Summary
Background
Exacerbated by an aging population, musculoskeletal diseases are a chronic and growing problem in the United States that impose significant health and economic burdens. The objective of this study was to analyze the correlation between the burden of diseases and the federal funds assigned to health-related research through the National Institutes of Health (NIH).
Methods
An ecological study design was used to examine the relationship between NIH research funding and disease burden for 60 disease categories. We used the Global Burden of Disease (GBD) Study 2019 to measure disease burden and the NIH Research, Condition, and Disease Categories (RCDC) data to identify 60 disease categories aligned with available GBD data. NIH funding data was obtained from the RCDC system and the NIH Office of Budget. Using linear regression models, we observed that musculoskeletal diseases were among the most underfunded (i.e., negative residuals from the model) with respect to disease burden.
Findings
Musculoskeletal diseases were underfunded, with neck pain being the most underfunded at only 0.83% of expected funding. Low back pain, osteoarthritis, and rheumatoid arthritis were also underfunded at 13.88%, 35.08%, and 66.26%, respectively. Musculoskeletal diseases were the leading cause of years lived with disability and the third leading cause in terms of prevalence and disability-adjusted life years. Despite the increasing burden of these diseases, the allocation of NIH funding to the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) has remained low compared to other institutes.
Interpretation
Despite the increasing health burden and economic cost of $980 billion annually, the allocation of NIH funding to the NIAMS has remained low compared to other institutes. These findings suggest that the NIH may need to reassess its allocation of research funding to align with the current health challenges of our country. Furthermore, these clinically relevant observations highlight the need to increase research funding for musculoskeletal diseases and improve their prevention, diagnosis, and treatment.
Funding
No funding.
Keywords: Musculoskeletal health, NIH, Research funding, Burden of disease, Years lived with disability, Disability-adjusted life years
Research in context.
Evidence before this study
Before undertaking this study, we conducted an extensive literature review to identify existing evidence related to the relationship between disease burden and research funding, particularly focusing on the National Institutes of Health (NIH) and the Global Burden of Disease (GBD) data. Our search was conducted across PubMed, Embase, and Web of Science databases, using the keywords: disease burden, research funding, NIH, GBD, relationship, funding patterns, epidemiology, health research, DALY, YLD, YLL, health services, NIH RCDC system, linear regression analysis, statistical significance, literature review, funding disparities, health outcomes, public health, epidemiological studies, health disparities, medical research, and bibliometric analysis. This encompassed studies and reports examining disease burden, funding patterns, epidemiology, health research, and their interplay without language or date restrictions. Additionally, we explored the reference lists of relevant publications for comprehensive coverage. The criteria for inclusion in our review included studies employing epidemiological methods, investigating funding disparities, health outcomes, and health disparities, and utilising statistical analyses such as linear regression. Our search also considered medical research, bibliometric analyses, and the implications of Disability-Adjusted Life Years (DALYs), Years Lived with Disability (YLDs), and Years of Life Lost (YLLs) on public health. The goal was to gather insights into the existing body of knowledge surrounding disease burden and research funding disparities to inform our ecological study design and analysis methodology.
Existing evidence primarily highlighted the importance of understanding the allocation of research funding in addressing global health challenges. While some studies had examined the relationship between disease burden and research funding in specific contexts, our study sought to comprehensively analyse NIH funding across 60 matched disease categories and their alignment with recent GBD data.
Added value of this study
Our study significantly adds to the existing evidence base by employing an ecological study design to comprehensively assess the relationship between NIH research funding and disability-adjusted life years (DALYs) for 60 diseases. The unique contribution of our research lies in bridging the gap between disease burden data from the GBD 2019 and NIH funding patterns, specifically for the years 2019 and 2021. The selection of these years was based on two considerations: Firstly, it aligns with the most current GBD data available. Secondly, it allows for a two-year interval to account for the anticipated gap in burden availability before any budgetary adjustments are implemented.
While previous research had highlighted the relevance of understanding this relationship, our study extends knowledge in the field by conducting a recent quantitative analysis of the alignment between funding allocation and disease burden, providing a practical reflection of the correlation between funding allocation and the dynamic nature of disease burden. We utilise the GBD Study 2019, which offers comprehensive insights into global disease burden and NIH funding data, providing a novel perspective on the funding landscape in the context of national health priorities.
Implications of all the available evidence
Our findings have crucial implications for both policy and practice. By demonstrating the association between NIH funding and disease burden across various disease categories, our study highlights opportunities for optimizing research investments. Policymakers and funding agencies like the NIH should consider realigning funding priorities to address health conditions that contribute significantly to the global disease burden.
Furthermore, our study underscores the importance of ongoing monitoring and evaluation of research funding patterns. As the global disease burden evolves, funding allocations should adapt to address emerging health challenges effectively. This research also paves the way for future studies to explore the impact of research funding on disease outcomes and intervention effectiveness, offering valuable insights for evidence-based decision-making in global health research and policy.
Introduction
Global health morbidity has shifted in recent decades from communicable, maternal, neonatal, and nutritional (CMNN) diseases to non-communicable diseases (NCDs).1 As death rates decline, non-fatal injuries and NCDs have become a significant concern for health systems. In addition, since 2000, the global population has increased by 1.7 billion,2 and life expectancy has risen by 6.3 years.3 However, the health-adjusted life expectancy, the average number of years that a person can expect to live in full health, has only increased by 4.9 years,3,4 indicating a shift in disease burden from childhood illness and infectious disease to unhealthy adulthood. The trend of lower fertility rates, older population, and increased healthy life expectancy may present unique challenges for healthcare systems unprepared to address the shift from communicable to non-communicable diseases.5
Musculoskeletal disorders encompass diverse conditions affecting bones, joints, muscles, and connective tissues.6 As a result of an aging population, musculoskeletal diseases are an emerging cause of health and financial burden in the United States,1 where they affect more than one in three people in the U.S., approximately 127.4 million individuals.7 In 2016, they were the leading driver of healthcare spending with an estimated direct cost of $380.9 billion, exceeding diabetes ($309.1 billion), cardiovascular diseases ($255.1 billion), mental disorders ($180.7 billion), and cancer ($123.8 billion).8 Despite this, research funding has predominantly focused on diseases associated with death rather than those that cause disability.9
The National Institutes of Health (NIH) is the largest funding source for biomedical research,10 with a mission of enhancing health, lengthening life, and reducing illness and disability.11 NIH funding comes primarily from taxpayer dollars, and its allocation is influenced by factors such as public health needs, scientific opportunities, quality of research proposals, political influence, and disease-specific advocacy.12, 13, 14 Despite the burden of musculoskeletal disease, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) has historically received less than 2% of the NIH budget.15 A study in 2017 found that medical expenses from osteoarthritis were more significant than those from diabetes and similar to those from cancer, yet received 16 and 70 times less NIH funding, respectively.16 Additionally, an NIH report in 2017 assessing research funding and disease burden included 74 disease categories. Still, it did not include major musculoskeletal diseases such as low back or neck pain,17 despite being the leading causes of disability in the same year. Furthermore, the burden of trauma is often unaccounted for, despite the total cause of traumatic injuries in the U.S. estimated at $671 billion per year.18
Our objective was to evaluate the correlation between the U.S. burden of disease and NIH funding levels and assess the current burden of musculoskeletal disease in terms of disability and prevalence. We hypothesize that there would be a correlation between the burden of disease and the federal funds assigned to health-related research through the NIH.
