Abstract
Rationale & Objective
Itching is a frequent symptom experienced by people with chronic kidney disease (CKD). We investigated the associations of CKD-associated pruritus (CKD-aP) with clinical outcomes.
Study Design
This was a longitudinal cohort study.
Setting & Participants
Patients from Brazil, France, and the United States enrolled in the Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps) from 2013 to 2021, an international prospective cohort study of adults with nondialysis dependent CKD, and an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 were included.
Exposure
CKD-aP was self-reported by response to the question: “During the past 4 weeks, to what extent were you bothered by itchy skin?”
Outcomes
The outcomes were as follows: CKD progression, kidney replacement therapy (KRT) initiation, mortality, hospitalization, cardiovascular events, infection events.
Analytical Approach
Associations with time-to-event outcomes were investigated using Cox proportional hazards models adjusted for potential confounders.
Results
There were 4,410 patients from 91 clinics with a median age of 69 years and a median eGFR at patient questionnaire completion of 29 (21-38) mL/min/1.73 m2. The proportion of patients not at all, somewhat, moderately, very much, and extremely bothered by itchy skin was 49%, 27%, 13%, 7%, and 3%, respectively. Patients with more advanced stages of CKD, older age, and greater comorbidities reported to be more likely bothered by itchy skin. Among patients at least moderately bothered, 23% were prescribed at least 1 pharmacotherapy (35% in the United States, 19% in France, 4% in Brazil), including antihistamine (10%), gabapentin (6%), topical corticosteroids (4%), pregabalin (3%), or sedating antihistamine (3%). The HR (95% CI) for patients extremely (vs not at all) bothered was 1.74 (1.11-2.73) for all-cause mortality, 1.56 (1.11-2.18) for all-cause hospitalization, and 1.84 (1.22-2.75) for cardiovascular events. As CKD-aP severity increased, patients also had higher rates of infection events (P = 0.04); CKD-aP severity was not associated with KRT initiation (P = 0.20) or CKD progression (P = 0.87).
Limitations
The limitations were 25% nonresponse rate, recall bias, and residual confounding factors.
Conclusions
These results demonstrate a strong association between severe itch and clinical outcomes, providing the nephrology community new insights into the possible adverse consequences of CKD-aP in individuals with nondialysis CKD, and warrant further exploration.
Plain-Language Summary
Chronic kidney disease-associated pruritus (CKD-aP) is a common disturbing symptom of chronic kidney disease (CKD). This article analyzes longitudinal data from the Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps) to describe prevalence of CKD-aP in 4,410 individuals with nondialysis CKD, and its association with clinical outcomes. We found that 51% of the surveyed population were bothered by pruritus. CKD-aP was more prevalent in those with more advanced stages of CKD, older age, and with more comorbid conditions. Compared to those not at all bothered by pruritus, those who were extremely bothered had a higher risk of all-cause mortality, hospitalizations, and cardiovascular events. Severity of CKD-aP was not associated with CKD progression or initiation of kidney replacement therapy.
Index Words: Chronic kidney disease, hospitalization, mortality, pruritus
Itching is a common symptom of chronic kidney disease (CKD) that can significantly affect an individual’s quality of life. Cross-sectional data from over 5,000 patients enrolled in the Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps) showed that CKD-associated pruritus (CKD-aP) is not only bothersome, but also frequent, with a reported 24% prevalence of moderate-to-extreme pruritus across all CKD stages, including 10%-13% with severe-to-extreme pruritus. This same study showed associations of CKD-aP with depression and poor sleep.1 In those receiving hemodialysis as kidney replacement therapy (KRT), CKD-aP is associated with fatigue, feelings of frustration, or depression; a negative impact on vocational or social interactions; poor sleep quality; decreased sleep quantity; lower adherence to treatment plans; and missed hemodialysis sessions.2,3
Associations with CKD-aP with clinical outcomes in the hemodialysis population has also been described because increasing severity of CKD-aP in this population has been shown to increase the risk of mortality, cardiovascular- and infection-related deaths, hospitalizations, dialysis withdrawal, and missed hemodialysis sessions.3 Despite its importance, these associations have not been described in people with CKD not receiving KRT.
Although the discomfort associated with CKD-aP can be significant, it is often underreported by patients and underrecognized by providers. This lack of identification can lead to challenges in studying its impact and treatment. Among 35,452 patients enrolled in the Dialysis Outcomes and Practice Patterns Study (DOPPS), only 17% of those nearly always or always bothered by itch reported their CKD-aP symptoms to staff, whereas 69% of medical directors underestimated the prevalence of severe CKD-aP in their units.2 Furthermore, treatment is variable, partly because of unclear pathophysiology. Although difelikefalin was approved in 2021 for treatment of CKD-aP in adults undergoing hemodialysis, there are currently no approved treatments for those with CKD-aP not receiving dialysis.4, 5, 6, 7, 8 Presently, standard of care treatments include emollients to keep the skin hydrated and a variety of off-label systemic treatments with varied degrees of effectiveness and potential side effects, such as gabapentinoids.9
As health care systems strive for person-centered delivery models, an individual’s subjective experience of illness, such as symptoms, will have a significant role in shared decision making, formulating of treatment plans, and interactions with physicians. This approach will require data focused on the disease experience as well as clinical outcomes and is synergistic with patient priorities. When asked, patients with CKD have prioritized reduction of symptoms as a research focus in kidney disease.10,11 Pruritus, given its debilitating consequences, is a symptom in which better understanding of its impact can possibly alter the experience of kidney disease for many.
The objectives of this article are to expand on the cross-sectional CKDopps findings reported by Sukul et al1 and leverage longitudinal data from the CKDopps to describe the association of self-reported severity of pruritus in patients with nondialysis CKD with time-to-event outcomes including the following: CKD progression to KRT; all-cause mortality; all-cause hospitalization; cardiovascular events; and infection.
Methods
Data Source
The CKDopps is an ongoing international prospective cohort study of adult nondialysis patients with CKD under nephrology care in Brazil, France, Germany, and the United States. The details of the study design and protocol have been published previously.12 Samples of nephrologist-run CKD clinics in each country were randomly selected after stratification of clinics by geographic region and key clinic characteristics (ie, size and public vs private). Participants with estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 at screening were randomly selected within each participating nephrology clinic.
CKDopps was approved by an independent institutional review board (E&I Review Services) along with national and/or local ethics committees, as required by local ethics regulations. The study was conducted with adherence to the Declaration of Helsinki. Written, informed consent was obtained from all patients eligible for study participation. Protocols, data storage, and data use were compliant with international data privacy laws.
