Highlights
-
•
Pattern-of-care studies are essential approaches to assess real-world utilization.
-
•
Reported overall utilization varies widely: around 40% in NSCLC, stage I NSCLC 26–42%
-
•
SBRT utilisation varies widely globally and over time (8–63%)
-
•
Actual utilisation reported is notably lower than evidence-based optimal utilisation.
-
•
Promote wider POC use for better understanding of evidence-based treatment application.
Keywords: Radiation Oncology, Lung Cancer, Patterns of care, Utilisation
Abstract
Background and purpose
The aim of this study was to review the published studies on the utilisation of radiotherapy in lung cancer (both small and non-small cell lung cancer, SCLC and NSCLC) patients in European countries with a population-based perspective.
Material and methods
A literature search since January 2000 until December 2022 was carried out. Only English-published papers were included, and only European data was considered. PRISMA guidelines were followed. A scoping narrative review was undertaken due to the hetereogeneity of the published papers.
Results
38 papers were included in the analysis, with the majority from the Netherlands (52.6%) and the UK (18.4%). Large variability is observed in the reported radiotherapy utilisation, around 40% for NSCLC in general and between 26 and 42% in stage I NSCLC. Stereotactic body radiotherapy (SBRT) shows a wide range of utilisation across countries and over time, from 8 to 63%. Similary, in stage III lung cancer, chemoradiotherapy (CRT) utilisation varied considerably (11–70%). Eleven studies compared radiotherapy utilisation between older and younger age-groups, showing that younger patients receive more CRT, while the opposite applies for SBRT. An widespreadlack of data on relevant covariates such as comorbidty and health-services related variables is observed.
Conclusion
The actual utilisation of radiotherapy for lung cancer reported in patterns-of-care studies (POCs) is notably lower than the evidence-based optimal utilisation. Important variability is observed by country, time period, stage at diagnosis and age. A wider use of POCs should be promoted to improve our knowledge on the actual application of evidence-based treatment recommendations.
Introduction
With 2,2 million new cases per year, lung cancer (LC) is the second most frequent cancer type worldwide. Unfortunately, it remains unparalleled in terms of mortality, accounting for 18 % of global cancer deaths [1]. In Europe, it is a major healthcare concern as well, with 477,500 new lung cancers being diagnosed in 2020, of which 3 out of 4 occur in males. This translates in LC ranking second in incidence and first in mortality in men in Europe, respectively third in incidence and second in mortality in women [2].
Radiotherapy is a central pillar in the multidisciplinary treatment approach of LC, as well in the most common type, non-small cell lung cancer (NSCLC), as in small-cell lung cancer (SCLC), and both in the curative and the palliative setting. According to evidence-based guidelines, radiotherapy should optimally be utilised in about 4 out of 5 of all patients diagnosed with LC, 77 % globally and between 76,9 and 81,9% in Europe [3], [4], [5]. This optimal utilisation is defined as the percentage of cancer patients that should receive radiotherapy at least once in the course of their disease [4]. Striving to assure optimal radiotherapy utilisation is crucial in view of obtaining the best outcomes for cancer patients, especially so in a cancer as frequent and with such a high clinical impact from radiotherapy as LC [6].
However, it is well acknowledged that the application of evidence-based guidelines is not always straightforward, the adherence for LC patients being estimated to range only between 44 and 52 % [7]. Non-adherence translates into an important gap between the optimal and actual utilisation of radiotherapy, as shown in population-based studies [8]. Major factors associated with the lack of guideline adherence in clinical practice, not in the least in the context of LC, are found to be increasing age and the presence of comorbidities, indicating that guidelines, and their supporting evidence, should be tailored to the clinical status of the patients [7], [8], [9], [10]. In addition to patient-related factors, health system-related aspects, such as the distance to the radiation oncology department, have also been associated with a lower proportion of patients in whom radiotherapy is used [11], [12].
The need to assess the actual use of radiotherapy in specific cancer types or clinical settings through patterns-of-care (POC) analyses becomes especially important if new therapeutic interventions have demonstrated a positive impact on patient outcomes. A clear example in LC is the introduction of stereotactic body radiotherapy (SBRT) in early-stage NSCLC patients not amenable to a surgical intervention. The positive survival impact was actually first established through real-world population-level data [13], only afterwards confirmed in a randomised clinical trial [14], [15]. However, this evidence has yet to be uniformly translated into clinical practice. Data from Australia, for instance, comparing the actual versus the optimal utilisation of SBRT in early-stage NSCLC, show a remaining gap in its use, related to both patient and health system factors [16].
The aim of this study was to review the literature published on the actual utilisation of external beam radiotherapy for LC patients, both NSCLC and SCLC, from a population-based perspective in European countries, since 2000. In addition, the evolution over time and potential factors associated with the utilisation, were evaluated.
Material and methods
Search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17]. The search strategy is shown in the Supplementary material 1.
We searched the PubMed and Web of Science databases in the period between January 1st, 2000 up to December 31st, 2022. This date range was intended to capture current real-world data on LC radiotherapy POCs, and to account for changes in treatment patterns since 2000. Therefore, studies only reporting data prior to 2000 were excluded. Only English language studies published in peer-reviewed journals were considered. Studies that did not describe population or registry-based studies, such as case reports, general reviews, clinical trials, conference abstracts and letters were excluded. To qualify for inclusion, at least part of the patient population, consisting of LC patients of any pathology or stage, had to be treated with radiotherapy. Finally, for this analysis, only papers pertaining to the European setting were considered.
Study selection, data extraction and analysis
Study titles, abstracts and full texts were screened independently by two reviewers (JC and JMB) for conformity with the inclusion and exclusion criteria. Any discrepancies between reviewers were solved by a third reviewer (YL). Studies satisfying the selection criteria were retrieved and their full text was analysed. One reviewer (JC) recorded all available data on a dedicated data-extraction form regarding year of publication, study population, geographical setting, period covered, number of patients included. More specifically, the actual utilisation of radiotherapy for LC, both overall and by multidisciplinary treatment approach (radiotherapy alone, or with concomitant or sequential chemotherapy), radiotherapy-specific aspects (such as conformal (3D-CRT), or SBRT, fractionation) and patient-related factors (comorbidity, age and sex) were retrived. Finally, other non-clinical or demography associated factors affecting radiotherapy utilisation were recorded. Due to the heterogeneity in data collected across the studies, a narrative synthesis was performed.
Results
A total of 209 references were identified: 198 through database searching, while 11 additional references were found through other sources. Based on title and abstract, 147 studies were found potentially eligible, yet only 58 were European-based. These were all evaluated on full text, resulting in 38 studies that were included in this review (Fig. 1).
Fig. 1.