Methods
Study design
We used an ecological study design to examine the relationship between NIH research funding and disability-adjusted life years (DALYs) for 60 diseases. The selected diseases were those for which estimates of NIH funding and burden data were available. The study period was chosen to reflect a two-year expected gap in burden data availability. Disease burden data were obtained from the Institute of Health Metrics and Evaluation (IHME) through 2019. Per the Common Rule, our study did not involve human subjects and was exempt from the requirement for informed consent and institutional review board review. This report adhered to STROBE reporting guidelines.19
Metrics of disease burden
To measure disease burden, we used the Global Burden of Disease Study (GBD) 2019, which provides the most comprehensive analysis of publicly available data on disease burden and presents the most recent GBD estimates through 2019.1 The disability-adjusted life year (DALY) is the sum of years of life lost to disability (YLD) and years of life lost due to premature mortality (YLL), where one DALY represents the loss of the equivalent of one year of full health.20
DALYs provide a more holistic view by accounting for fatal and non-fatal conditions, including the social values placed on mental, physical, and social function.21,22 In the context of an aging population, we included YLDs to account for the shift of the health burden to disability. We also included prevalence to evaluate the current U.S. disease burden and understand the demand for health services in disease management.
We identified the U.S. disease burden using the GBD Results Tool.7 Detailed methodologies of GBD 2019 have been previously described.1
Disease categories
We used GBD 2019, which provided point estimates and 95% uncertainty intervals for every listed cause from 1990 to 2019, and the NIH Research, Condition, and Disease Categories (RCDC)23 data to identify 60 disease categories aligned with available GBD data.
Subcategories within musculoskeletal diseases were selected based on available data from Level 3 disease causes within GBD 2019, including rheumatoid arthritis, osteoarthritis, low back pain, and neck pain. However, it is important to note that specific matched burden/funding data were unavailable for trauma and common conditions such as knee pain, ligament/tendon injury, and fractures. Therefore, they were not included in the analysis.
NIH funding
The funding data for specific diseases in 2019 and 2021 was sourced from the NIH RCDC system, while institute-specific funding data for 2019 was acquired from the NIH Office of Budget.15
These particular years were selected based on several considerations: Firstly, they aligned with the most up-to-date GBD data available, extending only up to 2019. Secondly, they allowed for a two-year interval to account for the anticipated gap in burden availability before any budgetary adjustments were implemented.
Statistical analysis
We used linear regression analysis to estimate the association between NIH funding and DALYs for the 60 disease categories. We log-transformed both variables to reduce skewness in the original data and mitigate the impact of outliers. Finally, we used the fitted linear regression model to derive the predicted funding levels (by back-transforming the outcome to the original scale) and residual values (the difference between the observed and predicted funding). We ranked them according to relative underfunding (i.e., negative residual values) and overfunding (i.e., positive residual values). We assessed model fit by examining residual plots to demonstrate a random distribution of residual values with no discernible pattern. Statistical significance was determined using p-value <0.05. Statistical significance was determined using a p-value <0.05. All statistical analysis was conducted with GraphPad (GraphPad Software 10.1.0, San Diego, CA, USA).
Role of funding source
No funding.
Results
NIH funding and musculoskeletal disease burden
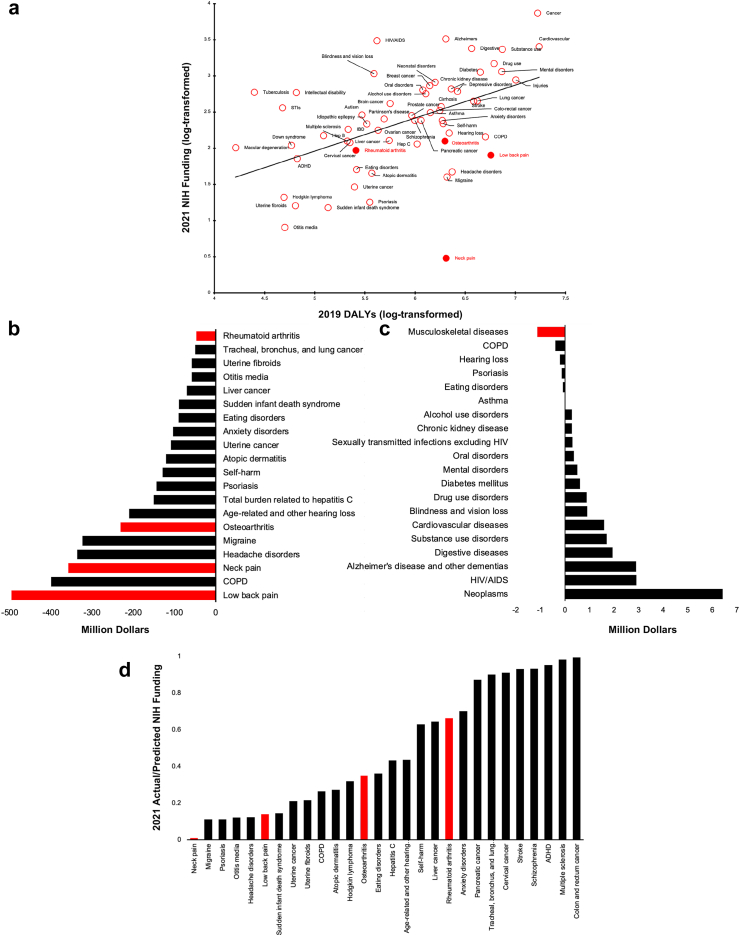
There was a positive association between the 2019 disease burden (measured in DALYs) and 2021 NIH funding levels (Fig. 1a), with 23.4% of funding variation driven by DALYs (correlation coefficient = 0.49, p-value <0.001). However, all musculoskeletal diseases (filled red dots) were underfunded compared to their societal/economic burden (Fig. 1a and b), with low back pain receiving the least relative funding, at $496.4 (231.6–761.2, 95% CI) million less funding than predicted (Fig. 1c). These findings were consistent with data from the 2017–2019 DALYs and the 2019–2021 NIH funding (Supplementary Figure S1). Quantitative data for the selected conditions in Fig. 1 can be found in Supplementary Tables S1–S3 and Figure S2.
Fig. 1.
a) 2021 NIH Funding and 2019 Burden of Disease in Disability-Adjusted Life Years; b) Difference Between Actual and Predicted 2021 NIH Funding of the 20 Most Underfunded Conditions; c) Difference Between Actual and Predicted 2021 NIH Funding Based on 2019 Disease Burden of 20 Disease Categories; and d) Ratio of Actual to Predicted 2021 NIH Funding of Underfunded Diseases. Abbreviations: DALY, disability-adjusted life year; STI, sexually transmitted infection; HIV, human immunodeficiency virus; COPD, chronic obstructive pulmonary disease; ADHD, attention-deficit/hyperactivity disorder.
