Abstract
Corneal allogenic intrastromal ring segments (CAIRS) refer to the intracorneal placement of fresh, unprocessed, processed, preserved, or packaged allogenic rings/segments of any type/length. We described uniform-thickness CAIRS previously. We now describe a new technique of customized CAIRS to personalize the flattening effect as per individual topography. A prospective interventional case series of patients with pericentral/ paracentral decentered cones and gradation of keratometry with one side steeper than the other was conducted. Individually customized tapered CAIRS with variable volume, arc length, taper length, and gradient of taper were implanted. In total, 32 eyes of 29 patients with at least 1-year follow-up were included. Special double-bladed trephines and a CAIRS customizer template allowed the creation of individually customized CAIRS. Mean uncorrected distance visual acuity (UDVA) and spectacle-corrected distance visual acuity improved from 0.22 to 0.47 (P = 0.000) and from 0.76 to 0.89 (P = 0.001), respectively. Significant improvement was seen in K1, K2, Km, Kmax, topographic astigmatism, Q-value, sphere, cylinder, spherical equivalent, Root Mean Square (RMS), Higher Order Aberrations (HOA), and vertical coma (P < 0.01, 0.05). There was no significant change in the width or height of CAIRS between 1 month and last visit on anterior-segment optical coherence tomography. Five eyes continued to remain at the same UDVA, 27 eyes had at least 2 lines, and 13 eyes had at least 3 or more lines improvement in UDVA. The maximum improvement in UDVA was 7 lines. A significant difference in flattening was obtained at different zones across the tapered CAIRS. Thus, differential flattening was achieved across the cone based on the customization plan. Personalized customization was possible for each cornea, unlike limited models of progressive-thickness synthetic segments. Allogenic nature, greater customizability, efficacy, and absent need for large inventories are advantages compared to synthetic segments.
Keywords: Asymmetric ICRS, asymmetric CAIRS, contact lens-assisted corneal crosslinking, CAIRS, corneal allogenic intrastromal ring segment, corneal crosslinking, CACXL, customized CAIRS, ectasia, ICRS, intra corneal ring segment, INTACS, kerarings, keratoconus, progressive thickness CAIRS, shaped CAIRS, tapered CAIRS, variable thickness CAIRS
Corneal allogenic intrastromal ring segments (CAIRS)[1] was first performed in 2015, and as described by us, refers to the intracorneal placement of ring segments of allogenic tissue of any source such as fresh cut segments, unprocessed, processed, preserved, or packaged segments.[1] These are being performed with greater frequency[2,3,4,5,6,7,8,9,10] because of the refractive and topographic effects they provide similar to but with greater range of effect than with synthetic intracorneal ring segments (ICRS). The allogeneic nature also decreases the risk of complications such as overlying melt, necrosis, intrusion, extrusion, and migration.[11,12,13,14,15,16,17,18] Though CAIRS has previously been used as uniformly cut longitudinal segments,[1,2,3,4,5,6,7,8,9,10] some ectatic corneas may benefit from greater customization. In this article, we describe a new technique that we call as customized or personalized or asymmetric CAIRS to achieve tailored segments better suited to individual topographic characteristics. This was done by using the principle of Barraquer's thickness law, which states that corneal flattening is achieved by adding tissue to the periphery. We customized CAIRS to have varying volumes in different meridia to achieve variation in the amount of flattening achieved. This technique of customized CAIRS was described by one of the authors (SJ).
Technique
This prospective interventional case series was approved by the institutional review board and the procedure conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients. Patients with keratoconus between Amsler–Krumeich stage 1 and 4 with pericentral or paracentral decentered cones who showed gradation of keratometric values with one side being steeper than the other were included in this study. Exclusion criteria for the purpose of this study were those with severe allergies, autoimmune and immunodeficiency syndromes, previous viral keratitis, central or paracentral scarring, corneas too thin to allow accelerated contact lens-assisted corneal crosslinking (A-CACXL), (<320 microns minimum pachymetry),[19,20,21] and history of any previous ocular surgery other than corneal cross-linking.
The decision to insert customized CAIRS was made based on the presence of zonal asymmetry within the steep areas of the keratometric map. The gradation of keratometric values in the ectatic zone on the axial curvature map was evaluated, and in case of asymmetry across meridia, an individualized topographical plan was created for each patient regarding total arc length and the length of the tapered zone. Thus, asymmetry from one side to the other was looked for within the area of the cone, and if found, the option of customized CAIRS was chosen. The area of the cone was then divided by three imaginary lines (A, B, and C): A and B demarcated the area of maximum keratometric values, and C demarcated the end of the decreasing gradient of the cone. The CAIRS segment was customized so that the area between A and B was planned as uniform thickness and a taper was given to the part of the segment planned between B and C. In effect, more volume of tissue was added to the meridia requiring greater flattening, and less volume was added where less flattening was required. The steepest part of the cone thus received more tissue, and the segment was thinned down in the flatter part of the cone. All surgeries were performed by a single surgeon (SJ).
