Abstract
Recent studies have implicated cytokines associated with Th2 cells in the genetic resistance to murine Lyme borreliosis. Because the B7/CD28 costimulatory pathway has been shown to influence the differentiation of Th-cell subsets, we investigated the contribution of the B7 molecules CD80 and CD86 to the Th2 cytokine profile and development of arthritis in BALB/c mice infected with Borrelia burgdorferi. Effective blockade of CD86/CD28 interaction was demonstrated by elimination of interleukin 4 (IL-4) and upregulation of gamma interferon (IFN-γ) responses by B. burgdorferi-specific T cells and by reduction of B. burgdorferi-specific immunoglobulin G. Despite the shift toward a Th1 cytokine pattern, which others have associated with disease susceptibility, the severity of arthritis was unchanged. Moreover, combined CD80/CD86 blockade by using anti-CD80 and anti-CD86 monoclonal antibodies or CTLA-4Ig enhanced IFN-γ production over that seen with CD86 blockade alone, yet augmentation of this Th1-associated cytokine did not enhance disease. These results demonstrate that IL-4 production by T cells in B. burgdorferi-infected BALB/c mice is dependent upon CD86/CD28 interaction and that this cytokine does not contribute significantly to host resistance to the development of arthritis. In addition, combined CD80/CD86 blockade resulted in preferential expansion of IFN-γ-producing T cells in B. burgdorferi infection, suggesting that costimulatory pathways other than B7/CD28 may contribute to T-cell activation during continuous antigen stimulation. These studies may provide insight into the role of the B7/CD28 pathway in other infectious and autoimmune diseases in which deviation of Th cell immune responses occurs and antigen is persistently present.
Lyme disease is a multisystem illness due to infection with the tick-transmitted spirochete Borrelia burgdorferi. Experimental infection of laboratory mice with B. burgdorferi results in acute arthritis and carditis that reproducibly peak at 2 to 4 weeks of infection and then resolve within 3 months despite spirochete persistence (4). Studies using SCID mice, which lack functional T and B cells, have demonstrated that disease is due to the innate immunity of the host and can occur in the absence of specific immune responses (6, 28). The persistent and progressive nature of disease manifestations in SCID mice underscores the importance of T and B cells in initiating disease regression (5, 6, 28). Recent studies support the additional role of specific immunity in modulating disease severity via direct effects on spirochete burden through B. burgdorferi-specific antibodies (5) and indirectly through Th cell-associated cytokines that influence the activation of innate immune cells (14, 23). In particular, the dominance of Th1-type responses, which support macrophage activation, in patients with chronic Lyme arthritis has implicated this T-cell phenotype in the development and perpetuation of severe inflammatory disease (32, 37). Th1-type responses have also been observed during B. burgdorferi infection of C3H mice, a disease-susceptible strain, whereas Th2 responses, which promote B-cell functions, can be detected in BALB/c mice, a comparatively disease-resistant strain (14, 23). Despite the greater inflammatory response in C3H mice, their pathogen burden as assessed by quantitative PCR of spirochete DNA remains higher than that of disease-resistant mouse strains (36), suggesting that the recruitment of innate immune cells is appropriate yet ineffective at controlling infection (29).
In addition to signals provided by T-cell antigen receptor engagement, the interaction of costimulatory molecules present on antigen-presenting cells (APCs) with their ligands on T cells is believed to be necessary for the initial priming of naive T cells. In particular, the B7/CD28 costimulatory pathway has been implicated in the differentiation of naive Th0 cells into Th1 and Th2 subsets (33). The mechanisms by which these molecules assist in the priming of the T-cell immune response are complex and poorly understood. Two members of the B7 family have been characterized, CD80 and CD86 (also known as B7-1 and B7-2, respectively), and differ not only in their binding properties to CD28 on T cells but also in the timing of their appearance on conventional APCs during the initiation of an immune response (11). CD86 appears earlier on the surface of mitogen-activated APCs and has a lower affinity for CD28 than does CD80. Once activated, T cells express CTLA-4, a second receptor to which both CD80 and CD86 bind with greater affinity than they bind CD28 (21). Interaction of CD80/CD86 with CTLA-4 can downregulate the T-cell immune response (35). Blockade of CD86 during the initiation of a T-cell response results in an immune response oriented toward a Th1 phenotype, whereas a similar blockade of CD80 does not consistently favor a Th2 phenotype (20). Experiments using mutant mice deficient in CD80 and/or CD86 reveal the important role of these molecules in sustaining a Th-cell phenotype and, in the case of CD86 expression, in the development of a Th2 response (20). Costimulation through the B7/CD28 pathway contributes to the expansion of autoimmune disease processes seen in experimental autoimmune encephalitis (17, 27), a predominantly Th1-associated disease, and autoimmune diabetes (19). Studies using a soluble recombinant form of CTLA-4 designated CTLA-4Ig have supported many of the observations made with anti-B7 antibodies (13, 19, 26).
