Abstract
Listeria monocytogenes infection of endothelial cells upregulates surface expression of adhesion molecules and stimulates neutrophil adhesion to infected cell monolayers. The experiments presented here tested the roles of specific bacterial virulence factors as triggers for this inflammatory phenotype and function. Human umbilical vein endothelial cell (HUVEC) monolayers were infected with wild-type L. monocytogenes or L. monocytogenes mutants; then surface expression of E-selectin and neutrophil adhesion were measured. The results showed that Δhly and prfA mutants were the most crippled, requiring 100-fold more mutant bacteria than wild-type bacteria for analogous stimulation. By comparison, L. monocytogenes mutants with deletions of actA, inlA, inlB, inlAB, plcA, and plcB resembled their parent strains, and a ΔplcA ΔplcB mutant displayed decreased intracellular growth rate but only a minor decrease in stimulation of E-selectin or neutrophil adhesion. Other experiments showed that cytochalasin D-treated HUVEC monolayers bound bacteria, but internalization and increased surface E-selectin and intercellular adhesion molecule-1 expression were profoundly inhibited. However, cytochalasin D had no effect on the HUVEC response to stimulation with lipopolysaccharide or tumor necrosis factor alpha. These data suggest that listeriolysin O production by infecting L. monocytogenes contributes to increased expression of surface E-selectin and intercellular adhesion molecule-1, but neither it nor intracellular replication are directly responsible for this event. Nonetheless it is possible that listeriolysin O potentiates the effect(s) of an other molecule(s) that directly triggers this response. Additionally, cellular invasion by L. monocytogenes appears to be critical for initiating the HUVEC response, potentially by providing a signal which results in upregulation of the necessary bacterial genes.
Interactions between vascular endothelial cells and pathogenic bacteria are common events in many infectious diseases and often result in endothelial cell stimulation and enhance leukocyte adhesion to infected cells (1). Such interactions are comprised of two components: endothelial cell stimulation by bacterial products and direct microbial infection of the endothelial cell. Bacterial products can stimulate endothelial cells in the absence of cellular infection, or the two processes can act in concert when bacteria invade endothelial cells. Bacterial products that stimulate cells without infection include the gram-negative cell wall component, lipopolysaccharide (LPS), the phospholipase C and perfringolysin O of Clostridium perfringens, and listeriolysin O (LLO) and the phosphoinositol-specific phospholipase C of Listeria monocytogenes (4, 16, 27, 33, 40, 41). As mentioned above, several different pathogenic bacteria have been shown to bind or invade endothelial cells and to stimulate them in the process (9, 14, 15, 38, 39, 44, 50). Products that could stimulate cells during binding and invasion include the outer membrane protein A of Borrelia burgdorferi, peptidoglycan from Leptospira icterohemorhagiae, and certain bacterial heat shock proteins (9, 21, 48, 49). Endothelial cell stimulation by either of these processes has profound effects on expression of endothelial cell adhesion molecules as well as cytokine and chemokine production and ultimately plays a critical role in the inflammatory process and host defenses.
L. monocytogenes is a pathogenic facultative intracellular bacterium able to invade and replicate within mammalian cells (14, 18, 35). Several L. monocytogenes genes involved in cellular invasion and intracellular parasitism have been identified and their function and products studied in detail (reviewed in reference 36). These include the pleiotropic regulator of the virulence gene cluster prfA, members of the gene cluster (plcA, hly, mpl, actA, and plcB), and the inl family of invasion genes (5, 19). Products with roles in phagosomal lysis and escape into the cytoplasm include LLO, a pore-forming toxin encoded by hly, and two C-type phospholipases, a phosphoinositol-specific phospholipase C encoded by plcA and a broad-spectrum phospholipase C encoded by plcB that cleaves phosphatidylcholine (PC-PLC) (18, 30, 35, 42). These enzymes act with LLO to facilitate phagosomal escape and cell-to-cell spread and also may be involved in stimulating intracellular signaling in the eukaryotic target. The mpl gene encodes an enzyme that processes the immature form of PC-PLC into a mature form (10, 30, 32). Intracellular motility and subsequent cell-to-cell spread is dependent upon the ActA protein, which is essential for polymerization of host F-actin (11, 26). The recently described inl family of genes encode internalin A and internalin B proteins that are involved in binding and invasion of eukaryotic cells (13, 14, 20, 29).
As a pathogenic microbe, L. monocytogenes is a well-known cause of bacteremia and central nervous system infections of immunocompromised humans and of domesticated animals (22, 31). The predilection of L. monocytogenes to invade the central nervous system from the bloodstream led to the hypothesis that infection of vascular endothelial cells was an important event in the pathophysiology of listeriosis (2, 14, 37). Previous work from this laboratory showed that L. monocytogenes can infect and replicate within human umbilical vein endothelial cells (HUVEC) (14). In response to infection, there was upregulated surface expression of the adhesion molecules E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) and stimulation of neutrophil (polymorphonuclear leukocyte [PMN]) adhesion to infected monolayers (15). Induction of this inflammatory phenotype and function did not occur following infection with the nonpathogenic Listeria innocua and Listeria welshimeri or following incubation of infected HUVEC with uninfected cells separated by a permeable membrane or with sterile-filtered supernatants from infected cells. These results suggested that specific bacterial virulence factors and direct contact of L. monocytogenes with HUVEC were required to trigger the HUVEC response. The experiments presented here studied the roles of specific virulence factors as stimuli for endothelial cell adhesion molecule expression and PMN adhesion.
MATERIALS AND METHODS
Antibodies.
