Abstract
Background
Chronic low back pain, common from the sixth decade, negatively impacts the quality of life of patients and health care systems. Recently, mesenchymal stem cells (MSCs) have been introduced in the management of degenerative discogenic pain. The present study summarizes the current knowledge on the effectiveness of MSCs in patients with discogenic back pain.
Sources of data
We performed a systematic review of the literature following the PRISMA guidelines. We searched PubMed and Google Scholar database, and identified 14 articles about management of chronic low back pain with MSCs injection therapy. We recorded information on type of stem cells employed, culture medium, clinical scores and MRI outcomes.
Areas of agreement
We identified a total of 303 patients. Ten studies used bone marrow stem cells. In the other four studies, different stem cells were used (of adipose, umbilical, or chondrocytic origin and a pre-packaged product). The most commonly used scores were Visual Analogue Scale and Oswestry Disability Index.
Areas of controversy
There are few studies with many missing data.
Growing points
The studies analysed demonstrate that intradiscal injections of MSCs are effective on discogenic low-back pain. This effect may result from inhibition of nociceptors, reduction of catabolism and repair of injured or degenerated tissues.
Areas timely for developing research
Further research should define the most effective procedure, trying to standardize a single method.
Keywords: mesenchymal stem cells, bone marrow, back pain, intradiscal injection
Introduction
Chronic low back pain is extremely common and mainly affects patients over 60, with a prevalence of about 70%,1–4 worsening the quality of life of patients and imposing negative economic consequences on health care systems.1,5
Recently, biological therapy with mesenchymal stem cells (MSCs) has been introduced in the management of discogenic pain and degenerative disc disease (DDD).6
Back pain of discogenic origin has a multifactorial pathogenesis, and genetic factors, age, body mass index (BMI), smoking, work activity and trauma contribute to the development of the pathology.7–15
Aging is accompanied by profound modifications of the intervertebral disc, including alterations of the normal anabolic/catabolic balance, which normally keeps the intervertebral disc intact.16 The nucleus pulposus loses water, and calcific areas induce a lower capacity to distribute load with a reduction of the intervertebral space.13,17 The lower synthesis of type I collagen, the main constituent of the fibrous annulus, progressively reduces the elastic properties of the nucleus pulposus, favouring protrusion and herniation.18–21 In addition to collagen, age-related changes also affect proteoglycans and the extra-cellular matrix (ECM). Generally, the ratio of chondroitin sulphate to keratan sulphate is in favour of the former; with age, this ratio is reversed, reducing hydrophilia.22–24
The metalloproteinases of the ECM are less subject to inhibitory control; in addition, degenerative processes induce an acidic environment that further promotes the activation of these enzymes, which participate in the degenerative processes of the disc.25 All the alterations to the disc, together with the continuous mechanical stresses to which the spine is subjected, affect the adjacent nerve structures and manifest with the appearance of pain.26 A high BMI increases the load on the discs, with possible earlier onset of discogenic pain.27
The management of discogenic low back pain can be conservative or surgical.28 Generally, the initial approach is conservative and includes nonsteroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, opioids and physiotherapy.29,30
In most patients, conservative management should be attempted before surgical treatment, as local and systemic complications may occur following surgery, including deep vein thrombosis, infection and myocardial infarcts.31–33
In spinal fusion, for example, in addition to the risks of non-union and hardware failure, alterations to the adjacent upper and lower vertebral segments are common due to abnormal load distribution.34
Recently, stem cell therapies have been increasingly studied to promote regeneration of the disc structures that determine the onset of symptoms. Degenerative discopathy seems to be responsible for 40% of low back pain.35
The intervertebral disc has its own multipotent stem cells, with progenitor cells both in the nucleus pulposus and the annulus fibrosus, with markers typical of MSCs.36
These stem cells can differentiate and participate in regenerative processes.37–40 With age, these cells progressively reduce, affecting the repair capabilities of the intervertebral disc. In the annulus fibrosus, progenitor cells can differentiate into different cell lines, such as adipocytes, chondrocytes, osteoblasts and endothelial cells.41
Other stem cells, both adipose and medullary, can differentiate into cells with characteristics similar to those of the nucleus pulposus under appropriate stimuli.42–44 In vitro, inoculated MSCs can develop phenotypic features similar to the disc own cells, capable of synthesizing the different matrix components when stimulated by growth factors such as Transforming Growth Factor-β (TGF-β),45–47 growth differentiation factor 5 (GDF5) and growth differentiation factor 6 (GDF6) belonging to the TGF family. In these studies, GDF-5 favoured the phenotypic differentiation of bone marrow (BM) stem cells into cells of the nucleus pulposus by promoting the synthesis of type II collagen,48 but it did not stimulate the production of proteoglycans, as TGF-β1 did.49–51
Therefore, in stem cell therapy, it is important to consider both the type of stem cells and the growth factors used in combination with them, as well as the use of scaffolds.
Patients in whom stem cell therapy would be indicated present with early disc degeneration and mild to moderate pain, and failure of conservative therapy. Ideal patients are those with degenerative involvement of a single Pfirrmann Grade III–IV disc.52
This review defines the current knowledge on the effectiveness of biological therapy using MSCs in patients with discogenic back pain.