All patients underwent pre- and postoperative slit-lamp examination, uncorrected distance visual acuity (UDVA), spectacle-corrected distance visual acuity (SCDVA) assessment (reported in decimal equivalent), dilated funduscopy, rigid gas permeable contact lens trial, Pentacam® (Oculus Optikgeräte GmbH, Wetzlar, Germany) imaging, and anterior-segment optical coherence tomography (AS-OCT, MS-39, CSO, Fl, Italy). Postoperative evaluations were at days 1, 7, and 30 and thereafter at 6 months and 1 year.
Preparation of customized CAIRS
As mentioned earlier, any allogenic tissue source may be used to prepare CAIRS and the cuts used to create the segments may be made using any technique. We used preserved donor corneoscleral rim denuded of epithelium and endothelium and a special double bladed trephine in our series. Only non-edematous donor cornea was chosen for creating CAIRS. Endothelial cell count was, however, not considered. The donor was laid on a concave Teflon block with the posterior stromal side facing up, and the double bladed Jacob CAIRS trephineTM (Madhu Instruments, India) [Fig. 1a] of either 6.5/8 mm or 7.75/8.75 mm (inner/outer) diameter was used to punch a circular stromal ring [Fig. 1b]. The Bowman's membrane (BM) side of the CAIRS was inked using a surgical marker pen to facilitate later identification of the side [Fig. 1c]. The circular CAIRS was then removed from the trephine and cut to obtain a strip of tissue [Fig. 1d]. This strip was flattened out to lay the BM on one side and the posterior stroma on the other side [Fig. 2a]. A special dome-shaped customizer template instrument (Jacob CAIRS customizerTM, Epsilon Instruments, USA) that was designed to facilitate accurate customization was then used. This convex titanium disc has circular optic zones from 3 to 12 mm as well as radial clock hours or the 12 major meridia marked on it [Fig. 2b]. The CAIRS strip with the BM side facing centrally was aligned on the desired optic zone of the CAIRS customizer as per the plan created for the eye. For our series, we chose an optic zone of 4.6 mm and hence the CAIRS strip was aligned against the outer edge of the 4.5-mm optic zone mark on the CAIRS customizerTM [Fig. 2c]. An inked fine Sinskey hook was used to apply two radial marks corresponding to the total required arc length and an additional radial mark at the meridian where tapering was to be initiated [Fig. 2d]. The zone of the taper was additionally inked intermittently to avoid confusion regarding the side of the taper once it was taken off the customizer. The inked tissue strip was then laid back on the Teflon block, and the desired total arc length and length of taper were cut using a sharp 15° side-port blade [Fig. 2e]. The prepared customized CAIRS was then carefully kept aside on a Teflon punch [Fig. 2f].
Figure 1.
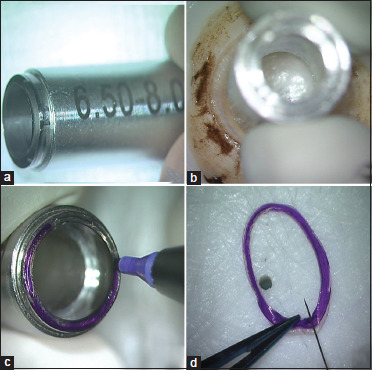
(a) Special double-bladed trephine (Jacob CAIRS trephineTM, Madhu Instruments, India); (b) CAIRS Trephine used to punch allogenic tissue (here, donor cornea denuded of epithelium and endothelium); (c) Bowman's membrane (BM) side of CAIRS inked using surgical marker pen to facilitate later side identification; (d) Circular CAIRS removed from trephine and cut to obtain strip of tissue
Figure 2.
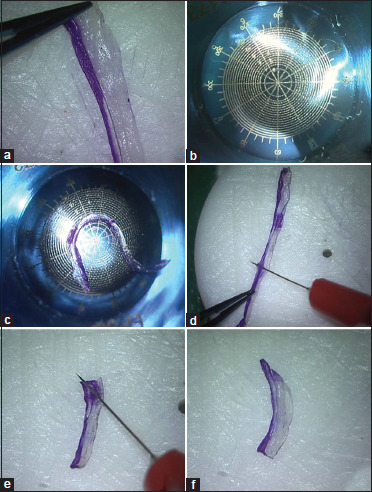
(a) CAIRS strip flattened out to lay BM on one side and posterior stroma on the other; (b) Jacob CAIRS customizerTM (Epsilon Instruments, USA) has circular optic zones from 3 to 12 mm and radial clock hours or 12 major meridia marked; (c) Tissue kept on CAIRS customizer and total arc length and taper length marked; (d and e) Inked tissue strip laid on Teflon block and desired total arc length and taper length cut using a sharp 15° side-port blade; (f) A tapered CAIRS seen prepared and ready for insertion
Intra-stromal channel creation in patient's cornea
Next, in the seated position, 0° and 180° marks were applied on the patient's cornea by using the horizontal slit-lamp beam to correct for potential cyclotorsion in the lying down position. The remaining corneal marks were placed with the patient lying down under the microscope of the VisuMax 500-kHz femtosecond platform (Carl Zeiss Meditec, Jena, Germany). The coaxially sighted corneal light reflex (CSCLR) was marked [Fig. 3a]. In case it lay outside the pupil, a point between the pupillary center and the CSCLR was marked. The Jacob clock gauge markerTM and the Jacob single-blade radial markerTM (Epsilon Instruments, USA) were used to mark the clock hours/meridia as per the individual patient's topographic plan for total arc length and taper transition [Fig. 3b and c]. The femtosecond laser was then centered on the marked CSCLR and used to create a laser-assisted intracorneal tunnel with an inner diameter of 4.6 mm and a tunnel width of approximately 1.5 mm. The tunnel depth was programmed to be at 50% of the minimum stromal thickness in the zone of implantation up to a maximum depth of 280 microns. For CAIRS with arc lengths up to 160°, two opposing access incisions were created with the femtosecond laser. For CAIRS with longer arc lengths, only one access incision was made with the femtosecond laser, and a second incision was created manually beyond the expected arc length of the planned CAIRS by cutting down with a 15° blade over a curved rod inserted into the channel.
