Abstract
Liver cancer, a common and intractable liver-related disease, is a malignant tumor with a high morbidity, which needs a high treatment cost but still lacks perfect clinical treatment methods. Looking for an effective platform for liver cancer study and drug screening is urgent and important. Traditional analytical methods for liver disease studies mainly rely on the 2D cell culture and animal experiments, which both cannot fully recapitulate physiological and pathological processes of human liver. For example, cell culture can only show basic functions of cells in vitro, while animal models always hold the problem of species divergence. The organoids, a 3D invitro culture system emerged in recent years, is a cell-bound body with different cell types and has partial tissue functions. The organoid technology can reveal the growth state, structure, function and characteristics of the tissue or organ, and plays an important role in reconstructing invitro experimental models that can truly simulate the human liver. In this paper, we will give a brief introduction of liver organoids and review their applications in liver cancer research, especially in liver cancer pathogenesis, drug screening, precision medicine, regenerative medicine, and other fields. We have also discussed advantages and disadvantages of organoids, as well as future directions and perspectives towards liver organoids.
Keywords: Liver cancer, Organoids, Pathogenesis, Drug screening, Precision medicine
1. Introduction
As the largest gland in the human body, the liver is an important organ functioning of metabolism, digestion, detoxification, immunity, and so on. It is also known as the largest “chemical factory” of the human body and is composed of more than 20 types of cells, including hepatocytes, cholangiocytes, hepatic endothelial cells, hepatic stellate cells, Kupffer cell [1] etc., which work together to perform the basic metabolic function of the liver. The liver receives a unique dual blood supply from hepatic portal vein and hepatic artery. Approximately 80 % of the blood from the liver comes from the hepatic portal vein and 20 % from the hepatic artery. On one hand, the double blood supply gives the liver powerful functions, such as providing nutrition and filtering blood. On the other hand, the rich blood supply provides a conductive environment for the tumor growth, making it easy to be transferred with the blood tract and metastasize in the body.
Liver cancer is one of the most common malignancies with high morbidity and mortality, of which risk factors mainly are hepatitis B virus, hepatitis C virus, fatty liver, alcohol-related cirrhosis, aflatoxin infection, smoking, obesity, diabetes, iron overload and various unhealthy diets [[2], [3], [4], [5], [6]]. It can cause about 800,000 people diagnosed worldwide and more than 700,000 deaths per year [7]. In China, the mortality of liver cancer ranks the third following lung cancer and gastric cancer. Its high cost and desperate feeling make a great burden on families all over the world.
Liver cancer can be divided into two categories: primary liver cancer and secondary liver cancer. Primary liver cancer is a malignant tumor with gene mutation of liver cells or intrahepatic bile duct epithelial cells. It mainly contains hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC) and mixed cell carcinoma, among which HCC accounts for more than 80 % of primary liver cancer cases in the world [8]. The main intra-tumoral feature of HCC is highly heterogeneous at histological and molecular level. Therefore, different types of HCC usually have different clinical features and carcinogenic mechanisms, leading to different responses to drugs [9].
Up to now, the clinical treatment methods of liver cancer include drug treatment, surgical treatment, and radiation treatment. Drug treatment is the common treatment choice in the early stage of liver cancer. However, its prognosis is always poor [10]. For examples, due to the side effects of drug toxicity, such as the drug-induced liver damage and kidney damage [11], most drug candidates are abolished in the phase I clinical approval process [12]. Therefore, looking for an effective and safe drug for liver cancer treatment is still an urgent and important thing at present.
As usually, before clinical research, the drug screening process mainly is done based on the cell culture technique and animal experiments. These two traditional methods have their own unique advantages, but both have some limitations and cannot simulate the same disease development and drug response with those in human body completely. Therefore, to understand the physiology and pathology of liver-related diseases accurately, it becomes particularly important to develop new research models that can fully reconstitute key features of the human liver.
Organoid technology is an emerging technique in recent years, in which different types and functions of cells and extracellular matrix are biologically self-aggregated and bound so as to build a 3D invitro organ-mimic system. Compared with the traditional 2D cell culture technique and animal experiments, organoids have highly similar properties with human-derived tissues or organs in both structure and function [13]. In 1975, Rheinwald and Green [14] obtained a keratinocyte cell line XB from a transplantable mouse teratoma and cultured the tumor cells in vitro for the first time, which laid a foundation for the following scientific researches. In 1992, Petersen and Bissell successfully cultured the first invitro 3D cell model to simulate mammary gland structures in both cancerous and non-cancerous cases [15,16], which was the rudiment of organoids. Compared with 2D monolayer cells, 3D culture systems have the advantages of natural cell-cell and cell-matrix interactions and can improve the efficiency of spatiotemporal signaling communication between cells, a crucial part in cell proliferation and function [17]. Because of shorter modeling time, stronger value-added power, closer to the original tumor and more economic benefits, organoids have been widely applied in the fields of drug screening and evaluation, regenerative medicine, tumor pathogenesis, etc. [18]. Therefore, in this paper, we will summarize and emphasize construction methods of organoids and their applications in liver cancer to provide a new thinking and a good reference for future liver-related studies.
2. Establishment of liver organoids
Liver organoids, composed of ductal cells and hepatocytes, are differentiated from endodermal progenitors. They are usually cultured in two ways [19]: one is culturing liver organoids using pluripotent stem cells (inducing pluripotent stem cells to form hepatic endodermal cells and then adding a variety of growth factors to induce differentiation into “cyst-like” liver organoids), and the other one is culturing liver organoids using organ-specific tissue (bile duct-like tissue is obtained by direct digestion, and cytokines, such as dexamethasone, HGF and EGF, are added to the culture medium) [19]. The growth process of liver organoids contains three stages: separation, proliferation, and differentiation. According to the source of cells, organoids can be divided into three categories, namely, adult stem cell-derived organoids, tumor-derived organoids, and pluripotent stem cell-derived organoids [20].
2.1. Adult stem cell-derived organoids
Adult stem cells (ASCs) are multipotential stem cells (present in different tissues of fetal) and adult cells, which can replicate themselves and produce different types of mature cells with specific phenotypes and functions. They can maintain the stability of the body function, and play a physiological role in cell renewal and tissue damage repairment [21]. Proliferation and differentiation of patients' own derived hepatocytes in vitro are in clinical practice, which will provide enough biological materials for scientific research to better understand the development and changes of the diseases.
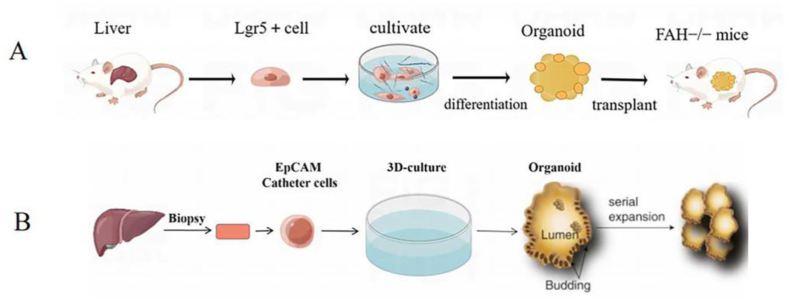
In 2009, Clever group [22] took intestinal stem cells from the crypts of the small intestine and cultured them in vitro to produce intestinal organoids. This study is the first work showing that ASCs can be used to grow and form organoids in vitro. In 2013, Huch et al. [23] successfully constructed the first liver organoid using Lgr5+ hepatocytes, which maintained the ability of proliferation and differentiation. They could be transferred into functional hepatocytes in vitro, and even be re-transplanted into mice to perform normal liver function (Fig. 1A). In 2015, Huch et al. [24] put up with a culture protocol for humanized liver organoids, which could stably maintain humanized liver cells to proliferate and differentiate for a long time, providing a good platform for model establishment, drug screening and disease treatment (Fig. 1B).