Predicted funding levels and residual values (difference between observed and predicted funding) were derived to assess the adequacy of funding for various diseases. Subsequently, the ratios of actual to predicted 2021 NIH funding for diseases characterized as underfunded (where the ratio <1) were determined (Fig. 1d, Supplementary Table S4). Musculoskeletal diseases (red) were underfunded relative to the disease burden, with neck pain receiving the least funding at 0.83% of the predicted amount. Low back pain, osteoarthritis, and rheumatoid arthritis were underfunded at 13.88%, 35.08%, and 66.26% of predicted amounts, respectively. To be commensurate with their total burden, funding for neck pain, low back pain, and osteoarthritis would need to increase 120-fold, 7-fold, and 3-fold, respectively.
In 2019, musculoskeletal diseases were underfunded relative to conditions with similar or lesser disease burdens (Table 1). In the context of prevalence, spending per patient for musculoskeletal diseases and injuries was substantially lower than for other diseases.
Table 1.
NIH spending and prevalence for selected diseases, 2019.
| Disease area | DALYs | Funding ($M) | U.S. prevalence |
Spending per patient ($) | |
|---|---|---|---|---|---|
| Proportiona | Number | ||||
| Cardiovascular | 17,266,977 | 2394 | 12.75% | 39,669,218 | 60.35 |
| Cancer | 16,651,972 | 6520 | 8.50% | 26,448,947 | 246.51 |
| Injuries | 10,129,022 | 897 | 36.25% | 112,745,086 | 7.96 |
| Musculoskeletal | 9,984,897 | 351 | 40.96% | 127,411,125 | 2.75 |
| Drug use disorders | 6,121,628 | 1621 | 3.44% | 10,699,858 | 151.50 |
| Low back pain | 5,697,152 | 170 | 16.75% | 52,105,428 | 3.26 |
| COPD | 5,021,538 | 112 | 6.48% | 20,147,917 | 5.56 |
| Diabetes | 4,461,171 | 1099 | 12.49% | 38,858,416 | 28.28 |
| Lung cancer | 4,186,491 | 419 | 0.14% | 444,083 | 943.52 |
| Hearing loss | 2,187,374 | 163 | 22.73% | 70,709,407 | 2.31 |
| Neck pain | 2,043,518 | 2 | 6.81% | 21,184,349 | 0.09 |
| Alzheimer's | 2,026,882 | 2398 | 1.58% | 4,902,695 | 489.12 |
| Osteoarthritis | 1,986,343 | 85 | 16.67% | 51,865,889 | 1.64 |
| Anxiety disorders | 1,872,338 | 233 | 6.51% | 20,241,173 | 11.51 |
| HIV | 415,325 | 3037 | 0.56% | 1,743,128 | 1742.27 |
| Rheumatoid arthritis | 257,884 | 94 | 0.52% | 1,622,773 | 57.93 |
| Multiple sclerosis | 211,385 | 111 | 0.13% | 409,217 | 271.25 |
Funding values are represented as dollars in millions and rounded. Prevalence percentages in the table are calculated as a proportion of the total cases (311,050,916) and are presented alongside the actual number of cases. Spending per Patient ($) is determined by dividing funding (in millions and rounded) by the number of cases in 2019 U.S. prevalence.
Abbreviations: DALY, disability-adjusted life year; COPD, chronic obstructive pulmonary disease.
The denominator utilized for prevalence is 311,050,916.
U.S. burden of disease
We compared prevalence, DALYs, and YLDs among 22 disease classifications (Table 2). In 2019, musculoskeletal diseases were the leading cause of disability years, contributing 25.71% of total YLDs. Musculoskeletal diseases were the third leading cause of DALYs, contributing 12.50% of the total, and were the third most prevalent disease, with 40.96% prevalence. Additionally, the burden of trauma in the U.S. from 2000 to 2019 has largely increased in both prevalence and years lived with disability (Table 3).
Table 2.
U.S. Burden of Disease, 2019.
| Cause | Prevalence |
DALY |
YLD |
|||
|---|---|---|---|---|---|---|
| Percent | Rank | Percent | Rank | Percent | Rank | |
| Other non-communicable diseases | 67.77% | 1 | 4.63% | 9 | 6.00% | 7 |
| Neurological disorders | 46.46% | 2 | 5.28% | 8 | 6.61% | 4 |
| Musculoskeletal disorders | 40.96% | 3 | 12.50% | 3 | 25.71% | 1 |
| Skin and subcutaneous diseases | 29.67% | 4 | 1.97% | 15 | 3.89% | 11 |
| Unintentional injuries | 28.37% | 5 | 3.90% | 10 | 5.21% | 8 |
| Digestive diseases | 27.46% | 6 | 3.31% | 11 | 1.54% | 13 |
| Sense organ diseases | 22.13% | 7 | 2.45% | 13 | 5.13% | 9 |
| HIV/AIDS and STIs | 21.39% | 8 | 0.42% | 18 | 0.33% | 20 |
| Diabetes and kidney diseases | 20.33% | 9 | 6.08% | 7 | 6.60% | 5 |
| Mental disorders | 17.03% | 10 | 6.56% | 5 | 13.73% | 2 |
| Respiratory infections and TB | 16.58% | 11 | 1.49% | 17 | 0.77% | 16 |
| Chronic respiratory diseases | 15.86% | 12 | 6.31% | 6 | 6.31% | 6 |
| Cardiovascular diseases | 12.75% | 13 | 15.59% | 1 | 4.57% | 10 |
| Neoplasms | 8.50% | 14 | 15.05% | 2 | 2.00% | 12 |
| Substance use disorders | 5.89% | 15 | 6.66% | 4 | 7.10% | 3 |
| Nutritional deficiencies | 5.59% | 16 | 0.37% | 20 | 0.63% | 17 |
| Transport injuries | 4.63% | 17 | 2.39% | 14 | 1.40% | 14 |
| Self-harm and interpersonal violence | 3.70% | 18 | 2.83% | 12 | 0.50% | 19 |
| Maternal and neonatal disorders | 1.46% | 19 | 1.50% | 16 | 1.20% | 15 |
| Enteric infections | 0.79% | 20 | 0.40% | 19 | 0.51% | 18 |
| Other infectious diseases | 0.37% | 21 | 0.22% | 21 | 0.09% | 22 |
| NTD and malaria | 0.36% | 22 | 0.09% | 22 | 0.18% | 21 |
Percentages for Prevalence, DALYs, and YLDs were calculated based on specific denominators corresponding to each health metric in 2019. The prevalence percentage represents the proportion of cases out of a total prevalence of 311,050,916. Similarly, the DALY percentage is derived from a denominator of 111,074,469, while the YLD percentage is calculated based on a denominator of 53,316,827.
