Abstract
Chronic endobronchiolitis compounded by recurring Pseudomonas aeruginosa infections is the major cause of morbidity and mortality in patients with cystic fibrosis (CF). In this study, a mouse model of repeated respiratory exposure to P. aeruginosa was established to facilitate investigations of factors contributing to P. aeruginosa persistence and associated inflammatory processes in the lung. While a single exposure to P. aeruginosa aerosols resulted in only mild histopathological changes, repeated exposure caused significant lung pathology in C57BL/6J mice. The peak of histopathological changes and inflammation in C57BL/6J mice was characterized by subacute lymphohistiocytic bronchopneumonia and persistent elevation of tumor necrosis factor alpha and macrophage inflammatory protein 2 in the lung but not in the serum. When isogenic nonmucoid (mucA+) and mucoid (mucA22) P. aeruginosa strains were compared, the mucoid cells were cleared several-fold less efficiently than the parental nonmucoid strain during the initial stages of the aerosol exposure regimen. However, the microscopic pathology findings and proinflammatory cytokine levels were similar in mice exposed to nonmucoid and mucoid P. aeruginosa throughout the infection. We also tested lung histopathology and proinflammatory cytokines in interleukin 10 (IL-10)-deficient transgenic (IL-10T) mice. Significant mortality was seen in IL-10T mice on initial challenge with P. aeruginosa, although no histopathological differences could be observed in the lungs of C57BL/6J and surviving IL-10T mice after a single exposure. However, increased pathology was detected in IL-10T mice relative to C57BL/6J after repeated challenge with P. aeruginosa. This observation supports the proposals that anti-inflammatory cytokines may play a role in suppressing P. aeruginosa-induced tissue damage during chronic infection.
Chronic lung infections with Pseudomonas aeruginosa and associated inflammation are the major causes of morbidity and mortality in patients with cystic fibrosis (CF) (17, 52). A number of concurrent proposals (4, 6, 16, 24, 39, 46, 55) addressing possible relationships between a defect in the CFTR gene and the respiratory complications in CF have recently been offered. For example, it has been suggested that reduced sialylation of glycoconjugates on the surface of epithelial cells promotes P. aeruginosa adhesion in CF (55). More recently, it has been reported that CF epithelial secretions display reduced bactericidal properties due to altered salt content (16, 46). CFTR has also been implicated in P. aeruginosa uptake by respiratory epithelial cells, which may play a role in the putative process of clearance by desquamation (39). These models are reliant for the most part on the known functions of CFTR as a chloride channel (9) and on its suspected pleiotropic effects (4). Other studies, focusing on cytokine profiles in bronchoalveolar lavage fluids of CF patients, have suggested that the excessive inflammation in the CF lung may be attributed to endogenously increased levels of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 8 (IL-8), and IL-1 (6). According to some reports, the levels of these cytokines may be altered in CF subjects even before a bacterial infection can be documented (24). Intriguingly, reduced levels of the anti-inflammatory cytokine IL-10 have been recently reported in CF patients (6).
Despite these promising leads, our understanding of host-pathogen interactions in CF is incomplete at present. It is likely that the chronic presence of P. aeruginosa, the most common pathogen in CF (17, 36, 52), contributes to the overall progression of the disease. One of the prominent properties of P. aeruginosa encountered in CF is its mucoid, alginate-overproducing phenotype (17, 36). The emergence of mucoid variants occurs at variable times upon the initial colonization with nonmucoid strains (12, 19, 29, 36, 37). Conversion to mucoidy in P. aeruginosa is linked to the establishment of chronic infection in CF (25, 36). The molecular mechanism of conversion to mucoidy has recently been elucidated (54). The majority of CF mucoid isolates carry mucA mutations (7, 30, 31) which allow transcription of alginate biosynthetic genes, resulting in a mucoid phenotype. Based on studies in surrogate models in vitro, it has been suggested that mucoidy may be a virulence factor in CF (36). Alginate production inhibits opsonic and nonopsonic phagocytosis (48), protects cells from reactive oxygen intermediates (28, 45), and plays additional roles associated with biofilm phenomena (27). Despite the general belief that alginate production plays a role in the persistence of P. aeruginosa in CF (17), direct in vivo evidence supporting its role in the pathogenesis of respiratory infections is currently not available. The dearth of in vivo results is further complicated by studies suggesting equal clearance of mucoid and nonmucoid P. aeruginosa upon intrabronchial instillation in guinea pigs (5).
Several useful models of acute and forced chronic P. aeruginosa infection have been described in normal, neutropenic, neonatal, or burned mice and in other animals (5, 8, 10, 21, 38, 47, 49–51, 53). Recently, an aerosol model of respiratory infections with Staphylococcus aureus and Burkholderia cepacia has been developed in the CFTRm1HGU transgenic mouse with a mild defect in the CFTR gene (11). A reduced clearance of these pathogens and more pronounced lung disease in the CFTRm1HGU animals have been reported. However, these studies did not address colonization with P. aeruginosa. In the present study, in order to investigate P. aeruginosa endobronchial infections in mice, we adopted bacterial aerosol delivery technology for the deposition of the pathogen in distal airways. Using this approach, we developed a model of prolonged respiratory infection with P. aeruginosa, based on a regimen of repeated exposure to P. aeruginosa aerosols, and investigated bacterial clearance and inflammatory response in the context of mucoid and nonmucoid status of P. aeruginosa. We also tested the role of IL-10 in lung inflammation, using IL-10-deficient mice, and observed increased histopathology in the lungs of the IL-10-deficient transgenic (IL-10T) animals relative to those of normal controls.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. aeruginosa was grown on Pseudomonas isolation agar (Difco) or Luria broth. P. aeruginosa PAO1 is the standard genetic nonmucoid strain (mucA+) (22, 31). PAO381 (14) is a leucine auxotroph derived from PAO1 (leu mucA+; nonmucoid) (30). PAO578I (leu mucA22; mucoid) is a mucoid derivative of PAO381 (14) carrying the previously characterized mucA22 allele, which has the most common mutation, ΔG440 (7, 31). PAO578I, unlike many mucoid derivatives which also carry a second site sup-2 mutation (7) and show medium-dependent alginate expression, is always mucoid, independent of growth condition. CF strains were fresh clinical isolates from patients diagnosed with CF. To prepare infection doses for nebulization, P. aeruginosa was grown in 200 ml of Luria broth at 37°C for 12 h and harvested by centrifugation at 4,000 rpm for 15 min at 4°C. Pellets (wet-cell mass, 1.2 g) were washed once in cold 1% Proteose Peptone (Difco)–phosphate-buffered saline (pH 7.4) and resuspended in cold phosphate-buffered saline. Cell density was adjusted to 1.0 × 1011 CFU/ml, and 5 ml of this suspension was used for nebulization.