Figure 3.
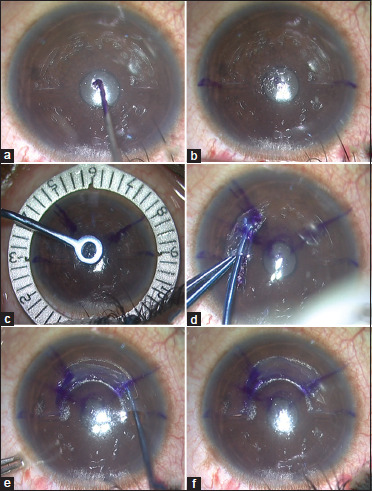
(a) Coaxially sighted corneal light reflex (CSCLR) marked; (b) 0° and 180° marked on patient's cornea by using horizontal slit-beam in sitting-down position; (c) Jacob clock gauge markerTM and Jacob single-blade radial markerTM (Epsilon Instruments, USA) used to mark clock hours/meridia as per individual patient's topographic plan for total arc length and taper transition; (d and e) Right and left Jacob curved Y-rodsTM and Jacob curved reverse Sinskey hooksTM (Epsilon Eye Instruments, USA) used to insert CAIRS into femtosecond channel created in patient's cornea; (f) Customized CAIRS segment seen lying within cornea, the total arc length and taper zone well aligned and matching with marks placed on patient's cornea as per the topographic plan
Insertion of customized CAIRS
CAIRS were inserted into the circular channel by using the right and left Jacob curved Y-rodsTM and Jacob curved reverse Sinskey hooksTM (Epsilon Eye Instruments, USA) – the former for pushing the segment forwards and the latter for pulling it in further from the opposite incision [Fig. 3d and e]. To facilitate ease of insertion, the broad edge of the CAIRS was introduced as the leading end and the taper trailed behind in a clockwise or anticlockwise direction depending on the plan. The curved instruments helped to easily slide the segments into the femtosecond channel in the patient's cornea and segment dehydration prior to implantation was not used in any case. The inked BM side of the CAIRS was placed facing internally toward the pupil. The inked mark also served as a guide to ensure that the CAIRS was inserted uniformly and without any twists in the segment. CAIRS were positioned to finally lie as per the planned total arc length and taper transition marked on the patient's cornea [Fig. 3f].
Corneal cross-linking
After segment insertion, all patients showing progression defined as an increase in simulated keratometry or maximum keratometry values greater than 0.75 diopters (D) in the preceding 6 months underwent accelerated corneal cross-linking (A-CXL) at 10 mW/cm2 for 9 minutes – either without contact lens or as CACXL[19,20,21,22,23] – depending on the minimum corneal thickness. In already cross-linked or non-progressive cases, cross-linking was omitted.
Postoperatively, all patients were maintained on dexamethasone eye drops six times per day for 2 weeks, tapered and stopped over the next 2 weeks. Antibiotic eye drops were applied for the initial 2 weeks [Video Clip 1].
Statistical methods
The data were analyzed using IBM SPSS statistics software (Version 20) in Chicago, IL, USA. All continuous variables were expressed using mean ± standard deviation or median (interquartile range). Normality assumption for clinical parameters and change in clinical parameters were assessed using the Shapiro–Wilk test. The comparison between the change in clinical parameters before and after treatment was done using a paired-sample t test or Wilcoxon signed-rank test based on the normality assumption. The difference in change in clinical parameters between the treatment group was tested using an independent sample t test or Mann–Whitney U test. P values <0.05 were considered significant.
