Abstract
The relationship between serum uric acid and lung function has been controversial. This study aims to determine whether there is an independent relationship between serum uric acid and lung function in the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2012. Serum uric acid was considered the exposure variable, and lung function (FEV1 and FVC) was the outcome variable. Multivariable linear regression was conducted with adjustments for potential confounders. The total number of participants from NHANES (2007–2012) was 30,442, of which 7514 were included in our analysis after applying exclusion criteria. We observed that serum uric acid was negatively associated with FEV1 and FVC after adjusting for confounders (β for FEV1 [− 24.77 (− 36.11, − 13.43)] and FVC [− 32.93 (− 47.42, − 18.45)]). Similarly, serum uric acid showed a negative correlation with FEV1 and FVC after adjusting for confounding variables both in male and female populations. The relationship between serum uric acid and FEV1 and FVC remained consistent and robust in various subgroups within both male and female populations, including age, race, BMI, alcohol consumption, smoking status, and income-poverty ratio. Serum uric acid is negatively associated with FEV1 and FVC in the US general healthy population. This negative relationship is significant in both the male and female populations.
Subject terms: Biomarkers, Health care, Medical research
Introduction
Lung function gradually decreases over time and varies greatly among individuals1. Individuals with impairment of lung function are more likely to suffer from chronic respiratory diseases and have an increased risk of all-cause death2. Therefore, it is of particular interest to identify biomarkers associated with this impairment3.
Uric acid (UA) is the final decomposition product of purine degradation and is present in the plasma and epithelial lining fluid of the respiratory tract4,5. Previous studies have indicated that serum UA is associated with cardiovascular diseases, including hypertension, stroke, coronary heart disease, and congestive heart failure6–9. Similarly, there is also a variety of research investigating the relationship between serum UA and respiratory disease, including chronic obstructive pulmonary disease (COPD), pulmonary hypertension and obstructive sleep apnea. Significant correlation has been found10–12.
Serum UA has been reported to be linked to lung function, but controversial results have been observed in an epidemiological setting: a few studies have shown a negative association between serum UA and lung function in the general healthy population13–15. Meanwhile, only in the female population, the negative association between serum UA and lung function was found in the study by Jeong et al.16. Nevertheless, a positive relationship between serum UA and lung function was observed in a healthy Korean population17. Consequently, this study aimed to investigate the association between serum UA and lung function in the US population using data from the National Health and Nutrition Examination Survey (NHANES 2007-2012).
Methods
Population research
Data was obtained from the National Health and Nutrition Examination Survey (NHANES) (2007–2012). NHANES is a complex, stratified, multistage probability sample survey of the non-institutionalized US population. These cross-sectional surveys are used to assess the severity and prevalence of various diseases, as well as to explore potential new directions for medical research and public health policies.
Inclusion and exclusion criteria
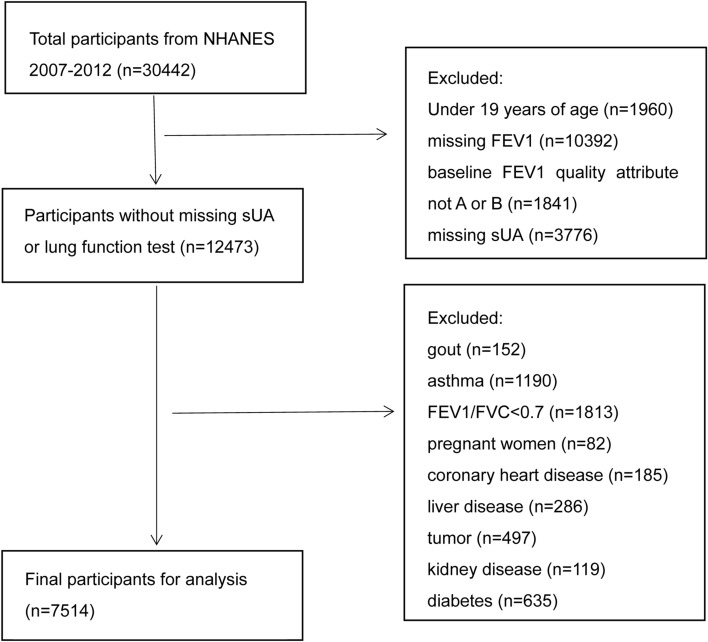
The population included participants with complete data on serum UA and lung function (specifically forced expiratory volume in one second (FEV1) and forced vital capacity (FVC)). The total number of participants from NHANES (2007–2012) was 30,442. After excluding subjects under 19 years old (n = 1960), those with missing FEV1 (n = 10,392), baseline FEV1 Quality Attribute not A or B (n = 1841), missing serum UA (n = 3776), gout (n = 152), asthma (n = 1190), FEV1/FVC < 0.7 (n = 1813), pregnant women (n = 82), coronary heart disease (n = 185), liver disease (n = 286), tumor (n = 497), kidney disease (n = 119), and diabetes (n = 635), a total of 7514 subjects remained for the final analysis (Fig. 1).