Fig. 1.
(A) Construction of liver organs by Lgr5+ hepatocytes; (B) Growing Liver Organoids from Ductal Cells.
2.2. Tumor-derived organoids
Compared with the traditional 2D monolayer cell culture technique, 3D cancer culture methods can better represent biological features of the original tumor. Tumor organoids have advantages of stable genome stability, strong value-added force and short culture time. And they can also retain the heterogeneity of tumor tissue in parental tumor genome, histological structure and tumorigenesis mechanism [25]. The common tumor organoids establishment methods mainly include gene editing of normal cells and direct culture of tumor tissue [26]. The preparation method is disassembling the tumor tissue into single cells, collecting these cancer cells and self-organizing into stable spheroids in suspension or matrix in autonomous culture. For example, in 2017, Duarte et al. directly used tumor tissue for fabricating organoids and then conducted drug resistance studies for cancer. Compared to traditional cell lines or mammary spheres, organoids are able to replicate, proliferate and differentiate efficiently in vitro and present epithelial morphology and original tumor drug response, meaning the ability of in situ transplantation of tumors. Notably, tumor-derived organoids can be genetically modified easily, making them a powerful tool for tumor biology and the genetics of drug resistance [27].
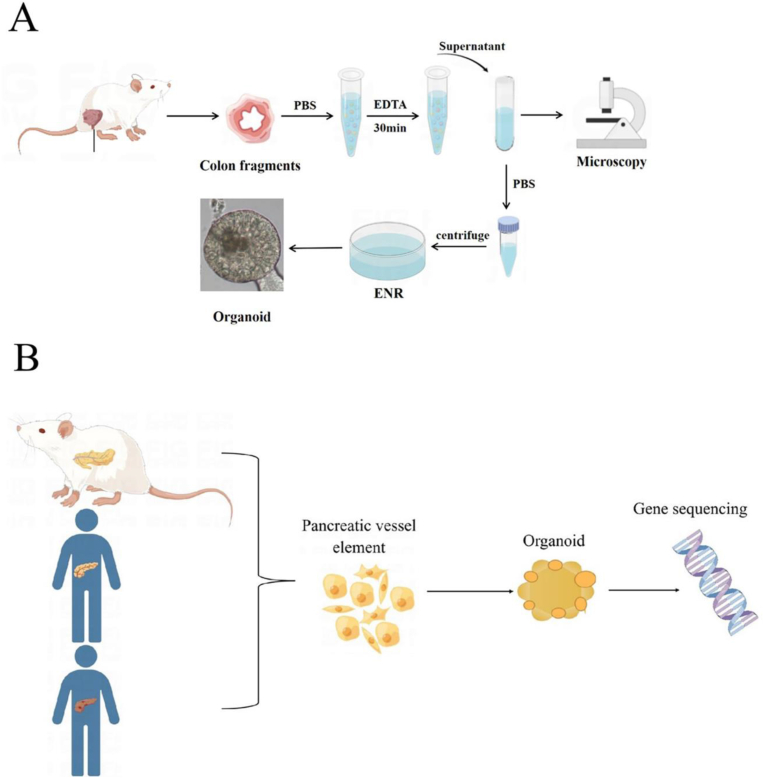
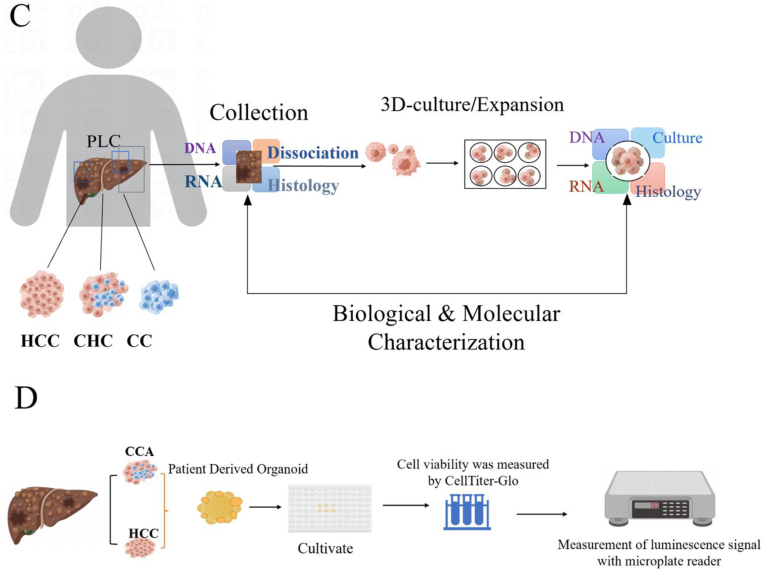
The establishment of organoids using tumor cells dates back to 2011, when Stange et al. [28] firstly manufactured organoids based on mouse colon crypts. Through adjustment and optimization of the culture parameters, they successfully developed organoids with the culture of colon adenoma, adenocarcinoma, and Barrett's esophagus (Fig. 2A). It was observed that mature goblet cells and enteroendocrine cells were produced and mouse small intestinal epithelial cells cultured under WENR (recombinant human Wnt-3A or Wnt-3A conditioned medium + ENR (mouse EGF, mouse brain protein, human R-spondin-1)) could be differentiated into different epithelial cell types with stem cell self-renewal. In 2015, Boj et al. [29] constructed pancreatic tumor organoids and sequenced more than 2000 tumor-related genes, confirming that tumor organoids had cancer-related gene mutations and could maintain the physiological structural characteristics of tumors (Fig. 2B). In 2017, Broutier et al. [30] successfully cultured three different subtypes of primary liver cancer cells into organoids (Fig. 2C), and found that the cultured liver cancer organoids could preserve the tissue structure and genomic composition of the original tumor. They also found that different tumor tissues and subtypes could be distinguished easily after long-term culture and the physiological structural characteristics of tumors could be maintained after transplantation of cultured liver cancer organoids into mice. Subsequently, in 2019, Li et al. [31] established 27 tumor organoid systems of primary hepatocellular carcinoma and cholangiocarcinoma (Fig. 2D), demonstrating that organoids could exhibit molecular and morphological features of cancer tissue. Correspondingly, drug screening studies showed a high heterogeneity of the organoids, which favored anti-tumor drugs screening effectively. Therefore, the hepatoma organoids cultured in vitro have the ability of high proliferation and, at the same time, maintaining the original tumor properties. They can act as a simple, stable and cheap experimental tool for tumor research, and have great application potential in regenerative medicine, anti-tumor drug screening and development.
