Summary
Platform trials bring the promise of making clinical research more efficient and more patient centric. While their use has become more widespread, including their prominent role during the COVID-19 pandemic response, broader adoption of platform trials has been limited by the lack of experience and tools to navigate the critical upfront planning required to launch such collaborative studies. The European Union-Patient-cEntric clinicAl tRial pLatform (EU-PEARL) initiative has produced new methodologies to expand the use of platform trials with an overarching infrastructure and services embedded into Integrated Research Platforms (IRPs), in collaboration with patient representatives and through consultation with U.S. Food and Drug Administration and European Medicines Agency stakeholders. In this narrative review, we discuss the outlook for platform trials in Europe, including challenges related to infrastructure, design, adaptations, data sharing and regulation. Documents derived from the EU-PEARL project, alongside a literature search including PubMed and relevant grey literature (e.g., guidance from regulatory agencies and health technology agencies) were used as sources for a multi-stage collaborative process through which the 10 more important points based on lessons drawn from the EU-PEARL project were developed and summarised as guidance for the setup of platform trials. We conclude that early involvement of critical stakeholder such as regulatory agencies or patients are critical steps in the implementation and later acceptance of platform trials. Addressing these gaps will be critical for attaining the full potential of platform trials for patients.
Funding
Innovative Medicines Initiative 2 Joint Undertaking with support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Keywords: Adaptive designs, Master protocols, Patient-centred, Clinical research, Integrated research platform
Introduction
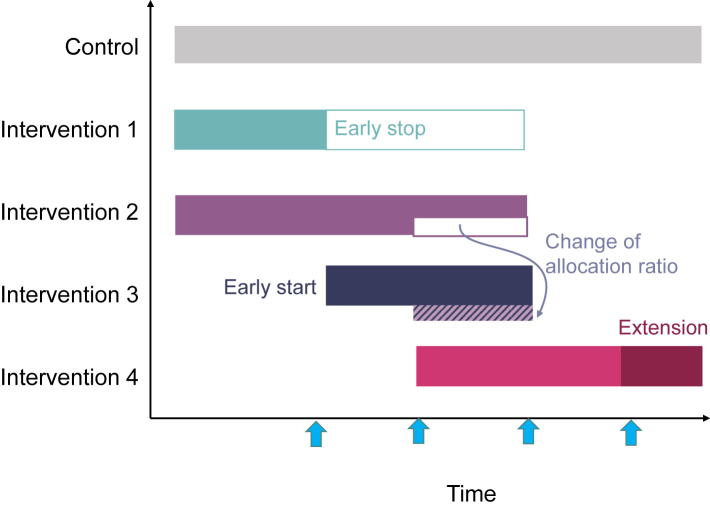
Platform trials are increasingly used in clinical research and drug development in particular.1 They are a form of adaptive design clinical trials that allow testing multiple interventions simultaneously and adding new treatments as they become available in the same trial structure within multiple subtrials (developed through intervention specific appendices-ISA), which can be either added or discontinued based on the results of interim analyses. Platform trials benefit from sharing trial infrastructure and resources, e.g., by sharing control data and joint committees. These elements are crucial in boosting both (1) the efficiency (i.e., the chances that a particular compound is graduated to the next clinical drug development phase if kept after an interim analysis, which also reduces the costs associated to carry on with the investment on a non-promising compound) and (2) the benefits for participants—i.e., increases the chances of participants being allocated to an intervention rather than placebo and the likelihood of receiving efficacious drugs as the platform trial progresses through interim analyses (see Fig. 1).
Search strategy and selection criteria.
To complete each section, besides selected outputs from EU-PEARL, a literature review was conducted. References for this review were identified through searches of PubMed with the search terms “platform trials”, “adaptive trial designs”, “master protocols” and “multi-arm multi-stage trials” from database inception until May 2023. Articles were also identified through searches of relevant grey literature (e.g., guidance from regulatory agencies and health technology agencies). Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review. Further details can be found in Supplementary Table S2.
Outstanding questions.
-
•
What are the most suitable indicators and sources to assess the feasibility of implementing a platform trial for a certain disease at a certain point in time—considering aspects such as the availability of approved drugs, the number and characteristics of compounds or other interventions under development, the difficulties in recruitment and the interest/willingness of drug/intervention owners to get involved?
-
•
Can common pathways for early consultation with international regulatory agencies be put in place for platform trials?
-
•
What specific training need patients and their representatives to contribute to the design and implementation of platform trials?
-
•
How to decide the best type of sponsorship for cross-company platform trials?
-
•
What types of tools can be used to leverage electronic health records to optimise potential candidates and automatise data collection in a large network of participating sites?
-
•
How to best design a business plan from the early stages depending on the type of sponsorship, characteristics of the master protocol, number of sites, and expected initial duration of the platform trial?
-
•
What is the most adequate manner to implement intellectual property and data sharing agreements in cross-company platform trials that provide enough assurance to companies regarding their legitimate interest, while allowing for transparency and proper use of data to conduct interim analyses?
Fig. 1.
Platform Trials: Simultaneously, sequentially and adaptively. A simplified illustration of a platform trial over time. It starts with a control and 2 treatment arms. The blue arrows indicate the timing of pre-planned adaptive interim analyses. Interim decisions may include early stopping (either for efficacy or futility—see intervention 1) or design adaptations such as the change of allocation ratio (see intervention 2 and 3), sample size reassessment (see intervention 4) or subgroup selection (not illustrated). For example, intervention 1 is stopped after the first interim analysis and at the same time a new arm is added (intervention 3). At the second interim analysis the allocation ratio is changed, e.g., by allocating more patients to intervention 3 compared to 2. At the interim analysis for intervention 4 a sample size re-assessment is performed (the extension is indicated by the darker colour).
Since they began in the early 2000s and until the onset of the COVID-19 pandemic, platform trials have been mostly used in the field of oncology; however, the number has increased significantly during the pandemic as rapid deployment of collaborative structures became essential to develop treatments and vaccines against SARS-CoV-2.2 A recent study showed that infectious disease has already become the primary field of platform trial application (52%), compared to only 29% in oncology.3 As of today, platform trials are mostly carried out in a handful of countries, the vast majority in high-income countries and led by either a United Kingdom or United States-based organisation.3 Moreover, cross-company collaboration has still not been widely adopted to conduct this type of clinical trials. Therefore, there is still room to advance the field of platform trials to optimise drug development in Europe and globally. The European Union-Patient-cEntric clinicAl tRial pLatform (EU-PEARL) is an Innovative Medicines Initiative (IMI) 2 Joint Undertaking funded project (2019–2023), and a strategic public and private sector alliance, which aims to support the transformation of clinical development to be more efficient and patient-centric.4 The EU-PEARL project used a multi-stakeholder, mixed methods approach focused on developing the concept of an Integrated Research Platform (IRP), which provides a framework centred on a platform trial. IRPs are designed to be a sustainable and scalable global solution, consisting of an infrastructure, workflows, and guidance on how to meet complex regulatory, ethical, legal, statistical, and data requirements. The IRP framework was consolidated both in a disease-agnostic way (applicable to any disease) and evaluated in four diverse indications with significant unmet medical needs, namely major depressive disorder (MDD), tuberculosis (TB), non-alcoholic steatohepatitis (NASH) and neurofibromatosis (NF). The four indications have been chosen by IMI in its initial call. Lessons from COVID-19 platforms were also incorporated into the EU-PEARL IRP framework. The main outputs of EU-PEARL can be accessed in Supplementary Table S1.