Figure 1.
Flowchart of the inclusion of participants.
Ethical approval
Participants aged ≥ 18 years furnished informed consent on their own. The NCHS Ethics Review Board approved the conduct of NHANES, and written informed consent was obtained from all participants.
Study variables
The principal variables of this study were lung function (dependent variable) and serum UA (independent variable). Serum UA was measured using a Beckman Synchron LX20 (Beckman Coulter, Inc., Brea, CA). Lung function was measured using Ohio 822/827 dry-rolling seal volume spirometers.
The following covariates were included: age, gender, race, income-poverty ratio, body mass index, alcohol drinking, smoke, systolic blood pressure, diastolic blood pressure, blood urea nitrogen, creatinine, calcium, total cholesterol, total protein, fractional exhaled nitric oxide (FeNO) and total bilirubin. Details regarding the measurement process of serum UA and lung function, as well as the acquisition process for other covariates, are available at www.cdc.gov/nchs/nhanes.
Statistical analyses
NHANES sample weights were taken into account when calculating all estimates. Continuous variables are presented as mean ± standard deviation (SD) or medians (25th percentile–75th percentile), while categorical variables are presented as frequency (%). This research utilized chi-square tests for categorical variables and linear regression models for continuous variables to assess significant differences in these variables. Dummy variables were used to indicate missing covariate values. After adjusting for confounders, multivariable linear regression models were constructed to determine the independent relationship between serum UA and lung function. Generalized additive models and smooth curve fittings were employed to evaluate any non-linear relationship between serum UA and lung function. Stratified and interaction analyses were performed to assess whether covariates influenced the association between serum UA and lung function, ensuring the robustness of data analysis. A 2-tailed P < 0.05 was considered statistically significant for all analyses. Data analysis was conducted using the statistical software packages R (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com).
Results
Baseline characteristics of selected participants
A total of 7514 participants were included in the final analysis. The mean age was 40.85 ± 14.28 years old. The mean serum UA levels were 5.34 ± 1.34 mg/dl. Table 1 presents the weighted baseline characteristics according to the serum UA tertiles. All variables showed statistically significant differences among the different serum uric acid groups. Except for the income-poverty ratio, race, and female gender, all other variables exhibited an increasing trend according to the tertiles of serum uric acid (Table 1).
Table 1.
Description of 7514 participants included in the present study.
| Characteristic | All(n = 7514) | Tertile of serum uric acid (mg/dl) | P value | ||
|---|---|---|---|---|---|