Fig. 2.
Research on the construction of organoids using tumor cells. (A) Establishment of the colon organoid model; (B) Gene sequencing of pancreatic tumor organs; (C) Three different subtypes of primary liver cancer cells were cultured as organ-analogous models; (D) 27 tumor organs of primary hepatocellular carcinoma and cholangiocarcinoma were used for drug screening.
Tumor organoids are miniature tumor models formed by isolating tumor cells (including cancer stem cells) from patients' tumor tissues and adding certain cytokines and small molecules to establish a microenvironment similar to that in vivo for 3D culture. Cancer stem cells are considered to be one of the important causes of tumor heterogeneity [32], and they are a group of cells with the potential for self-renewal, proliferation and differentiation. These cells are closely related to tumor formation, metastasis, and recurrence, and can differentiate into carcinoid cells in the primary tumor. This characteristic of cancer stem cells is an important reason why tumor organoids have similar structural characteristics and functional specificity to the source tissues, and can grow stably in an in vitro 3D culture system.
Most malignant tumors originate from epithelial cells. The traditional research method for organoids construction mainly relies on Hubrecht Organoid Technology, which only contains tumor cells and is difficult to co-culture with other tumor microenvironment cells for a long time. However, tumor microenvironment cells, including stromal cells, immune cells, chemokines, cytokines, and blood vessels, plays an important role in tumor initiation, development, and metastasis [33]. Therefore, in recent years, people have optimized tumor organoid culture technology to reconstruct the tumor microenvironment. Some new methods, such as Submerged matrigel culture [[34], [35], [36]], Microfluidic 3D culture [37], Air-liquid interface [38,39], provide a more mature research model for the interaction study between tumor microenvironment and tumor cells. For example, co-culturing T47D human breast cancer cells with immortalized human mammary fibroblasts (HMFs) as well as different combinations of various ECM components in a microfluidic device found that HMFs facilitated the growth of breast cancer cell clusters compared to the situation that cancer cells were cultured alone [40]. It allows experimentalists to generalize the multicellular architecture, the tissue-tissue interface and the physiologically relevant physical microenvironment of cancers grows within living human organs while maintaining vascular perfusion in vitro.
In order to avoid the normal cells being cultured as organoids, the tissue cells can be sorted by flow cytometry or magnetic beads, and then the organoids can be cultured after the tumor cells are obtained. The following three methods is always used to purify organoid populations. The first one is manual screening under the microscope based on morphological differences between normal organoids and Patient Derived Organoids (PDOs). For example, normal gastric organoids are spherical in shape, composed of a single layer of epithelial cells, and have a large lumen, while tumor organoids are usually sac-shaped and have a multi-layered cell morphology [41]. The second one is screening by conditioning the components in the medium. For example, most gastric cancers exhibit a TP53 mutation, which causes PDOs to be more tolerant to Nutlin-3 than normal organoids [41]. The third one is to digest the organoids into single cells and then sorting the tumor cells by flow cytometry.
2.3. Pluripotent stem cell-derived organoids
Pluripotent stem cells (PSCs), a kind of multipotential cells capable of self-renewal and self-replication, can differentiate into all cell types and further corresponding tissues and organs in the body. They can be divided into embryonic stem cells and induced pluripotent stem cells (iPSCs). The establishment of organoids based on PSCs requires the formation of specific germ layers (endoderm, mesoderm, or ectoderm) and then cocultured with essential growth factors and cytokines to induce directional cell differentiation and maturation.
First proposed by Takahashi et al. [42] in 2006, iPSCs can be developed into a patient-specific disease model and used for personalized medicine [43]. In 2013, Takebe et al. [44] firstly reported the construction of liver organoids with iPSCs that reconstituted the key vascular and functional features of human liver in vitro. In their work, they cocultured PSCs-induced endodermal cells with stromal cells and then transplanted them into immunodeficient mice to form liver buds (iPSC-LBs), which finally recapitulated tissue morphology and function of the liver. In 2019, Wang et al. [45] constructed a liver-analogous organ based on human PSCs using a new serum-free and feeder-free medium, which could stably maintain the phenotypic characteristics of bidirectional potential liver stem cells and differentiated into functional hepatocytes or bile duct cells. It proved to be able to survive for 20 generations in vitro and met the needs of large-scale use of cells in industry or clinical. In 2020, Takeishi et al. [46] reprogrammed human amnion cells and embryonic fibroblasts to create an iPSC line. The obtained iPSCs were then differentiated into different liver cell lines and further functional artificial mini livers in different growth environments. Due to the symptoms of portal vein thrombosis, intestinal obstruction, and poor blood flow after organoids transplantation into rats, this results in rats only living for 4 days. It may be due to the fact that organoids do not have blood vessels and cannot absorb nutrients, thus affecting the growth and function of organoids. Therefore, the study of iPSCs is of great significance in not only basic researches but also applications in organ regeneration, repair, and disease treatment.
In addition to the organoids obtained from the forementioned single cell culture method, multiple cell coculture technology can also produce organoids in vitro [47]. Multicellular coculture, also known as hybrid culture, is the culture method with two or more kinds of cells cocultured in the same system at the same time. Compared with single cell type culture, coculture with multiple cell types is more favorable for the construction of accurate physiological or pathological processes of human organs in vitro and can be further used to observe cell-cell and cell-matrix interactions. For example, Li et al. [31] established several human primary hepatocellular organoids derived from resected hepatocellular carcinoma tissue, and confirmed that the PDO culture showed a similar marker profile to the original primary human tumor through the identification of biomarkers such as epithelial markers (EPCAM) and bile duct markers cytokeratin 19 and 7 (CK19 and CK7). Takebe et al. [48] seeded hepatic endoderm cells from human iPSCs (iPSC-HEs) with human umbilical vein endothelial cells (HUVEC) and human mesenchymal stem cells (MSCs) into culture dishes with precured Matrigel. The mixture formed macroscopic visible 3D cell aggregates after 48 h. Besides, a variety of cells, including liver, kidney, lung, intestine, brain, heart, and cancer (HepG2) cells, can also be cocultured with HUVEC and MSC, and then vascularized organ buds can be generated by transplantation, which is a common approach for cultivating vascularized, complex organ buds [49].
3. Application of organoids in liver cancer
Organoids can be applied into the study of tumor pathogenesis, drug screening and evaluation, regenerative medicine research, and precision medicine. Compared with traditional organ models, organoids have superiorities in modeling time, proliferation extent, experimental price and tumor characteristic reservation [18], which make the organoids an excellent experimental tool for the studies and treatment of liver cancer.
3.1. Tumor pathogenesis
Globally, liver cancer is the most common pathogenic malignancy [50] with the two major representations of hepatocellular carcinomas (HCCs) and intrahepatic cholangiocarcinomas (ICCs) [51]. In general, pathogenic factors of liver cancer vary from toxins, virus to poor diet, and only less than 30 % of patients can be diagnosed at an early stage of liver cancer [52]. Recent studies have described a broad mutational spectrum of the hepatocellular carcinoma, showing an average of 30–40 mutations for each kind of tumors [52]. The main mutations are located in the promoter region of TERT, TP53, CTNNB1, CDKN2A and AXIN 1 [53]. However, the oncogenic addiction loop hasn't been well-defined in hepatocellular carcinoma [52]. Therefore, it is extremely crucial to develop experimental models that mimic the early stage of the human liver cancer development to analyze HCC and ICC initiation.
