Abstract
The authors report a case of pathologically proven intracardiac bronchogenic cyst embedded within the interatrial septum of a 30-year-old woman presenting with chest pain and first-degree AV block. Multimodality imaging played an essential role in the discovery, investigation, and diagnosis of this extremely rare entity.
Keywords: Cysts, Echocardiography, Cardiac, CT-angiography, Neoplasms-Primary, MR Imaging
Introduction
Intracardiac bronchogenic cysts (IBCs) are very rare cardiac tumors thought to be related to aberrant budding of ventral foregut during embryogenesis [1,2]. To the best of our knowledge, less than 30 cases of IBCs have been reported in the English language peer-reviewed medical literature with only around 16 being pathologically proven. We report a case of pathologically proven IBC, illustrating typical imaging features and underlying the importance of multimodality imaging in the diagnosis of this rare entity.
Case Presentation
A 30-year-old Hispanic female, with prior history of anxiety presented to the emergency department with sudden, sharp, and intermittent right chest pain radiating to the back for 6 days. She also complained of left-sided discomfort under her ribcage and shortness of breath during daily activities. She denied prior relevant cardiac history. Physical examination was unremarkable. She denied trauma, fever, or prior symptoms of chest pain or discomfort predating the current presentation. Initial work-up in the emergency room revealed normal troponin level and first-degree AV block on EKG. Orally administered naproxen, followed by intravenous hydromorphone, were unsuccessful in relieving the patient's chest pain. Additional lab work showed anemia with a hemoglobin level at 11.7 g/dL, elevated d-dimer at 0.88 mcg/mL fibrinogen equivalent units and a repeat EKG confirmed a first-degree AV block. A chest radiograph was normal. A CT angiogram of the chest with a pulmonary embolism protocol showed no pulmonary emboli but depicted an indeterminate mass in the interatrial septum of the heart which was then assessed by an EKG-gated cardiac CT angiogram (Fig. 1).
Fig. 1.
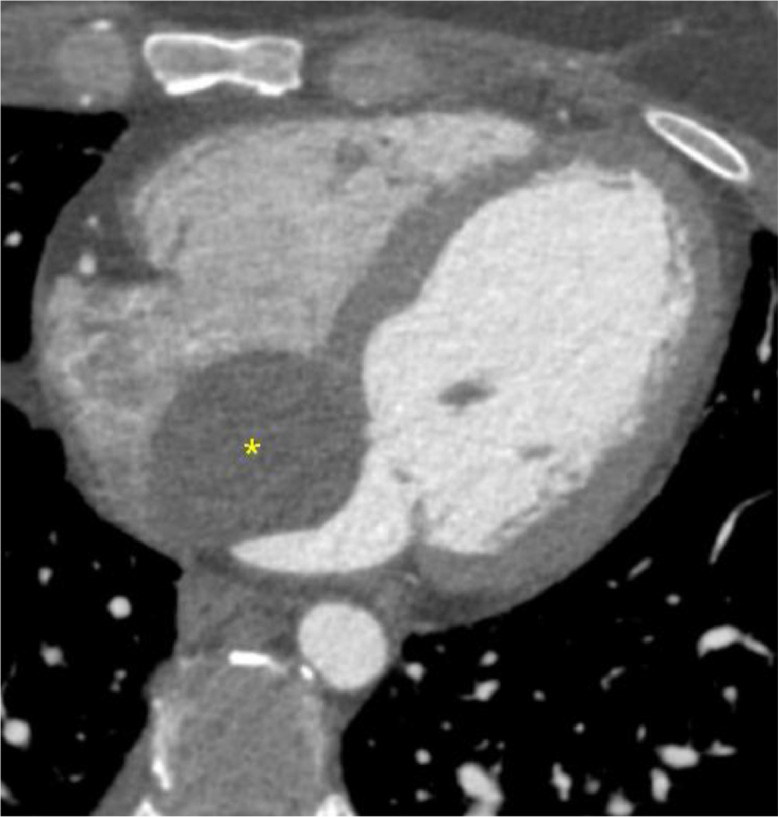
EKG gated cardiac CT angiogram shows a well-circumscribed oval-shaped cystic mass (20 Hounsfield units) measuring 47 × 38 mm centered at the interatrial septum (*). No change in density was noted between the cardiac CT calcium score performed without iodinated contrast (not shown) and the CT angiogram, indicating absence of enhancement.
