Abstract
Objective
In this study, we aimed to investigate whether age first had sexual intercourse (AFSI) and lifetime number of sexual partners (LNSP) have a direct causal effect on cervical cancer by Mendelian randomization (MR) analysis.
Methods
Four approaches were used for MR Analysis, including MR-Egger, weighted method, weighted median, and inverse variance weighted (IVW). MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) as well as MR-Egger regression analysis were conducted to detect whether there was pleiotropy between IVs and outcome, and the outlier SNPs can be detected by MR-PRESSO. The presence or absence of heterogeneity among IVs was suggested according to Cochran's Q statistic. Leave-one-out sensitivity analysis was performed to identify and remove SNPs which could independently change the results. We corrected the results using Bonferroni correction.
Results
From the results of IVW, AFSI had a negative effect on cervical cancer (OR = 0.996, 95 % CI: 0.995, 0.998 P = 1.70E-07), which still persisted after Bonferroni correction. However, no causal effect of LNSP on cervical cancer was found according to the IVW results (OR = 1.003, 95 % CI: 1.000, 1.007, P = 0.071). From the results of MR-PRESSO and MR-Egger, no SNP with horizontal pleiotropy between cervical cancer was detected and no SNP was identified as an outlier SNP. Cochran's Q statistic suggested that no heterogeneity existed among IVs of AFSI and LNSP. According to Leave-one-out analysis, the results of MR did not change after excluding any single IV.
Conclusion
This MR study reveals that early AFSI has a causal effect on cervical cancer.
Keywords: Cervical cancer, Age first had sexual intercourse, Lifetime number of sexual partners, Genetics, Mendelian randomization
This study found that early AFSI has a causal effect on cervical cancer while LNSP has no causal effect using Mendelian randomization analysis.
1. Introduction
As the most prevalent malignancy in the reproductive system of women, cervical cancer is caused by the formation of malignant cells in the cervix. Compared with younger women, women in the 40–60 age group had a higher prevalence of cervical cancer [1]. As the fourth most common cancer among female of reproductive age worldwide, it was estimated that nearly 13,960 new cases of invasive cervical cancer (ICC) and 4310 related deaths will occur in the United States by 2023 [2]. Although the pathogenesis of cervical cancer was not fully elucidated, some studies revealed that the incidence of cervical cancer may be associated with the following factors, including early age at first sexual intercourse (AFSI), early marriage, multiple childbearing, HPV infection, poor hygiene during the puerperium and a large lifetime number sexual partners (LNSP) et al. [1,3]. Among these factors, AFSI and LNSP attracted much attention.
Mounting evidence suggested that AFSI and LNSP are influencing factors for cervical cancer. A case-control study including 1864 patients with cervical cancer and 1719 controls from 8 developing countries revealed that early AFSI is closely related to an increased risk of ICC [3]. Recently, an observational study found that among 10,494 Japanese women younger than 40 years, those with ICC had a significantly younger AFSI as compared to those with cervical intraepithelial neoplasia grade (CIN) 2–3 and carcinoma in situ [4]. A study including 128,173 women aged 15–49 years from 8 west African countries reported that young AFSI is closely related to an increased risk of cervical cancer [5]. In addition, a study analyzed 21 epidemiological studies and found that low AFSI and more LNSP is positive related to the risk of ICC [6]. More LNSP was observed to be positive associated to the risk of carcinoma in situ and CIN3 [6]. LNSP was also proposed that can predict spontaneous regression of CIN 2 and CIN 3 [7]. A survey of more than 100,000 Scandinavian women aged 18–45 years from Danish, Norwegian and Swedish showed that young AFSI and more LNSP are associated with a higher incidence of cervical cancer [8].
Clarifying the causal relationship between AFSI/LNSP and cervical cancer is helpful to accurately determine the high-risk population of cervical cancer, contribute to the publicity and education of cervical cancer, early prevention and intervention of cervical cancer, and also guide the formulation of cervical cancer vaccination strategy and implementation. However, majority of the above conclusions between AFSI/LNSP and cervical cancer were from observational studies, which may be biased by confounding factors [9]. In addition, due to the ethical issues of AFSI and LNSP and the serious consequences of cervical cancer, conducting a randomized controlled trial (RCT) to investigate the direct causal association between AFSI/LNSP and cervical cancer may involve ethical issues. Therefore, the relationship between AFSI/LNSP and cervical cancer still needs to be further researched using an advanced method.
