Abstract
Background
Using the published survival statistics from cancer registration or population‐based studies, we aimed to describe the global pattern and trend of lung cancer survival.
Methods
By searching SinoMed, PubMed, Web of Science, EMBASE, and SEER, all survival analyses from cancer registration or population‐based studies of lung cancer were collected by the end of November 2022. The survival rates were extracted by sex, period, and country. The observed, relative, and net survival rates of lung cancer were applied to describe the pattern and time changes from the late 1990s to the early 21st century.
Results
Age‐standardized 5‐year relative/net survival rate of lung cancer was typically low, with 10%–20% for most regions. The highest age‐standardized relative/net survival rate was observed in Japan (32.9%, 2010–2014), and the lowest was in India (3.7%, 2010–2014). In most countries, the five‐year age‐standardized relative/net survival rates of lung cancer were higher in females and younger people. The patients with adenocarcinoma had a better prognosis than other groups. In China, the highest 5‐year overall relative/net survival rates were 27.90% and 31.62% in men and women in Jiangyin (2012–2013).
Conclusion
Over the past decades, the prognosis of lung cancer has gradually improved, but significant variations were also observed globally. Worldwide, a better prognosis of lung cancer can be observed in females and younger patients. It is essential to compare and evaluate the histological or stage‐specific survival rates of lung cancer between different regions in the future.
Keywords: cancer registry, lung cancer, observed survival rate, population‐based survival study, relative survival rate
This study collected globally published data on observed and relative survival rates of lung cancer from population‐based cancer registration. Over the past decades, the prognosis of lung cancer has gradually improved. However, region, period, sex, and age might affect the survival rate of lung cancer patients. The observed and relative survival rate of lung cancer patients varies greatly among different histological types and stages.
INTRODUCTION
Lung cancer is the second most common cancer incidence and the leading cause of cancer death in 2020, with an estimated 2.2 million new cases and 1.8 million deaths, representing approximately one in 10 (11.4%) cancers diagnosed and one in five (18.0%) deaths. 1 It is the most frequently occurring cancer (14.3%) and the third cancer (8.4%) in men and women; and the leading cause of cancer death (18.0%) and the second (13.7%) in men and women. 1 Regarding the regions, more than half of these cases occurred in developed countries. Incidence and mortality of lung cancer in high human development index (HDI) regions were at least three times higher compared with low HDI regions in both sex. 1
As the foundation of cancer prevention and control, population‐based cancer registration reflects the cancer burden of the entire population through obtaining comprehensive, accurate, and timely information on cancer incidence, mortality, and survival. 2 The long‐term survival rate of cancer patients might not be available for the countries or regions that have systematically reported data on cancer incidence and/or mortality. There were three different sources of survival data: clinical studies, hospital‐based follow‐up studies, and population‐based cancer registration. They are disparate in research aims and applications. Population‐based survival data include the survival information of all patients in the population, which can provide valuable indicators such as relative survival rate (RSR) for the effectiveness of cancer control and reflect the prospects of cure in a country or region. 3
In the present study, we performed a systematic review of survival analysis from population‐based cancer registration and extracted the relative, net, and observed survival rates. The aim of the study was to describe the global pattern and chronological changes in survival rates in lung cancer patients in different populations or regions during the 1990s and into the early 21st century.
METHODS
Data source
A literature search of related studies up to November 30, 2022, was conducted using the databases of SinoMed, PubMed, Web of Science, EMBASE, and SEER, with the following keywords: “lung cancer”, “pulmonary neoplasm”, “cancer registry”, “population‐based survival studies”, “relative survival”, “observed survival”, and “net survival”. Lung cancer was defined by using the 10th revision of the International Classification of Diseases (ICD‐10) codes of C33‐34, and the histopathological type of tumor was coded by the International Classification of Diseases‐Oncology third edition (ICD‐O‐3).
Two researchers (JHB, JYT) collected the data independently according to the search criteria, and 576 full‐text articles were reviewed based on the initial titles and abstracts retrieved. Studies were included if they met the following criteria: (1) available survival indicators, such as relative survival rate (RSR), observed survival rate (OSR), or net survival rate of patients with lung cancer, and (2) data from population‐based studies or cancer registration. In addition, we excluded duplicate, incomplete or unavailable estimates in the original articles. After our screening, 138 studies were included, 17 of which were in Chinese, and the remaining 121 were in English (Figure 1). The present study only displays data from the 1990s.
FIGURE 1.
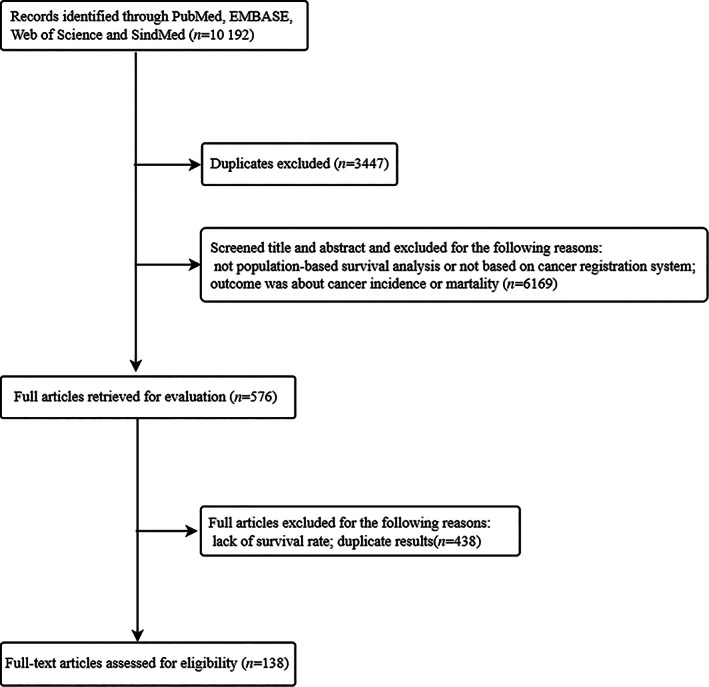
Study selection process.
Statistical analysis
Estimates of OSR, RSR, and net survival rates were extracted from the published studies. RSR is the ratio of the absolute survival rate of cancer patients to the expected survival rate of a group of people of the corresponding sex and age in the general population. Net survival is the cumulative probability of surviving up to a given time since diagnosis (e.g., 5 years) after correcting other causes of death (background mortality). Both net survival and RSR refer to the cumulative survival probabilities in a given period after excluding other causes of death, and we presented them if one or both were available in our study. 4 , 5 , 6 Survival generally depends on age at diagnosis, and the age distribution of cancer patients may vary over time in any one area or country and will almost certainly differ among geographical areas. Age‐standardized survival ensures age comparability of survival among different countries and regions. Standard cancer patient population is commonly used to compare the survival of lung cancer. 7 We used overall and age‐standardized 5‐year RSR or net survival rate to describe and compare the results in different countries or regions, age groups, and sex.