Mouse monoclonal antibodies directed against human ICAM-1 (CD54, immunoglobulin G1 [IgG1]) and E-selectin (CD62E, IgG1) were obtained from Serotec USA (Washington, D.C.). Horseradish peroxidase-conjugated goat anti-mouse IgG was obtained from Bio-Rad (Hercules, Calif.).
Bacteria.
L. monocytogenes EGD, originally obtained from G. B. Mackaness, was a gift from Priscilla Campbell (National Jewish Center, Denver, Colo.). This strain has been passed through mice to maintain virulence and is post-10th passage. Mutants of L. monocytogenes containing in-frame deletions of inlA (BUG 947), inlB (BUG 1047), and inlAB (BUG 949) and the wild-type parent strain, EGD (designated EGD-Pasteur), were gifts from P. Cossart (13). The nonhemolytic, avirulent L. monocytogenes strain 43250, which harbors a mutation in the prfA gene, was purchased from the American Type Culture Collection (ATCC) (Rockville, Md.) (28, 34). L. monocytogenes containing in-frame deletion mutants of hly (DP-L2161), plcA (DP-L1552), plcB (DP-L1935), actA (DP-L1942), and plcA plcB (DP-L1936) and the wild-type parent strain, 10403s, were gifts from D. Portnoy (30, 42). The L. monocytogenes prfA mutant EGD prfA1 was the gift of T. Chakraborty (5).
Cultures containing 109 bacteria/ml were stored at −70°C in tryptose phosphate broth or brain heart infusion (BH1) (Difco, Detroit, Mich.) containing 15% glycerol. For each experiment, a fresh culture of bacteria was prepared by inoculating 10 μl of stock culture into 4 ml of broth. The prfA1 mutant was cultured in broth containing erythromycin (5 μg/ml) (5). Cultures were incubated overnight at 37°C with shaking or, in the cases of DP-L2161, DP-L1552, DP-L1942, and DP-L1936, at 30°C without shaking. Bacteria were washed by centrifugation at 12,000 × g for 3 min followed by resuspension and vortex mixing in Hanks’ balanced salt solution (HBSS).
Cells.
Cultures of primary normal HUVEC were purchased from the ATCC or from Clonetics (San Diego, Calif.). Cells from ATCC were cultured in F-12K medium (Gibco BRL, Grand Island, N.Y.) containing 10% fetal calf serum (Hyclone Laboratories, Logan, Utah), 50 μg of endothelial cell growth supplement (Collaborative Biomedical Products, Bedford, Mass.)/ml, 100 μg of heparin/ml, and 0.05 mM ascorbic acid (Gibco). The lyophilized growth supplement contained streptomycin at a final concentration of 19.5 μg/ml in the complete medium. No additional antibiotics were used. ATCC cells were used between passages 15 and 20. They were maintained in 100-mm-diameter dishes, fed twice weekly, and split 1:2 or 1:3 weekly into 24- or 96-well plates as needed for experiments. Cells purchased from Clonetics were cultured in Clonetics EGM-2 medium in the absence of antibiotics. These cells were used between passages 3 and 10. Cells from the two sources responded similarly to L. monocytogenes infection with respect to upregulation of surface expression of adhesion molecules, stimulation of PMN adhesion, and responses to L. monocytogenes mutants. The only differences were that Clonetics cells had a longer useful life span and grew to a higher cell density than cells from the ATCC.
HUVEC infection.
Intracellular growth of Listeria in HUVEC was measured by a standard gentamicin protection assay as described previously and adapted for use with HUVEC (14, 19). Medium (0.25 ml) containing 105 bacteria/ml was centrifuged for 10 min at 1,000 × g onto HUVEC cultured in 24-well plates, followed by a 60-min incubation at 37°C in a CO2 incubator. Next, the cells were washed, covered with medium containing gentamicin (10 μg/ml) to kill extracellular bacteria, and then incubated for an additional 60 min. This time point is designated time zero. The cells were washed twice, and bacteria remaining with the monolayer, presumably intracellular, were collected by the addition of 1.0 ml of sterile distilled H2O containing 0.5% saponin plus 3 mM EDTA. The cell lysates were collected with a Pasteur pipette, and CFU from triplicate wells were quantified by serial dilution in sterile distilled H2O and plating on tryptic soy agar (Difco). The remaining wells were incubated for another 4 h (time plus 4 h) at 37°C in medium containing 10 μg of gentamicin/ml, and then Listeria CFU were measured as described above. Intracellular growth of bacteria during the 4-h incubation was calculated as follows: log10 CFU at time plus 4 h − log10 CFU at time zero.
In some experiments cytochalasin D (Sigma Chemical Co., St. Louis, Mo.) was used to inhibit bacterial invasion of HUVEC. Endothelial cells in duplicate plates were incubated with twofold dilutions of cytochalasin D for 60 min and then infected with 105 bacteria as described above. Cells and bacteria were cultured for 60 min at 37°C and washed, and CFU bound to the cells were quantified by serial dilution and plating. Next, gentamicin (final concentration, 50 μg/ml) was added to the remaining plate, and it was incubated for another 60 min. The wells were washed again, and intracellular bacterial CFU were quantified by serial dilution and plating.
Measurement of adhesion molecule expression.
HUVEC cultured in 96-well plates were left uninfected or were infected with serial dilutions of wild-type L. monocytogenes or L. monocytogenes mutants. Bacterial inocula were measured by serial dilution and plating. HUVEC were cultured for 60 min, washed, and covered with medium containing gentamicin (10 μg/ml). The cells were cultured for another 4 h and washed, and surface E-selectin or ICAM-1 expression was measured by whole-cell enzyme-linked immunosorbent assay (ELISA) as previously described (15). Relative E-selectin expression for each bacterial strain or mutant was expressed as a percentage of the E-selectin absorbance elicited by the indicated wild-type strain at an inoculum of 104 CFU/well, calculated as follows: (E-selectin absorbance after infection with the test bacterium/E-selectin absorbance after infection with the wild type at 104 CFU per well) × 100.
