Abstract
Parasitic flatworms cause various clinical and veterinary infections that impart a huge burden worldwide. The most clinically impactful infection is schistosomiasis, a neglected tropical disease caused by parasitic blood flukes. Schistosomiasis is treated with praziquantel (PZQ), an old drug introduced over 40 years ago. New drugs are urgently needed, as while PZQ is broadly effective it suffers from several limitations including poor efficacy against juvenile worms, which may prevent it from being completely curative. An old compound that retains efficacy against juvenile worms is the benzodiazepine meclonazepam (MCLZ). However, host side effects caused by benzodiazepines preclude development of MCLZ as a drug and MCLZ lacks an identified parasite target to catalyze rational drug design for engineering out human host activity. Here, we identify a transient receptor potential ion channel of the melastatin subfamily, named TRPMMCLZ, as a parasite target of MCLZ. MCLZ potently activates Schistosoma mansoni TRPMMCLZ through engagement of a binding pocket within the voltage-sensor-like domain of the ion channel to cause worm paralysis, tissue depolarization, and surface damage. TRPMMCLZ reproduces all known features of MCLZ action on schistosomes, including a lower activity versus Schistosoma japonicum, which is explained by a polymorphism within this voltage-sensor-like domain–binding pocket. TRPMMCLZ is distinct from the TRP channel targeted by PZQ (TRPMPZQ), with both anthelmintic chemotypes targeting unique parasite TRPM paralogs. This advances TRPMMCLZ as a novel druggable target that could circumvent any target-based resistance emerging in response to current mass drug administration campaigns centered on PZQ.
Keywords: Ca2+ signaling, ion channel, TRP channel, parasite, flatworm
Neglected tropical diseases caused by parasitic flatworms exert a considerable healthcare burden. For example, infection with worms from the genus Schistosoma causes schistosomiasis (Bilharzia), a disease that afflicts over 200 million people worldwide (1). Mature, adult blood flukes residing within the vasculature lay eggs that lodge in host tissues and trigger a local inflammatory reaction and formation of focal granulomas. Chronic infections are associated with fibrosis and obstructive, hypertensive pathologies in periportal and gastrointestinal tissues (Schistosoma mansoni, Schistosoma japonicum) or genitourinary disease (Schistosoma haematobium). The resulting anemia, undernutrition, and the heightened risk for other comorbidities places a considerable disease burden, especially on children, in countries where this infection is endemic.
The current clinical therapy for schistosomiasis, and the majority of other infections caused by parasitic flatworms, is the drug praziquantel (PZQ). Administered as the racemate ((±)-PZQ), the active enantiomer (R)-PZQ paralyzes worms and damages their surface, aiding immunological clearance from the body (2, 3, 4). (±)-PZQ is a cheap, safe, effective therapy (60–90% cure rates at clinical dosing (2, 5)). However, there are several features of (±)-PZQ that could be improved. These include the high clinical dosage and the bitter taste of the (S)-PZQ enantiomer, drawbacks being targeted through development of an enantiopure pediatric (R)-PZQ formulation (6). Additionally, PZQ is poorly effective against immature worms (7), which may lead to noncurative outcomes after single-dose treatment. Therefore, there is a need to develop agents that show efficacy against both juvenile and adult worms.
One intriguing anthelmintic is the benzodiazepine (S)-meclonazepam ((S)-MCLZ; 3-methylclonazepam, Ro11-3128). (S)-MCLZ has been the subject of recurring interest, over a period of 45 years since the antischistosomal efficacy of this agent was first recognized (8). Like PZQ, (S)-MCLZ triggers a rapid (<1 min), spastic paralysis of adult S. mansoni worms (9, 10) and pronounced damage to the worm tegument (11). However, unlike PZQ, (S)-MCLZ displays efficacy against juvenile worms (8, 12). While immature worms rapidly contract on exposure to either (S)-MCLZ or (±)-PZQ, juvenile worms fail to recover from exposure to (S)-MCLZ (12). This is an attractive feature as in vivo treatment will more likely be curative, eliminating worms at all stages of maturation (8, 13). Indeed, in a preliminary clinical evaluation (14), (S)-MCLZ cured S. mansoni and S. haematobium infections, although the therapeutic window associated with treatment was unacceptably poor. This is because (S)-MCLZ retains host central nervous system (CNS) activity. Subjects administered (S)-MCLZ exhibited dose-dependent drowsiness, dizziness, slurred speech, ataxia, reduced mental alertness, muscle weakness, hypotension, and bradycardia (14), consistent with the known sedative and psychomotor depressive action of benzodiazepines. These dangerous side effects prevent clinical development of (S)-MCLZ, although there have been various attempts over the years to circumvent this challenge either by blocking host CNS activity (coadministration of an antagonist (15)) or via efforts to engineer out host activity by identifying nonsedative analogues that retain schistocidal activity (16, 17)).
The latter approach would be catalyzed by identification of the parasite target(s) of (S)-MCLZ, which has remained an orphan drug for over 4 decades (8). Notably, GABAA channels that mediate CNS effects of benzodiazepines in humans (18, 19) are absent in parasitic trematodes (17). Identification of the target of MCLZ within the parasite would then enable a rational drug development approach to maximize parasite versus host selectivity.
Here, we report the discovery of a parasitic flatworm target for (S)-MCLZ: a transient receptor potential ion channel of the melastatin family.
Results
Addition of (R)-PZQ (1 μM) or (S)-MCLZ (1 μM), but not vehicle alone, to adult S. mansoni worms effected a rapid, spastic paralysis (Fig. 1A). Inhibition of motility was concentration dependent (IC50 = 0.25 ± 0.07 μM for (R)-PZQ, and IC50 = 1.54 ± 0.09 μM for (S)-MCLZ; Fig. 1B), consistent with prior reports (10, 20, 21). Both drugs (10 μM) caused a sustained depolarization (Fig. 1C), resolved by the loss of muscle layer potentials when individual worms were impaled in current clamp mode (11). In control worms, the trans-surface electrical potential was −22.7 ± 1.6 mV (Fig. 1D). However, this potential difference was lost after incubation with (±)-PZQ (−3.9 ± 0.6 mV) or (S)-MCLZ (−3.6 ± 0.4 mV). Damage to the worm tegument was also seen following treatment with either drug (Fig. 1E), quantified by measuring the permeability of a fluorescent marker (Fig. 1F). This triad of phenotypes, worm paralysis, depolarization, and surface damage, are cardinal features of PZQ and MCLZ action seen in various parasitic flatworms (3, 4).
Figure 1.