| Tertile 1 (< 4.70) (n = 2498) | Tertile 2 (4.70–5.70) (n = 2322) | Tertile 3 (> 5.70) (n = 2694) | |||
| Age (years) | 40.85 ± 14.28 | 40.26 ± 13.76 | 41.12 ± 14.77 | 41.15 ± 14.32 | 0.0418 |
| Gender, n (%) | < 0.0001 | ||||
| Male | 3690 (49.11) | 366 (14.66) | 1132 (48.77) | 2152 (79.88) | |
| Female | 3824 (50.89) | 2132 (85.34) | 1190 (51.23) | 542 (20.12) | |
| Race, n (%) | 0.0098 | ||||
| Mexican American | 732 (9.74) | 243 (9.73) | 242 (10.43) | 248 (9.21) | |
| Other Hispanic | 458 (6.09) | 177 (7.07) | 152 (6.54) | 131 (4.85) | |
| Non-Hispanic White | 5009 (66.66) | 1650 (66.05) | 1496 (64.42) | 1858 (68.97) | |
| Non-Hispanic Black | 793 (10.55) | 261 (10.46) | 261 (11.25) | 271 (10.07) | |
| Other Race | 524 (6.97) | 167 (6.69) | 171 (7.36) | 186 (6.90) | |
| Income-poverty ratio, n (%) | 0.0081 | ||||
| Poor | 1530 (20.36) | 522 (20.89) | 523 (22.54) | 489 (18.17) | |
| Nearly poor | 606 (8.06) | 189 (7.56) | 191 (8.22) | 226 (8.38) | |
| Not poor | 4929 (65.60) | 1643 (65.76) | 1464 (63.04) | 1818 (67.47) | |
| Missing | 449 (5.98) | 145 (5.79) | 144 (6.19) | 161 (5.98) | |
| Smoke, n (%) | < 0.0001 | ||||
| Yes | 2888 (38.44) | 879 (35.19) | 902 (38.83) | 1105 (41.01) | |
| No | 4449 (59.21) | 1566 (62.71) | 1352 (58.23) | 1533 (56.89) | |
| Missing | 177 (2.35) | 52 (2.10) | 68 (2.94) | 57 (2.10) | |
| Alcohol drinking, n (%) | < 0.0001 | ||||
| Yes | 5620 (74.80) | 1751 (70.10) | 1708 (73.57) | 2153 (79.93) | |
| No | 1319 (17.56) | 521 (20.86) | 444 (19.11) | 361 (13.40) | |
| Missing | 574 (7.64) | 226 (9.04) | 170 (7.31) | 180 (6.67) | |
| Body Mass Index (kg/m2) | 28.18 ± 6.30 | 25.94 ± 5.44 | 28.31 ± 6.13 | 30.08 ± 6.49 | < 0.0001 |
| SBP (mmHg) | 119.34 ± 14.65 | 116.06 ± 14.79 | 119.54 ± 14.41 | 122.09 ± 14.12 | < 0.0001 |
| DBP (mmHg) | 71.26 ± 11.26 | 69.76 ± 10.67 | 70.75 ± 10.88 | 73.00 ± 11.82 | < 0.0001 |
| Calcium (mg/dL) | 9.42 ± 0.34 | 9.36 ± 0.34 | 9.43 ± 0.34 | 9.47 ± 0.34 | < 0.0001 |
| Total bilirubin (mg/dL) | 0.77 ± 0.30 | 0.69 ± 0.26 | 0.75 ± 0.29 | 0.85 ± 0.33 | < 0.0001 |
| Total Protein (g/dL) | 7.15 ± 0.44 | 7.08 ± 0.45 | 7.15 ± 0.43 | 7.21 ± 0.43 | < 0.0001 |
| Total cholesterol (mg/dL) | 196.51 ± 39.49 | 194.20 ± 39.26 | 194.05 ± 39.30 | 200.50 ± 39.50 | < 0.0001 |
| Creatinine (mg/dL) | 0.85 ± 0.18 | 0.74 ± 0.15 | 0.85 ± 0.16 | 0.95 ± 0.17 | < 0.0001 |
| Blood urea nitrogen (mg/dL) | 12.30 ± 4.01 | 11.24 ± 3.62 | 12.34 ± 4.07 | 13.20 ± 4.06 | < 0.0001 |
| Serum UA (mg/dl) | 5.34 ± 1.34 | 3.91 ± 0.54 | 5.19 ± 0.32 | 6.73 ± 0.82 | < 0.0001 |
| FeNO (ppb) | 13.50 (9.00–19.50) | 12.50 (8.50–17.50) | 13.50 (9.00–19.50) | 14.00 (9.50–20.50) | < 0.001 |
| FVC (ml) | 4248.72 ± 1054.30 | 3816.56 ± 818.60 | 4206.57 ± 1090.78 | 4664.66 ± 1049.27 | < 0.0001 |
| FEV1 (ml) | 3436.98 ± 863.91 | 3113.14 ± 679.10 | 3399.32 ± 898.03 | 3753.45 ± 870.25 | < 0.0001 |
Data are presented as n (%), means ± SD or medians (25th percentile-75th percentile). BMI: body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; UA: uric acid; FeNO: fractional exhaled nitric oxide.