PRISMA flowchart. Abbreviations: LC: lung cancer, RT: radiotherapy.
Table 1 provides a detailed overview of all studies, with the extracted data organised per patient population: any LC (studies including different stages and/or histological subtypes); NSCLC only, with further subdivision into early-stage and locally-advanced NSCLC, and SCLC. It provides details on how the radiotherapy utilisation was analysed in the studies: overall utilisation, by treatment and/or patient and/or other characterics. Treatment characteristics pertain to the utilisation of radiotherapy in the multidisciplinary context (radiotherapy all or not concurrent with or sequential to chemotherapy) and to radiotherapy-specific aspects (e.g. technique or fractionation). Age is by far the most analysed patient characteristic, next to health status and sex. Only two papers specifically reports on curative versus palliative intent [18], [19]; the same holds for health services-related aspects (i.e. availability of radiotherapy in the hospital [20].
Table 1.
Actual radiotherapy utilisation in lung cancer: by patient population in different countries, overall and by treatment and patient characteristics.
| Publication |
Country /region |
Study population |
Years assessed |
Cases analysed |
Analysis radiotherapy utilisation |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
overall |
by treatment characteristics |
by patient characteristics |
other |
||||||||
| author, year | pathology, stage (other determinants) | year or period | numberof cases | multi- modality cCRT, sCRT, RT | Radiotherapy SBRT vs. RT, fractionation, PCI | health status WHO, comorbidity | age | sex | |||
| Any lung cancer, different subtypes and/or stages | |||||||||||
| Vulto, 2006 | NL (South) | LC, NSCLC, SCLC | 1988–2002 | 8842 | x | x | |||||
| 1988–92 | 2783 | ||||||||||
| 1993–97 | 2940 | ||||||||||
| 1998–2002 | 3119 | ||||||||||
| Vulto, 2006 | NL (South) | NSCLC, SCLC | 1995–2002 | 8946 | x | x | x | ||||
| (≥50y, amenable to RT) | |||||||||||
| Erridge, 2008 | UK (South-East Scotland) | LC, NSCLC, SCLC | 1995, 2002 | 1998 | x | x (not reporting for St III) | x# | ||||
| 1995 | 1995: 927 | ||||||||||
| 2002 | 2002: 971 | ||||||||||
| Berglund, 2012 | UK (EN, South-East) | LC, St IIIa | 2006–08 | 2771 | x | ||||||
| Henson, 2018 | UK (EN) | NSCLC | 2013–14 | 35,510 | x (not reporting for St III) | x | x | x | x## | ||
| SCLC | NSCLC: 30,431 | ||||||||||
| SCLC: 5,079 | |||||||||||
| Moller, 2018 | UK (EN) | LC in general | 2010–14 | 176,225 | x | x (not reporting for St III) | |||||
| Rodríguez-Barranco, 2019 | ES (Huelva, Granada) | LC in general | 2010–15 | 1196 | x | x (not reporting for St III) | |||||
| Solberg, 2019 | NO | LC in general | 2001–17 | 43,137 | x | x (SBRT vs. RT, not reporting for St I-II) |
|||||
| Non-small cell lung cancer, different stages | |||||||||||
| Myrdal, 2009 | SE (Central) | NSCLC | 1995–2003 | 4345 | x | x | |||||
| Berglund, 2010 | SE (Central) | NSCLC | 1996–2004 | 3370 | x### | ||||||
| Koning, 2011 | NL (4 regions: Twente, Amsterdam, West, South)c | NSCLC, St I-III | 1997–2008 | 24,185 | x | x (not reporting for St III) | |||||
| Driessen, 2017 | NL | NSCLC | 1990–2014 | 187,315 | x | x | |||||
| St I: 37,777 | |||||||||||
| St II: 13,151 | |||||||||||
| St III: 59,871 | |||||||||||
| St IV: 69,964 | |||||||||||
| Verleye, 2017 | BE | NSCLC | 2010–11 | 9817 | x | ||||||
| St I: 1107 | |||||||||||
| St II: 619 | |||||||||||
| St IIIA: 1197 | |||||||||||
| St IIIB: 790 | |||||||||||
| St IV: 3875 | |||||||||||
| Brada, 2019 | UK (EN) | NSCLC, St I-III | 2012–13 | 25,659 | x | x (SBRT, not reporting for St I-II) |
x | x | x# | ||
| Willén, 2019 | SE | NSCLC | 2002–16 | 39,671 | x | x (SBRT vs. RT) |
x### | ||||
| St IA-IIB: 10,004 | |||||||||||
| St IIIA: 3713 | |||||||||||
| St IIIB-IV: 25,353 | |||||||||||
| Evers, 2021 | NL | NSCLC, St I-III | 2008–18 | 61,582 | x | x (SBRT vs. RT) |
x | ||||
| St I: 25,405 | |||||||||||
| St II: 9272 | |||||||||||
| St III: 26,905 | |||||||||||
| Karlsson, 2021 | NO (South-East) | NSCLC (brain metastases) | 2006–18 | 2140 | x (SRT) |
||||||
| Willén, 2022 | SE | NSCLC | 2002–16 | 40,026 | x | x (SBRT vs. RT) |
x | ||||
| St IA-IIB: 9904 | |||||||||||
| St IIIA: 3762 | |||||||||||
| St IIIB-IV: 25,749 | |||||||||||
| Willén, 2022b | SE | NSCLC | 2002–16 | 40,075 | x | x (SBRT vs. RT) |
x#### | ||||
| St IA-IIB: 9941 | |||||||||||
| St IIIA: 3765 | |||||||||||
| St IIIB-IV: 25,767 | |||||||||||
| Non-small cell lung cancer, early stage | |||||||||||
| Palma, 2010 | NL | NSCLC St I (>75 years) |
1999–2007 | 875 | x | x (SBRT vs. RT) |
x | ||||
| 1999–2001 | 274 | ||||||||||
| 2002–04 | 254 | ||||||||||