Effects of (±)-PZQ and (S)-MCLZ on schistosome worms.A, images of adult, male schistosome worms in the absence of drug (top) or 1 min after addition of either (R)-PZQ (red, 1 μM) or (S)-MCLZ (blue, 1 μM). Structures of (R)-PZQ and (S)-MCLZ are shown. The scale bar represents 500 μm. B, concentration–response relationships for (R)-PZQ (red) and (S)-MCLZ (blue) detailing effect on worm motility, normalized to control, shown as mean ± SD. C, representative traces of electrical potentials registered upon penetration of the dorsal surface of S. mansoni male worms in either control conditions (black, 0.1% dimethyl sulfoxide) or in the presence of (R)-PZQ or (S)-MCLZ (10 μM). D, electrical potentials (mean ± SEM) in the indicated conditions (n ≥ 6 individual worms). ∗∗∗p < 0.001 analyzed using Tukey’s statistical test. E, fluorescence images of control worms or worms treated for 17 h with (R)-PZQ (10 μM) or (S)-MCLZ (10 μM). The scale bar represents 100 μm. F, corrected total cell fluorescence (CTCF) values for control and drug-treated worms. Individual values are shown, as well as population mean ± SD, from three independent regions captured from three different worms; ∗p < 0.05; ∗∗∗p < 0.01).
The gross similarity between the phenotypes observed with (R)-PZQ and (S)-MCLZ, as well as a previously noted resemblance between the structures of (R)-PZQ and (S)-MCLZ (10), prompted us to screen (S)-MCLZ against different schistosome TRPM family members (22). This is because one member of the TRPM family (TRPMPZQ) was recently identified as a target of PZQ (20). PZQ activates TRPMPZQ via engagement of a ligand-binding pocket mapped at the base of the transmembrane voltage-sensor-like domain (VSLD) of the ion channel (23).
(S)-MCLZ did not activate Sm.TRPMPZQ (Fig. 2A) but did activate a different TRPM channel (Smp_333650, (24)) to cause a concentration-dependent elevation in cytoplasmic Ca2+ (Fig. 2B). The TRPM channel activated by (S)-MCLZ was named TRPM-meclonazepam (TRPMMCLZ) to distinguish it from TRPMPZQ but preserve our naming terminology for these orphan parasite ion channels. (±)-PZQ, an activator of Sm.TRPMPZQ (Fig. 2C), did not activate Sm.TRPMMCLZ (Fig. 2D). (±)-PZQ activated Sm.TRPMPZQ with an EC50 = 0.51 ± 0.04 μM (Fig. 2E), and (S)-MCLZ activated Sm.TRPMMCLZ with an EC50 = 1.1 ± 0.14 μM (Fig. 2F). The change in fluorescence signal resolved by saturating concentrations of either agonist was similar (Fig. 2, B and C). No response to either drug was seen in untransfected HEK cells (“control,” Fig. 2, E and F). Similar results were obtained by confocal Ca2+ imaging, where (S)-MCLZ caused sustained Ca2+ transients in cells expressing Sm.TRPMMCLZ but not untransfected cells (Fig. S1). Finally, both drugs were screened as antagonists at either channel. Addition of high concentrations of (S)-MCLZ (>30 μM) partially attenuated PZQ activation of Sm.TRPMPZQ (Fig. 2G). In contrast, (±)-PZQ failed to inhibit (S)-MCLZ activation of Sm.TRPMMCLZ (Fig. 2H). In sum, (±)-PZQ and (S)-MCLZ act as potent activators of distinct schistosome TRPM channels.
Figure 2.
Activity of PZQ and (S)-MCLZ at distinct TRPM channels.A–D, traces show fluo-4 fluorescence in HEK293 cells expressing (A and C) Sm.TRPMPZQ or (B and D) Sm.TRPMMCLZ monitored prior and after addition (∼20 s) of increasing concentrations of (±)-PZQ (red) or (S)-MCLZ (blue). Drug concentrations span 1 nM to 100 μM. E and F, concentration–response curves for (E) (±)-PZQ or (F) (S)-MCLZ in untransfected HEK293 cells (filled gray circles) or HEK293 cells expressing either Sm.TRPMPZQ (circles) or Sm.TRPMMCLZ (squares). G, competition experiment of (S)-MCLZ versus (±)-PZQ (0.6 μM) at Sm.TRPMPZQ. H, similar competition experiment using (±)-PZQ versus (S)-MCLZ (1 μM) in cells expressing Sm.TRPMMCLZ. Concentration–response curves are depicted as mean ± SEM of biological triplicates performed in technical duplicate. RFU, raw fluorescence units.
Characteristics of the response of Sm.TRPMMCLZ to (S)-MCLZ were then examined. The (S)-MCLZ-evoked Ca2+ signal depended on Ca2+ entry across the plasma membrane, as chelation of extracellular Ca2+ with EGTA (1 mM) abolished the ability of (S)-MCLZ to elevate cytoplasmic Ca2+ (Fig. 3A). Addition of metal salts to the extracellular medium also inhibited (S)-MCLZ action, with the rank order of blockade being La3+ (IC50 = 407 ± 49 μM) > Ba2+ (IC50 = 1.7 ± 0.27 mM) > Mg2+ (Fig. 3B).
Figure 3.
Pharmacological profiling of Sm.TRPMMCLZ.A, (S)-MCLZ-evoked Ca2+ signals depend on Ca2+ entry. Response to (S)-MCLZ (100 μM) in HEK293 cells expressing Sm.TRPMMCLZ in normal HBSS (blue) or Ca2+-free HBSS (gray, HBSS supplemented with 1 mM EGTA). B, effect of divalent cations on Sm.TRPMMCLZ activity. Concentration–response curves were obtained by treating cells with (S)-MCLZ (1 μM) and increasing concentrations of LaCl3, BaCl2, or MgCl2. C, pharmacological signature of Sm.TRPMMCLZ. Effects of various ion channel antagonists (left, 100 μM) on the peak magnitude of (S)-MCLZ (1 μM) signals at Sm.TRPMMCLZ, or peak amplitude of response to various agonists (right, 10 μM). Data show individual data points (mean ± SD) from a total of three independent transfections. D, effect of different benzodiazepines on acute contraction of S. mansoni male worms. Compounds were incubated for 20 min at a 3 μM dose. The scale bar represents 0.5 cm. E, concentration–response curves profiling various benzodiazepines at Sm.TRPMMCLZ. All concentration–response curves are depicted as mean ± SEM of biological triplicates performed in technical duplicate. 5-HT, 5-hydroxytryptamine; AITC, allyl isothiocyanate.
Various compounds were screened for effects on Sm.TRPMMCLZ. Preincubation (5 min) with various mammalian voltage-operated calcium channel (Cav) blockers (nifedipine, nicardipine, methoxyverapamil) and a benzodiazepine antagonist of human GABAA receptors, flumazenil (all at 100 μM), weakly antagonized (S)-MCLZ (1 μM) action (Fig. 3C). In terms of activators, several vertebrate TRP channel ligands (menthol, allyl isothiocyanate, icilin, and capsaicin) and various neuroactive molecules (5-hydroxytryptamine, dopamine, carbachol, and GABA, all at 10 μM) were inactive at Sm.TRPMMCLZ. However, the vertebrate voltage-operated Ca2+ channel (Cav channel) ligands nimodipine and FPL-64176 (both at 10 μM) activated Sm.TRPMMCLZ (Fig. 3C) but not Sm.TRPMPZQ (Fig. S2). FPL-64176 was previously proposed as a schistosome voltage-operated Ca2+ channel agonist (25).