Under the circumstance, Mendelian randomization (MR), as a method with strong causal determination, can be used to assess the causal relationship between AFSI/LNSP and cervical cancer. In MR analysis, genetic variants of exposures, which are randomly assigned and not be influenced by possible confounding factors, were used as instrumental variables (IVs) [10]. Due to the stability of IV and the one-way relationship that genes affect traits but diseases do not change genotypes, the causal associations observed by MR can be protected from confounding factors and reverse causal bias [11]. In addition, compared with RCT, the data in MR analysis is obtained from the existing large-scale genome-wide association study (GWAS) open data, which not only saves time and human resources, but also does not have medical ethical issues [11].
In this study, we aimed to investigate whether AFSI and LNSP have a direct causal effect on cervical cancer by MR analysis. To the best of our knowledge, there is a dearth of MR studies investigating the causal effect of AFSI and LNSP on cervical cancer. This study fills this gap in the literature. The result of this study can not only provide a theoretical basis for the development of prevention and care strategies for patients with cervical cancer, but also help to evaluate the risk of cervical cancer in women with early AFSI or more LNSP.
2. Materials and methods
2.1. Study design and data source
The possible causal effect of AFSI and LNSP on cervical cancer was detected by two-sample MR analysis. The GWAS data of AFSI, LNSP and cervical cancer were all obtained from the IEU [12], which included 9,851,867participants (Supplement Table 1). Using this GWAS database, we extracted IVs strongly associated with AFSI and LNSP, and then extracted relevant data from the GWAS database of cervical cancer for MR Analysis. This study is a pooled analysis of previous GWAS data, which is a secondary analysis and does not require ethical permission and informed consent.
2.2. SNP selection
Firstly, we chose SNPs which were strongly (P < 5 × 10−8) associated with AFSI and LNSP and with a minor allele frequency (MAF) ≥ 0.01 from the GWAS data. Secondly, the clustering process (R2 < 0.001 and clumping distance = 10,000 kb) was conducted to eliminate the linkage disequilibrium (LD) between SNPs. Thirdly, we matched the screened SNPs in GWAS data of cervical cancer, and if the SNP was not present in the GWAS data, it was replaced by its proxy SNP with high LD (r2 > 0.8). Finally, palindrome SNPs were removed and the remaining SNPS can be used as IVs.
2.3. Statistical analyses
In this study, we conducted MR analysis using four approaches, including MR-Egger, weighted method, weighted median, and inverse variance weighted (IVW), to ensure that the results were more comprehensive and stable. Among these four methods, IVW was the most important basis for interpretation of the results. MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) as well as MR-Egger regression analysis were conducted to detect whether there was pleiotropy between IVs and outcome, and the outlier SNPs were also identified by MR-PRESSO. Then we deleted these abnormal IVs and re-conducted the analysis until no abnormal IVs were detected [13,14]. Meanwhile, the presence or absence of heterogeneity among IVs was suggested according to Cochran's Q statistic [15]. In order to identify and remove SNPs with significant effects on the results, Leave-one-out sensitivity analysis was performed. Due to that two exposures (AFSI and LNSP) were analyzed in this study, the P-value of the Bonferroni correction was 0.025 (0.05 was divided by 2*1) [16,17]. Therefore, P < 0.025 was considered statistically significant. All statistical analyses were done with the packages “TwoSampleMR” as well as “MRPRESSO” included in R version 4.1.1.
3. Results
3.1. Instrumental variables (IVs) selection
Based on the clumping process results, 200 SNPs and 63 SNPs strongly related (P < 5 × 10−8) to AFSI and LNSP were screened as IVs, respectively and there was no LD between these IVs. Then, we excluded SNPs with MAF less than 0.01. There were 10 IVs of AFSI and 2 IVs of LNSP which were identified as palindromic SNPs and removed in MR analysis. A total of 41 IVs of AFSI and 13 IVs of LNSP were not found in outcome GWAS data, and no proxy SNPs of them can be found in outcome GWAS data (Supplementary Tables 2–3). Ultimate there were 149 IVs of AFSI and 48 IVs of LNSP available in MR analysis.