As mouse and human hepatocytes exist species divergence and have different physiological characteristics (e. g. transcription factor networks) [54], organoids with highly similar properties to human tissues or organs have undoubtedly become an effective method for studying liver cancer development. Sabharwal et al. inactivated p53 and RB in human-induced hepatocytes (HiHeps) using SV40LT, creating organoids with liver structure and function. HiHep organoids were genetically engineered to simulate the initial alterations of human liver cancer. On one hand, malignant HCC morphology was triggered by c-Myc in HiHeps to result in higher levels of mitochondrial Ca2+ uptake, making important effects on mitochondrial respiration, oxidative stress responses and tumorigenesis [55]; On the other hand, 10 selected ICC-enriched oncogenes in ICC patients were transformed into hepatocytes by ICC-enriched mutations. The findings showed that some individual ICC-enriched oncogenes, especially RASG12V, could drive the conversion of hepatocytes to ICC [25]. Additionally, related research injected mice with NICD and AKT plasmids to overexpress them and used a mouse model of hepatocyte fate tracing. The results revealed that NOTCH and AKT signaling co-operated to transform normal hepatocytes into biliary cells that act as precursors of fast progressing, lethal ICCs, which is critical for malignancy and progression [56,57]. It is of great importance for the pathogenesis study, early detection and spatial interaction studies of the liver cancer. In the future, introducing other liver cancer-related surroundings, such as immune system and cellular stroma, maybe a brand-new research thread and direction.
3.2. Drug screening
Drug screening is an experimental operation to obtain compounds with specific physiological activity in the process of modern drug development. It is a key step in the development of new drugs. Liver organoids have been demonstrated to be capable of assisting drug discovery and efficacy evaluation of clinical drugs in liver cancer studies.
Liver organoids are currently being used for drug development in a variety of benign and malignant liver diseases [58] (Table 1). For instance, in 2018, Nuciforo et al. [59] used varied concentrations of sorafenib [60] in HCC and ICC organoids to assess the feasibility of preclinical drugs in liver cancer treatment. They found that HCC organoids’ growth was highly dependent on the dose of sorafenib by monitoring cell viability. They also tested the efficacy of sorafenib against three ICC organoids, among which the rare lymphoepithelioma-analogous ICC organoid responded to sorafenib obviously in vitro and its semi-inhibitory concentration value was comparable to that of the sorafenib-sensitive HCC organoid. The result indicated that organoids derived from primary liver cancer biopsies could be used to test drug specificity and sensitivity to the tumor. In 2019, Li et al. used liver cancer organoids for cancer drug screening. They established a total of 27 liver cancer organoid models according to different regions of bile duct cancer surgical specimens, and then tested 129 antitumor drugs [31]. The result showed that most anticancer drugs were effective against the few organoid cell lines, while only few drugs showed broad effectiveness against all organoid cell lines. This study also supported the usage of cancer organoids for drug effect evaluation in the field of drug discovery [61].
Table 1.
Examples of drug validation and testing based on liver organoids.
| Disease | Drug name | Evaluation Index | Ref. |
|---|---|---|---|
| Hepatocellular carcinoma and Intrahepatic cholangiocellular carcinoma | Sorafenib | Cell viability | [31] |
| Hepatocellular carcinoma | Cisplatin, Gemcitabine et al. | Cell viability | [25] |
| Wolman disease | FGF-19 | Lipid accumulation | [63] |
| HEV | Brequinar Homoharringtonine | Luciferase activity | [64] |
The Pharmaprojects January 2023 data shows that the number of anti-tumor drugs in the global research continues to rise, accounting for 39.8 % of the number of global research and development of drugs, an increase of 9.1 % over last year. Currently, the models used for new drug research mainly include 2D cultured cell models and animal models. However, 2D cultured cells have certain limitations in evaluating drug efficacy [62]and can't faithfully reflect invivo conditions. And species differences is the main reason for the high failure rate of new drug research for animal tests. Patient-derived organoids, to be honest, can highly recapitulate the characteristics of the tumor source and have higher sensitivity, heterogeneity, and stability.
Advantages of organoids are that both normal and tumor organs can be cultured in a petri dish in vitro using the Hubrecht organoid technique and that it can be used as an invitro culture model for large-scale screening. At the same time, the reliance on animal-derived Matrigel, which can cause variability between organoids due to the uncertainty of its composition, batch-to-batch variation, and local variations in mechanical properties during the Matrigel formation process, and the requirement of, such as, growth factors, hormones and special growth environment, make organoid culture more expensive and restrict its practical application to some extent.
3.3. Precision medicine
Precision medicine, also known as individualized medicine, is an innovative treatment program that tailors disease prevention and treatment based on individualized physiological or pathological characteristics. The concept of “precision medicine” was first introduced in 2011 by the American medical community to minimize the source of damage, minimize the cost of care, and maximize the recovery of patients [65]. The derived organoids can be used for biomarker identification and drug screening test, which dramatically improves the efficiency of clinicians in achieving precision medicine for different patients. Compared with conventional experimental methods of precision medicine (e. g. gene expression testing and drug sensitivity testing), the introduction of organoids can shorten experimental time obviously and facilitate a more accurate and real-time assessment of the individual patient's physical condition [66].
Liver cancer is a highly heterogeneous disease with characteristics of environmental or genetic susceptibility, morphological diversity, signaling network disorders and microenvironmental differences [67,68]. Presently, the anti-tumor therapy used in clinical is a universal treatment regimen based on extensive clinical trials. However, owing to the neglection of tumor heterogeneity, the same therapeutic drug lacks specificity toward different liver cancer patients and sometimes results in different therapeutic outcomes. Culturing liver organoid can help solve this problem with individual experimental design and test. For example, Steele et al. constructed a sample panel from seven gastric cancer (GC) patients. By testing the reaction sensitivity of several standard chemotherapeutic agents (epirubicin, oxaliplatin, and 5-fluorouracil) on different patients' organoids, they had provided a favorable scientific reference for the future implementation of personalized precision medicine for patients with liver cancer [69]. Furthermore, organoids cultured from patient tissues can preserve the tissue structure feature, gene expression and genomic panorama of the original cancer. Even under the same culture condition, organoids still can retain the characteristics of different cancer tissues and subtypes [70]. Surprisingly, the development of emerging gene editing technology has brought tremendous changes to the study of human biology and provided new hope for precision medicine [20]. The combination of tumor organoids and gene editing technology enables precise monitoring of the influence of single gene mutations on tumor cells, thereby establishing genotype-phenotype correlations [71].