This narrative review provides a summary of the main lessons drawn from EU-PEARL to facilitate broader adoption of platform trials by supporting drug developers, patients and their communities, investigators and regulators involved in planning and design of platform trial.
Writing approach of this narrative review
We followed a semi-structured procedure to write this paper. First, a writing committee was set up. Members of the writing committee included the project leader (AVD) and the coordinator (JG), as well as work package leaders, therefore representing the scientific committee. Then, we held weekly meetings for 10 weeks. The first two meetings were devoted to agreeing on the 10 messages we wanted to convey, how to phrase them as a subheading, and the main bullet points that should be included in each of the ten lessons. We also assigned each of the article sections (the introduction and each of the ten points) to the writing committee members. We worked offline in the assigned sections, and the following three sessions (3, 4 and 5) were used to discuss and agree on the changes to be made in each section. We reached full consensus in each point discussed. The following two sessions were devoted to deciding the shape and contents of the tables and figures. Sessions 8 and 9 were focused on the discussion and concluding remarks. Then two of authors (DM and JMP) worked on copyediting, referencing and formatting. Finally, during the last session we discussed minor formatting aspects and annexes.
Integrated research platforms are centred on a platform trial master protocol
Platform trials are one type of master protocols, i.e., one overarching protocol designed to answer multiple questions within the same overall trial structure. There are different types of master protocols (MP): basket, umbrella, and platform studies.5 According to Woodcock and LaVange5 basket trials study a single therapy in multiple diseases or disease subtypes, umbrella trials study multiple therapies in single disease, and platform trials allow to study multiple therapies in a perpetual way (therapies can leave and enter the trial when it is already running). The main strength of a MP and methodology is that it centres around a disease and enables continuous learning. This methodology has significant advantages for patients. The integration of multiple potential new treatments in one trial offers the possibility to share control data. Which in turn means that there is a lower likelihood for patients to be allocated to the standard of care or control arm. In addition, platform trials are seldom designed for a particular duration but to serve as a potentially perpetual structure over which sequential trials can be developed.
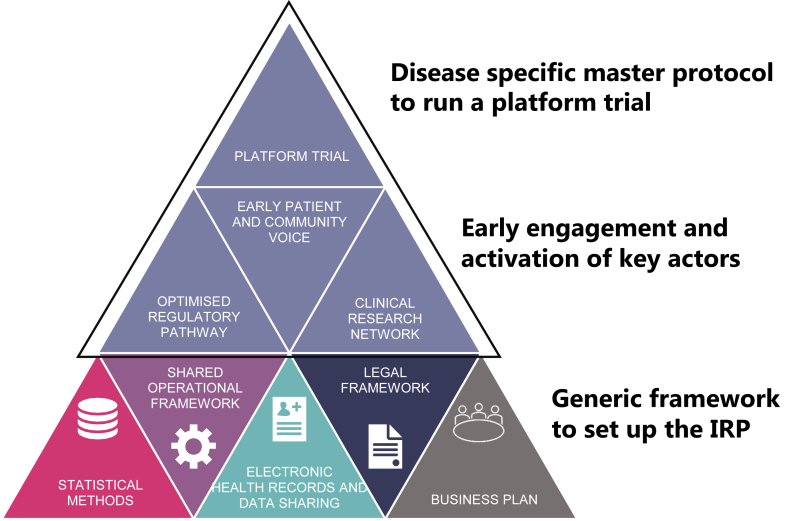
In order to picture the concept and uses of an IRP it is necessary to know its basic elements and their interactions (Fig. 2). IRPs support the development of platform trials through a sustainable infrastructure including well characterised patient cohorts, connected investigational sites and a data infrastructure which allows data to be pulled from various sources such as electronic health records (EHR). The EU-PEARL consortium defined an IRP as a common enabling framework to conduct platform trials, encompassing a MP, hospitals’ infrastructure and federated patient data while ensuring an optimised regulatory pathway, to accommodate the testing of multi-sourced interventions.
Fig. 2.
Elements of Integrated Research Platforms for drug development in the context of EU-PEARL. Three different layers describe the foundation which should be put in place and are the basis to conduct a platform trial for a certain disease. Layer one encompasses the generic framework to develop a platform trial, which are the same in any Integrated Research Platform (IRP) or disease. Layer two indicates the stakeholders which should be engaged in the building of the IRP very early but are disease specific. On top is the disease specific master protocol for the platform trial, e.g., in EU-PEARL both generic templates and (illustrated by the shared operational framework) and disease specific templates (top circle) have been developed for major depressive disorder (MDD), tuberculosis (TB), non-alcoholic steatohepatitis (NASH), and neurofibromatosis (NF).
Platform trials offer valuable tools to overcome the barriers for multistakeholder collaboration in clinical drug development.6 Besides the methodological advantages of platform trials such as the increased efficiency in cross-arm comparisons,7 which lead to reduced economic costs compared to standalone clinical trials, in EU-PEARL we used platform trials to underpin patient-centric aspects other than those intrinsic to the methodological characteristics of MP by incorporating the insight and needs of patients and their communities from early stages and all along the duration of the platform trial. Another benefit of platform trials is that they provide a re-useable clinical trial infrastructure that enhances operational aspects such as reducing accrual rates or simplifying complex logistics through a centralised governance, all of which is optimised by a tailored legal and regulatory pathway.8,9
Despite their advantages, platform trials cannot be universally applied to all diseases at any time, place or phase of clinical development. Similarly, though valuable tools to boost platform trials, IRPs are not enough by themselves to make a platform trial successful. There are various elements that need to be present when considering implementing a platform trial in certain diseases. A platform trial can be perfectly designed and planned yet only be successfully implemented at the correct time in the appropriate place/s with the correct stakeholders involved in a certain population. Table 1 shows the key aspects to be considered in a particular disease to assess whether the field can benefit from setting up platform trials to accelerate drug development in the short term.
Table 1.
