Abstract

3,5-Dinitrobenzylsulfanyl tetrazoles and 1,3,4-oxadiazoles, previously identified as having high in vitro activities against both replicating and nonreplicating mycobacteria and favorable cytotoxicity and genotoxicity profiles were investigated. First we demonstrated that these compounds act in a deazaflavin-dependent nitroreduction pathway and thus require a nitro group for their activity. Second, we confirmed the necessity of both nitro groups for antimycobacterial activity through extensive structure–activity relationship studies using 32 structural types of analogues, each in a five-membered series. Only the analogues with shifted nitro groups, namely, 2,5-dinitrobenzylsulfanyl oxadiazoles and tetrazoles, maintained high antimycobacterial activity but in this case mainly as a result of DprE1 inhibition. However, these analogues also showed increased toxicity to the mammalian cell line. Thus, both nitro groups in 3,5-dinitrobenzylsulfanyl-containing antimycobacterial agents remain essential for their high efficacy, and further efforts should be directed at finding ways to address the possible toxicity and solubility issues, for example, by targeted delivery.
Introduction
Although tuberculosis (TB) is a curable and preventable disease, it remains among the top causes of death worldwide and indeed recently became the second leading infectious killer after COVID-19. Moreover, the COVID-19 pandemic has reduced the access to TB diagnosis and treatment, which resulted in an increase in TB deaths. Only 6.4 million people newly diagnosed with TB were reported in 2021 from an estimated 10.6 million people who developed the disease, and the number of TB-related deaths increased from 1.4 million in 2019 to 1.6 million in 2021.1 The COVID-19 pandemic also reduced the number of people provided with treatment for drug-resistant TB by approximately 15%, and only one in three people with drug-resistant TB received treatment in 2020, with a slight recovery in 2021 (7.5% increase). Globally, approximately 3–4% of newly diagnosed TB cases are classified as multidrug-resistant strains (MDR-TB), and in the case of patients previously treated for TB, the proportion of MDR-TB is higher than 18%. Current therapy for drug-resistant TB has a low success rate (about 60% in 2019) and consists of prolonged multidrug regimens, which can last up to 24 months of taking five or more different anti-TB drugs.1 Such treatment regimens have many unpleasant side effects and drug–drug interactions (especially with antiretroviral drugs in the case of HIV co-infection) which cause poor compliance and hamper the coadministration of antiretroviral and anti-TB drugs. This, together with the fact that the most affected regions are those with relatively poor medical care, increases the risk of the formation and spread of MDR and extensively drug-resistant (XDR) strains. Therefore, addressing the availability and effectiveness of treatment for drug-resistant TB remains a major concern, and new, highly efficient, and better-tolerated drugs are needed. Recently, two nitro group-containing agents, delamanid2 and pretomanid,3 have been approved for the treatment of MDR/XDR-TB. Both these agents have a nitro group-dependent mechanism of action as they are bioreductively activated by deazaflavin-dependent nitroreductase (Ddn) in mycobacteria.4 Other compounds with nitro group-dependent antimycobacterial activity are the benzothiazinones,5 which are inhibitors of mycobacterial decaprenylphosphoryl-β-d-ribofuranose 2′-oxidase (DprE1).6,7 Two benzothiazinone derivatives, BTZ-043 and PBTZ-169 (macozinone), are currently undergoing evaluation in the clinic.8
Our research group has developed several structural types of new antitubercular agents with high and selective antimycobacterial activity. These compounds typically contain a five-membered heterocycle and a 3,5-dinitrophenyl9−11 or 3,5-dinitrobenzylsulfanyl moiety.12,13 The latter group, 3,5-dinitrobenzylsulfanyl tetrazoles (1) and oxadiazoles (2) (Figure 1), showed excellent activity against both drug-susceptible and drug-resistant strains. The best oxadiazole derivatives of structure 2 had minimum inhibitory concentrations (MIC) of 0.03 μM against replicating Mycobacterium tuberculosis (M.tb.) strains and were also highly effective against the nonreplicating M.tb. SS18b-Lux strain.13 Interestingly, despite the structural similarity to known DprE1 inhibitors,6,14 3,5-dinitrobenzylsulfanyl oxadiazoles 2 did not affect the function of this enzyme, and the actual mechanism of action remained elusive.13 The in vitro antimycobacterial efficiency of tetrazole derivatives 1 was lower compared to their oxadiazole counterparts; their MIC values reached 1 μM concentration.12 Despite the presence of two nitro groups in the molecules, these lead compounds did not suffer from cytotoxicity to various cell lines, including isolated human hepatocytes, and did not exhibit genotoxicity in several assays.
