Abstract
Objective
Disparities in colorectal cancer (CRC) screening prevalence across United States neighborhoods may reflect social inequities that create barriers to accessing and completing preventive health services. Our objective was to identify whether neighborhood social vulnerability was associated with a change in CRC screening prevalence in Boston neighborhoods during the COVID-19 pandemic.
Methods
Adults ages 50–74 years due for CRC screening who received primary care at one of 35 primary care practices affiliated with Massachusetts General Hospital or Brigham and Women’s Hospital (Boston, MA), 3/1/2020 to 3/1/2022. The Social Vulnerability Index (SVI) is an aggregate measure of neighborhood social factors often used by public health authorities to examine neighborhood susceptibility to many health outcomes.
Results
In 2020, 74.9 % of eligible individuals were up to date with CRC screening and this fell to 67.4 % in 2022 (p < 0.001). In 2020, 36.2 % of eligible patients lived in a neighborhood above the 80th percentile of SVI, consistent with high social vulnerability, while the same value was 35.1 % in 2022. There was no association between the change in screening prevalence and SVI: a decrease of 5.5 % screened in neighborhoods with SVI ≤ 80 compared to a decrease of 3.6 % in neighborhoods with SVI > 80 (p = 0.79).
Conclusions
The COVID-19 pandemic equalized the prevalence of CRC screening across Boston-area neighborhoods despite pre-existing geographic disparities in screening prevalence and SVI. Strategies to ensure equitable participation in CRC screening to promote health equity should be considered to promote equitable pandemic recovery.
Keywords: Colorectal cancer, Screening, Population health, Community health, Disparities, Socioeconomic status
1. Background
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States (US). CRC screening can reduce mortality, yet screening participation varies by patient socioeconomic status, health insurance status, education, gender, and race and ethnicity. (Siegel et al., 2023).
In 2020, Massachusetts had one of the highest CRC screening rates in the US with 70 % of eligible individuals up to date. (Siegel et al., 2023) However, important geographic disparities exist between adjacent Massachusetts counties. (Methodology for the Model-based Small Area Estimates of Cancer Risk Factors and Screening Behaviors. https://sae.cancer.gov/nhis-brfss/methodology.html. Accessed March 26, 2023) Although Boston and its surrounding areas have some of the highest CRC screening rates in Massachusetts, (Methodology for the Model-based Small Area Estimates of Cancer Risk Factors and Screening Behaviors. https://sae.cancer.gov/nhis-brfss/methodology.html. Accessed March 26, 2023) neighborhood screening rates vary by social factors and differences in social vulnerability were magnified by the COVID-19 pandemic. (Bauer et al., 2022, Dooling, 2020).
To elucidate and support the most vulnerable communities during public health emergencies, the Centers for Disease Control developed the Social Vulnerability Index (SVI). (Glance, 2022) SVI is an aggregate measure of 16 US census variables, including socioeconomic status (below 150 % federal poverty level, unemployed, housing cost burden, no high school diploma, no health insurance), household characteristics (aged 65 or older, aged 17 or younger, civilian with a disability, single-parent household, English language proficiency), racial and ethnic minority status, and housing type and transportation. Each census tract in the US is ranked on these social factors such that higher SVI indicates higher social vulnerability.
Previous research has examined the relationship between SVI and CRC screening at the county-level, (Bauer et al., 2022) but the combined effect of the pandemic and SVI on CRC screening in smaller geographic units such as neighborhoods is unknown. During the pandemic, the City of Boston observed profound differences in COVID-19 incidence by neighborhood. (Dooling, 2020) The objective of this study was to identify whether the social vulnerability of neighborhood of residence was associated with a change in CRC screening prevalence in Boston-area neighborhoods during the pandemic.
2. Methods
Electronic health records (EHR) were used to identify individuals who received primary care at one of 35 primary care practices affiliated with Massachusetts General Brigham (MGB), a large integrated delivery system in the Boston area. CRC screening completion was calculated for adults ages 50–75 at two timepoints: 3/1/2020 (the start of pandemic) and 3/1/2023 (during pandemic). (Museum, 2023) The study was approved by the MGB Human Subjects Committee. For eligible individuals, up to date with CRC screening was defined as completion of a fecal immunochemical test (FIT) within 1 year, FIT-DNA within 3 years, sigmoidoscopy within 5 years, or colonoscopy within 10 years. Individuals newly eligible for CRC screening (ages 45–49) due to the 2021 USPSTF screening recommendations (US Preventive Services Task Force, 2021) were not included in this study to maintain similar population estimates.