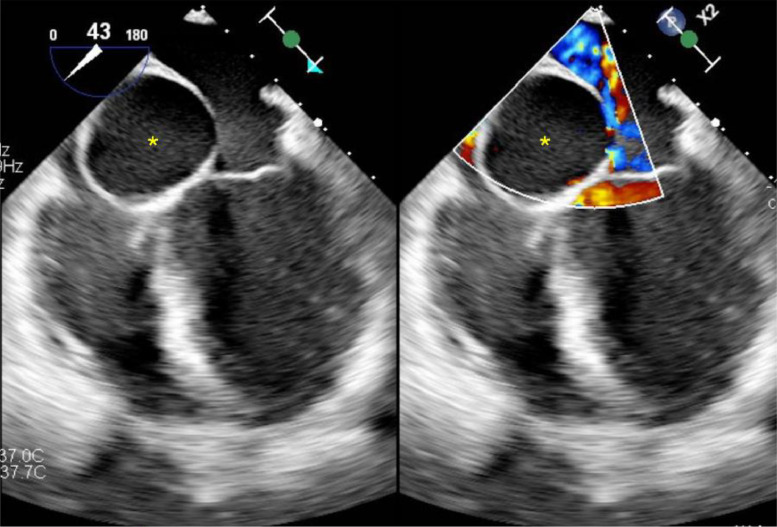
The patient was subsequently hospitalized for the newly discovered interatrial septal mass. Transesophageal echocardiography (Fig. 2) showed a left ventricular ejection fraction of 60%. It also confirmed an anechoic cystic mass within the interatrial septum abutting the tricuspid and mitral annulus.
Fig. 2.
Transesophageal echocardiogram. Side-by-side images without doppler (on the left) and with doppler (on the right) show the presence of a well-defined anechoic cystic mass centered at the interatrial septum (*). Absence of doppler flow confirms the cystic nature of the lesion (image on the right).
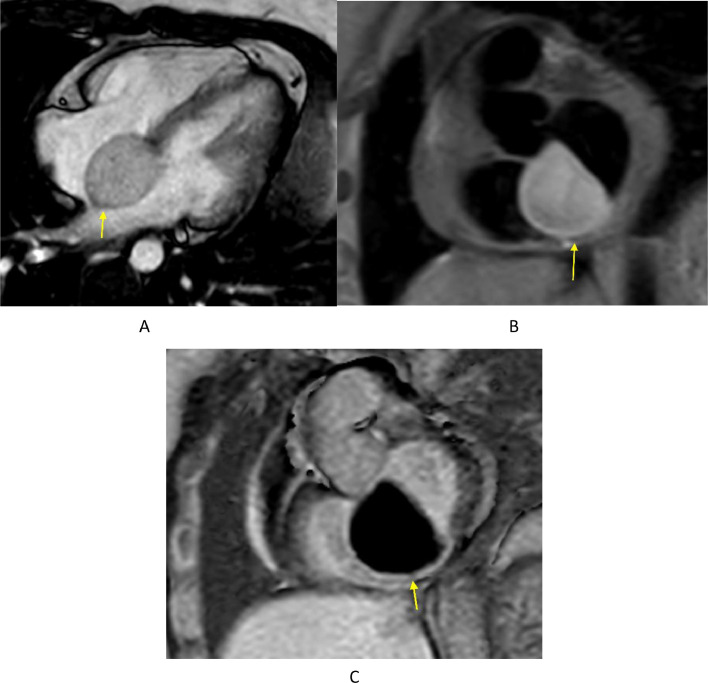
A cardiac MRI (Fig. 3) was performed to complete the multimodality assessment of the interatrial mass. It confirmed the well-defined cystic nature of the lesion centered at the interatrial septum, absence of intralesional fat and absence of enhancing nodule or septation consistent with an interatrial bronchogenic cyst.
Fig. 3.
Cardiac MRI (1.5 Tesla) images showing the well-defined ovoid interatrial lesion (yellow arrow). (A) Balanced turbo field echo (BTFE) 4 chamber cine image (10 mm thickness) confirms the well-defined nature of the lesion with a homogenous slightly hyperintense signal. (B) Black blood proton density short axis image (8 mm thickness) with fat saturation shows the homogenous slightly hyperintense signal of the lesion. The lack of signal loss with fat saturation confirms the lack of fat contents within the lesion and excludes lipomatous lesions and lipomatous hypertrophy from the differential diagnosis. (C) 2D Phase sensitive inversion recovery (PSIR) single shot short axis image (10 mm thickness) acquired 10 minutes following injection of intravenous gadolinium confirms lack of enhancement and cystic nature of the lesion.
