Abstract
Activation of Wnt/β-catenin signaling supports the self-renewal of mouse embryonic stem cells. We aimed to understand the effects of Wnt signaling activation or inhibition on chicken embryonic stem cells (chESCs), as these effects are largely unknown. When the glycogen synthase kinase-3 β inhibitor CHIR99021—which activates Wnt signaling—was added to chESC cultures, the colony shape flattened, and the expression levels of pluripotency-related (NANOG, SOX2, SOX3, OCT4, LIN28A, DNMT3B, and PRDM14) and germ cell (CVH and DAZL) markers showed a decreasing trend, and the growth of chESCs was inhibited after approximately 7 d. By contrast, when the Wnt signaling inhibitor XAV939 was added to the culture, dense and compact multipotent colonies (morphologically similar to mouse embryonic stem cell colonies) showing stable expression of pluripotency-related and germline markers were formed. The addition of XAV939 stabilized the proliferation of chESCs in the early stages of culture and promoted their establishment. Furthermore, these chESCs formed chimeras. In conclusion, functional chESCs can be stably cultured using Wnt signaling inhibitors. These findings suggest the importance of Wnt/β-catenin signaling in avian stem cells, offering valuable insights for applied research using chESCs.
Key words: CHIR99021, embryonic stem cells, small molecule inhibitor, Wnt signaling, XAV939
INTRODUCTION
Embryonic stem cells (ESCs) can differentiate into 3 germ layers and exhibit self-renewal capacity. Mouse embryonic stem cells (mESCs) were the first ESCs to be established in 1981 using the inner cell mass of mice (Evans and Kaufman, 1981). Leukemia inhibitory factor (LIF) is an important factor that maintains undifferentiated mESCs in vitro (Williams et al., 1988). LIF is a member of the interleukin-6 (IL-6) cytokine family and binds to the LIF receptor-β (LIFRβ) and glycoprotein 130 (gp130) heterodimer (Taga and Kishimoto, 1997). When ligands bind to receptors, Janus kinase (JAK)—a tyrosine kinase—is activated, which supports the maintenance of mESC pluripotency and self-renewal capacity via the phosphorylation of signal transducer and activator of transcription 3 (STAT3; Niwa et al., 1998). In 2008, a culture system involving the inhibition of mitogen-activated protein kinase (MAPK)-mediated differentiation signals and glycogen synthase kinase-3 β (GSK3β) was reported to strongly support mESC culture (Ying et al., 2008).
ESCs have been established in various mammals, including humans, through advances in regenerative medicine and animal genetics. However, the proliferative signals of ESCs in rodents, including mice, rats, and other animal species, are different. In rodents, JAK/STAT signaling is important for ESC self-renewal, whereas fibroblast growth factor 2 (FGF2), a member of the FGF family, is important for human ESCs (Amit et al., 2000).
Research on chicken embryonic stem cells (chESCs) has progressed. Embryogenesis differs between chickens and mammals because no stage corresponding to that of the inner cell mass of the blastocyst has been observed in chickens. Transplantation experiments conducted using blastoderms have demonstrated that blastoderms can differentiate into somatic and germ cells (Petitte et al., 1990), and chESCs have been established by collecting blastoderms and culturing them in vitro (Pain et al., 1996). In 2004, the chicken LIF (chLIF) was cloned (Horiuchi et al., 2004), and STAT3 phosphorylation by chLIF was reported to be important for maintaining chESCs (Yamashita et al., 2006). Interestingly, chESCs maintain pluripotency by activating JAK/STAT signaling (Nakanoh et al., 2017) and form chimeras upon transfer to early embryos (Nakano et al., 2011). Furthermore, RNA-seq analysis has revealed a higher degree of similarity between chESCs and mESCs (Jean et al., 2015) than between mouse epiblast stem cells (mEpiSCs; Tesar et al., 2007). However, germline transmission, which is a characteristic of mESCs, has rarely been observed in chESCs (Van De Lavoir et al., 2006a). Moreover, PouV, a gene homologous to OCT4—a central pluripotency factor in mammalian ESCs—may not be associated with the maintenance of pluripotency in chickens (Nakanoh et al., 2015). Based on these findings, chESCs are considered to possess unique characteristics.
To further understand chESCs, we focused on Wnt signaling, a representative signaling pathway that supports the proliferation of mESCs. The Wnt gene family encodes glycoproteins that regulate embryonic development (Moon, 2005). When Wnt/β-catenin signaling is activated, β-catenin migrates to the nucleus, binds to the T-cell factor (TCF) and lymphoid enhancer-binding factor (LEF) families, and functions as a transcription factor. The activation of Wnt/β-catenin signaling reportedly maintains undifferentiated mESCs (Sato et al., 2004; Hao et al., 2006). However, various studies have reported different functions of Wnt/β-catenin signaling in human ESCs (hESCs; Sato et al., 2004; Davidson et al., 2012).
Many studies have been conducted in mammals to determine the effect of Wnt/β-catenin signaling on mammalian ESCs. However, the effect of Wnt/β-catenin signaling on chESCs, which play an important role in mammalian ESCs, remains unknown. In this study, we investigated the effects of Wnt/β-catenin signaling on chESCs. The knowledge gained from our findings will be useful for applied research on pluripotent chicken stem cells.
MATERIALS AND METHODS
Experimental Animals
White Leghorn chickens were used in this study. Fertilized white leghorn eggs were purchased from Akita Foods (Fukuyama, Japan). Chicken blastodermal cells (chBCs) were collected from fertilized eggs at embryonic stage X, as described by Eyal-Giladi and Kochav (1976), following a previously reported protocol (Horiuchi et al., 2006).
The animal experiments conducted in this study were approved by the Animal Experimentation Committee of Hiroshima University (approval number: C21-45-4) and were conducted in accordance with Hiroshima University guidelines.
Culture of Chicken Embryonic Stem Cells
The chESCs used in this study were established from individual fertilized eggs. Three types of chESCs established from different fertilized eggs were tested for culture conditions to account for individual differences in fertilized eggs. chBCs were cultured in 6-well plates (Corning Inc., Corning, NY) or 60-mm dishes (Corning) seeded with feeder cells at 38°C, 5% CO2, and 3% O2. Sandoz inbred mouse-derived thioguanine- and ouabain-resistant (STO) cells (American Type Culture Collection, Manassas, VA) were used as feeder cells. STO cells were cultured in Dulbecco's modified Eagle medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Sartorius AG, Göttingen, Germany) at 37°C and 5% CO2. Cultured STO cells were treated with 10 μg/mL mitomycin C (Sigma-Aldrich, St. Louis, MO) and used as feeder cells. Feeder cells were seeded at a density of 1.5 × 104 cells/cm2 in 6-well plates or 60-mm dishes coated with 0.1% gelatin (Nacalai Tesque, Kyoto, Japan)-distilled water.
The chESCs were cultured in chicken embryonic stem cell medium supplemented with 20% Knockout Serum Replacement, 2% chicken serum, 1 × sodium pyruvate, 1 × modified Eagle's medium nonessential amino acid solution (all from Thermo Fisher Scientific), 1 × EmbryoMax nucleosides (Merck, Darmstadt, Germany), 1 × GlutaMAX (Thermo Fisher Scientific), 1 × antibiotic-antimycotic mixed stock solution (Nacalai Tesque), and 1 × monothioglycerol (Fujifilm Wako Pure Chemical, Osaka, Japan). In the passaging, chESCs were washed twice with PBS and incubated with TrypLE Express (Thermo Fisher Scientific) for 3 to 5 min at 37°C. XAV939-added chESCs were then separated into single cells by pipetting and pelleted by centrifugation at 200 × g for 3 min. However, chESCs to which XAV939 was not added were not amenable to single-cell passage and were passaged via clamp passage. The chESCs were transferred at 1/3 to 1/2 passaging ratio to plates seeded with feeder cells. Half of the medium was replaced daily with a fresh medium. Cryopreservation of chESCs was performed according to standard protocols using a STEM-CELLBANKER GMP grade (TaKaRa Bio, Shiga, Japan).