We have recently reported that the Th2 response of B. burgdorferi-infected BALB/c mice is preceded by a Th1 response and that the presence of interleukin 4 (IL-4) is associated with accelerated resolution of arthritis (12). A hind-foot inoculation route was used in that study so that T-cell responses could be examined in lymph nodes adjacent to joints afflicted with arthritis. We demonstrated that this route of inoculation induces moderately severe arthritis in BALB/c mice at day 14 of infection that undergoes more rapid regression than the arthritis seen in similarly infected C3H mice, in which IL-4 responses are not detectable. Previous studies have shown that treatment of mice with anti-IL-4 monoclonal antibody (MAb) exacerbates arthritis in BALB/c mice assessed at intervals corresponding to the plateau and resolution phases of disease, providing evidence that IL-4 modulates the severity of established arthritis (14, 23). The influence of Th2 cell effector functions on the development of arthritis remains unknown. In the current study, we have examined the effects of interruption of Th2 cell differentiation by B7/CD28 blockade with anti-CD80 and/or anti-CD86 MAb or CTLA-4Ig on the cytokine profiles and development of arthritis in BALB/c mice infected with B. burgdorferi.
MATERIALS AND METHODS
Reagents.
Purified anti-CD80 (1G10, rat immunoglobulin G2a [IgG2a]) and anti-CD86 (2D10, rat IgG2b) MAbs were graciously provided by Gordon Powers (Hoffmann-La Roche, Inc., Nutley, N.J.). CTLA-4Ig and L6 (control) were kindly provided by Peter Linsley (Bristol-Myers Squibb, Seattle, Wash.). The CTLA-4Ig fusion protein comprises the extracellular domain of murine CTLA-4 linked to the Cγ2α chain of murine immunoglobulin (10, 34). L6 is a control murine IgG2a MAb against a human carcinoma antigen (9).
Mice.
Specific-pathogen-free, 4- to 5-week-old, female BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) or the National Institutes of Health (Bethesda, Md.) and housed in filter frame cages at the Yale School of Medicine Animal Care Facility. The mice were administered autoclaved food and water ad libitum and killed by carbon dioxide asphyxiation.
Spirochetes.
A low-passage clone of B. burgdorferi N40 (cN40) with previously verified infectivity and pathogenicity was used in all experiments. A frozen aliquot of cN40 was thawed and expanded in modified Barbour-Stoenner-Kelly (BSK II) medium for each experiment (2). Spirochetes grown to mid-log phase were assessed for viability and counted by dark-field microscopy immediately prior to use.
Infection and B7 blockade of mice.
Mice were infected by hind-foot intradermal inoculation with 105 spirochetes in 50 μl of BSK II medium. The number of mice used in each experiment ranged from 5 to 10 per treatment group. For B7/CD28 blockade, the mice received an intraperitoneal injection of 100 μg of 1G10, 2D10, both MAbs, or the control rat IgG daily beginning 3 days before infection and continuing until time of sacrifice at day 14. In some experiments, 100 μg of CTLA-4Ig or the L6 control was administered on infection days 0, 5, and 10. Fourteen days after infection, the mice were killed, and popliteal lymph nodes were harvested and processed for cytokine assays. To confirm the infection status of the animals, their urinary bladders were incubated in BSK II medium for 14 days, after which time the presence of viable spirochetes was assessed by dark-field microscopy. Mouse joints were processed for histopathology as described below.
Cytokine analysis.
Harvested popliteal lymph nodes from individual mice were processed into single-cell suspensions, washed, and resuspended in Click’s medium supplemented with 10% fetal calf serum (FCS), 200 mM l-glutamine, 100 U of penicillin per ml, 100 mg of streptomycin per ml, and 5 × 10−5 M 2-mercaptoethanol (10% Click’s medium). A total of 8 × 105 lymph node cells (LNCs) were aliquoted into flat-bottom 96-well plates and stimulated with B. burgdorferi lysate at a final concentration of 35 μg/ml in a total volume of 200 μl. After 72 h of incubation at 37°C, culture supernatants were harvested and analyzed for gamma interferon (IFN-γ) and IL-4 by sandwich enzyme-linked immunosorbent assay (ELISA) using MAbs specific for IFN-γ and IL-4 according to the manufacturer’s recommendations (Pharmingen, San Diego, Calif.). Control standard curves were generated by using recombinant IFN-γ and IL-4 (Pharmingen) to quantitate cytokine responses.