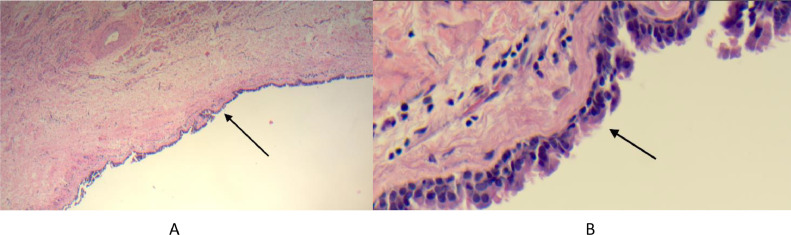
Following patient consent, surgical resection was planned via median sternotomy. After full cardiopulmonary bypass was established, cross clamp was applied, and diastolic arrest was achieved. The mass was accessed via an oblique right atriotomy allowing optimal visualization of the interatrial mass near the septal portion of the tricuspid annulus. Initial aspiration confirmed gelatinous content. A trans septal incision was then made revealing the proximity of the lesion to the A3 portion of the mitral annulus. Complete excision of the mass would have required a complex reconstruction of the mitral annulus and replacement of a normal functioning native mitral valve. The cystic mass was therefore unroofed from the right atrial side instead, evacuated of its contents and irrigated, and the floor of the cyst was fulgurated with electrocautery to prevent refilling of the cyst. There was no intra or postoperative complications. The roof of the cystic mass was sent for pathological assessment and consisted of unoriented, irregular, tan-white, and rubbery 1.7×1.2×0.2 cm tissue fragment with respiratory epithelial lining consistent with a bronchogenic cyst (Fig. 4). Analysis also confirmed the absence of neoplastic cells. The patient was discharged home on postoperative day 7 without complications, without persistent AV block and following resolution of presenting symptoms.
Fig. 4.
Microphotographs at low magnification (A) and high magnification (B) showing the surface of the cystic lesion covered by pseudostratified ciliated epithelial lining (arrow).
Discussion
Cardiac tumors are rare and account for about 0.02% of all tumors detected on autopsy [3]. Such tumors can be malignant (primary, or metastases) or benign and should be differentiated from pseudo-tumors such as thrombi, vegetations, Lambl excrescences or anatomical variants such as coumadin ridge, prominence of the crista terminalis or Chiari network [4,5]. Cardiac tumors are usually found and investigated through a variety of imaging modalities, including echocardiography (transthoracic and transesophageal), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) [5,6]. Cardiac metastases most commonly arise from lungs, accounting for approximately 36% to 39% of cases of all cardiac metastases. This is followed by metastases from breast cancer and hematologic malignancies which represent approximately 10% to 12% and 10% to 21% of cardiac metastases, respectively [7].
70% of primary tumors of the heart are benign [8]. Common benign cardiac tumors include myxomas, lipomas and lipomatous hypertrophy of the interatrial septum, rhabdomyomas, papillary fibroelastomas, angiomas, and fibromas [6]. Myxomas are the most common primary cardiac tumors and usually located in the left atrium, attached to the fossa ovalis. They are almost always solid, whereas cystic myxomas are rare with only a few cases documented in the literature [4,5].
Intracardiac bronchogenic cysts are extremely rare and have occasionally been initially detected through echocardiography following detection of a murmur but can also be found in largely asymptomatic individuals [9]. There are less than 30 reports of intracardiac bronchogenic cysts in adults found in the right ventricle, left ventricle, right atrium, left atrium, and interatrial septum. The pathology of an IBC is usually related to a benign intracardiac outgrowth following improper embryological development of the primitive ventral foregut [1,2]. Imaging features of IBCs are identical to those of the more common mediastinal bronchogenic cysts. On CT imaging, typical bronchogenic cysts are seen as well-defined nonenhancing spheroid or ovoid thin-walled lesions of variable attenuation, depending on their proteinaceous content, but most commonly with homogenous near-water attenuation. The same morphological features are seen on ultrasound and MRI. On Ultrasound, they typically appear as homogenous anechoic non-enhancing lesions. On MRI they tend to have a homogenous signal with high T2 signal intensity due to fluid within the cyst and a variable T1 signal depending on their proteinaceous contents. MRI also confirms the absence of enhancement and intralesional fat, especially in atypical cases and in cases with atypical locations, such as the one presented here, effectively excluding the much more common myxomas and lipomatous lesions and pseudo-lesions from the differential diagnosis [10]. Intracardiac blood cysts can also be considered in the differential diagnosis. They are rare intracardiac benign cystic neoplasms of uncertain origin, usually found in newborns and infants, disappearing spontaneously during growth. They usually involve the cardiac valves, with the anterior leaflet of the mitral valve being the most affected structure. They can appear identical to IBCs on all imaging modalities. Important differentiating features on imaging include their typical valvular location, occasional formation of intralesional microbubbles on contrast enhanced real-time echocardiography, occasional enhancement and contrast uptake on MRI and occasional mirroring of the blood pool signal on T1 and T2 weighted images, thought to be due to the presence of channels allowing communication with the intracardiac blood pool [11,12].