3.2. Causal effect of AFSI/LNSP on cervical cancer
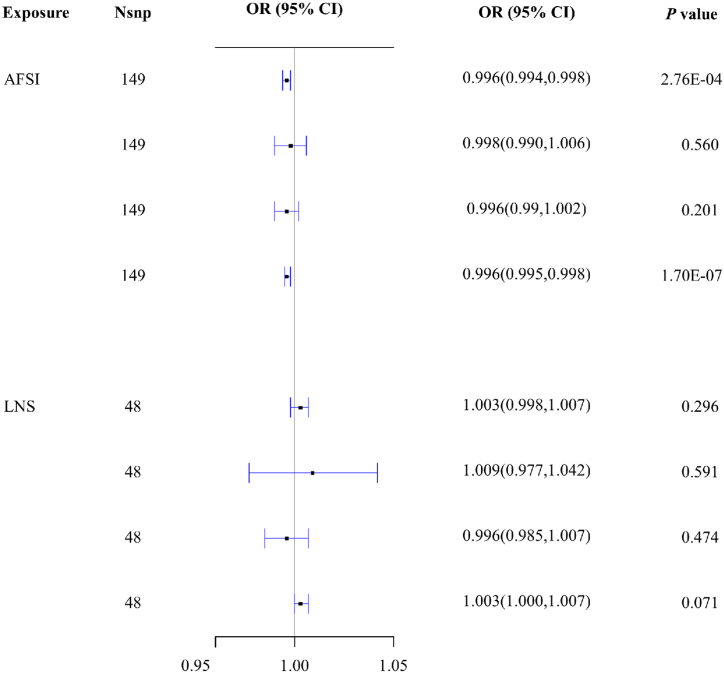
From the results of IVW, AFSI was negatively correlated with the risk of cervical cancer (OR = 0.996, 95 % CI: 0.995, 0.998 P = 1.70E-07), and the correlation remain existed after Bonferroni correction (Fig. 1, Supplementary Table 4). No causal effect of LNSP on cervical cancer was found according to the IVW results (OR = 1.003, 95 % CI: 1.000, 1.007, P = 0.071) (Fig. 1, Supplementary Table 4).
Fig. 1.
MR estimate results of AFSI and LNSP on cervical cancer.
3.3. Pleiotropy and Sensitivity Analysis
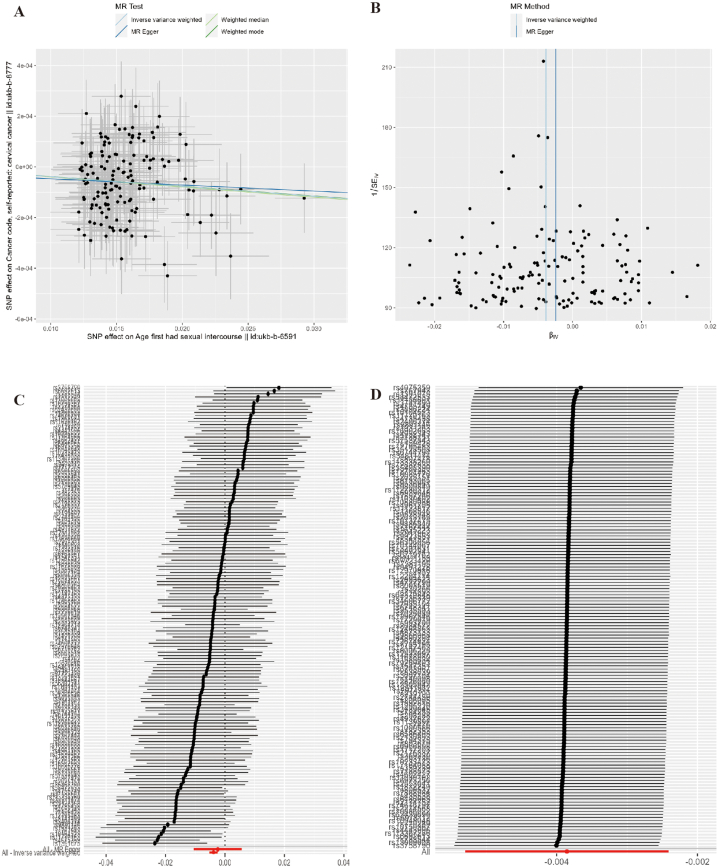
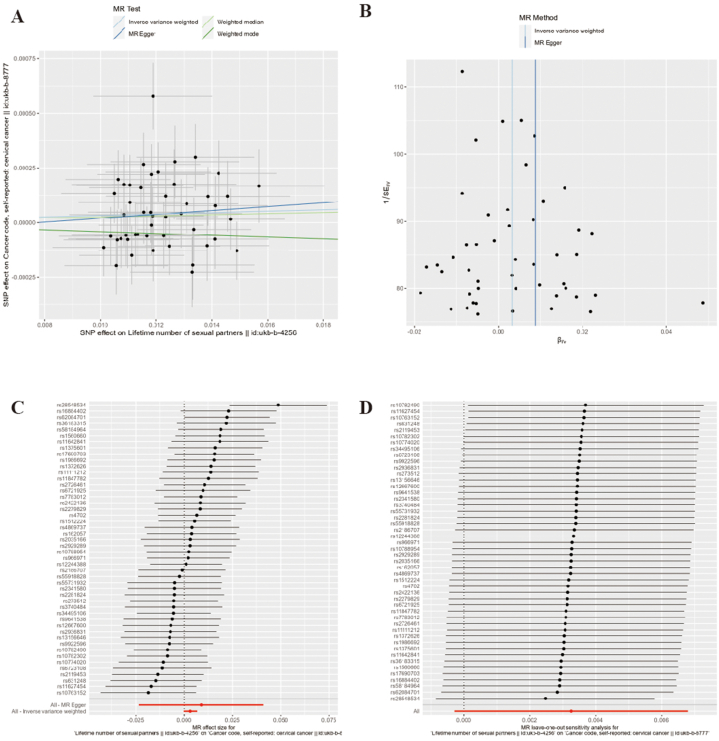
According to the results of MR-PRESSO and MR-Egger, no SNP with horizontal pleiotropy between cervical cancer was detected and no SNP was identified as an outlier SNP (all P > 0.05). Cochran's Q statistic suggested that no heterogeneity existed among IVs of AFSI and LNSP (Q = 132.079, P = 0.822) and LNSP (Q = 54.586, P = 0.208). Leave-one-out analysis showed that the results of MR did not change after excluding any single IV ((Fig. 2, Fig. 3, Supplementary Table 4)).
Fig. 2.
Pleiotropy and Sensitivity Analysis of AFSI on cervical cancer. Note: A: Scatter plot; B: Funnel plot; C: Forest plot; D: Leave-one-out plot.
Fig. 3.
Pleiotropy and Sensitivity Analysis of LNSP on cervical cancer
Note: A: Scatter plot; B: Funnel plot; C: Forest plot; D: Leave-one-out plot.
4. Discussion
The results of IVW suggested that LNSP had no causal effect on cervical cancer, while AFSI was still negative correlated with the risk of cervical cancer after Bonferroni correction, which provide a theoretical basis to further confirm the effect of AFSI on cervical cancer.