Abbreviations: DALY, disability-adjusted life year; YLD, years of life lost to disability; STI, sexually transmitted disease; TB, tuberculosis; NTD, neglected tropical disease.
Table 3.
U.S. trauma burden, 2000–2019.
| Injuries | Prevalence |
YLD |
||||
|---|---|---|---|---|---|---|
| 2000 | 2019 | Change | 2000 | 2019 | Change | |
| Fractures | 24.50% | 25.75% | 5.31% | 1,028,957 | 1,554,927 | 51.1% |
| Open wound | 8.56% | 8.97% | 4.79% | 66,691 | 100,799 | 51.1% |
| Muscle/tendon injuries | 3.52% | 3.64% | 3.41% | 19,369 | 28,870 | 49.1% |
| Contusion | 3.35% | 3.52% | 5.07% | 18,177 | 27,500 | 51.3% |
| Head injuries | 1.97% | 1.88% | −4.57% | 208,147 | 283,146 | 36.0% |
| Spinal injuries | 1.70% | 1.81% | 6.47% | 339,174 | 515,833 | 52.1% |
| Hip dislocation | 0.95% | 1.03% | 8.99% | 10,632 | 16,611 | 56.2% |
| Multiple injuries | 0.60% | 0.62% | 4.37% | 33,986 | 49,460 | 45.5% |
| Crush injury | 0.31% | 0.33% | 3.50% | 28,582 | 42,360 | 48.2% |
| Internal hemorrhage in abdomen and pelvis | 0.31% | 0.33% | 5.18% | 29,940 | 43,675 | 45.9% |
| Severe chest injury | 0.16% | 0.16% | −0.64% | 21,745 | 29,089 | 33.8% |
| Knee dislocation | 0.09% | 0.10% | 7.45% | 7604 | 11,627 | 52.9% |
| Shoulder dislocation | 0.06% | 0.05% | −5.45% | 2647 | 3630 | 37.1% |
Percentages for YLD in 2000 and 2019 are calculated using denominators of 39,255,640 and 53,316,826, respectively. Prevalence percentages for 2000 and 2019 are based on denominators of 262,090,459 and 311,050,916, respectively, reflecting the total prevalent cases for those years. Proportions displayed were rounded, and percent change was calculated using unrounded values.
Abbreviation: YLD, years of life lost to disability.
Burden of disease in DALYs
From 1990 to 2019, the CMNN disease burden decreased in the U.S. and globally while the NCD burden increased. However, at the same time, the musculoskeletal disease burden steadily increased (Table 4).
Table 4.
The U.S. and Global Proportion of Burden of Disease in Disability-Adjusted Life Years (DALYs) for CMNN, NCD, and MSK, 1990–2019.
| Cause | 1990 | 2000 | 2010 | 2019 | Percent change, 1990–2019 |
|---|---|---|---|---|---|
| United States | |||||
| CMNN | 7.5% | 5.9% | 5.1% | 4.5% | −40.4% |
| NCD | 80.6% | 84.0% | 85.3% | 86.4% | 7.2% |
| MSK | 10.6% | 11.5% | 12.0% | 12.5% | 17.9% |
| Global | |||||
| CMNN | 46.4% | 41.5% | 34.1% | 26.4% | −43.1% |
| NCD | 43.2% | 48.1% | 55.3% | 63.8% | 47.7% |
| MSK | 3.3% | 3.8% | 4.9% | 5.9% | 81.2% |
Values are displayed as percentages and represent the proportion of total DALYs. Percent change was calculated as the difference in the proportion of total DALYs between 1990 and 2019 divided by the proportion of total DALYs in 1990.
Abbreviations: CMNN, communicable, maternal, neonatal, and nutritional; NCD, non-communicable disease; MSK, musculoskeletal.
Musculoskeletal diseases accounted for approximately 14 million DALYs, making them the third leading cause of U.S. DALYs in 2019. There was a 5.13% increase in the proportion of total DALYs from 2009 to 2019 (Supplementary Table S5). Low back pain was the third leading cause of DALYs, with an 8.49% increase from 2009 to 2019. There was a significant increase in the disease burden of neck pain, with an 86.3% increase in the DALY rate and a 99.5% increase in total DALYs (Supplementary Table S6).
Research funding for musculoskeletal diseases is disproportionately low compared to most other diseases. Total 2018–2022 funding for cancer ($34.9 billion) was almost double the funding for all musculoskeletal-related conditions combined ($18.5 billion), which included 20 different categories such as osteoarthritis, injuries, and pain research (Supplementary Table S7). Back pain ($416 million) and neck pain ($9 million) were among the lowest, ranked for total funding at 201 and 307 out of 309 categories. Relative musculoskeletal research funding is well below the $13.6–$83.4 billion for the top 25 NIH research areas.
Musculoskeletal disease burden and NIH funding
We compared the proportion of total YLDs and DALYs to NIH institute-specific funding. For disease burden, we selected 20 categories representing 86.10% of total DALYs and 86.59% of total YLDs in 2019. In addition, we selected 12 of the 26 NIH-funded institutes/centers, which comprised 68.20% of the total NIH-allocated funding dollars in 2019. Detailed data can be found in the Supplementary Table S8.
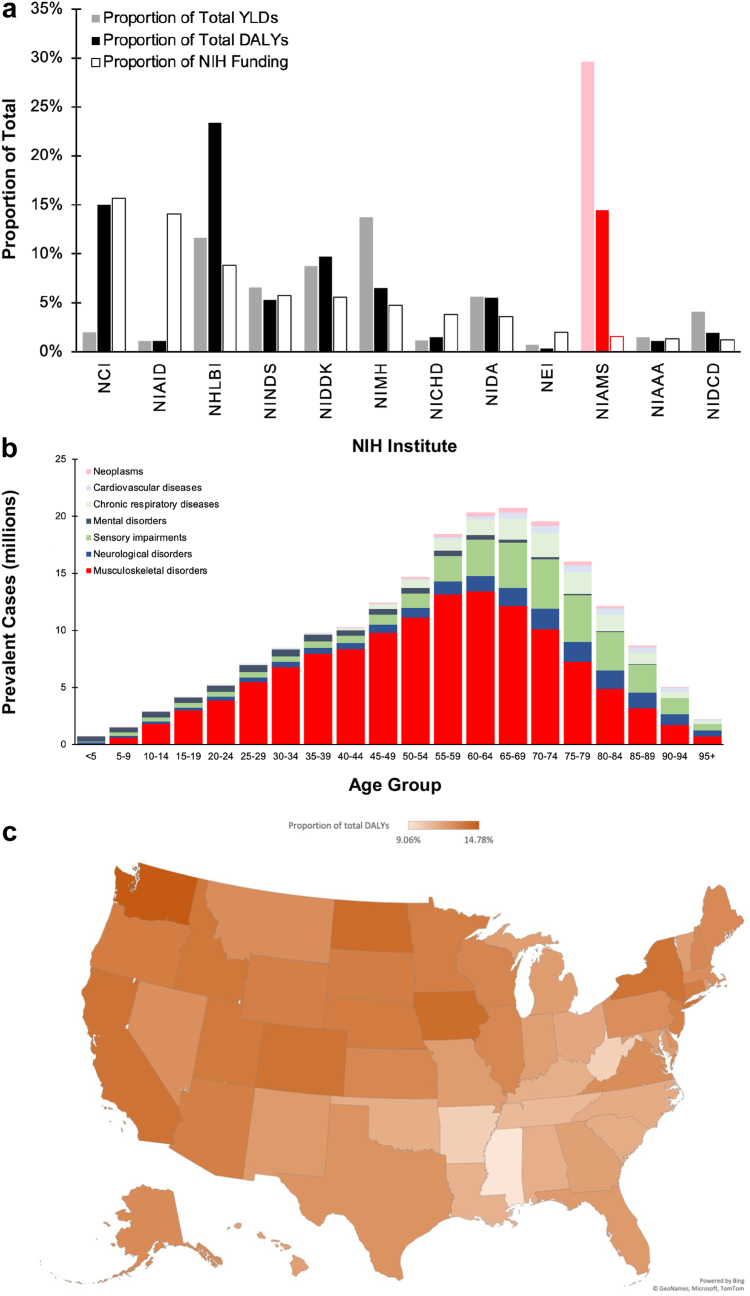
In 2019, musculoskeletal and skin diseases comprised 14.47% and 29.60% of total DALYs and YLDs, respectively (Fig. 2a). The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) received only 1.54% of the NIH funding. NIAMS had the lowest ratio of NIH funding to Disease burden (DALY or YLD).