Association between serum UA and lung function (FEV1 and FVC) in the total population
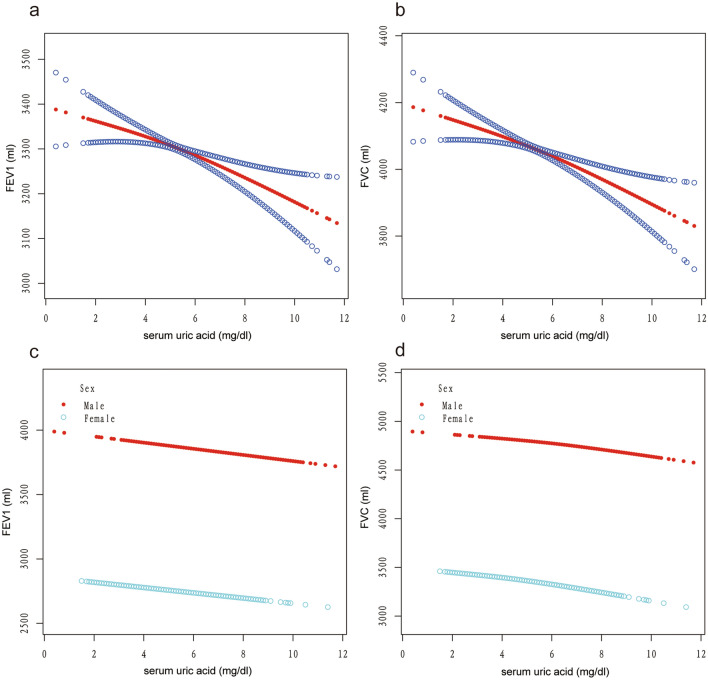
The relationship between serum UA and lung function was explored using a generalized additive model. The adjusted smoothed plots indicated a linear relationship between serum UA and lung function both in the total population and male or female populations (Fig. 2). Three models were constructed to analyze the independent role of serum UA in FEV1 and FVC in different populations: the general population, the male population, and the female population. Model 1 involved no modification variables, Model 2 included adjustments for gender or age and race, and Model 3 incorporated adjustments for the covariates presented in Table 1 (Tables 2 and 3). In the general population, Model 1 revealed a positive association between serum UA and FEV1 and FVC, whereas Models 2 and 3 identified a negative association between serum UA and FEV1 and FVC. Model 3 showed a significant negative association between serum UA and FEV1 (β = − 24.77; 95% CI − 36.11 to − 13.43) and FVC (β = − 32.93; 95% CI − 47.42 to − 18.45) (Table 2). Each 1 mg/dl increase in serum UA was associated with a 24.77 ml decline in FEV1 and a 32.93 ml decline in FVC. Sensitivity analysis using tertiles of serum UA as a categorical variable yielded similar results, with negative effect sizes for tertiles 2 and 3 in Models 2 and 3. The p-values for trend in all models were significant (all p < 0.05) (Table 2). In both the male and female populations, except for a small magnitude positive β value in Model 1 for FVC in males (with a p-value > 0.05), all other models showed negative effect sizes. Furthermore, all p-values in Model 3 were significant, indicating a negative relationship between serum UA and lung function in both male and female populations. When serum UA was categorized into tertiles as a categorical variable, the results remained consistent with the findings obtained when serum UA was treated as a continuous variable (Table 3).
Figure 2.
Correlation between serum uric acid and FEV1 (a and b) and FVC (c and d) in the male and female population. Correlation between serum uric acid and lung function test (FEV1 and FVC) in the total population (a and b) and stratified by gender (c and d). Each black point represents a sample. The area between two blue dotted lined is expressed as a 95% CI. Each point shows the magnitude of the serum uric acid and is connected to form a continuous line. Age, gender, race, income-poverty ratio, BMI, alcohol drinking, smoke, systolic blood pressure, diastolic blood pressure, blood urea nitrogen, total cholesterol, creatinine, calcium, total protein, FeNO and total bilirubin were adjusted.
Table 2.