Given the activity of (S)-MCLZ at Sm.TRPMMCLZ, other benzodiazepine ligands were profiled (Fig. 3D). Clonazepam, which caused acute worm paralysis (Fig. 3D, (10, 21)), was an agonist of Sm.TRPMMCLZ (EC50 = 2.1 ± 0.2 μM, Fig. 3E). Similar activity was seen in confocal imaging experiments (Fig. S1). However, benzodiazepines that did not evoke worm contraction (diazepam, flurazepam, flunitrazepam) and N-Me-(S)-MCLZ (Fig. 3D, (10, 17)) were not Sm.TRPMMCLZ agonists (Fig. 3E).
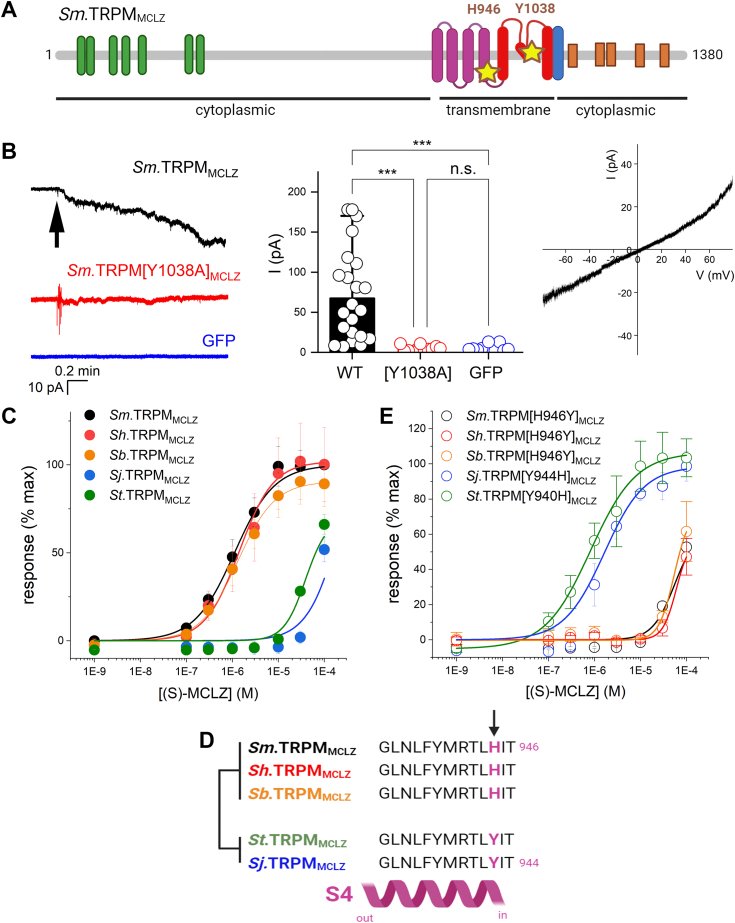
TRPMMCLZ is a member of the TRP melastatin (TRPM) subfamily of TRP ion channels, a well-represented family within flatworm genomes (26). TRPMMCLZ is present in genomes from other parasitic flatworms (Fig. S3). Sm.TRPMMCLZ is encoded within three exons, with the full-length protein displaying an architecture characteristic of TRPM channels (Fig. 4A). This ion channel possesses a long NH2-terminal cytoplasmic domain containing multiple “ankyrin-like” repeats, followed by six predicted transmembrane domains. The first four transmembrane helices (S1–S4) form the VSLD, followed by an ion channel–forming domain, with a pore-forming reentry loop between S5 and S6. A conserved juxtamembrane TRP helix precedes the cytoplasmic COOH terminal domain. TRPMMCLZ has previously been classified as a TRPM7-like channel (22, 27), although overall sequence similarity is low (∼23% with human TRPM7). Instead, the properties of flatworm TRPM channels manifest as a mélange of properties displayed by the TRPM channels of terrestrial vertebrates, which radiated independently, necessitating individual characterization of each parasite channel.
Figure 4.
Functional analysis of Sm.TRPMMCLZ.A, domain organization of Sm.TRPMMCLZ. Schematic shows the NH2-terminal region containing multiple ankyrin-like repeat domains (green), six transmembrane spanning helices (S1–S6) comprising the voltage-sensor-like domain (VSLD, purple) and pore-forming domain (red), the TRP domain (blue), as well as several cytoplasmic helices (orange) within the COOH-terminal domain. The locations of the pore mutant Sm.TRPM[Y1038A]MCLZ and VSLD-binding pocket mutant Sm.TRPM[H946Y]MCLZ are highlighted (star). B, left, Representative cell-attached current traces from HEK293 cells transiently transfected with Sm.TRPMMCLZ and GFP, Sm.TRPMMCLZ [Y1038A] and GFP, or GFP alone. Holding voltage −60 mV. Bath and pipette solutions: HBSS with 20 mM HEPES. Arrow indicates addition of 10 μM (S)-MCLZ to the bath. Middle, cumulative dataset. Tukey’s statistical test was used to compare changes in steady-state currents evoked by 10 μM (S)-MCLZ. ∗∗∗p < 0.001. Right, current–voltage (I-V) relationship obtained from a voltage ramp of 10 mV/s recorded in cell-attached mode from a HEK293 cell expressing Sm.TRPMMCLZ and GFP exposed to 10 μM (S)-MCLZ. C, concentration–response curves evaluating sensitivity of S. mansoni TRPMMCLZ (Sm.TRPMMCLZ, black), S. haematobium TRPMMCLZ (Sh.TRPMMCLZ, red), S. japonicum (Sj.TRPMMCLZ, blue), S. bovis (Sb.TRPMMCLZ, orange), and S. turkestanicum (St.TRPMMCLZ, green) to (S)-MCLZ. D, schematic showing alignment of the distal part of the S4 helix from various schistosome TRPMMCLZ orthologs. The variant residue, conserved between the low- and high-sensitivity groups is shown in purple. E, concentration–response curves for reciprocal point mutants in TRPMMCLZ orthologs in response to (S)-MCLZ.
Electrophysiological characterization of Sm.TRPMMCLZ was therefore performed. Cell-attached currents were measured in HEK293 cells expressing either GFP alone or GFP and Sm.TRPMMCLZ (Fig. 4B). In cells expressing GFP alone, addition of (S)-MCLZ (10 μM) did not evoke currents (0/10 cells examined). In contrast, in HEK cells expressing Sm.TRPMMCLZ and GFP, addition of (S)-MCLZ (10 μM) evoked an inward current in the majority of cells tested (17/21 cells, holding potential of 60 mV). However, responses to (S)-MCLZ (10 μM) were strongly attenuated in HEK293 cells expressing a Sm.TRPMMCLZ point mutant (Sm.TRPMMCLZ [Y1038A]) designed proximal to the pore filter of the channel (Fig. 4, A and B). The current–voltage (I-V) relationship of Sm.TRPMMCLZ in response to (S)-MCLZ was linear with a measured reversal potential of ∼0 mV (Fig. 4B).