The patient questionnaire containing the variable of interest (CKD-aP) was not distributed in CKDopps-German facilities. This analysis thus includes data from Brazil, France, and the United States in CKDopps phase 1 (2013-2018) along with US data from CKDopps phase 2 (2018-2021).
Variables
The eGFR was calculated using the CKD-Epi formula based on serum creatinine level. Routine laboratory and medication-related data were collected longitudinally throughout the study. Demographics, comorbid conditions, and clinical data were transcribed from medical records at study enrollment. A patient questionnaire, which collected data on self-reported health measures using validated instruments regarding CKD symptoms, health-related quality of life, and depression symptoms, was distributed to patients on enrollment and annually.
The exposure variable was patients’ response to a question from the Kidney Disease Quality of Life 36-item survey (KDQOL-36) in the patient questionnaire: “During the past 4 weeks, to what extent were you bothered by itchy skin?” The 5 response options were not at all bothered, somewhat, moderately, very much, and extremely. The response from the first available patient questionnaire was used for the analysis.
Study outcomes included KRT (ie, maintenance dialysis or preemptive kidney transplant) initiation, CKD progression, all-cause mortality, all-cause hospitalization, and composite (death or hospitalization) outcomes of cardiovascular (CV) events and infection events. CKD progression was defined as in Zee et al13 as having KRT initiation or reaching a surrogate endpoint of sustained low eGFR (ie, eGFR < 15 mL/min/1.73 m2) or 40% eGFR decline from the time of patient questionnaire completion. The eGFR-based endpoints required at least 2 eGFR measurements. The low eGFR was considered sustained if there was another confirmatory eGFR < 15 more than 4 weeks after the initial eGFR < 15 mL/min/1.73 m2 value; if no subsequent eGFR value was available, it was considered sustained if there was a KRT event or death event or patient lost to follow-up within 4 weeks after the initial eGFR < 15 mL/min/1.73 m2 value. The 40% eGFR decline was determined based on linear regression models using all historic eGFRs during follow-up after the time of patient questionnaire completion. Hospitalization admission dates, diagnosis codes, and procedure codes are captured throughout follow-up by the study coordinator; cause of death is recorded when patients depart the study. A listing of cause-specific death and hospitalization diagnosis and/or procedure for cardiovascular and infection events is presented in Table S1.
The age and CKD duration of patients were updated to the time of patient questionnaire completion. The contemporaneous laboratory measures and medication prescriptions were selected based on closest proximity to the time of patient questionnaire completion; laboratory data (120 days) and medication data (185 days) were restricted to dates closest to patient questionnaire completion.
Statistical Analysis
Descriptive statistics were used to summarize demographic and clinical characteristics of patients at patient questionnaire completion, by degree of self-reported pruritus severity. Cause-specific Cox proportional hazards models were used to investigate the association between pruritus severity and time-to-event outcomes, stratified by country and CKDopps study phase, and accounting for facility clustering using a robust sandwich covariance estimator. Time at risk started at the time of patient questionnaire completion until the event of interest or end of patient follow-up (due to clinic transfer, KRT initiation, death, loss to follow-up, or administrative study end), whichever occurred first. Models were adjusted for the following potential confounders: age, sex (identified gender or sex assigned at birth), African American race, 13 summary comorbid conditions (coronary artery disease, cerebrovascular disease, congestive heart failure, other cardiovascular disease, peripheral vascular disease, hypertension, diabetes, nonskin cancer, gastrointestinal bleeding, lung disease, neurologic disease, any psychiatric disorder, and recurrent cellulitis and/or gangrene), and eGFR at patient questionnaire completion. Sensitivity analyses included additional adjustment for serum albumin level and albuminuria. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported for each exposure category compared to the reference group of “Not at all” bothered. To test whether increasing severity of CKD-aP was associated with outcomes, we also tested for a linear trend across exposure categories by coding them from 1 (not at all bothered) to 5 (extremely bothered).
Missing data for all independent variables in Cox proportional hazards models were multiply imputed by chained equations, and results from 20 imputed data sets were combined for the final analysis using Rubin's formula.14,15 The proportion of missing data was below 10%. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Patient Characteristics
A total of 4,410 patients from 91 CKD clinics were included in the final analytic cohort: 496 (11%) from 15 clinics in Brazil, 2,586 from 41 clinics in France, and 1,328 from 35 clinics in the United States. The median age [interquartile range (IQR)] was 69 (61-77) years, and 41% were female. The predominant primary etiologies of CKD were hypertensive kidney disease (26%) and diabetes (26%). The median (IQR) eGFR at patient questionnaire completion was 29 (21-38) mL/min/1.73 m2.
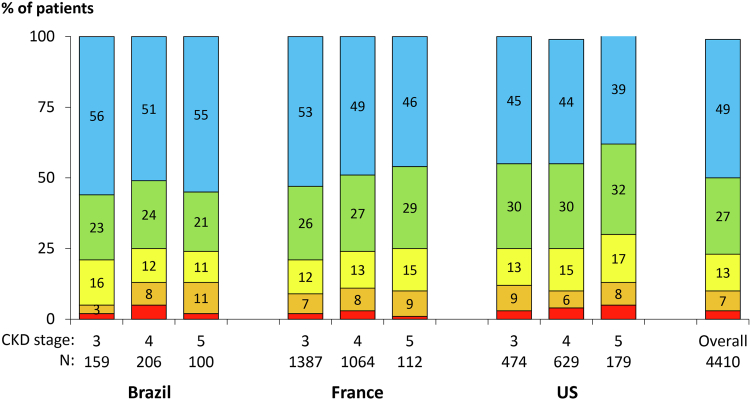
Over half (51%) of patients self-reported being at least somewhat bothered by itchy skin. The proportion of patients not at all, somewhat, moderately, very much, and extremely bothered by pruritus was 49%, 27%, 13%, 7%, and 3%, respectively. Patients with more advanced stages of CKD were likely more bothered by itchy skin (Fig 1). Patient characteristics by degree of self-reported pruritus are summarized in Table 1; patients more bothered by itchy skin tended to be slightly older with more comorbid conditions and had somewhat lower hemoglobin and serum albumin levels. The proportion of patients who self-reported being at least moderately bothered by dry skin was 34% overall and ranged from 16% among those not at all bothered by itchy skin to 90% among those extremely bothered by itchy skin.
Figure 1.
Self-reported extent bothered by itchy skin, by country and CKD stage. The stratified sample sizes do not add up to the total sample because 100 patients did not have a GFR measured within 120 days of questionnaire completion. Abbreviations: CKD, chronic kidney disease; GFR, glomerular filtration rate.
Table 1.
