T2-BLEND, T3-AMIL and T4-BLEND+AMIL, but it was higher in the T1-CON control. We also verified the highest CAT activity (P = 0.052) in the liver of the T4-BLEND+AMIL animals when compared to the other treatments. As for meat, we found that animals from T4-BLEND+AMIL (P = 0.094) tended towards a higher proportion of ROS than T3-AMIL and T1-CON, in contrast to meat from steers from T4-BLEND+AMIL (P = 0.001) showed higher GST activity when compared to T3-AMIL and control. PSH levels (P = 0.050) were higher in T3-AMIL when compared to T4-BLEND+AMIL and T2-BLEND; while the highest SOD activity (P = 0.045) was higher in T4-BLEND+AMIL and T2-BLEND when compared to control T1-CON.
Table 8.
Mean and standard error (SEM) of oxidative status in the liver and meat of steers supplemented with amylase and blend enzymes.
| Tissue | Treatments | TBARS | ROS | GST | PSH | SOD | CAT |
|---|---|---|---|---|---|---|---|
| Liver(n = 6 per group) |
T1 Control | 1.436a | 4.623a | 1082 | 579.7 | 3.976a | 2.044ab |
| T2 Blend | 1.183b | 3.235b | 1188 | 464.0 | 1.281b | 2.454ab | |
| T3 Amylase | 1.273ab | 2.206c | 1119 | 566.0 | 2.061b | 2.375ab | |
| T4 Blend + Amylase | 1.270ab | 2.434c | 1087 | 488.1 | 1.729b | 2.880a | |
| SEM | 0.025 | 0.067 | 4.142 | 8.369 | 0.042 | 0.013 | |
| p-value | 0.050 | 0.001 | 0.657 | 0.241 | 0.015 | 0.052 | |
| Meat(n = 6 per group) |
T1 Control | 2.704 | 1.119ab | 2832b | 757.2c | 9.080b | 1.505 |
| T2 Blend | 2.702 | 1.498a | 3202ab | 803.6b | 13.04a | 1.662 | |
| T3 Amylase | 2.442 | 0.699b | 2858b | 853.6a | 12.60ab | 1.185 | |
| T4 Blend + Amylase | 3.110 | 1.350a | 3478a | 808.0b | 14.38a | 1.876 | |
| SEM | 0.169 | 0.056 | 6.521 | 5.978 | 1.006 | 0.240 | |
| p-value | 0.147 | 0.094 | 0.001 | 0.050 | 0.045 | 0.109 |
Note: ROS (U DCFH/mg protein); TBARS (nmol MDA/mL), GST (µmolCDNB/min/mg protein), PSH (nmol SH/mg protein), SOD (U SOD/mg protein), CAT (U CAT/mg of protein).
1Treatments were: T1 Control- Control treatment, T2 blend– Treatment with 0.5 g of blend per kg of DM in the diet, T3 Amylase- Treatment with 0.5 g of amylase per kg of DM in the diet, T4 blend + Amylase- Treatment with 0.5 g enzyme blend+ 0 0.5 g amylase per kg of DM in the diet.
a,b,c Note: P ≤ 0.05 (different) and P ≥ 0.05 to ≤ 0.1 (trend) were illustrated by different letters (a,b,c) on the same column.
4. Discussion
Addition of exogenous enzymes amylase, protease, cellulase, xylanase and beta glucanase positively affected weight gain, but without affecting the final weight. Similar results were verified by Rodríguez-Carías et al. [32] who used a commercial product based on exogenous enzymes beta-glucanase, xylanase, pectinase, mannanase, xyloglucanase, laminarase, β-glucosidase, β-xylosidase, α-l-arabinofuranosidase, amylase and protease in lambs, and found an effect on animal performance. These authors explain that this greater weight gain occurs due to the improvement in nutrient digestibility, since the product did not affect DM intake [32]. It is believed that when using proteolytic enzymes they act by removing protein structures from the cell wall of the forage, allowing faster access for ruminal microorganisms [33], which leads us to justify the gains in performance when using the enzymes in combination.
Researchers found that the inclusion of amylase promotes rapid release of starch oligosaccharides that are used by both amylolytic and non-amylolytic bacteria [34], for this reason starch is fermented quickly. This was observed in our study, in both groups of steers that consumed amylase in the diet, the starch digestibility coefficient was higher; as well as increasing the concentration of total volatile fatty acids in the rumen; but this was not enough when the exogenous enzyme was added to the diet to enhance weight gain. The reasons for this are not known and complementary results do not help explain the mechanisms involved.