Association of serum uric acid with lung function in different models in general population.
| Variable | Model 1 (n = 7514) | Model 2 (n = 7514) | Model 3 (n = 7514) | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| For FEV1 (ml) | ||||||
| UA (mg/dl) | 201.50 (187.60, 215.41) | < 0.0001 | − 45.71 (− 58.75, − 32.68) | < 0.0001 | − 24.77 (− 36.11, − 13.43) | < 0.0001 |
| UA (mg/dl) in tertile | ||||||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 286.18 (239.18, 333.18) | < 0.0001 | − 127.94 (− 166.72, − 89.16) | 0.0001 | − 47.08 (− 77.48, − 16.67) | 0.0024 |
| Tertile 3 | 640.31 (596.01, 684.60) | < 0.0001 | − 151.41 (− 192.96, − 109.86) | 0.0001 | − 59.93 (− 94.77, − 25.09) | 0.0008 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
| For FVC (ml) | ||||||
| UA (mg/dl) | 263.97 (247.14, 280.80) | < 0.0001 | − 49.11 (− 64.42, − 33.81) | < 0.0001 | − 32.93 (− 47.42, − 18.45) | < 0.0001 |
| UA (mg/dl) in tertile | ||||||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 390.01 (333.20, 446.83) | < 0.0001 | − 132.38 (− 177.95, − 86.82) | 0.0001 | − 49.04 (− 87.87, − 10.20) | 0.0134 |
| Tertile 3 | 848.11 (794.56, 901.65) | < 0.0001 | − 150.61 (− 199.43, − 101.79) | < 0.0001 | − 73.29 (− 117.79, − 28.78) | 0.0013 |
| P for trend | < 0.001 | < 0.001 | 0.001 | |||
Model 1, no covariates were adjusted.
Model 2, gender was adjusted.
Model 3, Age, gender, race, income-poverty ratio, BMI, alcohol drinking, smoke, systolic blood pressure, diastolic blood pressure, blood urea nitrogen, total cholesterol, creatinine, calcium, total protein, FeNO and total bilirubin were adjusted.
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; UA: uric acid.
Table 3.
Association of serum uric acid with lung function in different models in male and female population.
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| For FEV1 (ml) in male (n = 3684 in each model) | ||||||
| UA (mg/dl) | − 0.91 (− 20.86, 19.04) | 0.9285 | − 16.63 (− 32.43, − 0.83) | 0.0392 | − 24.07 (− 41.37, − 6.77) | 0.0064 |
| UA (mg/dl) in tertile | ||||||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 10.11 (− 74.44, 94.67) | 0.8147 | − 53.68 (− 120.54, 13.17) | 0.1156 | − 66.51 (− 132.55, − 0.46) | 0.0485 |
| Tertile 3 | 19.77 (− 59.06, 98.60) | 0.6231 | − 51.83 (− 114.34, 10.67) | 0.1042 | − 74.38 (− 138.38, − 10.39) | 0.0228 |
| For FVC (ml) in male (n = 3684 in each model) | ||||||
| UA (mg/dl) | 1.36 (− 22.42, 25.14) | 0.9107 | − 19.31 (− 39.58, 0.95) | 0.0618 | − 30.06 (− 52.26, − 7.85) | 0.0080 |
| UA (mg/dl) in tertile | ||||||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 14.07 (− 86.72, 114.85) | 0.7844 | − 58.74 (− 144.50, 27.01) | 0.1795 | − 68.86 (− 153.66, 15.93) | 0.1115 |
| Tertile 3 | 41.38 (− 52.58, 135.34) | 0.3881 | − 54.72 (− 134.89, 25.46) | 0.1811 | − 82.29 (− 164.45, − 0.12) | 0.0497 |
| For FEV1 (ml) in female (n = 3830 in each model) | ||||||
| UA (mg/dl) | − 94.31 (− 110.91, − 77.72) | < 0.0001 | − 32.93 (− 45.31, − 20.55) | < 0.0001 | − 32.57 (− 46.96, − 18.17) | < 0.0001 |
| UA (mg/dl) in tertile | ||||||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | − 146.57 (− 187.55, − 105.59) | < 0.0001 | − 61.78 (− 91.95, − 31.61) | < 0.0001 | − 53.83 (− 85.05, − 22.62) | 0.0007 |
| Tertile 3 | − 281.94 (− 334.68, − 229.19) | < 0.0001 | − 95.76 (− 135.06, − 56.45) | < 0.0001 | − 83.52 (− 126.59, − 40.45) | 0.0001 |
| For FVC (ml) in female (n = 3830 in each model) | ||||||
| UA (mg/dl) | − 103.87 (− 122.91, − 84.82) | < 0.0001 | − 48.71 (− 64.51, − 32.91) | < 0.0001 | − 44.44 (− 62.65, − 26.23) | < 0.0001 |
| UA (mg/dl) in tertile | ||||||
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | − 147.21 (− 194.29, − 100.13) | < 0.0001 | − 68.43 (− 106.97, − 29.89) | 0.0005 | − 51.26 (− 90.76, − 11.75) | 0.0110 |
| Tertile 3 | − 306.34 (− 366.94, − 245.74) | < 0.0001 | − 141.44 (− 191.65, − 91.24) | < 0.0001 | − 113.05 (− 167.55, − 58.55) | < 0.0001 |
Model 1, no covariates were adjusted.