Broth from overnight cultures was tested for E-selectin-stimulating activity. Bacterial cultures in BHI broth were centrifuged, sterile filtered, and added to HUVEC in twofold increments to achieve final dilutions of 1:10 to 1:400. The cells were incubated for 6 h, and surface E-selectin expression was measured as before. A 10% dilution of sterile-filtered control BHI broth had no detectable effect on cellular viability or baseline surface E-selectin expression compared with those of control cells.
PMN adhesion to HUVEC.
Human peripheral blood PMN were obtained by venipuncture from healthy, human immunodeficiency virus-negative donors. Whole blood was drawn into EDTA-containing Vacutainer tubes (Becton Dickinson, Lincoln Park, N.J.), and PMNs were separated by centrifugation through Neutrophil Isolation Medium (Cardinal Associates, Santa Fe, N.Mex.) as previously described (14). HUVEC in 96-well plates were infected and incubated for 5 h. Immediately prior to incubation with HUVEC, PMNs (107/ml) were loaded with 5 μM calcein AM (Molecular Probes, Eugene, Oreg.) in phosphate-buffered saline at room temperature for 30 min and then were washed twice with 10 ml of HBSS without Mg2+ or Ca2+ to remove excess label (8). Infected HUVEC were washed twice with HBSS containing Mg2+ and Ca2+, and then 105 calcein AM-loaded PMNs in 100 μl of RPMI 1640 (Gibco) plus 5% fetal calf serum were added to the wells. Plates containing HUVEC and PMNs were incubated for 30 min at 37°C, and then HUVEC were washed five times with divalent cation-containing HBSS to remove unbound PMNs. Fluorescence from adherent PMNs was measured in a CytoFluor 4000 (PerSeptives Biosystems, Framingham, Mass.) fluorescence microplate reader with excitation and emission wavelengths of 530 and 485 nm, respectively. PMN adhesion is expressed as mean relative fluorescence units ± standard deviation (SD) from quadruplicate wells.
RESULTS
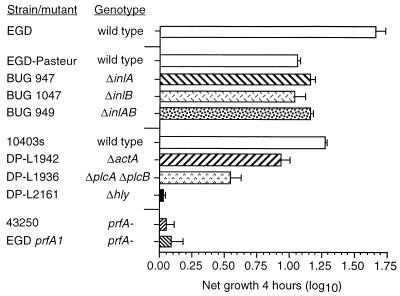
L. monocytogenes strains with mutations in specific virulence genes were used to study the roles of L. monocytogenes virulence factors in HUVEC stimulation. First, we measured the ability of various wild-type bacteria and mutants to replicate within HUVEC. Figure 1 shows that wild-type bacteria replicated rapidly within HUVEC. By comparison, Δhly and prfA mutants did not replicate but did avoid being killed by gentamicin, suggesting an intracellular location. Bacteria lacking either inlA or inlB or both replicated as readily as their parent strain once they had entered the cells. The ΔactA mutant grew slightly less rapidly than its parent strain, 10403s, whereas the ΔplcA ΔplcB mutant demonstrated a more significant, slower net replication in the monolayer, presumably due to defects in escape from the primary vacuole and cell-to-cell spread (30, 42).
FIG. 1.
Growth of wild-type L. monocytogenes and L. monocytogenes mutants within HUVEC. Confluent HUVEC monolayers were infected with 104 CFU. Cells and bacteria were cocultured for 60 min, washed, and then incubated for another 60 min in medium containing gentamicin to kill extracellular bacteria. Triplicate wells from one plate were lysed, and intracellular bacterial CFU at time zero were quantified by serial dilution and plating. The second plate was incubated for another 4 h (time plus 4 h), and CFU were quantified as before. Intracellular growth during the 4-h interval was calculated as follows: log10 CFU at time plus 4 h − log10 CFU at time zero. Results are shown as the mean (±SEM) log10 growth from three experiments.