In recent years, many studies which were mostly observational studies, have focused on the relationship between AFSI and cervical cancer and revealed that early AFSI can increase the risk of cervical cancer [[3], [4], [5], [6]], which was also confirmed by our study. This may be because that people with low AFSI were young when they first have sex, the reproductive system is not fully developed, and the knowledge and protection awareness of cervical cancer are low [18,19]. They may be less inclined to seek or less informed about cervical screening and less likely to receive cervical cancer vaccination. Low AFSI also means that they may have more sexual risk behaviors and are more likely to be infected with HPV, which ultimately leads to a higher risk of cervical cancer [[19], [20], [21], [22], [23]].
A study analyzed 28 studies to explore the casual relationship between AFSI and the health of adolescent girls and found that girls with AFSI younger than 14 years were less likely to use contraception, had longer duration of sexual activity before using contraception, and were at higher risk of cervical cancer. Moreover, girls with AFSI ≥16 were at a better physical and mental state than those with a young AFSI [19]. It was also reported that women with AFSI ≥18 were almost twice as likely to use contraception as compared with women with AFSI <15 [22].
LNSP was also proposed that can predict spontaneous regression of CIN 2 and CIN 3 [7]. A survey of more than 100,000 Scandinavian women aged 18–45 years from Danish, Norwegian and Swedish showed that young AFSI and more LNSP are associated with a higher incidence of cervical cancer [8]. It has been reported that more LNSP is a disadvantage factor for cervical cancer, and women with more LNSP have a higher risk of cervical cancer [[6], [7], [8],24]. However, no causal effect of LNSP on cervical cancer was observed in this study, which is inconsistent with previous studies, suggesting that the association found in previous observational studies may not be a direct causal relationship.