Model 2, Age and race was adjusted.
Model 3, Age, race, income-poverty ratio, BMI, alcohol drinking, smoke, systolic blood pressure, diastolic blood pressure, blood urea nitrogen, total cholesterol, creatinine, calcium, total protein, FeNO and total bilirubin were adjusted.
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; UA: uric acid.
Subgroup analyses
The role of other covariates on the association between serum UA and FEV1 and FVC was further examined in the male and female populations. The association between serum UA and FEV1 and FVC remained consistent and robust in both male and female populations across various subgroups, including age, race, BMI, alcohol drinking, smoke, and income-poverty ratio. Furthermore, all effect sizes of these subgroups were negative, and there was no significant p-interaction (Figs. S1, S2 in the Supplementary Appendix).
Discussion
The primary objective of this study was to investigate the independent association between serum UA and lung function in healthy US adults. Our findings revealed a negative association between serum UA and lung function (FEV1 and FVC) after adjusting for potential confounding factors. This relationship was consistent both in males and females. Further research is required to explore whether there exists a causal, pathophysiological mechanism linking serum UA and lung function, as well as to determine the potential utility of serum UA as a biomarker for identifying impaired lung function.
The positive relationship between serum UA and lung function was observed when no covariates were adjusted. However, after only adjusting for gender, the association changed to a negative one. Moreover, even after adjusting for other covariates, the negative association between serum UA and lung function remained. The observational analysis found a correlation between high plasma urate levels and low lung function. Furthermore, our study found a robust negative relationship between serum UA and lung function across various subgroups, including age, race, BMI, smoke, and alcohol consumption, in the overall study population. This negative relationship was also observed within the male and female subgroups mentioned above.
Possible explanations for the negative association between serum UA and lung function can be identified as follows. Firstly, the relationship might be attributed to reverse causation. Previous research has indicated that serum UA levels increase in hypoxic conditions, such as chronic heart failure and COPD18. It has also been suggested that pulmonary hypoxia triggers the breakdown of purines, resulting in the elevated production of serum UA 19. However, it remains uncertain whether the mild hypoxia observed in the general healthy population could impact serum UA levels, as our study excluded individuals with evident clinical conditions such as asthma, airflow limitation, and coronary heart disease. Secondly, the formation of uric acid necessitates the catalytic activity of xanthine oxidase, a process that occurs in the epithelial lining fluid of the respiratory tract and is accompanied by the generation of superoxide20. As superoxide is a free radical, it has the potential to induce oxidative damage to biological molecules. Therefore, it can be speculated that lung tissue damage may occur in the presence of high xanthine oxidase activity due to oxidative stress.
Our study makes a valuable contribution to the existing literature by demonstrating a consistent negative association between serum uric acid (UA) and lung function in both male and female individuals in the US general healthy population. Previous studies have attempted to assess the link between serum UA and lung function in the general population, but the results have been inconsistent. For instance, Hong et al. found a negative relationship between hyperuricemia and FEV1% or FVC% in a sample of 2901 participants from the Korean general population15. Similar findings were reported by Aida et al. and Kobylecki et al.13,14, which align with our own findings. However, Song et al. reported a potential positive effect of serum UA on lung function in middle-aged, healthy populations17. We speculate that the discrepancy in results could be attributed to several factors: First, participant exclusion criteria differed between the studies. Song et al. excluded individuals with chronic lung disease or abnormal chest radiograph findings, but the definition of these diseases was vague, and details were not provided. As such, it is challenging to determine the influence of these excluded participants on the overall conclusion. In contrast, our study excluded participants with asthma and FEV1/FVC < 0.7, and in these individuals, a negative relationship between serum UA and lung function was observed21,22. Thus, our conclusion is more robust, given the exclusion of these participants. Second, Song et al. did not assess the adjusted non-linear relationship between serum UA and lung function, nor did they conduct subgroup analyses as a sensitivity analysis for their conclusion. In our study, all effect sizes of subgroups were found to be negative, indicating the robustness of our findings. Third, the study populations differed, with our study focusing on US individuals while Song et al. targeted individuals from South Korea. Fourth, compared to our research, Song et al. did not consider the effects of FeNO, total bilirubin, and income-poverty ratio when adjusting for covariates in the relationship between serum UA and lung function. However, previous studies have confirmed that these variables are associated with lung function23–25.