In 2017, Broutier et al. cultured primary liver cancer (PLC)-derived organoids which preserved characteristics of tumor cells and allowed the identification of novel genes. They reported that the biomarkers of C19ORF48, UBE2S and DTYMK (for HCC) and C1QBP (for ICC) were linked with poor PLC prognosis. Therefore, the utility of PLC-derived organoids could open up opportunities for disease diagnosis and treatment evaluation [30]. Then, Broutier et al. further investigated the sensitivity of taserisib, AZD8931, SCH772984 and other drugs using liver cancer organoids, demonstrating a great potential of organoids for testing drug sensitivity and resistance in liver cancer studies and functioning as a new therapy tool for personalized medicine [30].
3.4. Regenerative medicine
As an emerging interdisciplinary technique with practical clinical application [72], regenerative medicine (RM) is dedicated to repairing, regenerating, or replacing damaged cells, tissues or organs for structural repair and functional restoration [73,74], with the great promise of treating cancer diseases. Meanwhile, RM is a paradigm for the future healthcare and the emerging regenerative technologies has unprecedented potential in expanding the range of individual treatment approaches [75]. In fact, the combination of RM and liver organoids are becoming more and more popular in liver-related study fields and is considered as a useful biological tool for organ replacement, disease modeling, toxicology studies and drug discovery [76].
Since 1983, orthotopic liver transplantation (OLT) has been considered the only medical solution for patients with liver failure. Partial liver transplant is frequently considered, which can realize several patient treatments from one single liver donor at the same time. However, about 1 %–5 % new transplanted livers present poor liver function, leading to about 7 % death. In addition, many procedure-related complications may occur during the transplanting process, such as hepatic artery thrombosis (2–5%), biliary complications (15 %) and/or infections [77]. However, liver organoids, derived from hepatic cells of the patients, can be a potentially effective pathway to the liver transplantation because of weak organ rejection.
In 2017, Mastrogiovanni et al. trans-proliferated healthy primary hepatocytes into liver organoids, whose functions and properties were analogous to those of the liver [30]. In 2021, Yang et al. established liver organoids using HepaRG cells and 3D bioprinting technology, which were able to successfully exhibit some liver functions, such as albumin secretion, drug metabolism and glycogen storage, after 7 days of proliferation and differentiation in vitro. Subsequently, transplantation of liver organoids into the peritoneal cavity of Fah−/−rag2−/− mice enabled the mice to acquire human-specific drug metabolism capabilities. The transplanted liver organoids formed a functional vascular system and remarkably improved the survival rate of the mice after liver transplantation. The work successfully demonstrated that liver organoids printed in vitro could be used as transplantation donors to replace the original liver and promote organism survival and growth [78]. Currently, liver organoids have been transplanted into different parts of the body, such as the liver, mesentery, peritoneum and omentum, but complete replacement of liver function hasn't been realized at present. Now, many surgical techniques have been developed that can be applied to transplant immunodeficient organoids to prevent graft rejection [79]. And liver transplantation has shown many superiorities for liver cancer patients who were not suitable for surgical resection [80]. The gradual maturation of liver organoids undoubtedly provides a potential direction for restoring partial liver function by liver transplantation in liver cancer patients, and liver organoids may serve as a rescue bridge for the transition from liver damage to liver regeneration, or as a complement to extensive hepatectomy and temporary maintenance of the liver during transplantation waiting.
Liver organoids, as a tool of liver regeneration, prove to have a great promising in live disease treatment. Capable of replacing damaged lives and increase survival rate of mammals, liver organoids will also have a potential in liver cancer treatment with the development of regenerative medicine in the future.
Except for cancer research, the organoid technique is also well established in the study of other common clinical liver diseases, such as hepatitis [[81], [82], [83]], cirrhosis [84], liver abscess [85], liver fibrosis [86], etc. In 2020, Ramli et al. cultured H1 human embryonic stem cells and iPSCs with serum-free medium and then induced them into liver organoids. They cultured the obtained liver organoids with a mixture of oleic and palmitic acids for 14 days [87] and then analyzed the transcriptomic expression. The result revealed that bile duct-like structures were found in the organoids and the organoids had the same gene expression profile with liver tissue from nonalcoholic steatohepatitis patients [88]. In 2022, Zhao et al. established the first model of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection using human hepatic duct organoids. Though using the infected hepatic duct organoids, the researchers explored the mechanism of liver injury under viral attack in detail. They verified that SARS-CoV-2 was susceptible to liver cells expressing ACE2 and TMPRSS2, and further caused direct damage to the human liver. They also identified that the impairment of the bile duct cell barrier and bile acid transport function was achieved through regulating genes involved in tight junction formation and bile acid transport. This study suggests that liver organoids could be considered as a research tool for the study of liver injury caused by viral infection [89].
With the development of various new organoids, organoid transplantation has also been practiced in a series of tissues and organs such as liver [90], extrahepatic biliary tract [91], and brain [92]. Due to its unique proliferative ability and tissue specificity, organoid transplantation is able to restore and repair structures in vivo without malformations or tumors [93]. This provides a good basis for organoids in the research of regenerative medicine for the treatment of cancer.
4. Summary and perspective
The liver is a complex organ with multiple functions in the body and responsible for physiological processes such as human metabolism, protein secretion, detoxification and bile production [94]. Liver cancer is the most common malignant tumor of the digestive system with a high mortality and poor prognosis. Therefore, how to find new treatment options to improve the survival rate of patients is always an urgent scientific and practical problem in the treatment of liver cancer [95].
The organoid technology, one of the emerging technologies in recent years, preserves histological properties and gene expression characteristics of original tissues in vitro and prove to be a useful alternative to traditional 2D cell culture and animal models [96]. It is powerful in studying disease development, regenerating tissues, deciphering pathogenesis and exploring treatment tools [97]. Organoid models of the colon [98], breast [99], prostate [100,101], heart [102], liver [23,103,104] and other organs or tissues have been successfully constructed, showing a great application potential in the fields of growth and development, disease pathology, drug screening, toxicological evaluation, regenerative medicine, and biomaterials, etc [19].
In this paper, we have summarized and emphasized the development of liver organoids and their applications in liver cancer. Liver organoids can be used to study the molecular mechanism of tumor development in vitro, discover new tumor targets and promote drug optimization to assist solve the problem of drug resistance [18]. At the same time, they also facilitate the exploration of liver cancer pathogenesis and the identification of tumor biomarkers [105]. With the development of the organoid technology, it can realize invitro modeling of rare diseases and achieve high-throughput drug screening, which is of great benefit in observing the early human development process timely [106]. The combination of organoids with real-time imaging technology or 3D printing technology have made further efforts for drug efficacy evaluation and cancer treatment.
Although organoid technology has the unique advantage of highly similar physiological structure and function with original tissues, it is still in the raw and development stage with decade history. The biggest challenge in organoid research is the limited maturation, which can only form fetal-like tissues rather than adult tissues. The lack of important physiological processes means organoids can only reproduce partial organ functions and can't replicate human organs completely in vivo [107]. In addition, further problems, such as potential carcinogenicity, standardized culture protocols, and accurate and effective detection methods, need to be considered and solved in the organoid studies [108]. But with the development of techniques, in the future, it is great promising in realizing invitro three-dimensional coculture of liver organoids with other tissues and strengthening the feasibility of entire organism reproduction.