Other additives included 20 ng/mL chLIF (Horiuchi et al., 2004), 1 μM PD0325901 (Axon Medchem, Groningen, the Netherlands), 10 μM Y-27632 (Fujifilm Wako Pure Chemical), 3 μM CHIR99021 (Focus Biomolecules, Plymouth Meeting, PA), 5 μM XAV939 (Fujifilm Wako Pure Chemical), 5 μM IWP-2 (Fujifilm Wako Pure Chemical), and 2 μM Gö6983 (Fujifilm Wako Pure Chemical).
Counting of Small Colonies
A colony formation assay was performed to determine the percentage of high-density colonies (Nakanoh et al., 2013). ChESCs were cultured under various conditions. After colony formation, 10 random fields of view were captured using an IX71 microscope (Olympus, Tokyo, Japan). Compact colonies with a high density in the field of view (approximately 2.2 mm2) were counted, and the total colony count (percentage) was calculated.
Alkaline Phosphatase Staining
ChESCs cultured in 6-well plates were subjected to alkaline phosphatase (AP) staining. At the time of staining, the culture medium was removed, and the chESCs were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA; Fujifilm Wako Pure Chemical). The fixed chESCs were stained using a Stemgent AP Staining Kit II (ReproCELL, Kanagawa, Japan) according to the manufacturer's instructions.
Proliferation Assay of chESCs
To examine the growth efficiency of chESCs under each culture condition, the collected chBCs were seeded onto 12-well plates at a density of approximately 3.0 × 105 cells/well (approximately half of one epiblast). Upon reaching confluency, cells were counted using a blood cell counter. For each culture condition, chESCs were cultured in 3 different wells, and cell counts were determined by the average of the 3 wells. chESCs cultured for more than 30 d were seeded into 12-well plates at approximately 1.5 × 105 cells/well, and cell counts were determined at each passaging point. Doubling time was calculated using the online algorithm “Doubling Time” (www.doubling-time.com/).
Reverse Transcriptase Quantitative PCR
Total RNA was extracted from the chESCs using a FastGene RNA Premium Kit (Nippon Genetics Co., Ltd., Tokyo, Japan). The extracted RNA was rapidly transcribed into cDNA using SuperScript IV Reverse Transcriptase (Thermo Fisher Scientific). Reverse transcriptase quantitative PCR (RT-qPCR) was performed using the KOD SYBR qPCR Mix (TOYOBO, Osaka, Japan). The PCR conditions were as follows: initial denaturation step: 98°C for 2 min; amplification steps: 98°C for 10 s, 65°C for 10 s, and 68°C for 30 s for 40 cycles; and melting curve analysis: 95°C for 15 s, 60°C for 1 min, and 99°C for 15 s. The target genes were β-actin and pluripotency-related (NANOG, SOX2, SOX3, OCT4, LIN28A, DNMT3B, and PRDM14) and germ cell (CVH and DAZL) markers. The primer sequences are shown in Table S1. Gene expression levels were normalized to the expression level of β-actin.
Immunofluorescence of chESCs
The expression of pluripotency markers in chESCs was analyzed using immunofluorescence. chESCs were fixed with 4% PFA-PBS at 20°C to 25°C for 30 min and washed with PBS. Subsequently, 0.1% TritonX-100-PBS was added and chESCs were incubated at 20°C to 25°C for 5 min. After washing with PBS, the chESCs were blocked with 3% bovine serum albumin (BSA)-PBS for 15 min at 20°C to 25°C. After washing with 0.1 % BSA-PBS, the chESCs were incubated with primary antibody (anti-NANOG; Nakano et al., 2011, diluted 1:25 using 1% BSA-PBS), an anti-stage-specific embryonic antigen-1 (SSEA-1) antibody (sc-21702; Santa Cruz Biotechnology, Dallas, TX; diluted 1:100 using 1% BSA-PBS) for 40 min at 37°C. After washing with 0.1% BSA-PBS, the chESCs were incubated with secondary antibodies (Alexa Fluor 488 goat anti-mouse immunoglobulin M [IgM; µ chain]; Ref: A21042; Thermo Fisher Scientific; diluted 1:200 using 1% BSA-PBS), goat anti-rabbit IgG (Alexa Fluor 594 goat anti-rabbit immunoglobulin G [IgG; H + L]; Ref: A11037; Thermo Fisher Scientific; diluted 1:200 using 1% BSA-PBS) for 40 min at 37°C. After washing, the samples were sealed with 4′,6-diamidino-2-phenylindole (DAPI) in VECTASHIELD Mounting Medium (Vector Laboratories, Burlingame, CA). Fluorescence was observed using a fluorescence microscope (BX53; Olympus, Tokyo, Japan).
Measurement of Stage-Specific Embryonic Antigen-1-Positive Cell Ratio Using Flow Cytometry
The percentage of SSEA-1-positive cells among the chESCs cultured under various conditions was determined using a MA900 cell sorter (Sony Biotechnology, San Jose, CA). The chESCs were separated into single cells using TrypLE Express and washed with a wash buffer (0.5% BSA and 0.1% N3Na-PBS). After washing, the chESCs were incubated with SSEA-1 (480) Alexa Fluor 488 (sc-21702 AF488; Santa Cruz Biotechnology; diluted 1:100 with wash buffer) for 40 min on ice. After incubation, the samples were washed with wash buffer and fixed with 1% PFA-PBS. The cells were washed twice with wash buffer before the assay.
Western Blot
Western blotting was performed to analyze the Wnt/β-catenin signal. Samples included chBCs treated with CHIR99021, XAV939, and IWP-2 at concentrations of 1, 5, and 10 μM. Control samples were supplemented with DMSO. chBCs were collected from fertilized eggs and seeded onto 12 well plates at a density of approximately 3.0 × 105 cells/well (approximately half of one epiblast). After 72 h, cytoplasmic/nuclear extracts were prepared using the Nuclear/Cytosol Fractionation Kit (ab289882; Abcam, Cambridge, UK). The cytoplasmic/nuclear extracts were then subjected to protein concentration estimation using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Each sample was heat-treated at 70°C for 10 min after the addition of 2-mercaptoethanol at a concentration of 2.5%. Five hundred ng/lane was subjected to SDS-PAGE to separate the contained proteins. Separated proteins were transferred onto a membrane (Immun-BlotPVDF Membrane for Protein Blotting 0.2 mm; Bio-Rad Laboratories, Hercules, CA). The membrane was blocked with PVDF Blocking Reagent for Can Get Signal (TOYOBO) at 4°C for approximately 10 h. Primary antibodies were anti-β-catenin antibody (GTX101435; beta-Catenin antibody [N1N2-2], N-term; dilution 1:350l GeneTex, Irvine, CA), anti-α -Tublin (ab4074; anti-alpha Tublin antibody (pAb); dilution 1:1,000; Abcam), anti-Histon H3 (39064; Histon H3 antibody (mAb); dilution 1:1,000; Active Motif, Carlsbad, CA) and anti-Axin1 (16541-1-AP; AXIN1 polyclonal antibody; dilution 1:600; Proteintech, Rosemount, IL) were used. These primary antibodies were diluted with Solution1 (Can GetSignal Immunoreaction Enhancer Solution, TOYOBO). The primary antibody reaction was performed at 20°C to 25°C for 1 h. After washing with 0.1% PBST, the secondary antibody reaction was performed. Secondary antibodies included HRP-labeled anti-mouse IgG (474-1806; Affinity Purified Antibody Peroxidase Labeled Goat anti-mouse IgG (H + L); Kirkegaard & Perry Laboratories, Gaithersburg, MD) and HRP-labeled anti-rabbit IgG (474-1516; Affinity Purified Antibody Peroxidase Labeled Goat anti-rabbit IgG (H + L); Kirkegaard & Perry Laboratories). These secondary antibodies were diluted at 1:100,000 with Solution2 (Can GetSignal Immunoreaction Enhancer Solution, TOYOBO). Secondary antibody reactions were performed at 20°C to 25°C for 1 h. SuperSignal West Atto Ultimate Sensitivity Substrate (Thermo Fisher Scientific) was used as the chemiluminescence detection reagent. Chemiluminescence was detected by Amersham Imager 680 (GE Healthcare, Chicago, IL).