Ingredients for a successful Integrated Research Platform and maturity of the Disease-specific IRPs developed during EU-PEARL.
| Ingredient | Description | MDD | TB | NASH | NF |
|---|---|---|---|---|---|
| Pipeline | Number of assets under development in pre-clinical and clinical phases. Existence of regulatory-approved drug treatment. | Partly, challenges in the pipeline for new assets. But approved treatment available. | Yes, both pharma and academic pipelines and consortia underway. Approved treatment pipelines and consortia. | Yes, dozens of compounds both in preclinical and clinical phases, but not FDA/EMA-approved pharmacological treatment. | Partly, treatment used to be mainly surgical until recent years. Some drugs available, particularly for NF1. |
| Network of sites | Clinical sites that are used to collaborating in clinical trials in the disease field | Partly, Single country level but not at European level. |
Yes, TBnet, RESIST-TB, UNITE4TB |
Partly, existing in the US (i.e., Pinnacle Clinical Research). | No, not for NF specifically but for rare diseases. ERICA, ERN-RND and EURORDIS |
| Endpoints | Consensus on endpoints as best indicators of the natural history of the disease and response to treatment | No, consensus on the definition and phenotypes (including biomarkers) of partially and totally-resistant MDD. | No, lack of efficacious and easily available (particularly in low-resource settings) treatment monitoring tools and tailored endpoints for treatment-resistant TB. | No, there is no agreement on non-invasive biomarkers as primary endpoints. FDA/EMA require histologic endpoints for non-cirrhotic population. | No |
| Strongly developed patient advocacy | Well-organised patients' organisations that are cognisant of the issues derived from lack of (adequate) treatments and advocate for clinical research in areas with unmet needs, particularly in underserved communities | No, not particularly. | Yes, build on the CABs from HIV Multiple efforts: TB Alliance, Unite4TB. |
No, no organised community in Europe. More so in the US (e.g., Fatty Liver Foundation). | Yes, several organisations e.g., CTF |
| Significant trial recruitment challenges | Slow or unpredictable trial enrollment driving the need to find alternatives | Yes, due to the difficulties associated to the lack of clear definitions and phenotypes of treatment resistant MDD. | Yes, clinical trial capability needs to be developed in countries most affected by the disease | No, not challenging to recruit in countries affected most | Yes, rare disease and vulnerable population such as children; multiple manifestations |
Footnote: CABs: community advisory boards; CTF: Children Tumor Foundation; EMA: European Medicines Agency; ERICA: European Rare Disease Research Coordination and Support Action consortium; ERN-RND: European Rare Disease Network; EURORDIS: European Organisation for Rare Diseases; FDA: US Food and Drug Administration; MDD: major depressive disorder; NASH: non-alcoholic steatohepatitis; NF: neurofibromatosis; RESIST-TB: https://www.resisttb.org/; TB: tuberculosis; TBnet: https://www.tbnet.eu/; UNITED4TB: https://www.unite4tb.org/; US: United States of America.
Early consultation with regulators can yield an optimised regulatory pathway
The COVID-19 pandemic resulted in societal pressure to expedite development of therapeutic options and resulted in an acceleration of adoption of clinical trial innovations, including platform trial designs. To ensure acceptability of the data of complex innovative trial designs, early interaction with regulators is a critical step in the design process. Two routes for interaction have proven to be an effective way for early engagement and consultation: the European Medicines Agency (EMA) Innovation Task Force (ITF) and the US Food and Drug Administration (FDA) Critical Path Innovation Meeting (CPIM). These discussion meetings are a forum for early dialogue on innovative approaches and allow an informal discussion about the design. The MDD IRP and NASH IRP clinical teams each discussed their critical design elements with the ITF, whereas the NASH team also consulted with CPIM. FDA and EMA also offer opportunities for further consultation and scientific advice as protocol development progresses and investigational medicinal products have been identified.
Besides the scientific consultations, the regulatory submission package and structuring for MP need careful upfront considerations and planning. The EU-Clinical Trials Information System (CTIS) was implemented as of January 2022.10 It is likely that the design features of this system were based on the most prevalent and traditional protocols. At this moment there is insufficient experience to understand whether the system accommodates the modular and adaptive platform trial. It is recommended to discuss the structure of the platform trial and submission package upfront. The EU-PEARL team approached the Heads of Medicines Agencies’ Clinical Trials Coordination Group and received consolidated and early feedback on the generic templates, demonstrating the interest in finding opportunities to improve the efficiencies for both reviewees and reviewers.
Platform trials enable patient-centric approaches from their inception
The European Patients' Academy (EUPATI) roadmap for patient engagement defines multiple points at which patients can actively participate and play a constructive role in the drug development process: from the definition of research priorities and patient-relevant endpoints to the optimisation of the clinical trial design and conduct, and even the dissemination of the research conducted.11 Also, trial participants’ experience can be used to inform regulatory decisions.12 The involvement of participants early into the design, planning and execution of clinical trials is critical not only for trial success but also for the improvement of the patient experience. Platform trials are not an exception and involving patients early in their design not only ensures that new therapies address the real needs of patients, but also improves recruitment rates since patients are more likely to enrol in trials if they see clear benefits of participation.12,13 Platform trials are particular patient centric as they allow to find a (best) treatment for patients rather to study a single intervention. Especially some adaptive design elements might be of particular interest for patients, e.g., early futility stopping of non-promising arms or use of response adaptive methods to increase the allocation ratio of more promising treatments.
Training plays a vital role in extending the practice of patient and community engagement in platform trials. On the one hand, patients and patient advocates need new skills and knowledge to play a full part in medicines development and, on the other hand, healthcare professionals, regulators, health technology assessment bodies, industry, and others need to be educated in good patient and community engagement practices.12
Since the collaborative platform trial field is somewhat new and complex, and there is still a relative unfamiliarity among the patient and patient advocacy community, EU-PEARL developed educational materials to facilitate greater understanding. These materials are hosted in the Patient Engagement Synapse Platform (see Supplementary Table S1), constituting the first European platform for Patient and Community Engagement in Platform Trials (PaCEPT).14
Clinical research networks provide the infrastructure on which the IRP can be established
The selection of investigational sites is a critical step in any clinical trial start up. The quality of the sites and staff determine the data quality and integrity of the trial outcomes to a great extent. Significant time and resources are spent identifying, assessing and selecting sites. Site staff and investigators need to be well trained on protocol and protocol procedures.15 For complex trials, especially if they are perpetual, ongoing and constant training is even more important, e.g., to account for inevitable staff turnover in long running platform trials. Clinical Research Networks (CRN) in which trialists work collaboratively enable large-scale, high-quality clinical trials to be run efficiently. This includes the involvement of clinical trial units to enable an effective design and delivery of such complex trials. Although the benefits of CRN are largely known, establishing a CRN can be complex. There are many factors for clinicians and researchers to consider.16 The EU-PEARL teams have identified building CRNs as a foundational step in the transformation towards IRPs, and therefore a wealth of working materials have been developed to identify the quality criteria of sites to join a platform trial, both in general and in particular for the four studied diseases (see Supplementary Table S1).
Improving efficiency through adaptive clinical trial design
Platform trials allow for various options of design and analysis features, e.g., adding/dropping of treatment arms, change of allocation ratios, population enrichment and sharing of data. Therefore, power and sample size calculations become more difficult compared to standard 2-arm randomised controlled clinical trials (RCTs). Furthermore, the performance of platform trial is characterised by multiple performance indicators, as the overall power, the power at sub-study level, or the time until inefficient treatment arms are dropped due to futility or promising treatments are detected.
Assessing the set of important operating characteristics of platform trial requires clinical trial simulations17 (see Table 2) to enable trial design discussion among all stakeholders involved. To assess the impact of different design features and adaptation strategies, one should start with a basic design and add advanced design features step-by-step. The basic design acts as benchmark to assess if the efficiency of the design is increased by these additional features. Based on the feedback of the trial stakeholders, several iterations might be needed until an optimal design addressing the various needs is found. Different aspects and risk minimisation can be of interest, e.g., the type 1 error rate on platform or sub-study level might be of regulatory interest,18 the average and maximum sample sizes required are relevant for budgeting and portfolio optimisation.
Table 2.