It was revealed that obtaining a new sexual partner, a large number of LNSP, and having non-marital male sexual partners were the main risk factors for HPV infection in women [25]. A study that analyzed 576,281 women from 280 studies also found that women with more LNSP had a higher risk of HPV infection [26]. The previous observed association may be because that women with multiple sexual partners are more susceptible to HPV infection, and their acceptance of cervical cancer screening is lower than that of women with single sexual partners, thus making observational studies vulnerable to misinterpretation of the direct association of LNSP with cervical cancer [6,20,27,28]. The results of this study suggest that there is no direct causal relationship between LNSP and cervical cancer. The association between LNSP and cervical cancer observed in previous observational studies may be a spurious causal association caused by other factors, such as HPV infection, and the association between LNSP and cervical cancer still needs to be further studied.
The results of this study suggest that we should provide sexual education, popular science of sexual knowledge and health education of cervical cancer in young children to reduce the probability of young sexual behavior and high-risk sexual behavior. It also suggests that we should improve the awareness and expand the popularization of cervical cancer screening in women, especially for women with high risk factors.
5. Advantages and disadvantages
Our study design had several advantages. Firstly, to the best of our knowledge, this is the first time that MR has been used to investigate the direct causal effect of AFSI and LNSP on cervical cancer. Secondly, we used the largest GWAS databases available for AFSI, LNSP and cervical cancer, with exposures and outcomes derived from the same GWAS database. Third, all the results of heterogeneity and sensitivity analysis ensure the stability of the MR results. Moreover, Bonferroni correction was used to ensure the stability of the conclusion.
However, there were also some limitations of this study. First, all GWAS data were restricted to individuals of European descent, resulting in that the extrapolation of the results to the whole population is limited. Second, whether some possible confounding factors, such as HPV infection, are involved in the causal relationship and their mediating effects still need to be further studied.
6. Conclusion
Our findings suggest a potential causal relationship between early AFSI and cervical cancer. The results of this study provided a theoretical basis for the development of prevention, health care, and treatment strategies for patients with cervical cancer.
Data availability statement
The GWAS data used in this MR analysis is obtained from the existing large-scale GWAS open data as shown in Supplement Table 1 and the data generated in this study are included in the article/Supplementary material.
Funding
Not applicable.
CRediT authorship contribution statement
Yuan-yuan Zhou: Writing – original draft. Man Chang: Writing – review & editing, Formal analysis. Chuan-ping Li: Software, Data curation. Xi-ling Han: Software, Data curation. Ping Fang: Visualization, Software. Xiao-ping Xia: Project administration, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23758.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Yu L., Lanqing G., Huang Z., Xin X., Minglin L., Fa-Hui L., et al. T cell immunotherapy for cervical cancer: challenges and opportunities. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1105265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Society A.C. American Cancer Society; 2023. Key Statistics for Cervical Cancer. [Google Scholar]
- 3.Louie K.S., de Sanjose S., Diaz M., Castellsagué X., Herrero R., Meijer C.J., et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br. J. Cancer. 2009;100(7):1191–1197. doi: 10.1038/sj.bjc.6604974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakazawa H., Yamaguchi S., Onuki M., Kitai M., Yahata H., Aoki Y., et al. Age at first sexual intercourse among young women with invasive cervical cancer: implications for routine vaccination against human papillomavirus in Japan. Jpn. J. Clin. Oncol. 2023;53(6):530–533. doi: 10.1093/jjco/hyad017. [DOI] [PubMed] [Google Scholar]
- 5.Olajide N., Robb K., Niedzwiedz C., Jani B. Cervical cancer risk factors in eight west African countries: cross-sectional analysis of the demographic and health survey 2017-20. Lancet. 2022;400(Suppl 1):S68. [Google Scholar]
- 6.Cervical carcinoma and sexual behavior: collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies. Cancer Epidemiol. Biomarkers Prev. 2009;18(4):1060–1069. doi: 10.1158/1055-9965.EPI-08-1186. [DOI] [PubMed] [Google Scholar]