Endnote X9 and Excel 2016 were used for literature management and data analysis.
RESULTS
Global pattern and trends
Table 1 shows the overall observed survival rates (OSRs) of lung cancer in China, 8 , 9 , 10 , 11 , 12 , 13 , 14 Japan, 15 India, 16 , 17 Peru, 18 the USA, 19 , 20 , 21 and the European countries. 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 The differences in 1, 3, and 5‐year OSRs between different regions were distinctive. The highest 1‐year OSR was in Taiwan (2010–2014, China) with 57.39%. 14 The highest 5‐year OSRs were in California (2004–2009, USA) 19 with 31.10%, followed by Haining (2003–2015, China) 13 with 24.14%. However, in Denmark (2006–2008), 22 Sihui (2007–2009, China), 8 and Taiwan (2002–2007, China), 14 the 5‐year OSRs were poorer, at 12.00%, 10.35%, and 9.37%, respectively.
TABLE 1.
Overall observed survival rates of lung cancer in selected regions during 1990–2016.
| Region | Period | 1‐year | 3‐year | 5‐year | |
|---|---|---|---|---|---|
| China | Guangdong, Sihui 8 | 1997–2006 | ‐ | ‐ | 4.76 |
| 2007–2009 | ‐ | ‐ | 10.35 | ||
| Hebei, Cixian 9 | 2000–2002 | 18.55 | 9.17 | 5.76 | |
| Jiangsu, Qidong 10 | 1993–1997 | 14.52 | 4.79 | 3.69 | |
| 1998–2002 | 19.95 | 6.69 | 5.13 | ||
| 2003–2007 | 21.68 | 8.72 | 6.32 | ||
| 2008–2011 | 26.28 | 8.62 | ‐ | ||
| Shandong, Zhaoyuan 11 | 2009–2013 | 48.83 | 19.51 | 13.47 | |
| Shanghai 12 | 2002–2006 | 42.53 | 19.19 | 13.75 | |
| Zhejiang, Haining 13 | 2003–2015 | 42.69 | 26.13 | 24.14 | |
| Taiwan 14 | 2002–2007 | 37.81 | 14.66 | 9.37 | |
| 2010–2014 | 57.39 | 29.55 | 17.34 | ||
| Japan 15 | 1993–1996 | 52.10 | 25.90 | 19.50 | |
| India | Chennai 16 | 1990–1999 | 7.00 | ||
| Mumbai 17 | 1992–1994 | 29.90 | 15.90 | 12.50 | |
| Denmark 22 | 2000–2002 | 31.00 | ‐ | 10.00 | |
| 2003–2005 | 34.00 | ‐ | 10.00 | ||
| 2006–2008 | 34.00 | ‐ | 12.00 | ||
| 2009–2011 | 37.00 | ‐ | 13.00 | ||
| Finland 23 | 1990–1992 | ‐ | ‐ | 12.00 | |
| Germany 24 | 2000–2004 | ‐ | ‐ | 13.00 | |
| Hungary 27 | 2011–2016 | 42.33 | ‐ | 14.75 | |
| Portugal 28 | 2009–2011 | 41.40 | 18.90 | 13.60 | |
| Spain 25 , 29 | 2000–2007 | ‐ | ‐ | 9.30 | |
| 2008–2013 | ‐ | ‐ | 12.10 | ||
| UK | Scotland 26 | 1995 | 23.40 | 8.30 | ‐ |
| 2002 | 29.10 | 10.50 | ‐ | ||
| Peru | Lima 18 | 2004–2005 | ‐ | ‐ | 8.20 |
| USA | Total 21 | 2002–2008 | ‐ | ‐ | 18.30 |
| California 19 | 1992–1997 | ‐ | ‐ | 19.10 | |
| 1998–2003 | ‐ | ‐ | 24.00 | ||
| 2004–2009 | ‐ | ‐ | 31.10 | ||
| Florida 20 | 1996–2007 | 39.90 | 18.20 | 12.10 | |
Note: ‐ No report or nonavailable in the original articles.
Figure 2 demonstrates the age‐standardized 5‐year relative or net survival rates in selected countries and regions from Africa, 30 , 31 , 32 America, 30 , 32 , 33 Asia, 30 , 32 Europe, 32 , 33 , 34 , 35 and Oceania. 33 Age‐standardized 5‐year relative or net survival rate of lung cancer was typically low, with 10%–20% for most regions, both in the developed and developing countries. The highest age‐standardized 5‐year relative or net survival rate was 32.9% in Japan (2010–2014), followed by 21.4% in Australia (2010–2014). 32 The lowest was only 3.7% in India (2010–2014). 32 The age‐standardized 5‐year relative or net survival rates of lung cancer increased with time in most countries, with the most pronounced increase in Denmark of more than 10% between 1990 and 2014. 33 , 34 However, in some countries, such as the Czech Republic and France, age‐standardized 5‐year relative or net survival rates increased by less than 5% between 1990 and 2014. 32 , 34
FIGURE 2.
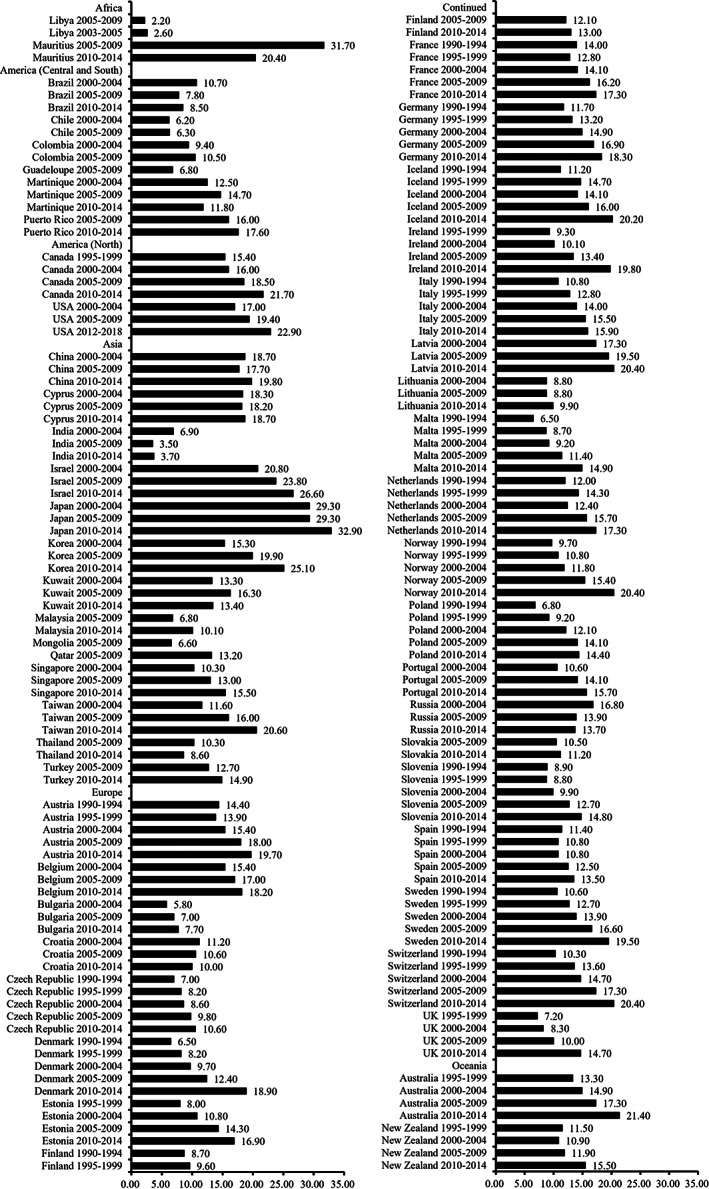
Age‐standardized 5‐year relative/net survival rates of lung cancer in selected regions during 1990–2018.