Patient Characteristics by Self-Reported Extent Bothered by Itchy Skin (All Data Extracted From Patient Questionnaires Completed at Enrollment)
| Overall | Self-Reported Extent Bothered by Itchy Skin |
|||||
|---|---|---|---|---|---|---|
| 1: Not At All Bothered | 2: Somewhat Bothered | 3: Moderately Bothered | 4: Very Much Bothered | 5: Extremely Bothered | ||
| Patients, N | 4,410 | 2,167 (49%) | 1,198 (27%) | 595 (13%) | 329 (7%) | 121 (3%) |
| Age at (y) | 69 [6-77] | 68 [6-76] | 70 [6-78] | 71 [6-78] | 70 [6-77] | 69 [6-77] |
| Female, n (%) | 1,812 (41%) | 868 (40%) | 487 (41%) | 254 (43%) | 136 (41%) | 67 (55%) |
| African American race, n (%) | 430 (10%) | 213 (10%) | 118 (10%) | 51 (9%) | 36 (11%) | 12 (10%) |
| eGFRa(mL/min/1.73 m2) | 29.0 [20.8-38.3] | 29.7 [21.3-38.9] | 28.8 [20.5-38.3] | 28.6 [20.8-36.5] | 28.1 [20.1-37.8] | 25.1 [19.5-33.2] |
| CKD duration at (y) | 4.85 [2.26-8.93] | 4.88 [2.21-9.32] | 4.98 [2.37-8.71] | 4.94 [2.31-9.93] | 4.05 [2.03-7.44] | 4.57 [2.37-8.23] |
| Reported cause of CKD, n (%) | ||||||
| Diabetes | 1,098 (26%) | 517 (25%) | 294 (25%) | 156 (27%) | 93 (29%) | 38 (34%) |
| Hypertension | 1,100 (26%) | 539 (26%) | 301 (26%) | 163 (28%) | 75 (23%) | 22 (20%) |
| GN | 580 (14%) | 312 (15%) | 140 (12%) | 70 (12%) | 42 (13%) | 16 (14%) |
| TIN | 428 (10%) | 225 (11%) | 112 (10%) | 50 (9%) | 30 (9%) | 11 (10%) |
| Polycystic | 208 (5%) | 106 (5%) | 68 (6%) | 25 (4%) | 4 (1%) | 5 (5%) |
| Other | 658 (15%) | 304 (14%) | 190 (16%) | 92 (16%) | 57 (18%) | 15 (14%) |
| Unknown | 193 (5%) | 97 (5%) | 48 (4%) | 22 (4%) | 22 (7%) | 4 (4%) |
| Comorbid conditions at baseline, n (%) | ||||||
| Coronary artery disease | 1,115 (26%) | 492 (23%) | 314 (27%) | 173 (30%) | 98 (30%) | 38 (32%) |
| Cerebrovascular disease | 463 (11%) | 222 (10%) | 120 (10%) | 65 (11%) | 39 (12%) | 17 (14%) |
| Congestive heart failure | 610 (14%) | 275 (13%) | 163 (14%) | 81 (14%) | 69 (21%) | 22 (18%) |
| Other cardiovascular disease | 1,047 (24%) | 504 (23%) | 282 (24%) | 137 (23%) | 91 (28%) | 33 (28%) |
| Peripheral vascular disease | 822 (19%) | 387 (18%) | 215 (18%) | 113 (19%) | 76 (23%) | 31 (26%) |
| Hypertension | 3,983 (91%) | 1,944 (91%) | 1,088 (92%) | 538 (92%) | 302 (92%) | 111 (93%) |
| Diabetes | 2,002 (45%) | 923 (43%) | 545 (45%) | 291 (49%) | 180 (55%) | 63 (52%) |
| Cancer (nonskin) | 827 (19%) | 402 (19%) | 226 (19%) | 124 (21%) | 61 (19%) | 14 (12%) |
| Gastrointestinal bleeding | 125 (3%) | 49 (2%) | 37 (3%) | 23 (4%) | 10 (3%) | 6 (5%) |
| Lung disease | 194 (11%) | 68 (8%) | 57 (11%) | 32 (12%) | 24 (18%) | 13 (20%) |
| Neurologic disease | 165 (4%) | 68 (3%) | 47 (4%) | 24 (4%) | 19 (6%) | 7 (6%) |
| Any psychiatric disorder | 491 (11%) | 202 (10%) | 129 (11%) | 83 (14%) | 50 (15%) | 27 (23%) |
| Recurrent cellulitis/gangrene | 76 (4%) | 23 (3%) | 25 (5%) | 10 (4%) | 14 (11%) | 4 (6%) |
| Cirrhosis of the liver | 53 (1%) | 21 (1%) | 11 (1%) | 9 (2%) | 10 (3%) | 2 (2%) |
| Laboratorya | ||||||
| Hemoglobin (g/dL) | 12.6 (1.8) | 12.7 (1.7) | 12.6 (1.8) | 12.4 (1.8) | 12.4 (1.9) | 12.2 (1.7) |
| Serum ferritin level (ng/mL) | 134 [73-247] | 134 [74-244] | 140 [75-245] | 132 [67-266] | 120 [68-223] | 141 [65-282] |
| Serum albumin level (g/dL) | 3.97 (0.50) | 3.98 (0.50) | 4.00 (0.47) | 3.93 (0.53) | 3.90 (0.53) | 3.83 (0.60) |
| Serum phosphorous level (mg/dL) | 3.71 (0.84) | 3.68 (0.85) | 3.70 (0.83) | 3.75 (0.80) | 3.81 (0.84) | 3.88 (0.80) |
| Total calcium (mg/dL) | 9.35 (0.58) | 9.36 (0.56) | 9.36 (0.56) | 9.33 (0.58) | 9.29 (0.69) | 9.30 (0.57) |
| Albuminuria or equivalentb | ||||||
| Normal | 1,030 (32%) | 547 (34%) | 269 (32%) | 121 (29%) | 70 (29%) | 23 (29%) |
| Mild | 919 (29%) | 458 (28%) | 252 (30%) | 108 (26%) | 76 (31%) | 25 (32%) |
| Severe | 1,262 (39%) | 619 (38%) | 329 (39%) | 186 (45%) | 97 (40%) | 31 (39%) |
| Glycated hemoglobinc, % | 7.2 (1.3) | 7.1 (1.3) | 7.3 (1.3) | 7.2 (1.2) | 7.2 (1.3) | 7.4 (1.6) |
| Patient-reported outcome | ||||||
| Dry skind | 1,474 (34%) | 353 (16%) | 341 (29%) | 405 (69%) | 266 (82%) | 109 (90%) |
Notes: Results are presented as mean (standard deviation), median [Q1-Q3], or N (%).