Results
Thirty-two eyes of 29 patients with at least 1-year follow-up who underwent customized tapered or asymmetric CAIRS implantation between 2019 and 2020 for keratoconus with pericentral or paracentrally decentered cones who showed gradation of keratometric values within the cone with one side being steeper than the other were included in this study. Sixteen male and 13 female patients with K-max ranging from 52 to 74 D were included. CAIRS implantation was combined with accelerated cross-linking in 31 eyes (CACXL - 10 eyes, CXL - 21 eyes), while one previously cross-linked eye underwent CAIRS without simultaneous cross-linking. In all patients, the customized CAIRS corresponded accurately to the corneal marks placed on the patient's eye. The mean arc length of the CAIRS inserted was 144.87 ± 17.63° (range: 90°–180°). Mean UDVA improved from 0.22 to 0.47 (P = 0.000), and mean SCDVA improved from 0.76 to 0.89 (P = 0.001). Significant improvement was seen in K1, K2, Km, Kmax, topographic astigmatism, Q value, sphere, cylinder, spherical equivalent, Root Mean Square (RMS), Higher Order Aberrations (HOA), and vertical coma (P < 0.01, 0.05). Five eyes continued to remain at the same UDVA, 27 eyes had at least 2 lines, and 13 eyes had at least 3 or more lines of improvement in UDVA. The maximum improvement in UDVA obtained was by 7 lines. Tables 1a and 1b discuss results in greater detail.
Table 1a.
Evaluation of preoperative and postoperative parameters
| Parameters (n=32) | Mean±SD | Median | Min, Max | P |
|---|---|---|---|---|
| UDVA Pre | 0.22±0.11 | 0.25 | 0.08, 0.50 | 0.000** |
| UDVA Post | 0.47±0.24 | 0.42 | 0.17, 1.00 | |
| SCDVA Pre | 0.76±0.19 | 0.67 | 0.33, 1.00 | 0.001** |
| SCDVA Post | 0.89±0.17 | 1 | 0.50, 1.00 | |
| SPHERE Pre | −1.95±2.8 | −1.38 | −10.0, 1.5 | 0.000** |
| SPHERE Post | −0.62±2.09 | 0 | −7.0, 2.5 | |
| CYLINDER Pre | −3.97±1.34 | −3.5 | −6.5, −1.0 | 0.001** |
| CYLINDER Post | −2.78±1.59 | −3 | −5.5, 0.0 | |
| Spherical Equivalent Pre | −3.94±2.81 | −3.13 | −11.75, −0.38 | 0.000** |
| Spherical Equivalent Post | −2.01±2.25 | −1.75 | −8.25, 1.00 | |
| K1 – front Pre | 46.53±2.82 | 46.1 | 41.7, 54.2 | 0.000** |
| K1 – front Post | 43.56±2.76 | 43.3 | 39.7, 52.7 | |
| K2 – front Pre | 51.91±3.5 | 51.7 | 46.6, 60.5 | 0.000** |
| K2 – front Post | 47.96±3.3 | 48.1 | 40.9, 57.7 | |
| Km – front Pre | 49.07±3.06 | 48.8 | 44.4, 57.1 | 0.000** |
| Km – front Post | 45.63±2.91 | 45.3 | 40.8, 55.1 | |
| Astigmatism Front Pre | 5.23±1.44 | 5.4 | 1.9, 7.8 | 0.001* |
| Astigmatism Front Post | 4.3±1.67 | 3.75 | 0.2, 7.8 | |
| Q-value Front Pre | −1.08±0.42 | −1.08 | −2.16, −0.48 | 0.000** |
| Q-value Front Post | −0.49±0.46 | −0.36 | −1.82, 0.22 | |
| K Max Pre | 58.9±5.54 | 58.25 | 52, 74 | 0.000** |
| KMax Post | 54.95±4.71 | 53.55 | 47, 67 | |
| Thinnest Pre | 449.88±35.99 | 457 | 375, 513 | 0.66 |
| Thinnest Post | 455.94±45.74 | 458 | 375, 548 | |
| RMS HOA (µm) Pre | 2.85±0.78 | 2.89 | 1.58, 4.42 | 0.040* |
| RMS HOA (µm) Post | 2.59±0.8 | 2.69 | 1.02, 4.05 | |
| Vertical coma (µm) Pre | −2.09±0.96 | −2.1 | −3.81, 0.56 | 0.001** |
| Vertical coma (µm) Post | −1.4±1.21 | −1.55 | −3.56, 1.12 | |
| Horizontal coma (µm) Pre | −0.02±1.08 | −0.07 | −1.97, 2.49 | 0.695 |
| Horizontal coma (µm) Post | 0.05±0.98 | 0.06 | −1.80, 2.47 | |
| AS-OCT segment width 1 month | 1734.53±129.95 | 1737 | 1524, 2007 | 0.822 |
| AS-OCT segment width Last Visit | 1725.63±144.11 | 1697 | 1414, 2065 | |
| AS-OCT segment height 1 month | 391.38±82.21 | 384.5 | 251, 578 | 0.112 |
| AS-OCT segment height Last Visit | 378.84±74.44 | 384 | 250, 540 |
**P<0.01 (Wilcoxon signed rank test), UDVA=Uncorrected Distance Visual Acuity, SCDVA: Spectacle Corrected Distance Visual Acuity, SD=Standard Deviation, Pre=Preoperative, Post=Postoperative
Table 1b.