The 5-digit ZIP code associated with each individual’s home address was cross-walked to a Zip Code Tabulation Area and then mapped to one of 27 neighborhoods (historical Boston neighborhoods (Neighborhoods. https://www.boston.gov/neighborhoods. Accessed March 23, 2023) and Boston-adjacent neighborhoods). Data from the COVID-19 Healthcare Coalition’s SVI dashboard were used to characterize each neighborhood. (Population, 2023) The association of SVI on change in CRC screening prevalence from before to during the COVID-19 pandemic was determined by testing the interaction between time and SVI in a logistic regression model. A Generalized Estimating Equations approach was used to account for repeated measures from the same subjects. The figures were created using Proc GMAP from SAS and the categories of SVI and CRC screening were determined based on the distributions.
3. Results
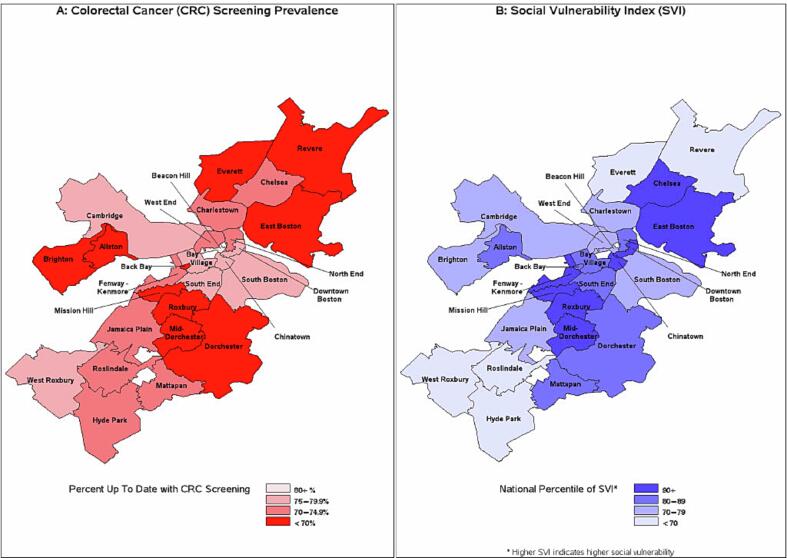
Pre-pandemic disparities in CRC screening prevalence by neighborhood were apparent (Fig. 1a). The up-to-date rate in 3/2020 ranged from 65.8 % (mid-Dorchester) to 84.5 % (Beacon Hill). Across Boston neighborhoods, SVI ranged from 40.9 (Roslindale) to 97.7 (mid-Dorchester), with a median SVI of 74.0 (Fig. 1b). In 3/2020, 36.2 % of eligible patients lived in a neighborhood above the 80th percentile of SVI, consistent with high social vulnerability, while the same value was 35.1 % in 3/2023. Of the 29,187 eligible patients in 3/2020, 21,074 (72.2 %) were up to date with CRC screening. Of the 33,692 eligible patients in 3/2023, 22,708 (67.4 %) were up to date.
Fig. 1.
Colorectal Cancer Screening Prevalence and Social Vulnerability Index in March 2020 by Boston-Area Neighborhood.
Table 1 shows the changes in screening prevalence between 3/2020 and 3/2023 by neighborhood. The overall change in screening prevalence from 3/2020 and 3/2023 was significant (72.2 % to 67.4 %, p < 0.001). However, there was no significant relationship between the change in screening prevalence and SVI: a decrease of 5.5 % screened in neighborhoods with SVI ≤ 80 compared to a decrease of 3.6 % in neighborhoods with SVI > 80 (p = 0.79).
Table 1.
Social Vulnerability and Colorectal Cancer Screening in Boston-Area Neighborhoods, 2020 to 2023.