| 2005–07 | 347 | ||||||||||
| Haasbeek, 2012 | NL | NSCLC, St I (>75 years)b |
2001–09 | 4605 | x | x | |||||
| 2001–03 | 1324 | ||||||||||
| 2004–06 | 1556 | ||||||||||
| 2007–09 | 1725 | ||||||||||
| Detillon, 2018 | NL (South) | NSCLC, St I (>65 years) | 2004–13 | 2168 | x | x (SBRT vs. RT) |
x | ||||
| 2004–08 | 1068 | ||||||||||
| 2009–13 | 1100 | ||||||||||
| Driessen, 2019 | NL | NSCLC, St I-II (>65 years) | 2010–15 | 12,182 | x (SBRT vs. RT) |
x | |||||
| St I: 8742 | |||||||||||
| St II: 3439 | |||||||||||
| Ostheimer, 2019 | DE | NSCLC, St I | 2000–14 | 16,292 |
x |
||||||
| 2000–03 | 1809 | ||||||||||
| 2004–07 | 2552 | ||||||||||
| 2007–14 | 11,931 | ||||||||||
| Phillips, 2019 | UK (EN) | NSCLC, St I | 2015–16 | 12,384 | x (SBRT vs. RT) |
||||||
| Damhuis, 2021 | UK (EN) | NSCLC, St I | 2015–16 | EN: 12,627 | x (SBRT vs. RT) |
x | |||||
| NO | NO: 980 | ||||||||||
| NL | NL: 5120 | ||||||||||
| Non-small cell lung cancer, locally-advanced stage | |||||||||||
| Wouters, 2010 | NL | NSCLC, St III | 2001–06 | 43,544 | x | ||||||
| St III: 13,744 | |||||||||||
| St IIIA: 4,938 | |||||||||||
| St IIIB: 8,806 | |||||||||||
| Dickhoff, 2016 | NL | NSCLC, St IIIA | 2010–13 | 4816 | x | ||||||
| Dickhoff, 2017 | NL | NSCLC, St IIIB | 2010–14 | 4401 | x | x | |||||
| Driessen, 2018 | NL | NSCLC, St III (>65 years) | 2009–13 | 7039 | x | x | |||||
| 65–74: 3,876 | |||||||||||
| >75: 3,163 | |||||||||||
| Adizie, 2019 | UK (EN) | NSCLC, St III | 2016 | 6276 | x | ||||||
| St IIIA: 3827 | |||||||||||
| St IIIB: 2449 | |||||||||||
| van den Ven, 2019 | NL | NSCLC, St III (and IV) |
2016 | 4096 | x | ||||||
| Joosten, 2020 | NL | NSCLC, St IIIA | 2010–16 | 9591 | x | x | x | ||||
| Dieleman, 2022 | NL | NSCLC St III, curative chemoradiation | 2015–18 | 2942 | x | x | |||||
| Small cell lung cancer, different stages | |||||||||||
| Janssen-Heijnen, 2007 | NL (South, Eindhoven) | SCLC | 1995–2002 | 1664 | x (only reporting for limited disease) | x | |||||
| Damhuis, 2018 | NL | LD-SCLC, St I-III (concurrent CRT only) | 2010–14 | 1635 | x | x (fractionation, BID) |
x | ||||
| Evers, 2021 | NL | SCLC, St I-III | 2008–19 | 6578 | x | x (SBRT vs. RT, PCI) |
x | x | x##### | ||
| Tomassen, 2021 | NL | SCLC | 2010–18 | 10,264 | x (PCI) |
||||||
Abbreviations and notes:
LC: lung cancer; NSCLC: non-small cell lung cancer; SCLC: small cell lung cancer; LD-SCLC: limited disease small cell lung cancer.
SRT: Stereotactic radiosurgery; BID: Twice-daily radiotherapy; PCI: Prophylactic cranial irradiation
BE: Belgium; DE: Germany; EN: England; ES: Spain; NL: The Netherlands; NO: Norway; SE: Sweden; UK: United Kingdom;
aincludes both NSCLC and SCLC Stage III; b different periods based on SBRT availability; c 4 regions defined by comprehensive cancer centres.
#curative vs. palliative, ## ethnicity, ### education level, ####Nordic and Non-Nordic immigrants compared to Swedish-born, ##### availability of radiotherapy in the hospital.
Fig. 2 shows the data per geographical setting, including the total patient numbers and the period covered. While 8 papers report on a mixture of LC populations, the vast majority of studies focuses on NSCLC, whereas only 4 studies on SCLC. It is remarkable that 71.1 % (n = 27) of the studies were conducted in the Netherlands (52.6 %, n = 20) and the United Kingdom (18.4 %, n = 7), representing 46.5 % respectively 30.8 % of the total number of patients reported. This geographical dominance pertains to all study populations, with additional large reports on specific patient populations coming from the Nordic countries (e.g. Norway for overall LC, Sweden for NSCLC), Belgium (on NSCLC) and Germany (for stage I NSCLC).
Fig. 2.
Available data on actual utilisation of radiotherapy in lung cancer, per geographical setting, patient population and period covered. * Damhuis 2022: use of data from England, Norway and The Netherlands. This study is included in the total of patients, but not in the number of studies per country.
Table 2 presents the 13 studies that report the overall percentage of actual radiotherapy utilisation, i.e. the number of patients who received radiotherapy in the population considered. Only some of these specify curative, radical or primary radiotherapy. For NSCLC in general the radiotherapy utilisation hovers around 40 %, with a lower value of 26 % in the study of Myrdal et al. in Sweden [21]. In stage I NSCLC, the utilisation ranges between 26 and 42 % depending on the time period studied, except for the study conducted by Ostheimer et al. [22], which shows much lower figures.
Table 2.
Overall percentage of actual radiotherapy utilisation in lung cancer, per study population.
