Abstract
Carfilzomib (CFZ), a chemotherapeutic agent used for multiple myeloma treatments reported to cause high incidence of cardiac events either new onset and/or exacerbate formerly diagnosed heart failure with ventricular and myocardial dysfunction.
Purpose: Current research designed to explore and examine the preventive effect of oxyphenbutazone in the CFZ -instigated cardiotoxicity. Methodology: Female Wistar Rats weighing 200–250 g selected randomly and grouped as follows: Group 1 designated as the Normal control and receive normal saline only. Group 2 served toxic control and exposed to CFZ (4 mg/kg, intraperitoneally [i.p.]). Group 3 & 4 served as treatment groups and administered with CFZ concomitantly orally fed with oxyphenbutazone at doses of 35 and 70 mg/kg/three times a week, respectively. The total duration of experimental protocol was of 21 days. After completion of the experiments animals subjected to blood collection using light ether anesthesia and serum was separated for biochemical analysis further. The serum levels of Mg+2, Ca+2 and cardiac enzymes (aspartate transaminase (AST), lactate dehydrogenase (LDH), creatine kinase (CK) and creatine kinase-MB (CK-MB) levels were estimated. Later animals sacrificed and heart tissue isolated for further examinations. Intracellular proteins NFkB and IkBα were estimated by western blot. Results: The serum analysis revealed that CFZ administration significantly elevated the levels of LDH, CK and CKMB in CFZ exposed animals when compared to normal animals while administration of oxyphenbutazone significantly reduced these biochemical changes, Intracellular antioxidant enzymes and NF-kB in treatment groups as compared to disease control animals. Conclusion: Findings of the research protocol suggests significant injuries to cardiac tissues when animals exposed to CFZ and Oxyphenbutazone protected the cardiac tissues.
Keywords: Cardiotoxicity, Carfilzomib, Oxyphenbutazone, Chemotherapy, Inflammation
1. Introduction
As the number of cancer survivors growing progressively, it becomes important for health care practitioners to manage satisfactorily the cardiac adverse events of chemotherapy (Mariotto et al., 2010; Elsayed et al., 2022, Aboubakr et al., 2023a, Aboubakr et al., 2023b). Numerous cardiovascular adverse effects of chemotherapies recognized. The current definitions of cardiotoxicity covers narrowly resting myocontractility alterations, such as left ventricle force of contraction and ejection volume and development to heart failure (HF). Although drugs and radiations not only affect resting ejection volume but also have wide variety of effects on heart and vascular system. However, altered ejection fractions are the marker test to confirm chemotherapy-induced cardiotoxicity, still. Now it’s the need of time to broaden the cardiotixicity definition that must encompass directed or indirect influences on cardiocyte morphology markers (fibrosis, myocardial relaxation, electrical conductivity and dysrrhythmias), vasculature (hemodynamics including systemic and pulmonary blood vascular abnormalities), blood markers (hemostasis with thrombosis) and immune responses of cardiocytes to injury and stress. Altered myocyte strain, fewer other specific biomarkers (eg, troponin isoforms, Creatinine kinase (CK) and CK-MB) during cancer chemotherapy can finely depict about cardiovascular system and prognosis of HF before a drop in LVEF (Sawaya et al., 2011, Geisberg et al., 2013, Cardinale and Sandri, 2010). Therefore, cardiotoxicity encompasses alterations in normal cardiac function tests and hemodynamics of cardiovascular system. Hemodynamics is of concern mainly because cancer survivor faces reduced ability to physical workouts which affect quality of life considerably (Koelwyn et al., 2014). Conclusive goals of the cardio-oncologist remains to recognize and manage cardio toxicities related to chemotherapy without impeding chemotherapy and begin pharmacological or lifestyle interventions sooner to improve survivals of cancerous patients.
The ubiquitin–proteasome system monitors cell signaling, protein turnover, growth and survival of cells in cancerous states. Chemotherapy targeting ubiquitin proteasome system found associated with cardiac dysfunction leading to heart failure (HF). The proteasome has an elementary function in perpetuation of cardiac morphology and physiology (Mearini et al., 2008). However, the patients treated with proteosome inhibitor tolerate this treatment very well, it is astonishing. Bortezomib was first proteasome inhibitor approved for clinical use to treat multiple myeloma (Esparís-Ogando et al., 2005). An irreversible proteasome inhibitor CFZ, is a second line drug for intermittent multiple myeloma. Though, CFZ is more potent and effective in resistant myeloma cases than earlier therapeutic agents, but found to be linked with severe cardiac events, from HF to sudden cardiac deaths (Herndon et al., 2013).
Phenylbutazones were first used for therapeutic purpose in 1949, belongs to anti-inflammatory drug (nonsteroidal) category and used in sudden onset as well as chronic inflammation, most importantly for arthritis (Von Rechenberg, 2005).