Figure 1.

General structure of 3,5-dinitrobenzylsulfanyl tetrazole (1) and 1,3,4-oxadiazole (2) lead compounds.12,13 Compounds 1a–e and 2a–e served as parent and reference compounds in this study.
The results of the above-mentioned studies indicated that the 3,5-dinitrobenzyl moiety is the fragment responsible for high in vitro antimycobacterial activity. It was found that 2,4-dinitrobenzyl isomers had substantially lower antimycobacterial activity compared to 3,5-dinitro compounds, and 3-nitro-5-(trifluoromethyl)benzyl or 3-amino-5-nitrobenzyl analogues lost antimycobacterial activity altogether.12,13,15 However, the presence of two nitro groups could be the main obstacle to the further development of these potent antimycobacterial agents. Despite the long history of nitro-containing drugs and recent findings of bioreductive activation,16 medicinal chemists typically try to avoid nitro groups in drug design due to concerns about toxicity and solubility.
Therefore, the first aim of this work was to elucidate the mode of action of oxadiazoles 2 to (a) determine whether the presence of a nitro group is essential for antimycobacterial activity and (b) rationalize the design of new analogues. Second, the structure–activity relationships were explored. In Part A (Figure 2A), one nitro group in the two lead compounds 3,5-dinitrobenzylsulfanyl tetrazole (1) and oxadiazole (2) was replaced by other electron withdrawing groups. Thus, chloro-, fluoro-, bromo-, cyano-, methoxycarbonyl-, carbamoyl-, and pyrrol-1-yl- analogues of tetrazoles 1a–e and/or oxadiazoles 2a–e were prepared, and their in vitro antimycobacterial activities were evaluated. Trifluoromethyl analogues were also prepared to complete the series.13 The 3,5-dinitrobenzyl moiety was also replaced by a heterocyclic (5-nitropyridin-3-yl)methyl or (5-nitrofuran-2-yl)methyl group.
Figure 2.
General structures of investigated tetrazole and 1,3,4-oxadiazole derivatives with replaced nitro group (A), with shifted nitro group (B), and their trifluoromethyl analogues (C) or those with methyl- or methoxy-substituted 3,5-dinitrobenzyl moiety (D). Tetrazoles 1a–e and oxadiazoles 2a–e served as the lead compounds in this study.
Parts B and C of the structure–activity relationship study focused on the position of the nitro groups on the benzyl moiety, which appeared to be crucial for the antimycobacterial activity of compounds 1 and 2. In addition to the previously investigated 2,4-dinitrobenzyl analogues, in this work we shifted just one nitro group of the parent compounds 1a–e and 2a–e and prepared their 3,4- and 2,5-dinitrobenzyl analogues (Figure 2B). As the preliminary experiments showed high in vitro antimycobacterial activity of the compounds with the 2,5-dinitrobenzyl moiety, their mononitro analogues with 2-nitro-5-(trifluoromethyl)benzyl and 5-nitro-2-(trifluoromethyl)benzyl groups were also synthesized (Figure 2C).
In part D, a methyl or methoxy group was introduced to the 3,5-dinitrobenzyl moiety to explore the effect of additional substitution and steric hindrance of one or both neighboring nitro groups on the antimycobacterial activity of lead compounds (Figure 2D). Structure–activity relationships with respect to the substituent R on the tetrazole or oxadiazole core have been fully elucidated in our previous studies;11−13 thus in this work we selected five lipophilic substituents R (a–e, Figure 2) and used them in all series prepared and studied in this work to obtain easily comparable results.