Figure S1 also shows the time trends of the overall 5‐year relative or net survival rate of lung cancer worldwide.
Subgroup analysis of lung cancer survival
Table 2 presents the recent reports of the sex‐specific age‐standardized 5‐year relative or net survival rates in selected regions including China, 36 Japan, 37 Singapore, 38 the USA, 37 and the European countries. 25 , 29 , 37 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 The 5‐year relative or net survival rates were higher in females in many regions except Germany (1990–1994). 40 The dissimilarity of the sex‐specific age‐standardized 5‐year relative or net survival rates in different areas was striking. For example, the highest 5‐year survival rates were 27.00% and 43.30% in males and females in Japan (2006–2008), 37 while the poorer 5‐year survival rates were 8.10% and 10.90% in the Azores (2005–2009, Portugal), and 8.00% and 9.90% in England (2000–2007, UK), for males and females, respectively. 37 , 45 In addition, we found that sex differences in lung cancer survival have become more significant, for example in China, as shown in Table S1.
TABLE 2.
Sex‐specific age‐standardized 5‐year relative/net survival rates of lung cancer in selected regions during 1990–2016.
| Region | Period | Male | Female | |
|---|---|---|---|---|
| China 36 | 2003–2005 | 15.40 | 17.40 | |
| 2006–2008 | 14.50 | 18.30 | ||
| 2009–2011 | 15.20 | 19.90 | ||
| 2012–2015 | 16.80 | 25.10 | ||
| Japan 37 | 1993–1996 | 20.80 | 27.10 | |
| 2006–2008 | 27.00 | 43.30 | ||
| Singapore 38 | 1998–2002 | 9.70 | 11.60 | |
| 2003–2007 | 9.60 | 14.10 | ||
| Austria 39 , 40 | 1990–1994 | 13.40 | 16.00 | |
| 2000–2002 | 13.50 | 17.80 | ||
| Belgium 39 , 41 | 2000–2002 | 15.00 | 19.90 | |
| 2000–2004 | 14.00 | 18.00 | ||
| Czech Republic 40 | 1990–1994 | 6.30 | 8.20 | |
| Denmark 43 , 48 | 1994–1998 | 7.00 | 7.00 | |
| 1999–2003 | 8.00 | 9.00 | ||
| 2005–2009 | 13.00 | 16.00 | ||
| 2000–2012 | 12.00 | 14.00 | ||
| Estonia 40 , 42 , 47 | 1990–1994 | 6.80 | 11.90 | |
| 1995–2000 | 7.00 | 13.00 | ||
| 2001–2006 | 10.00 | 16.00 | ||
| 2003–2009 | 12.00 | 17.00 | ||
| 2010–2016 | 15.00 | 20.00 | ||
| Finland 43 | 1994–1998 | 8.00 | 12.00 | |
| 1999–2003 | 8.00 | 13.00 | ||
| France 37 , 40 , 41 , 46 | 1990–1994 | 13.10 | 15.90 | |
| 1995–1999 | 12.10 | 16.80 | ||
| 2000–2004 | 13.00 | 17.00 | ||
| 2005–2010 | 16.00 | 20.00 | ||
| Germany 37 , 40 , 44 | 1990–1994 | 10.80 | 10.50 | |
| 1995–1999 | 13.00 | 13.80 | ||
| 2000–2007 | 14.50 | 18.50 | ||
| 2007–2010 | 15.50 | 20.30 | ||
| Iceland 43 | 1994–1998 | 11.00 | 13.00 | |
| 1999–2003 | 11.00 | 15.00 | ||
| Italy 37 , 40 , 41 | 1990–1994 | 9.80 | 10.50 | |
| 1995–1999 | 12.00 | 15.40 | ||
| 2000–2004 | 13.00 | 17.00 | ||
| 2000–2007 | 13.20 | 17.30 | ||
| Netherlands 40 | 1990–1994 | 11.70 | 12.40 | |
| Norway 43 | 1994–1998 | 9.00 | 12.00 | |
| 1999–2003 | 10.00 | 13.00 | ||
| Poland 39 , 40 | 1990–1994 | 6.10 | 6.80 | |
| 2000–2002 | 9.50 | 11.00 | ||
| Portugal | Total 41 | 2000–2004 | 9.00 | 17.00 |
| Azores 45 | 1997–2000 | 5.60 | 7.00 | |
| 2001–2004 | 4.20 | 16.00 | ||
| 2005–2009 | 8.10 | 10.90 | ||
| 2010–2016 | 10.80 | 23.30 | ||
| Slovakia 40 | 1990–1994 | 6.90 | 12.00 | |
| Slovenia 39 , 40 | 1990–1994 | 8.00 | 9.30 | |
| 2000–2002 | 9.30 | 12.00 | ||
| Spain 25 , 29 , 40 | 1990–1994 | 12.40 | 12.80 | |
| 1995–1999 | 10.20 | 13.40 | ||
| 2008–2013 | 12.70 | 17.60 | ||
| 2000–2007 | 10.10 | 14.70 | ||
| Sweden 43 | 1994–1998 | 11.00 | 15.00 | |
| 1999–2003 | 11.00 | 15.00 | ||
| Switzerland 39 , 40 , 41 | 1990–1994 | 9.70 | 16.20 | |
| 2000–2002 | 14.90 | 15.30 | ||
| 2000–2007 | 15.00 | 17.00 | ||
| Ireland 39 | 2000–2002 | 10.00 | 12.30 | |
| UK | England 37 , 40 | 1990–1994 | 7.40 | 7.70 |
| 1995–1999 | 8.00 | 9.10 | ||
| 2000–2007 | 8.00 | 9.90 | ||
| Northern Ireland 39 | 2000–2002 | 9.60 | 12.00 | |
| Scotland 39 , 40 | 2000–2002 | 7.80 | 8.90 | |
| 1990–1994 | 7.00 | 6.80 | ||
| Wales 39 , 40 | 1990–1994 | 8.00 | 7.50 | |
| 2000–2002 | 9.60 | 11.30 | ||
| USA 37 | 1997 | 13.50 | 16.60 | |
| 2004 | 15.00 | 19.00 | ||
Although few publications provided the survival data by stages, women also had a significant survival advantage over men irrespective of stages (Table S2). Localized lung cancer patients had a better prognosis than other groups. As for the histological types, patients with adenocarcinoma experienced better survival than other types both in men and women. The survival advantage among females was the most prominent in adenocarcinoma in selected countries. The lower 5‐year RSRs were observed in the large cell, small cell, and unknown groups except for adenocarcinoma and squamous cell carcinoma (Table S3).