| Publication |
Analysis radiotherapy utilisation |
|
|---|---|---|
|
Overall radiotherapy utilisation (%) |
Definition of utilisationa |
|
| Any lung cancer, different subtypes and/or stages | ||
| Vulto, 2006 | 41.2 % 41 % in NSCLC |
Proportion of patients diagnosed with lung cancer receiving RT within 6 months of diagnosis in the period 1998–2002. |
| Proportion of patients diagnosed with non-small cell lung cancer receiving RT within 6 months of diagnosis in 2002. | ||
| Patients who are planned to receive RT after several courses of chemotherapy (so the start of radiotherapy may actually be after 6 months) are also included as having received RT6mo. | ||
| Erridge, 2008 | 43 % | Proportion of patients diagnosed with lung cancer receiving RT within 6 months of diagnosis in 2002. |
| An exception was made for consolidation radiotherapy after chemotherapy in limited stage SCLC as this is part of the “initial treatment package,” but can commence during the seventh month after diagnosis. | ||
| Berglund, 2012 | 38 % | Proportion of patients diagnosed with Stage III NSCLC or SCLC receiving RT as primary initial treatment (surgical resection, radiotherapy and chemotherapy) in the period 2006–2008. |
| Moller, 2018 | 8.1 % | Proportion of patients diagnosed with lung cancer receiving radical RT (as initial active cancer treatment) from 3 months before diagnosis to 12 months after in the period 2010–2013. |
| Rodríguez-Barranco, 2019 | RT in all: Huelva 46.6 %, Granada 25.4 % RT as first treatment: Huelva 23.5 % Granada 9.8 % |
Proportion of incident first invasive primary lung cancer cases diagnosed in the period 2010–2011, with follow-up until April 2015, and who received RT. |
| Proportion of incident first invasive primary lung cancer cases diagnosed in the period 2010–2011, with follow-up until April 2015, and who received RT as initial treatment (surgery, chemotherapy, radiotherapy, targeted treatment, and their combination). | ||
| Solberg, 2019 | 2001: 6.7 % RT (normofractionated RT), 0 % SBRT 2016: 8.5 % RT, 8.8 % SBRT |
Proportion of lung cancer patients given RT as curative treatment in the period 2001–2016. |
| Non-small cell lung cancer, different stages | ||
| Myrdal, 2009 | 26 % | Proportion of patients diagnosed with NSCLC receiving RT as main type of treatment (surgery, chemotherapy, radiotherapy) in the period 1995–2003. |
| Koning, 2011 | 1997–2008: ‘About 40 %’b | Proportion of patients diagnosed with non-metastatic NSCLC receiving primary RT within about 6 months of diagnosis in the period 1997–2008. |
| 2004: 38 % | ||
| 2008: 42 % | ||
| Brada, 2019 | 40.4 % | Proportion of patients diagnosed with stage 0–III NSCLC receiving RT to the lung in the period 2012–2013. |
| Non-small cell lung cancer, early stage | ||
| Palma, 2010 | 34 % overall | Proportion of patients diagnosed with Stage I NSCLC receiving RT as primary treatment (surgery, radiotherapy, neither) in the period 1999–2007. |
| 1999–2001: 26 % | ||
| 2002–04: 32 % | ||
| 2005–07: 42 % | ||
| Haasbeek, 2012 | 34.1 % overall | Proportion of patients diagnosed with Stage I NSCLC receiving RT as primary treatment (surgery, radiotherapy, no curative treatment) in the period 2001–2009, with a follow-up until February 2011. |
| 2001–03: 31 % | ||
| 2004–06: 33 % | ||
| 2007–09: 38 % | ||
| Detillon, 2018 | 30 % | Proportion of patients >= 65 years diagnosed with clinical stage I NSCLC receiving RT as primary treatment in the period 2004–2013. |
| Treatment was classified as surgery, including thoracotomy and VATS; radiotherapy, including conventional radiotherapy and SBRT or neither of these. | ||
| Ostheimer, 2019 | 2000–03: 5,9% | Proportion of patients diagnosed with Stage I NSCLC receiving RT as primary treatment in the period 2000–2014. |
| 2004–07: 5 % | ||
| 2008–14: 8,8% | ||
| Cases with both RT and surgery as the treatment or with missing treatment variables were not included. | ||
Abbreviations and notes:
RT: radiotherapy; SBRT: stereotactic body radiotherapy.
Definition is adapted from the methodology described in each study.
As defined in text.
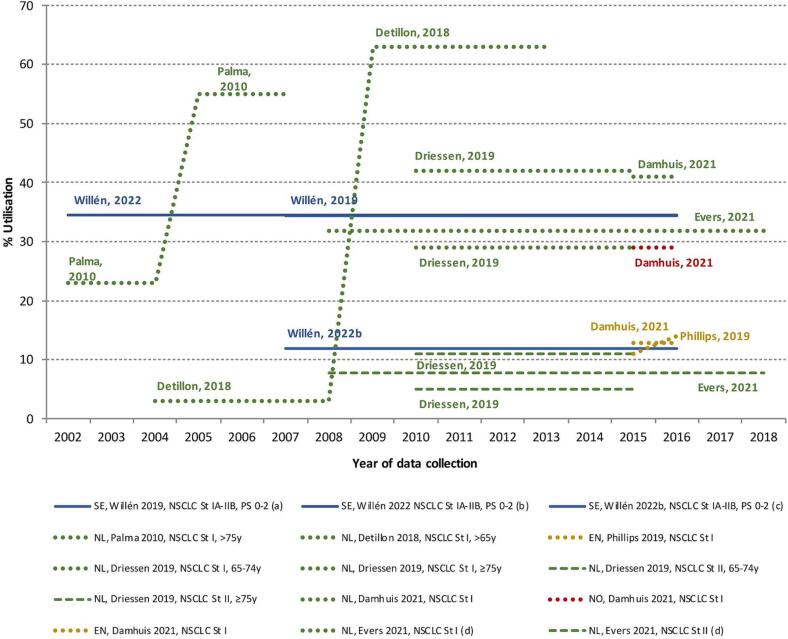
There are 11 studies that reported SBRT utilisation in NSCLC, of which 9 reported on its utilisation in early-stage (Table 1, and Supplementary Table 3a). Although study populations slightly differ across studies, in the most recent periods, typically higher SBRT utilisation is reported in the Netherlands (8–63 %), followed by Sweden (around 34 %), Norway (29 %), and England (around 12 %). In Driessen et al. [23], it was found that both SBRT and conventionally fractionated radiotherapy were used less often in patients 65–74 years compared to the group of patients older than 75 years in the Netherlands during the period 2010–15. Similarly, SBRT and radiotherapy utilisation increased with age in Sweden [24].
A total of 23 studies reported on the combined utilisation of chemotherapy and radiotherapy, with 17 focusing on stage III, primarily in NSCLC, but also 2 studies pertaining to SCLC, and 1 to both. For the most recent years, except for the findings presented in the study of Wouters et al. [25], the Netherlands reported chemoradiotherapy (CRT) to be used in around 37–70 % of all patients diagnosed with both locally-advanced NSCLC and SCLC (Table 1, and Supplementary Table 3b), whilst two studies conducted in England and Belgium reported a lower utilisation of CRT in the population. Damhuis et al. [26] in the Netherlands reported the percentage of CRT out of those receiving combined modality treatment, being 63 % and 53 % for stages IIIA and IIIB, respectively. Similarly, in Sweden, the reported CRT utilisation for stage IIIB (34 %) was lower compared to stage IIIA (46 %) [27]. The study carried out by Evers et al. [20] reported 46 % of concurrent CRT for stage III in 2019 in the Netherlands. They also reported that the use of concurrent CRT versus sequential chemoradiotherapy in stages II-III increased over time and was strongly associated with lower age, WHO performance status 0 and diagnosis in a hospital with in-house radiotherapy availability. Other studies also confirmed that CRT was more common in younger patients [23], [24], [28].
Fig. 3a, Fig. 3b present the percentage of SBRT and CRT utilisation by time period of data collection, showing the evident difference in utilisation between studies and countries that provided data. While the adoption of new standards-of-care typically evolve progressively, the comparison between different time periods in one study using the same methodology illustrate the drastic changes from one period to another, especially evidenced in a few Dutch studies.