- 7.Chan J.K., Monk B.J., Brewer C., Keefe K.A., Osann K., McMeekin S., et al. HPV infection and number of lifetime sexual partners are strong predictors for 'natural' regression of CIN 2 and 3. Br. J. Cancer. 2003;89(6):1062–1066. doi: 10.1038/sj.bjc.6601196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen B.T., Kjaer S.K., Arnheim-Dahlström L., Liaw K.L., Juul K.E., Thomsen L.T., et al. Age at first intercourse, number of partners and sexually transmitted infection prevalence among Danish, Norwegian and Swedish women: estimates and trends from nationally representative cross-sectional surveys of more than 100 000 women. Acta Obstet. Gynecol. Scand. 2020;99(2):175–185. doi: 10.1111/aogs.13732. [DOI] [PubMed] [Google Scholar]
- 9.Sekula P., Del Greco M.F., Pattaro C., Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. : JASN (J. Am. Soc. Nephrol.) 2016;27(11):3253–3265. doi: 10.1681/ASN.2016010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden J., Holmes M.V. Meta-analysis and Mendelian randomization: a review. Res. Synth. Methods. 2019;10(4):486–496. doi: 10.1002/jrsm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IEU 2023. https://gwas.mrcieu.ac.uk/
- 13.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greco M.F., Minelli C., Sheehan N.A., Thompson J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015;34(21):2926–2940. doi: 10.1002/sim.6522. [DOI] [PubMed] [Google Scholar]
- 16.Sedgwick P. Multiple hypothesis testing and Bonferroni's correction. BMJ. 2014;349:g6284. doi: 10.1136/bmj.g6284. [DOI] [PubMed] [Google Scholar]
- 17.Curtin F., Schulz P. Multiple correlations and Bonferroni's correction. Biol. Psychiatr. 1998;44(8):775–777. doi: 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
- 18.Paneru B., Karmacharya A., Bharati A., Makaju S., Adhikari B., Kafle D., et al. Association between cancer stigma and cervical cancer screening uptake among women of Dhulikhel and Banepa, Nepal. PLoS One. 2023;18(5) doi: 10.1371/journal.pone.0285771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lara L.A.S., Abdo C.H.N. Age at time of initial sexual intercourse and health of adolescent girls. J. Pediatr. Adolesc. Gynecol. 2016;29(5):417–423. doi: 10.1016/j.jpag.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi M., Sekine M., Hanley S.J.B., Kudo R., Hara M., Adachi S., et al. Risk factors for HPV infection and high-grade cervical disease in sexually active Japanese women. Sci. Rep. 2021;11(1):2898. doi: 10.1038/s41598-021-82354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plummer M., Peto J., Franceschi S. Time since first sexual intercourse and the risk of cervical cancer. Int. J. Cancer. 2012;130(11):2638–2644. doi: 10.1002/ijc.26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnusson B.M., Masho S.W., Lapane K.L. Early age at first intercourse and subsequent gaps in contraceptive use. J. Womens Health (Larchmt) 2012;21(1):73–79. doi: 10.1089/jwh.2011.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandfort T.G., Orr M., Hirsch J.S., Santelli J. Long-term health correlates of timing of sexual debut: results from a national US study. Am J. Public Health. 2008;98(1):155–161. doi: 10.2105/AJPH.2006.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z.C., Liu W.D., Liu Y.H., Ye X.H., Chen S.D. Multiple sexual partners as a potential independent risk factor for cervical cancer: a meta-analysis of epidemiological studies. Asian Pac. J. Cancer Prev. 2015;16(9):3893–3900. doi: 10.7314/apjcp.2015.16.9.3893. [DOI] [PubMed] [Google Scholar]
- 25.Chelimo C., Wouldes T.A., Cameron L.D., Elwood J.M. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J. Infect. 2013;66(3):207–217. doi: 10.1016/j.jinf.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Vinodhini K., Shanmughapriya S., Das B.C., Natarajaseenivasan K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch. Gynecol. Obstet. 2012;285(3):771–777. doi: 10.1007/s00404-011-2155-8. [DOI] [PubMed] [Google Scholar]
- 27.Nilima N., Mani K., Kaushik S., Rai S.N. Cervical cancer screening and associated barriers among women in India: a generalized structural equation modeling approach. Cancers. 2022;14(13) doi: 10.3390/cancers14133076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Were E., Nyaberi Z., Buziba N. Perceptions of risk and barriers to cervical cancer screening at Moi teaching and referral hospital (MTRH), Eldoret, Kenya. Afr. Health Sci. 2011;11(1):58–64. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GWAS data used in this MR analysis is obtained from the existing large-scale GWAS open data as shown in Supplement Table 1 and the data generated in this study are included in the article/Supplementary material.