Comparison in gradient of flattening between different zones
| Groups | Mean±SD | Median | Min, Max | P |
|---|---|---|---|---|
| Zone A | 9.99±3.26 | 10.75 | 4.3, 14.5 | 0.000** |
| Zone B | 6.06±2.30 | 5.65 | 2.6, 11.2 | |
| Zone B | 6.06±2.30 | 5.65 | 2.6, 11.2 | 0.000** |
| Zone C | 2.53±1.03 | 2.40 | 0, 6.1 | |
| Zone C | 2.53±1.03 | 2.40 | 0, 6.1 | 0.000** |
| Zone A | 9.99±3.26 | 10.75 | 4.3, 14.5 |
**P<0.01 (Mann–Whitney U test)
The gradient of flattening obtained by the tapered CAIRS was analyzed using the Mann–Whitney U test by studying three different zones: Zone A – through the maximum thickness, Zone B – through the tapered zone, and Zone C through the edge of the taper. Zones A, B, and C showed an average flattening of 9.99 ± 3.26, 6.06 ± 2.30, and 2.53 ± 1.03 D, respectively. There was a significant difference in the flattening obtained at Zone A versus B (P = 0.000), Zone B versus C (P = 0.000), and Zone C versus A (P = 0.000). There was no significant difference between the height and width of the segments measured by AS-OCT at 1 month and at last visit. Mild opacification of the CAIRS was noted in seven patients on slit-lamp examination, but this was not seen visibly in room light. No other complications were seen.
Discussion
The initial as well as subsequent reports of CAIRS have described uniform thickness segments.[1,2,3,4,5,6,7,8,9,10,11] However, many topographical characteristics make customization of intrastromal segments more desirable.[24,25,26,27,28,29,30,31] In this case series, we created tapered segments for patients with pericentral or paracentral decentered cones who showed gradation of keratometric values with one side being steeper than the other. We attempted to do this by varying the volume of tissue implanted in different zones – adding more volume where greater flattening was required and less where less flattening was required. As per Barraquer's thickness law,[32,33,34] adding tissue to the periphery flattens the central cornea. As expected, by implanting less volume of tissue by tapering the CAIRS thickness over a specific area of the cornea, we were able to achieve an asymmetric effect that led to less stromal flattening in this area [Fig. 4a-d]. While creating the CAIRS segment, to avoid falling short of expected results, only non-edematous donor cornea was chosen for creating the segments. Endothelial cell count was, however, not considered as a selection criterion.
Figure 4.
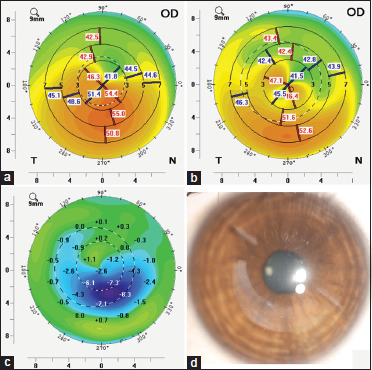
(a) Preoperative keratometric map; (b) Postoperative keratometric map; (c) Difference showing a gradient of flattening induced by customized tapered CAIRS closely following the morphological pattern of the inserted CAIRS; (d) Slit lamp image of the customized CAIRS
Our results showed significant improvements in UDVA and SCDVA; a significant decrease in sphere, cylinder, and higher-order aberrations; and a significant improvement in most parameters of the topographic map. The CAIRS customizerTM allowed accurate sizing and transitions as per individualized topography. The presence of different optic zones engraved on the instrument allowed easy and accurate translation of the planned topographic map to the patient's eye. It thus helps make the procedure easy and repeatable for anyone around the world.
Asymmetric synthetic ICRS have previously been reported to regularize the corneal shape and result in appreciable visual improvement in patients with a significant difference between corneal topographic and comatic axes.[25,26,27,28,29,30,31] These asymmetric segments are available as fixed combinations of arc length, diameter, thickness variation, width variation, and in clockwise and anti-clockwise versions. Moreover, the thickness changes at a uniform rate from one end to the other, without varying stretches of uniform thickness as we were able to do in the case of CAIRS. Therefore, although such fixed combinations in the case of synthetic segments result in having to limit the choice to the best possible one, even if less than ideal for the individual patient, CAIRS offers infinite possibilities to customize allowing true personalization to each patient. For instance, in our cases, we used neither a fixed arc length or thickness nor a uniform taper from one end to the other but chose different total arc lengths and thickness with differing zones of taper that was planned as per the keratometric map.