Phenylbutazones use restricted in the 1980 s clinically. Later, brief history of them is as follows: (1) 200 mg solid dosage form (tablets) use restricted for commercial causes in 1984; (2) In mid-1984: the product manufacture was restricted for treating spondylitis (inpatients only) and (3) In late 2002, 100 mg tablets withdrawn due to commercial motives. Peak use documented in 1974 with 14 million prescriptions, which declined to 2 million in 1984, in the same year the safety studies conducted by advisory committee of USA Food and Drugs Administration (FDA) (Faich, 1987). NSAIDs are chemo preventive in hepatocarcinogenic models. These decrease the number and mass of cancers in livers of rats kept on choline-deficient diet and restricts tumor growth in thepost-initiation phase. The result depicts involvement of prostaglandins in liver cancers (Tong, 2006).
In 1980 s phenylbutazone restricted because of blood disorders over all bone marrow depression suggested (aplastic anaemia, leukopenia, agranulocytosis and thrombocytopenia) and sometimes death. serum sickness also reported as allergic reaction. Fatalities observed clinically when used as recommended by manufacturer dose rates. Although used therapeutically still, in special disease treatment, under regulated conditions and blood profile monitoring. Thus, still some nations, licensed to generic manufacture of its production (Lees and Toutain, 2013).
Neoplasm growth assisting influences of phenylbutazone examined using DONRYU transgenic rats by administering in diet at different dose levels for 2 years (Maekawa et al., 2013). The authors concluded that phenylbutazone do not exert any carcinogenic effect on prolonged administrations.
Scientists conducted experiments using experimental rats (2 years chronic studies) and documented carcinogenic effects (renal tubular cell adenomas and carcinomas) of phenylbutazone in male F344/N rats. Some evidence of carcinogenic activity for female F344/N rat also reported by other research scientist in the form of transitional cell carcinomas at high dose levels (Kari et al., 1995).
So it's quite dubious to say, whether the phenylbutazones are working as anticancer or to promote the cancers. Moreover, some researchers are concluding from their findings that, phenylbutazones do not have any mutagenicity (Giri, and Mukhopadhyay, 1998). Moreover, Kirkland and colleague (2010) depicted that phenylbutazone cann’t be predicted to be a mutagen as Computer Software analysis does not support its interaction with DNA (Kirkland and Fowler, 2010). In our previous research oxyphenbutazone showed protection in diethyl nitrosamine and phenobarbital induced cancer model in rats by altering PG-E2 and Wnt/β-catening signaling (Shakir et al., 2019). Further various researchers have extensively studied the Pharmacology of oxyphenbutazone and it was found to have anti-inflammatory (Omneya, 2012), anti-oxidant (Venkatesh et al., 2012) anti-cancer (Ly et al., 2012) and antitumor (Insuastyet al., 2010) activities. The current objective was to study oxyphenbutazone in CFZ induced cardiotoxic rodents.
2. Methodology
2.1. Animals
Female Wistar albino rats (200–250 g) procured from experimental animal care center, and protocol was approved (IAEC No-TRS/PT/021/009) from institutional animals ethics committee. All animals were housed (12:12 h light and dark cycle) at an optimum temperature (22–25 °C) and relative humidity (45–55 %). Animals provided with free access to normal pellet diet and water ad libitium throughout. All procedures conducted, following the ethical guidelines of the animal care and use board of the centre.
2.2. Chemicals
Carfilzomib, Oxyphenbutazone were procured from (Sigma ahldrich USA), Normal saline was used as vehicle to administer the drugs.
2.3. Experimental design
The considered experimental rats were divided randomly and grouped into four (n = 6). Group 1: normal control was fed with normal saline (NS) for 3 weeks. Group 2: disease (toxic) control administered with CFZ for 3 weeks, twice in a week (4 mg/kg, intraperitoneally [i.p.]) for 3 weeks. Group 3 and 4: designated as treatment groups administered with CFZ following the same schedule as group-2 and treated with oxyphenbutazone (35/75 mg/kg/thrice a week, p.o. respectively) for 3 weeks (Saleem et al., 2018). After 21 days of treatment protocol, experiment terminated and blood sample were collected under light ether anesthesia later animals sacrificed by cervical dislocation method, the heart tissue isolated and utilized for intracellular protein estimations.
2.4. Biochemical estimations
Biochemical estimation of Ca2+, Mg2+, AST, LDH, CK and CK-MB was done using standard kits and biochemistry autoanalyzer (Dimension RxL MAX; Siemens, Malvern, PA, USA).
2.5. Preparation of tissue homogenates
Dissected the rat’s heart with clean tool and quickly perfused with ice-cold normal saline to prevent degradation by proteases. With the help of Potter Elvehjem homogenizer, homogenates the small pieces of heart in chilled 0.1 M phosphate buffer (pH 7.4) consisting potassium chloride (KCl) (1.17 % w/v), for the separation of nuclear debris the homogenate centrifuged at 700 × g and 4 °C for 10 min. Filtered supernatant separated which was again centrifuged at 9,000 × g and 4 °C for 20 min and post-mitochondrial supernatant were isolated (Omneya, 2012). Gene and protein expression determinations were done by using left over cardiac tissue.