Abbreviations: ACR, albumin-to-creatinine ratio; AER, albumin excretion rate; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; TIN, tubulointerstitial nephritis; PCR, protein to creatinine ratio; PER, protein excretion rate.
Within 120 days from the first patient questionnaire with the completion of the pruritus survey question.
Normal: ACR < 30 mg/g or AER < 30 mg/24 h or PCR < 150 mg/g or PER < 150 mg/24 h or dipstick; Mild: 30 ≤ ACR < 300 mg/g or 30 ≤ AER < 300 mg/24 h or 150 ≤ PCR < 500 mg/g or 150 ≤ PER < 500 mg/24 h or dipstick trace to 1+; Severe: ACR ≥ 300 mg/g or AER ≥ 300 mg/24 h or PCR ≥ 500 mg/g or PER ≥ 500 mg/24 h or dipstick 1+ or greater.
Among patients with diabetes.
At least moderately bothered by dry skin.
Pruritus Medication Use
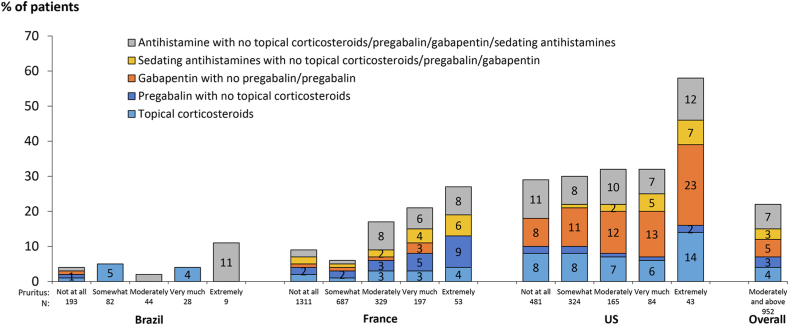
Table 2 presents the proportion of patients on medications commonly prescribed for treatment of pruritus, stratified by degree of self-reported pruritus. Generally, patients who reported they were more bothered by itchy skin were more likely to be prescribed these medications. Among patients who were at least moderately bothered by itchy skin, 9%, 5%, 3%, 4%, and 3% were taking an antihistamine, gabapentin, pregabalin, topical corticosteroids, or sedating antihistamine, respectively; 23% were prescribed at least one of these medications. Among patients bothered by itchy skin, pruritus medication use was higher in the United States compared to Brazil and France (Fig 2). The prescription of pruritus medications was quite rare (<5%) in Brazil even among patients with moderate-to-extreme pruritus.
Table 2.
Prescription of Medications Often Used to Treat CKD-aPa, by Self-Reported Extent Bothered by Itchy Skin
| Overall | Self-Reported Extent Bothered by Itchy Skin |
|||||
|---|---|---|---|---|---|---|
| 1: Not At All Bothered | 2: Somewhat Bothered | 3: Moderately Bothered | 4: Very Much Bothered | 5: Extremely Bothered | ||
| Patients, N | 4,410 | 2,167 | 1,198 | 595 | 329 | 121 |
| Antihistamine, n (%) | 228 (6%) | 93 (5%) | 45 (4%) | 51 (9%) | 24 (8%) | 15 (14%) |
| Gabapentinoids, n (%) | 243 (6%) | 94 (5%) | 68 (6%) | 38 (7%) | 26 (8%) | 17 (16%) |
| Gabapentin, n (%) | 158 (4%) | 56 (3%) | 50 (5%) | 25 (5%) | 16 (5%) | 11 (10%) |
| Pregabalin, n (%) | 88 (2%) | 39 (2%) | 20 (2%) | 13 (2%) | 10 (3%) | 6 (6%) |
| Topical corticosteroids, n (%) | 136 (3%) | 59 (3%) | 37 (3%) | 21 (4%) | 11 (4%) | 8 (8%) |
| Sedating antihistamines, n (%) | 69 (2%) | 24 (1%) | 14 (1%) | 12 (2%) | 13 (4%) | 6 (6%) |
| Any prescription of above, n (%) | 614 (15%) | 247 (12%) | 151 (14%) | 109 (20%) | 67 (22%) | 40 (38%) |
Note: Prescription (yes or no) was captured, but not the indication or condition for the medication. N (%) patients shown.
Within 185 days from the first patient questionnaire with the completion of the pruritus survey question.
Figure 2.
Prescription of medications often used to treat CKD-aP, by country and self-reported extent bothered by itchy skin. Prescription (yes or no) was captured, but not the indication or condition for the medication. Abbreviation: CKD-aP.
Pruritus and Outcomes
Over the median (IQR) follow-up time of 2.8 (1.2-4.8) years, the event rate per 100 patient-years was 6.7 for KRT initiation, 11.7 for CKD progression, 4.2 for all-cause mortality, 26.1 for all-cause hospitalization, 6.4 for CV events, and 4.1 for infection events. Sample sizes, event counts, and event rates for each outcome are presented in Table 3, stratified by CKD-aP.
Table 3.
Sample Sizes, Event Counts, and Event Rates, by Self-Reported Extent Bothered by Itchy Skin
| Self-Reported Extent Bothered by Itchy Skin |
|||||
|---|---|---|---|---|---|
| 1: Not At All Bothered |
2: Somewhat Bothered |
3: Moderately Bothered |
4: Very Much Bothered |
5: Extremely Bothered |
|
| KRT initiation | |||||
| No. of patients | 2,167 | 1,198 | 595 | 329 | 121 |
| No. of events | 390 | 240 | 121 | 71 | 27 |
| Event rate, per 100 patient-years | 6.1 | 6.9 | 7.3 | 7.8 | 9.5 |
| CKD progression | |||||
| No. of patients | 2,082 | 1,143 | 562 | 316 | 116 |
| No. of events | 633 | 369 | 196 | 108 | 43 |
| Event rate, per 100 patient-years | 10.9 | 11.7 | 13 | 12.8 | 16.2 |
| All-cause death | |||||
| No. of patients | 2,167 | 1,198 | 595 | 329 | 121 |
| No. of events | 239 | 140 | 85 | 47 | 24 |
| Event rate, per 100 patient-years | 3.8 | 4 | 5.1 | 5.1 | 8.5 |
| All-cause hospitalization | |||||
| No. of patients | 1,980 | 1,096 | 545 | 277 | 110 |
| No. of events | 995 | 569 | 291 | 194 | 64 |
| Event rate, per 100 patient-years | 23.7 | 25.5 | 28.3 | 39.7 | 40.8 |
| CV-related mortality or hospitalization | |||||
| No. of patients | 2,156 | 1,190 | 589 | 322 | 119 |
| No. of events | 334 | 204 | 98 | 69 | 33 |
| Event rate, per 100 patient-years | 5.7 | 6.4 | 6.6 | 8.5 | 13.6 |
| Infection-related mortality or hospitalization | |||||
| No. of patients | 2,158 | 1,191 | 589 | 325 | 118 |
| No. of events | 219 | 130 | 75 | 51 | 16 |
| Event rate, per 100 patient-years | 3.7 | 4 | 4.8 | 5.9 | 6.3 |
Abbreviations: CKD, chronic kidney disease; CV, cardiovascular; KRT, kidney replacement therapy.