Three species of schistosomes are responsible for the majority of human disease worldwide—S. mansoni, S. haematobium, and S. japonicum. Therefore, responses to (S)-MCLZ were also profiled at S. haematobium TRPMMCLZ (Sh.TRPMMCLZ) and S. japonicum (Sj.TRPMMCLZ). One reason for doing this is that (S)-MCLZ is known to be poorly active against S. japonicum worms (8, 9, 12). Consistent with this known observation, (S)-MCLZ proved to be a poor activator of Sj.TRPMMCLZ (EC50>50 μM) compared with Sh.TRPMMCLZ (EC50 = 1.4 ± 0.4 μM) or Sm.TRPMMCLZ (EC50 = 1.1 ± 0.1 μM), which displayed a similar sensitivity toward (S)-MCLZ (Fig. 4C). This disparity in sensitivity was also observed at other schistosome TRPMMCLZ orthologs. Schistosoma turkestanicum TRPMMCLZ (St.TRPMMCLZ) required much higher concentrations of (S)-MCLZ (EC50>50 μM) compared with Schistosoma bovis TRPMMCLZ (Sb.TRPMMCLZ), which exhibited good sensitivity toward (S)-MCLZ (EC50 = 1.3 ± 0.3 μM, Fig. 4C).
To investigate the basis for this differential sensitivity, the sequences of each of the schistosome TRPMMCLZ channel were scrutinized focusing on 23 residues previously established to line the (R)-PZQ-binding pocket in the VSLD of TRPMPZQ (23). One residue was found to be different between the two groups of TRPMMCLZ channel sensitivity but conserved within each group. This residue was at the cytoplasmic base of the S4 helix (Fig. 4D) and was represented by a histidine residue in the higher (S)-MCLZ sensitivity group (H946 in Sm.TRPMMCLZ) and by a tyrosine residue in the lower sensitivity clade (Y944 in Sj.TRPMMCLZ). Reciprocal mutations of this residue switched the sensitivity profile of TRPMMCLZ (Fig. 4E). Sm.TRPM[H946Y]MCLZ resulted in a decreased sensitivity to (S)-MCLZ (EC50>50 μM), and Sj.TRPM[Y944H]MCLZ displayed much higher sensitivity to (S)-MCLZ (EC50 = 1.6 ± 0.3 μM). Similar mutations in the other TRPMMCLZ orthologs replicated this same pattern: St.TRPM[Y940H]MCLZ displayed increased sensitivity to (S)-MCLZ (EC50 = 0.8 ± 0.2 μM), whereas Sh.TRPM[H946Y] and Sb.TRPM[H946Y] resulted in a lower sensitivity of response (Fig. 4E). These data demonstrate that (S)-MCLZ sensitivity of the wildtype channels is regulated by the identity of this residue at this S4 position in the VSLD.
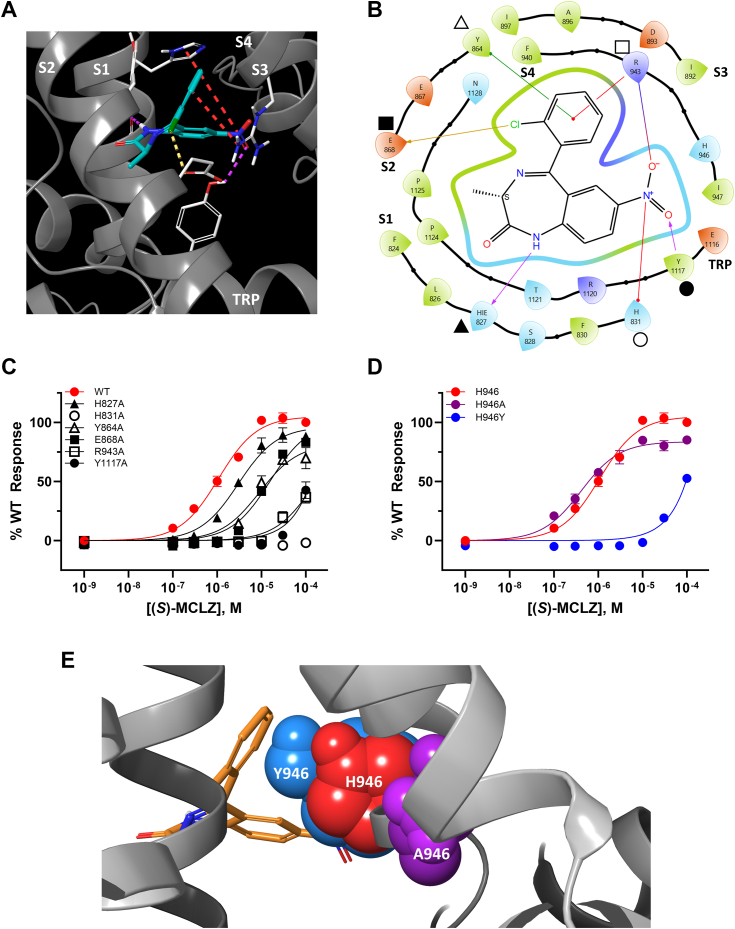
To investigate this further, a binding pose of (S)-MCLZ in the VSLD of Sm.TRPMMCLZ was determined from a combination of modeling approaches (Fig. S4). (S)-MCLZ was predicted to interact with the S1 helix, S2 helix, S4 helix, and the TRP helix of TRPMMCLZ (Fig. 5, A and B), with the frequency of engagement of particular residues supported by molecular dynamics (Fig. S5).
Figure 5.
Interactions of (S)-MCLZ with Sm.TRPMMCLZ.A, in silico binding pose of (S)-MCLZ (teal) in the VSLD of Sm.TRPMMCLZ. (S)-MCLZ interacts with the S1 helix (H831 and the backbone of H827), S2 (E868), S3 (R943), and the TRP helix (Y1117) of Sm.TRPMMCLZ. Key interactions are shown as follows: hydrogen bonds, pink dashed lines; halogen bonds, yellow dashed lines; salt bridges and cation–π interactions, red dashed lines. B, two-dimensional depiction of the binding pose from A. The residues denoted by various shapes correspond to binding pocket mutants described in C. HIE reflects a histidine residue protonated on the epsilon nitrogen of the imidazole ring. C, concentration–response curves for (S)-MCLZ activation of Sm.TRPMMCLZ (solid red circles), Sm.TRPM[H827A]MCLZ (solid triangles), Sm.TRPM[H831A]MCLZ (open circles), Sm.TRPM[Y864A]MCLZ (open triangles), Sm.TRPM[E868A]MCLZ (solid squares), Sm.TRPM[R943A]MCLZ (opensquares), and Sm.TRPM[Y1117A]MCLZ (solid black circles). These symbols are mapped to the corresponding mutated residue in B. D, concentration–response curves for (S)-MCLZ activation of Sm.TRPMMCLZ (red circles) compared with Sm.TRPM[H946A]MCLZ (purple circles) and Sm.TRPM[H946Y]MCLZ (blue circles). E, space-filling overlay of (S)-MCLZ in the VSLD of Sm.TRPMMCLZ depicting the size difference WT channel (H946, red) compared with H946Y (blue) and A946Y (purple). All concentration–response curves in this figure are depicted as mean ± SEM of biological triplicates performed in technical duplicate.