Feed efficiency, feed conversion and dry matter intake showed no treatment effect; which is a consequence of the greater digestibility of the EE and starch nutrients, which may be related to the greater weight gain of T4-BLEND+AMIL cattle. Rose et al. (2010) found similar results when testing a product based on protease and amylase in the diet of Guzerat cattle in confinement, in which there was also no influence on the same productive parameters mentioned above. Andreazzi et al. [35] when testing amylase in dairy cows, found that there was no change in dry matter intake, therefore, in milk production, it generated an increase with diets of at least 30 % starch. In the 90 s, researchers already said that the use of exogenous enzymes could be more efficient in diets with low moisture content, less than 30 %, than in diets with high moisture content [36], as when corn silage is present, which presents approximately 70 %, which explains the results obtained in our study, since the silage had a high moisture content.
With the blood count results, we noticed a high percentage of hematocrit, with interaction of treatment × day for T4-BLEND+AMIL, which was really not expected and we have no explanation of the mechanisms involved. According to Santos [37], normal blood hematocrit levels are 30 to 35 %, in this work we noticed that there was a positive effect when using the enzymes in a combined form T4-BLEND+AMIL and a negative effect at times when using only amylase (T3-AMIL), as the percentage was less than 30, indicating mild anemia, which may have contributed to the body weight not differing.
There is a tendency for treatment to affect glucose levels, with animals in the T2-BLEND and T3-AMIL groups having lower serum concentrations; however, we expected that the animals that consumed the enzyme diet would have a higher concentration of glucose in the blood. This increase in glucose concentration in amylase treatments may have resulted from increased ruminal starch fermentation and propionate uptake for gluconeogenesis [38]. According to Denardin Silva [39], starch is composed of amylose and amylopectin, formed by glucose joined by a-1,4 and a-1,6 bonds, part of which is broken down into glucose by hydrolysis and another part by the enzymatic action of amylase. As mentioned by Vigne et al. [14], when using amylase-based enzyme complexes in feedlot steers, there was an improvement in starch digestion. We also observed that the addition of α-amylase improved the digestibility of starch and ether extract, which demonstrates the action of this enzyme in the degradation of these compounds. As a result of the greater utilization of starch, a higher concentration of short-chain fatty acids was observed, especially propionic acid, the main precursor of glucose in ruminants. However, the concentration of glucose in the blood tended to be lower when the animals consumed the enzymes individually; which may be related to the difficulty of measuring this enzyme in ruminants, since the animal is rarely fasting; as well as the rate of passage of feed through the gastrointestinal tract differ between animals.
Rocha et al. [40] concluded that the optimal level of starch in cattle supplemented with essential oils and amylase is 35 %, thanks to the possibility that the amylase remains active until the intestine. Furthermore Toseti et al. [41] observed potential for increased microbial protein with combined supplementation of amylase and essential oils. Upregulation of several enzymes that promote carbohydrate degradation in glycolic pathways, gluconeogenesis and oxidative phosphorylation was observed in cattle supplemented with amylase and essential oils combined [40]. This justifies the increase in starch digestibility and increase in body weight gain when amylase was given in combination. Salem et al. [42] observed a 16 % increase in weight gain when providing exogenous enzymes. Cattle supplemented with an amylase-based enzymatic complex [43] concluded that starch from corn caused improvements in subcutaneous fat thickness in feedlot super-early steers. Another point that we can observe was the concentration of mineral matter, which was higher in the meat of cattle that consumed the enzymatic combination T4-BLEND+AMIL, but it was not evaluated which minerals were deposited in the muscle tissue.
Another point that we can observe is the concentration of mineral matter, which was higher in the meat of cattle that consumed the enzymatic combination T4-BLEND+AMIL, but it was not evaluated which minerals were deposited in the muscle tissue. Vigne et al. [44] used a similar enzyme complex in feedlot steers consuming diets with high starch content, and also observed greater fat deposition in the carcass, a consequence of better feed conversion and increased starch digestibility. As can be seen, the addition of exogenous enzymes can alter lipid metabolism in meat from cattle confined with high percentage of concentrate. In addition to greater fat deposition, treatments influenced the fatty acid profile in the meat. We emphasize that the lower amount of saturated fatty acids in the meat of cattle that consumed the mixture of T4-BLEND+AMIL enzymes is an excellent result for the consumer, since saturated fats must be avoided, since in the lipid metabolism it can increase the levels of LDL (known as bad cholesterol), which is a low-density lipoprotein, capable of carrying cholesterol particles from the liver and other places to the arteries, that is, when in excess in the circulation, it causes accumulation in the vessels that can, over time, clog or form thrombi [45]. The benefits of reducing LDL cholesterol in humans are observed on a large scale in the literature [46].