Table S4 compares the age‐specific 5‐year relative or net survival rates of lung cancer in China, 12 , 49 India, 17 Japan, 15 Europe, 50 Canada, 51 and the USA 52 between 1992 and 2016. The 5‐year survival rates decreased with age. The rates of lung cancer patients aged 15–44, followed by those aged 45–54, were higher than other age groups, while the prognosis of patients aged 75 or older was the poorest. In the age group of 15–44, the rates in the USA (2010–2016) were markedly higher than those in other regions. 52 All available results are only shown because some reports did not provide age‐specific survival data or adopted different age groups.
Lung cancer survival in China
Table 3 shows the detailed figures of population‐based overall and age‐standardized 5‐year relative or net survival rates of lung cancer in China. It mainly includes the survival data of lung cancer from the nation, 36 , 53 Fujian Province (Xiamen), 54 , 55 Macao, 56 Shanghai, 12 Taiwan, 32 Guangdong Province (Zhongshan and Sihui), 8 , 57 , 58 Hebei Province (Cixian), 9 Jiangsu Province (Qidong and Jiangyin), 59 , 60 Shandong Province (Linqu and Zhaoyuan), 11 , 49 and Zhejiang Province (Haining and Jiashan). 61
TABLE 3.
Overall and age‐standardized 5‐year relative/net survival rates of lung cancer in some areas of China during 1992–2018.
| Region | Period | Overall rates | Age‐standardized rates | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |||
| China 36 , 53 | 2003–2005 | ‐ | ‐ | ‐ | 16.10 | 15.40 | 17.40 | |
| 2006–2008 | ‐ | ‐ | ‐ | 15.80 | 14.50 | 18.30 | ||
| 2009–2011 | ‐ | ‐ | ‐ | 16.80 | 15.20 | 19.90 | ||
| 2012–2015 | ‐ | ‐ | ‐ | 19.70 | 16.80 | 25.10 | ||
| Fujian | Total 54 | 2012–2014 | 15.33 | 13.44 | 19.94 | 14.98 | 13.36 | 19.33 |
| Xiamen 55 | 2011–2014 | 11.98 | 9.44 | 18.86 | 11.32 | 9.00 | 18.07 | |
| 2015–2018 | 14.17 | 12.15 | 18.70 | 13.83 | 12.21 | 18.55 | ||
| Guangdong | Zhongshan 57 , 58 | 1995–1999 | ‐ | ‐ | ‐ | 7.10 | ‐ | ‐ |
| 2000–2004 | ‐ | ‐ | ‐ | 10.40 | ‐ | ‐ | ||
| 2005–2009 | ‐ | ‐ | ‐ | 12.80 | ‐ | ‐ | ||
| 2010–2013 | ‐ | ‐ | ‐ | 16.80 | ‐ | ‐ | ||
| Sihui 8 | 2007–2009 | 12.22 | 10.55 | 16.26 | ‐ | ‐ | ‐ | |
| Hebei | Cixian 9 | 2000–2002 | 7.23 | 6.96 | 7.73 | ‐ | ‐ | ‐ |
| Jiangsu | Qidong 49 , 60 | 1992–1996 | ‐ | 5.51 | 6.74 | ‐ | ‐ | ‐ |
| 1997–2000 | ‐ | 8.87 | 13.95 | ‐ | ‐ | ‐ | ||
| 2001–2007 | 12.73 | 11.73 | 15.21 | ‐ | ‐ | ‐ | ||
| Jiangyin 59 | 2012–2013 | 27.51 | 27.90 | 31.62 | ‐ | ‐ | ‐ | |
| Macao 56 | 2003–2005 | 21.00 | 20.00 | 23.00 | ||||
| Shandong | Linqu 49 | 1993–1999 | ‐ | 9.00 | 9.40 | ‐ | ‐ | ‐ |
| Zhaoyuan 11 | 2009–2013 | 15.46 | 14.57 | 18.01 | ||||
| Shanghai 12 | 2002–2006 | 20.23 | 20.27 | 22.11 | ‐ | ‐ | ‐ | |
| Zhejiang | Haining and Jiashan 61 | 2003–2006 | 14.20 | 14.10 | 14.60 | 14.80 | ‐ | ‐ |
| 2007–2010 | 13.40 | 11.70 | 18.10 | 14.20 | ‐ | ‐ | ||
| 2011–2014 | 18.10 | 15.20 | 25.00 | 16.40 | ‐ | ‐ | ||
| Taiwan 32 | 2000–2004 | ‐ | ‐ | ‐ | 11.60 | ‐ | ‐ | |
| 2005–2009 | ‐ | ‐ | ‐ | 16.00 | ‐ | ‐ | ||
| 2010–2014 | ‐ | ‐ | ‐ | 20.60 | ‐ | ‐ | ||
Note: ‐ No report or nonavailable in the original articles.
As shown in Table 3, the overall 5‐year relative or net survival rates of lung cancer gradually increased over time. The age‐standardized 5‐year relative or net survival rates in women were higher than those in men, especially in China during 2012–2015. 36 The highest overall 5‐year overall relative or net survival rates were 27.90% and 31.62% in men and women in Jiangyin (Jiangsu Province) during 2012–2013. 59 Since 2000, the overall 5‐year RSR in Cixian (Hebei Province) was the lowest, with only 7.23% in 2000–2002. 9
DISCUSSION
In the current study, we systematically collected and evaluated the survival data of lung cancer from population‐based cancer registration. Major indicators of survival were selected in our study, such as “observed survival rate, relative survival rate, net survival rate, age‐standardized relative survival rate, and age‐standardized net survival rate”. 62 First, we presented the global pattern and trends of lung cancer survival and compared of survival rates of lung cancer by the characteristics of a diagnostic period, region, sex, stage, pathology, and age group. Meanwhile, international comparisons of the age‐standardized and the overall survival rates were performed to have a better understanding of the global pattern and trends. 7 Furthermore, we detailed the survival rates of lung cancer in China.