| March 2020 |
March 2023 |
|||||
|---|---|---|---|---|---|---|
| Neighborhood | Social Vulnerability Index (percentile)* | Number eligible for screening | CRC Screening Prevalence (%) |
Number eligible for screening | CRC Screening Prevalence (%) |
Change in CRC Screening Prevalence (%) |
| Roslindale | 40.9 | 1,749 | 72.8 | 2,006 | 68.9 | −3.8 |
| West Roxbury | 44.1 | 1,859 | 76.3 | 2,124 | 71.5 | −4.8 |
| Everett | 54.8 | 1,467 | 68.6 | 1,729 | 63.6 | −5.0 |
| Hyde Park | 66.4 | 1,606 | 72.4 | 1,821 | 66.4 | −6.0 |
| Revere | 67.7 | 2,975 | 69.7 | 3,377 | 64.6 | −5.2 |
| Charlestown | 73.5 | 1,528 | 70.5 | 2,135 | 58.9 | −11.6 |
| Beacon Hill | 73.6 | 330 | 84.5 | 370 | 80.3 | −4.3 |
| Brighton | 73.9 | 574 | 67.8 | 653 | 63.9 | −3.9 |
| Jamaica Plain | 74 | 2,022 | 73.3 | 2,263 | 69.9 | −3.4 |
| Cambridge | 75.3 | 2,205 | 76.2 | 2,545 | 72.5 | −3.8 |
| West End | 77.7 | 794 | 74.7 | 908 | 71.7 | −3.0 |
| South Boston | 78.3 | 909 | 76.8 | 1,047 | 73.0 | −3.8 |
| North End | 79.2 | 614 | 72.3 | 877 | 60.1 | −12.2 |
| Back Bay | 81.8 | 69 | 66.7 | 69 | 72.5 | 5.8 |
| Dorchester | 86.2 | 2,190 | 69.7 | 2,479 | 67.4 | −2.3 |
| Allston | 86.9 | 157 | 67.5 | 167 | 68.9 | 1.3 |
| Mattapan | 87.8 | 624 | 71.5 | 742 | 66.4 | −5.0 |
| South End | 87.9 | 705 | 75.9 | 789 | 70.6 | −5.3 |
| Bay Village | 87.9 | 946 | 77.1 | 1,052 | 73.8 | −3.3 |
| Downtown Boston | 91.4 | 220 | 75.0 | 282 | 64.9 | −10.1 |
| Chinatown | 93.9 | 202 | 77.7 | 221 | 71.0 | −6.7 |
| East Boston | 93.9 | 889 | 69.1 | 1,022 | 65.6 | −3.5 |
| Fenway - Kenmore | 94.6 | 583 | 72.2 | 658 | 68.4 | −3.8 |
| Chelsea | 96 | 2,033 | 71.3 | 2,208 | 68.2 | −3.2 |
| Roxbury | 96.4 | 907 | 67.6 | 1,005 | 62.9 | −4.7 |
| Mission Hill | 96.7 | 338 | 68.6 | 373 | 65.7 | −3.0 |
| Mid-Dorchester | 97.7 | 692 | 65.8 | 770 | 61.4 | −4.3 |
| Overall | 74.9 | 29,187 | 72.2 | 33,692 | 67.4 | −4.8 |
*Higher SVI indicates higher vulnerability.
4. Discussion
Our findings reveal that the COVID-19 pandemic uniformly depressed CRC screening prevalence across Boston-area neighborhoods despite geographic disparities in CRC screening prevalence and SVI. While previous research has shown that US counties with higher social vulnerability had significantly lower rates of cancer screening (Bauer et al., 2022) and that communities with higher SVI were disproportionately affected by the pandemic, (Gray et al., 2020) our results indicate that there was no association between social vulnerability and change in CRC screening prevalence during the first two years of the pandemic. This suggests that there may be neighborhood characteristics which are not captured in SVI that contribute to changes in CRC screening disparities during this period.
Health system data may not accurately estimate neighborhood CRC screening rates as it does not capture individuals who seek care through other health systems or do not seek care at all. Earlier work showed that these data provide population-based estimates for tobacco use in the Boston area comparable to those derived from the Behavioral Risk Factor Surveillance Survey with more timely availability. (Linder et al., 2013) Public health agencies are increasingly using EHR data to do public health surveillance. (Klompas et al., 2017, Tatem et al., 2017).
As health systems and communities recover from the pandemic, effective strategies should be implemented to ensure more equitable participation in screening regardless of neighborhood social vulnerability. The US Department of Health and Human Services has set the 2030 target for CRC screening at 74.4 %. (People, 2030)As of March 2023, only 1 of the 27 Boston-area neighborhoods meet this target. To reach screening targets and avoid variations in CRC screening rates by neighborhood in the future, population characteristics not included in SVI, such as access to a primary care physician, food insecurity, neighborhood safety, and the spread of CRC screening information within neighborhoods should be considered.(Mayhand et al., 2021, Layne et al., 2023) Public health measures that address neighborhood-level barriers to cancer screening, particularly for neighborhoods that demonstrated lower screening rates prior to the pandemic, are essential to address the impact of the COVID-19 pandemic on CRC screening.