Statistical approaches and methods for the design of platform trials.
| Topic | Short description |
|---|---|
| Clinical trial simulations |
|
| Vanilla-sprinkle concept |
|
| Platform simulation software |
|
| Multiplicity |
|
| Sharing of data |
|
| Concurrent and non-concurrent control data |
|
| Allocation ratio |
|
| Adaptations |
|
Platform trials offer increased statistical efficiency compared to separate clinical trials. Importantly shared control groups reduce the number of required trial participants per tested intervention. An important question is whether to use data only from concurrent or also from non-concurrent controls for treatment-control comparisons. Non-concurrent controls are trial participants, allocated to the control group, who were recruited before a specific experimental treatment has entered the platform trial. Using such non-concurrent controls for treatment control comparisons can substantially increase the statistical power. However, potential trends affecting response over time, leading to biased treatment effect estimates and statistical tests are a matter of concern. In platform trials these effects can be estimated and adjusted for such time trends using either Bayesian or frequentist model-based approaches.19,20 However, these approaches rely on specific model assumptions, as homogeneous time trends across treatment arms. Therefore, the use of non-concurrent controls remains controversial particularly from a regulatory perspective.21,22 Still, in specific experimental settings, like rare diseases, or paediatric trials, non-concurrent controls can be a more reliable alternative to historic controls as they are recruited within the same trial infrastructure. Finally, the decision to use non-concurrent controls relies on the expected bias-variance trade-off and the anticipated risk of time trends.
Current guidance23 requires control of the studywise error rate for all primary hypotheses defining study success. However, for platform trials, it is now commonly understood that no multiplicity adjustment is required across independent sub-studies. By contrast, multiplicity adjustment is still required within a sub-study for confirmatory inference on multiple endpoints, doses of the same drug, subgroups, or interim analyses. In addition, from a public health perspective, quantifying the risk of false positive conclusions at the platform level is essential and can be achieved with tailored, so called online multiple testing procedures.24,25
Master protocols provide the framework for reusable clinical research infrastructures
In traditional trials there is usually a single study protocol with accompanying documents. When comparing a single experimental treatment to a control the structure of such a document is quite standard. The use of templates to develop regulatory documents is a standard practice and is well accepted by health authorities, industry, and academia. For clinical studies, the use of a protocol template provides a common structure which benefits sponsors, investigators, sites, vendors, and regulators by providing consistency, clarity, and ease of use.
When developing the initial study protocol for a platform trial it is usually not known which and when new treatment arms might enter the platform in the future. Simply updating a single study protocol by amendments and adding additional sections for new arms can be burdensome and the unique needs of platform trials are not foreseen by standard protocol templates. Such a document would become exceedingly long, and when looking for information about a certain ISA, one would have to navigate through sections which are no longer relevant, e.g., for treatments related to ISAs which have been already closed.26 A cover letter overviewing which information can be found and where is useful to guide regulators and ethics committee reviewers is also an important part of the platform trial documentation. Common information such as the information on the control arm will be put in the protocol, whereas the more specific information related to an experimental treatment will be placed in the respective ISA. EU-PEARL developed five templates for trialists: a protocol template, an ISA template, a template for the statistical analysis plan, a data monitoring committee charter template adjusted for the purpose of conducting a platform trial, as well as a guidance for supplementary information to the clinical trial regulator (CTR) cover letter.26
The core of the study design is articulated in the protocol. From an operational perspective, this makes operational standardisation and re-use of the full clinical trial infrastructure possible and efficient. Platform trials need excellent planning to optimise the full potential of efficiencies and maintain oversight. In collaborative platform trials there is additional focus on aligning the needs and expectations of all participating organisations. To help teams plan a platform trial and prepare for challenges, we have compiled a spreadsheet of topics to consider for each operational sub-team in the early planning phase of a platform trial (Supplementary Table S1).
Electronic health records can be leveraged to assess trial feasibility and site selection
A thorough trial feasibility assessment is the initial stage of successful execution. One important aspect is the upfront determination of how many patients will potentially be eligible to take part in the study, taking into account the specific inclusion and exclusion criteria of the study. Regional and local differences can eventually determine where the study should be executed. The availability of potential trial participants which fit the protocol criteria informs the protocol development process from the early stages, including decisions regarding the inclusion of specific study arms or subprotocols. The EU-PEARL consortium envisions the IRP as a learning system, utilising care data for research. To achieve this the site network will need to be able to use the data routinely collect in the EHR to assess patient eligibility for the study. A federated interoperable data model that could be leveraged without the need to make additional investments is the Observational Medical Outcome Partnership (OMOP) Common Data Model (CDM) format. During EU-PEARL, the Observational Health Data Science and Informatics (OHDSI) Atlas tool was used to assess OMOP in three use cases (MDD, NASH and NF-1), and also a general disease-agnostic guidance was created based upon these exploratory assessments. To assess the maturity, quality, and suitability of hospital EHR systems to set up federated networks, the EU-PEARL team concluded that more work needs to be done to create awareness about the opportunities these approaches can offer to accelerate research. EU-PEARL developed a set of guidance tools to leverage such opportunities.
Legal frameworks enable effective collaboration across the diverse parties participating in IRPs
Beyond the scientific and operational challenges to conduct a platform trial, another challenge cannot be underestimated: collaboration. One of the most important challenges in the drug development enterprise for platform trials is how different companies can partner on a common platform, with common controls, and how issues like intellectual property and data sharing occur, which remains largely unsolved. The increasingly larger use of platform trials as the preferred approach in drug development will bring solutions and toolkits to overcome such issues. In the meantime, establishing a robust legal framework to support the development of platform trials become key for IRPs success.
By design, IRPs enable multiple organisations to derive the benefits of a shared platform trial infrastructure; however, different stakeholders may have legitimate conflicting interests. For example, owners of investigational assets in the trial desiring to seek marketing authorisation may need to carefully plan dissemination of trial results to maintain trial integrity, while investigators may have an interest to publish emerging clinical insights. Upfront planning is required to reconcile the interests of IRP stakeholders, as well as to ensure all statutory requirements are met.
In a traditional company-sponsored clinical trial, the sponsor and owner of the investigational medicinal product (IMP) are the same organisation. In a platform trial, the sponsor and IMP owner(s) may be distinct to ensure that confidential information is not disseminated. The sponsor could be either a non-profit organisation or the clinical trial unit of an academic hospital. For example, the Pancreatic Cancer Action Network is the sponsor of the Precision Promise platform trial,27 whereas the REMAP-CAP platform trial28 has a regional sponsorship structure with University Medical Center Utrecht in Europe, Monash University in Australia, the Medical Research Institute of New Zealand in New Zealand, and Unity Health Toronto in Canada.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6 (R1) Good Clinical Practice and national regulations such as Clinical Trials Regulation (Regulation EU No 536/2014)15 prescribe responsibilities to the sponsor organisation. While some activities such as clinical trial monitoring may be contracted or delegated to a contract research organization (CRO), overall accountability as a sponsor cannot be delegated. Prospective sponsor organisations should carefully assess their readiness to meet these requirements.
IMP owners invest in clinical development to seek marketing authorisation and commercialisation of their innovation. Intellectual Property (IP) rights related to the IMP will be an obvious consideration; however, IP may also arise from elements of the platform trial which are shared such as insights arising from a common control arm or biological samples not involving IMP.