Thus, customized CAIRS, while allowing fixed combinations, also allows the ability to freely customize to obtain the exact desired combination of arc length, volume, transition zone, and taper. It can be easily customized based on the presenting clinical and topographic situation. This advantage of a high level of customization would not be possible with synthetic asymmetric ICRS. Even with the disadvantage of only fixed combinations being available, a large inventory of synthetic segments is needed to be maintained, with different arc lengths and optic zones, thicknesses and widths,[25,26,27,28,29,30,31] whereas customized CAIRS can be easily cut on the spot by the surgeon or rapidly provided pre-cut as per plan by eye banks.
Further studies are needed to clarify the exact mechanisms of action of CAIRS. It might be assumed that unless the segment is wider than the channel, inserting a segment of variable thickness into a channel of uniform width would not have the effect desired from variable thickness. However, this was not the case as seen in our study. This is because even if the channel is of uniform size, a segment of variable thickness would still exert a variable effect as it acts mainly by Barraquer's law of tissue addition. The channel would collapse over the thin part of the segment and thus would fit both parts of the segment. The effect of mild volume changes on localized topography has also been seen in complicated partial lenticular extraction during SMILE surgery[35] and in studies on myopic and hyperopic lenticule addition for keratoconus patients.[36,37] The change in profile created by these lenticules placed within pockets larger than the lenticule illustrates the effect of mild volume changes translating into contrasting results.
Furthermore, CAIRS offers additional advantages of being allogenic and is, therefore, less likely to lead to complications such as anterior or posterior stromal necrosis, migration, melt, extrusion, and intrusion,[6,7,8,9] unlike synthetic segments. AS-OCT did not show any significant change between 1 month and last visit; however, longer studies with serial follow-up AS-OCT and topography are required to ascertain this.
Preoperative K1, K2, and Kmax ranged from 41.7–54.2 D, 46.6–60.5 D, and 52–74 D, respectively, in our study. Some patients with mild keratoconus might be eligible for topography-guided treatment combined with CXL. However, even in mild cases, CAIRS has the advantage over this in being an additive procedure and thereby avoiding the risk of possible destabilization. It has obvious advantages in moderate to advanced keratoconus. In the future, however, a comparison between these two techniques for mild keratoconic patients would be desirable.
The limitation of this report is the lack of a nomogram to predict outcomes more accurately. Randomized controlled trial with matched patients is required to study the magnitude of the effect of customization as compared to standard uniform thickness CAIRS. However, this study does serve as a proof-of-concept to determine the feasibility of customizing CAIRS according to individual patient topography. In the described cases, we created single-edge tapered CAIRS; however, other types of customized CAIRS are also possible, such as double-edged tapered, broad-edged tapered, and central tapered. A larger trial with a defined nomogram, greater number of patients, the inclusion of all phenotypes of keratoconus, and a longer follow-up is underway to better understand the clinical implication of different CAIRS customization modalities.
To conclude, customized CAIRS is a distinct type of CAIRS, designed based on the individual patient's topography and refractive requirement. It can be symmetric or asymmetric; specially shaped, selectively tapered, or with sharp transitions; with variable/progressive thickness and/or variable/progressive width; with the type, direction, thickness, volume, arc length, and location of change adjustable by the surgeon. Our report does not intend to define all possible types of customizations or to create algorithms and nomograms but serves to report the initial results of customization as a proof-of-concept showing the possibility of creating such customized segments with CAIRS to obtain topographic effects tailored to the individual patient.
Financial support and sponsorship:
Nil.
Conflicts of interest:
Dr. Soosan Jacob: patent granted for special trephines and devices used to create these segments; patents pending for CAIRS segments and various types of shaped corneal segments; Madhu Instruments, Ziemer Ophthalmic Systems. None of the other authors have any financial interests.