On the S1 helix, the NH of the benzodiazepine was predicted to hydrogen bond to the backbone carbonyl of H827 and a salt bridge was predicted to exist between the nitro group and H831. On S4, a cation–π interaction between R943 and the chloroarene and a hydrogen bond between R943 and the nitro group were predicted. A hydrogen bond between Y1117 of the TRP helix and the aromatic nitro group was also predicted as a key interaction. Other ancillary interactions were observed between (S)-MCLZ and the S2 helix: a halogen bond between E868 and the aryl chloride and π–π stacking between Y864 and the chloroarene.
This in silico binding pose was interrogated experimentally by studying Sm.TRPMMCLZ mutants (Fig. 5C). Consistent with the in silico pose, Sm.TRPM[H831A]MCLZ, Sm.TRPM[R943A]MCLZ, and Sm.TRPM[Y1117A]MCLZ showed little activation by (S)-MCLZ. Sm.TRPM[H827A]MCLZ resembled the wildtype channel, consistent with the ligand interacting with the helix backbone rather than the histidine side chain. A backbone interaction is further supported through from structure-activity relationship data (Fig. 3, D and E) in that benzodiazepines possessing an alkylated nitrogen did not activate Sm.TRPMMCLZ. Mutating the S2 residues (Sm.TRPM[E868A]MCLZ and Sm.TRPM[Y864A]MCLZ) had much less of an effect (Fig. 5C), consistent with the weaker predicted interactions. Dose–response curves for alanine mutants of all residues predicted to be within 5 Å of (S)-MCLZ in the VSLD are presented in Fig. S6.
What about the impact of the variant S4 residue observed between Sm.TRPMMCLZ and Sj.TRPMMCLZ? One possibility is that the H-to-Y residue change abolishes a key histidine–ligand interaction. No interaction with this residue was predicted (Fig. 5B). Consistent with this, the alanine mutant (Sm.TRPM[H946A]MCLZ) displayed slightly increased potency toward (S)-MCLZ rather than any loss of activity (Fig. 5D). This suggested that the difference in (S)-MCLZ activity is not due to the loss of a key interaction between the histidine residue and (S)-MCLZ. Rather, steric considerations are key. The increase in the size of the tyrosine side chain compared with histidine interferes with (S)-MCLZ occupancy of the VSLD-binding pocket. A space-filling model suggests that the tyrosine residue has a greater steric clash with (S)-MCLZ than histidine, and alanine clashes the least (Fig. 5E). The potency of (S)-MCLZ at TRPMMCLZ was therefore inversely correlated with the size of this S4 residue, providing a molecular explanation for the differential activity of (S)-MCLZ against different schistosome species.
Discussion
Here, we identified a schistosome TRPM channel activated by (S)-MCLZ. The properties of this parasite ion channel (TRPMMCLZ) match the known features of (S)-MCLZ action on schistosome worms.
First, the phenotypic features of (S)-MCLZ action on worms were recapitulated by TRPMMCLZ. The overall sensitivity of TRPMMCLZ to (S)-MCLZ (EC50∼1 μM, Fig. 1F) matched the sensitivity of schistosome worms to (S)-MCLZ (IC50 in range of 2–3 μM, (10, 21)). The kinetics of the (S)-MCLZ response at TRPMMCLZ matched the rapid worm contraction evoked by (S)-MCLZ, and the nondesensitizing nature of the TRPMMCLZ response resembled the long-lasting spastic paralysis triggered by (S)-MCLZ. Chelation of extracellular Ca2+ blocked (S)-MCLZ action on worms as well as (S)-MCLZ-mediated cytoplasmic Ca2+ elevations mediated by TRPMMCLZ (9, 10).
Second, the pharmacological profile of TRPMMCLZ was also consistent with the action of (S)-MCLZ on worms. The partial inhibition of Ca2+ permeability through TRPMMCLZ by Mg2+ (Fig. 3B) was consistent with the phasic nature of worm muscle contraction by (S)-MCLZ in the presence of high Mg2+ (9). The Cav channel blockers nicardipine and nifedipine weakly attenuated TRPMMCLZ-mediated Ca2+ signals (Fig. 3C) and (S)-MCLZ-evoked contraction (10, 12). However, most telling was the congruence in benzodiazepine structure-activity relationship. Benzodiazepines that did not cause S. mansoni contraction did not activate Sm.TRPMMCLZ (Fig. 3, D and E). However, a benzodiazepine, clonazepam, that caused S. mansoni contraction was a Sm.TRPMMCLZ agonist (Fig. 3, D and E).
Third, (S)-MCLZ was a poor activator of Sj.TRPMMCLZ, compared with either Sm.TRPMMCLZ or Sh.TRPMMCLZ (Fig. 4C). This is consistent with long-standing observations that (S)-MCLZ is poorly effective against S. japonicum ex vivo and in vivo (8, 9, 12). Our data suggest that the sensitivity of schistosome TRPMMCLZ falls into “high” and “low” sensitivity groups determined by the identity of a specific S4 helix residue. The identity of this residue correlates with the evolutionary relationship of different schistosome species within the generalized “African” or “Asian” pedigree (28). This S4 residue abuts the (S)-MCLZ-binding pose causing steric interference with the optimal orientation of (S)-MCLZ within the TRPMMCLZ VSLD ligand-binding pocket. It will also be important to test whether other schistosome TRPM channels, which have yet to be successfully heterologously expressed, show sensitivity toward (S)-MCLZ. (S)-MCLZ did not, however, activate Sm.TRPMPZQ (Fig. 2A).
Similarities in the responses of schistosomes to (R)-PZQ and (S)-MCLZ have long been noted, encompassing phenotypes associated with their action (long-lasting paralysis, sustained tissue depolarization, tegumental damage) and also conformational similarity between the structures of both drugs (10). Because of these commonalities, it has previously been speculated that these compounds may share a similar mechanism of action. Our discovery of (S)-MCLZ action at TRPMMCLZ validates this proposal, as both anthelmintic agents are now shown to target a TRPM channel, albeit distinct TRPM family members. (R)-PZQ activates TRPMPZQ, whereas (S)-MCLZ activates TRPMMCLZ (Fig. 2). Elegant competition experiments performed by Pica-Mattoccia over 15 years ago presciently predicted this eventuality (12). These authors demonstrated that excess PZQ did not block MCLZ action on juvenile S. mansoni worms and reciprocally excess MCLZ did not block PZQ action on S. japonicum worms (12). As correctly surmised from these worm competition assays, PZQ and MCLZ were unlikely to share the same receptors, which is consistent with the demonstration here of the unique selectivity of (R)-PZQ toward TRPMPZQ and (S)-MCLZ for TRPMMCLZ.