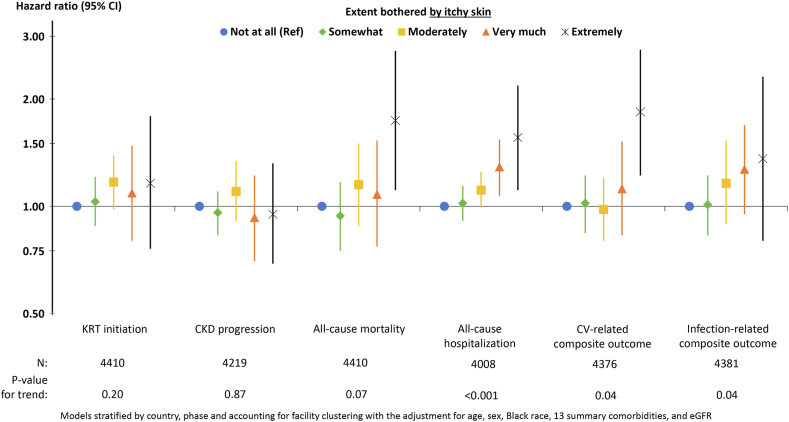
The adjusted associations between self-reported pruritus and time-to-event outcomes are illustrated in Fig 3. Compared to patients who reported being not at all bothered by itchy skin, patients who were extremely bothered had a higher rate of all-cause mortality (HR, 1.74; 95% CI, 1.11-2.73), all-cause hospitalization (HR, 1.56; 95% CI, 1.11-2.18), and CV events (HR, 1.84; 95% CI, 1.22-2.75). As the severity of self-reported pruritus increased, patients had higher rates of all-cause hospitalization (P < 0.001), CV events (P = 0.04), and infection events (P = 0.04); severity of itching was not associated with KRT initiation (P = 0.20) or CKD progression (P = 0.87). Results were largely unchanged in sensitivity analyses additionally adjusting for serum albumin level and albuminuria (Table 4).
Figure 3.
Adjusted association (HR, 95% CI) between self-reported extent bothered by itchy skin and outcomes. Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 4.
Adjusted Association (HR, 95% CI) Between Self-Reported Extent Bothered by Itchy Skin and Outcomes, Impact of Progressive Adjustment
| Self-Reported Extent Bothered by Itchy Skin |
|||||
|---|---|---|---|---|---|
| 1: Not At All Bothered | 2: Somewhat Bothered | 3: Moderately Bothered | 4: Very Much Bothered | 5: Extremely Bothered | |
| KRT initiation | |||||
| Model 1 | 1 (reference) | 1.03 (0.88-1.21) | 1.17 (0.98-1.39) | 1.09 (0.80-1.48) | 1.16 (0.76-1.79) |
| Model 2 | 1 (reference) | 1.04 (0.89-1.22) | 1.15 (0.96-1.37) | 1.09 (0.80-1.47) | 1.18 (0.78-1.80) |
| Model 3 | 1 (reference) | 1.08 (0.91-1.27) | 1.09 (0.91-1.32) | 1.15 (0.85-1.56) | 1.17 (0.76-1.80) |
| CKD progression | |||||
| Model 1 | 1 (reference) | 0.96 (0.83-1.10) | 1.10 (0.91-1.34) | 0.93 (0.70-1.22) | 0.95 (0.69-1.32) |
| Model 2 | 1 (reference) | 0.96 (0.83-1.11) | 1.09 (0.90-1.33) | 0.91 (0.69-1.20) | 0.96 (0.70-1.31) |
| Model 3 | 1 (reference) | 0.98 (0.85-1.14) | 1.03 (0.85-1.25) | 0.94 (0.71-1.23) | 0.93 (0.66-1.31) |
| All-cause mortality | |||||
| Model 1 | 1 (reference) | 0.94 (0.75-1.17) | 1.15 (0.88-1.50) | 1.08 (0.77-1.53) | 1.74 (1.11-2.73) |
| Model 2 | 1 (reference) | 0.96 (0.77-1.19) | 1.15 (0.88-1.51) | 1.08 (0.77-1.53) | 1.76 (1.14-2.74) |
| Model 3 | 1 (reference) | 0.96 (0.77-1.19) | 1.12 (0.86-1.47) | 1.08 (0.76-1.53) | 1.75 (1.12-2.75) |
| All-cause hospitalization | |||||
| Model 1 | 1 (reference) | 1.02 (0.91-1.14) | 1.11 (0.99-1.25) | 1.29 (1.07-1.54) | 1.56 (1.11-2.18) |
| Model 2 | 1 (reference) | 1.03 (0.91-1.16) | 1.11 (0.99-1.25) | 1.29 (1.07-1.55) | 1.57 (1.14-2.15) |
| Model 3 | 1 (reference) | 1.03 (0.91-1.16) | 1.10 (0.98-1.23) | 1.29 (1.07-1.55) | 1.56 (1.13-2.13) |
| CV-related mortality or hospitalization | |||||
| Model 1 | 1 (reference) | 1.02 (0.84-1.22) | 0.98 (0.80-1.20) | 1.12 (0.83-1.52) | 1.84 (1.22-2.75) |
| Model 2 | 1 (reference) | 1.03 (0.85-1.25) | 0.98 (0.80-1.20) | 1.12 (0.83-1.53) | 1.81 (1.22-2.69) |
| Model 3 | 1 (reference) | 1.03 (0.85-1.25) | 0.96 (0.78-1.18) | 1.12 (0.82-1.53) | 1.82 (1.24-2.69) |
| Infection-related mortality or hospitalization | |||||
| Model 1 | 1 (reference) | 1.01 (0.83-1.22) | 1.16 (0.89-1.53) | 1.27 (0.95-1.69) | 1.36 (0.80-2.31) |
| Model 2 | 1 (reference) | 1.02 (0.84-1.24) | 1.17 (0.89-1.54) | 1.26 (0.94-1.68) | 1.36 (0.81-2.30) |
| Model 3 | 1 (reference) | 1.02 (0.84-1.25) | 1.15 (0.88-1.52) | 1.27 (0.94-1.70) | 1.37 (0.81-2.31) |
Note: Models stratified by country, phase, and accounting for facility clustering.