2.6. Western blot assay
Firstly, the protein was extracted from the tissue homogenates of rat heart. The extracted protein sample were segregated using 10 % SDS - polyacrylamide gel electrophoresis (PAGE) following transfer into a PVDF membrane (Millipore, USA). This membrane was used for primary detection of antibodies (Abcam, UK) blocked and. treatment with 5 % skim milk followed by culturing it for overnight at 4 °C. Later on the membrane was cleanse thrice with Tris Buffered Saline + Tween 20 (TBS-T) followed by culturing it with secondary antibody at 24 °C for an hour. The western blot were scanned and graphed using ECL reagent (Pierce, USA) followed by density verification using image software (Imam et al., 2016, Al-Harbi, 2016).
2.7. Estimation of tissue malondialdehyde (MDA) content
MDA, represent membrane lipid peroxidation intracellularly, it was measured in cardiac tissue by modified procedure of Okhawa (Ohkawa et al., 1979). The MDA fractions expressed as nmoles of MDA/mg of protein. Lowry’s procedure followed to estimation total tissue proteins (Lowry et al., 1951).
2.8. Determination of reduced glutathione (GSH)
Quantification of GSH in cardiac tissue was done using procedure described by Sedlak and Lindsay (Sedlak and Lindsay, 1968). UV spectrophotometer was used to record the optical density of reaction mixture within 5 min at 412 nm after addition of dithiobis-2-ni-trobenzoic acid against blank.
2.9. Statistical analysis
All results are displayed as means ± SEM (n = 6). Statistical significance between two or more groups was analyzed using one-way ANOVA followed by Tukey-Kramer post-hoc test by InStat GraphPad Prism version. 5.0 (GraphPad InStat Software 5.0, La Jolla, CA, USA). Differences recorded statistically significant when P < 0. 05.
3. Results
3.1. Effects of oxyphenbutazone and CFZ on serum Ca2+, Mg2+, AST, LDH, and CK and CK-MB levels
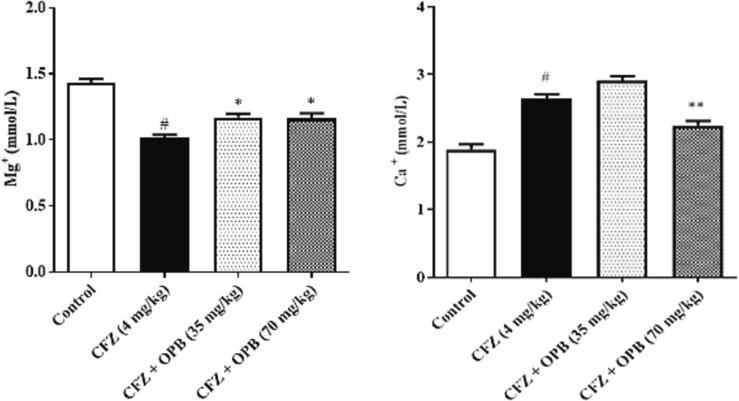
Exposure to CFZ considerably declined (P < 0.001) serum Mg2+ levels while no significant alter-ations observed in serum Ca2+ levels. Administration of Oxyphenbutazone significantly increased serum Mg2+ and reverted them to normal (control) levels.
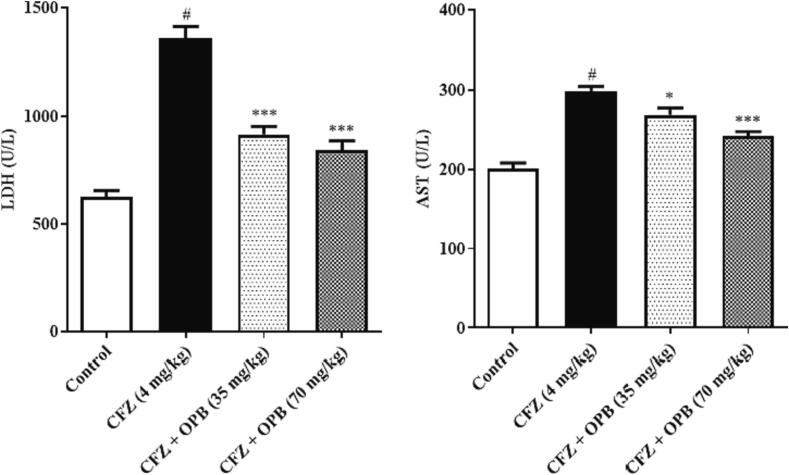
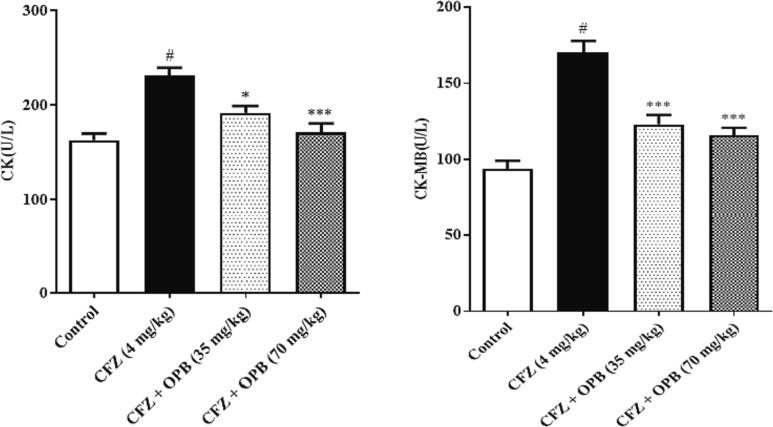
The cardiotoxic biomarker enzymes AST, LDH, CK, and CKMB levels were significantly (P < 0.001) inclined in animals received CFZ relative to the other groups. In contrast, oxyphenbutazone treatment restored these enzymes towards normal (control) levels (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Effects of Oxyphenbutazone (35, and 70 mg/kg) treatment on CFZ-induced changes in serum Mg++ and Ca ++ concentration in cardiotoxicity model in rats. The biochemical assay in serum was performed to assess the alteration in electrolytes concentration in all treated groups following CFZ-induced cardiotoxicity. All data were expressed as mean ± SEM. #p < 0.05 compared to control group; *p < 0.05, and **p < 0.01 compared to CFZ-treated group.