Over the last decades, the improvements in lung cancer survival have probably been a direct consequence of major health reforms and technological advances, including changes in smoking habits, improvements in medical insurance, the promotion of screening, earlier diagnosis, treatment advances, creation of incentives for clinical research, and better patient management than in previous periods. 33 , 63 , 64 However, these factors varied greatly in different regions, leading to geographical differences in lung cancer survival. In addition, population‐based survival studies may use different methods to collect survival data on cancer cases, which can affect the accuracy and comparability of the results.
Globally, the 5‐year relative or net survival rates in Africa (such as Libya) and South America (such as Chile and Brazil) are much poorer than the countries in Europe and North America during the same period. Nevertheless, the 5‐year survival rates of lung cancer are also diverse across regions in Europe. It has been consistently reported that poorer survival has been detected in cancer patients from socioeconomically disadvantaged groups than in advantaged groups. 65 , 66 Socioeconomic status (SES) is a broad term for the social standing or “class” of an individual or group of people. It is often measured based on the highest attained education, income, and occupation. A pooled analysis of 17 021 cases and 20 885 controls found that, after adjusting for smoking, low SES was associated with increased risk of lung cancer by 84% and 54% in men and women. 67 Cancer diagnosis and treatment practices can vary widely between different regions and healthcare systems, which can affect the survival rates of patients. People with high SES groups may have better access to advanced diagnostic tools or treatments, while others may have limited resources or expertise.
The presence of lung cancer screening and early detection by low‐dose computed tomography (LDCT) and advanced treatments can decrease mortality rates and improve survival outcomes. 68 The National Lung Screening Trial found screening via LDCT is the most effective way to reduce mortality in lung cancer. 69 Some organized and opportunistic lung cancer screenings have been established in many countries and regions, such as China, the USA, and Europe. 68 , 70 , 71 In addition, surgery, radiotherapy, systemic therapy, and the concept of multidisciplinary treatment of lung cancer have improved the outcome of lung cancer patients. 71 Therefore, the survival rate of lung cancer has continuously improved in most countries and regions.
The disparities of sex and age in lung cancer survival were also reviewed in our study. The survival rates of lung cancer in women were higher than in men in most countries and regions, whereas the pattern of squamous cell carcinoma in Japan and South Korea appears contrary. The survival advantage among women was most pronounced in patients diagnosed with adenocarcinoma (Table S4). In 1990, the benefit for lung cancer survival in women over men, irrespective of histological type, was reported in the USA. 72 Since then, several studies of sex differences in lung cancer survival have been reported worldwide. 47 , 73 , 74 , 75 Previous studies indicated that women have a survival advantage within all histological subsets except squamous cell carcinoma. 47 , 73 , 75 Smoking is an independent factor affecting the prognosis of lung cancer, while the disparity in smoking between men and women leads to s disparity in lung survival. 74 , 76 Second, the different distribution of epidermal growth factor receptor (EGFR) mutations between sexes might have contributed to prognostic advantages in lung cancer survival for females. In addition, unequal clinical management between men and women might offer an alternative explanation. A study published on sex equality in health care suggested that superior health awareness and healthcare utilization in women might contribute to the less advanced stage at diagnosis, which could directly manifest itself as improved survival. 77 Sex‐specific distinctions in the survival rate of lung cancer subtypes require more population‐based follow‐up studies. For the age at cancer diagnosis, survival was highest among patients in the 35–44 age group, followed by the 45–54 age group, and lowest for the 75 or older age group. This may be partially due to elderly patients being treated less aggressively because of their vulnerability to comorbidities and various chronic diseases. No frameworks have been established in terms of new targeted therapies for older people, which require special treatment considerations. 78
When comparing survival rates in different countries, periods, populations, and so on, the following points need to be considered. First, it is worth noting estimates used in each original study, such as observed, relative, and net survival rates used in this review. However, descriptive indicators of cancer survival also included cause‐specific survival, and so on. The estimation methods and their interpretations may be completely different and cannot be substituted for each other. Next, close attention to additional comorbidities or variables (such as age, sex, ethnicity, etc.) used in survival estimation in the study is required. For example, as in this review, some studies excluded patients aged under 15 14 , 22 , 25 , 32 , 39 , 40 , 44 , 47 , 50 , 51 , 73 , 79 , 80 , 81 , 82 , 83 , 84 or 18. 20 Furthermore, a large number of studies excluded DCO (death certificate only) cases or autopsy cases, 10 , 17 , 25 , 26 , 30 , 32 , 41 , 46 , 47 , 49 , 51 , 53 , 56 , 57 , 58 , 60 , 61 , 73 , 74 , 79 , 80 , 81 , 85 , 86 , 87 , 88 which would affect outcomes. These might be cautious or particular needs when comparing cancer survival rates between different regions or populations.
In conclusion, in the present study, we summarized 1–5 years observed, relative, and net survival rates of lung cancer worldwide, markedly distinct between different countries or periods in the same regions. The implications of this data are that the region, period, sex, and age might affect the survival rate of lung cancer patients. Therefore, the highest priority to improve survival globally in lung cancer prevention remains the hope that all countries will advance initiatives to reduce smoking, encourage screening, conduct more translational research and application on new technological treatments, and so on.
AUTHOR CONTRIBUTIONS
Yong‐Bing Xiang designed the research and obtained funding. Jing‐Hao Bi and Yong‐Bing Xiang conducted the study. Jing‐Hao Bi and Jia‐Yi Tuo collected publications and abstract data. Jing‐Hao Bi and Yong‐Bing Xiang prepared and wrote the first draft of the manuscript. Jing‐Hao Bi, Jia‐Yi Tuo, Yu‐Xuan Xiao, Dan‐Dan Tang, Xiao‐Hui Zhou, Yu‐Fei Jiang, Xiao‐Wei Ji, Yu‐Ting Tan, Hui‐Yun Yuan, and Yong‐Bing Xiang reviewed and approved the final version of the manuscript; and Yong‐Bing Xiang has primary responsibility for final content.
FUNDING INFORMATION
This work was supported by the National Key Project of Research and Development Program of China (2021YFC2500404, 2021YFC2500405).
CONFLICT OF INTEREST STATEMENT
All authors declare there are no conflicts of interest.
Supporting information
Figure S1. Overall 5‐year relative/net survival rates of lung cancer in selected regions during 1990–2018.
Table S1. Sex‐specific overall 5‐year relative/net survival rates of lung cancer in selected regions during 1990–2018.
Table S2. Sex‐specific and stage‐specific 5‐year relative survival rates of lung cancer in selected regions during 1993–2018.
Table S3. Sex‐specific and histological 5‐year relative survival rates of lung cancer in selected regions during 1993–2018.
Table S4. Age‐specific 5‐year relative/net survival rates of lung cancer in selected regions during 1992–2016.