To confirm a T-cell source of cytokines, LNCs were depleted of T cells by using the anti-Thy1.2 MAb 30 H-12 (18). Briefly, LNCs were washed in 2% Click’s medium and resuspended at 107 cells/ml in 30 H-12 hybridoma supernatant supplemented with 50 μg of anti-CD3 and anti-T-cell receptor αβ MAb (Pharmingen). The cells were incubated for 30 min at 4°C, centrifuged, and resuspended at 107 cells/ml in rabbit serum diluted 1:6 in phosphate-buffered saline (PBS). Labelled cells were then incubated for 30 min at 37°C and washed three times in 2% Click’s medium to remove debris, and unlysed cells were subjected to a second round of antibody labelling and complement-mediated cell lysis. The resultant T-cell-depleted population of cells was resuspended in 10% Click’s medium at 4 × 106 cells/ml and stimulated for cytokine analysis as described above.
Quantitation of B. burgdorferi-specific antibodies.
ELISA microtiter plates (ICN Biomedicals, Inc., Aurora, Ohio) were coated with 50 μl of B. burgdorferi lysate (50 μg/ml) at 4°C for 12 h. The plates were washed three times with PBS and then blocked for 1 h at room temperature (RT) with 100 μl of PBS containing 10% FCS (PBS–10% FCS) per well. The plates were washed once with PBS, and test sera diluted 1:5 in PBS–10% FCS were added to duplicate wells. The plates were incubated at RT for 2 h and then washed three times with PBS. Experimental and standard plates were incubated with 50 μl of alkaline phosphatase-conjugated goat anti-mouse IgG (1:1,000), goat anti-mouse IgG1 (1:500, γ1 chain specific; Southern Biotechnology, Birmingham, Ala.), or goat anti-mouse IgG2a (1:500, γ2a chain specific) in PBS–10% FCS. After incubation for 2 h at RT, the plates were washed three times in PBS and developed with p-nitrophenyl phosphate at 1 mg/ml in glycine buffer (pH 10.4). Optical densities were measured at 405 nm with a Titertek Multiscan (Flow Laboratories).
Histopathology.
Joints (knees and tibiotarsal joints) were immersion fixed in 10% formalin, decalcified, and stained with hematoxylin and eosin as described previously (6). Histopathology was evaluated by an observer blinded to the experimental protocol, and severity of joint inflammation was scored on a scale of 0 to 3 (with 3 representing the most severe) as previously reported (6).
RESULTS
In vivo blockade of B7/CD28 interaction modifies the Th cytokine profile produced by B. burgdorferi-specific T cells.
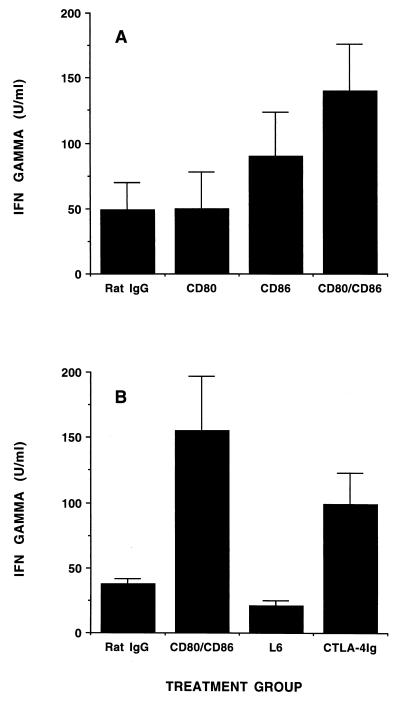
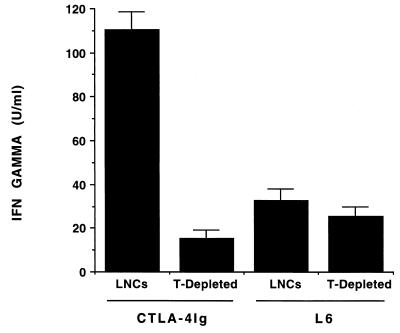
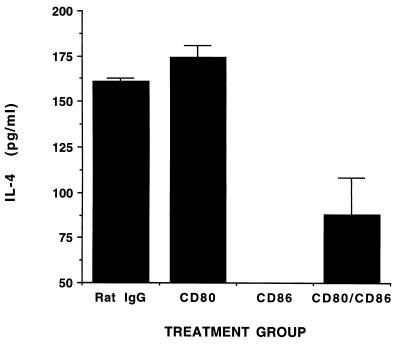
Because the B7/CD28 pathway is critical for the differentiation and maintenance of Th2 subsets, we examined the effect of blockade of this pathway on the subsequent cytokine profiles of reactive LNCs from infected BALB/c mice. Treatment with anti-CD80 MAb alone did not significantly affect the secretion of IFN-γ by T cells derived from 14-day-infected mice, whereas treatment with anti-CD86 MAb modestly increased its production (Fig. 1A). A striking and consistent finding was that LNCs from mice treated with both anti-CD80 and anti-CD86 MAbs produced a greater amount of IFN-γ than those from mice treated with anti-CD86 MAb alone (Fig. 1A). A similar enhancement of IFN-γ production was found when CTLA-4Ig was used to block B7 interaction with CD28 or CTLA-4 on T cells (Fig. 1B). The increase in IFN-γ production was not reflected in the relative proportion of T cells, or that of the CD4+- and CD8+-T-cell subsets, among the treatment groups as assessed by fluorescence-activated cell sorter (FACS) analysis (data not shown). Depletion of T cells from LNCs reduced IFN-γ production below the limits of assay detection in both treated and control mice (Fig. 2), confirming the T-cell origin of this cytokine. Consistent with the role of CD86 in Th2-cell differentiation, treatment