Video available on: https://journals.lww.com/ijo
References
- 1.Jacob S, Patel SR, Agarwal A, Ramalingam A, Saijimol AI, Raj JM. Corneal allogenic intrastromal ring segments (CAIRS) combined with corneal cross-linking for keratoconus. J Refract Surg. 2018;34:296–303. doi: 10.3928/1081597X-20180223-01. [DOI] [PubMed] [Google Scholar]
- 2.Parker JS, Dockery PW, Jacob S, Parker JS. Preimplantation dehydration for corneal allogenic intrastromal ring segment implantation. J Cataract Refract Surg. 2021;47:e37–9. doi: 10.1097/j.jcrs.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 3.Dapena I, Parker JS, Melles GRJ. Potential benefits of modified corneal tissue grafts for keratoconus: Bowman layer ‘inlay’ and ‘onlay’ transplantation, and allogenic tissue ring segments. Curr Opin Ophthalmol. 2020;31:276–83. doi: 10.1097/ICU.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 4.Parker JS, Dockery PW, Parker JS. Flattening the curve: Manual method for corneal allogenic intrastromal ring segment implantation. J Cataract Refract Surg. 2021;47:e31–3. doi: 10.1097/j.jcrs.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 5.Parker JS, Dockery PW, Parker JS. Trypan blue-assisted corneal allogenic intrastromal ring segment implantation. J Cataract Refract Surg. 2021;47:127. doi: 10.1097/j.jcrs.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 6.Daoud RC, Sammouh FK, Baban TA, Warrak JE, Warrak EL. Allogenic corneal tissue transplantation in substitution for extruded intracorneal rings: A case series. J Fr Ophtalmol. 2019;42:1090–3. doi: 10.1016/j.jfo.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Jarade E, Issa M, Chanbour W, Warhekar P. Biologic stromal ring to manage stromal melting after intrastromal corneal ring segment implantation. J Cataract Refract Surg. 2019;45:1222–5. doi: 10.1016/j.jcrs.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Carpel EF, Santilli C, Maltry A. Long-term viability of allogenic donor stroma. Indian J Ophthalmol. 2020;68:3057–9. doi: 10.4103/ijo.IJO_1008_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozhaya K, Mehanna CJ, Jacob S, Saad A, Jabbur NS, Awwad ST. Management of anterior stromal necrosis after polymethyl-methacrylate corneal ring segments: Explantation versus exchange with corneal allogenic intrastromal ring segments (CAIRS) J Refract Surg. 2022;38:256–63. doi: 10.3928/1081597X-20220223-01. [DOI] [PubMed] [Google Scholar]
- 10.Awwad ST, Jacob S, Assaf JF, Bteich Y. Extended dehydration of corneal allogenic intrastromal ring segments to facilitate insertion: The corneal jerky technique. Cornea. 2023 doi: 10.1097/ICO.0000000000003328. doi: 10.1097/ICO.0000000000003328. [DOI] [PubMed] [Google Scholar]
- 11.Kanellopoulos AJ, Pe LH, Perry HD, Donnenfeld ED. Modified intracorneal ring segment implantations (INTACS) for the management of moderate to advanced keratoconus: Efficacy and complications. Cornea. 2006;25:29–33. doi: 10.1097/01.ico.0000167883.63266.60. [DOI] [PubMed] [Google Scholar]
- 12.Coskunseven E, Kymionis GD, Tsiklis NS, Atun S, Arslan E, Siganos CS, et al. Complications of intrastromal corneal ring segment implantation using a femtosecond laser for channel creation: A survey of 850 eyes with keratoconus. Acta Ophthalmol. 2011;89:54–7. doi: 10.1111/j.1755-3768.2009.01605.x. [DOI] [PubMed] [Google Scholar]
- 13.Jarade E, Dirani A, Fadlallah A, Antonios R, Cherfan G. New technique of intracorneal ring segments suturing after migration. J Refract Surg. 2013;29:855–7. doi: 10.3928/1081597X-20130723-01. [DOI] [PubMed] [Google Scholar]
- 14.Diakonis VF, Kankariya VP, Woreta F, Yoo SH, Lubahn JG, Kymionis GD, et al. Refractive and topographic fluctuations due to intracorneal ring segments motility. J Refract Surg. 2014;30:140–2. doi: 10.3928/1081597X-20131112-01. [DOI] [PubMed] [Google Scholar]
- 15.Kugler LJ, Hill S, Sztipanovits D, Boerman H, Swartz TS, Wang MX. Corneal melt of incisions overlying corneal ring segments: Case series and literature review. Cornea. 2011;30:968–71. doi: 10.1097/ICO.0b013e3182031ca0. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Ramamurthi S, Ramaesh K. Late dislocation of intrastromal corneal ring segment into the anterior chamber. J Cataract Refract Surg. 2010;36:2003–5. doi: 10.1016/j.jcrs.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Cosar CB, Sridhar MS, Sener B. Late onset of deep corneal vascularization: A rare complication of intrastromal corneal ring segments for keratoconus. Eur J Ophthalmol. 2009;19:298–300. doi: 10.1177/112067210901900222. [DOI] [PubMed] [Google Scholar]
- 18.Moshirfar M, Bean AE, Desautels JD, Birdsong OC. Corneal hydrops secondary to intrastromal corneal ring intrusion into the anterior chamber 7 years after implantation: A case report. Ophthalmol Ther. 2017;6:373–9. doi: 10.1007/s40123-017-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob S, Kumar DA, Agarwal A, Basu S, Sinha P, Agarwal A. Contact lens-assisted collagen cross-linking (CACXL): A new technique for cross-linking thin corneas. J Refract Surg. 2014;30:366–72. doi: 10.3928/1081597X-20140523-01. [DOI] [PubMed] [Google Scholar]