Overall, we think the organoid technology will become a more powerful platform for understanding human diseases and discovering new drugs because of its intrinsic advantages. It is also great promising in treating cancer and improving patient prognosis, which endows the organoids with broader development prospects and more practical implications.
Ethical statement
There are no animal experiments carried out for this article.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgements
Thanks to General Program of National Natural Science Foundation of China (82174039), General project of Shandong Natural Science Foundation (ZR2020MH371), Shandong Taishan Scholars Young Expert Project (tsqn202103110), Supporting plan for leading talents above provincial level in Yantai, Shandong Province Science and Technology Small and Medium-sized Enterprises Innovation Ability Improvement Project (2023TSGC0912).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Ting Cao, Email: tingcao@zju.edu.cn.
Jiayu Zhang, Email: zhangjiayu0615@163.com.
References
- 1.Trefts E., Gannon M., Wasserman D.H. The liver. Curr Biol. 2017 Nov 6;27(21):R1147–R1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arzumanyan A., Reis H.M., Feitelson M.A. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013 Feb;13(2):123–135. doi: 10.1038/nrc3449. [DOI] [PubMed] [Google Scholar]
- 3.Yang S.F., Chang C.W., Wei R.J., Shiue Y.L., Wang S.N., Yeh Y.T. Involvement of DNA damage response pathways in hepatocellular carcinoma. BioMed Res Int. 2014 Apr 28;2014 doi: 10.1155/2014/153867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smela M.E., Currier S.S., Bailey E.A., Essigmann J.M. The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis. 2001 Apr;22(4):535–545. doi: 10.1093/carcin/22.4.535. [DOI] [PubMed] [Google Scholar]
- 5.Chuang S.C., La Vecchia C., Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009 Dec 1;286(1):9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Center M.M., Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011 Nov;20(11):2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 7.Chen W., Zheng R., Zhang S., Zeng H., Xia C., Zuo T., et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017 Aug 10;401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag H.B., Rudolph K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007 Jun;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 9.Li L., Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016 Sep 1;379(2):191–197. doi: 10.1016/j.canlet.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Anwanwan D., Singh S.K., Singh S., Saikam V., Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020 Jan;1873(1) doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballet F. Hepatotoxicity in drug development: detection, significance and solutions. J Hepatol. 1997;26(Suppl 2):26–36. doi: 10.1016/s0168-8278(97)80494-1. [DOI] [PubMed] [Google Scholar]
- 12.Horvath P., Aulner N., Bickle M., Davies A.M., Nery E.D., Ebner D., et al. Screening out irrelevant cell-based models of disease. Nat Rev Drug Discov. 2016 Nov;15(11):751–769. doi: 10.1038/nrd.2016.175. [DOI] [PubMed] [Google Scholar]
- 13.Sun C.P., Lan H.R., Fang X.L., Yang X.Y., Jin K.T. Organoid models for precision cancer immunotherapy. Front Immunol. 2022 Apr 5;13 doi: 10.3389/fimmu.2022.770465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rheinwald J.G., Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975 Nov;6(3):317–330. doi: 10.1016/0092-8674(75)90183-x. [DOI] [PubMed] [Google Scholar]
- 15.Habanjar O., Diab-Assaf M., Caldefie-Chezet F., Delort L. 3D cell culture systems: tumor application, advantages, and disadvantages. Int J Mol Sci. 2021 Nov 11;22(22) doi: 10.3390/ijms222212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen O.W., Rønnov-Jessen L., Howlett A.R., Bissell M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G., David B.T., Trawczynski M., Fessler R.G. Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev Rep. 2020 Feb;16(1):3–32. doi: 10.1007/s12015-019-09935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y.X., Xu F.S., Wang T., Yang C.Y., Zhou X.B., Ren X.S. Application and prospects of organoid culture in cancer. Chin Pharmacol Bull. 2021 Sep;37(9):1197–1201. [Google Scholar]
- 19.Li T.R., Zhao R.B., Zhang Q., Kong X.D. Progress of research on organoid and its applications. Prog Biochem Biophys. 2019 Jun;46(8):737∼750. [Google Scholar]
- 20.Yu D.H., Cao H., Wang X.R. Advances and applications of organoids: a review. Sheng Wu Gong Cheng Xue Bao. 2021 Nov 25;37(11):3961–3974. doi: 10.13345/j.cjb.200764. [DOI] [PubMed] [Google Scholar]
- 21.Slack J.M. Stem cells in epithelial tissues. Science. 2000 Feb 25;287(5457):1431–1433. doi: 10.1126/science.287.5457.1431. [DOI] [PubMed] [Google Scholar]
- 22.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009 May 14;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 23.Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013 Feb 14;494(7436):247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M., et al. 24. Cell. 2015 Jan 15;160(1–2):299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L., Wang Y., Cen J., Ma X., Cui L., Qiu Z., et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat Cell Biol. 2019 Aug;21(8):1015–1026. doi: 10.1038/s41556-019-0359-5. [DOI] [PubMed] [Google Scholar]
- 26.Ma C.J., Yang Y., Zhang B.L., Zhou X.N., Dai R., Lin Q. Research status and application prospects of organoids. J Kunming Med University. 2019 Nov;40(6):1–5. [Google Scholar]
- 27.Duarte A.A., Gogola E., Sachs N., Barazas M., Annunziato S., R de Ruiter J., et al. BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat Methods. 2018 Feb;15(2):134–140. doi: 10.1038/nmeth.4535. [DOI] [PubMed] [Google Scholar]
- 28.Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011 Nov;141(5):1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Boj S.F., Hwang C.I., Baker L.A., Chio, Engle D.D., Corbo V., et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015 Jan 15;160(1–2):324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broutier L., Mastrogiovanni G., Verstegen M.M., Francies H.E., Gavarró L.M., Bradshaw C.R., et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017 Dec;23(12):1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L., Knutsdottir H., Hui K., Weiss M.J., He J., Philosophe B., et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019 Jan 24;4(2) doi: 10.1172/jci.insight.121490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapoor-Narula U., Lenka N. Cancer stem cells and tumor heterogeneity: deciphering the role in tumor progression and metastasis. Cytokine. 2022 Sep;157 doi: 10.1016/j.cyto.2022.155968. [DOI] [PubMed] [Google Scholar]
- 33.Arneth B. Tumor microenvironment. Medicina (Kaunas) 2019 Dec 30;56(1):15. doi: 10.3390/medicina56010015. PMID: 31906017; PMCID: PMC7023392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Driehuis E., Kolders S., Spelier S., Lõhmussaar K., Willems S.M., Devriese L.A., et al. Oral mucosal organoids as a potential platform for personalized cancer therapy. Cancer Discov. 2019 Jul;9(7):852–871. doi: 10.1158/2159-8290.CD-18-1522. [DOI] [PubMed] [Google Scholar]