of mice with anti-CD86 MAb eliminated IL-4 production by T cells in response to B. burgdorferi (Fig. 3). A small but substantially reduced amount of IL-4 could be elicited from T cells of mice treated with anti-CD80 and anti-CD86 MAbs in combination or with CTLA-4Ig (Fig. 3 and data not shown). Treatment with anti-CD80 MAb alone had no significant effect on IL-4 production (Fig. 3). The cytokines produced by lymphocytes derived from control infected mice were not influenced by the addition of excess anti-CD80 and/or anti-CD86 MAb to cultures (data not shown), indicating that these antibodies could not alter responses of primed T cells to B. burgdorferi antigens and that the cytokine patterns detected were not an effect of in vitro immune deviation. Moreover, the cytokine responses observed were dependent upon in vivo priming of T cells by B. burgdorferi infection since neither IFN-γ nor IL-4 could be detected in similar assays using LNCs from uninfected mice (data not shown).
FIG. 1.
Enhanced IFN-γ production by LNCs from B. burgdorferi-infected BALB/c mice treated with anti-CD80 and/or anti-CD86 MAb or CTLA-4Ig. (A) BALB/c mice were treated with antibodies to the indicated molecules or with control rat IgG as described in Materials and Methods. Bars represent the mean amount of IFN-γ present in the supernatants of pooled LNCs stimulated with B. burgdorferi lysates, as determined by ELISA, + standard error of the mean. These results are representative of three separate experiments. (B) BALB/c mice were treated with anti-CD80 and anti-CD86 MAbs, rat IgG, CTLA-4Ig, or L6 (control) as described in Materials and Methods. Bars represent the mean amount of IFN-γ produced by LNCs after restimulation with B. burgdorferi lysate, as determined by ELISA, + standard error of the mean. These results are representative of two separate experiments.
FIG. 2.
T cells are required for IFN-γ production by LNCs of infected BALB/c mice treated with CTLA-4Ig. LNCs of infected BALB/c mice treated with the indicated reagents were restimulated in vitro after depletion of T cells by antibody- and complement-mediated cell lysis. Control cells were sham treated with antibody and complement. The bars represent the mean amount of IFN-γ produced after 72 h of incubation with B. burgdorferi lysate, as determined by ELISA, + standard error of the mean.
FIG. 3.
Treatment of BALB/c mice with anti-CD86 MAb inhibits the development of IL-4-producing T cells. LNCs from mice treated with MAbs as described in the legend to Fig. 1A were stimulated in vitro with B. burgdorferi lysate, and levels of IL-4 were quantitated in supernatants by ELISA. The lower limit of IL-4 detection by this assay was 50 pg/ml. These results are representative of three independent experiments.
B. burgdorferi-specific IgG titers are diminished with B7/CD28 blockade.
Because both Th1 and Th2 cells assist in B-cell isotype switching to IgG, alteration of the cytokine profiles of responding Th cells during the course of B. burgdorferi infection could affect the level of antigen-specific IgG. Sera were therefore analyzed for total IgG, IgG1, and IgG2a subclasses by using a B. burgdorferi-specific ELISA. IgG1 and IgG2a subclasses reflect B-cell responses due to Th2 and Th1 cell help, respectively. All treatment regimens that led to blockade of CD86/CD28 interaction led to a statistically significant reduction in B. burgdorferi-specific IgG1 (Table 1), consistent with the association of this antibody subtype with Th2 cell effector functions. In contrast, CD80/CD28 blockade had no significant effect on any IgG subclass. Treatment with CTLA-4Ig reduced the total IgG as well as IgG1 and IgG2a levels, suggesting that the effect of this molecule on antibody responses is greater than the combined effects of anti-CD80 and anti-CD86 MAbs.
TABLE 1.