IRP stakeholders may also have legitimate conflicting interests regarding how and when data can be accessed or published: maintaining platform trial integrity to avoid bias may require temporarily embargoing dissemination of information while another stakeholder seeks early publication.
Collaboration Agreements between the sponsor and IMP owners can be utilised to align interests and facilitate a successful collaboration. These agreements may describe governance structure, selection of CRO, decision to add new trial arms, IP ownership and data access rights, safety reporting and liability, management of audits and inspections, and termination. The sponsor must also negotiate Clinical Trial Agreements (CTA) with institutions and investigators participating in execution of the clinical trial. In addition to common commercial terms, the CTA may address: (i) term and termination; (ii) data privacy; (iii) confidentiality; (iv) payment; (v) notification; (vi) independent contractors; (vii) sub-contracting; (viii) governing law and jurisdiction; (ix) agreement modifications; (x) assignment; (xi) waiver; (xii) survival; (xiii) warranty; (xiv) authorised representatives; (xv) conflict between agreement and annexes.
To be sustainable, platform trials require a business plan which creates value for all trial stakeholders
The dominant business model for drug development requires each organisation interested in executing a clinical trial to invest in setting up the trial infrastructure, utilising and maintaining the infrastructure during the trial, and dismantling the infrastructure at the conclusion of the trial. This model is both inefficient and underserves patients when viewed at a longer time horizon than an individual clinical trial.
To address these challenges, a platform trial provides a reusable trial infrastructure accessible to multiple organisations and patients seeking access to multiple research opportunities. The business plan enabling the platform trial must deliver on this value proposition with a funding mechanism to support establishing the platform trial and sustaining it as new interventions to be tested continuously enter and exit the platform. Intervention testing in steady-stage may be funded in a fee-for-service model from intervention owners; however, startup costs require alternative funding mechanisms or sources (e.g., philanthropy, government, or a consortium of drug developers) to be a trusted and an attractive development pathway for asset owners. Perpetual platform trials require constant efforts to ensure sufficient resources to address the perpetual funding requirements.
Academic medical centres are well positioned to lead platform trials due to scientific expertise required to guide the research infrastructure and their continuous engagement with patients who may be interested in participating in clinical research. Indeed, 89% of platform trials until mid-2022 had an academic sponsor.3 Leading a platform trial as the trial sponsor changes the position of the academic medical centre within the value chain of clinical research. The business plan enables the academic medical centre to transition from operating as a contracted investigational site in a series of clinical trials to become a sustainable business offering access to its research infrastructure and optimised intervention evaluation services.
Platform trials and IRPs must address key gaps in drug development
After considering the prior aspects, EU-PEARL proposed phase II designs for the four diseases studied to evaluate their applicability to overcoming key drug development challenges in a broad range of diseases. These designs screen therapeutic interventions for promising therapies which could be confirmed as safe and effective in separate phase 3 studies. Amongst the four diseases considered, three (i.e., MDD, TB and NASH) are global public threats, whereas NF is a rare disease.
The work on MDD29 was focused on a frequent form of the disease, namely treatment-resistant MDD. A main caveat when designing clinical trials for MDD relies on the difficulty to establish clear phenotypes and response to treatment is a particularly challenging feature. Defining and collecting objective, quantitative data in a homogenous way on treatment resistance, including partial resistance, has posed serious challenges to drug development in MDD.30 In the case of TB, this showcased a very prevalent and lethal disease, considered as a neglected tropical disease for its particularly high burden in low-income countries, which do have available therapies, but these require very long durations of therapy, which is frequently associated with secondary effects and development of antimicrobial resistance.31 In addition, the efficacy endpoints used as of today in clinical development and the treatment monitoring techniques are far from optimal.32
NASH has several factors which indicate that a platform trial approach could be an efficient clinical development approach.3 NASH is a highly prevalent disease, so recruiting patients is not generally a constraint, while the logistic and statistical constrains associated to the use of liver biopsy-dependent endpoint entails very large sample sizes, thus leading to logistically complex and costly trials.33 Moreover, there are no FDA/EMA-approved drugs for NASH. Thus, there is a delicate balance in deciding whether to try to change the landscape to a more collaborative one, with the interest of companies in sharing data being one of the key elements in the decision.34
NF is a rare disease with various forms of clinical presentation which commonly has an early onset, often during childhood. Drug development in this area is characterised by large community involvement, however challenging due to the difficulties to recruit patients and the long-life necessity of treatments. Most existing trials focused on NF1, so there is an unmet need in NF2/schwannomatosis.35 Moreover, most trials are single-country and in the case of NF2, single-arm.36 Already successful experiences of collaborative platform trials in pediatrics exist, which allow to overcome/mitigate some of the barriers inherent to clinical research in pediatric patients, which are particularly challenging in the field of rare diseases, remarkably small sample sizes,37 as well as fostering international collaboration.
Future directions and remaining challenges
Readiness of non-traditional sponsors
Recent successful experiences with the HEALEY ALS38 (US), RECOVERY39 (UK), EU-RESPONSE40 (EU), and REMAP-CAP28 (Global, including European Union, EU) platform trials2 have highlighted the role of academic hospitals as the leaders and regulatory sponsors of platform trials. However, this approach has been more common in the US and UK. Within Europe, an academic hospital faces the additional burden of building capacity to lead a multi-national clinical trial, including navigating clinical trial approval in each EU country. While this challenge can be overcome, as demonstrated by REMAP-CAP, additional administrative and regulatory affairs resources may be needed to make trials available at the scale of the European population. European funding support could strengthen this capacity for specific diseases; however, a more transformational change of the clinical trial approval process could be more effective to position academic hospitals, which are centres of excellence within Europe, to have led European clinical research beyond national borders.
The objectives of a certain platform trial may differ across stakeholder groups. Consequently, the type of sponsorship might potentially affect relevant aspects of the platform trial set up such as registration, health technology assessment, or how to adapt the protocols according to stakeholders’ priorities. Experience overseeing multi-national clinical trial to fulfil all regulatory sponsor duties is a critical requirement for any potential sponsor of a platform trial, as well as handling multi-source funding for the set-up of a multinational, multifaceted clinical trial infrastructure. As highlighted by one reviewer, a challenge of perpetual platform trials is many of these activities have to be performed simultaneously, e.g., finding new interventions, designing new ISAs, regulatory registrations and initiation of new arms, performing interim analysis or preparing results of closed arms for reporting. The simultaneous execution of these tasks may pose an unanticipated challenge for teams lacking prior experience with platform trials.