Fig. 2.
a) Proportion of US Burden of Disease vs. NIH Institute Allocated Funding, 2019; b) Prevalence of Major Disease Categories by Age Group, 2019; and c) Proportion of the total DALYs from Musculoskeletal Disease by State, 2019. Abbreviations: DALY, disability-adjusted life year; YLD, years of life lost to disability; NCI, National Cancer Institute; NIAID, National Institute of Allergy and Infectious Diseases; NHLBI, National Heart, Lung, and Blood Institute; NINDS, National Institute on Neurological Disorders and Stroke; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIMH, National Institute of Mental Health; NICHD, National Institute of Child Health and Human Development; NIDA, National Institute on Drug Abuse; NEI, National Eye Institute; NIAMS, National Institute of Arthritis and Musculoskeletal and Skin Diseases; NIAAA, National Institute on Alcohol Abuse and Alcoholism; NIDCD, National Institute on Deafness and Other Communication Disorders.
From 2015 to 2019, the percentage of total DALYs from musculoskeletal diseases increased by 4.34%, while the proportion of NIH funding allocated to NIAMS decreased by 10.57%.
In 2019, nearly 83 million adults in the United States aged 15–64 had a musculoskeletal disorder. Musculoskeletal disorders comprise two-thirds of the prevalent cases in ages 10–74 (Fig. 2b). Musculoskeletal prevalence was most significant in adults aged 60–64, with over 13.4 million cases. In the U.S., the percentage of total YLDs from musculoskeletal diseases was an average of 12.40% (Fig. 2c).
Discussion
Our analysis of 60 diseases indicated a positive association between NIH funding and disease burden. However, our results revealed that funding for musculoskeletal diseases is disproportionately low despite their significant and growing burden.
The disease burden is increasingly defined by chronic conditions rather than premature mortality.1 In the past decade, musculoskeletal disorders have become the leading cause of years lived with disability in the U.S. and worldwide.7 According to a recent analysis, in 2020, the global prevalence of low back pain was estimated to affect 619 million individuals, with projections suggesting a rise to 843 million cases by 2050.24 In the same year, 595 million people globally had osteoarthritis, equal to 7.6% of the global population, marking a significant increase of 132.2% in total cases since 1990.25 Additionally, these numbers represent increases in case totals from 2020 to 2050 of 74.9% for knee osteoarthritis, 48.6% for hand osteoarthritis, 78.6% for hip osteoarthritis, and 95.1% for other types of osteoarthritis.
Musculoskeletal (MSK) disorders often lead to mental health decline, increased risk of developing other chronic health conditions, and increased risk of all-cause mortality.26 They also threaten healthy aging by limiting functional ability and reducing mental and physical capacity. Furthermore, the U.S. Food and Drug Administration (FDA) has categorized osteoarthritis as a “serious disease”.27 However, the lack of effective therapies limits our ability to manage these conditions. With modern medicine, people live longer but more years with disabling diseases. Therefore, there is a need for special attention to the intensifying burden of health issues that cause pain, impair mobility, and prevent individuals from living full and healthy lives.1,28
U.S. healthcare spending is rising, accounting for 18% of the country’s economy.29 A study from 2020 found that low back and neck pain, other musculoskeletal disorders, and osteoarthritis are among the top conditions with the highest healthcare spending, totalling $344.3 billion.8 The annual cost of musculoskeletal diseases is estimated at $980 billion or 5.8% of the U.S. gross domestic product.30 These diseases also lead to indirect costs such as lost wages,30 commonly affecting individuals during their peak earning years. In addition, musculoskeletal diseases are the leading cause of disability, responsible for one-third of worker’s compensation claims31 and over 200 million workdays lost.30 They also contribute to the need for rehabilitation services and are the main reason for premature exit from the workforce.32 In 2019, injuries accounted for 9% of the U.S. disease burden,7 yet trauma was funded just over 2% of the NIH budget.23 Despite the high economic cost and prevalence of musculoskeletal diseases and trauma, funding for research and treatment remains low33 compared to other conditions, such as cancer and cardiovascular diseases.
Globally, national health priorities have been found to have low correlations with GBD estimates on disease burden.34 The NIH allocation process, which determines U.S. funding for different diseases, has been criticized for being arbitrary and not taking into account the burden of disease.35, 36, 37, 38 Previous research has shown that some diseases receive more funding than is proportionate to their burden, while other underfunded conditions continue to remain underfunded.39 Despite recommendations to improve the criteria for funding prioritization,37 there has been little improvement in the correlation between funding and disease burden.14,39,40 A 2013 study suggested a relationship between funding and disease burden.41 Still, it only measured disease burden by deaths and hospitalizations, which did not fully reflect the burden of disability. It also assessed the twelve institutes with the most funding, of which NIAMS was not one. Despite representing a significant portion of the disease burden, musculoskeletal diseases have been largely excluded from previous studies.14,17,39,40,42 Our study addressed the gap in previous research by examining DALYs and including musculoskeletal diseases, making a substantive contribution to the literature.