Methods
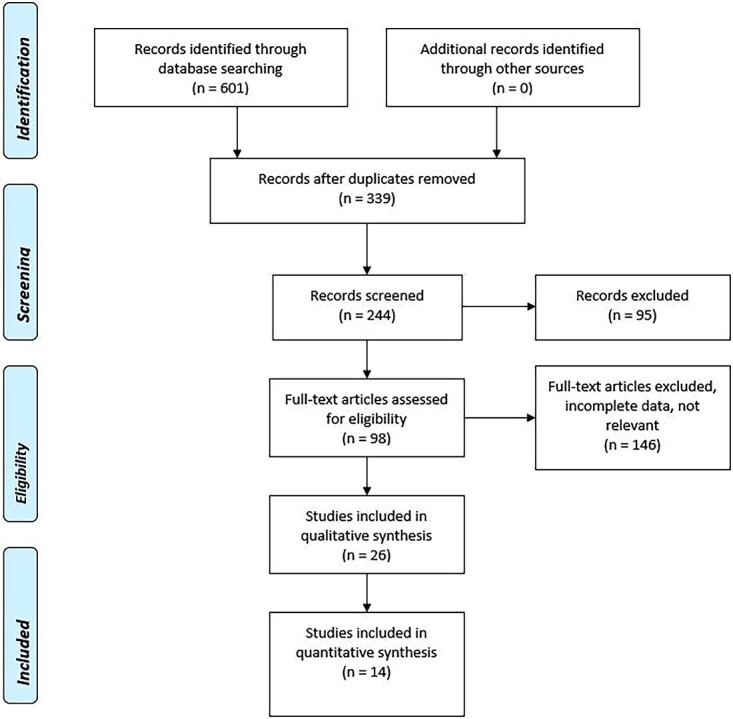
This study and its procedures were organized, conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines53 (Fig. 1).
Fig. 1.
Correlation between MCMS and year of publication.
Eligibility criteria
We searched studies about the use of stem cells in the management of discogenic back pain. Studies included in the search are case reports and case series, clinical trials and systematic reviews. We excluded animal studies, editorials, narrative reviews and articles in which stem cells were used in combination with confounding factors that could affect the outcome such as PRP.
Data sources and search
We performed an exhaustive search of all databases associated with PubMed and Scopus up to April 2023, using the following key words: MSCs, stem cells, back pain, discogenic back pain, intervertebral disc degeneration.
Study selection
The articles resulting from the search were evaluated independently by two orthopaedic residents. A researcher experienced in systematic review solved cases of doubt. The initial selection of articles was based on the title and reading of the abstract. In accordance with inclusion and exclusion criteria previously reported, the articles considered relevant to the aim of the study were selected. Subsequently, these articles were read in their entirety to ascertain their actual relevance to the purposes of this review.
Data collection
The data extracted from reading the articles included in the present systematic review were collated in an Excel database. Doubts and inconsistencies were followed and solved by discussion. The features analysed include:
Type of stem cells employed
Characteristics of the culture medium
Clinical scores
MRI outcomes
Methodological assessment
We used the Modified Coleman Methodology Score (MCMS)54 criteria to assess the studies reviewed (Table 1). A score from 0 to 100 is assigned to each study; a score of 100 indicates a study in which there are no confounding factors or bias. The MCMS was correlated with publication year to examine the chronological trend in methodology.54
Table 1.
MCMS
| Score | ||
|---|---|---|
| Part A: Only one score to be given for each of the 7 sections | ||
| 1. Study size: number of patients | ||
| <30 | 0 | |
| 30–50 | 4 | |
| 50–100 | 7 | |
| >100 | 10 | |
| 2. Mean follow-up | ||
| <12 months | 0 | |
| 12–36 months | 4 | |
| 37–60 months | 7 | |
| >61 months | 10 | |
| 3. Surgical approach | ||
| Different approach used and outcome not reported separately | 0 | |
| Different approach used and outcome reported separately | 7 | |
| Single approach used | 10 | |
| 4. Type of study | ||
| Retrospective cohort study | 0 | |
| Prospective cohort study | 10 | |
| Randomized controlled trial | 15 | |
| 5. Description of diagnosis | ||
| Described without percentage specified | 0 | |
| Described with percentage specified | 5 | |
| 6. Description of surgical technique | ||
| Inadequate (not stated, unclear) | 0 | |
| Fair (technique only stated) | 5 | |
| Adequate (technique stated, details of surgical procedure given) | 10 | |
| 7. Description of postoperative rehabilitation | ||
| Described | 5 | |
| Not described | 0 | |
| Part B: Scores may be given for each option in each of the 3 sections if applicable | ||
| 1. Outcome criteria | ||
| Outcome measures clearly defined | 2 | |
| Timing of outcome assessment clearly stated | 2 | |
| Use of outcome criteria that has reported reliability | 3 | |
| General health measure included | 3 | |
| 2. Procedure of assessing outcomes | ||
| Participants recruited | 5 | |
| Investigator independent of surgeon | 4 | |
| Written assessment | 3 | |
| Completion of assessment by patients themselves with minimal investigator assistance | 33 | |
| 3. Description of subject selection process | ||
| Selection criteria reported and unbiased | 5 | |
| Recruitment rate reported | ||
| >90% | 5 | |
| <90% | 0 | |
Results
The initial search produced a total of 601 articles. After removal of duplicates, we obtained 339 articles. After the first abstract and title analysis, we excluded 95 articles. From the 244 remaining articles, we excluded 146 articles after full-text assessment. A total of 14 articles were included in the present review (Table 2).
Table 2.