Effect of B7/CD28 or B7/CTLA-4 blockade on the development of B. burgdorferi-specific IgG in infected mice
| Treatment | Mean OD at 405 nma
|
||
|---|---|---|---|
| Total IgG | IgG1 | IgG2a | |
| Expt 1 | |||
| Rat IgG | 0.751 | 0.242 | 0.260 |
| Anti-CD80 | 0.639 | 0.166 | 0.200 |
| Anti-CD86 | 0.539 | 0.081b | 0.180 |
| Anti-CD80 + anti-CD86 | 0.563 | 0.075b | 0.090 |
| Expt 2 | |||
| Rat IgG | 0.916 | 0.233 | 0.214 |
| Anti-CD80 + anti-CD86 | 0.440 | 0.163c | 0.141 |
| L6 | 0.740 | 0.234 | 0.195 |
| CTLA-4Ig | 0.543b | 0.098b | 0.100b |
Values represent the means of optical density (OD) readings at 405 nm from ELISA analysis of the indicated IgG measured in serum specimens of individual mice. The effect of each treatment on B. burgdorferi-specific IgG responses was analyzed by one-way analysis of variance. Dunnett’s t test was used to compare control group values to treatment group values in some cases. P levels of <0.05 were accepted as evidence of significant group differences.
Values are significantly different from values for controls.
This result was not significantly different from the control value because of one outlying OD value.
Blockade of B7/CD28 has a minimal effect on arthritis in BALB/c mice infected with B. burgdorferi via hind-foot inoculation.
Because both Th cytokine patterns and B. burgdorferi-specific IgG responses were altered with B7/CD28 blockade, we examined the effects of anti-CD80 and/or anti-CD86 MAb and CTLA-4Ig treatment on arthritis severity as assessed by histopathology (Table 2). Control mice inoculated with 105 spirochetes and treated with rat IgG alone had moderately severe arthritis at day 14 of infection (Table 2). Although none of the treatment regimens employed led to significant changes in arthritis severity, a trend toward arthritis attenuation was observed in two separate experiments for mice treated with anti-CD86 MAb alone or in combination with anti-CD80 MAb (Table 2). Arthritis was not affected, however, when CTLA-4Ig was used to block B7/CD28 interaction, despite similarities in cytokine profiles and B. burgdorferi-specific IgG levels between mice treated with CTLA-4Ig and those treated with anti-CD80 and anti-CD86 MAbs.
TABLE 2.
Effect of B7/CD28 or B7/CTLA-4 blockade on development of arthritis in mice infected with B. burgdorferi
| Expt no. | Treatment | Avg arthritis severitya |
|---|---|---|
| 1 (8 mice/group) | Rat IgG | 2.2 ± 0.7 |
| Anti-CD80 | 2.6 ± 0.4 | |
| Anti-CD86 | 1.7 ± 0.6 | |
| Anti-CD80 + anti-CD86 | 1.4 ± 0.7 | |
| 2 (5 mice/group) | Rat IgG | 2.5 ± 0.5 |
| Anti-CD80 + anti-CD86 | 1.5 ± 0.4b | |
| L6 | 2.0 ± 0 | |
| CTLA-4Ig | 1.8 ± 0.4 | |
| 3 (10 mice/group) | L6 | 2.1 ± 0.3 |
| CTLA-4Ig | 2.0 ± 0.2 |
The highest tibiotarsal joint score for each mouse was used to calculate the severity score for each treatment group, which is given as the mean ± standard deviation. Statistical significance was calculated by Student’s t test. Only differences yielding P values of <0.05 were considered significant.
Significantly different from value obtained for rat IgG (P < 0.01).
DISCUSSION
A fundamental importance of the B7/CD28 costimulatory pathway in immune response deviation toward Th2-cell responses has been suggested by murine models of infectious and autoimmune diseases, particularly those in which cell-mediated immunity contributes to host defense or autoimmune pathology (8, 20). In the case of B. burgdorferi infection, control of spirochete burden is believed to be accomplished largely through the combined effects of phagocytes (24) and specific antibody (5). Th-cell responses that enhance borreliacidal activity by either of these mechanisms could contribute to host defense. The effects of Th2 responses are most apparent during the plateau and resolution phases of B. burgdorferi-induced arthritis, with IL-4 presence correlating with accelerated disease resolution (12) and its depletion correlating with more pronounced joint swelling (23). In this study, we blocked the B7/CD28 costimulatory pathway to reduce or eliminate the Th2 response that normally arises after B. burgdorferi infection of BALB/c mice. The marked reduction in IL-4 production by T cells of B. burgdorferi-infected mice treated with anti-CD86 MAb supports a primary role for CD86/CD28 costimulation in the development of a Th2 response. In contrast, anti-CD80 MAb had no substantial effect on either IFN-γ or IL-4 production, suggesting that the CD80 molecule participates minimally in initiating or perpetuating Th-cell responses elicited by B. burgdorferi infection.