Animals.
All mice in this study were 8 weeks old (21.0 ± 1.2 g) (mean ± standard deviation [SD]) at the inception of the experiment. C57BL/6J and IL-10T mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). IL-10T mice were backcrossed 10 times with C57BL/6J mice to ensure similar genetic backgrounds. All animals were housed under specific-pathogen-free conditions within the animal care facility at the University of Michigan until the initiation of aerosol exposure experiments. Once the animals had been exposed to P. aeruginosa aerosols, they were transferred to conventional housing. During the housing of IL-10T mice, a few syncytial cells were sometimes seen in the alveoli of unexposed control animals. In the course of repeated exposure, the animals did not lose weight relative to unexposed controls. However, the wet-lung weight continuously increased relative to that of the age-matched unexposed control but was equal regardless of the mucoid or nonmucoid status of bacteria.
Apparatus and aerosol exposure.
Equipment for generation of bacterial aerosols was manufactured by Glas-Col (Terre Haute, Ind.). The Glas-Col inhalation exposure system was chosen in this study based on the following characteristics (35): (i) it is suitable for delivering nebulized pathogenic agents in the form of aerosols consisting of dried droplet nuclei (average size, 2 μm), which are deposited in distal airways; (ii) the initial deposition of aerosolized bacteria is uniform throughout the lungs; (iii) nebulization, cloud decay, and decontamination are controlled by a microprocessor; (iv) the inoculum is evenly distributed among all animals simultaneously exposed (relative error, 9.4%; SD, ±5.2), yielding low mouse-to-mouse variation; and (v) a HEPA filter, germicidal UV lamps, and an outlet incinerator serve as a means of biological containment and decontamination. The main component of the inhalation exposure equipment is a nebulizer unit into which 5 ml of bacterial suspension was introduced. Mice were placed in a compartmentalized mesh basket (five chambers, each with a capacity for 20 mice). Parameters for the standard aerosol exposure cycle were 30 min for nebulization, 30 min for cloud decay, 5 min for decontamination (UV irradiation). For determination of initial bacterial deposition in the lungs, animals were sacrificed immediately after the standard exposure cycle. For single-exposure experiments, animals were sacrificed 18 h after the completion of the standard cycle, unless stated otherwise. For repeated exposure, animals were subjected to a new cycle of aerosol delivery every 72 h and sacrificed 18 h after the last exposure.
Lung histopathology.
Lungs, heart, thymus, and trachea were removed en bloc. The left lobe of the lung was clamped with a hemostat at the trachea bifurcation and removed for homogenization, followed by microbiological and cytokine assays. The right lobe of the lung was insufflated in alcoholic formalin and fixed in 10% neutral buffered formalin. Tissue processing and paraffin embedding were carried out by conventional methods. The lung was sectioned along the long axis of the lobe catching the primary bronchus for a variable distance. Tissue sections (5 to 6 μm) were stained with hematoxylin-eosin (H-E) or Alcain Blue/periodic acid-Schiff stain. Stained sections were scored for (i) pulmonary septal (capillary and intra-alveolar) inflammation, (ii) inflammatory cell accumulation in bronchioles, (iii) exudate, (iv) airway epithelial cell hyperplasia, (v) perivascular and peribronchiolar lymphoid hyperplasia, and (vi) goblet cell hyperplasia. Pathology scoring indices were as follows: 0, no change (normal tissue); 1, <10% of parenchyma affected; 2, 30% of parenchyma affected; 3, 50% of parenchyma affected; 4, >50% of parenchyma affected.
Bacterial clearance.
Pulmonary clearance of P. aeruginosa was monitored by plate counts of viable bacteria in lung homogenates (left lobe) 18 h after the last exposure, unless stated otherwise. The numbers were corrected for left-lung weight. Initial bacterial deposition was from 2.0 × 106 to 8.5 × 106 CFU/lung and was monitored after each exposure by including and sacrificing two additional mice along with the experimental group.
Dynamics of P. aeruginosa removal from the lungs.
All infected mice displayed vigorous ability to clear P. aeruginosa from the lung following exponential (PAO1) or nearly exponential (auxotrophic mutants) kinetics of reduction in bacterial load. The remaining fractions of the initially deposited bacteria at different time points for PAO1 and PAO381, respectively, were (i) 160% ± 15% (mean ± SD) and 11.1% ± 2% (4 h postinfection), (ii) 25.9% ± 3% (13 h) and 0.27% ± 0.04% (18 h), and (iii) 0.05% ± 0.005% and 0.002% ± 0.0002% (48 h). The prototroph PAO1 was cleared less efficiently than its auxotrophic derivatives (e.g., PAO381), a result which was attributed to its ability to proliferate immediately upon the initial deposition, a feature not noted with auxotrophic mutants and fresh CF isolates. CF isolates showed high strain-to-strain variability and were inferior to PAO strains in their ability to survive in the murine lung (usually completely cleared at 48 h postinfection). In order to avoid high strain-to-strain variability among CF isolates and to allow comparisons of genetically defined isogenic mucoid and nonmucoid strains, PAO derivatives were used in this model.
Cytokine measurements.
TNF-α, macrophage inflammatory protein 2 (MIP-2), and IL-10 in lung tissue (left-lung lobe homogenates) and sera were measured by enzyme-linked immunosorbent assay kits (R & D Systems, Minneapolis, Minn.) according to the manufacturer’s instructions. Detection limits for murine TNF-α, MIP-2, and IL-10 were 5.0, 1.5, and 4.0 pg/ml, respectively. Absorbance was monitored at 450 nm with a wavelength correction at 540 nm in a Bio-Rad microplate reader model 550. All determinations were performed in triplicate.
Statistical analysis.
Analysis of variance (ANOVA), t test, and posthoc pairwise comparisons with the Student-Newman-Keuls test were performed with SuperANOVA (version 1.11; Abacus Concepts) and SPSS statistics software (PowerMac advanced version 6.1). A Kruskal-Wallis nonparametric ANOVA was carried out with StatView (version 4.5; Abacus Concepts).
RESULTS
Pulmonary clearance of P. aeruginosa from the murine lung in the model of repeated exposure to bacterial aerosols.