Studies included and main features
| Study | Year of publication | Type of study | No. of patients | Stem Cells | Conclusions |
|---|---|---|---|---|---|
| Lewandrowski et al.55 | 2023 | Retrospective | 33 | Allogenic BM MSCs | The injection of allogeneic MSCs to treat patients with painful intermediate-stage degenerative disc disease has merit. |
| Amirdelfan et al.56 | 2021 | Randomized Controlled Trial | 100 | Allogenic BM MSCs | Intradiscal injection of MPCs could be a safe, effective, durable and minimally invasive therapy for subjects who have CLBP associated with moderate DDD. |
| Wolff et al.57 | 2020 | Retrospective | 33 | Autologous Bone Marrow Concentrate | Autologous BMCs are a logical strategy to alleviate discogenic pain and restore patient function with the goal of providing a restorative therapy, which provides long-term benefits of reduced pain and improved disc health and function |
| Ju et al.58 | 2020 | Randomized Controlled Trial | 13 | Cell-based stem cell treatment (MPC-06-ID, Mesoblast) | No difference in outcomes between therapeutic intradiscal agents and the control saline arm. In all groups, patient reported pain and disability scores decreased significantly |
| Centeno et al.59 | 2017 | Pilot study | 33 | Autologous Bone Marrow Concentrate | The intradiscal injection of culture expanded MSCs to treat DDD with symptomatic disc bulge produced encouraging results: reduced pain, increased function and reduced disc bulge size in most patients |
| Kumar et al.60 | 2017 | Single-arm clinical trial | 10 | AT-MSCs | The study confirmed the safety and tolerability of coinjection of AT-MSCs and a HA derivative in patients with intervertebral disc degeneration |
| Pettine et al.61 | 2017 | Prospective, open-label, non-randomized, single-arm study | 26 | Autologous Bone Marrow Concentrate | These results indicate that injection of BM concentrate has the potential to provide a non-surgical option for patients with chronic discogenic low back pain |
| Elabd et al.62 | 2016 | Case Series | 5 | Autologous BM MSCs | Intra-discal injection of autologous, hypoxic cultured BM-derived MSCs demonstrated safety and feasibility in five patients diagnosed with DDD. |
| Noriega et al.63 | 2016 | Randomized Controlled Trial | 12 | Allogenic BM MSCs | The therapy with expanded allogeneic BM-derived MSC results in significant relief of pain and disability, and quantitative MRI evidence suggests partial disc healing. The healing effects appear to be smaller than those reported for treatment with autologous MSC. |
| Mochida et al.64 | 2015 | Prospective Clinical Study | 9 | NP cells co-cultured in direct contact with autologous BMA-MSCs. | The study confirmed the safety of activated NP-cell transplantation and provided promising findings that suggest the minimal efficacy of this treatment to slow the further degeneration of human intervertebral discs |
| Pang et al.65 | 2014 | Clinical Trial | 2 | Human umbilical cord tissue-derived mesenchymal stem cells (HUC-MSCs) | The study indicates that HUC-MSC transplantation is a favourable alternative method for the treatment of chronic discogenic low back pain. |
| Coric et al.52 | 2013 | Prospective Study | 15 | NuQu® allogeneic juvenile chondrocytes (ISTO Technologies) | Preliminary safety was demonstrated, and clinical results were encouraging, with statistically significant improvements in ODI, NRS and SF-36 scores |
| Orozco et al.66 | 2011 | Clinical Trial | 10 | Autologous BM MSCs | The therapy with BM-derived MSCs may be a valid alternative treatment for chronic back pain caused by DDD. Advantages over current gold standards include simpler and more conservative intervention without surgery, preservation of normal biomechanics and same or better pain relief |
| Yoshikawa et al.67 | 2010 | Case Report | 2 | Autologous BM MSCs | The intervertebral disc regeneration therapy using MSC brought about favourable results. It seems to be a promising minimally invasive treatment |
Ten of 14 studies used stem cells derived from the BM. Three of these studies used Bone Marrow Concentrate. In one of these studies, stem cells were cultured next to the nucleus pulpous (NP). In the remaining four studies, different stem cells were used [adipose, umbilical, chondrocytarian origin (NuQu® allogeneic juvenile chondrocytes)] and a pre-packaged product, Mesoblast (MPC-06-ID, Mesoblast), was also employed.
The details of the culture are reported in Table 3.
Table 3.
Injection characteristics
| Study | Stem cells | Media | Injected solution | Injection site | Injection volume |
|---|---|---|---|---|---|
| Lewandrowski et al.55 | BM allogenic | Dulbecco’s modified eagle medium (DMEM) | Hyaluronic acid derived from immunoselected umbilical cord stem cells | Intradiscal | 2 ml |
| Amirdelfan et al.56 | BMA | – | Hyaluronic acid | Intradiscal | 2 ml |
| Wolff et al.57 | BMC | Magellan Autologous Platelet Separator System (Isto Biologics, Hopkinton | MSCs, platelets, growth factors. | Intradiscal | 3 ml |
| Ju et al.58 | Mesoblast | – | Hyaluronic acid | Intradiscal | 2 ml |
| Centeno et al.59 | BMC | Platelet lysate; Doxycycline; Heparin; Hypoxic conditions |
Platelet lysate | Intradiscal | – |
| Kumar et al.60 | AT-MSCs | – | 1% Hyaluronic Acid Tissuefill® (HA derivative; CHA Meditech Co., Ltd, Daejeon, South Korea) | Intradiscal | 2 ml |
| Pettine et al.61 | BMC | ART BMC (Celling Biosciences, Austin, TX) | – | Intradiscal | 2–3 ml |
| Elabd et al.62 | BMA | Dulbecco’s modified eagle medium (DMEM) with 10% platelet lysate, 5 μg/ml doxycycline, and 2 IU/ml heparin in a 37°C/5% CO2/5% O2 incubator (hypoxic conditions) | BMA + autologous platelet lysate | Intradiscal | 0.25–1 ml |
| Noriega et al.63 | BM allogenic | Ringer Lactate | Saline solution | Intradiscal | 2 ml |
| Mochida et al.64 | BMA | Serum; NP cells | Saline solution | Intradiscal | 1 ml |
| Pang et al.65 | HUC-MSCs | Dulbecco’s modified Eagle’s medium (DMEM, Gibco) + 10% FBS | – | Intradiscal | 1 ml |
| Coric et al.52 | NuQu® allogeneic juvenile chondrocytes | Gentamicin, l-glutamine, growth factors, and l-ascorbate | Fibrin | Intradiscal | 1–2 ml |
| Orozco et al.66 | BMA | – | – | Intradiscal | – |
| Yoshikawa et al.67 | BMA | 15% autologous serum, gentamicin; 100 nmol/L estriol; 0.1% trypsin | Collagen sponge | Intradiscal | 10 ml |
Stem cells were mixed with other substances before injection. In three studies, a platelet lysate was used; in two, a saline solution; in four, hyaluronic acid; in one, fibrin; in 1one collagen sponges were used. The injection volume varied between 1 and 3 ml. Yoshikawa et al. used collagen sponges with a volume of 10 ml.67
All studies reported beneficial results of stem cell therapy, with improvements in pain, strength and return to daily and work activities.