Fig. 2.
Effects of Oxyphenbutazone (35, and 70 mg/kg) treatment on CFZ-induced changes in serum LDH and AST levels in cardiotoxicity model. The serum biochemical assay was performed to assess the alteration in LDH and AST enzymes levels in all treated groups following CFZ-induced cardiotoxicity. All data were expressed as mean ± SEM. #p < 0.05 compared to control group; *p < 0.05, and **p < 0.01 compared to CFZ-treated group.
Fig. 3.
Effects of Oxyphenbutazone (35, and 70 mg/kg) treatment on CFZ-induced changes in serum CK and CK-MB levels in cardiotoxicity model. The serum biochemical assay was performed to assess the alteration in LDH ans AST enzymes levels in all treated groups following CFZ-induced cardiotoxicity. All data were expressed as mean ± SEM. #p < 0.05 compared to control group; *p < 0.05, and **p < 0.01 compared to CFZ-treated group.
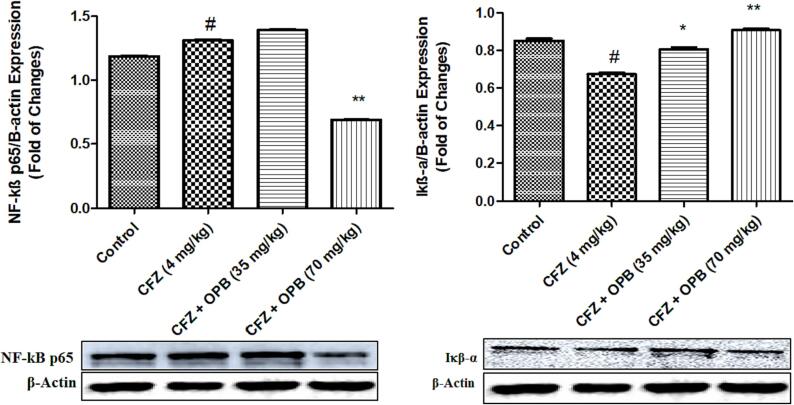
3.2. Effects of oxyphenbutazone and CFZ on intracellular NF-kB and IkB-α
The transcription factor NF-kB enhances expressions of several genes. To examine cardio protective role of NF-kB and the effect of oxyphenbutazone, the expressions of NF-kB and its inhibitory protein IkB-α were determined by Western blot assay. Significant difference recorded in NF-kB expressions between NC, CFZ and oxyphenbutazone treated groups (Fig. 2). Protein expressions of NF-kB documented significantly elevated in CFZ group, which was brought to normal by oxyphenbutazone treatment.
CFZ significantly decrease cytoplasmic inhibitor protein IkB-α level as compared to NC. When treated with oxyphenbutazone these levels were significantly increased at high dose 70 mg/kg then the low dose of 35 mg/kg as compared with disease controls. Noteworthy, oxyphenbutazone treatment prevented alteration in expressions of NF-kB and IkB-α. oxyphenbutazone treatment prevented the activation of NF-kB by increasing the expression of cytoplasmic inhibitor protein IkB-α (Fig. 4).
Fig. 4.
Effects of Oxyphenbutazone (35, and 70 mg/kg) treatment on CFZ-induced changes in expression of NF-kB and IkB-a protein in cardiotoxicity model. The western blot analysis was performed to assess the CFZ-induced changes in expression of NF-kB and IkB-a protein in all treated groups following CFZ-induced cardiotoxicity. All data were expressed as mean ± SEM. #p < 0.05 compared to control group; *p < 0.05, and **p < 0.01 compared to CFZ-treated group.