In addition, when isolated or combined enzymes were used in the bovine diet, we verified a higher percentage of unsaturated fatty acids, the fat considered ideal for human consumption, since we have the omegas that increased when consumed in a diet containing only the analysis, such as acid oleic. Observed effect on the concentration of polyunsaturated fatty acids (PUFA) in the T4-BLEND+AMIL group; which is extremely beneficial for human health with potential effect on cancer protection (Chikwabha et al. 2018). We believe that the mechanisms that led to changes in the fatty acid profile are indirect, altering the digestive process, and consequently the lipid metabolism; the mechanisms involved are unclear and need further research to avoid speculation.
With changes in the profile of fatty acids in meat, an effect on the oxidative status of liver and meat was already expected. This is because it is known that lipids are among the main tissues responsible for producing oxidative instability and raising levels of free radicals. Consumption of combined exogenous enzymes and isolated amylase had a positive effect on redox balance, mainly in meat, but also in liver. We verified less lipid peroxidation and reactive oxygen species, as well as greater activity of antioxidant enzymes. In the literature there is no explanation of how exogenous enzymes modulate the oxidative status, but it is known that proteases are able to improve the oxidative stability of feeds with fermented processed items [47].
5. Conclusion
The results allow us to conclude that the combination of digestive enzymes (amylase, protease, cellulase, xylanase and beta glucanase) in the bovine diet improves growth performance, in addition to increasing the amount of unsaturated fatty acids and reducing the amount of saturated fatty acids in the meat. Consumption of exogenous enzymes also has a positive effect on oxidative stability in meat.
Ethics committee
Project approved at CEUA/UDESC, protocol number 8717230921.
CRediT authorship contribution statement
Alexandre L. Simon: Writing – original draft, Methodology, Data curation, Conceptualization. Priscila M. Copetti: Validation, Methodology, Formal analysis. Rafael V.P. Lago: Writing – original draft, Methodology, Investigation, Data curation. Maksuel G. Vitt: Writing – original draft, Methodology, Investigation, Formal analysis. Aline L. Nascimento: Writing – original draft, Methodology, Investigation. Luiz Eduardo Lobo e Silva: Writing – original draft, Methodology. Roger Wagner: Writing – review & editing, Validation, Supervision, Methodology, Formal analysis, Conceptualization. Bruna Klein: Writing – review & editing, Validation, Supervision, Methodology. Camila Soares Martins: Writing – original draft, Methodology. Gilberto V. Kozloski: Writing – review & editing, Validation, Supervision, Resources, Methodology, Investigation, Data curation. Aleksandro S. Da Silva: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of Competing Interest
The authors declare no competing or financial interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.btre.2023.e00824.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- 1.Wedekin V.S.P., Bueno C.R.F., Amaral A.M.P. Informações Econômicas. Informações Econ. 1994;24:123–131. [Google Scholar]
- 2.L. Velloso, Terminação de bovinos em confinamento. slp, s. ed., 1984.
- 3.Queiroz R.C., Bergamaschine A.F., Bastoset J.F.P., et al. Uso de produto à base de enzima e levedura na dieta de bovinos: digestibilidade dos nutrientes e desempenho em confinamento. Rev. Bras. Zootec. 2004;33:1548–1556. [Google Scholar]
- 4.Guimarães Tiago Pereira. Exigências Proteicas para bovinos de corte. Multi-Sci. J. 2015;1(1):90–99. (ISSN 2359-6902)v.n. [Google Scholar]
- 5.De Souza Martins A., et al. Eficiência de síntese microbiana e atividade enzimática em bovinos submetidos à suplementação com enzimas fibrolíticas. R. Bras. Zootec. 2006;35:45–48. [Google Scholar]
- 6.De Souza Martins A., et al. Degradabilidade in situ e observações microscópicas de volumosos em bovinos suplementados com enzimas fibrolíticas exógenas1. R. Bras. Zootec. 2007;36(6):1927–1936. [Google Scholar]