Considering the life course approach to MSK conditions and how prevention and early treatment approaches can have lifelong individual and societal benefits, it is also imperative to assess pediatric orthopaedic needs. Children are a vulnerable population, as medical advances occur slowly as regulatory processes must balance their protection with the need for innovation and evolution of care. However, the pediatric population in the United States remains underserved, especially regarding access to devices specific to treating musculoskeletal pathology.43 Issues accounting for this failure are multi-factorial but largely due to the mismatch between the relatively small population of patients who would benefit from developing a new drug or device and the significant cost. Pharmaceutical and device manufacturers are often publicly held companies that answer to shareholders, investors, and the dictum of the market; the sizeable financial risk posed by pediatric medical product development makes it difficult to sell to investors. The resulting paucity of medical products specifically designed and approved for children means that the ideal medical product required to treat a pediatric-specific musculoskeletal pathology frequently does not exist, with little likelihood of being developed. As such, clinicians are forced to adapt existing adult products for use in children. However, as concern for medical liability escalates, physicians and surgeons have become increasingly hesitant to treat patients with a drug or device “off-label”. The costly and protracted FDA approval process poses yet another barrier. Obtaining FDA approval requires that a manufacturer demonstrate evidence of safety, efficacy, and probable clinical benefit. Multi-center prospective studies and randomized clinical trials are vital to evaluating pharmaceuticals and medical devices. Apart from the enormous expense of conducting these in-vivo, prospective studies, methodological barriers make it nearly impossible to study the repurposing of drugs and medical devices already approved for adults to be used “off-label” for identical indications in children since institutional review boards (IRB) are unable to approve studies that use a device or drug for an “off-label” indication. Further investigative hurdles include difficulty establishing appropriate control and comparative treatment groups as a child’s anatomy and function can vary considerably over the duration of a study as a consequence of non-linear growth and development; the random assignment of patients to “untreated” or “natural history” controls may be ethically unacceptable; and for specific pediatric musculoskeletal conditions, there is often no universally accepted “gold standard” against which a device or drug can be compared. While industry and medical specialty society-sponsored, multi-center study groups may compensate for the lack of power related to small patient cohorts, combining data from several study groups may be problematic because of a lack of consistent inclusion and exclusion criteria, inconsistent duration of patient follow-up, and non-uniform assessment of clinical, radiographic, and patient-specific outcome parameters. This “Catch 22” scenario makes it difficult to generate the “substantial evidence” required by the FDA to approve the safety and efficacy of a drug or device.
Limited market potential and vague government policies have stifled pediatric musculoskeletal product development. Bringing innovative products to market that improve patient care for this “orphan population” requires clinical and translational scientific research. Collaborations among all stakeholders (patients, providers, researchers, industry, and government agencies–NIH, FDA) are essential to identify specific musculoskeletal pathologies across pediatric age groups and to provide data on unmet clinical needs. Independent panels of experts can clarify language/definitions (e.g., probable benefit) and specify a framework for evaluating the safety and effectiveness of medical products designed specifically for children. Minimally acceptable performance standards or benchmarks for new and innovative products should be established a priori, based on a systematic review of published biologic, biomechanics, animal, cadaveric, and clinical studies, with appropriate statistical modelling and analysis. These metrics can also be applied to “off-label” applications to demonstrate that the performance of a product used in children is equivalent in safety and efficacy to its approved use in adults. Implementation requires dedicated funding through the NIH to encourage translational research for creating advanced products and treatments that target pediatric musculoskeletal pathology. The Pediatric Medical Device Safety and Improvement Act of 2007 established grants for non-profit pediatric device development consortia and lifted profit restrictions established in the original Humanitarian Device Exemption (HDE) Act, prompting an increase in industry-sponsored HDE applications.44,45 To foster the development of independent, prospective, pediatric patient registries that provide outcomes data with sufficient statistical power to systematically and objectively evaluate the “off-label” use of products requires pooled financial support from the pharmaceutical and device industries (derived by usage fees to the FDA). To facilitate the creation of these national registries, the NIH and FDA must establish universal IRB approval criteria for conducting prospective studies involving "off-label" product use. Instituting these research funding mechanisms and regulatory guidelines for creating and evaluating advanced medical products specific to treating musculoskeletal pathology in children requires all stakeholders to foster meaningful cross-disciplinary relationships.
NIH funding for musculoskeletal research is primarily directed to NIAMS, although some crossover funding occurs with the National Institute on Aging (NIA) and other Institutes. However, NIAMS has consistently received a small proportion of the NIH budget. In 2015, NIAMS was allocated 1.72% of the $30.3 billion NIH budget. Despite the increasing burden of musculoskeletal diseases, the allocation to NIAMS decreased to 1.54% in 2019 and 1.45% in 2022.15 The discrepancy between disease burden and NIAMS funding is further heightened when considering that a portion is allocated to skin diseases. The lack of funding and resources has significantly diminished opportunities for academic innovation and translational research. For instance, orthopaedic surgeons predominantly rely on three medications in their armamentarium, non-steroidal anti-inflammatory drugs, steroids, and opioids, with the over-prescription of the latter having contributed significantly to the opioid crisis. This has given rise to the marketing of various injectables with questionable efficacy in lieu of innovative and effective solutions, which require significant resources. The field also suffers from an overwhelming reliance on hardware solutions for immediate reduction and repair, little opportunity to advance and develop therapeutics and diagnostics to address a myriad of orthopaedic hard and soft tissue, local and systemic ailments and injuries, and the means to prevent and reverse degenerative conditions from taking hold. The lack of funding has also diminished the capacity for the field to conduct translational research, where an Advanced Research Projects Agency for Health (ARPA-H)-type approach is needed to address unmet musculoskeletal needs.
The lack of prioritization for academic innovation has resulted in direct downstream effects with other funding sources, such as pharma/biotech and venture capital, who do not find investment in MSK innovations financially rewarding given the extended time to market and limited resources to de-risk innovative solutions in preparation for technology transfer. For example, only 2%–7.5% of the pipeline of the top 10 pharma companies is allocated to musculoskeletal drugs.46 Furthermore, musculoskeletal-related FDA approvals do not reach the top 15 conditions (oncology with >30% and neurology with >10% of approvals based on 2022 figures).47 Despite the significant market size, this lack of prioritization and funding for MSK innovation ultimately contributes to continued pain and suffering for millions of patients who could benefit from effective, innovative solutions to their ailments and injuries.
The increased burden and cost of MSK conditions, particularly in the context of an aging population, merits reassessment of the national investment in research funding. This is particularly critical if one considers that even a moderate amount of increased funding could result in significant downstream, overall health benefits if earmarked for those conditions where preventive measures are the best way to decrease late morbidity. At present, the application of innovative treatments, as seen with conditions like osteopenia, hints at a potential future for joint preservation and the avoidance of osteoarthritis, a debilitating condition leading to approximately 1.2 million total knee and hip replacements performed annually in the U.S. In 2014, these procedures alone cost an estimated $20 billion, contributing significantly to substantial healthcare expenditures.48 From 2014 to 2030, primary total hip arthroplasty is projected to surge by 71%, resulting in 635,000 procedures, while primary total knee arthroplasty is projected to grow by 85%, reaching 1.26 million procedures.49 It becomes evident that even a modest 1% reduction in the prevalence of hip and knee arthroplasty signifies a substantial $200 million in potential cost savings, underscoring the urgent imperative for increased funding in MSK research to address this significant economic burden proactively.