Different scores were used. The most commonly used are VAS and ODI, used in 9 of 14 and 10 of 14 studies, respectively. Other scores were: SF-36, used in 5 of 14 studies; NRS, in 2 of 14; JOA, in 2 of 14. In relation to the VAS, 5 of 8 studies used a scale from 0 to 100, 2 of 8 from 0 to 10 and 1 study did not report such data.
Using the t student between ODI pre and post management, the P-value is 0.0004; similarly for the VAS score, the P-value is <0.0001.
The details of the different scores are reported in Table 4.
Table 4.
Scores
| Study | Scores | Pre-treatment | Post-treatment |
|---|---|---|---|
| Lewandrowski et al.55 | VAS ODI |
8.2 44.8 |
1.74 6.07 |
| Amirdelfan et al.56 | VAS ODI SF-36 WPAI |
70 48.5–– |
–––– |
| Wolff et al.57 | ODI NRS SF-36 |
36.7 5.2 53.4 |
––– |
| Ju et al.58 | VAS ODI |
66.5 20 |
21.8 9.3 |
| Centeno et al.59 | SANE NPS FRI |
– 5.2 60.5 |
53 3.3 30 |
| Kumar et al.60 | VAS ODI SF-36 |
6.5 42.8 |
2.9 16.8 |
| Pettine et al.61 | VAS ODI |
82.1 56.7 |
21.9 17.5 |
| Elabd et al.62 | – | – | – |
| Noriega et al.63 | VAS ODI SF-12 men SF-12 phy |
67 34 46 39 |
47 22 48 45 |
| Mochida et al.64 | JOA LBP |
14.2 1.2 |
27.2 2.7 |
| Pang et al.65 | VAS ODI |
7.5 51 |
2.5 12.5 |
| Coric et al.52 | ODI NRS SF-36 men SF-36 phy |
53.1 35.2 48.5 35.5 |
20.3 3.1 50.5 46.9 |
| Orozco et al.66 | VAS ODI SF-36 men SF-36 phy |
68.9 25 54.1 12.7 |
20 7.4 49.7 24.8 |
| Yoshikawa et al.67 | JOA VAS |
2.5 – |
17 – |
The MRI baseline characteristics of early stage patients were disc hydration, height, bulging or protrusions and annulus tears. The MRI was repeated at follow-up to identify any changes in these characteristics. In 7 of 14, studies, the water content of the disc was evaluated with the MRI T2-weighted sequence, evidencing that hydration had increased. The height of the disc was assessed in 8 of 14 studies, with encouraging results related to the conservation or increase of the height of the discs. Bulging was evaluated in 4 of 14 studies, with a reduction in at least 23% of cases. In 6 of 13 studies, the condition of the spine was graded using Modic criteria, from grade I to III; 6 of 14 studies used the Pfirrmann grading system, from grade I to V; finally in 2 of 14 studies the Modified Dallas Discogram Description from a grade 0 to IV was used.
In 3 of 14 studies, the most common adverse effect was injection pain, treated with NSAIDs and opioids. The use of subsequent surgical treatment was considered as failure of stem cell therapy; this occurred in 4 of 303 patients.
MCMSs
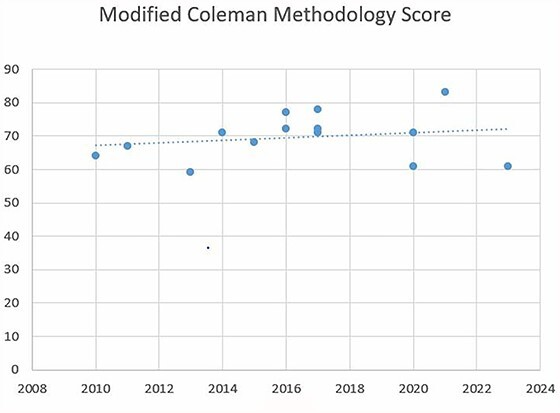
Calculating the Pearson’s correlation coefficient between MCMS and the year of publication (Fig. 2), we obtained a positive association (r = 0.48, P-value 0.1). In recent years, there was not an improvement in methodology.
Fig. 2.
PRISMA flow diagram 2009.
The mean MCMS score was 69.64. Table 5 reports mean, SD and range for each MCMS criteria.
Table 5.