Since respiratory infections with P. aeruginosa in CF are frequently associated with chronic presence of the pathogen, we investigated parameters of P. aeruginosa clearance from the lungs in animals continually exposed to this pathogen. A group of age-matched C57BL/6J mice (n = 64) was subjected to a course of repeated exposure to P. aeruginosa aerosols which included the administration of fresh bacterial challenge every 72 h. The strains used were PAO381 (mucA+; nonmucoid) and its genetically characterized (30, 31, 44) mucoid derivative PAO578I (mucA22) (14). In this protocol, a new bolus of aerosolized P. aeruginosa was deposited in the lung when the previous bacterial load was significantly reduced. During this regimen, the mice usually showed symptoms of slow responsiveness and piloerection within 4 to 6 h after each exposure but appeared healthy the next day, and no mortality was observed. This pattern was seen for all exposure points from the beginning to the termination of the experiment. The animals were sacrificed for bacteriological assessment after exposures 1, 8 (3 weeks), and 15 (6 weeks). The following observations were made with respect to the clearance of P. aeruginosa from the lung. For the groups of mice exposed one or eight times to P. aeruginosa, a statistically significant reduction in the survival of the mucoid (mucA22) strain PAO578I was observed compared to its nonmucoid (mucA+) parent, PAO381. Monitored after one or eight consecutive exposures, the nonmucoid parental strain PAO381 was cleared twofold faster 4 h postexposure when the fraction of the initial inoculum remaining in the lung was still above 20% (P < 1.6 × 10−4, ANOVA; data not shown). The survival of PAO578I (mucoid) was 6 to 8 times higher 18 h postinfection (P < 0.001, ANOVA; Table 1) than its nonmucoid parent PAO381, although the majority of the initial inoculum was cleared at that time point. Collectively, the survival data at 4 and 18 h suggest that in animals not previously exposed to P. aeruginosa or exposed repeatedly within a period of 3 weeks after the initiation of the experiment, the mucoid strain had an advantage over its nonmucoid parent in resisting pulmonary clearance. However, when the animals were examined 6 weeks after the initiation of the experiment, differences in survival between the mucoid and nonmucoid strains were abrogated (P = 0.25, ANOVA; Table 1). The loss of the advantage associated with mucoidy coincided with an overall improvement in pulmonary clearance (P = 0.005, ANOVA).
TABLE 1.
Differential pulmonary clearance of mucoid and nonmucoid P. aeruginosa in C57BL/6J micea
| No. of exposures | Strain | Phenotype | Initial deposition (CFU/lung)b | Viable counts at 18 h postexposure (CFU/g of lung tissue ± SE)c |
|---|---|---|---|---|
| 1 | PAO381 | Nonmucoid | 4.7 × 106 | 838 ± 32 |
| PAO578I | Mucoid | 4.6 × 106 | 7,043 ± 161 | |
| 8 | PAO381 | Nonmucoid | 4.5 × 106 | 633 ± 52 |
| PAO578I | Mucoid | 4.0 × 106 | 4,051 ± 180 | |
| 15 | PAO381 | Nonmucoid | 3.9 × 106 | 147 ± 45 |
| PAO578I | Mucoid | 3.8 × 106 | 232 ± 85 |
C57BL/6J mice (n = 64; 8 weeks old at the inception of the experiment) were singly or repeatedly exposed to aerosols of two isogenic nonmucoid and mucoid P. aeruginosa strains, the nonmucoid parent PAO381 (mucA+) and the mucoid mutant PAO578I (mucA22). For each exposure point, the mice were separated into three groups to determine the initial deposition of PAO381 and PAO578I in the lungs, and the viable remaining bacteria 18 h following the exposure were counted by plating. P. aeruginosa aerosols were generated, and the animals were exposed as described in Materials and Methods.
Initial deposition represents the mean value obtained with two C57BL/6J mice at each time point. Values for initial deposition per individual mouse (left-lung lobe) were as follows: at exposure 1, 4.4 × 106 and 5.0 × 106 for PAO381 and 4.4 × 106 and 4.8 × 106 for PAO578I; at exposure 8, 4.2 × 106 and 4.8 × 106 for PAO381 and 4.0 × 106 and 4.0 × 106 for PAO578I; at exposure 15, 4.4 × 106 and 3.4 × 106 for PAO381 and 4.1 × 106 and 3.5 × 106 for PAO578I.
Four mice were used at indicated experimental points for the determination of viable cell counts for each strain. P values (ANOVA) were 1.0 × 10−3 for exposure 1, 1.0 × 10−3 for exposure 8, and 0.25 for exposure 15.
Lung histopathology during extended exposure to P. aeruginosa.
The experiments described in the previous sections were aimed at determining potential differences between mucoid and nonmucoid strains in resistance to innate clearance mechanisms in the respiratory tract. A related goal in these studies was to monitor lung histopathology in mice repeatedly exposed to P. aeruginosa. For this purpose, while the left lung was homogenized for microbiology and additional analyses, the right lung was fixed and inspected for histopathological changes. A summary of histopathological findings is given in Fig. 1A. The lung histopathology at various stages of the repeated exposure is illustrated in Fig. 2. Only minor changes were observed after a single exposure (Fig. 2B), with some exudate detected in the large airways. In the later phases of the repeated-exposure experiment, the exudate subsided, but other microscopic histopathology parameters increased. The most prominent changes observed were peribronchial cuffing and bronchial epithelium hyperplasia. Although some goblet cell hyperplasia was detected on periodic acid-Schiff-stained sections (data not shown), this was not a prominent feature of the infected mice. Significantly more cells (P = 0.03, ANOVA) were observed in the alveoli after exposures 8, 15, and 22 (Fig. 2C through E) than following the initial exposure (Fig. 2B). Overall, the highest lung pathology (Fig. 1) coincided with exposures 8 and 15 (P = 0.011, Kruskal-Wallis nonparametric ANOVA). The histopathological findings were consistent with increased inflammation upon repeated exposure to P. aeruginosa for the period of time preceding the presumptive immune phase of the repeated-exposure experiment (Fig. 1A and 2F). However, no significant differences (P = 0.67, Kruskal-Wallis nonparametric ANOVA) between the mucoid and nonmucoid strains were observed in overall levels or details of lung histopathology (Fig. 1A). This finding suggests that both phenotypic forms of P. aeruginosa interacted with the host in a similar fashion and that the increased persistence of the mucoid strain PAO578I in our experiments (Table 1) was not sufficient to cause or induce additional damage to the lung in the model being tested.
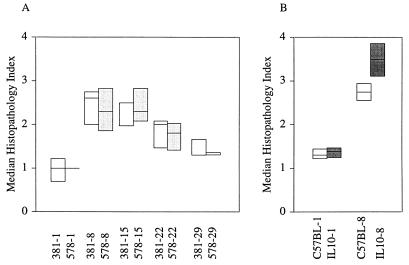
FIG. 1.