Funding
This work was supported by Stand Up To Cancer, a division of the Entertainment Industry Foundation (grant number 2021a002931) and the American Cancer Society (CRP-22–080-01-CTPS). None of the authors report a conflict of interest.
CRediT authorship contribution statement
Roopa S. Bhat: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. Suzanne Brodney: Writing – review & editing, Supervision, Project administration. Yuchiao Chang: Writing – review & editing, Software, Formal analysis, Conceptualization. Meghan Rieu-Werden: Writing – review & editing, Visualization, Software, Formal analysis, Data curation. Folasade P. May: Writing – review & editing, Funding acquisition, Conceptualization. Jennifer S. Haas: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Grant from Stand Up to Cancer to: Bhatt, Brodney, Chang, May and Haas.
Data availability
The data that has been used is confidential.
References
- Bauer C., Zhang K., Xiao Q., Lu J., Hong Y.R., Suk R. County-level social vulnerability and breast, cervical, and colorectal cancer screening rates in the US, 2018. JAMA Netw Open. 2022;5(9):e2233429. doi: 10.1001/jamanetworkopen.2022.33429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling S. Why Some Boston Neighborhoods Have Been Hit Harder By The Pandemic Than Others. WBUR. April 20, 2020, 2020.
- At A Glance: CDC/ATSDR Social Vulnerability Index. https://www.atsdr.cdc.gov/placeandhealth/svi/at-a-glance_svi.html. Published 2022. Accessed March 26, 2023.
- Gray D.M., 2nd, Anyane-Yeboa A., Balzora S., Issaka R.B., May F.P. COVID-19 and the other pandemic: populations made vulnerable by systemic inequity. Nat Rev Gastroenterol Hepatol. 2020;17(9):520–522. doi: 10.1038/s41575-020-0330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M., Cocoros N.M., Menchaca J.T., Erani D., Hafer E., Herrick B., Josephson M., Lee M., Payne Weiss M.D., Zambarano B., Eberhardt K.R., Malenfant J., Nasuti L., Land T. State and local chronic disease surveillance using electronic health record systems. Am J Public Health. 2017;107(9):1406–1412. doi: 10.2105/AJPH.2017.303874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne T.M., Agarwal P., Rapkin B.D., Jandorf L.H., Bickell N.A. Cancer beliefs and screening behaviors: the impact of neighborhood and other social determinants of health. Front Oncol. 2023;13:1072259. doi: 10.3389/fonc.2023.1072259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J.A., Rigotti N.A., Brawarsky P., Kontos E.Z., Park E.R., Klinger E.V., Marinacci L., Li W., Haas J.S. Use of practice-based research network data to measure neighborhood smoking prevalence. Prev Chronic Dis. 2013;10:E84. doi: 10.5888/pcd10.120132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhand K.N., Handorf E.A., Ortiz A.G., Gonzalez E.T., Devlin A., Sorice K.A., Esnaola N., Fisher S., Lynch S.M. Effect of neighborhood and individual-level socioeconomic factors on colorectal cancer screening adherence. Int J Environ Res Public Health. 2021;18(9) doi: 10.3390/ijerph18094398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methodology for the Model-based Small Area Estimates of Cancer Risk Factors and Screening Behaviors. https://sae.cancer.gov/nhis-brfss/methodology.html. Accessed March 26, 2023.
- CDC Museum COVID-19 Timeline. https://www.cdc.gov/museum/timeline/covid19.html. Accessed December 9, 2023.
- Neighborhoods. https://www.boston.gov/neighborhoods. Accessed March 23, 2023.
- Healthy People 2030. https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer/increase-proportion-adults-who-get-screened-colorectal-cancer-c-07. Accessed March 23, 2023.
- Tracking Vulnerable Population by Region. https://c19hcc.org/resource/vulnerable-population. Accessed Mar 23, 2023.
- Siegel R.L., Wagle N.S., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- Tatem K.S., Romo M.L., McVeigh K.H., Chan P.Y., Lurie-Moroni E., Thorpe L.E., Perlman S.E. Comparing prevalence estimates from population-based surveys to inform surveillance using electronic health records. Prev Chronic Dis. 2017;14:E44. doi: 10.5888/pcd14.160516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.