3.3. Effects of oxyphenbutazone and CFZ on oxidative stress markers
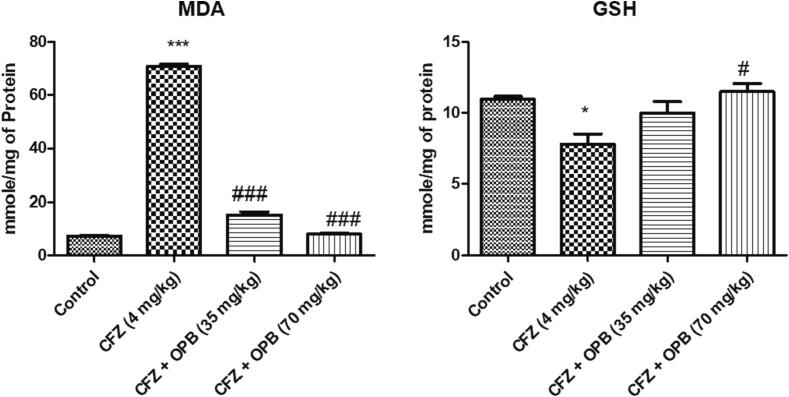
The results in Fig. 3 concluded that treatment of animals with CFZ exhibited a considerable (p < 0.05) elevation in rat heart MDA levels and similarly a significant decline in intracellular GSH levels as compared to the normal control animals. Considerable reverse exhibited in oxyphenbutazone treated animals in cardiocyte MDA levels after CFZ administration a (Fig. 5).
Fig. 5.
Effects of Oxyphenbutazone (35, and 70 mg/kg) treatment on CFZ-induced changes in MDA and GSH levels in cardiotoxicity model. The biochemical assay was performed to assess the changes in oxidative stress parameters in all treated groups following CFZ-induced cardiotoxicity. All data were expressed as mean ± SEM. #p < 0.05, and ##p < 0.01, and ###p < 0.001 compared to control group; *p < 0.05, **p < 0.01, and ***p < 0.001 compared to CFZ-treated group.
4. Discussion
Carfilzomib indicated for multiple myeloma and mechanistically act by inhibiting proteasomes (Méndez-Toro et al., 2020). Although the drug is well documented for its risk factor fin the establishment of cardiotoxicity especially those who have concurrent cardiovascular disorders (Méndez-Toro et al., 2020). For CFZ use in cancer chemotherapy cardiotoxicity considered as major limiting factor and chances of cardiotoxicity increases with already existing heart disease (Singal and Iliskovic, 1998, Yeh et al., 2004). Although the underlying mechanism to cardio-toxicity is not fully established yet, experimental studies conducted on animals suggested inhibition of proteasomes damage cardiomyocytes subsequently and induces apoptosis (Wei et al., 2008). Elevated systemic levels of proteasome inhibitor found to influence nitric oxide synthase enzyme negatively and reported to cause increased endothelial dysfunction and cardiac disease (Shah et al., 2018). Electrolytes and minerals are confirmed player of cardiovascular health, imbalances found frequently and potentially hazardous which may cause cardiovascular diseases (CVDs) development and progress (Mohammadifard et al., 2019). Treatment of multiple myeloma patients with Carfilzomib had confirmed electrolyte disbalance as adverse effect (Mushtaq et al., 2018). Findings of our current experiment are also in agreement with the previous researches and the results summarize that exposure of CFZ to rodents considerably decline the serum Mg + 2 levels (P < 0.001) while there were no significant alterations found in serum calcium ion levels. Treatment with oxyphenbutazone prevented these electrolyte misbalances in treatment groups. Which the cardioprotective influences of oxypenbutazone in CFZ induced cardiotoxicity. Earlier in response to cardiac toxicity elevation in serum AST, LDH, CK, CK-MB have been approved in various research protocols which were conducted to assess the effect of CFZ on heart (Al-Shabanah et al., 1998, El-Missiry et al., 2001, Imam et al., 2017). Our research findings attested to those researches. Administration of CFZ in disease control animals leaded a highly significant elevation in serum AST, LDH, CK, CK-MB levels. Treatment of rats with oxyphenbutazone at 35/75 mg/kg/ three times a week significantly decreased these altered levels of cardiac function tests. The better results were observed in the animals which were treated with 75 mg/kg dose of oxyphenbuatzone.