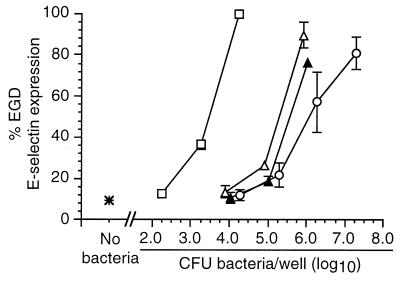
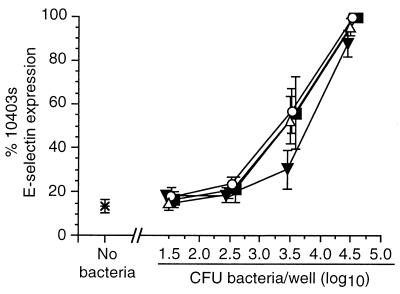
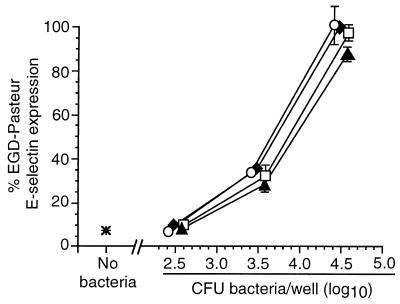
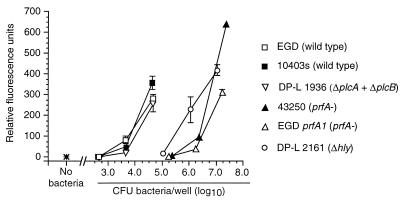
Next, HUVEC were infected with increasing numbers of wild-type L. monocytogenes or mutant bacteria, and relative surface E-selectin expression was measured by whole-cell ELISA. In these experiments the response to infection with mutant bacteria was represented as a percentage of the absorbance at 490 nm following infection with 104 wild-type L. monocytogenes organisms. Dose-response curves for each type of bacterium were generated that plotted percent E-selectin signal as a function of the inoculum for that bacterium that was used to infect HUVEC. Each of the wild-type bacteria stimulated E-selectin expression, and there were only minor differences between them when the inoculum and the ELISA signal were compared. By contrast, nonhemolytic prfA-negative or Δhly L. monocytogenes mutants were clearly separated from hemolytic bacteria (Fig. 2) in that infection with 104 CFU/well did not increase surface E-selectin expression over that of uninfected cells. However, when the inoculum of infecting nonhemolytic mutants was increased to 106 CFU/well, these bacteria stimulated relative E-selectin expression comparably to wild-type bacteria at 104 CFU/well. By comparison, the dose-response curve generated from three separate experiments with the mutant DP-L1942 (ΔactA) was superimposable upon that of its parent strain, 10403s, showing that intracellular motility is not an essential factor for HUVEC stimulation (data not shown). Individual deletions of the plcA and plcB genes had no significant effect on relative E-selectin expression, whereas the ΔplcA ΔplcB (DP-L1936) mutant was slightly less active than the parent strain, 10403s, but only at an inoculum of 3.5 log10 CFU/well (Fig. 3). Figure 4 shows that the ΔinlA, ΔinlB, and ΔinlAB mutants stimulated E-selectin expression similarly to their parent strain (EGD-Pasteur).
FIG. 2.
Nonhemolytic L. monocytogenes mutants are crippled in their ability to stimulate increased surface expression of E-selectin on endothelial cells. HUVEC were infected with increasing numbers of wild-type L. monocytogenes (□), prfA mutants 43250 (▴) or EGD prfA1 (▵), or a Δhly mutant (○). Bacterial inocula were measured by serial dilution and plating. After a 4-h incubation, surface E-selectin expression was quantified by whole-cell ELISA. Relative percent E-selectin expression for each bacterium was calculated as follows: absorbance at 490 nm following infection with test bacterium/absorbance at 490 nm following infection with 104 L. monocytogenes EGD organisms. The mean (±SEM) relative percent E-selectin expression from three to seven experiments is shown.
FIG. 3.
Stimulation of HUVEC surface E-selectin expression by L. monocytogenes ΔplcA, ΔplcB, and ΔplcA ΔplcB mutants. HUVEC were infected with increasing numbers of the parent wild-type L. monocytogenes 10403s (▪) or L. monocytogenes ΔplcA (▵), ΔplcB (○), and ΔplcA ΔplcB (▾) mutants. Bacterial inocula were measured by serial dilution and plating. After a 4-h incubation, surface E-selectin expression was quantified by whole-cell ELISA. Relative percent E-selectin expression for each bacterium was calculated as follows: absorbance at 490 nm following infection with test bacterium/absorbance at 490 nm following infection with 104 L. monocytogenes 10403s organisms. The mean (±SEM) relative percent E-selectin expression from three experiments is shown.
FIG. 4.
Stimulation of HUVEC E-selectin expression by L. monocytogenes ΔinlA, ΔinlB, and ΔinlAB mutants. HUVEC were infected with increasing numbers of the parent wild-type L. monocytogenes EGD-Pasteur (⧫) or L. monocytogenes ΔinlA (○), ΔinlB (▴), or ΔinlAB (□) mutants. Bacterial inocula were measured by serial dilution and plating. After a 4-h incubation, surface E-selectin expression was quantified by whole-cell ELISA. Relative percent E-selectin expression for each bacterium was calculated as follows: absorbance at 490 nm following infection with test bacterium/absorbance at 490 nm following infection with 104 L. monocytogenes EGD-Pasteur organisms. The mean (±SEM) relative percent E-selectin expression from three experiments is shown.
Subsequent experiments tested the relative abilities of these L. monocytogenes mutants to stimulate PMN adhesion to infected monolayers. Figure 5 shows that PMN adhesion in response to bacterial infection generally paralleled the expression of E-selectin. The nonhemolytic prfA and Δhly mutants stimulated very little PMN adhesion at an inoculum of 105 CFU/well. However, increasing the inocula of nonhemolytic prfA and Δhly mutants to 107 CFU/well stimulated PMN adhesion in excess of that caused by hemolytic bacteria at a 250- to 500-fold lower inoculum. The ΔplcA ΔplcB mutant was similar to wild-type EGD and only slightly less effective than 10403s. Similarly, the ΔinlA, ΔinlB, and ΔinlAB mutants were essentially comparable to their parent strain in stimulating PMN adhesion to infected monolayers (data not shown). These data show that loss of LLO is the single most important mutation that cripples the bacterium’s ability to stimulate E-selectin expression and PMN adhesion. However, the fact that at high inocula of nonhemolytic mutants HUVEC could stimulate these features indicates that LLO production is not an absolute requirement for endothelial cell stimulation.
FIG. 5.
PMN adhesion to endothelial cells infected by wild-type L. monocytogenes or L. monocytogenes mutants. HUVEC were infected with increasing numbers of wild-type L. monocytogenes or L. monocytogenes mutants and then cultured for another 4 h. Bacterial inocula were measured by serial dilution and plating. Infected monolayers were washed and then incubated for 30 min with 105 calcein-AM-loaded PMNs/well. The monolayers were washed again, and fluorescence emission was measured with a fluorescence microplate reader. PMN adhesion is represented as mean (±SD) relative fluorescence units from quadruplicate groups of wells from one of two experiments with identical results.