Areas of research in immediate need of increased funding include trauma care, post-traumatic conditions, and osteoarthritis. Trauma results in more deaths than tuberculosis, human immunodeficiency virus, and malaria combined every year in low-resource countries. Trauma can be addressed by research on health care policy, injury reduction measures, and the creation of sustainable trauma care delivery systems to improve access to acute care. Research on the pathogenesis and genetics of conditions such as osteoarthritis could lead to therapies capable of delaying the onset of end-stage osteoarthritis, resulting in incalculable savings worldwide and allowing the reallocation of healthcare resources to treat other conditions.
A comprehensive response is crucial to effectively address the global burden of musculoskeletal health issues, as emphasized by multiple sources.50, 51, 52, 53 One key aspect of this response is the need to increase the proportion of research funding allocated to MSK research and allocate additional funding leveraged through public-private partnerships.51 This advanced research investment will provide a strong foundation for addressing MSK health challenges. MSK health education is another integral component that should be prioritized to improve prevention and management efforts.50,51 Furthermore, extending global and national health and performance indicators beyond mortality reduction to consider the impact of disability on function and participation is essential for a holistic approach, which recognizes the impact of MSK health impairments on populations across the life course and existing disparities.51,52 Lastly, driving engagement and partnerships spanning from citizen and patient involvement to industry and government collaboration is fundamental.50,51 This inclusive approach can help mobilize resources, expertise, and support at various levels to tackle the multifaceted issues of MSK health. Particularly in low- and middle-income countries, where MSK health is often overlooked, strengthening health systems and prioritizing MSK health is essential to promoting healthier populations worldwide.54,55
Our report has several limitations that must be considered when interpreting the results. First, we did not consider other federal funding sources, such as the National Science Foundation and Department of Veterans Affairs, private industry, or non-profit funding. We acknowledge that there are limitations to the metrics used for disease burden, such as potential biases in reporting and coding errors, as well as limitations in capturing the full impact of a disease on healthcare and economic expenses. Our variables were chosen to demonstrate the contemporary use of DALYs to reflect the burden of disease, and we acknowledge that our regression model did not consider other metrics for disease burden beyond DALYs that could potentially explain the variability in funding. Additionally, we recognize the potential for funding overlap between disease category classifications. Furthermore, it should be noted that when comparing different conditions, the metrics used may not provide a complete picture of the deviations between them. Therefore, these data points should be considered as only one aspect of a broader understanding of the impact of disease burden.
Our analysis found an alignment between NIH funding and disease burden, but funding for musculoskeletal diseases is disproportionately low. To be commensurate with the burden, funding for musculoskeletal diseases must increase. Our findings reveal the need for the NIH to re-evaluate its funding priorities, particularly in light of the changing health landscape. A step in the right direction will be to match the proportion of the NIH funding allocated to the NIAMS with the proportion of musculoskeletal DALYs. This will result in an approximately ten-fold increase in the NIAMS budget from the 2022 level of $680.2M to roughly $6.77B; a move indeed to be welcomed by 127.4 million taxpayers who suffer from a variety of underfunded musculoskeletal ailments and injuries, daily. Such additional funding will drive innovation, which will prompt attention and investment on the part of pharma/biotech and venture capital to bring effective solutions to the market.
Contributors
Conception of the work: AN, MBH, and ATN; Data acquisition and verification: ATN and AN; Data interpretation: ATN, AN, and IMA; Methodology: ATN, AN, and IMA; Statistical analysis and verification: ATN, AN, and IMA. Literature search and original writing on pediatric orthopaedics by BDS. ATN wrote the manuscript with support from AN, IMA, BDS, MBH, JDK, MM, and EDR. All authors attest they meet the ICMJE criteria for authorship. All authors had full access to all the data reported in the study and reviewed, edited, and approved the final version.
Data sharing statement
All data used in these analyses are publicly available from The Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease (GBD) 2019 (https://ghdx.healthdata.org/gbd-2019), NIH Research Portfolio Online Reporting Tools (https://report.nih.gov/funding/categorical-spending#/), and NIH Office of Budget (https://officeofbudget.od.nih.gov).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
IMA has received grants from the American Heart Association and the National Institutes of Health. JDK serves on the Scientific Advisory Board for OnPoint Surgical. MM possesses the founder’s stock and options, has received consulting fees for Miach Orthopedics, and serves an uncompensated role of secretary with the Medical Publishing Board of Trustees of AOSSM.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100661.
Appendix A. Supplementary data
References
- 1.Vos T., Lim S.S., Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roser M., Ritchie H., Ortiz-Ospina E., Rodés-Guirao L. World population growth. 2013. Our world in data. [Google Scholar]
- 3.Wang H., Abbas K.M., Abbasifard M., et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1160–1203. doi: 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2022. Healthy life expectancy (Hale) at birth (years) [Google Scholar]
- 5.Holman H.R. The relation of the chronic disease epidemic to the health care crisis. ACR Open Rheumatol. 2020;2(3):167–173. doi: 10.1002/acr2.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBlanc K.E., LeBlanc L.L. Musculoskeletal disorders. Prim Care. 2010;37(2):389–406. doi: 10.1016/j.pop.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 7.IHME . Global health data exchange GBD results tool. 2019. [Google Scholar]
- 8.Dieleman J.L., Cao J., Chapin A., et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Nations General Assembly . 2018. Political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases. [Google Scholar]
- 10.Viergever R.F., Hendriks T.C.C. The 10 largest public and philanthropic funders of health research in the world: what they fund and how they distribute their funds. Health Res Policy Syst. 2016;14(1):12. doi: 10.1186/s12961-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institutes of Health (NIH) Mission and goals. 2015. [Google Scholar]
- 12.Health Sciences Policy Program . 1998. Scientific opportunities and public needs: improving priority setting and public input at the National Institutes of Health. [PubMed] [Google Scholar]
- 13.Best R.K. Disease politics and medical research funding. Am Sociol Rev. 2012;77(5):780–803. [Google Scholar]
- 14.Ballreich J.M., Gross C.P., Powe N.R., Anderson G.F. Allocation of National Institutes of Health funding by disease category in 2008 and 2019. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.34890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health (NIH). Office of budget. NIH appropriations history by Institute and Center.
- 16.Silvestre J., Ahn J., Levin L.S. National Institutes of Health funding to Departments of Orthopaedic Surgery at U.S. Medical Schools. J Bone Joint Surg. 2017;99(2) doi: 10.2106/JBJS.16.00088. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health (NIH). Report on NIH funding vs. global burden of disease, using data from 2017.