Abbreviations: CKD, chronic kidney disease; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio; KRT, kidney replacement therapy.
Model 1: adjusted for age, sex, African American race, 13 summary comorbid conditions, and eGFR.
Model 2: Model 1 + serum albumin level.
Model 3: Model 2 + albuminuria categories.
Discussion
To the best of our knowledge, this is the first time associations of CKD-aP with clinical outcomes have been described among people with nondialysis CKD. Among 4,401 patients with CKD surveyed in 3 countries, including France, the United States, and Brazil, a majority (51%) reported at least some bother by itch, with severity increasing with CKD progression in the US and French cohorts. Those with more severe pruritus were more likely to be treated pharmacologically. Of the 3 countries studied, pharmacological interventions were most common in the United States. Greater severity of self-reported pruritus was associated with a higher rate of all-cause mortality, all-cause hospitalization, and cardiovascular or infection-related events, but not KRT initiation or CKD progression.
Descriptions of CKD-aP in the nondialysis CKD population are limited. Previous studies show a prevalence range of 19% to approximately 50%.1,16,17 Fourteen percent of participants in our cohort responded that they were at least moderately bothered by pruritus, 8% were very much bothered, and 3% extremely bothered; another 26% of our population responded they were somewhat bothered by itch. Our study population had significant overlap with the CKDopps cohort analyzed by Sukul et al.1 Thus the CKD-aP prevalence, variation by country and CKD stage, and assessment of patient characteristics are very similar to those reported by Sukul et al.1 For example, those with more severe itch were more likely to be female, of older age, and have diabetic kidney disease, lung disease, peripheral vascular disease, and psychiatric disease. In addition, those who were very much bothered or extremely bothered by itch were more likely to have more advanced kidney disease compared to participants not at all bothered by itch. This is also similar to findings from a recent study reporting incident pruritus in nondialysis CKD (n = 1,951).17 We did not see an increase in pruritus in individuals with higher albuminuria.
Many of the demographic trends we observed have been reported in the general population. For example, pruritus is the most common skin disorder in the geriatric population. A recent meta-analysis of 17 studies showed a 12% (95% CI, 3.9%-24%) prevalence of pruritus for those aged 60-69 years.18 In our study, with a mean age of 69, there was a 51% prevalence of pruritus of some level of intensity, substantially higher than what was reported in the meta-analysis of older adults, supporting that the pruritus we observe in our cohort of nondialysis CKD is unlikely to be caused by aging alone. Other associations, including the higher prevalence of pruritus in those with lung disease and diabetes, have been previously described in those receiving hemodialysis.19 Additionally, it is not uncommon for those with psychiatric disorders to experience pruritus, as we also observed.20 It is important to recognize these associations when people present with pruritus because it can alter treatment plans.
We found that among individuals who were at least moderately bothered by pruritus, 25% were receiving medical therapies. Although our data do not allow us to record if the medications prescribed were intended to treat CKD-aP, many of those reported are often used to mitigate pruritus. For example, we found use of antihistamines and sedating medications that target pathways not clearly linked to the pathogenesis of CKD-aP and have potential for adverse effects.21 Patients with CKD often have multiple comorbid conditions and are at risk for polypharmacy. Studies have shown that CKD patients take a median of eight to 11 medications.21,22 A recent analysis of 1,117 CKD patients from Japan showed a higher risk of kidney failure, cardiovascular events, and all-cause mortality for those who used a higher number of medications.21 In our analysis, the US cohort used pharmacological management of pruritus more than any other country. There can be several reasons for this practice pattern. This may reflect a plan to manage a patient without dialysis and therefore to use pharmacological interventions alone. However, it can also reflect a practice of polypharmacy in high-risk patients who may suffer serious side effects from medications. It is important to note that less harmful approaches, such as topical corticosteroids, were used less frequently than systemic medication. Overall, our findings support the need for a better understanding of the mechanism of CKD-aP so treatments can be optimized while minimizing risks to patients. In 2021, the Food and Drug Administration approved an intravenous selective kappa opioid receptor agonist, difelikefalin, for the treatment of moderate-to-severe CKD-aP in adults undergoing dialysis, and oral difelikefalin has demonstrated efficacy in a phase 2 study of stage 3-5 patients with CKD.4,23 There are 2 ongoing late stage clinical studies evaluating the safety and efficacy of oral difelikefalin in advanced patients with CKD with moderate-to-severe pruritus (NCT05342623 and NCT05356403) as well as one testing a topical treatment (MC2-25, NCT05482698). Aside from these investigational efforts whose outcomes are still unknown, treatment of CKD-aP in those with nondialysis CKD is not well-studied and remains a significant unmet need.
In this study, we expanded on a prior CKDopps analysis of CKD-aP and PROs by Sukul et al1 by investigating clinical outcomes. This is unique because in our prior report we did not have a long enough follow-up time. We found that participants who were extremely bothered by pruritus, compared to those who were not bothered at all, had higher rates of all-cause mortality, hospitalization, and cardiovascular events. Additionally, rates of all-cause hospitalizations, CV events, and infection-related events all increased with severity of pruritus. In contrast, we did not observe an association of pruritus with KRT initiation or CKD progression. These findings are important because the associations of pruritus with increased mortality, infections, and hospitalizations have been described in the maintenance hemodialysis population but never in the CKD population. Among 1,773 hemodialysis patients studied by Narita et al,24 patients with severe pruritus had an increased rate of death (HR, 1.6; 95% CI, 1.2-2.4), even after adjustment for other risk factors including diabetes and hypoalbuminemia. Sukul et al1 found similar results in their analysis of hemodialysis patients, finding a 1.24 (95% CI, 1.08-1.41) adjusted mortality HR for patients extremely (vs not at all) bothered by itch.
There can be several explanations for these findings. The first is that those who reported being extremely bothered by pruritus also were more likely to have comorbid conditions that increase morbidity and mortality, such as coronary artery disease, cerebrovascular disease, and peripheral vascular disease. Although Cox proportional hazards models were adjusted for these comorbid conditions, the models may not have accounted for residual confounding not represented by these variables. However, it is also conceivable that the poorer quality of life, poorer sleep quality, and greater likelihood of depression seen with greater severity of CKD-aP may contribute to our findings of poor clinical outcomes. Our findings may also reflect the systemic inflammation seen in CKD-aP. Inflammatory markers such as C-reactive protein, interleukin-6, and other pro-inflammatory cytokines, have been shown to be elevated in CKD-aP, possibly explaining the increased mortality we observed.21,25,26 However, these studies were small and in hemodialysis patients. The similarity between our findings and those of Sukul et al1 in patients enrolled in DOPPS is notable. In both cohorts, CKD-aP was associated with increased mortality and increased CV, infection-related death, and hospitalizations with worsening severity of pruritus. One potentially unifying explanation is the low-grade inflammation associated with CKD, maintenance dialysis, and CKD-aP.27 This increased inflammation is associated with increased mortality and CV events, regardless of CKD stage. Although caution needs to be applied to any assumptions, these findings do suggest that initiation of dialysis in someone with CKD-aP may not reduce their excess mortality risk, and inflammatory markers may be the pathophysiology explaining these findings.