Patients may or may not experience symptoms resulting from IBCs. Our patient presented with significant chest pain and a first-degree AV block on EKG. Cardiac lesions are rare causes of AV blocks but have been implicated in AV blocks as extensive as permanent third-degree AV blocks, resulting from interference with the conduction pathways [[13], [14]]. We believe that our patient's first-degree AV block may have resulted from the interatrial IBC. Excision of IBCs is debatable and usually reserved for rapidly growing and/or symptomatic lesions [14], [15], [16]. A decision was made to proceed with surgical treatment following failure of pain relief in our patient. Histological analysis of IBCs reveals amber-colored mucous fluid within the cyst with a respiratory epithelial lining composed of pseudostratified cuboidal and columnar cells [17].
Conclusion
In conclusion, IBC are very rare tumors of the heart, making them unusual suspects during the initial evaluation of cardiac symptoms and cardiac masses. Their imaging features are identical to the more common extracardiac bronchogenic cysts typically discovered within the mediastinum. While further work is still needed to determine optimal management of such cysts, especially when asymptomatic, multimodality imaging is essential for diagnosis, treatment, and surgical planning as well as post-treatment surveillance.
Patient consent
Regarding patient written informed consent, authors confirm that it has been obtained from the relevant parties for the publication of this manuscript.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vaideeswar P, Agnihotri MA, Patwardhan AM. Intracardiac bronchogenic cyst. J Card Surg. 2011;26:266–268. doi: 10.1111/j.1540-8191.2011.01206.x. [DOI] [PubMed] [Google Scholar]
- 2.Fukada Y, Endo Y, Nakanowatari H, Kitagawa A, Tsuboi E, Irie Y. Bronchogenic cyst of the interatrial septum. Fukushima J Med Sci. 2020;66:41–43. doi: 10.5387/fms.2019-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 4.Tyebally S, Chen D, Bhattacharyya S, Mughrabi A, Hussain Z, Manisty C, et al. Cardiac tumors: JACC cardiooncology state-of-the-art review. JACC CardioOncology. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y, Wu W, Gao L, Ji M, Xie M, Li Y. Multimodality imaging of benign primary cardiac tumor. Diagnostics. 2022;12:2543. doi: 10.3390/diagnostics12102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pino Paolo Giuseppe, Moreo A, Lestuzzi Chiara. Differential diagnosis of cardiac tumors: general consideration and echocardiographic approach. J Clin Ultrasound. 2022;50:1177–1193. doi: 10.1002/jcu.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation. 2013;128:1790–1794. doi: 10.1161/circulationaha.112.000790. [DOI] [PubMed] [Google Scholar]
- 8.Amano J, Nakayama J, Yoshimura Y, Ikeda U. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen Thorac Cardiovasc Surg. 2013;61:435–447. doi: 10.1007/s11748-013-0214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogunkunle OO, Animashaun D. Intracardiac bronchogenic cyst in a 2-year-old Nigerian boy. Case Reports. 2012 doi: 10.1136/bcr.09.2011.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yacoub JH, Clark JA, Paal EE, Manning MA. Approach to cystic lesions in the abdomen and pelvis, with radiologic-pathologic correlation. Radiographics. 2021;41:1368–1386. doi: 10.1148/rg.2021200207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beale RA, Russo R, Beale C, Levin W, Atalay MK, Fingleton J, et al. Mitral valve blood cyst diagnosed with the use of multimodality imaging. CASE. 2021;5:173–176. doi: 10.1016/j.case.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bortolotti U, Vendramin I, Lechiancole A, Sponga S, Pucci A, Milano AD, et al. Blood cysts of the cardiac valves in adults: review and analysis of published cases. J Card Surg. 2021;36:4690–4698. doi: 10.1111/jocs.15992. [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Mateo V, Arias MA, Juárez-Tosina R, Rodríguez-Padial L. Permanent third-degree atrioventricular block as clinical presentation of an intracardiac bronchogenic cyst. Europace. 2008;10:638–640. doi: 10.1093/europace/eun056. [DOI] [PubMed] [Google Scholar]
- 14.Limaiem F, Mlika M. Bronchogenic Cyst. PubMed. 2023 https://pubmed.ncbi.nlm.nih.gov/30725658/ Available at: Accessed October 5, 2023. [PubMed] [Google Scholar]
- 15.Rumer GF, French S, Froede RC. Paraesophageal ciliated cysts of the mediastinum. Am J Surg. 1958;96:420–424. doi: 10.1016/0002-9610(58)90937-1. [DOI] [PubMed] [Google Scholar]
- 16.Phan AT, Hu J, Oganesian B, Williams SO. Symptomatic bronchogenic cyst in a lipomatous interatrial septum. Cardiol Res. 2023;14:315–318. doi: 10.14740/cr1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J, Cho G-Y, Park K-H, Oh I-Y. Intracardiac bronchogenic cyst. Circulation. 2014;130:1107–1109. doi: 10.1161/circulationaha.114.010992. [DOI] [PubMed] [Google Scholar]