Mean score for each MCMS criteria
| Methodology criterion | Mean score (SD) | Range |
|---|---|---|
| Part A | ||
| 1. Study size | 1.64 (2.4) | 0–7 |
| 2. Follow-up | 2.35 (3.2) | 0–10 |
| 3. N procedures | 10 (0) | 10 |
| 4. Type of study | 10 (4.8) | 0–15 |
| 5. Diagnostic certainly | 5 (0) | 5 |
| 6. Description of surgical technique | 9.28 (1.81) | 5–10 |
| 7. Rehabilitation & compliance | 0.35 (1.33) | 0–5 |
| Part B | ||
| 1. Outcome criteria | 9.35 (1.27) | 7–10 |
| 2. Outcome assessment | 12.0 (0) | 12 |
| 3. Selection process | 9.64 (1.33) | 5–10 |
| MCMS | 69.64 (6.92) | 59–83 |
Discussion
MSCs have been used for regenerative therapy in different musculo-skeletal conditions. MSCs have been shown to be effective and safe in osteoarthritis and meniscal, and tendon and ligament injuries.68 MSC can be obtained from different tissues: fat, BM and umbilical cord. Stem cells derived from the BM are the most commonly studied, although stem cells derived from adipose tissue are more numerous. Adipose tissue-derived mesenchymal stem cells (AT-MSCs) have a lower capability to differentiate in chondrocytes; in some studies, preculture with NP cells was performed to increase their regenerative capability.69 Stem cells derived from the umbilical cord are used for their low immunogenicity. Discogenic back pain is one of the most common conditions affecting individuals between the fifth and seventh decade, and it is estimated that in 2050 over 2 billion people will be over 60.70 There is no association between pain and MRI appearance.71
During the progression of this chronic condition, there is a shift from type I to type II collagen with progressive dehydration of the ECM and consequent reduction of the mechanical support capability of the disc.16
Cell transplant therapy, involving both MSC and NP, has resulted in increased water content in the disc and consequent height restoration in both in vivo and human studies.36,72–74 The percutaneous implantation of MSC may induce pain relief with three mechanisms: inhibition of nociceptors, reduction of catabolism and repair of tissues. Noriega et al.75 used stem cells derived from allogeneic marrow without adverse events. They quantified the slope of pain relief from baseline to compare between the various trials, and an efficiency of allogeneic of 0.28 versus autologous MSCs of 0.71 was documented. Some studies used NP cells to prevent ‘graft versus host disease’, but these cells had a poor capacity for ECM regeneration.64 Mochida et al.64 cultured NP cells together with MSC to increase the synthetic capacity of autologous NP cells and reduce the risk of GVHD. Umbilical MSCs could differentiate into NP when cultured with them.76
Coric et al.52 used allogeneic chondrocytes to avoid damage to the already damaged NP, further aggravating the pathology. Cells from young patients showed a greater ability to synthesize ECM, without causing GVHD.
One aspect to consider is the low-oxygen environment of the disc, which is also required for successful MSCs culture. Indeed, cells grown at normal oxygen concentrations induced an increase in disc hydration, but not in height.66 Several studies reported on the cross-talk between the injected MSC and the native NP cells, in particular the TGF-beta signalling system, hypothesizing a major role in the regeneration of ECM.77
Overall the studies included in this review indicate that percutaneous injection of intradiscal MSC was safe and resulted in a high success rate.
A multicentre study58 evaluated four types of therapies (Growth factor BMP-7, Active fibrin sealant, Growth factor rhGDF-5, MSC), comparing them to placebo (saline solution) and obtaining good results. A possible effect of the injection of saline solution is the dilution of the cytokines responsible for inflammation.78
Noriega et al.63 obtained interesting results in relation to the time of follow-up. In the control group, which received an injection of local anaesthetic, they obtained a decrease of VAS within 8 days from the administration, without further improvements; the ODI worsened during the year of follow-up. Instead, in the study group with MSC, the greatest effect was achieved at about 3 months and maintained at 6 and 12 months follow-up.
Different scores were used in the various studies to evaluate the state of degeneration of the disc and, consequently, the eligibility of patients for therapy. Patients with complete annular fissuration could not be treated because of disc incontinence. During the injection, Kumar et al.60 suspended the MSCs with a derivative hyaluronic acid, aiming to reduce or prevent the dispersion of stem cells and any differentiation in osteoblasts.
MSCs can differentiate into fibroblasts59 and strengthen the annulus, preventing herniation by depositing new collagen fibres. In fact, 85% of patients showed a reduction in posterior bulge. A reduction of at least 25% of the bulge decreased the pain significantly. Only one case of herniation that required surgery was reported after 5 months. This complication could have resulted from needle injection, excessive proliferation of MSCs or excessive production of ECM.
Among the complications related to the injection of MSCs is the formation of osteophytes in the tissues surrounding the injection site.79
When conservative therapy failed, it is possible to use different surgical methods,7,80 but these have several complications: dural lesions, infections and epidural hematomas.81–84 In stabilization of the spine, for example, by limiting the movements of the affected section of the spine, the stress imposed to the adjacent vertebrae is increased, contributing to the degeneration of those discs. Pettine et al. reported about the reduced length of hospital stay with MSC compared with surgical treatment, which involves 5 days in hospital.61 Despite this, some failure necessitated surgical treatment; for example, three patients were treated surgically between 6 and 12 months after implantation of MSC for persistent pain.52
New therapeutic approaches aim to induce the migration of MSCs to the damaged site and warrant further exploration.85,86
The limitations of this study are related to the low number of articles, the lack of data on patients, the aetiology of discogenic back pain, the type of culture medium and the solution injected, and the use of different clinical scores in the various studies. All these do not allow to obtain homogeneous results regarding treatment efficacy.
Conclusion
Stem cells are a promising potential resource to be exploited in the management of musculoskeletal conditions associated with aging, in which the cellular regenerative capabilities can be employed. Further research efforts should define the actual effectiveness of MSCs in the different areas of their use.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Luca Miranda, Department of Musculoskeletal Disorders, Faculty of Medicine and Surgery, University of Salerno, Via Salvador Allende, 43, Baronissi SA 84081, Italy; Clinica Ortopedica, Ospedale San Giovanni di Dio e Ruggi D’Aragona, Via San Leonardo, Salerno 84131, Italy.