Results and Discussion
Mode of Action of 3,5-Dinitrobenzylsulfanyl Oxadiazoles 2
Previously we proved that 3,5-dinitrobenzylsulfanyl oxadiazoles and thiadiazoles do not affect the mycobacterial DprE1 and may target the synthesis of mycobacterial nucleic acids.13 To elucidate the mechanism of action of these compounds, mutants of M.tb. Erdman resistant to 3,5-dinitrobenzylsulfanyl 1,3,4-oxadiazole T6030 (11i in ref (13)) and 1,3,4-thiadiazole T6053 (14g in ref (13)) were generated using concentrations 10 times and 20 times higher than their MIC values. Whole genome sequencing followed by bioinformatics analysis showed that all mutant colonies carried a different nonsynonymous single nucleotide polymorphism in the fgd1 gene (rv0407) encoding F420-dependent glucose-6-phosphate dehydrogenase (FGD1) (Table 1), similarly as in M.tb. mutants resistant to nitroimidazoles pretomanid and delamanid,17−19 FDA-approved anti-TB drugs. Mutations in FGD1 disrupt the reduction of cofactor F420 to F420-H2, which inhibits the function of Ddn and blocks the reductive activation of nitroimidazoles.17
Table 1. Resistance Profile and Mutations in M.tb. Erdman Mutants Exhibiting Resistance to 3,5-Dinitrobenzylsulfanyl Oxadiazole T6030 and Thiadiazole T6053.
| M.tb. strain | nucleotide change in fgd1 | amino acid change in FGD1 | T6030/T6053 MIC (μM) |
|---|---|---|---|
| Erdman | 0.1 | ||
| T6030-10× | g310c | Gly104Arg | 8.1 |
| T6030-20× | c949t | Gln317a | 6.7 |
| T6053-10× | g911t | Gly304Val | 5.6 |
| T6053-20× | c863a | Ser288a | 3.4 |
Truncated protein.
To further confirm that the antimycobacterial activity of compounds T6030 and T6053 rely on the Ddn-activation, we determined the MIC values in Ddn- and FbiC-deficient M.tb. mutants. We found that both mutant strains showed resistance to both T6030 and T6053 (>3- and 10-fold increase in MIC values, respectively, compared to wild-type M.tb. H37Rv), as well as to pretomanid.
These results indicated that 3,5-dinitrobenzylsulfanyl oxadiazoles 2 are activated in a similar way as nitroimidazoles pretomanid and delamanid and proved that their antimycobacterial activity is nitro group-dependent. This conclusion is in agreement with a recent study of van Calenbergh et al., who experimentally proved that the antimycobacterial activity of closely related quinazolinones bearing the key 3,5-dinitrobenzylsulfanyl group depends on the reductive activation of the 3,5-dinitrobenzyl moiety by Ddn as in the case of the nitroimidazoles (Figure 3).20
Figure 3.
3,5-Dinitrobenzylsulfanyl-substituted oxadiazole T6030, thiadiazole T6053, and previously studied quinazolinone (26 in ref (21)) whose Ddn-dependent mechanism of antimycobacterial activity was independently proven.
These findings proved that at least one nitro group must be maintained in the structure of 3,5-dinitrobenzylsulfanyl heterocycles such as tetrazoles 1 and oxadiazoles 2 and drove the design of their mononitro analogues prepared in this work.
Chemistry Part A
Synthesis of the compounds with one trifluoromethyl- (52a–e, 57a–e), chloro- (53a–e, 58a–e), fluoro- (54a–e, 59a–e), bromo- (55a–e, 60a–e), cyano- (56a–e, 61a–e), methoxycarbonyl- (62a–e), carbamoyl- (63a–e), or N-benzylcarbamoyl-group (64a–e) started with the preparation of the corresponding 3-nitro-5-substituted benzoic acids 3–8, followed by the reduction of the carboxylic acid group using borane in THF (Scheme 1 and 2).21
Scheme 1. Synthesis of Trifluoromethyl-, Fluoro-, Chloro-, Bromo-, and Cyano-Substituted Nitrobenzyl Alcohols (13–17).