Histopathology indices during repeated exposure to P. aeruginosa in normal (A) and IL-10T (B) mice. Histopathology scores ranged from 0 to 4 as described in Materials and Methods. Shown are the 25th, 50th (median), and 75th percentiles (Kruskal-Wallis nonparametric ANOVA). (A) Open boxes and 381-1, -8, -15, -22, and -29, nonmucoid (mucA+) parental strain PAO381 after 1, 8, 15, 22, and 29 exposures (n = 4 for each experimental point). Lightly shaded boxes and 578-1, -8, -15, -22, and -29, PAO578I, a mucoid mucA22 derivative of PAO381, after 1, 8, 15, 22, and 29 exposures (n = 4 for each experimental point). Unexposed age-matched control animals (n = 2) for each time point had a score of 0 (not shown). (B) Eight-week-old (at the inception of the experiment) C57BL/6J (n = 12) (open boxes) and IL-10T (n = 8) (filled boxes) mice were exposed to P. aeruginosa PAO1 once (-1) or 8 times (-8). Pairwise comparisons (Student-Newman-Keuls test) indicated that all histopathology indices relative to unexposed controls were significant (P < 0.05). Pairwise analyses showed no statistically significant difference between PAO381 and PAO578I for any of the exposures. Exposures 8 through 22 had a statistically significant increase (P < 0.05) relative to exposure number 1, but this significance was lost for exposure 29. The difference between C57BL/6J and IL-10T was not significant for exposure 1 but was significant for exposure 8 (P < 0.05).
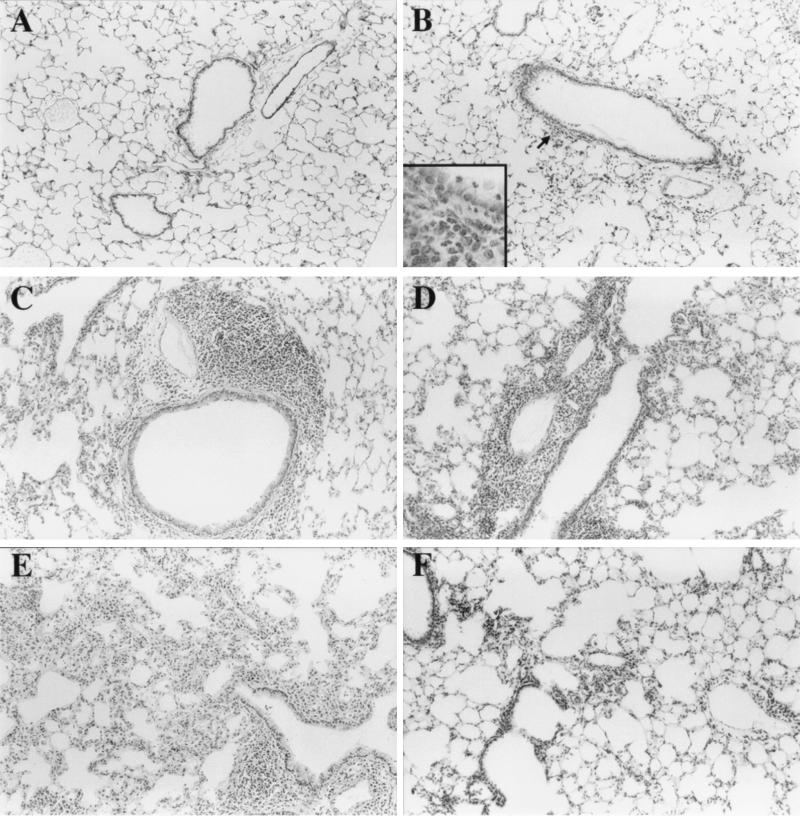
FIG. 2.
Lung histopathology in C57BL/6J mice during repeated exposure to P. aeruginosa. Two groups (n = 40) of 8-week-old C57BL/6J mice were repeatedly exposed to mucoid (PAO578I) or nonmucoid (PAO381) P. aeruginosa aerosols at 72-h intervals for a period of 12 weeks (see Materials and Methods). Shown are H-E-stained sections of the right lobe of the lungs at sequential exposure points. For each stage, only the mucoid (PAO578I) or nonmucoid (PAO381) strain is shown, but similar findings were obtained for both strains. (A) Lung from the unexposed control (at age corresponding to exposure 22 in the infected group). (B) Exposure 1 (strain PAO578I). Inset, inflammatory cells in peribronchial area. (C) Exposure 8 (strain PAO381). Note extensive peribronchial cuffing. Perivascular and peribronchial inflammation was characterized as subacute lymphohistiocytic bronchopneumonia. (D) Exposure 15 (strain PAO578I). (E) Exposure 22 (strain PAO578I). (F) Exposure 29 (strain PAO381). Note improved lung histology.
Cytokine profiles in the lungs and sera during repeated exposure to P. aeruginosa aerosols.
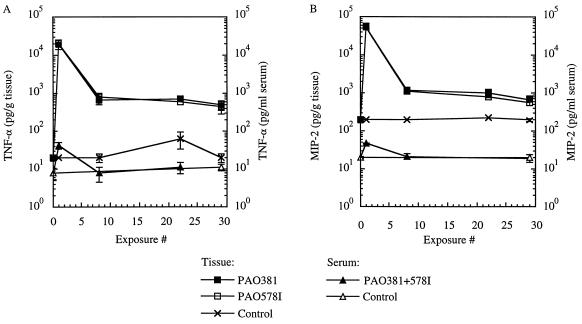
In order to examine profiles of a subset of inflammatory cytokines in animals exposed to P. aeruginosa, we measured levels of TNF-α and MIP-2, a chemokine considered to be a murine equivalent of human IL-8 (20, 42, 43), in the lungs and sera at exposure points coinciding with bacteriological and histological evaluations. The results of these experiments are shown in Fig. 3. Several observations were made. (i) Both TNF-α and MIP-2 remained increased in the lungs of repeatedly exposed animals for the duration of the experiment (P = 10−4, ANOVA). (ii) A maximum increase in inflammatory cytokines was observed following the initial exposure; the reduced levels at later exposures remained 22-fold (TNF-α) and 3-fold (MIP-2) higher than in the unexposed, age-matched control animals (Fig. 3B). (iii) In contrast to lung tissue cytokine levels, serum concentrations remained generally low, with only a slight increase coinciding with the initial maximum of TNF-α and MIP-2 production following the first exposure (Fig. 3A and B, exposure 1). These findings indicated that upon the repeated encounter with the pathogen, the mice adjusted to a new, intermediate level of inflammatory cytokine production which remained relatively constant throughout the experiment. The highest cytokine levels did not coincide with the peak of pathological findings in the lung, and only a relatively moderate elevation of proinflammatory cytokines seemed to be associated with the phase characterized by the most prominent morphological changes in the lung. In keeping with the results of the lung pathology evaluations, which appeared to be equal for both the mucoid and nonmucoid strains tested, we did not observe differences in TNF-α and MIP-2 levels between the animals exposed to PAO578I and those exposed to PAO381 (P = 0.35, ANOVA). These findings suggest that the production of alginate or the increased persistence of mucoid P. aeruginosa in the lungs infected with PAO578I was not sufficient to generate perceptible differences in cytokine levels.