Marco Quaranta, Department of Musculoskeletal Disorders, Faculty of Medicine and Surgery, University of Salerno, Via Salvador Allende, 43, Baronissi SA 84081, Italy; Clinica Ortopedica, Ospedale San Giovanni di Dio e Ruggi D’Aragona, Via San Leonardo, Salerno 84131, Italy.
Francesco Oliva, Department of Musculoskeletal Disorders, Faculty of Medicine and Surgery, University of Salerno, Via Salvador Allende, 43, Baronissi SA 84081, Italy; Clinica Ortopedica, Ospedale San Giovanni di Dio e Ruggi D’Aragona, Via San Leonardo, Salerno 84131, Italy.
Nicola Maffulli, Department of Musculoskeletal Disorders, Faculty of Medicine and Surgery, University of Salerno, Via Salvador Allende, 43, Baronissi SA 84081, Italy; Clinica Ortopedica, Ospedale San Giovanni di Dio e Ruggi D’Aragona, Via San Leonardo, Salerno 84131, Italy; Centre for Sports and Exercise Medicine, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, Mile End Hospital, 275 Bancroft Road, London E1 4DG, England; Guy Hilton Research Centre, Faculty of Medicine, School of Pharmacy and Bioengineering, Keele University, Thornburrow Drive, Hartshill, Stoke-on-Trent ST4 7QB, England.
Authors’ contributions
Luca Miranda (Conceptualization, Data curation, Methodology), Marco Quaranta (Conceptualization, Data curation, Formal analysis, Methodology), Francesco Oliva (Conceptualization, Formal analysis, Validation, Writing—original draft), Nicola Maffulli (Conceptualization, Data curation, Project administration, Supervision, Validation, Writing—review & editing).
Conflict of interest statement
The authors have no have no potential conflicts of interest.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
The authors have no financial or proprietary interests in any material discussed in this article.
Data availability
All data generated or analysed during this study are included in this published article.
References
- 1. Mathew J, Singh SB, Garis S, et al. Backing up the stories: the psychological and social costs of chronic low-back pain. Int J Spine Surg 2013;7:e29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine 2005;30:1082–5. [DOI] [PubMed] [Google Scholar]
- 3. Andersson GB. Epidemiological features of chronic low-back pain. Lancet 1999;354:581–5. [DOI] [PubMed] [Google Scholar]
- 4.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88Suppl 2:21–4. 10.2106/JBJS.E.01273. PMID: 16595438. [DOI] [PubMed] [Google Scholar]
- 5. Manchikanti L, Singh V, Datta S, et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009;12:E35–70. [PubMed] [Google Scholar]
- 6. Sakai D, Andersson GBJ. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol 2015;11:243–56. [DOI] [PubMed] [Google Scholar]
- 7. Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther 2003;5:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashley JW, Enomoto-Iwamoto M, Smith LJ, et al. Intervertebral disc development and disease-related genetic polymorphisms. Genes Dis 2016;3:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res 2016;34:1289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dario AB, Ferreira ML, Refshauge KM, Lima TS, Ordoñana JR, Ferreira PH. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. Spine J. 2015;15:1106–17. 10.1016/j.spinee.2015.02.001. Epub 2015 Feb 7. PMID: 25661432. [DOI] [PubMed] [Google Scholar]
- 11. Influence of lifestyle characteristics and VDR polymorphisms as risk factors for intervertebral disc degeneration: a case–control study. SpringerLink [Internet]. [cited 2021. Jun 1]. 10.1186/s40001-018-0309-x. [DOI] [PMC free article] [PubMed]
- 12. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population-based cohort: the Wakayama spine study. [Internet]. [cited 2021. Jun 1]. https://pubmed.ncbi.nlm.nih.gov/28089899/. [DOI] [PubMed]
- 13.Bogduk, Nikolai. Clinical anatomy of the lumbar spine and sacrum. Elsevier Health Sciences, 2005. [Google Scholar]
- 14. Postacchini F, Lami R, Pugliese O. Familial predisposition to discogenic low-back pain. An epidemiologic and immunogenetic study. Spine 1988;13:1403–6. [DOI] [PubMed] [Google Scholar]
- 15. DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med 2011;12:224–33. [DOI] [PubMed] [Google Scholar]
- 16.Gruber HE, Hanley EN. Observations on morphologic changes in the aging and degenerating human disc: Secondary collagen alterations. BMC Musculoskelet Disord 3, 9 2002. 10.1186/1471-2474-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001;26:1873–8. [DOI] [PubMed] [Google Scholar]
- 18. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix [Internet]. [cited 2021. Jun 1]. https://pubmed.ncbi.nlm.nih.gov/15564918/. [DOI] [PubMed]
- 19. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. PubMed [Internet]. [cited 2021. Jun 1]. https://pubmed.ncbi.nlm.nih.gov/26384579/. [DOI] [PubMed]
- 20. Cornefjord M, Olmarker K, Rydevik R, et al. Mechanical and biochemical injury of spinal nerve roots: a morphological and neurophysiological study. Eur Spine J 1996;5:187–92. [DOI] [PubMed] [Google Scholar]
- 21. Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res 1987;5:198–205. [DOI] [PubMed] [Google Scholar]
- 22.Richardson SM, Freemont AJ, Hoyland JA. Pathogenesis of Intervertebral Disc Degeneration. In: Shapiro I, Risbud M. (eds) The Intervertebral Disc. Springer, Vienna. 2014. 10.1007/978-3-7091-1535-0_11. [DOI] [Google Scholar]
- 23. Bushell GR, Ghosh P, Taylor TF, et al. Proteoglycan chemistry of the intervertebral disks. Clin Orthop Relat Res 1977;129:115–23. [DOI] [PubMed] [Google Scholar]