Next we tested whether bacterial products secreted into culture medium during growth could elicit E-selectin expression. Concentrations of BHI broth of >1% from cultures of the hemolytic L. monocytogenes 10403s and DP-L1936 (ΔplcA ΔplcB) produced rapid cellular ballooning and death. In contrast, broth from cultures of prfA or Δhly L. monocytogenes mutants or L. innocua caused no obvious cytotoxicity at concentrations up to 10%. Surface E-selectin expression was only minimally increased, and there were no significant differences between hemolytic or Δhly L. monocytogenes and L. innocua at concentrations of ≤1% or between Δhly L. monocytogenes and L. innocua at concentrations up to 10% (data not shown). These data suggest that secreted LLO acting alone or in combination with other secreted bacterial products can cause cellular necrosis. However, these experiments did not detect differences between broth from L. monocytogenes and broth from the nonpathogenic L. innocua in stimulation of surface E-selectin despite radical differences in their abilities to stimulate HUVEC during cellular infection (15).
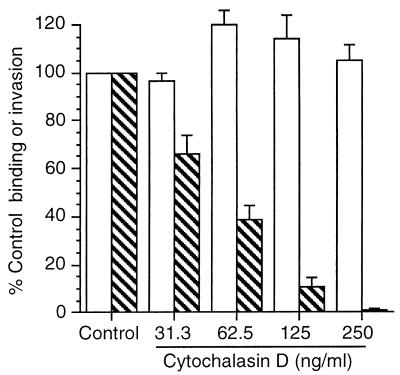
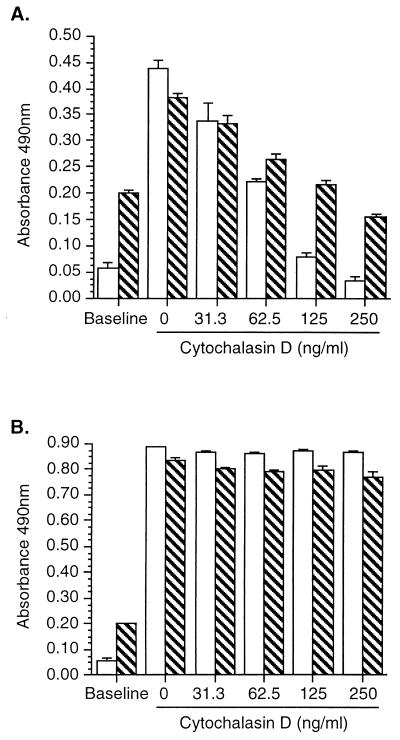
Previous work showed that addition of bacteriostatic antibiotics to HUVEC 30 min after infection did not affect subsequent surface E-selectin expression, suggesting that the cellular response was triggered early in the host-pathogen interaction (15). Thus, in a separate series of experiments we used cytochalasin D to separate the roles of binding and invasion to test whether binding of wild-type bacteria was sufficient to initiate upregulation of E-selectin. Untreated (control) HUVEC bound 4.66% ± 1.44% of the inoculum, and 34.66% ± 1.86% of bound bacteria invaded the cells (mean ± standard error of the mean (SEM); n = 3). Figure 6 shows that binding to HUVEC was increased modestly but internalization was reduced to as low as 1.2% of that of the control. Together with this, cytochalasin D caused a dose-dependent inhibition of L. monocytogenes-elicited E-selectin expression (Fig. 7). However, cytochalasin D did not alter increased surface expression of E-selectin in response to Salmonella typhimurium LPS (100 ng/ml) or recombinant human tumor necrosis factor alpha (data not shown), showing that HUVEC were still capable of responding to other inflammatory stimuli.
FIG. 6.
Cytochalasin D inhibits invasion of endothelial cells by L. monocytogenes. HUVEC were cultured with increasing amounts of cytochalasin D for 60 min and then were infected with 104 L. monocytogenes EGD organisms. Cells and bacteria were incubated for 60 min and washed, and then one set of triplicate wells were lysed and bound CFU were quantified by serial dilution and plating. The remaining cells were incubated with medium containing gentamicin for 60 min, and then intracellular bacterial CFU were quantified as before. Percent bacterial binding (open bars) was calculated as follows: CFU bound/CFU added. Percent invasion (hatched bars) was calculated as follows: intracellular bacterial CFU/CFU bound. Results presented are the mean (±SEM) percent control (no cytochalasin D added) binding and invasion from three experiments.
FIG. 7.
Cytochalasin D inhibits surface E-selectin and ICAM-1 upregulation in response to L. monocytogenes infection but not to stimulation with LPS. HUVEC were incubated with increasing concentrations of cytochalasin D for 60 min; then the cells were infected with 104 L. monocytogenes EGD organisms (A) or stimulated with 100 ng of LPS/ml (B). After 5 h E-selectin (open bars) and ICAM-1 (hatched bars) surface expressions were measured by whole-cell ELISA. Results presented are the mean (±SD) absorbance at 490 nm from quadruplicate wells from one of two experiments with similar results.