Preparation of Enhanced Green Fluorescent Protein-Expressing chESCs
Enhanced green fluorescent protein (EGFP)-expressing chESCs were generated using the PiggyBac system. The PiggyBac donor vector contained an elongation factor-1α (EF1α)-EGFP cassette. Two types of vectors expressing PiggyBac transposase (PBase) and EF1α-EGFP (VectorBuilder Japan, Inc., Kanagawa, Japan) were constructed and transferred into chESCs (Figure S1). Lipofectamine 3000 (Thermo Fisher Scientific) was used for gene transfer. Seven d after the gene transfer, EGFP-expressing chESCs were sorted using the cell sorter.
Generation of Chimeric Chickens Using EGFP-Expressing chESCs
Established EGFP-expressing chESCs (2.0 × 104 cells) were injected into the subgerminal cavity of White Leghorn stage X-fertilized eggs. The recipient embryos were then filled with egg whites and sealed with clear plastic wrap. After 5 d, the embryos were separated from the yolk, washed with PBS, and observed using a fluorescence stereomicroscope (Leica M165 FC; Leica Microsystems GmbH, Wetzlar, Germany).
Statistics
Statistical analyses were performed, and some graphical depictions were created using GraphPad Prism 10 software. Welch's t test was used to compare results obtained for the 2 groups, and Tukey's test was used for multiple comparisons (ns: not significant; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
RESULTS
Culture of chESCs Using chLIF and 2 Inhibitors
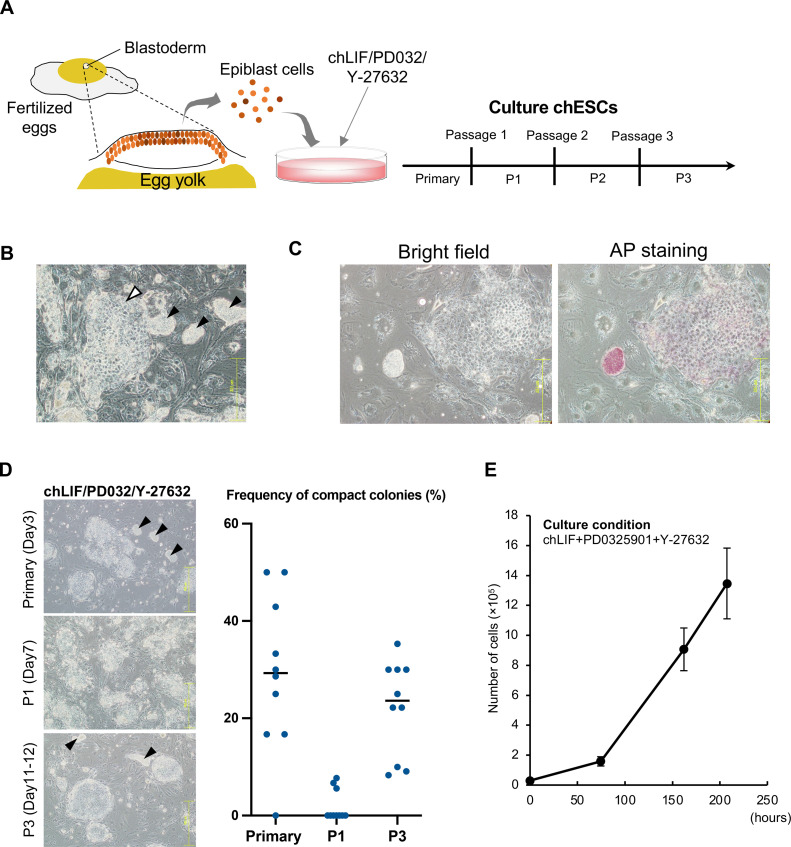
The feasibility of stably culturing chESCs under the conditions reported in previous studies was investigated. As the activation of JAK/STAT signaling and the inhibition of MAPK kinase are useful for the culture of chESCs (Horiuchi et al., 2004; Farzaneh et al., 2018), chBCs collected from fertilized eggs at stage X were cultured in the presence of chLIF and 2 small-molecule inhibitors (PD0325901 and Y-27632; 2i + LIF condition; Figure 1A). Notable, under the 2i + LIF condition, chESCs formed 2 types of colonies (Figure 1B): compact colonies with a high cell density similar to that of mESCs (black arrows), and flat colonies with distinct individual cells (white arrows). AP staining of the chESCs revealed that the small colonies were strongly stained, whereas the flat colonies were weakly stained (Figure 1C). This suggests that small colonies retained a higher degree of pluripotency than flat colonies. After the first passage (P1), the number of compact colonies decreased rapidly (Figure 1D). Depending on the fertilized eggs from which the chBCs were collected, differences in proliferative ability were observed after P1, with some chBCs losing their proliferative ability and dying. In contrast, chBCs that continued to proliferate after P1 showed stable growth. After the third passage (P3), the percentage of small colonies increased compared with that observed after P1 and stable growth and 2 colony morphologies (small and flat) were observed (Figure 1D and E). These results indicate that chBCs cultured under the 2i + LIF condition can establish chESCs, although they exhibit instability.
Figure 1.
Culture of chicken embryonic stem cells (chESCs) under the 2i + leukemia inhibitory factor (LIF) condition. (A) Collection of the blastoderms from fertilized eggs and culture of chESCs. The defined passage number of chESCs is shown. (B) 2 colony morphologies of chESCs are shown: compact colonies with high density (black arrows) and flat colonies (white arrows). (C) Alkaline phosphatase (AP) staining of chESCs on d 4 of culture after chicken blastodermal cells (chBCs) collection. (D) Examination of compact colonies. Percentages of compact colonies were calculated at primary culture stage, first passage (P1), and third passage (P3) after chBCs collection. The line indicates the mean value (n = 10). Compact colonies are indicated by black arrows. (E) Growth curve of chESCs under the 2i + LIF condition. Error bars represent SDs of the mean values. 2i + LIF condition: PD0325901 + Y27632 + chicken LIF (chLIF).
Effect of the Wnt Signal Activator on chESCs
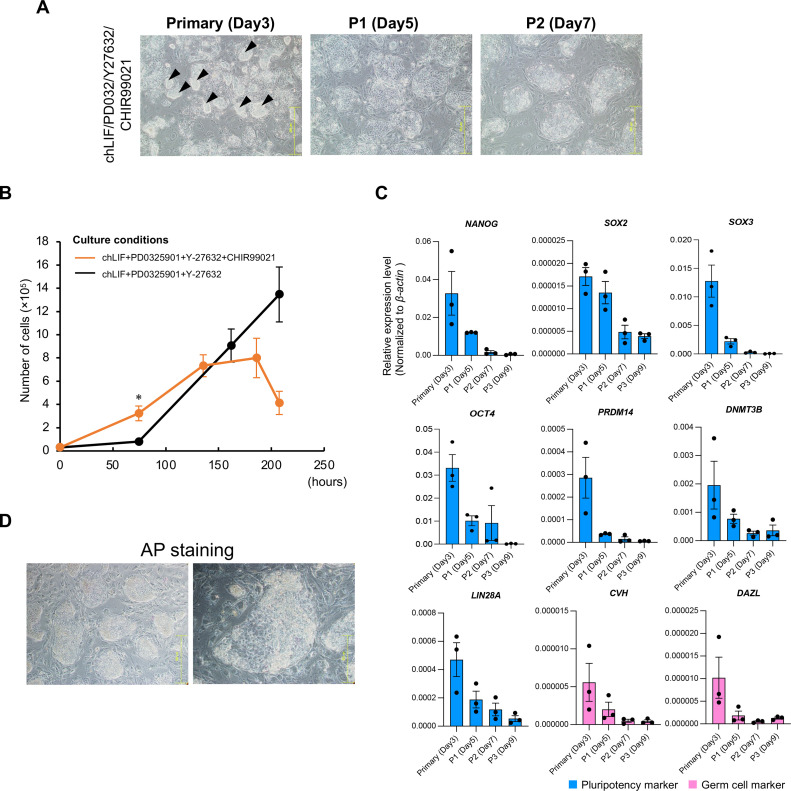
To activate Wnt signaling, a small-molecule inhibitor, CHIR99021, was added to the cultures. CHIR99021 is an aminopyrimidine derivative that inhibits GSK3α and GSK3β. In the primary culture, chESCs formed compact colonies similar to those observed under the 2i + LIF condition (Figure 2A, black arrows). However, the number of compact colonies decreased significantly after P1, as each cell formed flat colonies with distinct individual cells. Thereafter, compact colonies rarely formed, and most chESCs formed flat colonies. The chESCs to which CHIR99021 was added actively proliferated at around 3 d of incubation (Figure 2B, P < 0.05) compared to those under the 2i + LIF condition. However, the proliferative ability of chESCs decreased around 7 d of incubation, eventually leading to a complete cessation of proliferation.