- 24. Oegema TR, Bradford DS, Cooper KM. Aggregated proteoglycan synthesis in organ cultures of human nucleus pulposus. J Biol Chem 1979;254:10579–81. [PubMed] [Google Scholar]
- 25. Goupille P, Jayson MI, Valat JP, et al. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine 1998;23:1612–26. [DOI] [PubMed] [Google Scholar]
- 26. Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997;350:178–81. [DOI] [PubMed] [Google Scholar]
- 27. Liuke M, Solovieva S, Lamminen A, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes 2005;29:903–8. [DOI] [PubMed] [Google Scholar]
- 28. Longo UG, Loppini M, Romeo G, et al. Evidence-based surgical management of spondylolisthesis: reduction or arthrodesis in situ. J Bone Joint Surg Am 2014;96:53–8. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen JT, Nguyen JL, Wheatley MJ, et al. Muscle hernias of the leg: a case report and comprehensive review of the literature. Can J Plast Surg 2013;21:243–7. [PMC free article] [PubMed] [Google Scholar]
- 30. Migliorini F, Maffulli N. Choosing the appropriate pharmacotherapy for nonspecific chronic low back pain. J Orthop Surg Res 2022;17:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baliga S, Treon K, Craig NJA. Low back pain: current surgical approaches. Asian Spine J 2015;9:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basques BA, Diaz-Collado PJ, Geddes BJ, et al. Primary and revision posterior lumbar fusion have similar short-term complication rates. Spine 2016;41:E101–6. [DOI] [PubMed] [Google Scholar]
- 33. Ma Y, Passias P, Gaber-Baylis LK, et al. Comparative in-hospital morbidity and mortality after revision versus primary thoracic and lumbar spine fusion. Spine J 2010;10:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee JC, Choi SW. Adjacent segment pathology after lumbar spinal fusion. Asian Spine J 2015;9:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheung KMC, Karppinen J, Chan D, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009;34:934–40. [DOI] [PubMed] [Google Scholar]
- 36. The presence of stem cells in potential stem cell niches of the intervertebral disc region: an in vitro study on rats. [Internet]. [cited 2021. Jun 1]. 10.1007/s00586-015-4168-7. [DOI] [PubMed]
- 37. Henriksson H, Thornemo M, Karlsson C, et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine 2009;34:2278–87. [DOI] [PubMed] [Google Scholar]
- 38. Liu LT, Huang B, Li CQ, et al. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS One 2011;6:e26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feng G, Yang X, Shang H, et al. Multipotential differentiation of human anulus fibrosus cells: an in vitro study. J Bone Joint Surg Am 2010;92:675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Risbud MV, Guttapalli A, Tsai TT, et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine 2007;32:2537–44. [DOI] [PubMed] [Google Scholar]
- 41. Gruber HE, Riley FE, Hoelscher GL, et al. Human annulus progenitor cells: analyses of this viable endogenous cell population. J Orthop Res 2016;34:1351–60. [DOI] [PubMed] [Google Scholar]
- 42. Sive JI, Baird P, Jeziorsk M, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol 2002;55:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minogue BM, Richardson SM, Zeef LAH, et al. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum 2010;62:3695–705. [DOI] [PubMed] [Google Scholar]
- 44. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 45. Asai-Coakwell M, French CR, Ye M, et al. Incomplete penetrance and phenotypic variability characterize Gdf6-attributable oculo-skeletal phenotypes. Hum Mol Genet 2009;18:1110–21. [DOI] [PubMed] [Google Scholar]
- 46. Settle SH, Rountree RB, Sinha A, et al. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol 2003;254:116–30. [DOI] [PubMed] [Google Scholar]
- 47. Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2002;2:389–406. [DOI] [PubMed] [Google Scholar]
- 48. Le Maitre CL, Freemont AJ, Hoyland JA. Expression of cartilage-derived morphogenetic protein in human intervertebral discs and its effect on matrix synthesis in degenerate human nucleus pulposus cells. Arthritis Res Ther 2009;11:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peroglio M, Eglin D, Benneker LM, et al. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J 2013;13:1627–39. [DOI] [PubMed] [Google Scholar]
- 50. Stoyanov JV, Gantenbein-Ritter B, Bertolo A, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater 2011;21:533–47. [DOI] [PubMed] [Google Scholar]
- 51. Gantenbein-Ritter B, Benneker LM, Alini M, et al. Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur Spine J 2011;20:962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coric D, Pettine K, Sumich A, et al. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 joint spine section meeting. J Neurosurg Spine 2013;18:85–95. [DOI] [PubMed] [Google Scholar]
- 53. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;29:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coleman BD, Khan KM, Maffulli N, et al. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Scand J Med Sci Sports 2000;10:2–11. [DOI] [PubMed] [Google Scholar]
- 55. Lewandrowski KU, Dowling A, Vera JC, et al. Pain relief after allogenic stem cell disc therapy. Pain Physician 2023;26:197–206. [PubMed] [Google Scholar]
- 56.Amirdelfan K, Bae H, McJunkin T, DePalma M, Kim K, Beckworth WJ, Ghiselli G, Bainbridge JS, Dryer R, Deer TR, Brown RD. Allogeneic mesenchymal precursor cells treatment for chronic low back pain associated with degenerative disc disease: a prospective randomized, placebo-controlled 36-month study of safety and efficacy. Spine J. 2021;21:212–230. 10.1016/j.spinee.2020.10.004. Epub 2020 Oct 9. PMID: 33045417. [DOI] [PubMed] [Google Scholar]