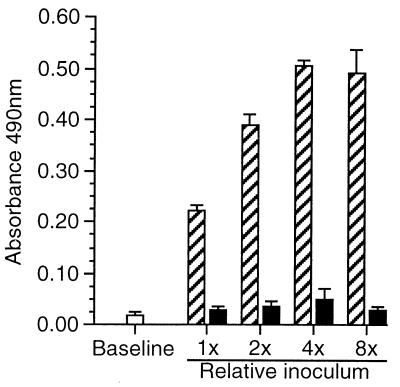
Alternative interpretations of these results are that the critical factor for HUVEC stimulation was either the event of invasion or the bacterial load of infected cells when adhesion molecule expression was measured. If the second interpretation is correct, increasing the inoculum of bacteria should produce an incremental increase in surface E-selectin expression on cytochalasin D-treated HUVEC. However, Fig. 8 shows that an eightfold increase in added CFU stimulated surface E-selectin expression on control cells but not on cytochalasin D-treated cells. However, there was no difference in surface E-selectin or ICAM-1 (data not shown) between cytochalasin D-treated cells infected with 1× or 8× inocula of bacteria, presumably because a threshold number of invading bacteria was not achieved. Taken together these data show that L. monocytogenes invasion of HUVEC is dependent upon microfilament function and suggest that binding of L. monocytogenes to HUVEC is not sufficient to trigger increased surface expression of E-selectin or ICAM-1. They also indicate a potential role for bacterial invasion as a critical step in HUVEC stimulation.
FIG. 8.
Increasing the bacterial inoculum does not increase surface E-selectin on cytochalasin D-treated cells. HUVEC were incubated with 250 μg of cytochalasin D/ml for 60 min (black bars) or left untreated (hatched bars) prior to infection with twofold increments of L. monocytogenes EGD. The mean (±SD) inoculum (1×) was 1.72 × 103 ± 0.23 × 103 CFU of bacteria/well. Cells were cultured for 60 min, gentamicin was added, and the cells were incubated for another 4 h. E-selectin expression was measured by whole-cell ELISA. Results presented are the mean (±SEM) absorbance at 490 nm from three experiments.
DISCUSSION
PMNs occupy a critical role in the early host response to infection by L. monocytogenes as well as to infection by other pathogenic bacteria (6, 7, 23). Recruitment of PMNs to foci of infection and inflammation is a multifacted process, in which increased surface expression of adhesion molecules on vascular endothelial cells occupies a critical role (reviewed in reference 45). Recent data from this laboratory and others have shown that endothelial cell infection with L. monocytogenes or exposure to L. monocytogenes products can stimulate PMN adhesion to HUVEC monolayers and that it increases surface expression of the adhesion molecules E- and P-selectin, ICAM-1, and VCAM-1 (15, 27). Data from this laboratory showed that PMN adhesion to infected HUVEC monolayers paralleled the surface expression of E-selectin: monoclonal antibodies against E-selectin and CD18 blocked approximately 75% and 35% of PMN adhesion to infected monolayers, respectively (15). The study presented here used mutants of L. monocytogenes to test the roles of its virulence factors as stimuli for upregulating HUVEC surface expression of E-selectin and stimulating PMN adhesion to infected monolayers.
The most obvious distinction among the various bacteria tested was that Δhly and prfA mutants were profoundly crippled in their stimulatory capacity. L. monocytogenes Δhly mutants are deficient only in the production of LLO, whereas prfA mutants are defective for transcription of all the prfA-dependent virulence genes, including hly; however there can be low-level prfA-independent transcription, resulting in contact hemolysis (12). As a result, these mutants are severely crippled in their capacity for intracellular parasitism and were not able to replicate within HUVEC. Nonetheless, HUVEC could be stimulated when the inocula of these nonhemolytic mutants were increased at least 100-fold over that used for wild-type bacteria. This is a clear contrast to the nonhemolytic, avirulent Listeria species, L. innocua and L. welshimeri, which did not stimulate HUVEC even when 108 CFU/well was used for infection (15).
None of the other L. monocytogenes mutants demonstrated such an extreme loss of stimulatory capacity as that caused by the single loss of LLO production. Deletions of plcA and plcB had no detectable inhibitory effect, whereas deletion of both plcA and plcB led to a decreased intracellular growth rate but caused little change in HUVEC activation. The enzymes encoded by these genes, a phosphoinositol-specific phospholipase C and a broad-spectrum phospholipase C, participate with LLO in lysis of primary phagosomes and during cell-to-cell spread, and they can mediate LLO-independent phagosomal lysis in some cells (30, 42). The phosphoinositol-specific phospholipase C has also been shown to participate with LLO in stimulation of HUVEC phosphoinositide hydrolysis and diacylglycerol formation (41). Data presented here indicate that L. monocytogenes phospholipases are not directly involved in stimulating E-selectin expression or PMN adhesion. The lack of intracellular growth by nonhemolytic mutants suggests that LLO-independent phagosomal lysis does not occur in HUVEC, or at least is infrequent compared with Henle 407 cells (30). If such an event did occur, it is possible that ΔplcA Δhly or ΔplcB Δhly mutants would be more crippled than single Δhly mutants for stimulating E-selectin and PMN adhesion, suggesting a role for early phagosomal lysis in triggering these events. Another set of mutants, ΔinlA, ΔinlB, and ΔinlAB, were comparable to the wild type in intracellular growth, stimulation of E-selectin expression, and stimulation of PMN adhesion; however, invasion rates for these mutants were not measured. Nevertheless, these results suggest that under serum-containing conditions, which do not favor internalin-mediated binding (14), inlA and inlB are not required for HUVEC stimulation.