The treatment regimens we employed to block B7/CD28 interaction during the initial phases of B. burgdorferi infection did not completely inhibit the activation of antigen-specific T cells. T cells from treated mice could still be stimulated in vitro to produce cytokines in recall assays. The treatment regimens used in our studies to achieve B7 blockade were similar to those used in other experimental systems, and the complete elimination of IL-4 in mice treated with anti-CD86 MAb alone along with the associated reduction of IgG1 levels indicated that the dosing schedule was sufficient to profoundly inhibit Th2 responses. It was not possible by FACS analysis to show effects of anti-CD80 and/or CD86 MAb on CD80 and CD86 surface expression due to their already low level of expression on LNCs from control infected mice. Evidence of continued T-cell priming in infected mice in which the B7/CD28 interaction had been interrupted by using both anti-CD80 and anti-CD86 MAbs or CTLA-4Ig suggests the involvement of other molecules in the activation of naive T cells by B. burgdorferi antigens. Several other molecules have been implicated in the activation of T cells, including heat-stable antigen, the chondroitin sulfate form of invariant chain, CD40, CD70, VCAM-1, and ICAM-1 (31). In our experiments, one or several of these molecules, some of which may be upregulated during infection, could have provided the necessary costimulatory signals for the activation of naive T cells upon encounter with B. burgdorferi antigens.
A striking observation in our studies was the marked enhancement in IFN-γ production detected in mice treated with both anti-CD80 and anti-CD86 MAbs or with CTLA-4Ig. This finding was not unique to BALB/c mice infected with B. burgdorferi, since treatment of disease-susceptible C3H mice with the combination of anti-CD80 and anti-CD86 MAbs caused a comparable (ca. threefold) increase in IFN-γ production (data not shown). We believe that T cells are the principal source of IFN-γ in these experiments because their depletion with a cocktail of anti-T-cell MAb eliminated this increase. Such depletion would also eliminate CD4+-T-cell subsets with NK markers, which could potentially also contribute to the production of IFN-γ in recall assays. However, previous studies examining the effects of anti-CD80 and/or anti-CD86 MAb on T-cell responses elicited by immunization with conventional antigen did not demonstrate enhanced IFN-γ production (20), suggesting that continued antigen stimulation provided by active infection is required for this effect to be observed. It is possible that signals provided by other costimulatory molecules, which may be upregulated after infection, could lead to T-cell activation and favor Th1 responses, although our data also show some degree of Th2 priming as well. This hypothesis is indirectly supported by studies with other experimental disease models in which Th1 responses are important. In murine leishmaniasis, BALB/c mice develop Th2 responses and rapidly succumb to infection with this intracellular pathogen. Treatment of BALB/c mice with CTLA-4Ig conferred resistance to disease, whereas similar treatment of disease-resistant C57BL/6 mice, which develop a Th1 type of response, did not alter the course of Leishmania major infection (8). Those results suggested a requirement for B7 costimulation in the development of Th2, but not Th1, responses in L. major infection. Similarly, frequent CTLA-4Ig administration to mice immunized with myelin basic protein resulted in more severe manifestations of experimental allergic encephalitis, a disease due to Th1 cells specific for myelin basic protein (26). It is possible that the continued presence of CTLA-4Ig could prevent CTLA-4 interaction with CD80/CD86 molecules and subsequent downregulation of the T-cell response during the disease process. However, an inability to downregulate an established T-cell response cannot account for the resistance of BALB/c mice to L. major infection conferred by treatment with CTLA-4Ig or the enhanced IFN-γ production seen in our experiments, since CD80/CD86 blockade was instituted prior to infection and T-cell priming.
Alterations in cytokine levels observed in mice treated with anti-CD80 and/or anti-CD86 MAb or CTLA-4Ig were associated with changes in B. burgdorferi-specific IgG; this was reflected to the greatest degree in the Th2-associated IgG1 subclass. This effect was largely attributable to blockade of CD86/CD28 interaction. Recent studies using conventional antigen have provided evidence that CD86 also promotes IgG2a production under some experimental conditions (7). A modest, although not statistically significant, reduction in IgG2a was apparent in our study. Despite the enhanced IFN-γ production noted with combined CD80/CD86 blockade, levels of the Th1-associated IgG2a subclass remained low. This finding suggests that although B. burgdorferi-specific T cells are present, they are inefficient at providing B-cell help for isotype switching, perhaps through downregulation or absence of CD40 ligand on the T-cell population producing the IFN-γ. It is unlikely that IgG levels were diminished because of B-cell depletion, since FACS analysis revealed no substantial change in the B-cell population among the different treatment groups. Moreover, previous studies have shown that CTLA-4Ig, which contains a complement-binding murine IgG2a element, has no effect on the absolute number of circulating B cells (10). Since arthritis severity can be modulated with B. burgdorferi-specific IgG, reduction in IgG subclasses could impair antibody-mediated destruction of spirochetes, but arthritis was not enhanced in our study (5). The relative importance of IgG2a or IgG1 antibodies in controlling spirochete numbers is not known, since both opsonization and complement-dependent mechanisms contribute to borreliacidal activity during the first few weeks of B. burgdorferi infection (15, 16, 29, 30).