Fig. 3a.
Trends in the percentage of stereotactic body radiotherapy utilisation in early-stage NSCLC. Abbreviations: SBRT: stereotactic body radiotherapy, NSCLC: non-small cell lung cancer, NL: the Netherlands, NO: Norway, SE: Sweden, EN: England. Note: For better visualisation, different colours are used for differentiating the country of the study (green: NL; blue: SE; yellow: EN; red: NO), while different types of lines are used for differentiating sub-groups of NSCLC (full lines: St IA-IIB; dotted lines: St I; dashed lines: St II). a Willen et al 2019: Study period: 2002–2016. SBRT available from 2007. b Willen et al 2022: If cases without treatment information are excluded from denominator (N/A), we estimated that SBRT utilization would be 34.5%.. c Willen et al 2022b: Cases without treatment information (N/A) are included in denominator. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3b.
Trends in the percentage of chemoradiotherapy utilisation in NSCLC and SCLC Stage III. Abbreviations: CRT: chemoradiotherapy, NSCLC: non-small cell lung cancer, SCLC: small cell lung cancer, LS-SCLC: limited stage small cell lung cancer, BE: Belgium, NL: the Netherlands, SE: Sweden, EN: England. Note: For better visualisation, different colours are used for differentiating the country of the study (green: NL; blue: SE; yellow: EN; red: BE), while different types of lines are used for differentiating sub-groups of NSCLC and SCLC (full line or triangle: NSCLC St III; dotted line or dot: NSCLC St IIIA; dashed line or square: NSCLC St IIIB; dashed-dotted line or diamond: SCLC). a Willen et al 2019 and Willen et al 2022b: Data only available for 2007–2016. b Willen et al 2022: If cases without treatment information are excluded from denominator (N/A), we estimated that CRT utilization for SIIIA, P0-2 and SIIIB-IV, P0-2 would be 45.9% and 9.3% respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In 17 papers, some data on radiotherapy utilisation in function of age are presented, but only 11 out of these formally compare younger to older age groups. These are shown in Fig. 4, while the complete list of the 17 references can be found in Table 1 and in the Supplementary Table 2. In general, younger patient populations are more aggressively treated with multimodality approaches, with a greater proportion receiving CRT rather than radiotherapy alone or sequential chemoradiotherapy. In contrast, more SBRT utilisation is seen in the more advanced age groups, likely because younger patients are more typically amenable to surgery.
Fig. 4.
Radiotherapy utilisation by age, in different patient populations and indications.
Discussion
The aim of this paper was to assess the utilisation of external beam radiotherapy in LC patients over the past two decades, using population-based data in European countries.
One of the most striking findings of this review is the geographical concentration of this type of analysis in two countries, namely the Netherlands (52.6 % of the papers) and the UK (18.4 %). These two thirds of the papers also represent three quarters of the reported patients. Only a small proportion of the available data comes from Belgium, Germany, two Nordic countries and Spain, countries focusing on a topical question for a certain patient population. Previous bibliometric analysis on LC research however did not reveal the UK or the Netherlands as particularly high-output countries, even on the contrary, although the UK ranks amongst the countries most investing in health-services related topics for radiotherapy research as a whole [29], [30]. One can therefore conclude that there is a gap in Europe-wide knowledge on practice patterns of radiotherapy in LC. This may at least partly be the consequence of a limited availability of population-based data from cancer registries with good linkage to radiotherapy treatment data, at the level of European countries or regions.
Compared to the high evidence-based needs for radiotherapy in LC, with an estimated utilisation of radiotherapy in about 80 % of all patients diagnosed, the actual utilisation data remain disappointingly low. This may in part be related to the methodology of data capture and presentation in the papers, for instance only reporting radical treatments [31], [32]. But one should also acknowledge the typically slow diffusion of evidence from clinical trials into clinical practice. Timelines of 10 to 20 years for translating efficacy from trials into real-life effectiveness that reaches the patients are not an exception [33]. In the LC patient population, where data generation in clinical trials is particularly tedious as age and comorbidities often preclude patients from participation [10], these timelines are surely not better. Recommendations to use a more blended approach to evidence generation, with real-life data supplementing clinical trials, have been advocated for the context of radiation oncology and are equally appropriate for the LC population [34], [35]. In fact, the evidence on clinical benefit of SBRT in early-stage NSCLC was first revealed in population-based data, well before a small randomised clinical trial confirmed this [12], [14], [15]. It would definitively be beneficial to focus more on pragmatic clinical trials, better mimicking the real-life context and allowing for inclusion of patients that are more elderly or have more comorbidity, to support a quicker introduction into clinical practice [36], [37]. The same applies to coverage with evidence development programs, de facto connecting evidence generation and practice [38].
Turning back to the data in our analysis, the long timeframe evaluated allowed to discern some evolutions in POCs over time (cf. Fig. 3a, Fig. 3b). Although still not matching the optimal figures, later cohorts showed typically higher radiotherapy utilisation, more conform to the evidence-based needs. Moreover, a slow adoption of literature evidence started to emerge. In stage III NSCLC, a gradual higher utilisation of CRT was observed, especially for younger and more fit patients, while a similar increase was seen for SBRT, yet more in the elderly population with comorbidities. In both examples, the progressive dissemination into clinical practice is de facto the result of a multidisciplinary interaction, where the risks and benefits compared to the alternative treatment options – the sequential chemo-radiotherapy approach, and surgery, respectively – should be balanced. In the case of CRT practical considerations may also play, especially if the hospital where the chemotherapy is administered does not have a radiotherapy department [20]. On the other hand, the fact that in many countries maintenance durvalumab was only reimbursed after CRT, conform to the setting evaluated in the Pacific trial, may have stimulated a shift towards more frequent use of CRT [39]. Utilisation of SBRT, on the contrary, implies the implementation of this novel technique by the radiation oncology departments, hence the availability of high-end equipment and experienced personnel, additional training, and quality assurance. In a recent population-based study carried out in Australia, an important gap between evidence-based optimal utilisation and actual use was still observed [16]. Even if its use is increasing in Europe, our findings still showed a underutilisation of SBRT in early-stage NSCLC compared with the results reported in this study (24 % in stages I-II: 32 % in stage I all cases and 10 % stage II, and 82 % of stage I non operable NSCLC cases). Different international studies found a higher utilisation in academic hospitals with high volume and multidisciplinary meetings [40], [41].