FIG. 3.
TNF-α (A) and MIP-2 (B) levels in lung tissue and sera during the course of repeated exposure of C57BL/6J mice to P. aeruginosa PAO381 (nonmucoid) and PAO578I (mucoid). Determination of TNF-α and MIP-2 was by enzyme-linked immunosorbent assay as described in Materials and Methods.
Lung pathology in IL-10 transgenic mice infected with P. aeruginosa aerosols.
Lower levels of IL-10 have recently been reported in the bronchoalveolar fluids of CF patients relative to those of healthy controls (6). We intended to test the potential role of IL-10 in the inflammatory process associated with P. aeruginosa infection by comparing C57BL/6J and IL-10T mice in our infection model. Two groups of C57BL/6J and IL-10T mice (n = 12 per group) were exposed to aerosolized P. aeruginosa PAO1. Strain PAO1 was used in these experiments because it displayed retarded clearance relative to PAO381 in normal mice, and thus its increased presence could promote inflammatory changes. The first observation made in these experiments was that following the two initial exposures, 50% of the IL-10T animals died. No mortality was observed in the C57BL/6J group. The high death rate among IL-10T mice was in sharp contrast to the virtual absence of mortality in the C57BL/6J mice in any of our experiments, regardless of whether PAO1 or PAO381 was used (Table 2). Necropsy of the IL-10T mice that succumbed to the infection revealed marked to severe alveolar hemorrhages and numerous neutrophils in the alveoli and alveolar ducts (Fig. 4). Remarkably, there were no discernible differences in histopathological changes between C57BL/6J and the surviving IL-10T mice after a single exposure to P. aeruginosa. In order to assess whether differences in lung histopathology may become discernible in IL-10T mice relative to those in C57BL/6J mice during the prolonged presence of P. aeruginosa, the mice were subjected to a regimen of repeated aerosol challenge. The experiments were carried out up to the point coinciding with the peak of pathology (exposure 8, Fig. 1A) in previous experiments with C57BL/6J mice. The lung histopathology reached a higher index at exposure 8 in both groups of surviving mice (Fig. 1B). However, unlike after a single exposure, when no differences between the normal and IL-10-deficient mice could be detected, lung histopathology was significantly more severe in IL-10T than in C57BL/6J mice (P = 10−3, ANOVA) (Fig. 4). As in the experiments carried out with PAO381, the lungs of C57BL/6J mice exposed for 3 weeks to PAO1 had moderate to marked perivascular and peribronchial infiltrates of mononuclear cells (Fig. 4A) composed primarily of lymphocytes and scattered macrophages. The lungs of IL-10T mice exposed for 3 weeks had similar lesions; however, the amount of lung parenchyma involved was much greater (Fig. 4B), and the macrophage component was more prominent. In these experiments, there were no microscopic lesions found in the unexposed control C57BL/6J mice. One of the unexposed IL-10T controls had minimal lymphocytic cuffing of blood vessels and airways, and there was a minimal increase in goblet cells in the bronchi. These findings are reflected in the histopathology indices shown in Fig. 1B (P = 10−3, ANOVA). Thus, the model of repeated exposure to P. aeruginosa aerosols developed in this work permitted the detection of the effects of IL-10 on lung histopathology which were not detectable after a single exposure to the pathogen.
TABLE 2.
Mortality in IL-10T mice after exposure to P. aeruginosa aerosols
| Expt no. | Mouse strain | P. aeruginosa strain | Mortality (no. dead/total) |
|---|---|---|---|
| 1a | C57BL/6J | PAO1 | 0/8 |
| IL-10T | PAO1 | 4/8 | |
| IL-10T | None (control) | 0/4 | |
| 2b | C57BL/6J | PAO1 | 0/20 |
Two groups of 8-week-old C57BL/6J and IL-10T mice (n = 12 per group) were exposed to P. aeruginosa PAO1 aerosols. The initial deposition was 7.1 × 106 CFU/lung. Eighteen hours postexposure, four mice from each group were sacrificed for analyses of bacterial clearance, proinflammatory cytokine profiles, and histopathology. Of the remaining eight mice in each group, two animals from the IL-10T group died 60 h following exposure 1. Following the second exposure (bacterial deposition, 1.3 × 106 CFU/lung), two additional mice from the IL-10T group died 18 to 22 h postchallenge. Further exposures did not cause mortality among the surviving IL-10T mice, which were sacrificed at 18 h post-exposure 8 (Fig. 1 and 4). No mortality was observed in the control (unexposed) IL-10T group.
Eight-week-old C57BL/6J mice were exposed twice to P. aeruginosa PAO1. The bacterial depositions were 7.1 × 106 CFU/lung for the first exposure and 4.6 × 106 CFU/lung for the second exposure.
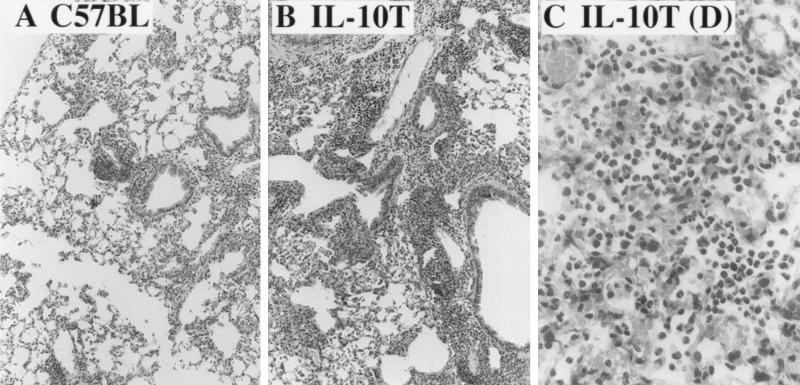
FIG. 4.