- 35.Pasch C.A., Favreau P.F., Yueh A.E., Babiarz C.P., Gillette A.A., Sharick J.T., et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clin Cancer Res. 2019 Sep 1;25(17):5376–5387. doi: 10.1158/1078-0432.CCR-18-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganesh K., Wu C., O'Rourke K.P., Szeglin B.C., Zheng Y., Sauvé C.G., et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019 Oct;25(10):1607–1614. doi: 10.1038/s41591-019-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aref A.R., Campisi M., Ivanova E., Portell A., Larios D., Piel B.P., et al. 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade. Lab Chip. 2018 Oct 9;18(20):3129–3143. doi: 10.1039/c8lc00322j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuki K., Cheng N., Nakano M., Kuo C.J. Organoid models of tumor immunology. Trends Immunol. 2020 Aug;41(8):652–664. doi: 10.1016/j.it.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chioni A.M., Bajwa R.T., Grose R. 3D organotypic culture model to study components of ERK signaling. Methods Mol Biol. 2017;1487:255–267. doi: 10.1007/978-1-4939-6424-6_19. [DOI] [PubMed] [Google Scholar]
- 40.Sontheimer-Phelps A., Hassell B.A., Ingber D.E. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. 2019 Feb;19(2):65–81. doi: 10.1038/s41568-018-0104-6. PMID: 30647431. [DOI] [PubMed] [Google Scholar]
- 41.Wallaschek N., Niklas C., Pompaiah M., Wiegering A., Germer C.T., Kircher S., et al. Establishing pure cancer organoid cultures: identification, selection and verification of cancer phenotypes and genotypes. J Mol Biol. 2019 Jul 12;431(15):2884–2893. doi: 10.1016/j.jmb.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 43.McCauley H.A., Wells J.M. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development. 2017 Mar 15;144(6):958–962. doi: 10.1242/dev.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013 Jul 25;499(7459):481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 45.Wang S., Wang X., Tan Z., Su Y., Liu J., Chang M., et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019 Dec;29(12):1009–1026. doi: 10.1038/s41422-019-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeishi K., Collin de l'Hortet A., Wang Y., Handa K., Guzman-Lepe J., Matsubara K., et al. Assembly and function of a bioengineered human liver for transplantation generated solely from induced pluripotent stem cells. Cell Rep. 2020 Jun 2;31(9) doi: 10.1016/j.celrep.2020.107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai W.J., Zhang Y., Chen X., Fu H., Li B., Li Z.S., et al. Metabolic activation system based on Co-culture system of human hepatocytes. J Toxicol. 2020 Apr;34(2):1002–3127. [Google Scholar]
- 48.Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013 Jul 25;499(7459):481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 49.Takebe T., Enomura M., Yoshizawa E., Kimura M., Koike H., Ueno Y., et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015 May 7;16(5):556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Anwanwan D., Singh S.K., Singh S., Saikam V., Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020 Jan;1873(1) doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita T., Kaneko S. [Liver cancer] Rinsho Byori. 2016 Jul;64(7):787–796. [PubMed] [Google Scholar]
- 52.Bruix J., Han K.H., Gores G., Llovet J.M., Mazzaferro V. Liver cancer: approaching a personalized care. J Hepatol. 2015 Apr;62(1 Suppl):S144–S156. doi: 10.1016/j.jhep.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakagawa H., Fujita M., Fujimoto A. Genome sequencing analysis of liver cancer for precision medicine. Semin Cancer Biol. 2019 Apr;55:120–127. doi: 10.1016/j.semcancer.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Odom D.T., Dowell R.D., Jacobsen E.S., Gordon W., Danford T.W., MacIsaac K.D., et al. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet. 2007 Jun;39(6):730–732. doi: 10.1038/ng2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014 Nov;14(11):709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan B., Malato Y., Calvisi D.F., Naqvi S., Razumilava N., Ribback S., et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012 Aug;122(8):2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sekiya S., Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012 Nov;122(11):3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meunier L., Larrey D. Drug-induced liver injury: biomarkers, requirements, candidates, and validation. Front Pharmacol. 2019 Dec;10:1482. doi: 10.3389/fphar.2019.01482. Published 2019 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nuciforo S., Fofana I., Matter M.S., Blumer T., Calabrese D., Boldanova T., et al. Organoid models of human liver cancers derived from tumor needle biopsies. Cell Rep. 2018 Jul 31;24(5):1363–1376. doi: 10.1016/j.celrep.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang W., Chen Z., Zhang W., Cheng Y., Zhang B., Wu F., et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Targeted Ther. 2020 Jun 10;5(1):87. doi: 10.1038/s41392-020-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y., Zhu Y., Wang H., Chen L. Advances and applications of liver organoids. JCB (J Cell Biol) 2021 May;43(6):1132–1141. [Google Scholar]
- 62.Drost J., Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018 Jul;18(7):407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 63.Brooks A., Liang X., Zhang Y., Zhao C.X., Roberts M.S., Wang H., et al. Liver organoid as a 3D in vitro model for drug validation and toxicity assessment. Pharmacol Res. 2021 Jul;169 doi: 10.1016/j.phrs.2021.105608. [DOI] [PubMed] [Google Scholar]
- 64.Li P., Li Y., Wang Y., Liu J., Lavrijsen M., Li Y., et al. Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids. Sci Adv. 2022 Jan;8(3) doi: 10.1126/sciadv.abj5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Research Council (US) Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. National Academies Press (US); Washington (DC): 2011. Committee on A Framework for developing a new taxonomy of disease. [PubMed] [Google Scholar]
- 66.Ye W., Luo C., Li C., Huang J., Liu F. Organoids to study immune functions, immunological diseases and immunotherapy. Cancer Lett. 2020 May;477:31–40. doi: 10.1016/j.canlet.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Tu T., Budzinska M.A., Maczurek A.E., Cheng R., Di Bartolomeo A., Warner F.J., et al. Novel aspects of the liver microenvironment in hepatocellular carcinoma pathogenesis and development. Int J Mol Sci. 2014 May 27;15(6):9422–9458. doi: 10.3390/ijms15069422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fattovich G., Stroffolini T., Zagni I., Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004 Nov;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 69.Steele N.G., Chakrabarti J., Wang J., Biesiada J., Holokai L., Chang J., et al. An organoid-based preclinical model of human gastric cancer. Cell Mol Gastroenterol Hepatol. 2019 Sep;7(1):161–184. doi: 10.1016/j.jcmgh.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Y.S., Yang X.L., Xin R., Wu T.M., Shi Y., Dan Zhang D., et al. The power and the promise of organoid models for cancer precision medicine with next-generation functional diagnostics and pharmaceutical exploitation. Transl Oncol. 2021 Aug;14(8) doi: 10.1016/j.tranon.2021.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujii M., Sato T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater. 2021 Feb;20(2):156–169. doi: 10.1038/s41563-020-0754-0. [DOI] [PubMed] [Google Scholar]