Engagement of a TRP channel by benzodiazepines is noteworthy—to the best of our knowledge—without precedent in the vertebrate literature. This again highlights the distinct pharmacological pedigree of schistosome TRP channels compared with their mammalian counterparts (27, 29). Validation of (S)-MCLZ action at TRPMMCLZ now enables target-based screening efforts (30) to identify other types of channel activator, exemplified by different vertebrate Cav channel ligands (Fig. 3C), which could potentially be modified to derive potent TRPMMCLZ activators. If these or other chemotypes are discovered, it will be important to evaluate whether they are effective against juvenile worms like (S)-MCLZ. Specifically, is activity against immature schistosomes an intrinsic property of this specific ion channel target (TRPMMCLZ) or does activity against juveniles correlate with worm exposure as defined by the pharmacokinetic attributes of different anthelmintics?
In sum, target identification of TRPMMCLZ now catalyzes drug design efforts to identify nonsedative benzodiazepines or other chemotypes active at TRPMMCLZ as potential anthelmintic leads. This discovery will hopefully realize new opportunities to combat the worldwide burden of schistosomiasis.
Experimental procedures
Reagents
Chemical reagents were from Sigma, with the exception of (S)-MCLZ, other benzodiazepines, and FPL-64176 (Cayman Chemical). N-Me-(S)-MCLZ was synthesized as reported (17). Cell culture reagents and the dye Image-IT DEAD Green Viability Stain were from Invitrogen. Lipofectamine 2000 was from Thermo Fisher.
Adult schistosome assays
Adult schistosomes were recovered by dissection of the mesenteric vasculature in female Swiss Webster mice previously infected with S. mansoni cercariae (NMRI strain) by the Schistosomiasis Resource Center at the Biomedical Research Institute. Animal experiments followed ethical regulations approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Harvested schistosomes were washed in DMEM high-glucose medium supplemented with HEPES (25 mM), pyruvate, and 5% heat-inactivated fetal bovine serum (FBS) (Gibco) and penicillin-streptomycin (100 units/ml) and incubated overnight (37 °C/5% CO2) in vented petri dishes (100 × 25 mm). For movement analyses, assays were performed using three male worms per well in a six-well dish. Video recordings were captured using a Zeiss Discovery v20 stereomicroscope with a QiCAM 12-bit cooled color CCD camera controlled by Image-Pro imaging software (v. 11). Recordings (60 s) of worm motility (1 image every 4 s) before and after addition of various drugs were analyzed as described (23). For analysis of tegument permeability, adult male worms were treated with either (±)-PZQ or (S)-MCLZ (both at 10 μM) for 17 h. The vital dye Image-IT DEAD Green Viability Stain (Invitrogen) was added (final concentration, 1 μM) and incubated for 30 min. The medium was removed, and worms were fixed in 4% paraformaldehyde and washed 3× with PBS. Worms were mounted in 80% glycerol and imaged on a Nikon Eclipse 80i. Composite images were generated using FIJI (Version 2.14.0/1.54f). Fluorescence intensity was analyzed using FIJI by measuring the area, integrated density, and mean gray value from three nonoverlapping images of each male worm. Fluorescence intensity was determined using corrected total cell fluorescence (CTCF) = integrated density – (area of selected cell x mean fluorescence of background readings, (31)). For each image, a background area was used to normalize against autofluorescence. Statistical analysis was performed using a one-way ANOVA with a post hoc Tukey honest significant difference test.
Molecular cloning
For cloning of Sm.TRPMMCLZ, total RNA was isolated from adult schistosome worm pairs using TRIzol and poly-A purified using a NucleoTrap mRNA mini kit. cDNA was synthesized using the High-Capacity RNA-to-cDNA kit (Invitrogen). Using the annotated Sm.TRPMMCLZ transcript as a template (Smp_333650.1), cDNA from transcribed sequences was amplified by PCR (Platinum Taq polymerase) and ligated into pGEM-T Easy (Promega) prior to confirmation by sequencing. The resulting sequence exhibited high identity with the annotated reference sequence, which was used for functional assays. Other schistosome TRPMMCLZ orthologs (Fig. S3) were synthesized and codon optimized (GenScript) from their curated annotations (S. turkestanicum, CAH8438365.1; S. bovis, CAH8481013.1, (24)). Sequence alignments were performed with Clustal Omega (32) and estimates of sequence identity and similarity made using the default settings of Ident and Sim (33).
Cell culture and transfection
HEK293 cells (ATCC CRL-1573.3) were cultured in DMEM supplemented with 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and L-glutamine (290 μg/ml). This cell line was authenticated by STR profiling (ATCC), and cells were evaluated for mycoplasma contamination by monthly scheduled testing (LookOut Mycoplasma PCR Detection Kit, Sigma). For screening Sm.TRPMMCLZ, Sm.TRPMMCLZ mutants, or Sm.TRPMPZQ, cDNAs were transiently transfected into HEK293 cells using Lipofectamine 2000 at a density of 3 × 106 cells per dish (100 mm).
Ca2+ reporter assays
Assays were performed using a Fluorescence Imaging Plate Reader (FLIPRTETRA, Molecular Devices) as described (20). In brief, HEK293 cells were seeded (20,000 cells/well) in a black-walled clear-bottomed poly-d-lysine-coated 384-well plate (Greiner Bio-One) in DMEM growth medium (supplemented with 10% dialyzed FBS). After 24 h, the growth medium was removed and cells were loaded with a fluorescent Ca2+ indicator (Fluo-4 NW, Invitrogen) by incubation (5 μl per well, 1 h at 37 °C) in Hank’s balanced salt solution (HBSS, 1.26 mM CaCl2, 0.49 mM MgCl2, 0.41 mM MgSO4, 5.33 mM KCl, 0.44 mM KH2PO4, 4.17 mM NaHCO3, 137.9 mM NaCl, 0.34 mM Na2HPO4, 5.55 mM D-glucose) supplemented with probenecid (2.5 mM) and HEPES (20 mM). Drug dilutions were prepared in assay buffer, without probenecid and dye, in F-form 384-well plates (Greiner Bio-One). After loading, the Ca2+ assay was performed at room temperature. Basal fluorescence was monitored for 20 s, then 5 μl of each drug was added, and the signal (raw fluorescence units) was monitored over an additional 250 s. For quantitative analyses, peak fluorescence increases in each well were normalized to maximum-fold increase over baseline. Except where indicated, all data are presented as mean ± SEM.