- 7.Edwards J.E., Huws S.A., Kim E.J., Kingston-Smith A.H. Characterization of the dynamics of initial bacterial colonization of nonconserved forage in the bovine rumen. FEMS Microbiol. Ecol., Oxford. 2007;62(1):323–335. doi: 10.1111/j.1574-6941.2007.00392.x. [DOI] [PubMed] [Google Scholar]
- 8.Giraldo L.A., Tejido M.L., Ranilla M.J., Ramos S., Carro M.D. Influence of direct feed fibrolytic enzymes on diet digestibility and ruminal activity in sheep fed a grass hay-based diet. J. Animal Sci., Champaign. 2008;86(7):1617–1620. doi: 10.2527/jas.2007-0343. [DOI] [PubMed] [Google Scholar]
- 9.Silvestre A.M., Millen D.D. The 2019 Brazilian survey on nutritional practices provided by feedlot cattle consulting nutritionists. Rev. Bras. Zootec. [S.L.] 2021;50 [Google Scholar]
- 10.Giuberti G., Gallo A., Masoero F., Ferraretto L.F., Hoffman P.C., Shaver R.D. Factors affecting starch utilization in large animal food production system: a review. Starch-Stärke. 2014;66:72–90. [Google Scholar]
- 11.Utrilla-Coello R.G., Hernández-Jaimes C., Carrillo-Navas H., González F., Rodríguez E., Bello-Pérez L.A., Vernon-Carter E.J., Alvarez-Ramirez J. Acid hydrolysis of native corn starch: Morphology, crystallinity, rheological and thermal properties. Carbohydr. Polym. 2014;103:596–602. doi: 10.1016/j.carbpol.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 12.OBERT C., CHIKWANHA C., et al. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. Food Res. Int., [S.L.] 2018;104:25–38. doi: 10.1016/j.foodres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 13.ROSA B.L., Alves J.B., Bergamaschine A.C., et al. Levels of concentrate and inclusion of probiotics for cattle Guzera in feedlot. Rev. Bras. de Saude e Prod. Anim. 2010;11:440–451. [Google Scholar]
- 14.Vigne G.L.D., et al. Digestibilidade do amido e comportamento ingestivo de novilhos confinados sob efeito de doses de complexo enzimático em dietas de alta densidade energética. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2019;71:1015–1026. v. [Google Scholar]
- 15.Feldman B.F., Zinkl J.G., Jain N.C. Vol. 1344. Williams & Wilkins; 2000. (Veterinary Hematology). [Google Scholar]
- 16.Tatsch E., Bochi G.V., Pereira R.D.S., Kober H., Oliveira J.R.D., Moresco R.N. Influence of anticoagulants and storage temperature on blood nitrite levels. J Bras Patol Med Lab. 2011;47:147–150. [Google Scholar]
- 17.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;(82):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 18.Habig W.H., Pabst M.J., Jakob W.B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 19.FAVERO A.L.R.BRUNETTO, F.F., BISSACOTTI B.F., et al. Phytogenic blend in the diet of growing Holstein steers: effects on performance, digestibility, rumen volatile fatty acid profile, and immune and antioxidant responses. Anim. Feed Sci. Technol. 2023;297:115595. [Google Scholar]
- 20.Mc Cord J.M., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 21.Nelson D.P., Kiesow L.A. Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solutions in the UV) Anal. Biochem. 1972;49(2):474–478. doi: 10.1016/0003-2697(72)90451-4. [DOI] [PubMed] [Google Scholar]
- 22.Jentzsch A.M., Bachmann H., Furst P., Biesalski H. Improved analysis of malondialdehyde in human body fluids. Free Radical Biol. Medicine. 1996;20:251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1978;95:351–358. doi: 10.1016/0003-2697(79)90738-3. 1978. [DOI] [PubMed] [Google Scholar]
- 24.Ali S.F., Lebel C.P., Bondy S.C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxico. 1992;113:637–648. [PubMed] [Google Scholar]
- 25.HALLIWELL B., GUTTERIDGE J.M.C. 4th edn. Oxford University Press; New York: 2007. Free Radicals in Biology and Medicine. [Google Scholar]
- 26.Maltez L.C., Barbas L.A.L., Nitz L.F., Pellegrin L., Okamoto M.H., Sampaio L.A., Monserrat J.M., Garcia L. Oxidative stress and antioxidant responses in juvenile Brazilian flounder Paralichthys orbignyanus exposed to sublethal levels of nitrite. Fish Physiol. Biochem. 2018;44:1349–1362. doi: 10.1007/s10695-018-0526-9. [DOI] [PubMed] [Google Scholar]