Data sharing challenges
In addition to operational efficiencies from a shared platform trial infrastructure, inferential efficiencies from fewer required patients or patients allocated to control while maintaining statistical power are also possible; however, enacting some of these potentialities may be limited by the need to share proprietary clinical data with competitors. Furthermore, while regulatory guidance on historic controls and adjustment for multiplicity is available,41 specific guidance for platform trials is still limited. Currently EMA is developing a guidance document on platform trials42 discussing requirements for Type I error control at the level of a platform trial and the individual sub-studies, and risks (due to the adaptive nature of platform trials and potential time trends) with regard to the interpretability of trial results.43 An important topic, also to be addressed in the guidance document,42 is the communication of trial results and related data sharing. Here two conflicting objectives arise. On the one hand, the need for transparency, with trial results being published when the trial or in this case of platform trial a treatment arm is completed; on the other hand, the requirement to preserve the integrity of the still ongoing sub-studies. For a platform trial, which potentially runs perpetually, publication of results when the platform is completed (which may be many years after a specific sub-study was completed) would not be acceptable. Rather, to maintain the publication standards of drug development programs based on separate trials, results would be expected to be published when the sub-study is completed. However, publication of results from sub-studies may affect the integrity of still ongoing arms. This holds because part of the published data from the control arms will also be used in the analysis of these still ongoing arms. This is of special concern if non-concurrent controls are used in the analysis. Besides the statistical aspects of data sharing and publication of trial data, especially in multi-sponsor clinical trials with commercial sponsors, sharing of data may also be difficult because of competition. For example, the statistical time trend adjustments discussed above depend on time trend estimates obtained from data of all experimental treatment groups and there needs to be an agreement between sponsors to allow to use this data in the analysis. A further potential of platform trials requiring data sharing is the opportunity to perform treatment comparisons across arms. While this may not be of interest to sponsors aiming at approval of new medicines, this information may be highly relevant for patients and payers. However, to allow for such comparisons based on individual patient data, corresponding data sharing agreements need to be in place.44
Regulatory requirements lag the regulatory vision
European policy makers can steer towards incentivising the build of IRP infrastructure for specific areas where the platform trial designs are most likely to justify the approach. The EU-PEARL demonstrators indicate that there are a few elements which are predictive. Although during the COVID-19 pandemic the regulators and policy makers demonstrated agility in expediting review processes and made the impossible possible, the process of evolving the regulation of clinical trials in Europe is lengthy. The recent evolution from the Clinical Trial Directive to the Clinical Trial Regulation introduced important changes, and many benefits, including greater certainty of clinical trial startup timeline across EU member states. However, CTR—ratified by the European Parliament on April 16, 2014, and effective on January 31, 2022,45 did not foresee the multi-compound, multi-drug developer clinical trial characteristic of platform trials, nor their expanded use during the COVID-19 pandemic.
Achieving EMA's Accelerating Clinical Trials in Europe (ACT EU) will require focused effort to overcome procedural and administrative obstacles introduced by CTR, such as limitations of number of protocol amendments and challenges segregating confidential information from different asset owners. EMA's paper on platform trial39 is an important step to facilitate platform trial implementation with great clarity on terminology, appropriate methodologies and use of platform trials for confirmatory studies.
It is possible to think about incentivising IRPs which host collaborative platform trials for areas in a high unmet need to tip the scale on the efficiency point of view. These incentives should address the funding structure across borders, the sponsorship framework and agreed regulatory pathway and cover more than the traditional 5-year horizon. The latter is dependent on how well the field is developed in terms of clinical research capabilities and scientific regulatory rigor and alignment on endpoints and design elements.
Conclusions
The implementation of platform trials through IRP holds great promise to boost drug development in a patient-centric and sustainable manner for a variety of diseases. Master protocols for platform trials, clinical research networks connected through federated patient data, early consultation with health authorities, and an optimised legal and regulatory pathway are key elements of such IRP. Shared infrastructure offers great potential for a more sustainable and collaborative effort between patients, investigators, regulators, academia and pharmaceutical drug developers. Moreover, innovative trial designs and statistical approaches allow for enhanced efficiency. However, several barriers should be overcome to bring the advantages of platform trials to the patients and their communities at a global scale and in a widespread manner for all potential medical indications. Platform trial designs are here to stay, it is just a matter of how fast we end up embracing them.
Contributors
Conceptualisation and design: FK, CS, SRN, DM, NM, JMP, MP; Drafting of the article: FK, CS, SRN, DM, JMP, MP; Collection of data: FK, CS, SRN, DM, NM, JMP, MP; FK, CS, DM and JMP accessed and verified the underlying data; Tables and figures: FK, CS, SRN, MP; Coordination: CS, DM, NM, JMP; Review and editing: FK, CS, SRN, DM, NM, AVD, JG, JMP, MP; Supervision: AVD, JG, MP; Final approval of the manuscript: FK, CS, SRN, DM, NM, AVD, JG, JMP, MP.
Declaration of interests
CS and AvD are former employees of Janssen, Johnson & Johnson, whereas DM is still currently employed. JMP reports having received consulting fees from Boehringer Ingelheim, MSD and Novo Nordisk; speaking fees from Gilead, Intercept, Novo Nordisk, Novartis and FLS; travel expenses from Gilead, Rubió, Pfizer, Astellas, MSD, CUBICIN, and Novo Nordisk; educational and research support from Madrigal, Gilead, Pfizer, Astellas, Accelerate, Novartis, Abbvie, ViiV, Echosens, Siemens, and MSD; and research funds from European Commission/EFPIA IMI2 853966-2, IMI2 777377, H2020 847989, ISCIII PI19/01898 and PI22/01770, Ajuntament de Barcelona/La Caixa, and Fundació Institut de Bioenginyeria de Catalunya (IBEC). MP reports grants to their institution from Merck KGaA, Baxter, Almirall, and Fresenius, and also reports Steering Committee Membership within the International Society of Biopharmaceutical Statistics. Other authors: no potential conflicts to disclose.
Acknowledgements
EU-PEARL has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 853966. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. This information represents only the authors' views. The Joint Undertaking is not responsible for any use that may be made of the information it contains. The authors wish to acknowledge to all the people that have participated or collaborated at any point to achieve the objectives of the EU-PEARL, which will largely benefit patients and trial participants. The funders had no role in the following: design, data collection, data analyses, interpretation, or writing of the report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102384.
Contributor Information
Juan M. Pericàs, Email: juanmanuel.pericas@vallhebron.cat.