The data show that LLO production contributes significantly to optimal stimulation of HUVEC surface E-selectin expression and PMN adhesion. However, the fact that nonhemolytic mutants could stimulate the cells shows that LLO production is not absolutely required for this event. Similarly, the response of HUVEC to nonhemolytic L. monocytogenes suggests that intracellular replication is also not a required trigger. This conclusion is consistent with our previous data showing that bacteriostatic antibiotics applied to HUVEC 30 min after infection did not inhibit the cells’ E-selectin response and with immunofluorescence microscopy showing the presence of infected cells which were E-selectin negative (15). The lack of a direct effect of LLO was also suggested by the finding that broth from L. monocytogenes cultures did not stimulate increased surface expression of E-selectin compared with broth from L. innocua at a similar dilution and that bacteria bound to cytochalasin D-treated cells did not stimulate them. Recent experiments by Krüll et al. directly tested the role of LLO as a stimulus for HUVEC adhesion molecule surface expression (27). They showed that purified LLO and genetically engineered L. innocua that overexpress LLO stimulated surface expression of P-selectin but not E-selectin, ICAM-1, or VCAM-1, suggesting the presence of an LLO-independent trigger for expression of these molecules. Thus, a mechanism(s) that accounts for L. monocytogenes stimulation of HUVEC E-selectin, ICAM-1, and VCAM-1 surface expression is not known. However, it is possible that LLO potentiates the effects of the L. monocytogenes virulence factor or factors that trigger these responses directly.
Other experiments used cytochalasin D to study the roles of wild-type L. monocytogenes binding and invasion as stimuli for adhesion molecule expression. Increasing concentrations of cytochalasin D slightly enhanced bacterial binding to HUVEC but inhibited internalization of bound bacteria profoundly. Along with this, increased surface expression of E-selectin and ICAM-1 was also prevented, suggesting that bacterial invasion, or at least rearrangement of the cytoskeleton, is a critical event. By comparison, cytochalasin D did not alter the effects of stimulation with LPS and tumor necrosis factor alpha showing that not all signaling pathways which upregulate E-selectin and ICAM-1, for example through CD14, were interrupted (33). It is possible that cytochalasin D interrupts cell signaling pathways triggered by bacterial binding that are important for invasion as well as adhesion molecule expression. Binding of L. monocytogenes to epithelial cells has been shown to stimulate mitogen-activated protein kinase through LLO and phosphoinositide 3-kinase activity through internalin B-mediated binding (24, 46, 47). However, these events were not influenced by cytochalasin D treatment of the target cells. This could indicate that if cytochalasin D blocks pathways in the HUVEC system which ultimately affect surface adhesion molecule expression, they are distal to activation of phosphoinositide 3-kinase.
Bacterial invasion of mammalian cells is a complex process which involves active participation by the microbe and the eukaryotic target (17, 20). Current models indicate that pathogenic bacteria use several different mechanisms to sense their microenvironment and adjust expression of their virulence factors accordingly (reviewed in reference 17). Thus, it is possible that blockade of invasion could prevent elaboration of bacterial products critical for stimulating adhesion molecule expression. Recent data show that expression of L. monocytogenes hly, plcB, actA, and plcA and production of their respective proteins is upregulated by incubation under stress conditions, such as a shift from rich to minimal medium, heat shock, or growth within mammalian cells (3, 25, 43). In the experiments presented here, these virulence factors were not directly responsible for HUVEC stimulation. Nevertheless, regulation of L. monocytogenes virulence genes in response to microenvironmental cues supports the concept that invasion could trigger expression of the gene or genes necessary for stimulating HUVEC.
ACKNOWLEDGMENTS
I am grateful for the excellent technical assistance of Sandra Wilson and to T. Chakraborty, P. Cossart, and D. Portnoy for providing L. monocytogenes mutants.
This work was supported by a WVU School of Medicine research grant.
REFERENCES
- 1.Beilke M A. Vascular endothelium in immunology and infectious disease. Rev Infect Dis. 1989;11:273–283. doi: 10.1093/clinids/11.2.273. [DOI] [PubMed] [Google Scholar]
- 2.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 3.Bohne J, Sokolovic Z, Goebel W. Transcriptional regulation of prfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol Microbiol. 1994;11:1141–1150. doi: 10.1111/j.1365-2958.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 4.Bryant A E, Stevens D L. Phospholipase C and perfringolysin O from Clostridium perfringens upregulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured umbilical vein endothelial cells. Infect Immun. 1996;64:358–362. doi: 10.1128/iai.64.1.358-362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty T, Leimeister-Wächter M, Doman E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of the virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czuprynski C J, Henson P M, Campbell P A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukocyte Biol. 1984;35:193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- 7.Czuprynski C J, Brown J F, Maroushek N, Wagner R D, Steinberg H. Administration of anti-granulocyte monoclonal antibody RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 8.De Clerk L S, Bridts C H, Mertens A M, Moens M M, Stevens W J. Use of fluorescent dyes for the determination of adherence of human leukocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods. 1994;172:115–124. doi: 10.1016/0022-1759(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 9.Dobrina A, Nardon E, Vecile E, Cinco M, Patriarca P. Leptospira icterohemorrhagiae and leptospire peptidoglycans induce endothelial cell adhesiveness for polymorphonuclear leukocytes. Infect Immun. 1995;63:2995–2999. doi: 10.1128/iai.63.8.2995-2999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domann E, Leimeister-Wächter M, Goebel W, Chakraborty T. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect Immun. 1991;59:65–72. doi: 10.1128/iai.59.1.65-72.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wächter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domann E, Wehland J, Niebuhr K, Haffner C, Leimeister-Wächter M, Chakraborty T. Detection of a prfA-independent promoter responsible for listeriolysin gene expression in the mutant Listeria monocytogenes strains lacking the PrfA regulator. Infect Immun. 1993;61:3073–3075. doi: 10.1128/iai.61.7.3073-3075.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 14.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drevets D A. Listeria monocytogenes infection of cultured endothelial cells stimulates neutrophil adhesion and adhesion molecule expression. J Immunol. 1997;158:5305–5313. [PubMed] [Google Scholar]