Electrophysiology
For current clamp measurements, the steady-state electrical potential was measured in single adult male S. mansoni worms by penetrating the dorsal surface of individual worms pinned to a Sylgard-coated petri dish. Electrophysiological assays were performed over a period of 10 days following worm isolation. Electrodes were pulled from borosilicate glass capillaries (#BF150-110-10, Sutter Instrument) on a vertical puller (Narishige, Model PC-10) and filled with 3 M KCl to a resistance of 2 to 5 MOhm. The bath solution was HBSS supplemented with 20 mM HEPES and 100 μM carbachol to impair muscle contractions. Electrode voltage was referenced to the grounded bath solution in the current-clamp recordings. The bath was perfused with buffer at 37 °C using an automatic temperature controller (TC324, Warner Instrument Corp). Ligands were added to the bath to a final concentration of 10 μM, 10 min prior to measurements. The control condition used was vehicle alone (0.1% dimethyl sulfoxide). A multiClamp 700B amplifier and Digidata 1440A digitizer (Molecular Devices) were used for recordings, and data were processed using Clampfit 11 software (Molecular Devices). For cell attached recordings of TRPMMCLZ, HEK293 cells were plated on glass coverslips and on the following day cotransfected with a plasmid encoding green fluorescent protein (GFP) alone, or GFP and Sm.TRPMMCLZ or Sm.TRPMMCLZ[Y1038A] mutant. Prior to recording, coverslips were secured within a recording chamber mounted on an Olympus BX51WI microscope. Pipette and bath solutions were HBSS with 20 mM HEPES. Pipette resistance was 8 to 10 MOhm. Signals were passed through an 8-pole Bessel low-pass filter at 1 kHz and sampled at 10 kHz. (S)-MCLZ was added directly to the bath from dimethyl sulfoxide stock solution up to 10 μM final bath concentration. In the voltage-clamp recordings (cell attached), the polarity of voltage and membrane currents were defined with respect to the virtual ground on the external side of the membrane.
Confocal Ca2+ imaging
For confocal Ca2+ imaging, HEK cells were loaded with Fluo-4-AM (4 μM) and Pluronic F127 (0.4%) for 25 min at room temperature. Cells were then washed twice with HBSS and incubated at room temperature for de-esterification (30 min). Experiments in U2OS cells were done using the genetically encoded calcium indicator, GCaMP6M. Fluorescence was imaged on a Leica DMi8 microscope, and fluorescence changes (λex = 488 nM, (λem = 513 ± 15 nm bandpass) were monitored using an Andor Dragonfly confocal equipped with an Andor Zyla sCMOS camera. Data were expressed as a ratio (F/F0) of fluorescence at any given time (F) relative to fluorescence prior to drug addition (F0).
Computational procedures
All computational modeling was performed in the Schrodinger Computational Suite (v. 2022-4) using the Maestro GUI (v. 13.1). Unless otherwise noted, default settings were used for all procedures. A workflow for these methods is provided in Fig. S4.
The AlphaFold model of Sm.TRPMMCLZ (Smp_333650.1, Accession # A0A5K4FCC0) was imported into the Maestro GUI, prepared with the Protein Preparation Wizard, and minimized in the OPLS4 force field at pH = 7.4. The output structure was used for subsequent modeling. To prepare (S)-MCLZ for modeling, the molecule was drawn in ChemDraw Professional (v. 21.0.0), imported into the Maestro GUI (v. 13.1), and prepared using the LigPrep tool in the OPLS force field at pH = 7.4. The output structure was used for subsequent modeling. A general binding area in the VSLD of Sm.TRPMMCLZ was determined by using the SiteMap feature within the Schrodinger Suite. A grid was then generated around this site, and (S)-MCLZ was docked into the grid using Glide, with SP-precision. The highest-ranked pose was used for Induced-Fit Docking (IFD).
For IFD, a grid was generated around the center of (S)-MCLZ in the output pose from Glide docking. Initial IFD of (S)-MCLZ was performed with the receptor and ligand van der Waals scaling set to 0.30. Residues were optimized within 8.0 Å of the ligand poses, and Glide redocking was performed with the SP protocol. Poses were manually examined, and the highest-ranking pose for each ligand that displayed interactions consistent with functional data was prioritized. Beginning with these initial poses, model refinement was performed for each ligand with IFD, using default scaling settings and the XP protocol, a more precise algorithm, for Glide redocking. This iterative refinement resulted in a reproducibly stable binding pose as reported in Figure 5.
Unbiased molecular dynamics were performed using Desmond within the Schrodinger Computational Suite. The consensus binding pose from IFD was inserted into a membrane bilayer using the System Builder workflow, aligning the membrane coordinates to PDB 6NR3 in the OPM database as previously described for Sm.TRPMPZQ. Solvation was treated explicitly using the SPC water model with 0.15 M NaCl, charges were neutralized by adding Na+ or Cl-ions when necessary, and the membrane was generated in the OPLS4 force field. The system was minimized to a protein RMSD <0.3 Å, and the minimized system was used as the starting pose for molecular dynamics simulations. Seven independent runs of 500 ns were completed. The simulations were run in the NPγT ensemble using both the Langevin thermostat (300 K) and semi-isotropic barostat (1.01325 bar). The system was relaxed before simulation and gradually brought to temperature with decreasing constrains as per the default series of Desmond simulations. Each simulation began from a random seed, the velocities were randomized, and frames were recorded at an interval of 50 ps, which allowed for the collection of 10,000 frames in each simulation. Each of the runs were independently clustered using the Trajectory Clustering Tool within the Maestro GUI. The poses of the most populated cluster were then superimposed and refined using the “Refine Protein-Ligand Complex” tool, and this resulted in a stable consensus pose consistent with that depicted in Figure 5. Ligand-Residue Interaction Counts were assessed individually for each run using the “Simulation Interactions Diagram” tool and plotted in Fig. S5.
Data Availability
All data necessary for evaluating the conclusions of this study are present within the article and the supporting information.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Schematic figures were created using Biorender.com. Schistosome-infected mice were provided by the NIAID Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, MD) through NIH-NIAID Contract HHSN272201000005I for distribution via BEI Resources.
Author contributions
J. S. M. conceptualization; S.-K. P., D. J. S., C. M. R., E. G. C., I. P., S. K. formal analysis; S.-K. P., D. J. S., C. M. R., E. G. C., I. P., S. K. investigation; J. S. M. writing – original draft; S.-K. P., D. J. S., C. M. R., E. G. C., I. P., S. K. writing – review & editing; J. S. M. supervision; J. S. M. funding acquisition.