Increased lung pathology in IL-10T mice after repeated exposure to P. aeruginosa. (A) C57BL/6J lung section after 3 weeks of repeated exposure to P. aeruginosa PAO1. Note perivascular, peribronchial, and interstitial inflammation. The mouse whose lung section is pictured in panel A was a specimen with maximal severity for the corresponding group. (B) IL-10T mouse lung section after 3 weeks of exposure to P. aeruginosa. Note increased severity of inflammatory changes compared to those in C57BL/6J. The mouse whose lung section is pictured in panel B was average for the group. (C) Lung necropsy of an IL-10T mouse that died after two exposures to P. aeruginosa aerosols. Note the numerous neutrophils. All sections were stained with H-E.
Bacterial clearance and cytokines in IL-10T mice challenged with P. aeruginosa.
Bacterial clearance and levels of proinflammatory cytokines TNF-α and MIP-2 were determined in the IL-10T and control mice that underwent a course of repeated exposure. Interestingly, IL-10T mice cleared P. aeruginosa somewhat better than C57BL/6J mice did upon initial exposure (4.4 × 104 ± 9 × 103 CFU [IL-10T] versus 1.4 × 105 ± 3.5 × 104 CFU [C57BL/6J] per mouse lung) (P = 7.6 × 10−3, ANOVA), with initial deposition of 7.1 × 106 CFU per lung. However, a threefold-lower level of TNF-α (19 ± 2.9 ng versus 54 ± 7.8 ng per g of lung tissue; P = 10−3, ANOVA) and a twofold-lower level of MIP-2 (75 ± 2.5 ng versus 140 ± 18 ng per g of lung tissue; P = 10−3, ANOVA) were found in the lungs of the IL-10T mice relative to those of the C57BL/6J mice at the time of bacteriological evaluation after the initial exposure. One explanation for the lower levels of detected proinflammatory cytokines in the IL-10T animals at the time of organ harvesting is that IL-10 mice cleared P. aeruginosa faster. The higher bacterial burden in C57BL/6J animals most likely continues to elicit more TNF-α and MIP-2 than in the IL-10T mice, which at the time of lung harvesting had significantly lower counts of P. aeruginosa cells. As in the experiments with repeated exposures described in previous sections, TNF-α and MIP-2 levels declined between exposures 1 and 8 but remained significantly higher compared to those in the unexposed age-matched control groups (data not shown). At the termination of the experiment (exposure 8), bacterial clearance improved in both groups relative to the single-exposure experiment and was equal in IL-10T and C57BL/6J mice. This result was accompanied by equal levels of TNF-α and MIP-2 in the two groups.
DISCUSSION
In this work, a mouse model of repeated aerosol challenge with P. aeruginosa has been described. This infection model was used to assess the role of several microbial and host factors in bacterial clearance and inflammation. In addition, normal and transgenic mice defective for the major anti-inflammatory cytokine IL-10 were studied. The main conclusions for the analyses presented here are as follows. (i) Mucoid P. aeruginosa displayed lower susceptibility to innate clearance mechanisms in the murine lung relative to its nonmucoid parent. However, the extent of lung histopathology and the levels of proinflammatory cytokines were similar in animals exposed to mucoid and nonmucoid P. aeruginosa. (ii) Unlike after a single exposure to P. aeruginosa, which caused only a marginal histopathology, significant lung pathology was observed during the repeated inhalation regimen. This feature of the repeated exposure model facilitated several comparative studies carried out in the present work. (iii) An increase in the histopathology in IL-10T mice was observed upon repeated challenge with P. aeruginosa. The IL-10-deficient animals displayed an array of differences relative to normal mice, including significant mortality upon the initial encounter of the pathogen and increased pathology of the lung detectable only in the model of repeated aerosol exposure described here.
The observations indicating that mucoid P. aeruginosa is more resistant than the nonmucoid form to the innate clearance mechanisms in the murine lung are in keeping with a recently detected retarded clearance of mucoid strains monitored 4 h postinfection with a single administration of bacterial aerosols (7). The latter study also employed a variety of mutants and growth conditions with a number of mucoid P. aeruginosa strains, including fresh CF isolates (7), aimed at demonstrating a direct role of alginate production, and such analyses were not repeated here. The nonmucoid parent PAO381 and its mucoid derivative, PAO578I, employed in the present study were leucine auxotrophs, and the dynamics of bacterial clearance and survival may have been affected by this property, although such effects are expected to be equal in both strains. It should also be noted that CF isolates frequently contain auxotrophic mutations (1). Taken together with the analyses using CF strains (7), our results support the findings of the previous extensive work carried out in vitro suggesting that the mucoid coating of P. aeruginosa may confer resistance against phagocytic and other bactericidal systems in the lung (2, 17, 25, 28, 36, 45). Our observations contrast with an earlier report by Blackwood and Pennington, who found that mucoid P. aeruginosa had no advantage over its nonmucoid revertant in a model of intratracheal instillation in guinea pigs (5), and are in keeping with an earlier report by Govan et al. (18) and a more systematic analysis by Boucher et al. (7).
The significant pathology observed in the respiratory tract of animals repeatedly exposed to P. aeruginosa supports the notion that this opportunistic pathogen can cause considerable damage to the host (provided that it is not eliminated from the lung), negating some views that P. aeruginosa may be just a bystander in CF. The considerable pathology detectable in the repeated-exposure model enabled us to compare the damage inflicted by mucoid and nonmucoid isogenic strains. However, the results of such analyses did not indicate that mucoid P. aeruginosa caused heightened pathological changes relative to its nonmucoid parent. Nevertheless, mucoid cells are cleared less efficiently and appear to linger in the lung longer than nonmucoid organisms. This finding suggests that mucoidy may confer an ability to resist innate clearance mechanisms in the lung and, along with other potentially contributing factors, could be the basis for selection of mucA mutants in CF. The results of an earlier study, with the rat agar bead model (53), suggest that mucoid strains emerge in vivo when P. aeruginosa persistence is assisted by agar beads for protection from phagocytic cells. A selection in vitro for mucA mutations, which are found in 85% of mucoid isolates from CF patients (7), remains, however, to be demonstrated in vivo in a situation in which P. aeruginosa is not artificially protected from natural clearance mechanisms in the lung. It would also be of interest to localize in the lung the residual fraction of mucoid cells observed in our studies. Current experiments with green fluorescence protein-expressing P. aeruginosa may help detect the pockets of bacteria remaining in the lung.
Furthermore, while we have not investigated the immune mechanisms later in the infection, it will be of interest to determine the nature of an apparent protection that was detectable at exposure 15 (Table 1) and subsequent stages (data not shown). Whatever immune processes and Pseudomonas targets are involved in these phenomena, the elicited protection appears to have been effective against both mucoid and nonmucoid cells. This may be important considering the previously published detection of specific opsonic antibodies in CF patients which escaped colonization with P. aeruginosa (40, 41). Future work will be needed to investigate the parameters of prolonged exposure to P. aeruginosa in immunized animals.