- 16.Essani N A, McGuire G M, Manning A, Jaeschke H. Endotoxin-induced activation of the nuclear transcription factor κB and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells and endothelial cells in vivo. J Immunol. 1996;156:2956–2963. [PubMed] [Google Scholar]
- 17.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 18.Gaillard J-L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 20.Galán J E. Interactions of bacteria with non-phagocytic cells. Curr Opin Immunol. 1994;6:590–595. doi: 10.1016/0952-7915(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 21.Galdiero M, Cipollaro de l’Ero G, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infect Immun. 1997;65:699–707. doi: 10.1128/iai.65.2.699-707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray M L, Killinger A H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory S H, Sagnimeni A J, Wing E J. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–2520. [PubMed] [Google Scholar]
- 24.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 25.Klarsfeld A D, Goossens P L, Cossart P. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE, and an arginine ABC transporter gene, arpJ. Mol Microbiol. 1994;13:585–597. doi: 10.1111/j.1365-2958.1994.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 26.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced F-actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 27.Krüll M, Nöst R, Hippenstiel S, Domann E, Chakraborty T, Suttorp N. Listeria monocytogenes potently induces up-regulation of endothelial adhesion molecules and neutrophil adhesion to cultured human endothelial cells. J Immunol. 1997;159:1970–1976. [PubMed] [Google Scholar]
- 28.Leimeister-Wächter M, Goebel W, Chakraborty T. Mutations affecting hemolysin production in Listeria monocytogenes located outside the listeriolysin gene. FEMS Microbiol Lett. 1989;65:23–30. doi: 10.1016/0378-1097(89)90360-1. [DOI] [PubMed] [Google Scholar]
- 29.Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect Immun. 1995;63:3896–3903. doi: 10.1128/iai.63.10.3896-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquis H, Doshi V, Portnoy D A. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLauchlin J. Human listeriosis in Britain, 1967–85, a summary of 722 cases. Epidemiol Infect. 1990;104:191–201. doi: 10.1017/s0950268800059355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mengaud J, Geoffroy C, Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991;59:1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noel R F, Jr, Sato T T, Mendez C, Johnson M C, Pohlman T H. Activation of human endothelial cells by viable or heat-killed gram-negative bacteria requires soluble CD14. Infect Immun. 1995;63:4046–4053. doi: 10.1128/iai.63.10.4046-4053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pine L, Weaver R E, Carlone G M, Pienta P A, Rocourt J, Goebel W, Kathariou S, Bibb W F, Malcolm G B. Listeria monocytogenes ATCC 35152 and NCTC 7973 contain a nonhemolytic, nonvirulent variant. J Clin Microbiol. 1987;25:2247–2251. doi: 10.1128/jcm.25.11.2247-2251.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prats N, Briones V, Blanco M M, Altimira J, Ramos J A, Dominguez L, Marco A. Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 1992;11:744–747. doi: 10.1007/BF01989983. [DOI] [PubMed] [Google Scholar]
- 38.Riley B S, Oppenheimer-Marks N, Radolf J D, Norgard M V. Virulent Treponema pallidum promotes adhesion of leukocytes to human vascular endothelial cells. Infect Immun. 1994;62:4622–4625. doi: 10.1128/iai.62.10.4622-4625.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellati T J, Burns M J, Ficazzola M A, Furie M B. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect Immun. 1995;63:4439–4447. doi: 10.1128/iai.63.11.4439-4447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sibelius U, Rose F, Chakraborty T, Darji A, Wehland J, Weiss S, Seeger W, Grimminger F. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect Immun. 1996;64:674–676. doi: 10.1128/iai.64.2.674-676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sibelius U, Chakraborty T, Krögel B, Wolf J, Rose F, Schmidt R, Wehland J, Seeger W, Grimminger F. The listerial exotoxins listeriolysin and phophatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J Immunol. 1996;157:4055–4060. [PubMed] [Google Scholar]
- 42.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokolovic Z, Riedel J, Wuenscher M, Goebel W. Surface-associated, PrfA-regulated proteins of Listeria monocytogenes synthesized under stress conditions. Mol Microbiol. 1993;8:219–227. doi: 10.1111/j.1365-2958.1993.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 44.Sporn L A, Lawrence S O, Silverman D J, Marder V J. E-selectin-dependent neutrophil adhesion to Rickettsia rickettsii-infected endothelial cells. Blood. 1993;81:2406–2412. [PubMed] [Google Scholar]
- 45.Springer T A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 46.Tang P, Rosenshine I, Finlay B B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Cell Biol. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang P, Rosenshine I, Cossart P, Finlay B B. Listeriolysin O activates mitogen-activated protein kinase in eukaryotic cells. Infect Immun. 1996;64:2359–2361. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdagaal E M E, Zegveld S T, van Furth R. Heat shock protein 65 induces CD62e, CD106, and CD54 on cultured human endothelial cells and increases their adhesiveness for monocytes and granulocytes. J Immunol. 1996;157:369–376. [PubMed] [Google Scholar]
- 49.Wooten R M, Modur V R, McIntyre T M, Weis J J. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor NF-κB and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 50.Yao L, Bengualid V, Lowy F, Gibbons J J, Hatcher V B, Berman J W. Internalization of Staphylococcus aureus by endothelial cells induces cytokine gene expression. Infect Immun. 1995;63:1835–1839. doi: 10.1128/iai.63.5.1835-1839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]