Treatment regimens to block B7 interaction with its ligands were administered prior to the onset of infection and continued until mice were analyzed 2 weeks later so that the effects of experimental interventions on preventing arthritis could be assessed by histopathology. This contrasts with other studies in which Th-associated cytokines or anticytokine antibodies were administered early in infection and disease was assessed by caliper measurement of hind-foot swelling during the plateau and resolution phases (after 4 weeks) (14, 23). B. burgdorferi lipoproteins are believed to be the principal spirochete products that elicit acute inflammatory responses detected as disease in infected mice (22, 25). B. burgdorferi infection of SCID mice demonstrates that these innate immune responses can occur in the absence of specific immunity. However, T-cell costimulatory pathways assist in the differentiation of Th cell subsets into those that contribute to the activation and those that contribute to the inactivation of cells of the innate immune system, thereby modulating the innate response. For B. burgdorferi, resistance to infection has been correlated with Th2 responses, and the early appearance of Th2-associated cytokines like IL-4 could serve to attenuate inflammation driven by spirochete lipoproteins. Our results suggest that Th2-associated effector functions do not contribute to disease expression within the first 2 weeks of infection, and immune deviation toward a Th1 response, as indicated by increased IFN-γ production, does not enhance disease during this period.
A recent report has suggested that B7/CD28 interaction may be one factor contributing to the association of arthritis severity and age of mice at the time of B. burgdorferi infection (1). C3H mice infected with B. burgdorferi develop severe arthritis when infected as weanlings but appear more resistant to arthritis if infected at 6 weeks of age (3). In that study (1), blockade of B7/CD28 interaction during B. burgdorferi infection of weanling C3H mice had no effect on the severe arthritis present at 2 weeks. In contrast, 6-week-old C3H mice treated with blocking antibodies to B7 developed more severe arthritis than age-matched controls. The mechanism leading to this difference in arthritis severity is obscure, since no appreciable changes in B. burgdorferi-specific antibody titers or Th cell-associated cytokine secretion could be seen. Our study differs substantially from this previous report in several critical aspects. First, Th2 cell responses have been found in BALB/c but not C3H mice; since the purpose of our study was to determine the contribution of Th2 cell effector functions to the development of arthritis, experiments were performed with BALB/c mice. Second, previous studies have shown that detection of Th cell-associated cytokine responses, particularly IL-4, is enhanced when spirochetes are introduced and lymph nodes adjacent to a site of disease are examined. For this reason, spirochetes in our study were inoculated intradermally into a hind foot, so that T-cell responses could be analyzed in the popliteal node draining the arthritic hind-limb joints. In the study by Anguita et al. (1), inoculation of spirochetes into the skin of the back and examination of cytokines in splenocyte mRNA may have contributed to the absence of detectable differences in cytokines among their treatment groups. Finally, the more intensive dosing schedule of blocking antibodies used in our study may have led to more complete B7/CD28 blockade throughout the infection period, as indicated by the detectable differences in B. burgdorferi-specific IgG subclasses in serum specimens.
In summary, our results demonstrate that IL-4 production by T cells in B. burgdorferi-infected BALB/c mice is dependent upon CD86/CD28 interaction and that Th2 responses do not contribute significantly to modulation of arthritis as it develops. In addition, our studies demonstrate that despite B7/CD28 blockade, evidence of T-cell priming was present during active infection with B. burgdorferi, suggesting that other T-cell costimulatory pathways assist in T-cell activation under conditions of continuous antigen stimulation. These results may provide insight into the role of B7/CD28 costimulation in other experimental infectious and autoimmune diseases in which deviation in the Th-cell immune response occurs and antigen is persistently present.
ACKNOWLEDGMENTS
This work was supported by grants from the Arthritis Foundation (to I. Kang and L. K. Bockenstedt), the National Institutes of Health (AR 07107, AR 42637, AI 45253, and AI 26815 to M.-C. Shanafelt, L. K. Bockenstedt, and S. W. Barthold), and the Mathers Foundation (to L. K. Bockenstedt).