The use of transgenic mice as in the infection model presented here provides other opportunities for investigating the role of inflammation and anti-inflammatory cytokines (e.g., IL-10). The choice of cytokines monitored in this study was based on (i) the specifics of cytokine profiles in CF (6, 24), (ii) the acknowledged roles of both TNF-α and MIP-2 as crucial factors in the innate response against bacterial pathogens, and (iii) the role of IL-10 in the suppression of inflammatory processes. TNF-α has been implicated in inflammation and in the clearance of P. aeruginosa from the murine lung (33), albeit our recent studies suggest that such effects may be highly strain dependent (7). TNF-α plays a dual role by upregulating adhesion molecules such as ICAM-1, a vital component of polymorphonuclear leukocyte recruitment, and by contributing to the restriction of microbial growth by amplifying the innate clearance mechanisms (3, 34). The proposed role for MIP-2 is as a mouse equivalent of human IL-8. While IL-8 is the major neutrophil chemotactic factor in the human lung, the mouse lacks this chemokine. Instead, MIP-2 and another similar murine chemokine, KC, have been proposed to act as neutrophil attractants in the mouse (20, 42, 43). MIP-2 can bind murine IL-8 type B receptor homolog with high affinity and has been associated with neutrophil influx and pulmonary inflammation. Increased levels of both TNF-α and IL-8 have been found in the bronchoalveolar fluid of CF patients (6). Similarly, we found increased levels of TNF-α and MIP-2 in the lungs of mice chronically exposed to P. aeruginosa. The increase in TNF-α and MIP-2 in the repeated aerosol challenge was for the most part limited to the lung tissue, indicating that the effects of infection were restricted to the lung, reminiscent of the strict confinement of P. aeruginosa to the respiratory tract, commonly seen in CF. Furthermore, P. aeruginosa was not found in the spleens and livers of infected animals at any stage of infection (data not shown).
IL-10 has both anti-inflammatory and immunosuppressive functions (32). Besides its role in contributing to the polarization of immune response by inhibiting the activity of Th1 cells (26, 32), IL-10 is an important suppressor of innate inflammatory response and downregulates the production of cytokines such as TNF-α (13). Since IL-10 protects mice from lipopolysaccharide-induced lethal shock (15, 23), the relatively high mortality among IL-10T mice exposed to P. aeruginosa aerosols observed in the present study may be explained by the lack of IL-10, although other, indirect effects cannot be excluded based on our experiments. While, as expected, no IL-10 was detected in IL-10T mice, IL-10 was produced in C57BL/6J mice in our experiments (data not shown), and its levels were increased 2.4-fold (P = 0.01, ANOVA) 18 h following exposure to PAO1, supporting its possible role in response to P. aeruginosa infection. We also know that the IL-10T mice that succumbed to P. aeruginosa infection did not die because of the systemic spread of bacteria, since no viable organisms were recovered from the spleens and livers of these animals. Intriguingly, no additional fatalities were observed in IL-10T mice after the second exposure cycle. The reasons for this phenomenon are not known at present. However, it is possible that the reduction in TNF-α levels during the later stages of the continuous exposure regimen, observed in all animals examined, may contribute to the improved survival of IL-10T mice past the initial encounter with the pathogen.
The usefulness of the repeated inhalation exposure model developed in this study is best illustrated in experiments with IL-10T mice. A single exposure to P. aeruginosa aerosol was not sufficient to detect any histological differences between the lungs of IL-10T and C57BL/6J mice. Based solely on histopathological evaluation after a single exposure to aerosol infection, one could conclude that IL-10 was not significant for lung inflammation induced with P. aeruginosa. However, in a comparison of IL-10T and C57BL/6J mice after a series of consecutive exposures to P. aeruginosa, increased lung damage and inflammation were detected in IL-10T animals relative to those in the identically exposed control animals capable of producing IL-10. These observations potentially underscore the significance of the finding that IL-10 levels are reduced in the lungs of CF patients relative to those in the lungs of healthy individuals (6) in terms of its possible relevance for the uncontrolled lung tissue destruction typically seen in CF. Based on our findings that IL-10 may be important for suppressing inflammation in the lung in the incessant presence of P. aeruginosa, future attempts to reverse inflammatory processes and tissue damage by the administration of exogenous recombinant IL-10 may provide a model to test the potential benefits of an IL-10-based therapy for CF.
ACKNOWLEDGMENTS
This work was supported by grants AI31139 from the National Institute of Allergy and Infectious Diseases and DERETI96PO from the Cystic Fibrosis Foundation. H. Yu was a Cystic Fibrosis Foundation postdoctoral fellow.