Our observations can have important clinical implications. Although the associations of pruritus with the clinical outcomes observed in the present study do not imply causality, they support the role of pruritus as a strong predictor of adverse outcomes in patients with CKD. This can affect the care of patients with CKD. Visits with nephrologists often focus on biomedical markers of disease progression rather than those that are reported by patients. Additionally, symptoms often present in clusters; therefore, an individual may have many unaddressed sources of distress. This highlights the significance of inquiring about symptoms and exploring their multidimensional impact, including on psychological and emotional well-being.
There are several limitations to this study. First, one-quarter of patients enrolled in CKDopps did not respond to the question asking about the extent they were bothered by itchy skin. As described in a previous analysis of CKDopps data, these nonresponders were different from responders—more likely women, more comorbid conditions, more advanced CKD—potentially limiting generalizability of our findings.1 Second, there may be a bias in responses because individuals may underreport or overreport the severity of their itch. Third, the data are associations, and do not necessarily provide causal explanations for why these findings exist. Fourth, differential diagnosis was not considered on conditions that can lead to pruritus and possibly confound results, such as eczema, lymphoma, and liver or thyroid disease. Fifth, limited data on longitudinal CKD-aP precluded us from investigating incident CKD-aP or otherwise analyzing CKD-aP as a time-updated variable. Finally, the survey did not collect the prescribed indications of medications; thus, the medications commonly used for pruritus may have been prescribed for other comorbid medical conditions.
Despite these limitations, we report for the first time the associations of CKD-aP with clinical outcomes in a nondialysis CKD population. These data, along with the patient-reported outcomes already known, can increase awareness of CKD-aP, its complications, and treatment. Importantly, this information can enhance education of patients with CKD, which physicians can communicate to those they treat, allowing for a more informed preparation for illness. Furthermore, these data highlight the importance of obtaining a better understanding of the pathophysiology of CKD-aP and the need to have more effective therapies available for treatment. In summary, these results provide clinicians with more information to convey to people with CKD about their disease, yet also expose gaps in the current understanding of CKD-aP and options for treatment, all warranting further investigation.
Article Information
CKDopps Investigators
CKDopps Steering Committee and Country Investigators: Antonio Lopes, Roberto Pecoits-Filho (Brazil); Christian Combe, Christian Jacquelinet, Ziad Massy, Benedicte Stengel (France); Johannes Duttlinger, Danilo Fliser, Gerhard Lonnemann, Helmut Reichel (Germany); Takashi Wada, Kunihiro Yamagata (Japan); Ron Pisoni, Bruce Robinson (United States). Additional CKDopps Research Group: Viviane Calice da Silva, Ricardo Sesso (Brazil); Elodie Speyer (France); Koichi Asahi, Junichi Hoshino, Ichiei Narita (Japan); Rachel Perlman, Friedrich Port, Nidhi Sukul, Michelle Wong, Eric Young, Jarcy Zee (United States).
Authors’ Full Names and Academic Degrees
Jennifer S. Scherer, MD, Charlotte Tu, PhD, Ronald L. Pisoni, PhD, Elodie Speyer, PhD, Antonio A. Lopes, MD, Warren Wen, PhD, Frederique Menzaghi, PhD, Joshua Cirulli, PharmD, Natalia Alencar de Pinho, PhD, Roberto Pecoits-Filho, MD, and Angelo Karaboyas, PhD
Authors’ Contributions
Research idea and study design: AAL, WW, RLP, JC, AK, FM, JSS, RPF; data acquisition: RLP, ES, NAdP, RPF; data analysis/interpretation: WW, RLP, JC, AK, ES, CT, FM, JSS, NAdP, RPF; statistical analysis: AK, CT; supervision or mentorship: AAL, RLP, AK, RPF. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. This manuscript was directly supported by Cara Therapeutics. In France, CKDopps is part of the CKD-REIN cohort, which is funded by the Agence Nationale de la Recherche through the 2010 “Cohortes-Investissements d’Avenir” program and by the 2010 national Programme Hospitalier de Recherche Clinique. CKD-REIN is also supported through a public-private partnership with Amgen, Fresenius Medical Care, and GlaxoSmithKline (GSK) since 2012; Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017; Lilly France since 2013; Otsuka Pharmaceutical since 2015; Vifor Fresenius since 2017; and Sanofi-Genzyme from 2012 to 2015. Dr Scherer received funding from NIH-NIDDK K23DK125840.
Financial Disclosure
Dr Scherer declares one time consulting/speaking fees for Vifor Pharmaceuticals and Cara Therapeutics and is on a Clinical Advisory Committee for Monogram Health; she is also an investigator for Cara Therapeutics. Drs Menzaghi, Cirulli, and Wen are employees of Cara Therapeutics and shareholder of Cara Therapeutics. Drs Tu, Pisoni, Pecoits-Filho, and Karaboyas are employees of Arbor Research Collaborative for Health, which administers the DOPPS Programs. In addition, Dr Pecoits-Filho reports honoraria paid to employer for consulting from Bayer, Astra Zeneca, GSK, Akebia, Boehringer, Lilly, and FMC as well as honoraria paid to employer for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bayer, Astra Zeneca, GSK, Akebia, Boehringer, Lilly, and FMC. Dr de Pinho declares financial support from the following pharmaceutical companies integrating the public-private partnership of the CKD-REIN cohort: Fresenius Medical Care, GlaxoSmithKline (GSK), Vifor France, and Boeringher Ingelheim; all grants are made to Paris-Saclay University. The remaining authors declare that they have no relevant financial interests.
Acknowledgments
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial support.
Data Sharing
The data that support the findings of this study are not publicly available to protect the privacy of research participants. A limited dataset may be made available on reasonable request to Arbor Research Collaborative for Health.
Peer Review
Received May 10, 2023. Evaluated by 2 external peer reviewers, with direct editorial input by the Statistical Editor and the Editor-in-Chief. Accepted in revised form August 25, 2023.