We thank Peter Linsley and Bristol-Myers Squibb for CTLA-4Ig and Peter Linsley for review of the manuscript. We also thank Kevin Feen, Deborah Beck, Rhonda Bangham, and Gordon Terwilliger for excellent technical assistance.
REFERENCES
- 1.Anguita J, Roth R, Samanta S, Gee R J, Barthold S W, Mamula M, Fikrig E. B7-2 and B7-2 monoclonal antibodies modulate the severity of murine Lyme arthritis. Infect Immun. 1997;65:3037–3041. doi: 10.1128/iai.65.8.3037-3041.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 4.Barthold S W, de Souza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;43:419–420. [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W., S. Feng, L. K. Bockenstedt, E. Fikrig, and K. Feen. 1997. Protective and arthritis-resolving activity in serum of mice infected with Borrelia burgdorferi. Clin. Infect. Dis. 25(Suppl. 1):S9–S17. [DOI] [PubMed]
- 6.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 7.Borriello F, Sethna M P, Boyd S D, Schweitzer A N, Tivol E A, Jacoby D, Strom T B, Simpson E M, Freeman G J, Sharpe A H. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 8.Corry D B, Reiner S L, Linsley P S, Locksley R M. Differential effects of blockage of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 9.Fell H P, Gayle M A, Yelton D, Lipsich L, Schieven G L, Marken J S, Aruffo A, Hellstrom K E, Hellstrom I, Bajorath J. Chimeric L6 anti-tumor antibody. Genomic construction, expression, and characterization of the antigen binding site. J Biol Chem. 1992;267:15552–15558. [PubMed] [Google Scholar]
- 10.Finck B K, Linsley P S, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 11.Hathcock K S, Laszlo G, Pucillo C, Linsley P, Hodes R J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T helper cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karandikar N J, Vanderlugt C L, Walunas T L, Miller S D, Bluestone J A. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane-Myers A, Nickell S P. Role of IL-4 and IFNγ in modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;155:2020–2028. [PubMed] [Google Scholar]
- 15.Kochi S K, Johnson R C, Dalmasso A P. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi. J Immunol. 1991;146:3964–3970. [PubMed] [Google Scholar]
- 16.Kochi S K, Johnson R C, Dalmasso A P. Facilitation of complement-dependent killing of the Lyme disease spirochete, Borrelia burgdorferi, by specific immunoglobulin G Fab antibody fragments. Infect Immun. 1993;61:2532–2536. doi: 10.1128/iai.61.6.2532-2536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 18.Ledbetter J A, Herzenberg L A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Lenschow D J, Ho S C, Sattar H, Rhee L, Gray G, Nabavi N, Herold K C, Bluestone J A. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 21.Linsley P S, Brady W, Urnes M, Grosmair L S, Damle N K, Ledbetter J A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Seiler K P, Tai K, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery R R, Malawista S E. Borrelia burgdorferi and the macrophage: routine annihilation but occasional haven? Parasitol Today. 1994;10:154–157. doi: 10.1016/0169-4758(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 25.Morrison T B, Weis J H, Weis J J. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J Immunol. 1997;158:4838–4845. [PubMed] [Google Scholar]
- 26.Perrin P J, Maldonado J H, Davis T A, June C H, Racke M K. CTLA-4 blockage enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 27.Racke M K, Scott D E, Quigley L, Gray G S, Abe R, June C H, Perrin P J. Distinct roles for B7-1 (CD80) and B7-2 (CD86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest. 1995;96:2195–2203. doi: 10.1172/JCI118274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaible U, Kramer M, Museteanu C, Zimmer H, Mossman H, Simon M M. The severe combined immunodeficient mouse: a laboratory model of Lyme arthritis and carditis. J Exp Med. 1989;170:1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaible U E, Kramer M D, Wallich R, Tran T, Simon M M. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with resistance and susceptibility to development of arthritis. Eur J Immunol. 1991;21:2397–2405. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz J L, Lovrich S D, Callister S M, Schell R F. Depletion of complement and effects of passive transfer of resistance to infection with Borrelia burgdorferi. Infect Immun. 1991;59:3815–3818. doi: 10.1128/iai.59.10.3815-3818.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharpe A H. Analysis of lymphocyte costimulation in vivo using transgenic and ’knockout’ mice. Curr Opin Immunol. 1995;7:389–395. doi: 10.1016/0952-7915(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 32.Simon A K, Seipelt E, Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci USA. 1994;91:8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson C B. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation. Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 34.Wallace P M, Johnson J S, MacMaster J F, Kennedy K A, Gladstone P, Linsley P S. CTLA-4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994;58:602–610. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K P, Thompson C B, Griesser H, Mak T W. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Weis J H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yssel H, Shanafelt M D, Sodenberg C, Schneider R, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]