Figure 2.
The activation of Wnt signaling by CHIR99021. (A) Colony morphology of chESCs cultured in the presence of CHIR99021. The chESCs are shown at primary culture stage, first passage (P1), and second passage (P2) after chBCs collection. Compact colonies with high density are indicated using black arrows. (B) Growth curves of chESCs in the presence or absence of CHIR99021. Error bars represent SDs of the mean values. Statistical analysis was performed using Welch's t test. *P < 0.05 (n = 3). (C) The relative expression levels of pluripotency-related (NANOG, SOX2, SOX3, OCT4, LIN28A, DNMT3B, and PRDM14) and germ cell markers (CVH and DAZL). The gene expression levels were normalized based on the expression levels of β-actin. Error bars represent standard errors (SEs) of the mean values. (D) AP staining of chESCs on d 7 after addition of CHIR99021.
To determine whether the addition of CHIR99021 effectively maintained the pluripotency of chESCs, the expression levels of pluripotency-related (NANOG, SOX2, SOX3, OCT4, PRDM14, DNMT3B, and LIN28A) and germ cell (CVH and DAZL) markers were examined. The expression of these markers showed a decreasing trend over time from d 5 to 9 after the addition of CHIR99021 (Figure 2C). The chESCs cultured with CHIR99021 were negative for AP staining (Figure 2D), which was consistent with the RT-qPCR results. These results suggested that the continuous activation of Wnt signaling did not promote the maintenance of pluripotency in chESCs.
Effects of Wnt Signaling Inhibitors on chESCs
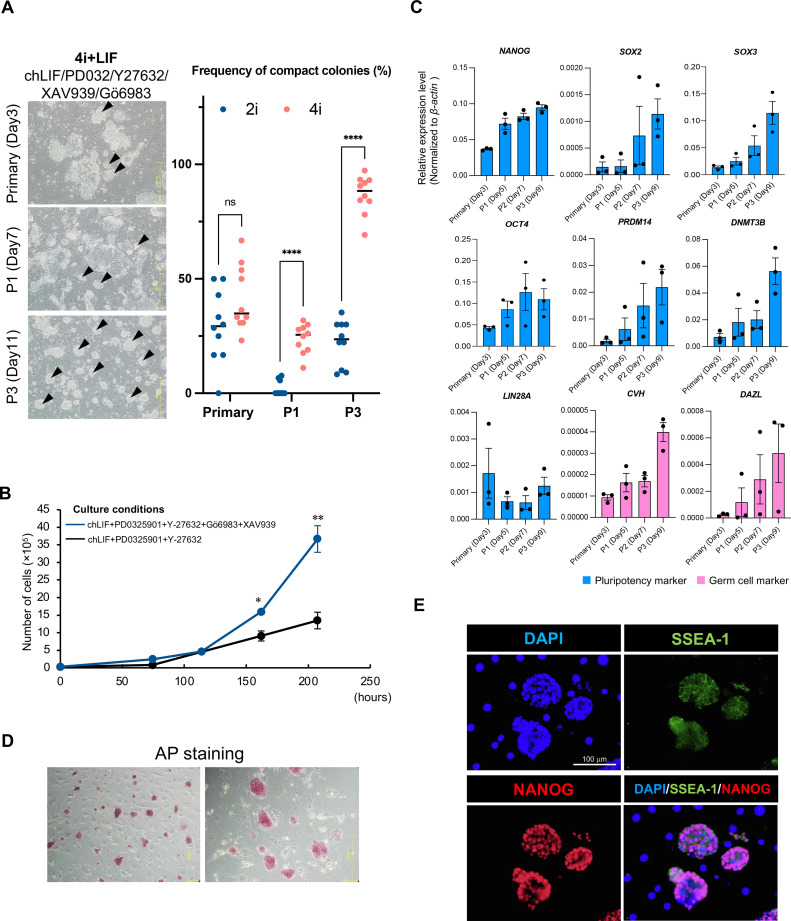
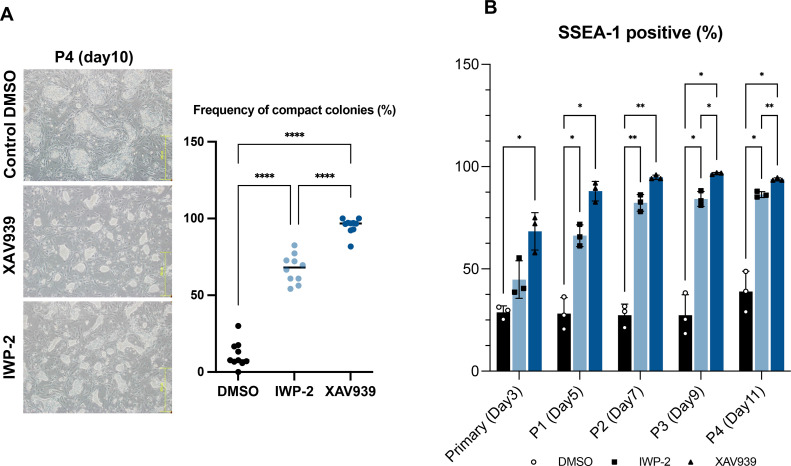
We attempted to inhibit Wnt signaling because the expression levels of pluripotency-related and germ cell markers decreased after the addition of CHIR99021; we used XAV939, a Wnt signaling inhibitor. Furthermore, to optimize the chESC culture conditions, we added the protein kinase C (PKC) inhibitor Gö6983. ChESCs were cultured in the presence of 4 inhibitors (PD0329501, Y-27632, XAV939, and Gö6983) and chLIF (4i + LIF). XAV939 increased the percentage of SSEA-1-positive cells at 2.5 to 10 μM concentrations (Figure S2). The higher the concentration of XAV939, the faster the increase in SSEA-1 positivity, but the difference in positivity became smaller as the duration of incubation increased. In the current experiment, a concentration of 5 μM was employed, which more stably increased SSEA-1 positivity. In the primary culture of chBCs, approximately 40% of the colonies were of the compact type (Figure 3A). Several flat colonies were observed after P1. However, after P1, the chESCs formed more compact colonies than those observed under 2i + LIF conditions (P < 0.0001). Furthermore, after P3, >80% of the chESCs formed compact colonies. The chESCs cultured under the 4i + LIF condition exhibited stable proliferation, with significantly higher cell numbers at around 7 d of culture compared to those under the 2i + LIF condition (Figure 3B; P < 0.05). Furthermore, the instability of chESCs under the 2i + LIF condition was not observed, and most chESCs exhibited stable growth. This result suggests that the 4i + LIF condition supports the establishment of chESCs by stabilizing their growth of chESCs in the early phase of culture, particularly when the culture of chESCs tends to become unstable. In addition, ChESCs cultured under 2i + LIF conditions were not able to undergo single-cell passage, whereas those under 4i + LIF conditions were capable of single-cell passage. To determine whether the chESCs maintained pluripotency under 4i + LIF conditions, the expression levels of pluripotency-related (NANOG, SOX2, SOX3, OCT4, PRDM14, DNMT3B, and LIN28A) and germ cell (CVH and DAZL) markers were analyzed; the expression levels of some these markers showed an increasing trend over time from d 5 to 9 (Figure 3C). AP staining results showed that most chESCs were strongly positive for AP staining, in contrast to when CHIR99021 was added (Figure 3D). In addition, the expression of SSEA-1 and NANOG, markers of pluripotency, was examined using immunofluorescence staining. The results showed that SSEA-1 and NANOG were localized on the membrane and nucleus, respectively, confirming the expression of pluripotency markers (Figure 3E). These results suggest that XAV939, a Wnt signaling inhibitor, and Gö68983, a PKC inhibitor, helped maintain the pluripotency of chESCs.