EU-PEARL Consortium:
Adrian Sánchez-Montalva, Ana Belén Estevez, Àlex Sánchez, Anna Sanjuan, Elena Sena, Emma Granados, Esther Arévalo de Andrés, Fátima Nuñez, Gara Arteaga, Gabriela Perez Fuentes Ruiz, Guillermo Fernández, Jesus Rivera-Esteban, Joan Comella, Josep Antoni Ramos-Quiroga, Joan Genescà, Juan Espinosa, Juan Manuel Pericàs, Lada Murcia, Lucinda Cash-Gibson, Maria de Valles Silvosa, María Fernanda Barroso de Sousa, Olga Sánchez-Maroto Carrizo, Pol Ibañez-Jiménez, Salvador Augustin, Santiago Perez-Hoyos, Sarai Rodríguez-Navarro, Sergio Muñoz-Martínez, Silvia Serres, Susana Kalko, Amelie Michon, Anton Ussi, Ben Lydall, Edwin van de Ketterij, Ignacio Quiles, Tamara Carapina, Constantin Kumaus, Dariga Ramazanova, Elias Laurin Meyer, Franz Koenig, Marta Bofill Roig, Martin Brunner, Martin Posch, Pavla Krotka, Sonja Zehetmayer, Charlotte Carton, Eric Legius, Amina Begum, Carmine Pariante, Courtney Worrell, Giulia Lombardo, Luca Sforzini, Mollie Brown, Nancy Gullet, Nare Amasi-Hartoonian, Rosalie Ferner, Melisa Kose, Andrea Spitaleri, Arash Ghodousi, Clelia Di Serio, Daniela Cirillo, Federica Cugnata, Francesca Saluzzo, Francesco Benedetti, Maria Giovanna Scarale, Michela Zini, Paola Maria Rancoita, Riccardo Alagna, Sara Poletti, Britt Dhaenens, Johan Van Der Lei, Jurriaan de Steenwinkel, Maxim Moinat, Rianne Oostenbrink, Witte Hoogendijk, Michael Hölscher, Norbert Heinrich, Christian Otte, Cornelia Potratz, Dario Zocholl, Eugenia Kulakova, Frank Tacke, Jelena Brasanac, Jonas Leubner, Maja Krajewska, Michaela Maria Freitag, Stefan Gold, Thomas Zoller, Woo Ri Chae, Christel Daniel, Leila Kara, Morgan Vaterkowski, Nicolas Griffon, Pierre Wolkenstein, Raluca Pais, Vlad Ratziu, David Voets, Christophe Maes, Dipak Kalra, Geert Thienpoint, Jens Deckerck, Nathan Lea, Peter Singleton, Kert Viele, Peter Jacko, Scott Berry, Tom Parke, Amelie Michon, Burç Aydin, Christine Kubiak, Jacques Demotes, Keiko Ueda, Mihaela Matei, Sergio Contrino, Claas Röhl, Estefania Cordero, Fiona Greenhalgh, Hannes Jarke, Juliana Angelova, Mathieu Boudes, Stephan Dressler, Valentina Strammiello, Quentin Anstee, Iñaki Gutierrez-Ibarluzea, Maximilian Otte, Natalie Heimbach, Benjamin Hofner, Cora Burgwinkel, Hue Kaestel, Katharina Hees, Quynh Nguyen, Daniel Prieto-Alhambra, Eng Hooi (Cheryl) Tan, Mario Raviglione, Pierpaolo de Colombani, Simone Villa, Eduard Maron, Gareth Evans, Adam J. Savitz, Ann Van Dessel, Anna Duca, Anne Kaminski, Bie Wouters, Brandon Porter, Catherine Charron, Cecile Spiertz, Christopher Zizzamia, Daniel Millar, Danny Hasselbaink, David Orr, Divya Kesters, Ellen Hubin, Emma Davies, Eva-Maria Didden, Gabriela Guz, Evelyn Verstraete, Gary Mao, George Capuano, Heddie Martynowicz, Heidi De Smedt, Ingela Larsson, Ines Bruegelmans, Isabelle Coste, Jesus Maria Gonzalez Moreno, Julia Niewczas, Jiajun Xu, Karin Rombouts, Katherine Woo, Kathleen Wuyts, Kathryn Hersh, Khrista Oldenburg, Lingjiao Zhang, Mark Schmidt, Mark Szuch, Marija Todorovic, Maartje Mangelaars, Melissa Grewal, Molli Sandor, Nick Di Prospero, Pamela Van Houten, Pansy Minnick, Polyana Bastos, Robert Patrizi, Salvatore Morello, Severijn De Wilde, Tao Sun, Timothy Kline, Tine de Marez, Tobias Mielke, Tom Reijns, Vanina Popova, Yanina Flossbach, Yevgen Tymofyeyev, Zeger De Groote, Alex Sverdlov, Alexandra Bobirca, Annekatrin Krause, Catalin Bobrica, Daniela Heintz, Dominic Magirr, Ekkehard Glimm, Fabienne Baffert, Federica Castiglione, Franca Caruso, Francesco Patalano, Frank Bretz, Guenter Heimann, Ian Carbarns, Ignacio Rodríguez, Ioana Ratescu, Lisa Hampson, Marcos Pedrosa, Mareile Hark, Peter Mesenbrink, Sabina Hernandez Penna, Sarah Bergues-Lang, Susanne Baltes-Engler, Tasneem Arsiwala, Valeria Jordan Mondragon, Hua Guo, Jose Leite Da Costa, Carl-Fredrik Burman, George Kirk, Anders Aaes-Jørgensen, Jorgen Dirach, Mette Skalshøi Kjær, Alexandra Martin, Diyan Hristov, Florent Rousseaux, Norbert Hittel, Robert Dornheim, Daniel Evans, Nick Sykes, Camille Couvert, Catherine Leuven, Loïc Notelet, Madhavi Gidh-Jain, Mathieu Jouannin, Nadir Ammour, Suzanne Pierre, Volker Haufe, Yingwen Dong, Catherine Dubanchet, Nathalie de Préville, Tania Baltauss, Zhu Jian, Sara Shnider, Tal Bar-El, Annette Bakker, Marco Nievo, Uche Iloeje, Almari Conradie, Ece Auffarrth, Leandra Lombard, Majda Benhayoun, Morounfolu Olugbosi, Stephanie S. Seidel, Berta Gumí, Claudia García Guzmán, Eva Molero, Gisela Pairó, Núria Machin, Raimon Cardelús, Saira Ramasastry, Saskia Pelzer, Andreas Kremer, Erno Lindfors, and Chris Lynch
Appendix A. Supplementary data
References
- 1.Park J.J.H., Siden E., Zoratti M.J., et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20:572. doi: 10.1186/s13063-019-3664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pericàs J.M., Derde L.P.G., Berry S.M., EU-PEARL and REMAP-CAP investigators Platform trials as the way forward in infectious disease' clinical research: the case of coronavirus disease 2019. Clin Microbiol Infect. 2023;29:277–280. doi: 10.1016/j.cmi.2022.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noor N.M., Love S.B., Isaacs T., Kaplan R., Parmar M.K.B., Sydes M.R. Uptake of the multi-arm multi-stage (MAMS) adaptive platform approach: a trial-registry review of late-phase randomised clinical trials. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EU-PEARL. EU-PEARL–innovative patient centric clinical trial platforms. Last accessed, May 6, 2023.
- 5.Woodcock J., LaVange L.M. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 6.Bronson A., Chase M.K., Fisher K., Millar D., Perlmutter J., Richardson N. Mobilizing the clinical trial ecosystem to drive adoption of master protocols. Clin Trials. 2022;19:690–696. doi: 10.1177/17407745221110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang T.J., Luedtke A. Improved efficiency for cross-arm comparisons via platform designs. Biostatistics. 2022 doi: 10.1093/biostatistics/kxac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Love S.B., Cafferty F., Snowdon C., et al. Practical guidance for running late-phase platform protocols for clinical trials: lessons from experienced UK clinical trials units. Trials. 2022;23:757. doi: 10.1186/s13063-022-06680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broglio K., Niewczas J., Wathen K. Who wants to go first? A simulation study of accrual in a stand-alone trial versus starting a platform trial. Contemp Clin Trials. 2022;123 doi: 10.1016/j.cct.2022.107000. [DOI] [PubMed] [Google Scholar]
- 10.CTIS Clinical Trials Information System | European Medicines Agency (europa.eu). Last accessed June 10, 2023.