- 18.Florence C., Haegerich T., Simon T., Zhou C., Luo F. Estimated lifetime medical and work-loss costs of emergency department-treated nonfatal injuries--United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(38):1078–1082. doi: 10.15585/mmwr.mm6438a5. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Disability-adjusted life years (DALYs) 2019. [Google Scholar]
- 21.Murray C.J. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72(3):429–445. [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow R.H., Bryant J.H. Health policy approaches to measuring and valuing human life: conceptual and ethical issues. Am J Public Health. 1995;85(10):1356–1360. doi: 10.2105/ajph.85.10.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institutes of Health (NIH) RePORT [Internet]. Estimates of funding for various research, condition, and disease categories (RCDC).
- 24.Ferreira M.L., de Luca K., Haile L.M., et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. 2023;5(6):e316–e329. doi: 10.1016/S2665-9913(23)00098-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinmetz J.D., Culbreth G.T., Haile L.M., et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Rheumatol. 2023;5(9):e508–e522. doi: 10.1016/S2665-9913(23)00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsang A., Von Korff M., Lee S., et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Katz J.N., Neogi T., Callahan L.F., et al. Disease modification in osteoarthritis; pathways to drug approval. Osteoarthr Cartil Open. 2020;2(2) doi: 10.1016/j.ocarto.2020.100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutayeb A. Handbook of disease burdens and quality of life measures. Springer New York; New York, NY: 2010. The burden of communicable and non-communicable diseases in developing countries; pp. 531–546. [Google Scholar]
- 29.Centers for Medicare & Medicaid Services 2023. https://www.cms.gov/data-research/statistics-trends-and-reports/national-health-expenditure-data/nhe-fact-sheet [PubMed]
- 30.United States Bone, Joint Initiative . The burden of musculoskeletal diseases in the United States (BMUS) 3rd ed. 2014. [Google Scholar]
- 31.U.S. Bureau of Labor Statistics . Survey of occupational injuries and illnesses data 2020. 2020. [Google Scholar]
- 32.Cieza A., Causey K., Kamenov K., Hanson S.W., Chatterji S., Vos T. Global estimates of the need for rehabilitation based on the global burden of disease study 2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10267):2006–2017. doi: 10.1016/S0140-6736(20)32340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd B., McKenney M., Boneva D., Elkbuli A. Disparities in National Institute of Health trauma research funding: the search for sufficient funding opportunities. Medicine. 2020;99(6) doi: 10.1097/MD.0000000000019027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira C.B., Ferreira G.E., Buchbinder R., Machado G.C., Maher C.G. Do national health priorities align with global burden of disease estimates on disease burden? An analysis of national health plans and official governmental websites. Public Health. 2023;222:66–74. doi: 10.1016/j.puhe.2023.06.038. [DOI] [PubMed] [Google Scholar]
- 35.Istook E. Research funding on major diseases is not proportionate to taxpayers’ needs. J NIH Res. 1997;9:26–28. [Google Scholar]
- 36.Anderson C. A new kind of earmarking. Science. 1993;260(5107):483. doi: 10.1126/science.8475380. [DOI] [PubMed] [Google Scholar]
- 37.Committee on NIH Research Priority-Setting Process . 1998. Scientific opportunities and public needs: improving priority setting and public input at the National Institutes of Health. [PubMed] [Google Scholar]
- 38.Hatziandreu E., Graham J.D., Stoto M.A. AIDS and biomedical research funding: comparative analysis. Rev Infect Dis. 1988;10(1):159–167. doi: 10.1093/clinids/10.1.159. [DOI] [PubMed] [Google Scholar]
- 39.Gillum L.A., Gouveia C., Dorsey E.R., et al. NIH disease funding levels and burden of disease. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0016837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gross C.P., Anderson G.F., Powe N.R. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340(24):1881–1887. doi: 10.1056/NEJM199906173402406. [DOI] [PubMed] [Google Scholar]
- 41.Sampat B.N., Buterbaugh K., Perl M. New evidence on the allocation of NIH funds across diseases. Milbank Q. 2013;91(1):163–185. doi: 10.1111/milq.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nimgaonkar A., Mughal A.Y., Heimer H., Nimgaonkar V., Greenstein D., Wright A. Exploring static and dynamic relationships between burden of disease and research funding in the United States. Health Res Policy Syst. 2022;20(1):60. doi: 10.1186/s12961-022-00837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheha E.D., Hammouri Q., Snyder B.D., Campbell R.M., Vitale M.G., POSNA/SRS Task Force for Pediatric Medical Devices Off-label use of pediatric orthopaedic devices: important issues for the future. J Bone Joint Surg Am. 2014;96(3) doi: 10.2106/JBJS.M.00288. [DOI] [PubMed] [Google Scholar]
- 44.Woodlee D. Understanding the US FDA’s custom device exemption: practical solutions for handling the sale of patient-specific devices in the USA. J Med Device Regul. 2011;8(3):3–11. [Google Scholar]
- 45.FDA requires device manufacturers to include information on pediatric populations. 2010. [Google Scholar]
- 46.Loyd I. Pharma R&D annual review 2022: navigating the landscape. Pharma Intelligence UK Limited; 2022. [Google Scholar]
- 47.Aitken M., Connelly N., Kleinrock M., Pritchett J. Parsippany; 2023. Global trends in R&D 2023: activity, productivity, and enablers. [Google Scholar]
- 48.Lam V., Teutsch S., Fielding J. Hip and knee replacements. JAMA. 2018;319(10):977. doi: 10.1001/jama.2018.2310. [DOI] [PubMed] [Google Scholar]
- 49.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg. 2018;100(17):1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 50.Briggs A.M., Schneider C.H., Slater H., et al. Health systems strengthening to arrest the global disability burden: empirical development of prioritised components for a global strategy for improving musculoskeletal health. BMJ Glob Health. 2021;6(6) doi: 10.1136/bmjgh-2021-006045. http://gh.bmj.com/content/6/6/e006045.abstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Briggs A., Slater H., Jordan J., et al. Towards a global strategy to improve musculoskeletal health. Global Alliance for Musculoskeletal Health; Sydney, Australia: 2021. [Google Scholar]
- 52.Briggs A.M., Betteridge N., Dreinhöfer K.E., et al. Towards healthy populations: a need to strengthen systems for musculoskeletal health. Semin Arthritis Rheum. 2023;58 doi: 10.1016/j.semarthrit.2022.152147. [DOI] [PubMed] [Google Scholar]
- 53.Briggs A.M., Jordan J.E., Kopansky-Giles D., et al. The need for adaptable global guidance in health systems strengthening for musculoskeletal health: a qualitative study of international key informants. Glob Health Res Policy. 2021;6(1):24. doi: 10.1186/s41256-021-00201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briggs A.M., Jordan J.E., Sharma S., et al. Context and priorities for health systems strengthening for pain and disability in low- and middle-income countries: a secondary qualitative study and content analysis of health policies. Health Policy Plan. 2023;38(2):129–149. doi: 10.1093/heapol/czac061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider C.H., Parambath S., Young J.J., et al. From local action to global policy: a comparative policy content analysis of national policies to address musculoskeletal health to inform global policy development. Int J Health Policy Manag. 2023;12:7031. doi: 10.34172/ijhpm.2022.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.