Figure 3.
The inhibition of Wnt signaling by XAV939. (A) Colony morphology of chESCs under the 4i + LIF condition. Compact colonies with high densities are indicated using black arrows. The percentages of compact colonies were calculated at primary culture stage, first passage (P1), and third passage (P3) after chBCs collection. The red plots show the 2i + LIF condition, and the blue plots show the 4i + LIF condition. The line indicates the mean value. Statistical analysis was performed using Welch's t test. ns: not significant; ****P < 0.0001 (n = 10). (B) Growth curves of chESCs under 4i + LIF and 2i + LIF conditions. Error bars represent SDs of the mean values. Statistical analysis was performed using Welch's t test. *P < 0.05, **P < 0.01 (n = 3). (C) The relative expression levels of pluripotency-related (NANOG, SOX2, SOX3, OCT4, LIN28A, DNMT3B, and PRDM14) and germ cell markers (CVH and DAZL). The gene expression levels were normalized based on the expression levels of β-actin. Error bars represent SEs of the mean values. (D) AP staining of chESCs cultured under the 4i + LIF condition. (E) Expression analysis of pluripotency markers (SSEA-1, NANOG) using immunofluorescence. DAPI was used for counterstaining, and MERGE images were composited from DAPI-, SSEA-1-, and Nanog-positive areas. 4i + LIF condition: PD0325901 + Y27632 + XAV939 + Gö6983 + chLIF.
Examination of Cytokines and Small-Molecule Inhibitors in the Culture
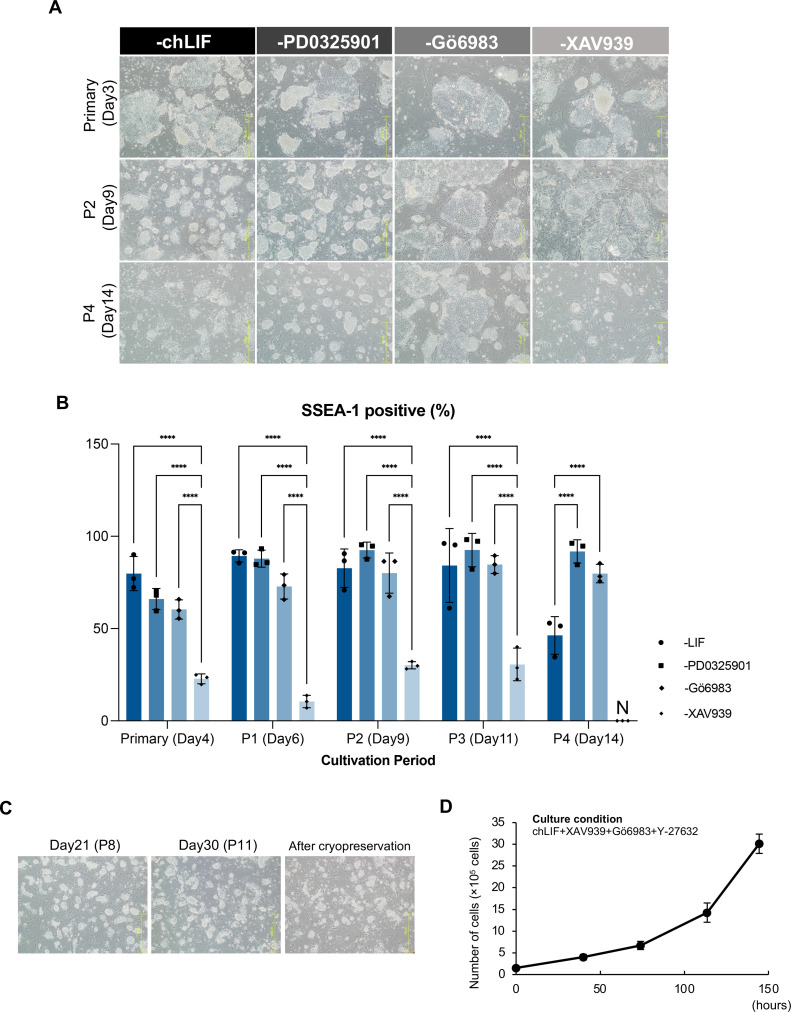
To identify the factors necessary for maintaining the pluripotency of chESCs under 4i + LIF conditions, the chESCs were cultured in the absence of each factor. In the absence of chLIF, compact colonies formed after the second passage (d 9). However, after the fourth passage (d 14) some colonies differentiated into fibroblast-like cells (Figure 4A). In the absence of PD0325901, the chESCs formed compact colonies and showed stable proliferation. Interestingly, the absence of Gö6983, a PKC inhibitor, promoted the formation of flat colonies with distinct individual cells. Importantly, in the absence of XAV939, compact colonies were rarely observed, and the cells eventually ceased to proliferate and died. The positivity rate of SSEA-1, a pluripotency marker, was analyzed using flow cytometry. The percentage of SSEA-1-positive cells increased significantly after the addition of XAV939 (P < 0.0001; Figure 4B). Figure 4A and B shows that XAV939, an inhibitor of Wnt signaling, promoted the maintenance of pluripotency in chESCs. The chESCs cultured without PD0325901 exhibited stable growth after more than 10 passages (Figure 4C). Following cryopreservation, chESCs maintained compact colony morphology, although differentiated cell morphology was observed in some of these cells. The chESCs cultured for more than 30 d showed stable proliferation akin to that during the initial culture, with a doubling time of 33.15 ± 1.57 h (Figure 4D). The culture conditions for chLIF, XAV939, Gö6983, and Y- 27632 were selected as the culture conditions for chESCs because they supported stable growth of chESCs for more than 30 d.
Figure 4.
Examination of culture additives. (A) The morphology of chESCs at primary culture stage (d 3), second passage (P2; d 9), and fourth passage (P4; d 14) after chBCs collection, cultured in the absence of cytokine, and small-molecule inhibitors (chLIF, PD0325901, Gö6983, and XAV939). (B) The percentage of stage-specific embryonic antigen-1 (SSEA-1)-positive cells cultured in the absence of culture additives. chESCs were examined at primary culture stage (d 4), first passage (P1; d 6), second passage (P2; d 9), third passage (P3; d 11), and fourth passage (P4; d 14) after chBCs collection. Error bars represent SDs of the mean values. Statistical analysis was performed using Tukey's test; ****P < 0.0001 (n = 3). “N” indicates not measured. (C) Morphology of chESCs after 14 d of culture. chESCs of d 21 (P8), d 30 (P11), and after cryopreservation are shown. (D) Growth curve of chESCs cultured for more than 30 d. The culture condition is chLIF + XAV939 + Gö6983 + Y-27632.
Subsequently, an assessment was conducted to ascertain whether chESC pluripotency was maintained by other Wnt signaling inhibitors. We used IWP-2, a Wnt signaling inhibitor with a different target of action from that of XAV939, after conducting inhibition studies using different concentrations of IWP-2 (Figure S3). Compact colonies were formed by chESCs cultured in the presence of IWP-2 instead of XAV939, and these colonies were similar to those formed by chESCs cultured in the presence of XAV939 (Figures 5A). However, the percentage of compact colonies was lower in the IWP-2 group than in the XAV939 group (P < 0.0001). Flow cytometric analysis revealed a significantly higher percentage of SSEA-1-positive cells in the IWP-2 group than in the control group (treated with dimethyl sulfoxide; P < 0.05; Figure 5B). However, the percentage of SSEA-1-positive cells was lower in the IWP-2 group than in the XAV939 group (P < 0.05). These results suggested that the inhibition of Wnt signaling promoted the maintenance of pluripotency in chESCs, and the extent of pluripotency maintenance varied depending on the type of inhibitor used.
Figure 5.