Kobylecki et al.14 conducted a Mendelian randomisation study to investigate the causal relationship between serum uric acid and lung function. Their observational analysis found a correlation between high plasma urate levels and worse lung function. However, genetically high plasma urate levels did not show a direct causal association with the outcomes. Likely potential explanations include reverse causation and unmeasured confounding factors. It is important to note that the study population mainly consisted of individuals of Danish descent and had specific inclusion and exclusion criteria that significantly differed from our study, as the United States is a multicultural country with diverse ethnicities. Further research is still required to assess the causal relationship between serum UA and lung function.
The study possesses several strengths. Firstly, a nonlinearity assessment was incorporated, providing a more comprehensive understanding of the relationship between serum uric acid (UA) and lung function. Secondly, additional confounding variables such as Fractional exhaled nitric oxide (FeNO), total bilirubin, and income-poverty ratio were adjusted for. Thirdly, various sensitivity and subgroup analyses were performed, and the findings consistently upheld their robustness. Lastly, the study had a relatively large sample size in comparison to previous studies with similar objectives.
The study has several limitations. Firstly, due to its cross-sectional design, a causal relationship between serum uric acid (UA) and lung function cannot be established. Secondly, the use of uric acid-lowering medications was not accounted for, although individuals with gout were excluded. Lastly, the study population was derived from NHANES (2007-2012), and certain exclusion criteria were applied, potentially limiting the generalizability and extrapolation of the findings.
In conclusion, serum uric acid is negatively associated with FEV1 and FVC in the US general healthy population. This negative relationship is significant in both the male and female populations. These outcomes emphasize the significance of serum uric acid as a potential mechanism underlying FEV1 and FVC decline. Further epidemiologic studies will still be required to confirm this reverse association.
Supplementary Information
Author contributions
The authors’ responsibilities were as follows—W.L., C.W., W.Y.W., Y.H.L., D.H.W.: contributed to data collection, analysis, and manuscript writing; Y.H.L., W.L., X.Y.Y., F.L.: devoted to research design and implementation. All authors declare that they have read and approved the manuscript and final version.
Funding
This study was supported by the Xiamen Health Care Guideline Project (3502Z20214ZD1005). The funder had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Data availability
Data described in the manuscript will not be made available because the data used in this study were from the NHANES database, which is a free and open database for all researchers around the world. The link to the database is https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wen Luo and Chen Wang.
Contributor Information
Dinghui Wu, Email: 903952438@qq.com.
Yihua Lin, Email: lyh7@xmu.edu.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-51808-y.
References
- 1.Ortega VE, Kumar R. The effect of ancestry and genetic variation on lung function predictions: What is "normal" lung function in diverse human populations? Curr. Allergy Asthma Rep. 2015;15:16. doi: 10.1007/s11882-015-0516-2. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am. J. Respir. Crit. Care Med. 2006;173:985–990. doi: 10.1164/rccm.200508-1344OC. [DOI] [PubMed] [Google Scholar]
- 3.Baughman P, et al. Combined effect of lung function level and decline increases morbidity and mortality risks. Eur. J. Epidemiol. 2012;27:933–943. doi: 10.1007/s10654-012-9750-2. [DOI] [PubMed] [Google Scholar]
- 4.van der Vliet A, et al. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. 1999;276:L289–296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- 5.Peden DB, et al. Uric acid is a major antioxidant in human nasal airway secretions. Proc. Natl. Acad. Sci. U. S. A. 1990;87:7638–7642. doi: 10.1073/pnas.87.19.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Hou W, Zhang X, Hu L, Tang Z. Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclerosis. 2014;232:265–270. doi: 10.1016/j.atherosclerosis.