Bi J‐H, Tuo J‐Y, Xiao Y‐X, Tang D‐D, Zhou X‐H, Jiang Y‐F, et al. Observed and relative survival trends of lung cancer: A systematic review of population‐based cancer registration data. Thorac Cancer. 2024;15(2):142–151. 10.1111/1759-7714.15170
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Hospital Chinese Academy of Medical Science . Chinese guideline for cancer registration. Beijing: People's Medical Publishing House (PMPH); 2016. p. 33–75. [Google Scholar]
- 3. Hakulinen T. Cancer survival corrected for heterogeneity in patient withdrawal. Biometrics. 1982;38(4):933–942. [PubMed] [Google Scholar]
- 4. Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics. 2012;68(1):113–120. [DOI] [PubMed] [Google Scholar]
- 5. Hakulinen T, Seppä K, Lambert PC. Choosing the relative survival method for cancer survival estimation. Eur J Cancer. 2011;47(14):2202–2210. [DOI] [PubMed] [Google Scholar]
- 6. Seppä K, Hakulinen T, Pokhrel A. Choosing the net survival method for cancer survival estimation. Eur J Cancer. 2015;51(9):1123–1129. [DOI] [PubMed] [Google Scholar]
- 7. Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40(15):2307–2316. [DOI] [PubMed] [Google Scholar]
- 8. Li YH, Lu YQ, Ling W, Lin EH, Yao JZ. Survival analysis of patients with malignant tumors in Sihui city between 1987 and 2009. China Cancer. 2017;26(8):596–600. [Google Scholar]
- 9. He YT, Zeng Y, Xu H, Song GH, Tian G, Chen C, et al. Survival rate among cancer patients in Cixian county, 2000‐2002. Chin J Public Health. 2011;27(9):1107–1110. [Google Scholar]
- 10. Yang J, Zhu J, Zhang YH, Chen YS, Ding LL, Kensler TW, et al. Lung cancer in a rural area of China: rapid rise in incidence and poor improvement in survival. Asian Pac J Cancer Prev. 2015;16(16):7295–7302. [DOI] [PubMed] [Google Scholar]
- 11. Zhai YT, Hao JH, Ning WW, Li W, Fu ZT, Jiang ZM, et al. Incidence, mortality and survival rate of lung cancer in Zhaoyuan, Shandong, 2008–2017. 2021;28(25):1125–1130. [Google Scholar]
- 12. Zhang ML, Wu CX, Gong YM, Peng P, Gu K, Shi L, et al. Survival analysis of patients with lung cancer in Shanghai. China Oncol. 2017;27(5):326–333. [Google Scholar]
- 13. Jiang CX, Shen YZ, Zhang ZH, Zhu LJ, Yang J. Analysis of incidence and survival rate of cancer among residents in Haining city from 2003 to 2015. China Cancer. 2018;27(4):267–272. [Google Scholar]
- 14. Lin HT, Liu FC, Wu CY, Kuo CF, Lan WC, Yu HP. Epidemiology and survival outcomes of lung cancer: a population‐based study. Biomed Res Int. 2019;2019:8148156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsukuma H, Ajiki W, Ioka A, Oshima A. Survival of cancer patients diagnosed between 1993 and 1996: a collaborative study of population‐based cancer registries in Japan. Jpn J Clin Oncol. 2006;36(9):602–607. [DOI] [PubMed] [Google Scholar]
- 16. Swaminathan R, Selvakumaran R, Esmy PO, Sampath P, Ferlay J, Jissa V, et al. Cancer pattern and survival in a rural district in South India. Cancer Epidemiol. 2009;33(5):325–331. [DOI] [PubMed] [Google Scholar]
- 17. Yeole BB. Respiratory cancer population‐based survival in Mumbai. India Asian Pac J Cancer Prev. 2005;6(4):449–454. [PubMed] [Google Scholar]
- 18. Stenning‐Persivale K, Savitzky Franco MJ, Cordero‐Morales A, Cruzado‐Burga J, Poquioma E, Nava ED, et al. The mortality‐incidence ratio as an indicator of five‐year cancer survival in metropolitan Lima. Ecancermedicalscience. 2018;12:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillman RO, McClure SE. Steadily improving survival in lung cancer. Clin Lung Cancer. 2014;15(5):331–337. [DOI] [PubMed] [Google Scholar]
- 20. Tannenbaum SL, Zhao W, Koru‐Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kish JK, Yu M, Percy‐Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in surveillance, epidemiology, and end results (SEER) registries. J Natl Cancer Inst Monogr. 2014;49:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deleuran T, Thomsen RW, Nørgaard M, Jacobsen JB, Rasmussen TR, Søgaard M. Comorbidity and survival of Danish lung cancer patients from 2000‐2011: a population‐based cohort study. Clin Epidemiol. 2013;5(Suppl 1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mäkitaro R, Pääkko P, Huhti E, Bloigu R, Kinnula VL. Prospective population‐based study on the survival of patients with lung cancer. Eur Respir J. 2002;19(6):1087–1092. [DOI] [PubMed] [Google Scholar]
- 24. Haberland J, Bertz J, Wolf U, Ziese T, Kurth BM. German cancer statistics 2004. BMC Cancer. 2010;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chirlaque MD, Salmerón D, Galceran J, Ameijide A, Mateos A, Torrella A, et al. Cancer survival in adult patients in Spain. Results from nine population‐based cancer registries. Clin Transl Oncol. 2018;20(2):201–211. [DOI] [PubMed] [Google Scholar]
- 26. Erridge SC, Murray B, Price A, Ironside J, Little F, Mackean M, et al. Improved treatment and survival for lung cancer patients in South‐East Scotland. J Thorac Oncol. 2008;3(5):491–498. [DOI] [PubMed] [Google Scholar]
- 27. Bogos K, Kiss Z, Tamási L, Ostoros G, Müller V, Urbán L, et al. Improvement in lung cancer survival: 6‐year trends of overall survival at hungarian patients diagnosed in 2011‐2016. Pathol Oncol Res. 2021;27:603937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guerreiro T, Forjaz G, Antunes L, Bastos J, Mayer A, Aguiar P, et al. Lung cancer survival and sex‐specific patterns in Portugal: a population‐based analysis. Pulmonology. 2021;18:52. [DOI] [PubMed] [Google Scholar]
- 29. Guevara M, Molinuevo A, Salmerón D, Marcos‐Gragera R, Carulla M, Chirlaque MD, et al. Cancer survival in adults in Spain: a population‐based study of the spanish network of cancer registries (REDECAN). Cancer. 2022;14:2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995‐2009: Analysis of individual data for 25 676 887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet. 2015;385(9972):977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El Mistiri M, Salati M, Marcheselli L, Attia A, Habil S, Alhomri F, et al. Cancer incidence, mortality, and survival in Eastern Libya: updated report from the Benghazi Cancer Registry. Ann Epidemiol. 2015;25(8):564–568. [DOI] [PubMed] [Google Scholar]