Reagents and conditions: (a) fuming HNO3, H2SO4, 0 °C → rt, overnight, 87%; (b) BH3·THF, THF, −20 °C → rt, overnight, 79–98%; (c) Na2S·H2O, NH4Cl, CH3OH, reflux, 17 h, 92%; (d) X = Cl: NaNO2, CuCl, HCl, H2O, −5 °C; 3 h, rt; 30 min, 65 °C; 78%; (e) X = F: NOBF4, CH3CN, argon, 5 °C; 48 h, rt; 1,2-Cl2C6H3, 170 °C, 40 min, 61%; (f) NBS, H2SO4, 60 °C, 2 h, 87%; (g) K4[Fe(CN)6]·3H2O, Pd(OAc)2, Na2CO3, DMAC, 120 °C, 6 h, 30%.
Scheme 2. Synthesis of Methyl 3-(Hydroxymethyl)-5-nitrobenzoate (18) and 3-(Hydroxymethyl)-5-nitrobenzamides 19 and 20 from 3-Methoxycarbonyl-5-nitrobenzoic Acid (8).
Reagents and conditions: (a) BH3·THF, THF, −20 °C→ rt, overnight, 76%; (b) R = H: NH3, CH3OH, autoclave reactor, 80 °C, 32 h, 71%; (c) R = CH2Ph: PhCH2NH2, CH3OH, autoclave reactor, 120 °C, 40 h, 64%.
3-Nitro-5-trifluoromethylbenzoic acid (3) was obtained by nitration of 3-trifluoromethylbenzoic acid in excellent yield (Scheme 1).13 Synthesis of 3-chloro- (5) or 3-fluoro-5-nitrobenzoic acid (6) started from 3,5-dinitrobenzoic acid. Its reduction by sodium sulfide hydrate in the presence of ammonium chloride provided 3-amino-5-nitrobenzoic acid 4, which, after diazotization and substitution with chlorine or fluorine gave acids 5 and 6, respectively. 3-Bromo-5-nitrobenzoic acid (7) was prepared via bromination of 5-nitrobenzoic acid with N-bromosuccinimide (NBS) in the presence of sulfuric acid in 87% yield.22 Final reduction of nitrobenzoic acids 3 and 5–7 led to the corresponding benzyl alcohols 13–16 in high yields (79–98%). 3-Cyano-5-nitrobenzyl alcohol 17 was prepared from 3-bromo-5-nitrobenzyl alcohol (16) by palladium-catalyzed cyanation (Scheme 1).23
The synthesis of 3-methoxycarbonyl and 3-carbamoyl 5-nitrobenzyl alcohols (18–20) started with partial esterification of 5-nitroisophthalic acid. 3-Methoxycarbonyl-5-nitrobenzoic acid (8) was obtained in a mixture with dimethyl 5-nitroisophthalate and 5-nitroisophthalic acid and therefore was isolated in modest yield (42%). Nitrobenzoic acid 8 underwent the reduction to provide methyl 3-(hydroxymethyl)-5-nitrobenzoate (18) in 76% yield. Aminolysis of methyl benzoate 18 with ammonia or benzylamine in an autoclave resulted in the desired carbamoyl derivatives 19 and 20, respectively. (Scheme 2).
The synthetic approach to 3-nitro-5-(1H-pyrrol-1-yl)benzyl alcohol (22) consisted of two steps. First, 3,5-dinitrobenzyl alcohol was partially reduced by sodium sulfide hydrate in the presence of ammonium chloride in methanol. In the second step, reaction of 3-amino-5-nitrobenzyl alcohol (21) with 2,5-dimethoxytetrahydrofuran led to the formation of the pyrrole derivative 22 in 68% yield (Scheme 3).
Scheme 3. Synthesis of 3-Nitro-5-(1H-pyrrol-1-yl)benzyl Alcohol (22).
Reagents and conditions: (a) Na2S·H2O, NH4Cl, CH3OH, reflux, 15 h, 70%; (b) 2,5-dimethoxytetrahydrofuran, THF/CH3COOH (2:1), reflux, 24 h, 68%.