- 72.Jacques E., Suuronen E.J. The progression of regenerative medicine and its impact on therapy translation. Clin Transl Sci. 2020 May;13(3):440–450. doi: 10.1111/cts.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ciccocioppo R., Cantore A., Chaimov D., Orlando G. Regenerative medicine: the red planet for clinicians. Intern Emerg Med. 2019 Sep;14(6):911–921. doi: 10.1007/s11739-019-02126-z. [DOI] [PubMed] [Google Scholar]
- 74.Edgar L., Pu T., Porter B., Aziz J.M., La Pointe C., Asthana A., et al. Regenerative medicine, organ bioengineering and transplantation. Br J Surg. 2020 Jun;107(7):793–800. doi: 10.1002/bjs.11686. [DOI] [PubMed] [Google Scholar]
- 75.Terzic A., Harper C.M., Jr., Gores G.J., Pfenning M.A. Regenerative medicine blueprint. Stem Cell Dev. 2013 Dec;22(Suppl 1):20–24. doi: 10.1089/scd.2013.0448. [DOI] [PubMed] [Google Scholar]
- 76.Lou Y.R., Leung A.W. Regenerative medicine blueprint. organoids for biomedical research and applications. Biotechnol Adv. 2018 Jan-Feb;36(1):132–149. doi: 10.1016/j.biotechadv.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Messina A., Luce E., Hussein M., Dubart-Kupperschmitt A. Pluripotent-stem-cell-derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration. Cells. 2020 Feb 12;9(2):420. doi: 10.3390/cells9020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H., Sun L., Pang Y., Hu D., Xu H., Mao S., et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut. 2021 Mar;70(3):567–574. doi: 10.1136/gutjnl-2019-319960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reza H.A., Okabe R., Takebe T. Organoid transplant approaches for the liver. Transpl Int. 2021 Nov;34(11):2031–2045. doi: 10.1111/tri.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Forner A., Reig M., Bruix J. Hepatocellular carcinoma. Lancet. 2018 Mar;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 81.Linder K.A., Malani P.N. Hepatitis A. JAMA. 2017 Dec;318(23):2393. doi: 10.1001/jama.2017.17244. [DOI] [PubMed] [Google Scholar]
- 82.Gregorio G.V., Mieli-Vergani G., Mowat A.P. Viral hepatitis. Arch Dis Child. 1994 Apr;70(4):343–348. doi: 10.1136/adc.70.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trépo C., Chan H.L., Lok A. Hepatitis B virus infection. Lancet. 2014 Dec;384(9959):2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 84.Heidarzadeh A., Moridani M.A., Khoshmanesh S., Kazemi S., Hajiaghabozorgi M., Karami M. Effectiveness of COVID-19 vaccines on hospitalization and death in Guilan, Iran: a test-negative case-control study. Int J Infect Dis. 2023 Mar;128:212–222. doi: 10.1016/j.ijid.2022.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khim G., Em S., Mo S., Townell N. Liver abscess: diagnostic and management issues found in the low resource setting. Br Med Bull. 2019 Dec;132(1):45–52. doi: 10.1093/bmb/ldz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eulenberg V.M., Lidbury J.A. Hepatic fibrosis in dogs. J Vet Intern Med. 2018 Jan;32(1):26–41. doi: 10.1111/jvim.14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramli M.N.B., Lim Y.S., Koe C.T., Demircioglu D., Tng W., Gonzales K.A.U., et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology. 2020 Oct;159(4):1471–1486. e12. doi: 10.1053/j.gastro.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 88.Huang S., Sun C., Hou Y., Tang Y., Zhu Z., Zhang Z., et al. A comprehensive bioinformatics analysis on multiple Gene Expression Omnibus datasets of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Sci Rep. 2018 May;8(1):7630. doi: 10.1038/s41598-018-25658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao B., Ni C., Gao R., Wang Y., Yang L., Wei J., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020 Oct;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu H., Gehart H., Artegiani B., Löpez-Iglesias C., Dekkers F., Basak O., et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018 Nov 29;175(6):1591–1606.e19. doi: 10.1016/j.cell.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 91.Sampaziotis F., Justin A.W., Tysoe O.C., Sawiak S., Godfrey E.M., Upponi S.S., et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017 Aug;23(8):954–963. doi: 10.1038/nm.4360. [DOI] [PubMed] [Google Scholar]
- 92.Mansour A.A., Gonçalves J.T., Bloyd C.W., Li H., Fernandes S., Quang D., et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018 Jun;36(5):432–441. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bredenoord A.L., Clevers H., Knoblich J.A. Human tissues in a dish: the research and ethical implications of organoid technology. Science. 2017 Jan 20;355(6322) doi: 10.1126/science.aaf9414. [DOI] [PubMed] [Google Scholar]
- 94.Stanger B.Z. Cellular homeostasis and repair in the mammalian liver. Annu Rev Physiol. 2015;77:179–200. doi: 10.1146/annurev-physiol-021113-170255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y., Li Y., Zhou T., Zheng J., Li S., Li H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients. 2016 Mar 10;8(3):156. doi: 10.3390/nu8030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun L., Hui L. Progress in human liver organoids. J Mol Cell Biol. 2020 Aug;12(8):607–617. doi: 10.1093/jmcb/mjaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clevers H. Modeling development and disease with organoids. Cell. 2016 Jun;165(7):1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 98.Töpfer E., Pasotti A., Telopoulou A., Italiani P., Boraschi D., Ewart M.A., et al. Bovine colon organoids: from 3D bioprinting to cryopreserved multi-well screening platforms. Toxicol Vitro. 2019 Dec;61 doi: 10.1016/j.tiv.2019.104606. [DOI] [PubMed] [Google Scholar]
- 99.Cheung K.J., Gabrielson E., Werb Z., Ewald A.J. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013 Dec 19;155(7):1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao D., Vela I., Sboner A., Iaquinta P.J., Karthaus W.R., Gopalan A., et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014 Sep 25;159(1):176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karthaus W.R., Iaquinta P.J., Drost J., Gracanin A., van Boxtel R., Wongvipat J., et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014 Sep 25;159(1):163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mills R.J., Titmarsh D.M., Koenig X., Parker B.L., Ryall J.G., Quaife-Ryan G.A., et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8372–E8381. doi: 10.1073/pnas.1707316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Au S.H., Chamberlain M.D., Mahesh S., Sefton M.V., Wheeler A.R. Hepatic organoids for microfluidic drug screening. Lab Chip. 2014 Sep 7;14(17):3290–3299. doi: 10.1039/c4lc00531g. [DOI] [PubMed] [Google Scholar]
- 104.Leite S.B., Roosens T., El Taghdouini A., Mannaerts I., Smout A.J., Najimi M., et al. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials. 2016 Feb;78:1–10. doi: 10.1016/j.biomaterials.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 105.Wang S., Gao D., Chen Y. The potential of organoids in urological cancer research. Nat Rev Urol. 2017 Jul;14(7):401–414. doi: 10.1038/nrurol.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huch M., Knoblich J.A., Lutolf M.P., Martinez-Arias A. The hope and the hype of organoid research. Development. 2017 Mar 15;144(6):938–941. doi: 10.1242/dev.150201. [DOI] [PubMed] [Google Scholar]
- 107.Bhaduri A., Andrews M.G., Kriegstein A.R., Nowakowski T.J. Are organoids ready for prime time? Cell Stem Cell. 2020 Sep 3;27(3):361–365. doi: 10.1016/j.stem.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen K.G., Mallon B.S., McKay R.D., Robey P.G. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014 Jan 2;14(1):13–26. doi: 10.1016/j.stem.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]