Addition of the small-molecule inhibitor IWP-2. (A) The morphology of chESCs cultured in the presence of IWP-2 at P4 (d 10) after chBCs collection. Cells cultured in the presence of dimethyl sulfoxide (DMSO), XAV939, and IWP-2 are shown. The plots indicate the percentage of compact colonies formed by chESCs cultured in the presence of DMSO (chLIF, Gö6983, and Y-27632), XAV939 (chLIF, XAV939, Gö6983, and Y-27632), and IWP-2 (chLIF, XAV939, Gö6983, and Y-27632). The line indicates the mean value. Statistical analysis was conducted using Tukey's test. ****P < 0.0001 (n = 10). (B) Percentage of SSEA-1-positive cells in chESCs, cultured in the presence of DMSO, XAV939, and IWP-2, at primary culture stage (d 3), P1 (d 5), P2 (d 7), P3 (d 9), and P4 (d 11) after chBCs collection. Error bars represent SDs of the mean values. Statistical analysis was performed using Tukey's test. *P < 0.05; **P < 0.01 (n = 3).
In addition, the effect of small molecule inhibitors used (CHIR99021, XAV939, and IWP-2) on the levels of β-catenin a mediator of Wnt/β-catenin signaling, was investigated. The results suggest that the addition of CHIR99021 stabilized β-catenin levels, while the addition of XAV939 and IWP-2 decreased β-catenin levels (Figure S4A). In addition, XAV939, which relatively strongly supported the pluripotency of chESCs compared to IWP-2, contributed to the stabilization of axis inhibition protein 1 (Axin1) (Figure S4B). Therefore, XAV939 may contribute to the regulation of Wnt/β-catenin signaling through the stabilization of Axin1.
Evaluation of the Differentiation Potential of chESCs
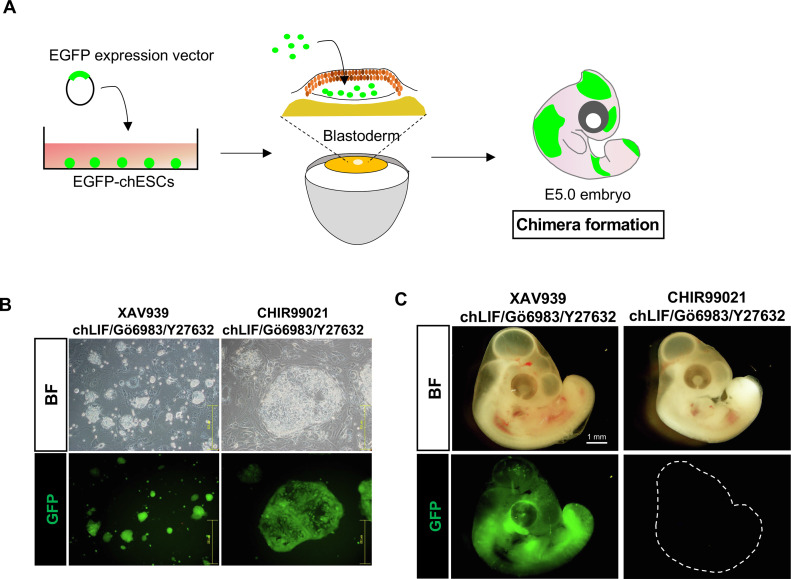
The in vivo differentiation potential of chESCs cultured in the presence of Wnt signaling activators and inhibitors was analyzed (Figure 6A). The chESCs were fluorescently labeled (Figure 5, Figure 6B). EGFP-chESCs (2.0 × 104 cells) were transferred into the subgerminal cavity of stage X-fertilized eggs and incubated for 5 d. EGFP fluorescence was observed in embryos harboring transferred-EGFP-chESCs cultured in XAV939 medium (Figure 6C). Some embryos exhibited EGFP fluorescence in over half of the body, whereas some embryos displayed almost no fluorescence. This discrepancy may be attributed to the chimera formation ability of the chESCs and the success rate of transfer to early embryos. In contrast, minimal EGFP fluorescence was observed in embryos harboring transferred-EGFP-chESCs cultured in the CHIR99021 medium, suggesting a loss of chimeric formation ability.
Figure 6.
Evaluation of in vivo differentiation of chESCs. (A) Schematic diagram depicting the process of analysis of chimera formation. (B) Fluorescently labeled chESCs cultured in the presence of XAV939 and CHIR99021. (C) Expression of enhanced green fluorescent protein (EGFP) in d 5-embryos. Embryos harboring chESCs cultured in the presence of XAV939 or CHIR99021 are shown.
DISCUSSION
In this study, the effect of Wnt/β-catenin signaling on chESCs was analyzed and the inhibition of Wnt/β-catenin signaling was shown to promote the maintenance of pluripotency in chESCs. In contrast, the activation of Wnt signaling over several days decreased pluripotency. Activation of Wnt signaling via inhibition of GSK3β promotes self-renewal in mice (Sato et al., 2004; Hao et al., 2006). In humans, the effect of Wnt signaling is not clearly known, and differing effects of Wnt signaling on hESCs have been observed in various studies (Davidson et al., 2012). Recent research has indicated that Wnt signaling has various effects that are regulated over time and does not have a specific effect (Huang et al., 2015). Overall, Wnt signaling is closely related to the regulation of development, and elucidation of its mechanism is important for advancing stem cell research.
The Wnt/β-catenin pathway is evolutionarily conserved. It controls development in chickens (Marvin et al., 2001) and promotes the proliferation of primordial germ cells (PGCs), a type of chicken stem cell (Lee et al., 2016). Wnt signaling activated flat colony formation and reduced pluripotency markers in chESCs, possibly due to differentiation. In this study, we examined the effect of Wnt signaling activation over several days. However, we could not examine the effects of short-term activation and stimulus at low concentration on Wnt signaling. In human ESCs, the short time (within 24 h) activation of Wnt signaling (Huang et al., 2015) and the addition of the Wnt signaling activator at low concentrations (Guo et al., 2017) promoted the proliferation of human pluripotent stem cells (PSCs). Therefore, Wnt signaling may have various functions in chESCs in time- and concentration-dependent manners. In the future, a more detailed examination may provide a better understanding of Wnt signaling in chESCs.
The chESCs exhibited 2 colony morphologies, and the ratio of cells exhibiting these morphologies varied. A previous study reported that chESCs exhibit multiple types of colony morphology (Nakanoh et al., 2015). In that study, different colonies formed from the epiblast and hypoblast layers. Only highly dense and compact colonies, derived from the epiblast layer, expressed NANOG. Consistent with this observation, our findings indicate that highly dense, compact colonies retain a high degree of pluripotency. Interestingly, regulation of Wnt signaling resulted in the formation of different types of colonies. Inhibition and activation of Wnt signaling resulted in the formation of compact and flat colonies, respectively. This was presumably due to the effects of E-cadherin and β-catenin. E-cadherin interacts with β-catenin, which functions as a central factor in Wnt/β-catenin signaling. mESCs form compact colonies via cell adhesion mediated by the E-cadherin–β-catenin complex (Pieters and van Roy, 2014). The morphology of chESCs, like that of mESCs, may be affected by the action of the E-cadherin–β-catenin complex.
β-Catenin not only promotes intercellular adhesion, but also functions as a transcription factor (Daniels and Weis, 2005). Therefore, Western blot analysis suggests that the regulation of Wnt signaling may have affected the maintenance of pluripotency in chESCs via the action of β-catenin. The phosphorylation of β-catenin by GSK3β is inhibited by CHIR99021, which induces the activation of Wnt signaling. As a result, β-catenin is not degraded and accumulates. XAV939, which inhibits Wnt signaling, is a tankyrase inhibitor that promotes β-catenin degradation by stabilizing the axin proteins (Huang et al., 2009). This mechanism may be similar in chESCs. Although the mechanism of action of IWP-2 was not analyzed in this study, a decreasing trend in β-catenin levels was observed. In mammals, IWP-2 inhibits Wnt signaling by suppressing β-catenin accumulation via inhibition of Porcupine (Chen et al., 2009). The small-molecule inhibitors used in this study may have altered the pluripotency of chESCs by regulating gene expression downstream of Wnt signaling via the accumulation and degradation of β-catenin. In contrast, stabilization of β-catenin by the activation of Wnt signaling is important for the proliferation of chicken PGCs (chPGCs) in vitro (Lee et al., 2016). These findings suggest that the regulation of Wnt signaling is important for research on chicken stem cells.