- 27.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 28.Giacomelli C.M., Marchiori M.S., Vedovatto M., DA Silva A.S. Encapsulated pepper blend in the diet of confined Holstein bullocks: effect on ruminal volatile fatty acid profiles, growth performance, and animal health. Trop. Anim. Health Prod. 2023;55:114–221. doi: 10.1007/s11250-023-03473-y. [DOI] [PubMed] [Google Scholar]
- 29.Hartman L., Lago R.C. Preparação rápida de ésteres de metila ácido graxo partir de lipídios. Pract. 1973;22(494):475–477. [Google Scholar]
- 30.Visentainer J.V., Franco M.R.B. Ácidos Graxos em Óleos e Gorduras: Identificação e Quantificação. 1a ed. Varela:; São Paulo: 2006. [Google Scholar]
- 31.Visentainer Aspectos analíticos da resposta do detector de ionização em chama para ésteres de ácidos graxos em biodiesel e alimentos. Química Nova. 2012;35:274–279. [Google Scholar]
- 32.RODRÍGUEZ-CARÍAS A.A., et al. Evaluación de a-amilasa y proteasa sobre el consumo y la digestibilidad de nutrientes de las dietas y parámetros fisiológicos en ovinos. J.Agric. Univ. Puerto Rico. 2017;101(1):63–78. v.n.1 jan. [Google Scholar]
- 33.Colombatto D., Beauchemin K.A. A proposed methodology to standardize the determination of enzymic activities present in enzyme additives used in ruminant diets. Canadian J. Animal Sci., Sherbrooke. 2003;83(3):559–568. [Google Scholar]
- 34.Tricarico J.M., Johnston J.D., Dawson K.A. Dietary supplementation of ruminant diets with an α-amylase from Aspergillus oryzae. Animal Feed Science and Technology, Amesterdão. 2008;145(1–4):136–150. [Google Scholar]
- 35.Andreazzi A.S.R., et al. Effect of exogenous amylase on lactation performance of dairy cows fed a high-starch diet. J. Dairy Sci. 2018;101(8):7199–7207. doi: 10.3168/jds.2017-14331. [DOI] [PubMed] [Google Scholar]
- 36.Beauchemin K.A., et al. Effects of fibrolytic enzymes in corn or barley diets on performance and carcass characteristics of feedlot cattle. Can. J. Anim. Sci. 1997;77(4):645–653. [Google Scholar]
- 37.Santos G.T. Efeito de diferentes volumosos sobre os constituintes sangüíneos de vacas da raça holandesa. Rev. Bras. Saúde Prod. An. 2008;9:35–44. [Google Scholar]
- 38.Reynolds C.K., et al. Net Metabolism of Volatile Fatty Acids, d-β-Hydroxybutyrate, Nonesterified Fatty Acids, and Blood Gasses by Portal-Drained Viscera and Liver of Lactating Holstein Cows. J. Dairy Sci. 1988;71(9):2395–2404. doi: 10.3168/jds.s0022-0302(88)79824-0. [DOI] [PubMed] [Google Scholar]
- 39.Denardin C.C., Da Silva L.P. Estrutura dos grânulos de amido e sua relação com propriedades físico-químicas. Ciência Rural. 2009;39(3):945–954. [Google Scholar]
- 40.Rocha L.C. et al., Feedlot diets containing different starch levels and additives change the cecal proteome involved in cattle’s energy metabolism and inflammatory response. Scientific Reports, [S.L.] 2022;12 doi: 10.1038/s41598-022-09715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toseti L.B., et al. Effects of a blend of essential oils and exogenous α-amylase in diets containing different roughage sources for finishing beef cattle. Animal Feed Sci. Technol., [S.L.] 2020;269 v.nov.Elsevier BV. [Google Scholar]
- 42.Salem A.Z.M., et al. Effects of exogenous enzymes on nutrient digestibility, ruminal fermentation and growth performance in beef steers. Livestock Sci., [S.L.] 2013;154(1–3):69–73. [Google Scholar]
- 43.Ítavo L.C.V., et al. Fontes de amido no concentrado de bovinos superprecoces de diferentes classes sexuais. Arq. Bras. Med. Vet. Zootec. 2014;66(4):1129–1138. [Google Scholar]
- 44.Vigne G.L.D., et al. Doses of enzyme complex in a high-energy diet on performance and carcass traits of feedlot steers. Revista Brasileira de Zootecnia. 2018;47(03) v.n. [Google Scholar]
- 45.Beatriz A., et al. Nutrição e exercício na prevenção e controle das doenças cardiovasculares. Rev. Bras. Med. Esporte. 2002;8(6):244–254. [Google Scholar]
- 46.Tobert J.A. LDL cholesterol-how low can we go? Endocrinol. Metab. Clin. North. Am. 2022;51(3):681–690. doi: 10.1016/j.ecl.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Broncano J.M., et al. Use of proteases to improve oxidative stability of fermented sausages by increasing low molecular weight compounds with antioxidant activity. Food Res. Int. 2011;44(9):2655–2659. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.