Further to confirm the defensive effect of oxyphenbutazone in CFZ leaded cardiotoxicity, the levels of inflammatory mediators were estimated by western blot assay. Previous research experiments conducted to study the effect of CFZ to induce cardiotoxicity have already confirmed the stimulation of NF-κB by booming IκB-α expressions. NF-κB associated with expressions of numerous genes which are involved confirmly in inflammation, cellular injuries and stress, and found to caste leading functions in regulation of cell survival. Stimulation of NF-κB signaling cascade confirmed to play role in the endothelial pathologies, in atherosclerotic plaque etiologies, sudden onset myocardial infarct and heart failure (Jones et al., 2003, Liu and Malik, 2006, Yu, et al., 2020, Tosaki, 2020, Hashimoto, et al., 2023, Yang et al., 2023). NF-κB contains p50 and p65 hetero-dimers remained inactivated in the cytosol when associated with inhibitory proteins called IKBs. Various stimulatory responses activates IKB kinase (IKK), causing IκB-α phosphorylation, ubiquitination and degradation by the proteasome. Later the separated p50–p65 complex translocates into the nucleus, binds to its DNA binding sites within the promoter regions of NF-κB target genes, and regulates genes transcription (Perkins and Gilmore, 2006, Ahmad et al., 2015). Findings of present experiments exhibit significantly elevated expressions of NF-κB due to CFZ exposure, which was restored by oxyphenbutazone treatment.
Oxidative free radicals significantly contribute to tissue injuries. Oxidative stress generates when the free radical’s generation is more and defensive antioxidant system become compromised. Over the time, continuously persisting oxidative free radicals cause organ malfunction. Hence to keep check the free radicals and to enhance the body’s antioxidant defense are the essential objectives to work upon (Zilinyi et al., 2018, Sallam et al., 2021, Wang et al., 2021, Behairy et al., 2023, Aboubakr et al., 2023a, Aboubakr et al., 2023b). Depletion of GSH intracellularly is one of the important factors to be considered in the cellular deleterious effects of reactive free radicals which permit lipid peroxidation (Konukoglu et al., 1998, Inoue, 2011) GSH regulates the levels of hydrogen peroxide and hydroperoxide as well by oxidation reactions in conversion of GSH to GSSG (Wu et al., 2004) and acts as first line defense against reactive and nutritive free radicals (Zindenberg et al., 1991). Therefore, in our experiment we assayed MDA and rGSH in heart tissue to gauge oxidative stress. The findings depicted, significantly elevated MDA and reduced GSH in cardiac tissue after CFZ administrations, which were reversed by oxyphenbutazone treatment.
5. Conclusion
Findings of our research experiment confirmed that CFZ at 4 mg/kg twice a week can cause cardiotoxicity in experimental rats successively. Treatment with oxyphenbutazone can be proved efficacious in management of cardiotoxicity induced by CFZ. Although more mechanistic pre-clinical confirmations and finally clinical researches are required to conclude it as cadioprotective with safety profile.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation. “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-474-1).
Funding: The project (project no. IFKSUOR3-474-1) was funded by the Deputyship for Research & Innovation, Ministry of Education, Saudi Arabia.
Contributor Information
Faisal Imam, Email: fimam@ksu.edu.sa.
Muhammad Afzal, Email: mohmmad.afzal@bmc.edu.sa.
Nehmat Ghaboura, Email: pharmacy8.jed@bmc.edu.sa.
Khalid Saad Alharbi, Email: khalid.alharbi9@qu.edu.sa.
Imran Kazmi, Email: ikazmi@kau.edu.sa.
Samiyah Alshehri, Email: saalshehri@ksu.edu.sa.
Sana Saeed Alqarni, Email: saalqarni@ksu.edu.sa.
Emine Guven, Email: eguven@msm.edu.
References
- Aboubakr M., et al. Protective effects of N acetylcysteine and vitamin E against acrylamide-induced neurotoxicity in rats. Pak. Vet. J. 2023;43(2):262–268. doi: 10.29261/pakvetj/2023.027. [DOI] [Google Scholar]
- Aboubakr M., Farag A., et al. Antioxidant and anti-apoptotic potency of allicin and lycopene against methotrexate-induced cardiac injury in rats. Environ. Sci. Pollut. Res. Int. 2023;30(38):88724–88733. doi: 10.1007/s11356-023-28686-4. Epub 2023 Jul 13 PMID: 37440131. [DOI] [PubMed] [Google Scholar]
- Ahmad S.F., et al. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-jb, STAT3 and pro-inflammatory mediators and enhancement of IjBa and anti-inflammatory cytokines. Inflammation. 2015;38(2):846–857. doi: 10.1007/s10753-014-9994-y. [DOI] [PubMed] [Google Scholar]
- Al-Harbi N.O. Carfilzomib-induced cardiotoxicity mitigated by dexrazoxane through inhibition of hypertrophic gene expression and oxidative stress in rats. Toxicol. Mech. Methods. 2016;26(3):189–195. doi: 10.3109/15376516.2016.1143071. [DOI] [PubMed] [Google Scholar]
- Al-Shabanah O., et al. Captopril ameliorates myocardial and hematological toxicities induced by adriamycin. Biochem. and Mol. Biol International. 1998;45:419–427. doi: 10.1080/15216549800202802. [DOI] [PubMed] [Google Scholar]
- Behairy, A., Elkomy, A., Elsayed, F. et al., 2023. Spirulina and Thymoquinone Protect Against Methotrexate-Induced Hepatic Injury in Rats. Rev. Bras. Farmacogn. Doi: 10.1007/s43450-023-00470-y.