From the above it is clear that both patient characteristics, especially comorbidities and performance status, and health service-related factors, such as the organisation of care but probably also related to the prevailing reimbursement, are important to facilitate utilisation. In the reviewed papers, age was frequently analysed regarding its impact on radiotherapy utilisation. While age may to a certain extent be a proxy for comorbidity [10] a thorough evaluation of the patients’ performance is crucial in the context of LC treatment, hence a broader inclusion of performance status in population-based data sets is recommended. An even bigger data deficit is observed for health services-related aspects, which were mentioned in only one recent study from the Netherlands [20]. It was precisely in the Netherlands that the first population-based evidence was generated on the benefit of SBRT in early-stage NSCLC patients not amenable to surgery [13]. Moreover, the Netherlands have a track-record in predicting the increase in human and capital needs ensuing from the increasing cancer incidence and evidence-based changes in radiotherapy practice [42]. In other countries, such as the UK and Belgium, coverage with evidence development programs were set up to provide early access to SBRT, while monitoring the patients’ outcome [43], [44]. Beyond initiatives stimulating access to novel interventions at national level, quality standards ascertained by a comprehensive and experienced multidisciplinary team are a crucial factor at the level of the institutions to translate evidence-based guidelines into practice [45].
Our analysis showed some limitations. Due to the large variability in the papers, with data spanning over more than two decades, it was not easy to analyse the data in a consistent manner. Specific problems encountered were changes occurring in the TNM staging system over time, data only reported in text and figures instead of more comprehensively in tables [25], [32], [46], [47], [48], [49], [50], [51], location and morphology codes not reported [13], [18], [20], [21], [24], [25], [27], [28], [46], [47], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], only radical radiotherapy utilisation or only first treatment delivered to the patient reported [18], [31], [32], [46], [60], SBRT data not available [61]. More specifically from a methodological point of view, treatment intent, radiotherapy approach and multimodality schemes were not described in detail in many studies. Lastly, due to the typical delay in practice after the evidence and guidelines have been published, no population-level data were available for combinations with targeted drugs or immunotherapy.
Despite these limitations, our analysis provides a comprehensive overview of the available population-based data on radiotherapy utilisation in LC in Europe. It however clearly demonstrates the considerable gap in knowledge on the matter in Europe, the data being dominated by only two countries. Moreover, information on comorbidities and health services-related aspects are scarce. The absence of population-based data hampers quantifying radiotherapy utilisation, and its underlying causes, both needed for policy decision-making and practice. This is especially important in a tumour as frequent and deadly as LC, for which a high percentage of patients should receive radiotherapy to guarantee their best outcome [6]. The recently launched initiative of the European Network of Cancer Registries, planning to collect national cancer treatment data in a standardised manner, might be a first step to improve on the actual situation [62].
Funding
This research has been supported by an unrestricted educational grant from Astra Zeneca to ESTRO.
CRediT authorship contribution statement
Julieta Corral: Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Josep M. Borras: Conceptualization, Methodology, Writing – original draft. Yolande Lievens: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The administrative support made by Meritxell Nomen is gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100717.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Dyba T., Randi G., Bray F., Martos C., Giusti F., Nicholson N., et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308–347. doi: 10.1016/j.ejca.2021.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atun R., Jaffray D.A., Barton M.B., Bray F., Baumann M., Vikram B., et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153–1186. doi: 10.1016/S1470-2045(15)00222-3. [DOI] [PubMed] [Google Scholar]
- 4.Barton M.B., Jacob S., Shafiq J., Wong K., Thompson S.R., Hanna T.P., et al. Estimating the demand for radiotherapy from evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112:140–144. doi: 10.1016/j.radonc.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Borras J.M., Barton M., Grau C., Corral J., Verhoeven R., Lemmens V., et al. The impact of cancer incidence and stage on optimal utilization of radiotherapy: Methodology of a population-based analysis by the ESTRO-HERO project. Radiother Oncol. 2015;116(1):45–50. doi: 10.1016/j.radonc.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Hanna T.P., Shafiq J., Delaney G.P., Vinod S.K., Thompson S.R., Barton M.B. The population benefit of evidence -based radiotherapy: 5-year local control and overall survival benefits. Radiother Oncol. 2018;126:191–197. doi: 10.1016/j.radonc.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Vinod S.K. Decision making in lung cancer – how applicable are the guidelines? Clin Oncol (r Coll Radiol) 2015;27(2):125–131. doi: 10.1016/j.clon.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Lievens Y., De Schutter H., Stellamans K., Rosskamp M. Van Eycken L; belgian college for physicians in radiation oncology. radiotherapy access in belgium: how far are we from evidence-based utilisation? Eur J Cancer. 2017;84:102–113. doi: 10.1016/j.ejca.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie P., Vajdic C., Delaney G., Comans T., Morris L., Agar M., et al. Radiotherapy utilisation rates for patients with cancer as a function of age: A systematic review. J Geriatr Oncol. 2023;14(3) doi: 10.1016/j.jgo.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 10.De Ruysscher D., Botterweck A., Dirx M., et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study. Ann Oncol. 2009;20(1):98–102. doi: 10.1093/annonc/mdn559. [DOI] [PubMed] [Google Scholar]
- 11.Chan J., Brown E., Dennis K., Milosevic M., Brundage M. Provincial variations in radiotherapy utilization as a measure of access: a pan-Canadian study. Radiother Oncol. 2022;167:122–126. doi: 10.1016/j.radonc.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg M.A., Deboever N., Mills A.C., Egyud M.R., Hofstetter W.L., Mehran R.J., et al. Impact of travel distance on receipt of indicated adjuvant therapy in resected non-small cell lung cancer. J Thorac Cardiovasc Surg. 2023;S0022–5223(23) doi: 10.1016/j.jtcvs.2023.08.049. [DOI] [PubMed] [Google Scholar]