- 20.Knyazer B, Kormas RM, Chorny A, Lifshitz T, Achiron A, Mimouni M. Corneal cross-linking in thin corneas: 1-year results of accelerated contact lens-assisted treatment of keratoconus. J Refract Surg. 2019;35:642–8. doi: 10.3928/1081597X-20190903-01. [DOI] [PubMed] [Google Scholar]
- 21.Srivatsa S, Jacob S, Agarwal A. Contact lens assisted corneal cross linking in thin ectatic corneas - A review. Indian J Ophthalmol. 2020;68:2773–8. doi: 10.4103/ijo.IJO_2138_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matlov Kormas R, Abu Tailakh M, Chorny A, Jacob S, Knyazer B. Accelerated CXL versus accelerated contact lens-assisted CXL for progressive keratoconus in adults. J Refract Surg. 2021;37:623–30. doi: 10.3928/1081597X-20210609-02. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Roozbahani M, Piccinini AL, Golan O, Hafezi F, Scarcelli G, et al. Depth-dependent reduction of biomechanical efficacy of contact lens-assisted corneal cross-linking analyzed by brillouin microscopy. J Refract Surg. 2019;35:721–8. doi: 10.3928/1081597X-20191004-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barraquer R, Alfonso J, Murta J. Keratoconus patterns and intrastromal segments. J Surg. 2012;90:s249. [Google Scholar]
- 25.Prisant O, Pottier E, Guedj T, Hoang Xuan T. Clinical outcomes of an asymmetric model of intrastromal corneal ring segments for the correction of keratoconus. Cornea. 2020;39:155–60. doi: 10.1097/ICO.0000000000002160. [DOI] [PubMed] [Google Scholar]
- 26.Baptista PM, Marques JH, Neves MM, Gomes M, Oliveira L. Asymmetric thickness intracorneal ring segments for keratoconus. Clin Ophthalmol. 2020;14:4415–21. doi: 10.2147/OPTH.S283387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kammoun H, Piñero DP, Álvarez de Toledo J, Barraquer RI, García de Oteyza G. Clinical outcomes of femtosecond laser-assisted implantation of asymmetric ICRS in keratoconus with no coincidence of topographic and comatic axes. J Refract Surg. 2021;37:693–9. doi: 10.3928/1081597X-20210712-04. [DOI] [PubMed] [Google Scholar]
- 28.Barugel R, David C, Kallel S, Borderie M, Cuyaubère R, Goemaere I, et al. Comparative study of asymmetric versus non-asymmetric intrastromal corneal ring segments for the management of keratoconus. J Refract Surg. 2021;37:552–61. doi: 10.3928/1081597X-20210526-01. [DOI] [PubMed] [Google Scholar]
- 29.Arbelaez JG, Arbelaez MC. Efficacy of progressive thickness intrastromal corneal ring segments in the treatment of duck phenotype keratoconus. Eur J Ophthalmol. 2021;31:2191–9. doi: 10.1177/11206721211001722. [DOI] [PubMed] [Google Scholar]
- 30.Cuiña Sardiña R, Arango A, Alfonso JF, Álvarez de Toledo J, Piñero DP. Clinical evaluation of the effectiveness of asymmetric intracorneal ring with variable thickness and width for the management of keratoconus. J Cataract Refract Surg. 2021;47:722–30. doi: 10.1097/j.jcrs.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 31.Vega-Estrada A, Chorro E, Sewelam A, Alio JL. Clinical outcomes of a new asymmetric intracorneal ring segment for the treatment of keratoconus. Cornea. 2019;38:1228–32. doi: 10.1097/ICO.0000000000002062. [DOI] [PubMed] [Google Scholar]
- 32.Burris TE, Ayer CT, Evensen DA, Davenport JM. Effects of intrastromal corneal ring size and thickness on corneal flattening in human eyes. Refract Corneal Surg. 1991;7:46–50. [PubMed] [Google Scholar]
- 33.Colin J, Cochener B, Savary G, Malet F, Holmes-Higgin D. INTACS inserts for treating keratoconus: One-year results. Ophthalmology. 2001;108:1409–14. doi: 10.1016/s0161-6420(01)00646-7. [DOI] [PubMed] [Google Scholar]
- 34.Rabinowitz YS, Li X, Ignacio TS, Maguen E. INTACS inserts using the femtosecond laser compared to the mechanical spreader in the treatment of keratoconus. J Refract Surg. 2006;22:764–71. doi: 10.3928/1081-597X-20061001-06. [DOI] [PubMed] [Google Scholar]
- 35.Ganesh S, Brar S, Lazaridis A. Management and outcomes of retained lenticules and lenticule fragments removal after failed primary SMILE: A case series. J Refract Surg. 2017;33:848–53. doi: 10.3928/1081597X-20171004-01. [DOI] [PubMed] [Google Scholar]
- 36.Mastropasqua L, Nubile M. Corneal thickening and central flattening induced by femtosecond laser hyperopic-shaped intrastromal lenticule implantation. Int Ophthalmol. 2017;37:893–904. doi: 10.1007/s10792-016-0349-6. [DOI] [PubMed] [Google Scholar]
- 37.Riau AK, Htoon HM, Alió Del Barrio JL, Nubile M, El Zarif M, Mastropasqua L, et al. Femtosecond laser-assisted stromal keratophakia for keratoconus: A systemic review and meta-analysis. Int Ophthalmol. 2021;41:1965–79. doi: 10.1007/s10792-021-01745-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