- Cardinale D., Sandri M.T. Role of biomarkers in chemotherapy-induced cardiotoxicity. ProgCardiovasc Dis. 2010;53:121–129. doi: 10.1016/j.pcad.2010.04.002. [DOI] [PubMed] [Google Scholar]
- El-Missiry M.A., et al. Attenuation of the acute adriamycin-induced cardiac and hepatic oxidative toxicity by N-(2-mercaptopropionyl) glycine in rats. Free Rad. Res. 2001;35:575–581. doi: 10.1080/10715760100301581. [DOI] [PubMed] [Google Scholar]
- Elsayed A., Elkomy A., et al. Ameliorating effect of lycopene and N-acetylcysteine against cisplatin-induced cardiac injury in rats. Pak. Vet. J. 2022;42(1):107–111. doi: 10.29261/pakvetj/2021.035. [DOI] [Google Scholar]
- Esparís-Ogando A., Alegre A., et al. Bortezomib is an efficient agent in plasma cell leukemias. Int. J. Cancer. 2005;114:665–667. doi: 10.1002/ijc.20793. [DOI] [PubMed] [Google Scholar]
- Faich G.A. Risks and indications of phenylbutazone: Another look. Pharmacotherapy. 1987;7:25–27. doi: 10.1002/j.1875-9114.1987.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Geisberg C.A., Abdallah W.M., et al. Circulating neuregulin during the transition from stage A to stage B/C heart failure in a breast cancer cohort. J. Card. Fail. 2013;19:10–15. doi: 10.1016/j.cardfail.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri A.K., Mukhopadhyay A. Mutagenicity assay in Salmonella and in vivo sister chromatid exchange in bone marrow cells of mice for four pyrazolone derivatives. Mutat. Res. 1998;420:15–25. doi: 10.1016/s1383-5718(98)00138-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., et al., 2023. Successful Treatment with Low-dose Crizotinib in a Patient with ROS1-rearranged Lung Cancer Who Developed Crizotinib-induced Heart Failure. Intern. Med. 62(2), 281-284. doi: 10.2169/internalmedicine.9157-21. Epub 2022 Jun 21. PMID: 35732445; PMCID: PMC9908384. [DOI] [PMC free article] [PubMed]
- Herndon T.M., Deisseroth A., Kaminskas E., et al. U.S. Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res. 2013;19:4559–4563. doi: 10.1158/1078-0432.CCR-13-0755. [DOI] [PubMed] [Google Scholar]
- Imam F., et al. Apremilast reversed carfilzomib-induced cardiotoxicity through inhibition of oxidative stress, NF-kB and MAPK signaling in rats. Toxicol. Mech. Methods. 2016;26(9):700–708. doi: 10.1080/15376516.2016.1236425. [DOI] [PubMed] [Google Scholar]
- Imam F., et al. Rutin Attenuates Carfilzomib-Induced Cardiotoxicity Through Inhibition of NF-κB, Hypertrophic Gene Expression and Oxidative Stress. Cardiovasc. Toxicol. 2017;17:58–66. doi: 10.1007/s12012-015-9356-5. [DOI] [PubMed] [Google Scholar]
- Inoue M. In: The Liver: Biology and Pathobiology. 5th ed. Arias I.M., Boyer J.L., Fausto N., Jokoby W.B., Schachter D.A., Shafritz D.A., editors. Raven Press; New York: 2011. Protective mechanisms against reactive oxygen species; pp. 443–459. [Google Scholar]
- Insuasty B., et al. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1- phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010;18:4965–4974. doi: 10.1016/j.bmc.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Jones W.K., Brown M., Ren X., et al. NF-kappaB as an integrator of diverse signaling pathways: the heart of myocardial signaling? Cardiovasc. Toxicol. 2003;3(3):229–254. doi: 10.1385/ct:3:3:229. [DOI] [PubMed] [Google Scholar]
- Kari F., et al. Long-term exposure to the antiinflammatory agent phenylbutazone induces kidney tumors in rats and liver-tumors in mice. Jpn. J. Cancer Res. 1995;86:252–263. doi: 10.1111/j.1349-7006.1995.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland D., Fowler P. Further analysis of Ames-negative rodent carcinogens that are only genotoxic in mammalian cells in vitro at concentrations exceeding 1 mM, including retesting of compounds of concern. Mutagenesis. 2010;25:539–553. doi: 10.1093/mutage/geq041. [DOI] [PubMed] [Google Scholar]
- Koelwyn G.J., Jones L.W., Moslehi J. Unravelling the causes of reduced peak oxygen consumption in patients with cancer: complex, timely, and necessary. J. Am. Coll. Cardiol. 2014;64:1320–1322. doi: 10.1016/j.jacc.2014.07.949. [DOI] [PubMed] [Google Scholar]
- Konukoglu D., et al. A study on the carotid artery intima-media thickness and its association with lipid peroxidation. Clin. Chim. Acta. 1998;277:91–98. doi: 10.1016/s0009-8981(98)00117-x. [DOI] [PubMed] [Google Scholar]