- 13.Palma D., Visser O., Lagerwaard F.J., Belderbos J., Slotman B.J., Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28:5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 14.Nyman J., Hallqvist A., Lund J.Å., Brustugun O.T., Bergman B., Bergström P., et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8. doi: 10.1016/j.radonc.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Ball D., Tao Mai G., Vinod S., et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494–503. doi: 10.1016/S1470-2045(18)30896-9. [DOI] [PubMed] [Google Scholar]
- 16.Ghandourh W., Holloway L., Batumalai V., Chlap P., Field M., Jacob S. Optimal and actual rates of stereotactic ablative body radiotherapy (SABR) utilisation for primary lung cancer in Australia. Clin Transl Radiat Oncol. 2022;34:7–14. doi: 10.1016/j.ctro.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erridge S.C., Murray B., Price A., Ironside J., Little F., Mackean M., et al. South-East Scotland cancer network lung cancer team. improved treatment and survival for lung cancer patients in South-East Scotland. J Thorac Oncol. 2008;3(5):491–498. doi: 10.1097/JTO.0b013e31816fca46. [DOI] [PubMed] [Google Scholar]
- 19.Brada M., Ball C., Mitchell S., Forbes H., Ashley S. Improving outcomes in non-small cell lung cancer; population analysis of radical radiotherapy. Radiother Oncol. 2019;132:204–210. doi: 10.1016/j.radonc.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Evers J., Hendriks L.E.L., De Jaeger K., Wijsman R., De Ruysscher D., Terhaard C., et al. Trends and variations in the treatment of stage I-III small cell lung cancer from 2008 to 2019: A nationwide population-based study from the Netherlands. Lung Cancer. 2021;162:61–70. doi: 10.1016/j.lungcan.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Myrdal G., Lamberg K., Lambe M., Ståhle E., Wagenius G., Holmberg L. Regional differences in treatment and outcome in non-small cell lung cancer: a population-based study (Sweden) Lung Cancer. 2009;63(1):16–22. doi: 10.1016/j.lungcan.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Ostheimer C., Evers C., Palm F., Mikolajczyk R., Vordermark D., Medenwald D. Mortality after radiotherapy or surgery in the treatment of early stage non-small-cell lung cancer: a population-based study on recent developments. J Cancer Res Clin Oncol. 2019;145(11):2813–2822. doi: 10.1007/s00432-019-03013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driessen E.J., Aarts M.J., Bootsma G.P., van Loon J.G., Janssen-Heijnen M.L. Trends in treatment and relative survival among Non-Small Cell Lung Cancer patients in the Netherlands (1990–2014): Disparities between younger and older patients. Lung Cancer. 2017;108:198–204. doi: 10.1016/j.lungcan.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Willén L., Berglund A., Bergström S., Isaksson J., Bergqvist M., Wagenius G., et al. Are older patients with non-small cell lung cancer receiving optimal care? a population-based study. Acta Oncol. 2022;61(3):309–317. doi: 10.1080/0284186X.2021.2000637. [DOI] [PubMed] [Google Scholar]
- 25.Wouters M.W., Siesling S., Jansen-Landheer M.L., Elferink M.A., Belderbos J., Coebergh J.W., et al. Variation in treatment and outcome in patients with non-small cell lung cancer by region, hospital type and volume in the Netherlands. Eur J Surg Oncol. 2010;36(Suppl 1):S83–S92. doi: 10.1016/j.ejso.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Damhuis R. Population-based results of chemoradiotherapy for limited stage small cell lung cancer in The Netherlands. Clin Oncol (r Coll Radiol) 2018;30(1):17–22. doi: 10.1016/j.clon.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Willén L., Berglund A., Bergström S., Bergqvist M., Öjdahl-Bodén A., Wagenius G., et al. Educational level and management and outcomes in non-small cell lung cancer a nationwide population-based study. Lung Cancer. 2019;131:40–46. doi: 10.1016/j.lungcan.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Dickhoff C., Dahele M., Smit E.F., Paul M.A., Senan S., Hartemink K.J., et al. Patterns of care and outcomes for stage IIIB non-small cell lung cancer in the TNM-7 era: results from the Netherlands cancer registry. Lung Cancer. 2017;110:14–18. doi: 10.1016/j.lungcan.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal A., Lewison G., Idir S., Peters M., Aldige C., Boerckel W., et al. The state of lung cancer research: a global analysis. J Thorac Oncol. 2016;11(7):1040–1050. doi: 10.1016/j.jtho.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal A., Lewison G., Rodin D., Zietman A., Sullivan R., Lievens Y. Radiation therapy research: a global analysis 2001–2015. Int J Radiat Oncol Biol Phys. 2018;101(4):767–778. doi: 10.1016/j.ijrobp.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Møller H., Coupland V.H., Tataru D., Peake M.D., Mellemgaard A., Round T., et al. Geographical variations in the use of cancer treatments are associated with survival of lung cancer patients. Thorax. 2018;73(6):530–537. doi: 10.1136/thoraxjnl-2017-210710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solberg S., Nilssen Y., Brustugun O.T., Grimsrud T.K., Haram P.M., Helbekkmo N., et al. Increase in curative treatment and survival of lung cancer in Norway 2001–2016. Eur J Epidemiol. 2019;34(10):951–955. doi: 10.1007/s10654-019-00536-z. [DOI] [PubMed] [Google Scholar]
- 33.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: Understanding time lags in translational research. J R Soc Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galson S., Simon G. NAM Perspectives; Discussion Paper, National Academy of Medicine, Washington, DC: 2016. Real-World Evidence to Guide the Approval and Use of New Treatments. https://doi.org/10.31478/201610b. [Google Scholar]
- 35.Borras J.M., Corral J., Aggarwal A., Audisio R., Espinas J.A., Figueras J., et al. Innovation, value and reimbursement in radiation and complex surgical oncology: time to rethink. Radiother Oncol. 2022;169:114–123. doi: 10.1016/j.radonc.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Burock S., Meunier F., Lacombe D. How can innovative forms of clinical research contribute to deliver affordable cancer care in an evolving health care environment? Eur J Cancer. 2013;49(13):2777–2783. doi: 10.1016/j.ejca.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Saesen R., Depreytere K., Krupianskaya K., Langeweg J., Verheecke J., Lacombe D., et al. Analysis of the characteristics and the degree of pragmatism exhibited by pragmatic-labelled trials of antineoplastic treatments. BMC Med Res Methodol. 2023;23(1):148. doi: 10.1186/s12874-023-01975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker S., Scupher M., Claxton K., Palmer S. Coverage with evidence development, only in research, risk sharing or patient access scheme?: A framework for coverage decision. Value Health. 2012;15:570–579. doi: 10.1016/j.jval.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018 Dec 13;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 40.Koshy M., Malik R., Spiotto M., Mahmood U., Weichselbaum R., Sher D. Disparities in treatment of patients with inoperable stage I non-small cell lung cancer: a population-based analysis. J Thorac Oncol. 2015;10(2):264–271. doi: 10.1097/JTO.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boxer M.M., Vinod S.K., Shafiq J., Duggan K.J. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer. 2011;117(22):5112–5120. doi: 10.1002/cncr.26149. [DOI] [PubMed] [Google Scholar]