Funding and additional information
This work was supported by the Marcus Family and National Institutes of Health (R01-AI145871 to J. S. M.). D. J. S. acknowledges support from National Institutes of Health (T32 HL134643) and the Medical College of Wisconsin Cardiovascular Center’s A.O. Smith Fellowship Scholars Program. I. P. was supported by a student summer research award (SSRA) from University College Dublin, School of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Mike Shipston
Supporting information
References
- 1.King C.H., Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 2.Cioli D., Pica-Mattoccia L., Basso A., Guidi A. Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 2014;195:23–29. doi: 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Chai J.Y. Praziquantel treatment in trematode and cestode infections: an update. Infect. Chemother. 2013;45:32–43. doi: 10.3947/ic.2013.45.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park S.K., Marchant J.S. The Journey to discovering a flatworm target of praziquantel: a long TRP. Trends Parasitol. 2020;36:182–194. doi: 10.1016/j.pt.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doenhoff M.J., Hagan P., Cioli D., Southgate V., Pica-Mattoccia L., Botros S., et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136:1825–1835. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- 6.N'Goran E.K., Odiere M.R., Assande Aka R., Ouattara M., Aka N.A.D., Ogutu B., et al. Efficacy, safety, and palatability of arpraziquantel (L-praziquantel) orodispersible tablets in children aged 3 months to 6 years infected with Schistosoma in Cote d'Ivoire and Kenya: an open-label, partly randomised, phase 3 trial. Lancet Infect. Dis. 2023;23:867–876. doi: 10.1016/S1473-3099(23)00048-8. [DOI] [PubMed] [Google Scholar]
- 7.Pica-Mattoccia L., Cioli D. Sex- and age-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Stohler H.R. Current Chemotherapy, (Proceedings of the 10th International Congress of Chemotherapy, Zurich, Switzerland, September 18-23, 1977) Vol. 1. American Society for Microbiology; Washington, DC: 1978. Ro 11-3128, a novel schistosomicidal compound; p. 147. [Google Scholar]
- 9.Pax R., Bennett J.L., Fetterer R. A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn Schmiedebergs Arch. Pharmacol. 1978;304:309–315. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- 10.Thibaut J.P., Monteiro L.M., Leite L.C., Menezes C.M., Lima L.M., Noel F. The effects of 3-methylclonazepam on Schistosoma mansoni musculature are not mediated by benzodiazepine receptors. Eur. J. Pharmacol. 2009;606:9–16. doi: 10.1016/j.ejphar.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Bricker C.S., Depenbusch J.W., Bennett J.L., Thompson D.P. The relationship between tegumental Disruption and muscle-contraction in Schistosoma mansoni exposed to various compounds. Z. Parasitenkd. 1983;69:61–71. doi: 10.1007/BF00934011. [DOI] [PubMed] [Google Scholar]
- 12.Pica-Mattoccia L., Ruppel A., Xia C.M., Cioli D. Praziquantel and the benzodiazepine Ro 11-3218 do not compete for the same binding sites in schistosomes. Parasitology. 2008;135:47–54. doi: 10.1017/S0031182007003514. [DOI] [PubMed] [Google Scholar]
- 13.Bickle Q.D., Andrews B.J. Resistance following drug attenuation (Ro 11-3128 or oxamniquine) of early Schistosoma mansoni infections in mice. Parasitology. 1985;90:325–338. doi: 10.1017/s0031182000051027. [DOI] [PubMed] [Google Scholar]
- 14.Baard A.P., Sommers D.K., Honiball P.J., Fourie E.D., du Toit L.E. Preliminary results in human schistosomiasis with Ro 11-3128. S. Afr. Med. J. 1979;55:617–618. [PubMed] [Google Scholar]
- 15.Hunkeler W., Mohler H., Pieri L., Polc P., Bonetti E.P., Cumin R., et al. Selective antagonists of benzodiazepines. Nature. 1981;290:514–516. doi: 10.1038/290514a0. [DOI] [PubMed] [Google Scholar]
- 16.O'Boyle C., Lambe R., Darragh A. Central effects in man of the novel schistosomicidal benzodiazepine meclonazepam. Eur. J. Clin. Pharmacol. 1985;29:105–108. doi: 10.1007/BF00547377. [DOI] [PubMed] [Google Scholar]
- 17.McCusker P., Mian M.Y., Li G., Olp M.D., Tiruveedhula V., Rashid F., et al. Non-sedating benzodiazepines cause paralysis and tissue damage in the parasitic blood fluke Schistosoma mansoni. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu S., Noviello C.M., Teng J., Walsh R.M., Jr., Kim J.J., Hibbs R.E. Structure of a human synaptic GABA(A) receptor. Nature. 2018;559:67–72. doi: 10.1038/s41586-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu S., Sridhar A., Teng J., Howard R.J., Lindahl E., Hibbs R.E. Structural and dynamic mechanisms of GABA(A) receptor modulators with opposing activities. Nat. Commun. 2022;13:4582. doi: 10.1038/s41467-022-32212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S.K., Gunaratne G.S., Chulkov E.G., Moehring F., McCusker P., Dosa P.I., et al. The anthelmintic drug praziquantel activates a schistosome transient receptor potential channel. J. Biol. Chem. 2019;294:18873–18880. doi: 10.1074/jbc.AC119.011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menezes C.M., Rivera G., Alves M.A., do Amaral D.N., Thibaut J.P., Noel F., et al. Synthesis, biological evaluation, and structure-activity relationship of clonazepam, meclonazepam, and 1,4-benzodiazepine compounds with schistosomicidal activity. Chem. Biol. Drug Des. 2012;79:943–949. doi: 10.1111/j.1747-0285.2012.01354.x. [DOI] [PubMed] [Google Scholar]
- 22.Bais S., Greenberg R.M. Schistosome TRP channels: an appraisal. Int. J. Parasitol. Drugs Drug Resist. 2020;13:1–7. doi: 10.1016/j.ijpddr.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S.K., Friedrich L., Yahya N.A., Rohr C.M., Chulkov E.G., Maillard D., et al. Mechanism of praziquantel action at a parasitic flatworm ion channel. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abj5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe K.L., Bolt B.J., Shafie M., Kersey P., Berriman M. WormBase ParaSite - a comprehensive resource for helminth genomics. Mol. Biochem. Parasitol. 2017;215:2–10. doi: 10.1016/j.molbiopara.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCusker P., Chan J.D. Anti-schistosomal action of the calcium channel agonist FPL-64176. Int. J. Parasitol. Drugs Drug Resist. 2019;11:30–38. doi: 10.1016/j.ijpddr.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bais S., Greenberg R.M. TRP channels in schistosomes. Int. J. Parasitol. Drugs Drug Resist. 2016;6:335–342. doi: 10.1016/j.ijpddr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bais S., Churgin M.A., Fang-Yen C., Greenberg R.M. Evidence for novel pharmacological Sensitivities of transient receptor potential (TRP) channels in schistosoma mansoni. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawton S.P., Hirai H., Ironside J.E., Johnston D.A., Rollinson D. Genomes and geography: genomic insights into the evolution and phylogeography of the genus Schistosoma. Parasit. Vectors. 2011;4:131. doi: 10.1186/1756-3305-4-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bais S., Berry C.T., Liu X., Ruthel G., Freedman B.D., Greenberg R.M. Atypical pharmacology of schistosome TRPA1-like ion channels. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chulkov E.G., Smith E., Rohr C.M., Yahya N.A., Park S.K., Scampavia L., et al. Identification of novel modulators of a schistosome transient receptor potential channel targeted by praziquantel. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCloy R.A., Rogers S., Caldon C.E., Lorca T., Castro A., Burgess A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 2014;13:1400–1412. doi: 10.4161/cc.28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., et al. Fast, scalable generation of high-quality protein multiple sequence alignments using clustal omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stothard P. The sequence manipulation suite: javascript programs for analyzing and formatting protein and DNA sequences. Biotechniques. 2000;28:1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data necessary for evaluating the conclusions of this study are present within the article and the supporting information.