Footnotes
Complete author and article information provided before references.
Table S1: List of Cause-Specific Death and Cause-Specific Hospitalization Diagnosis and/or Procedure.
Contributor Information
Jennifer S. Scherer, Email: Jennifer.scherer@nyulangone.org.
CKDopps Investigators:
Antonio Lopes, Roberto Pecoits-Filho, Christian Combe, Christian Jacquelinet, Ziad Massy, Benedicte Stengel, Johannes Duttlinger, Danilo Fliser, Gerhard Lonnemann, Helmut Reichel, Takashi Wada, Kunihiro Yamagata, Ron Pisoni, Bruce Robinson, Viviane Calice da Silva, Ricardo Sesso, Elodie Speyer, Koichi Asahi, Junichi Hoshino, Ichiei Narita, Rachel Perlman, Friedrich Port, Nidhi Sukul, Michelle Wong, Eric Young, and Jarcy Zee
Supplementary Material
Table S1.
References
- 1.Sukul N., Speyer E., Tu C., et al. Pruritus and patient reported outcomes in non-dialysis CKD. Clin J Am Soc Nephrol. 2019;14(5):673–681. doi: 10.2215/CJN.09600818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayner H.C., Larkina M., Wang M., et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12(12):2000–2007. doi: 10.2215/CJN.03280317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukul N., Karaboyas A., Csomor P.A., et al. Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med. 2021;3(1):42–53.e1. doi: 10.1016/j.xkme.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishbane S., Jamal A., Munera C., et al. A Phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222–232. doi: 10.1056/NEJMoa1912770. [DOI] [PubMed] [Google Scholar]
- 5.KORSUVATM (difelikefalin) injection, for intravenous use. United States Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214916s000lbl.pdf Accessed April 15, 2023.
- 6.Kapruvia (INN-Difelikefalin) 50 micrograms/mL solution for injection. European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/kapruvia-epar-product-information_en.pdf Accessed April 15, 2023.
- 7.Topf J., Wooldridge T., McCafferty K., et al. Efficacy of Difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis of KALM-1 and KALM-2 phase 3 studies. Kidney Med. 2022;4(8) doi: 10.1016/j.xkme.2022.100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishbane S., Wen W., Munera C., et al. Safety and tolerability of Difelikefalin for the treatment of moderate to severe pruritus in hemodialysis patients: pooled analysis from the phase 3 clinical trial program. Kidney Med. 2022;4(8) doi: 10.1016/j.xkme.2022.100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A.Y., Wong L.S. Uremic pruritus: from diagnosis to treatment. Diagnostics (Basel) 2022;12(5) doi: 10.3390/diagnostics12051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong A., Sainsbury P., Carter S.M., et al. Patients' priorities for health research: focus group study of patients with chronic kidney disease. Nephrol Dial Transplant. 2008;23(10):3206–3214. doi: 10.1093/ndt/gfn207. [DOI] [PubMed] [Google Scholar]
- 11.Hemmelgarn B.R., Pannu N., Ahmed S.B., et al. Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant. 2017;32(5):847–854. doi: 10.1093/ndt/gfw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariani L., Stengel B., Combe C., et al. The CKD Outcomes and Practice Patterns Study (CKDopps): rationale and methods. Am J Kidney Dis. 2016;68(3):402–413. doi: 10.1053/j.ajkd.2016.03.414. [DOI] [PubMed] [Google Scholar]
- 13.Zee J., Muenz D., McCullough K.P., et al. Potential surrogate outcomes for kidney failure in advanced CKD: evaluation of power and predictive ability in CKDopps. Kidney Med. 2022;4(2) doi: 10.1016/j.xkme.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghunathan T.E., Solenberger P.W., Van Hoewyk J. Institute for Social Research, University of Michigan; 2002. IVEware: Imputation and Variance Estimation Software: User Guide. [Google Scholar]
- 15.Little R.J.A., Rubin D.B. Wiley; 1987. Statistical Analysis with Missing Data. [Google Scholar]
- 16.Solak B., Acikgoz S.B., Sipahi S., Erdem T. Epidemiology and determinants of pruritus in pre-dialysis chronic kidney disease patients. Int Urol Nephrol. 2016;48(4):585–591. doi: 10.1007/s11255-015-1208-5. [DOI] [PubMed] [Google Scholar]
- 17.Wulczyn K.E., Rhee E.P., Myint L., Kalim S., Shafi T. Incidence and risk factors for pruritus in patients with nondialysis CKD. Clin J Am Soc Nephrol. 2023;18(2):193–203. doi: 10.2215/CJN.09480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S., Zhou F., Xiong Y. Prevalence and risk factors of senile pruritus: a systematic review and meta-analysis. BMJ Open. 2022;12(2) doi: 10.1136/bmjopen-2021-051694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisoni R.L., Wikström B., Elder S.J., et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21(12):3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 20.Lee H.G., Stull C., Yosipovitch G. Psychiatric disorders and pruritus. Clin Dermatol. 2017;35(3):273–280. doi: 10.1016/j.clindermatol.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Kimura H., Tanaka K., Saito H., et al. Association of polypharmacy with kidney disease progression in adults with CKD. Clin J Am Soc Nephrol. 2021;16(12):1797–1804. doi: 10.2215/CJN.03940321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason N.A. Polypharmacy and medication-related complications in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2011;20(5):492–497. doi: 10.1097/MNH.0b013e328349c261. [DOI] [PubMed] [Google Scholar]
- 23.Yosipovitch G., Awad A., Spencer R.H., Munera C., Menzaghi F. A phase 2 study of oral difelikefalin in subjects with chronic kidney disease and moderate-to-severe pruritus. J Am Acad Dermatol. 2023;89(2):261–268. doi: 10.1016/j.jaad.2023.03.051. [DOI] [PubMed] [Google Scholar]
- 24.Narita I., Alchi B., Omori K., et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626–1632. doi: 10.1038/sj.ki.5000251. [DOI] [PubMed] [Google Scholar]
- 25.Chen H.Y., Chiu Y.L., Hsu S.P., et al. Elevated C-reactive protein level in hemodialysis patients with moderate/severe uremic pruritus: a potential mediator of high overall mortality. QJM An Int J Med. 2010;103(11):837–846. doi: 10.1093/qjmed/hcq036. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel M., Alscher D.M., Dunst R., et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant. 2006;21(3):749–755. doi: 10.1093/ndt/gfi204. [DOI] [PubMed] [Google Scholar]
- 27.Nowak K.L., Chonchol M. Does inflammation affect outcomes in dialysis patients? Semin Dial. 2018;31(4):388–397. doi: 10.1111/sdi.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.