- 11.Geissler J., Ryll B., di Priolo S.L., Uhlenhopp M. Improving patient involvement in medicines research and development:: a practical roadmap. Ther Innov Regul Sci. 2017;51:612–619. doi: 10.1177/2168479017706405. [DOI] [PubMed] [Google Scholar]
- 12.Drug Information Association Patient engagement action plan. Seven steps to move the needle. Meeting Highlights: DIA Europe. diaglobal.org Last accessed 16 April 2023.
- 13.Geißler J., Isham E., Hickey G., Ballard C., Corbett A., Lubbert C. Patient involvement in clinical trials. Commun Med. 2022;2:94. doi: 10.1038/s43856-022-00156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarke H., Röhl C., Krause A., Boudes M., Greenhalgh F. Patient and community engagement in clinical platform trials. Open Res Europe. 2023;3:74. [Google Scholar]
- 15.European Medicines Agency . ICH Good clinical practice–scientific guideline E6 (R2). ICH E6 (R2) Good clinical practice–scientific guideline. European Medicines Agency (europa.eu); 2021. Last accessed 10 June, 2023. [Google Scholar]
- 16.Nemeh F., Buchbinder R., Hawley C.M., Nelson M.R., Waterkeyn J.G., Reid C.M. Activities supporting the growth of clinical trial networks in Australia. Trials. 2022;23:81. doi: 10.1186/s13063-021-05974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer E.L., Mielke T., Parke T., Jacko P., Koenig F. Simple–a modular tool for simulating complex platform trials. SoftwareX. 2023;23 doi: 10.1016/j.softx.2023.101515. [DOI] [Google Scholar]
- 18.Meyer E.L., Mesenbrink P., Dunger Baldauf C., Glimm E., Li Y., König F., EU-PEARL (EU Patient-cEntric clinicAl tRial pLatforms) Consortium Decision rules for identifying combination therapies in open-entry, randomized controlled platform trials. Pharmaceut Stat. 2022;21(3):671–690. doi: 10.1002/pst.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roig M.B., Krotka P., Burman C.F., et al. On model-based time trend adjustments in platform trials with non-concurrent controls. BMC Med Res Methodol. 2022;22:228. doi: 10.1186/s12874-022-01683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saville B.R., Berry D.A., Berry N.S., Viele K., Berry S.M. The Bayesian time machine: accounting for temporal drift in multi-arm platform trials. Clin Trials. 2022;19:490–501. doi: 10.1177/17407745221112013. [DOI] [PubMed] [Google Scholar]
- 21.Sridhara R., Marchenko O., Jiang Q., et al. Use of nonconcurrent common control in master protocols in oncology trials: report of an American statistical association biopharmaceutical section open forum discussion. Stats Biopharm Res. 2022;14:353–357. [Google Scholar]
- 22.Bofill Roig M., Burgwinkel C., Garczarek U., et al. On the use of non-concurrent controls in platform trials: a scoping review. Trials. 2023;24:408. doi: 10.1186/s13063-023-07398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EMA guidance. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-multiplicity-issues-clinical-trials_en.pdf
- 24.Zehetmayer S., Posch M., Koenig F. Online control of the False Discovery Rate in group-sequential platform trials. Stat Methods Med Res. 2022;31:2470–2485. doi: 10.1177/09622802221129051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson D.S., Wason J.M.S., König F., Posch M., Jaki T. Online error rate control for platform trials. Stat Med. 2023;42:2475–2495. doi: 10.1002/sim.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gidh-Jain M., Parke T., König F., Spiertz C., Mesenbrink P. Developing generic templates to shape the future for conducting integrated research platform trials. Res Square [Preprint] 2023 doi: 10.21203/rs.3.rs-3382348/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pancreatic Cancer Action Network. Precision PromiseSM. PanCAN’s precision promise adaptive clinical trial platform—pancreatic cancer action network. Last accessed June 10, 2023.
- 28.REMAP-CAP trial. REMAP-CAP Trial. remapcap.org Last accessed May 4, 2023.
- 29.Otte C., Gold S.M., Penninx B.W., et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 30.Sforzini L., Worrell C., Kose M., et al. A Delphi-method-based consensus guideline for definition of treatment-resistant depression for clinical trials. Mol Psychiatry. 2022;27:1286–1299. doi: 10.1038/s41380-021-01381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyckendorf J., Georghiou S.B., Frahm N., et al. Tuberculosis treatment monitoring and outcome measures: new interest and new strategies. Clin Microbiol Rev. 2022;35 doi: 10.1128/cmr.00227-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boeree M.J., Lange C., Thwaites G., et al. UNITE4TB: a new consortium for clinical drug and regimen development for TB. Int J Tuberc Lung Dis. 2021;25:886–889. doi: 10.5588/ijtld.21.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pericàs J.M., Di Prospero N.A., Anstee Q.M., et al. The need for more efficient and patient-oriented drug development pathways in NASH-setting the scene for platform trials. Aliment Pharmacol Ther. 2023;57:948–961. doi: 10.1111/apt.17456. [DOI] [PubMed] [Google Scholar]
- 34.Pericàs J.M., Tacke F., Anstee Q.M., et al. Platform trials to overcome major shortcomings of traditional clinical trials in non-alcoholic steatohepatitis? Pros and cons. J Hepatol. 2023;78:442–447. doi: 10.1016/j.jhep.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 35.Acar S., Nieblas-Bedolla E., Armstrong A.E., Hirbe A.C. A systematic review of recent and ongoing clinical trials in patients with the neurofibromatoses. Pediatr Neurol. 2022;134:1–6. doi: 10.1016/j.pediatrneurol.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Dhaenens B.A.E., Ferner R.E., Evans D.G., et al. Lessons learned from drug trials in neurofibromatosis: a systematic review. Eur J Med Genet. 2021;64 doi: 10.1016/j.ejmg.2021.104281. [DOI] [PubMed] [Google Scholar]
- 37.Nelson R.M., Conklin L.S., Komocsar W.J., Chen F., Williamson F., Crandall W.V. The role of master protocols in pediatric drug development. Ther Innov Regul Sci. 2022;56:895–902. doi: 10.1007/s43441-022-00448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.HEALEY ALS Platform Trial https://www.massgeneral.org/neurology/als/research/platform-trial
- 39.The RECOVERY trial. https://www.recoverytrial.net/
- 40.The EU-RESPONSE trial. https://eu-response.eu/
- 41.Burger H.U., Gerlinger C., Harbron C., et al. The use of external controls: to what extent can it currently be recommended? Pharm Stat. 2021;20:1002–1016. doi: 10.1002/pst.2120. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) Concept paper on platform trials (europa.eu); 2022. Concept paper on platform trials, EMA/CHMP/840036/2022. [Google Scholar]
- 43.Collignon O., Schiel A., Burman C.F., Rufibach K., Posch M., Bretz F. Estimands and complex innovative designs. Clin Pharmacol Ther. 2022;112:1183–1190. doi: 10.1002/cpt.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh J.A. Governance of adaptive platform trials. Wellcome Open Res. 2023;8:141. [Google Scholar]
- 45.European Commission . Clinical trials–regulation EU No 536/2014. Clinical trials–regulation EU No 536/2014 (europa.eu) 2021. Last accessed 8 May 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.