In chickens, chPGCs and chESCs are representative stem cells, each with distinct applications. chPGCs proliferate stably in vitro (Whyte et al., 2015) and contribute to the germ line in vivo (Van de Lavoir et al., 2006b), and are, therefore, actively used for the generation of genetically modified chickens. In contrast, chESCs are highly differentiated into somatic cells by transplantation into fertilized eggs (Van De Lavoir et al., 2006a). However, their germline differentiation is limited, and there is no precedent for obtaining offspring derived from ESCs cultured over an extended period. Therefore, ESCs are utilized for research on early development in birds (Nakanoh et al., 2017) and for the evaluation of the production of useful proteins (Zhu et al., 2005). Although we could not investigate germ line transmission in chESCs in the present study, it is noteworthy that the mRNA expression levels of germ cell markers were increased. Further analysis of the ability of chESCs cultured in optimized media to differentiate into germ cells may provide new insights.
Mammalian PSCs can either be in the “naïve” or “prime” state (Nichols and Smith, 2009). The naïve type is defined as an undifferentiated state, whereas the prime type is defined as an advanced stage of development. Therefore, research on the establishment of naïve PSCs for use in regenerative medicine and genetic engineering has been actively conducted in several animal species (Gafni et al., 2013; Chen et al., 2015; Kawaguchi et al., 2015). Several recent studies have suggested that naïve and primed states may also exist in birds. The developmental stages of fertilized eggs during oviposition differ between bird species. Zebra finches lay eggs at an earlier developmental stage than chickens at stages VI to VII. Finch blastoderms express more naïve-specific marker genes than do chicken blastoderms (Mak et al., 2015). In a comprehensive study of gene expression conducted using mESCs, mEpiSCs, and chESCs, the expression profile of chESCs was observed to be more similar to that of mESCs than mEpiSCs (Jean et al., 2015). In a recent study on avian-induced pluripotent stem cells (iPSCs), iPSCs from various bird species were found to exhibit different properties; in particular, Japanese ptarmigan-derived iPSCs showed properties similar to those of mESCs (Katayama et al., 2022). In our study, chESCs cultured in the presence of Wnt signaling inhibitors showed several characteristics similar to those of naïve mESCs (Nichols and Smith, 2009), including colony morphology, dependence on LIF signaling, chimera-formation ability, and high clonogenicity. However, it was not possible to define the status of chESCs due to the different developmental patterns between mammals and birds and the lack of clear markers known to define naïve and prime states in birds. Further detailed analyses may help clearly define the characteristics of avian ESCs.
In conclusion, we investigated the effects of Wnt/β-catenin signaling on chESCs. Using Wnt signaling inhibitors, we found that chESCs could be stably maintained in an undifferentiated state. This result indicates that Wnt/β-catenin signaling plays an important role in stem cell research not only in mammals but also in birds. Based on these findings, further progress in avian stem cell research is expected.
ACKNOWLEDGMENTS
The authors acknowledge Senior Research Associates: Yasuhiko Takahashi and Norio Sasaoka; and Research Associates: Masatoshi Yamamoto and Koji Asano for their help in interpreting the significance of the results of this study. This work was supported by JST, the Establishment of University Fellowships toward the Creation of Science Technology Innovation (grant number: JPMJFS2129). We thank Editage (http://www.editage.jp) for English language editing.
Data Availability Statement: Data supporting the findings of this study will be made available by the corresponding author upon reasonable request.
Author Contributions: Conceptualization: R. K. and H. H; Methodology: R. K., R. E., and K. I; Investigation: R. K., R. E., and K. I.; Writing - original draft: R. K.; Writing - review and editing: R. K., R. E., K. I., T. W., T. T., M. M., and H. H.; Supervision: H. H.; Project administration: H. H.; Funding acquisition: R. K. and H. H.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103361.
Appendix. Supplementary materials
Figure S1. Nucleotide sequence of the vector used. (A) hyPBase expression vector. (B) PiggyBac donor vector harboring elongation factor-1α (EF1α)-enhanced green fluorescent protein (EGFP).
Figure S2. Concentration study of XAV939. (A) The percentages of stage-specific embryonic antigen-1 (SSEA-1)-positive cells at XAV939 concentrations of 2.5, 5, 7.5, and 10 μM. chESCs were analyzed at primary culture stage (d 4), first passage (P1; d 6), second passage (P2; d 9), third passage (P3; d 11), and fourth passage (P4; d 13) after chicken blastodermal cells (chBCs) collection. (B) The morphology of chESCs at P3 (d 11).
Figure S3. Concentration study of IWP-2. (A) The percentages of SSEA-1-positive cells at IWP-2 concentrations of 5 and 10 μM. chESCs were analyzed at primary culture stage (d 3), first passage (P1; d 5), second passage (P2; d 7), third passage (P3; d 9), and fourth passage (P4; d 11) after chBCs collection. (B) The morphology of chESCs at P1 (d 5) and P3 (d 9).
Figure S4. Regulation of Wnt/β-catenin signaling by small-molecule inhibitors. (A) Detection of β-catenin protein in cytoplasmic and nuclear fractions using Western blot. Samples were treated with 3 small-molecule inhibitors (CHIR99021, XAV939, IWP-2) at 1, 5, and 10 μM. α-Tublin and Histon H3 were detected as loading controls. (B) Detection of Axin1 in the cytoplasmic fraction. Samples were treated with 3 small-molecule inhibitors (CHIR99021, XAV939, IWP-2) at 1, 5, and 10 μM.
REFERENCES
- Amit M., Carpenter M.K., Chu M.S., Harris C.P., Waknitz M.A., Itskovitz-Eldor J., Thomson J.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M.E., Tang W., Lu J., Ma Z., Fan C.W., Wei S., Hao W., Kilgore J., Williams N.S., Roth M.G. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Niu Y., Li Y., Ai Z., Kang Y., Shi H., Xiang Z., Yang Z., Tan T., Si W., Li W., Xia X., Zhou Q., Ji W., Li T. Generation of cynomolgus monkey chimeric fetuses using embryonic stem cells. Cell Stem Cell. 2015;17:116–124. doi: 10.1016/j.stem.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Daniels D.L., Weis W.I. β-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- Davidson K.C., Adams A.M., Goodson J.M., McDonald C.E., Potter J.C., Berndt J.D., Biechele T.L., Taylor R.J., Moon R.T. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. USA. 2012;109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H., Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. Dev. Biol. 1976;49:321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Farzaneh M., Zare M., Hassani S.N., Baharvand H. Effects of various culture conditions on pluripotent stem cell derivation from chick embryos. J. Cell. Biochem. 2018;119:6325–6336. doi: 10.1002/jcb.26761. [DOI] [PubMed] [Google Scholar]
- Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A., Rais Y., Shipony Z., Mukamel Z., Krupalnik V., Zerbib M., Geula S., Caspi I., Schneir D., Shwartz T., Gilad S., Amann-Zalcenstein D., Benjamin S., Amit I., Tanay A., Massarwa R., Novershtern N., Hanna J.H. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- Guo G., von Meyenn F., Rostovskaya M., Clarke J., Dietmann S., Baker D., Sahakyan A., Myers S., Bertone P., Reik W., Plath K., Smith A. Epigenetic resetting of human pluripotency. Development. 2017;144:2748–2763. doi: 10.1242/dev.146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Li T.G., Qi X., Zhao D.F., Zhao G.Q. WNT/β-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Horiuchi H., Furusawa S., Matsuda H. Maintenance of chicken embryonic stem cells in vitro. Methods Mol. Biol. 2006;329:17–34. doi: 10.1385/1-59745-037-5:17. [DOI] [PubMed] [Google Scholar]