- 32. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TML, Myklebust TA, et al. Progress in cancer survival, mortality, and incidence in seven high‐income countries 1995‐2014 (ICBP SURVMARK‐2): a population‐based study. Lancet Oncol. 2019;20(11):1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berrino F, De Angelis R, Sant M, Rosso S, Lasota MB, Coebergh JW, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995‐99: results of the EUROCARE‐4 study. Lancet Oncol. 2007;8(9):773–783. [DOI] [PubMed] [Google Scholar]
- 35. Innos K, Baburin A, Aareleid T. Cancer patient survival in Estonia 1995‐2009: time trends and data quality. Cancer Epidemiol. 2014;38(3):253–258. [DOI] [PubMed] [Google Scholar]
- 36. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003‐15: a pooled analysis of 17 population‐based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. [DOI] [PubMed] [Google Scholar]
- 37. Santucci C, Carioli G, Bertuccio P, Malvezzi M, Pastorino U, Boffetta P, et al. Progress in cancer mortality, incidence, and survival: a global overview. Eur J Cancer Prev. 2020;29(5):367–381. [DOI] [PubMed] [Google Scholar]
- 38. Lim GH, Chow KY, Lee HP. Singapore cancer trends in the last decade. Singapore Med J. 2012;53(1):3–9. quiz 10. [PubMed] [Google Scholar]
- 39. Brenner H, Francisci S, de Angelis R, Marcos‐Gragera R, Verdecchia A, Gatta G, et al. Long‐term survival expectations of cancer patients in Europe in 2000‐2002. Eur J Cancer. 2009;45(6):1028–1041. [DOI] [PubMed] [Google Scholar]
- 40. Sant M, Aareleid T, Berrino F, Lasota MB, Carli PM, Faivre J, et al. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94‐results and commentary. Ann Oncol. 2003;14:v61–v118. [DOI] [PubMed] [Google Scholar]
- 41. Bordoni A, Uhry Z, Antunes L. Trends in net survival lung cancer in six European Latin countries: results from the SUDCAN population‐based study. Eur J Cancer Prev. 2017;26:S70–S76. [DOI] [PubMed] [Google Scholar]
- 42. Innos K, Oselin K, Laisaar T, Aareleid T. Patterns of survival and surgical treatment in lung cancer patients in Estonia by histologic type and stage, 1996‐2016. Acta Oncol. 2019;58(11):1549–1556. [DOI] [PubMed] [Google Scholar]
- 43. Hakulinen T, Engholm G, Gislum M, Storm HH, Klint A, Tryggvadóttir L, et al. Trends in the survival of patients diagnosed with cancers in the respiratory system in the Nordic countries 1964‐2003 followed up to the end of 2006. Acta Oncol. 2010;49(5):608–623. [DOI] [PubMed] [Google Scholar]
- 44. Eberle A, Jansen L, Castro F, Krilaviciute A, Luttmann S, Emrich K, et al. Lung cancer survival in Germany: a population‐based analysis of 132,612 lung cancer patients. Lung Cancer. 2015;90(3):528–533. [DOI] [PubMed] [Google Scholar]
- 45. Forjaz G, Chen HS, Howlader N, Rego R, Rodrigues V, Mariotto AB. Measuring progress against cancer in the Azores, Portugal: incidence, survival, and mortality trends and projections to 2025. Cancer Epidemiol. 2020;69:101810. [DOI] [PubMed] [Google Scholar]
- 46. Cowppli‐Bony A, Uhry Z, Remontet L, Voirin N, Guizard AV, Trétarre B, et al. Survival of solid cancer patients in France, 1989‐2013: a population‐based study. Eur J Cancer Prev. 2017;26(6):461–468. [DOI] [PubMed] [Google Scholar]
- 47. Innos K, Padrik P, Valvere V, Aareleid T. Sex differences in cancer survival in Estonia: a population‐based study. BMC Cancer. 2015;15:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jakobsen E, Rasmussen TR, Green A. Mortality and survival of lung cancer in Denmark: results from the Danish Lung Cancer Group 2000‐2012. Acta Oncol. 2016;55:2–9. [DOI] [PubMed] [Google Scholar]
- 49. Zhu J, Zhang YH, Chen YS, Ding LL, Chen JG. Analysis of survival rate of lung cancer in Qidong city from 2001 to 2007. Chin J Lung Cancer. 2011;14(1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Francisci S, Minicozzi P, Pierannunzio D, Ardanaz E, Eberle A, Grimsrud TK, et al. Survival patterns in lung and pleural cancer in Europe 1999‐2007: results from the EUROCARE‐5 study. Eur J Cancer. 2015;51(15):2242–2253. [DOI] [PubMed] [Google Scholar]
- 51. Ellison LF. Measuring the effect of including multiple cancers in survival analyses using data from the Canadian Cancer Registry. Cancer Epidemiol. 2010;34(5):550–555. [DOI] [PubMed] [Google Scholar]
- 52. Surveillance Research Program, National Cancer Institute . SEER*Explorer: An interactive website for SEER cancer statistics. https://seer.cancer.gov/statistics-network/explorer/. Accessed 27 Sep 2022
- 53. Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, et al. Cancer survival in China, 2003‐2005: a population‐based study. Int J Cancer. 2015;136(8):1921–1930. [DOI] [PubMed] [Google Scholar]
- 54. Zhou Y, Xiang ZS, Ma JY, Lin YT, Chen YP, Jiang HJ, et al. Survival of cancer patients in Fujian, Southeast China: a population‐based cancer registry study. Neoplasma. 2021;68:892–898. [DOI] [PubMed] [Google Scholar]
- 55. Lin YL, Xv LS, Zhang JH, Wu XQ. Evaluation on population‐based survival rate of lung cancer by period analysis, Xiamen city. Prev Med Trib. 2021;27:889–896. [Google Scholar]
- 56. Lei WK, Yu XQ, Lam C, Leong WK. Survival analysis of 2003‐2005 data from the population‐based Cancer Registry in Macao. Asian Pac J Cancer Prev. 2010;11(6):1561–1567. [PubMed] [Google Scholar]
- 57. Wei KR, Liang ZH, Li ZM. Net survival of major cancers in Zhongshan city of Guangdong province from 2003 to 2013. China Cancer. 2020;29(2):103–107. [Google Scholar]
- 58. Wei KR, Liang ZH, Cen HS. Net survival of cancers in Zhongshan city, Guangdong province, 1995~2009. China Cancer. 2016;25(10):747–751. [Google Scholar]
- 59. Li Y, Zhang J, Zhu AP, Liu J. Survival rate of patients newly diagnosed with malignant cancers in Jiangyin city from 2012 to 2013. China Cancer. 2020;29(4):241–245. [Google Scholar]
- 60. Chen JG, Zhu J, Zhang YH. Analysis of Survival rate of main malignant tumors in Qidong city from 1972 to 2000. China. Cancer. 2006;15(9):575–578. [Google Scholar]
- 61. Li HZ, Du LB, Li QL, Jiang CX, Zhun YF, Yang JH, et al. Cancer survival in Haining and Jiashan cancer registry areas of Zhejiang province. China Cancer. 2020;29(1):14–21. [Google Scholar]