- 42.Slotman B.J., Vos P.H. Planning of radiotherapy capacity and productivity. Radiother Oncol. 2013;106:206–210. doi: 10.1016/j.radonc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Chalkidou A., MacMillan T., Grzeda M., Peacock J., Eddy S.J., et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021;22:98–106. doi: 10.1016/S1470-2045(20)30537-4. [DOI] [PubMed] [Google Scholar]
- 44.Lievens Y, Boesmans L, Engels H, Geets X, Jansen N, Janssens S, Lambrecht M, Remouchamps V, Roosens S, Stellamans K, Verellen D, Weltens C, Weytjens R, Van Damme N. Coverage with evidence development: generating real-life evidence on SBRT in Belgium. https://www.estro.org/Congresses/ESTRO-2022/585/highlightsofprofferedpapers-bestpapers/5389/interdisciplinarybestpaper-coveragewithevidencedev. [DOI] [PMC free article] [PubMed]
- 45.Berghmans T., Lievens Y., Aaapro M., Baird A.M., Beishon M., Calabrese F., et al. European Cancer Organization Essential Requirements for Lung Cancer. 2020;15:221–239. doi: 10.1016/j.lungcan.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Vulto A., Louwman M., Rodrigus P., Coebergh J.W. Referral rates and trends in radiotherapy as part of primary treatment of cancer in South Netherlands, 1988–2002. Radiother Oncol. 2006;78(2):131–137. doi: 10.1016/j.radonc.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Vulto A.J., Lemmens V.E., Louwman M.W., Janssen-Heijnen M.L., Poortmans P.H., Lybeert M.L., et al. The influence of age and comorbidity on receiving radiotherapy as part of primary treatment for cancer in South Netherlands, 1995 to 2002. Cancer. 2006;106(12):2734–2742. doi: 10.1002/cncr.21934. [DOI] [PubMed] [Google Scholar]
- 48.Janssen-Heijnen M.L., Lemmens V.E., van den Borne B.E., Biesma B., Oei S.B., Coebergh J.W. Negligible influence of comorbidity on prognosis of patients with small cell lung cancer: a population-based study in the Netherlands. Crit Rev Oncol Hematol. 2007;62(2):172–178. doi: 10.1016/j.critrevonc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Haasbeek C.J.A., Palma D., Visser O., Lagerwaard F.J., Slotman B., Senan S. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012;23(10):2743–2747. doi: 10.1093/annonc/mds081. [DOI] [PubMed] [Google Scholar]
- 50.Detillon D.D.E.M.A., Driessen E.J.M., Aarts M.J., Janssen-Heijnen M.L.G., van Eijck C.H.J., Veen E.J. Changes in treatment patterns and survival in elderly patients with stage I non-small-cell lung cancer with the introduction of stereotactic body radiotherapy and video-assisted thoracic surgery. Eur J Cancer. 2018;101:30–37. doi: 10.1016/j.ejca.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Karlsson A.T., Hjermstad M.J., Omdahl T., Aass N., Skovlund E., Hellebust T.P., et al. Overall survival after initial radiotherapy for brain metastases; a population based study of 2140 patients with non-small cell lung cancer. Acta Oncol. 2021;60(8):1054–1060. doi: 10.1080/0284186X.2021.1924399. [DOI] [PubMed] [Google Scholar]
- 52.Berglund A., Holmberg L., Tishelman C., Wagenius G., Eaker S., Lambe M. Social inequalities in non-small cell lung cancer management and survival: a population-based study in central Sweden. Thorax. 2010;65(4):327–333. doi: 10.1136/thx.2009.125914. [DOI] [PubMed] [Google Scholar]
- 53.Koning C.C., Aarts M.J., Struikmans H., Poortmans P.M., Lybeert M.L., Jobsen J.J., et al. Mapping use of radiotherapy for patients with non-small cell lung cancer in the Netherlands between 1997 and 2008. Clin Oncol (r Coll Radiol) 2012;24(2):e46–e53. doi: 10.1016/j.clon.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Dickhoff C., Dahele M., de Langen A.J., Paul M.A., Smit E.F., Senan S., et al. Population-based patterns of surgical care for stage IIIA NSCLC in the Netherlands between 2010 and 2013. J Thorac Oncol. 2016;11(4):566–572. doi: 10.1016/j.jtho.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Willén L., Berglund A., Bergström S., Isaksson J., Bergqvist M., Wagenius G., et al. Patterns of care and outcomes in immigrants with non-small cell lung cancer. a population-based study (Sweden) PLoS One. 2022;17(12) doi: 10.1371/journal.pone.0278706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joosten P.J.M., Damhuis R.A.M., van Diessen J.N.A., de Langen J.A., Belderbos J.S.A., Smit E.F., et al. Results of neoadjuvant chemo(radio)therapy and resection for stage IIIA non-small cell lung cancer in The Netherlands. Acta Oncol. 2020;59(7):748–752. doi: 10.1080/0284186X.2020.1757150. [DOI] [PubMed] [Google Scholar]
- 57.Damhuis R.A.M., Senan S., Khakwani A., Harden S., Helland Ȧ., Strand T.E. Age-related treatment patterns for stage I NSCLC in three European countries. J Geriatr Oncol. 2021;12(8):1214–1219. doi: 10.1016/j.jgo.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Tomassen M.L., Aarts M.J., Peters M., van Lindert A., De Ruysscher D.K.M., Verhoeff J.J.C., et al. Prophylactic cranial irradiation in patients with small cell lung cancer in The Netherlands: a population-based study. Clin Transl Radiat Oncol. 2021;27:157–163. doi: 10.1016/j.ctro.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dieleman E, van der Woude L, van Os R, van Bockel L, Coremans I, van Es C, De Jaeger K, Knol HP, Kolff W, Koppe F, Pomp J, Reymen B, Schinagl D, Spoelstra F, Tissing-Tan C, van der Voort van Zyp N, van der Wel A, Wijsman R, Dielwart M, Wiegman E, Damhuis R, Belderbos J. The Dutch Lung Cancer Audit-Radiotherapy (DLCA-R): Real-World Data on Stage III Non-Small Cell Lung Cancer Patients Treated With Curative Chemoradiation. Clin Lung Cancer. 2023;24(2):130-136. [DOI] [PubMed]
- 60.Berglund A., Lambe M., Lüchtenborg M., Linklater K., Peake M.D., Holmberg L., et al. Social differences in lung cancer management and survival in South East England: a cohort study. BMJ Open. 2012 May 25;2(3) doi: 10.1136/bmjopen-2012-001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verleye L., De Gendt C., Vrijens F., Schillemans V., Camberlin C., Silversmit G., et al. Patterns of care for non-small cell lung cancer patients in Belgium: a population-based study. Eur J Cancer Care (engl) 2018;2727 doi: 10.1111/ecc.12747. [DOI] [PubMed] [Google Scholar]
- 62.Giusti F., Martos C., Carvalho R., van Eycken L., Visser O., Bettio M. Quality indicators: completeness, validity and timeliness of cancer registry data contributing to the European Cancer Information System. Front Oncol. 2023;12 doi: 10.3389/fonc.2023.1219128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.