- Lees P., Toutain P.-L. Pharmacokinetics, pharmacodynamics, metabolism, toxicology and residues of phenylbutazone in humans and horses. Vet. J. 2013;196:294–303. doi: 10.1016/j.tvjl.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Liu S.F., Malik A.B. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mearini G., et al. The ubiquitinproteasome system in cardiac dysfunction. BBA. 2008;1782:749–763. doi: 10.1016/j.bbadis.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Méndez-Toro, et al. Carfilzomib induced cardiotoxicity in a multiple myeloma patient. Cardio-Oncology. 2020;6:17. doi: 10.1186/s40959-020-00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadifard N., et al. Trace minerals intake: Risks and benefits for cardiovascular health. Crit. Rev. Food Sci. Nutr. 2019;259(8):1334–1346. doi: 10.1080/10408398.2017.1406332. Epub 2017 Dec 13 PMID: 29236516. [DOI] [PubMed] [Google Scholar]
- Mushtaq A., et al., 2018. Efficacy and toxicity profile of carfilzomib based regimens for treatment of multiple myeloma: A systematic review. Crit. Rev. Oncol. Hematol. 125, 1-11. doi: 10.1016/j.critrevonc.2018.02.008. Epub 2018 Mar 2. PMID: 29650268; PMCID: PMC5901887. [DOI] [PMC free article] [PubMed]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Ana- Lytical Biochemistry. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Omneya K.M. Synthesis and anti-inflammatory activity of 1- acetyl/propanoyl-5-aryl-3-(4-morpholinophenyl)-4, 5- dihydro-1H pyrazole derivatives. Med. Chem. Res. 2012;21:3240–3245. [Google Scholar]
- Perkins N.D., Gilmore T.D. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13(5):759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Saleem S., Khan R., et al. Oxyphenbutazone promotes cytotoxicity in rats and Hep3B cells via suppression of PGE2 and deactivation of Wnt/β-catenin signaling pathway. Mol. Cell. Biochem. 2018;444(1–2):187–196. doi: 10.1007/s11010-017-3243-2. [DOI] [PubMed] [Google Scholar]
- Sallam A.O., et al. The Ameliorative Effects of L-carnitine against Cisplatin-induced Gonadal Toxicity in Rats. Pak. Vet. J. 2021;41(1):147–151. doi: 10.29261/pakvetj/2020.082. [DOI] [Google Scholar]
- Sawaya H., Sebag I.A., Plana J.C., et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein bound and non-protein bound sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Shah C., Bishnoi R., et al. Cardiotoxicity associated with carfilzomib : systematic review and meta-analysis. Leuk. Lymphoma. 2018:1–13. doi: 10.1080/10428194.2018.1437269. [DOI] [PubMed] [Google Scholar]
- Singal P.K., Iliskovic N. Doxorubicin-induced cardiomyopathy. New Eng. Jour. of Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- Tong Wu. Cyclooxygenase-2 in hepatocellular carcinoma CANCER. TREATMENT REVIEWS. 2006;32:28–44. doi: 10.1016/j.ctrv.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Tosaki A. Arrhythmo Geno Pharmaco Therapy. Front Pharmacol. 2020;211:616. doi: 10.3389/fphar.2020.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh P., Hari P.K., et al. Synthesis of coumarin fused pyrazoline-5- one derivatives and screening for their antimicrobial and antioxidant activity. J. Pharm. Res. 2012;5:875–877. [Google Scholar]
- Wang X., et al. Cryptotanshinone Ameliorates Doxorubicin-Induced Cardiotoxicity by Targeting Akt-GSK-3β-mPTP Pathway In Vitro. Molecules. 2021;26(5):1460. doi: 10.3390/molecules26051460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Yang T., et al. Effects of infliximab on oxidative stress and inflammation of H9c2 cells induced by H2O2: Effect of infliximab in myocardial infarction. Cell. and Mol. Biol. 2023;69(9):213–218. doi: 10.14715/cmb/2023.69.9.33. [DOI] [PubMed] [Google Scholar]
- Yeh E.T.H., et al. Review: Current perspective: Cardiovascular complications of cancer therapy diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- Yu H., et al., 2020. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020 5(1), 209. doi: 10.1038/s41392-020-00312-6. PMID: 32958760; PMCID: PMC7506548. [DOI] [PMC free article] [PubMed]
- Zilinyi R., et al. The Cardioprotective Effect of Metformin in Doxorubicin-Induced Cardiotoxicity: The Role of Autophagy. Molecules. 2018;23(5):1184. doi: 10.3390/molecules23051184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindenberg C.S., Olin K.L., Villarweva J. Ethanol induced changes in hepatic free radical defense mechanisms and fatty acid composition in the miniature pig. Hepatology. 1991;13:1185–1192. [PubMed] [Google Scholar]