- 62. Jiang YF, Li ZY, Ji XW, Shen QM, Tuo JY, Yuan HY, et al. Global pattern and trend of liver cancer survival: a systematic review of population‐based studies. Hepatoma Res. 2020;6:52. [Google Scholar]
- 63. Scagliotti G. Improving survival in lung cancer: commitment of The Lung Ambition Alliance. Am J Manag Care. 2019;25:SP387–SP389. [PubMed] [Google Scholar]
- 64. Shin A, Oh CM, Kim BW, Woo H, Won YJ, Lee JS. Lung cancer epidemiology in Korea. Cancer Res Treat. 2017;49(3):616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dalton SO, Olsen MH, Johansen C, Olsen JH, Andersen KK. Socioeconomic inequality in cancer survival–changes over time. A population‐based study, Denmark, 1987‐2013. Acta Oncol. 2019;58(5):737–744. [DOI] [PubMed] [Google Scholar]
- 66. Baili P, Di Salvo F, Marcos‐Gragera R, Siesling S, Mallone S, Santaquilani M, et al. Age and case mix‐standardised survival for all cancer patients in Europe 1999‐2007: results of EUROCARE‐5, a population‐based study. Eur J Cancer. 2015;51(15):2120–2129. [DOI] [PubMed] [Google Scholar]
- 67. Hovanec J, Siemiatycki J, Conway DI, Olsson A, Stücker I, Guida F, et al. Lung cancer and socioeconomic status in a pooled analysis of case‐control studies. PLoS One. 2018;13(2):e0192999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oudkerk M, Devaraj A, Vliegenthart R, Henzler T, Prosch H, Heussel CP, et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754–e766. [DOI] [PubMed] [Google Scholar]
- 69. Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 70. Yang D, Liu Y, Bai C, Wang X, Powell CA. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett. 2020;468:82–87. [DOI] [PubMed] [Google Scholar]
- 71. Gao S, Li N, Wang S, Zhang F, Wei W, Li N, et al. Lung cancer in People's Republic of China. J Thorac Oncol. 2020;15(10):1567–1576. [DOI] [PubMed] [Google Scholar]
- 72. Ferguson MK, Skosey C, Hoffman PC, Golomb HM. Sex‐associated differences in presentation and survival in patients with lung cancer. J Clin Oncol. 1990;8(8):1402–1407. [DOI] [PubMed] [Google Scholar]
- 73. Kinoshita FL, Ito Y, Morishima T, Miyashiro I, Nakayama T. Sex differences in lung cancer survival: long‐term trends using population‐based cancer registry data in Osaka, Japan. Jpn J Clin Oncol. 2017;47(9):863–869. [DOI] [PubMed] [Google Scholar]
- 74. Sagerup CM, Småstuen M, Johannesen TB, Helland Å, Brustugun OT. Sex‐specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax. 2011;66(4):301–307. [DOI] [PubMed] [Google Scholar]
- 75. Sakurai H, Asamura H, Goya T, Eguchi K, Nakanishi Y, Sawabata N, et al. Survival differences by gender for resected non‐small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese lung cancer registry study. J Thorac Oncol. 2010;5(10):1594–1601. [DOI] [PubMed] [Google Scholar]
- 76. Gallaway MS, Huang B, Chen Q, Tucker TC, McDowell JK, Durbin E, et al. Smoking and smoking cessation among persons with tobacco‐ and non‐tobacco‐associated cancers. J Community Health. 2019;44(3):552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Y, Freemantle N, Nazareth I, Hunt K. Gender differences in survival and the use of primary care prior to diagnosis of three cancers: an analysis of routinely collected UK general practice data. PLoS One. 2014;9(7):e101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. [DOI] [PubMed] [Google Scholar]
- 79. Gedvilaitė V, Danila E, Cicėnas S, Smailytė G. Lung cancer survival in Lithuania:changes by histology, age, and sex from 2003‐2007 to 2008‐2012. Cancer Control. 2019;26(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gondos A, Arndt V, Holleczek B, Stegmaier C, Ziegler H, Brenner H. Cancer survival in Germany and the United States at the beginning of the 21st century: an up‐to‐date comparison by period analysis. Int J Cancer. 2007;121(2):395–400. [DOI] [PubMed] [Google Scholar]
- 81. Chirlaque MD, Salmerón D, Ardanaz E, Galceran J, Martínez R, Marcos‐Gragera R, et al. Cancer survival in Spain: estimate for nine major cancers. Ann Oncol. 2010;21:iii21–iii29. [DOI] [PubMed] [Google Scholar]
- 82. Alawadhi E, Al‐Awadi A, Elbasmi A, Coleman MP, Allemani C. Cancer survival by stage at diagnosis in Kuwait: a population‐based study. J Oncol. 2019;2019:8463195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Afshar N, English DR, Thursfield V, Mitchell PL, Te Marvelde L, Farrugia H, et al. Differences in cancer survival by sex: a population‐based study using cancer registry data. Cancer Causes Control. 2018;29(11):1059–1069. [DOI] [PubMed] [Google Scholar]
- 84. Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, et al. The advantage of women in cancer survival: an analysis of EUROCARE‐4 data. Eur J Cancer. 2009;45(6):1017–1027. [DOI] [PubMed] [Google Scholar]
- 85. Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995‐2007 (the International Cancer Benchmarking Partnership): an analysis of population‐based cancer registry data. Lancet. 2011;377(9760):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Talbck M, Dickman PW. Predicting the survival of cancer patients recently diagnosed in Sweden and an evaluation of predictions published in 2004. Acta Oncol. 2012;51(1):17–27. [DOI] [PubMed] [Google Scholar]
- 87. Brenner H, Stegmaier C, Ziegler H. Long‐term survival of cancer patients in Germany achieved by the beginning of the third millenium. Ann Oncol. 2005;16(6):981–986. [DOI] [PubMed] [Google Scholar]
- 88. Matsuda T, Ajiki W, Marugame T, Ioka A, Tsukuma H, Sobue T. Population‐based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative Study. Jap J Clin Oncol. 2011;41(1):40–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overall 5‐year relative/net survival rates of lung cancer in selected regions during 1990–2018.
Table S1. Sex‐specific overall 5‐year relative/net survival rates of lung cancer in selected regions during 1990–2018.
Table S2. Sex‐specific and stage‐specific 5‐year relative survival rates of lung cancer in selected regions during 1993–2018.
Table S3. Sex‐specific and histological 5‐year relative survival rates of lung cancer in selected regions during 1993–2018.
Table S4. Age‐specific 5‐year relative/net survival rates of lung cancer in selected regions during 1992–2016.