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 7.Zuo T, et al. Hyperuricemia and coronary heart disease mortality: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2016;16:207. doi: 10.1186/s12872-016-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzas R, Tautu OF, Dorobantu M, Ivan V, Lighezan D. Serum uric acid and arterial hypertension-Data from Sephar III survey. PLoS One. 2018;13:e0199865. doi: 10.1371/journal.pone.0199865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Liu Y, Meng L, Wang L, Liu D. Effect of uric acid-lowering agents on patients with heart failure: A systematic review and meta-analysis of randomised controlled trials. Front. Cardiovasc. Med. 2021;8:639392. doi: 10.3389/fcvm.2021.639392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhaun N, et al. Endothelin antagonism and uric acid levels in pulmonary arterial hypertension: Clinical associations. J. Heart Lung Transpl. 2014;33:521–527. doi: 10.1016/j.healun.2014.01.853. [DOI] [PubMed] [Google Scholar]
- 11.Wan YF, et al. Uric acid levels in obstructive sleep apnea patients with atrial fibrillation. Arch. Med. Res. 2014;45:132–137. doi: 10.1016/j.arcmed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Rumora L, et al. Uric acid and uric acid to creatinine ratio in the assessment of chronic obstructive pulmonary disease: Potential biomarkers in multicomponent models comprising IL-1beta. PLoS One. 2020;15:e0234363. doi: 10.1371/journal.pone.0234363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aida Y, et al. The relationship between serum uric acid and spirometric values in participants in a health check: The Takahata study. Int. J. Med. Sci. 2011;8:470–478. doi: 10.7150/ijms.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobylecki CJ, Vedel-Krogh S, Afzal S, Nielsen SF, Nordestgaard BG. Plasma urate, lung function and chronic obstructive pulmonary disease: A Mendelian randomisation study in 114 979 individuals from the general population. Thorax. 2018;73:748–757. doi: 10.1136/thoraxjnl-2017-210273. [DOI] [PubMed] [Google Scholar]
- 15.Hong JW, Noh JH, Kim DJ. Association between serum uric acid and spirometric pulmonary function in Korean adults: The 2016 Korea National Health and Nutrition Examination Survey. PLoS One. 2020;15:e0240987. doi: 10.1371/journal.pone.0240987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong H, et al. Gender-specific association of serum uric acid and pulmonary function: Data from the Korea national health and nutrition examination survey. Medicina (Kaunas) 2021 doi: 10.3390/medicina57090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song JU, Hwang J, Ahn JK. Serum uric acid is positively associated with pulmonary function in Korean health screening examinees. Mod. Rheumatol. 2017;27:1057–1065. doi: 10.1080/14397595.2017.1285981. [DOI] [PubMed] [Google Scholar]
- 18.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417,734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS) J. Intern. Med. 2009;266:558–570. doi: 10.1111/j.1365-2796.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 19.Baker JE, et al. Nitrite confers protection against myocardial infarction: Role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. J. Mol. Cell Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinamonti S, et al. Xanthine oxidase activity in bronchoalveolar lavage fluid from patients with chronic obstructive pulmonary disease. Free Radic. Biol. Med. 1996;21:147–155. doi: 10.1016/0891-5849(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Wan C, Wen F. An unexpected role for serum uric acid as a biomarker for severity of asthma exacerbation. Asian Pac. J. Allergy Immunol. 2014;32:93–99. doi: 10.12932/AP0337.32.1.2014. [DOI] [PubMed] [Google Scholar]
- 22.Kahnert K, et al. Uric acid, lung function, physical capacity and exacerbation frequency in patients with COPD: A multi-dimensional approach. Respir. Res. 2018;19:110. doi: 10.1186/s12931-018-0815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegewald MJ, Crapo RO. Socioeconomic status and lung function. Chest. 2007;132:1608–1614. doi: 10.1378/chest.07-1405. [DOI] [PubMed] [Google Scholar]
- 24.Curjuric I, et al. Serum bilirubin is associated with lung function in a Swiss general population sample. Eur. Respir. J. 2014;43:1278–1288. doi: 10.1183/09031936.00055813. [DOI] [PubMed] [Google Scholar]
- 25.Coumou H, Westerhof GA, de Nijs SB, Zwinderman AH, Bel EH. Predictors of accelerated decline in lung function in adult-onset asthma. Eur. Respir. J. 2018 doi: 10.1183/13993003.01785-2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will not be made available because the data used in this study were from the NHANES database, which is a free and open database for all researchers around the world. The link to the database is https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.