- 57. Wolff M, Shillington JM, Rathbone C, et al. Injections of concentrated bone marrow aspirate as treatment for discogenic pain: a retrospective analysis. BMC Musculoskelet Disord 2020;21:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ju DG, Kanim LE, Bae HW. Is There Clinical Improvement Associated With Intradiscal Therapies? A Comparison Across Randomized Controlled Studies. Global Spine J. 2022;12:756–764. 10.1177/2192568220963058. Epub 2020 Oct 13. PMID: 33047622; PMCID: PMC9344499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Centeno C, Markle J, Dodson E, et al. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med 2017;15:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumar H, Ha DH, Lee EJ, et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res Ther 2017;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pettine KA, Suzuki RK, Sand TT, et al. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int Orthop 2017;41:2097–103. [DOI] [PubMed] [Google Scholar]
- 62. Elabd C, Centeno CJ, Schultz JR, et al. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med 2016;14:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Noriega DC, Ardura F, Hernández-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation 2017;101:1945–51. [DOI] [PubMed] [Google Scholar]
- 64. Mochida J, Sakai D, Nakamura Y, et al. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cell Mater 2015;29:202–12. [DOI] [PubMed] [Google Scholar]
- 65. Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Phys 2014;17:E525–30. [PubMed] [Google Scholar]
- 66. Orozco L, Soler R, Morera C, et al. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 2011;92:822–8. [DOI] [PubMed] [Google Scholar]
- 67. Yoshikawa T, Ueda Y, Miyazaki K, et al. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine 2010;35:E475–80. [DOI] [PubMed] [Google Scholar]
- 68. Law L, Hunt CL, van Wijnen AJ, et al. Office-based mesenchymal stem cell therapy for the treatment of musculoskeletal disease: a systematic review of recent human studies. Pain Med 2019;20:1570–83. [DOI] [PubMed] [Google Scholar]
- 69. Chun HJ, Kim YS, Kim BK, et al. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg 2012;78:364–71. [DOI] [PubMed] [Google Scholar]
- 70. Petering RC, Webb C. Treatment options for low back pain in athletes. Sports Health 2011;3:550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Komori H, Shinomiya K, Nakai O, et al. The natural history of herniated nucleus pulposus with radiculopathy. Spine 1996;21:225–9. [DOI] [PubMed] [Google Scholar]
- 72.Victor YL Leung, Darwesh MK Aladin, Fengjuan Lv, et al. Mesenchymal Stem Cells Reduce Intervertebral Disc Fibrosis and Facilitate Repair, Stem Cells 2014;32:2164–2177. 10.1002/stem.1717. [DOI] [PubMed] [Google Scholar]
- 73. Song K, Gu T, Shuang F, et al. Adipose-derived stem cells improve the viability of nucleus pulposus cells in degenerated intervertebral discs. Mol Med Rep 2015;12:4664–8. [DOI] [PubMed] [Google Scholar]
- 74. Erwin WM, Islam D, Eftekarpour E, et al. Intervertebral disc-derived stem cells: implications for regenerative medicine and neural repair. Spine 2013;38:211–6. [DOI] [PubMed] [Google Scholar]
- 75.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–31. 10.1016/s0140-6736(74)90884-8. PMID: 4139420. [DOI] [PubMed] [Google Scholar]
- 76. Differentiation of human Wharton’s jelly cells toward nucleus pulposus-like cells after coculture with nucleus pulposus cells in vitro. Tissue Eng Part A [Internet cited 2021 Jun 1. https://europepmc.org/article/med/21902606. [DOI] [PubMed] [Google Scholar]
- 77.Lehmann TP, Jakub G, Harasymczuk J, Jagodziński PP. Transforming growth factor β mediates communication of co-cultured human nucleus pulposus cells and mesenchymal stem cells. J Orthop Res. 2018;36:3023–3032. 10.1002/jor.24106. Epub 2018 Aug 2. PMID: 29999195. [DOI] [PubMed] [Google Scholar]
- 78. Fukui S, Iwashita N, Nitta K, et al. The results of percutaneous intradiscal high-pressure injection of saline in patients with extruded lumbar herniated disc: comparison with microendoscopic discectomy. Pain Med 2012;13:762–8. [DOI] [PubMed] [Google Scholar]
- 79. Vadalà G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 2012;6:348–55. [DOI] [PubMed] [Google Scholar]
- 80. Gruber HE, Hanley EN. Ultrastructure of the human intervertebral disc during aging and degeneration: comparison of surgical and control specimens. Spine 2002;27:798–805. [DOI] [PubMed] [Google Scholar]
- 81. Bibby SRS, Jones DA, Ripley RM, et al. Metabolism of the intervertebral disc: effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine 2005;30:487–96. [DOI] [PubMed] [Google Scholar]
- 82. Errico TJ. Why a mechanical disc? Spine J 2004;4:151S–7. [DOI] [PubMed] [Google Scholar]
- 83. Crevensten G, Walsh AJL, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 2004;32:430–4. [DOI] [PubMed] [Google Scholar]
- 84. Sakai D, Mochida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials 2003;24:3531–41. [DOI] [PubMed] [Google Scholar]
- 85. Pereira CL, Gonçalves RM, Peroglio M, et al. The effect of hyaluronan-based delivery of stromal cell-derived factor-1 on the recruitment of MSCs in degenerating intervertebral discs. Biomaterials 2014;35:8144–53. [DOI] [PubMed] [Google Scholar]
- 86. Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007;25:1737–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