REFERENCES
- 1.Barth A L, Pitt T L. Auxotrophic variants of Pseudomonas aeruginosa are selected from prototrophic wild-type strains in respiratory infections in patients with cystic fibrosis. J Clin Microbiol. 1995;33:37–40. doi: 10.1128/jcm.33.1.37-40.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer A S, Speert D P, Park S, Tu J, Witt M, Nast C C, Norman D C. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of Pseudomonas aeruginosa. Infect Immun. 1991;59:302–308. doi: 10.1128/iai.59.1.302-308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez E L, Young L S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988;140:3006–3013. [PubMed] [Google Scholar]
- 4.Biwersi J, Verkman A S. Functions of CFTR other than as a plasma membrane chloride channel. In: Dodge J A, Brock D J H, Widdicombe J H, editors. Cystic fibrosis—current topics. Vol. 2. Chichester, England: John Wiley & Sons Ltd.; 1994. pp. 155–171. [Google Scholar]
- 5.Blackwood L L, Pennington J E. Influence of mucoid coating on clearance of Pseudomonas aeruginosa from lungs. Infect Immun. 1981;32:443–448. doi: 10.1128/iai.32.2.443-448.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonfield T L, Panuska J R, Konstan M W, Hillard K A, Hillard J B, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 7.Boucher J C, Yu H, Mudd M H, Deretic V. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun. 1997;65:3838–3846. doi: 10.1128/iai.65.9.3838-3846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cash H A, Woods D E, McCullough B, Johanson W G, Bass J A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- 9.Collins F S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256:774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- 10.Cryz S J, Jr, Furer E, Germanier R. Simple model for the study of Pseudomonas aeruginosa infections in leukopenic mice. Infect Immun. 1983;39:1067–1071. doi: 10.1128/iai.39.3.1067-1071.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson D J, Dorin J R, McLachlan G, Ranaldi V, Lamb D, Doherty C, Govan J, Porteous D J. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nat Genet. 1995;9:351–357. doi: 10.1038/ng0495-351. [DOI] [PubMed] [Google Scholar]
- 12.Doggett R G, Harrison G M, Stillwell R N, Wallis E S. An atypical Pseudomonas aeruginosa associated with cystic fibrosis of the pancreas. J Pediatr. 1966;68:215–221. [Google Scholar]
- 13.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 14.Fyfe J A M, Govan J R W. Alginate synthesis in mucoid Pseudomonas aeruginosa: a chromosomal locus involved in control. J Gen Microbiol. 1980;119:443–450. doi: 10.1099/00221287-119-2-443. [DOI] [PubMed] [Google Scholar]
- 15.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele A, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 17.Govan J R W, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govan J R W, Fyfe J A M, Baker N R. Heterogeneity and reduction in pulmonary clearance of mucoid Pseudomonas aeruginosa. Rev Infect Dis. 1983;5:S874–S879. doi: 10.1093/clinids/5.supplement_5.s874. [DOI] [PubMed] [Google Scholar]
- 19.Govan J R W, Nelson J W. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48:912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 20.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicuddy D C, Standiford T J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 21.Hatano K, Goldberg J B, Pier G B. Biologic activities of antibodies to the neutral-polysaccharide component of the Pseudomonas aeruginosa lipopolysaccharide are blocked by O side chains and mucoid exopolysaccharide (alginate) Infect Immun. 1995;63:21–26. doi: 10.1128/iai.63.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holloway B W. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955;13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- 23.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin-10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan T Z, Wagener J S, Bost T, Martinez J, Accurso F J, Riches D W H. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 25.Koch C, Hoiby N. Pathogenesis of cystic fibrosis. Lancet. 1993;341:1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 27.Lam J, Chan R, Lam K, Costerton J W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980;28:546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Learn D B, Brestel E P, Seetharama S. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun. 1987;55:1813–1818. doi: 10.1128/iai.55.8.1813-1818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahenthiralingam E, Campbell M E, Speert D P. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin D W, Schurr M J, Mudd M H, Deretic V. Differentiation of Pseudomonas aeruginosa into the alginate-producing form: inactivation of mucB causes conversion to mucoidy. Mol Microbiol. 1993;9:497–506. doi: 10.1111/j.1365-2958.1993.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 31.Martin D W, Schurr M J, Mudd M H, Govan J R W, Holloway B W, Deretic V. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc Natl Acad Sci USA. 1993;90:8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore K W, O’Garra A, de Waal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1992;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 33.Morissette C, Francoeur C, Darmond-Zwaig C, Gervais F. Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect Immun. 1996;64:4984–4992. doi: 10.1128/iai.64.12.4984-4992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulligan M S, Vaporciyan A A, Miyasaka M, Tamatani T, Ward P A. Tumor necrosis factor regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol. 1993;142:1739–1744. [PMC free article] [PubMed] [Google Scholar]
- 35.Orme I M, Collins F M. Mouse model of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection and control. Washington, D.C: ASM Press; 1994. pp. 113–134. [Google Scholar]
- 36.Pedersen, S. S. 1992. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS 100(Suppl. 28):1–79. [PubMed]
- 37.Pedersen S S, Hoiby N, Espersen F, Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47:6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pier B B, Meluleni G, Neuger E. A murine model of chronic mucosal colonization by Pseudomonas aeruginosa. Infect Immun. 1992;60:4768–4776. doi: 10.1128/iai.60.11.4768-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaska J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pier G B, Saunders J M, Ames P, Edwards M S, Auerbach H, Goldfarb J, Speert D P, Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987;317:793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- 41.Pier G B, Small G J, Warren H B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science. 1990;249:537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- 42.Schall T J. The chemokines. In: Thomson A W, editor. The cytokine handbook. 2nd ed. San Diego, Calif: Academic Press; 1994. pp. 419–460. [Google Scholar]
- 43.Schmal H, Shanley T P, Jones M L, Friedl H P, Ward P A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996;156:1963–1972. [PubMed] [Google Scholar]
- 44.Schurr M J, Martin D W, Mudd M H, Deretic V. Gene cluster controlling conversion to alginate-overproducing phenotype in Pseudomonas aeruginosa: functional analysis in a heterologous host and role in the instability of mucoidy. J Bacteriol. 1994;176:3375–3382. doi: 10.1128/jb.176.11.3375-3382.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson J A, Smith S E, Dean R T. Scavenging by alginate of free radicals released by macrophages. Free Radical Biol Med. 1989;6:347–353. doi: 10.1016/0891-5849(89)90078-6. [DOI] [PubMed] [Google Scholar]
- 46.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:1–20. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 47.Sordelli D O, Garcia V E, Cerequetti C M, Fontan P A, Hooke A M. Intranasal immunization with temperature sensitive mutants protects granulocytopenic mice from lethal pulmonary challenge with Pseudomonas aeruginosa. Curr Microbiol. 1992;24:9–14. [Google Scholar]
- 48.Speert D P. Pseudomonas aeruginosa infections in patients with cystic fibrosis. In: Baltch A L, Smith R P, editors. Pseudomonas aeruginosa infections and treatment. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 183–236. [Google Scholar]
- 49.Starke J R, Edwards M S, Langston C, Baker C J. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987;22:698–702. doi: 10.1203/00006450-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Stieritz D D, Holander I A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975;131:688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- 51.Tang H, Kays M, Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect Immun. 1995;63:1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welsh M J, Tsui L-C, Boat T F, Beaudet A L. Cystic fibrosis. In: Scriver C R, Beaudet A L, Sly W S, Valle D, editors. The metabolic and molecular basis of inherited disease. III. New York, N.Y: McGraw-Hill, Inc.; 1995. pp. 3799–3876. [Google Scholar]
- 53.Woods D E, Sokol P A, Bryan L E, Storey D G, Mattingly S J, Vogel H J, Ceri H. In vivo regulation of virulence of Pseudomonas aeruginosa associated with genetic rearrangement. J Infect Dis. 1991;163:143–149. doi: 10.1093/infdis/163.1.143. [DOI] [PubMed] [Google Scholar]
- 54.Yu H, Schurr M J, Boucher J C, Martinez-Salazar J M, Martin D W, Deretic V. Molecular mechanism of conversion to mucoidy in Pseudomonas aeruginosa. In: Silver S, Nakazowa T, Haas D, editors. Molecular biology of pseudomonads. Washington, D.C: ASM Press; 1996. pp. 384–397. [Google Scholar]
- 55.Zar H, Saiman L, Quittell L, Prince A. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr. 1995;126:230–233. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]