- Horiuchi H., Tategaki A., Yamashita Y., Hisamatsu H., Ogawa M., Noguchi T., Aosasa M., Kawashima T., Akita S., Nishimichi N., Mitsui N., Furusawa S., Matsuda H. Chicken leukemia inhibitory factor maintains chicken embryonic stem cells in the undifferentiated state. J. Biol. Chem. 2004;279:24514–24520. doi: 10.1074/jbc.M313231200. [DOI] [PubMed] [Google Scholar]
- Huang T.S., Li L., Moalim-Nour L., Jia D., Bai J., Yao Z., Bennett S.A., Figeys D., Wang L. A regulatory network involving β-catenin, E-cadherin, PI3k/Akt, and slug balances self-renewal and differentiation of human pluripotent stem cells in response to Wnt signaling. Stem Cells. 2015;33:1419–1433. doi: 10.1002/stem.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.M.A., Mishina Y.M., Liu S., Cheung A., Stegmeier F., Michaud G.A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C.J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M.W., Lengauer C., Finan P.M., Tallarico J.A., Bouwmeester T., Porter J.A., Bauer A., Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- Jean C., Oliveira N.M., Intarapat S., Fuet A., Mazoyer C., De Almeida I., Trevers K., Boast S., Aubel P., Bertocchini F., Stern C.D., Pain B. Transcriptome analysis of chicken ES, blastodermal and germ cells reveals that chick ES cells are equivalent to mouse ES cells rather than EpiSC. Stem Cell Res. 2015;14:54–67. doi: 10.1016/j.scr.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama M., Fukuda T., Kaneko T., Nakagawa Y., Tajima A., Naito M., Ohmaki H., Endo D., Asano M., Nagamine T., Nakaya Y., Saito K., Watanabe Y., Tani T., Inoue-Murayama M., Nakajima N., Onuma M. Induced pluripotent stem cells of endangered avian species. Comm. Biol. 2022;5:1049. doi: 10.1038/s42003-022-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T., Tsukiyama T., Kimura K., Matsuyama S., Minami N., Yamada M. Generation of naïve bovine induced pluripotent stem cells using piggybac transposition of doxycycline-inducible transcription factors. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.C., Lim S., Han J.Y. Wnt/β-catenin signaling pathway activation is required for proliferation of chicken primordial germ cells in vitro. Sci. Rep. 2016;6:34510. doi: 10.1038/srep34510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak S.S., Alev C., Nagai H., Wrabel A., Matsuoka Y., Honda A., Sheng G., Ladher R.K. Characterization of the finch embryo supports evolutionary conservation of the naive stage of development in amniotes. eLife. 2015;4:e07178. doi: 10.7554/eLife.07178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin M.J., Di Rocco G., Gardiner A., Bush S.M., Lassar A.B. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R.T. Wnt/β-catenin pathway. Science's STKE. 2005;2005:cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- Nakano M., Arisawa K., Yokoyama S., Nishimoto M., Yamashita Y., Sakashita M., Ezaki R., Matsuda H., Furusawa S., Horiuchi H. Characteristics of novel chicken embryonic stem cells established using chicken leukemia inhibitory factor. J. Poult. Sci. 2011;48:64–72. [Google Scholar]
- Nakanoh S., Fuse N., Tadokoro R., Takahashi Y., Agata K. Jak1/Stat3 signaling acts as a positive regulator of pluripotency in chicken pre-gastrula embryos. Dev. Biol. 2017;421:43–51. doi: 10.1016/j.ydbio.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Nakanoh S., Fuse N., Takahashi Y., Agata K. Verification of chicken Nanog as an epiblast marker and identification of chicken PouV as Pou5f3 by newly raised antibodies. Dev. Growth Diff. 2015;57:251–263. doi: 10.1111/dgd.12205. [DOI] [PubMed] [Google Scholar]
- Nakanoh S., Okazaki K., Agata K. Inhibition of MEK and GSK3 supports ES cell-like domed colony formation from avian and reptile embryos. Zool. Sci. 2013;30:543–552. doi: 10.2108/zsj.30.543. [DOI] [PubMed] [Google Scholar]
- Nichols J., Smith A. Naïve and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Niwa H., Burdon T., Chambers I., Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain B., Clark M.E., Shen M., Nakazawa H., Sakurai M., Samarut J., Etches R.J. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development. 1996;122:2339–2348. doi: 10.1242/dev.122.8.2339. [DOI] [PubMed] [Google Scholar]
- Petitte J.N., Clark M.E., Liu G., Verrinder Gibbins A.M., Etches R.J. Production of somatic and germline chimeras in the chicken by transfer of early blastodermal cells. Development. 1990;108:185–189. doi: 10.1242/dev.108.1.185. [DOI] [PubMed] [Google Scholar]
- Pieters T., van Roy F. Role of cell-cell adhesion complexes in embryonic stem cell biology. J. Cell Sci. 2014;127:2603–2613. doi: 10.1242/jcs.146720. [DOI] [PubMed] [Google Scholar]
- Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D.G. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Van De Lavoir M.C., Mather-Love C., Leighton P., Diamond J.H., Heyer B.S., Roberts R., Zhu L., Winters-Digiacinto P., Kerchner A., Gessaro T., Swanberg S., Delany M.E., Etches R.J. High-grade transgenic somatic chimeras from chicken embryonic stem cells. Mech. Dev. 2006;123:31–41. doi: 10.1016/j.mod.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Van De Lavoir M.C., Diamond J.H., Leighton P.A., Mather-Love C., Heyer B.S., Bradshaw R., Kerchner A., Hooi L.T., Gessaro T.M., Swanberg S.E., Delany M.E., Etches R.J. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–769. doi: 10.1038/nature04831. [DOI] [PubMed] [Google Scholar]
- Whyte J., Glover J.D., Woodcock M., Brzeszczynska J., Taylor L., Sherman A., Kaiser P., McGrew M.J. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Rep. 2015;5:1171–1182. doi: 10.1016/j.stemcr.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.L., Hilton D.J., Pease S., Willson T.A., Stewart C.L., Gearing D.P., Wagner E.F., Metcalf D., Nicola N.A., Gough N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Tategaki A., Ogawa M., Horiuchi H., Nishida K., Akita S., Matsuda H., Furusawa S. Effect of novel monoclonal antibodies on LIF-induced signaling in chicken blastodermal cells. Dev. Comp. Immunol. 2006;30:513–522. doi: 10.1016/j.dci.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., van de Lavoir M.C., Albanese J., Beenhouwer D.O., Cardarelli P.M., Cuison S., Deng D.F., Deshpande S., Diamond J.H., Green L., Halk E.L., Heyer B.S., Kay R.M., Kerchner A., Leighton P.A., Mather C.M., Morrison S.L., Nikolov Z.L., Passmore D.B., Pradas-Monne A., Preston B.T., Rangan V.S., Shi M., Srinivasan M., White S.G., Winters-Digiacinto P., Wong S., Zhou W., Etches R.J. Production of human monoclonal antibody in eggs of chimeric chickens. Nat. Biotechnol. 2005;23:1159–1169. doi: 10.1038/nbt1132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Nucleotide sequence of the vector used. (A) hyPBase expression vector. (B) PiggyBac donor vector harboring elongation factor-1α (EF1α)-enhanced green fluorescent protein (EGFP).
Figure S2. Concentration study of XAV939. (A) The percentages of stage-specific embryonic antigen-1 (SSEA-1)-positive cells at XAV939 concentrations of 2.5, 5, 7.5, and 10 μM. chESCs were analyzed at primary culture stage (d 4), first passage (P1; d 6), second passage (P2; d 9), third passage (P3; d 11), and fourth passage (P4; d 13) after chicken blastodermal cells (chBCs) collection. (B) The morphology of chESCs at P3 (d 11).
Figure S3. Concentration study of IWP-2. (A) The percentages of SSEA-1-positive cells at IWP-2 concentrations of 5 and 10 μM. chESCs were analyzed at primary culture stage (d 3), first passage (P1; d 5), second passage (P2; d 7), third passage (P3; d 9), and fourth passage (P4; d 11) after chBCs collection. (B) The morphology of chESCs at P1 (d 5) and P3 (d 9).
Figure S4. Regulation of Wnt/β-catenin signaling by small-molecule inhibitors. (A) Detection of β-catenin protein in cytoplasmic and nuclear fractions using Western blot. Samples were treated with 3 small-molecule inhibitors (CHIR99021, XAV939, IWP-2) at 1, 5, and 10 μM. α-Tublin and Histon H3 were detected as loading controls. (B) Detection of Axin1 in the cytoplasmic fraction. Samples were treated with 3 small-molecule inhibitors (CHIR99021, XAV939, IWP-2) at 1, 5, and 10 μM.