Benzyl alcohols 13–20 and 22 were further converted to the corresponding benzyl halides 35–43, which were used for the alkylations of the corresponding 1-substituted 1H-tetrazole-5-thiols and 5-substituted 1,3,4-oxadiazole-2-thiols (Scheme 4). The alkylation reactions were carried out in acetonitrile using triethylamine as a base, with the final 3-substituted 5-nitrobenzylsulfanyl tetrazoles 52–56 and oxadiazoles 57–65 obtained in high yields (53–98%).
Scheme 4. Synthesis of 3-Nitro-5-(trifluoromethyl)benzyl Derivatives 52a–e and 57a–e, 3-Chloro-5-nitrobenzyl Derivatives 53a–e and 58a–e, 3-Fluoro-5-nitrobenzyl Derivatives 54a–e and 59a–e, 3-Bromo-5-nitrobenzyl Derivatives 55a–e and 60a–e, 3-Cyano-5-nitrobenzyl Derivatives 56a–e and 61a–e, 3-Methoxycarbonyl-5-nitrobenzyl Derivatives 62a–e, 3-Carbamoyl-5-nitrobenzyl Derivatives 63a–e, 3-(Benzylcarbamoyl)-5-nitrobenzyl Derivatives 64a–e, and 3-Nitro-5-(1H-pyrrol-1-yl)benzyl Derivatives 65a–e.
Reagents and conditions: (a) for 36, 37, and 43: SOCl2, Et3N, CH2Cl2, 0 °C–rt, 3 h, 74–79%; (b) for 38 and 39: PCl5, CHCl3, 0 °C–reflux, 24 h, 70–89%; (c) for 35, 40–42: NBS, Ph3P, CH2Cl2, 0 °C–rt, 30 min–2 h, 40–94%; (d) 1-substituted 1H-tetrazole-5-thiol or 5-substituted 1,3,4-oxadiazole-2-thiol, Et3N, CH3CN, rt, 0.5–1 h or overnight, 53–98%.
The synthesis of 2-alkyl/aryl-5-((5-nitropyridin-3-yl)methylsulfanyl)-1,3,4-oxadiazoles 82a–e started from commercially available 3-pyridinemethanol, which was converted to 3-acetoxymethylpyridine-N-oxide (31) via reactions with mCPBA in acetic anhydride.24 Nitration of N-oxide 31 using silver nitrate and 4-nitrobenzoyl chloride in dry dichloromethane gave the nitro-derivative 32 in 16% yield.25 The reduction of 3-acetoxymethyl-5-nitropyridine-N-oxide (32) by PCl3 followed by acid hydrolysis resulted in (5-nitropyridin-3-yl)methanol (34) in 67% yield over two steps. (5-Nitropyridin-3-yl)methanol 34 was converted to 3-(chloromethyl)-5-nitropyridine hydrochloride, which was directly used for the alkylation of 5-substituted 1,3,4-oxadiazole-2-thiols. The final (5-nitropyridin-3-yl)methylsulfanyl 1,3,4-oxadiazoles 82a–e were obtained in good yield (48–74%). The last series of studied compounds, 2-aryl-5-((5-nitrofuran-2-yl)methylsulfanyl)-1,3,4-oxadiazoles 83a–e, was prepared by the alkylation of 5-aryl-1,3,4-oxadiazole-2-thiols with commercially available 5-(bromomethyl)-2-nitrofuran in the presence of triethylamine in acetonitrile, with the final compounds 83a–e obtained in 43–73% yield (Scheme 5).
Scheme 5. Synthesis 2-Alkyl/Aryl-5-((5-nitropyridin-3-yl)methylsulfanyl)-1,3,4-oxadiazoles 82a–e and 2-Aryl-5-((5-nitrofuran-2-yl)methylsulfanyl)-1,3,4-oxadiazoles 83a–e.
Reagents and conditions: (a) acetic anhydride, rt, 30 min; 65 °C, 1 h; (b) mCPBA, rt, overnight, 75% (two steps); (c) 4-NO2PhCOCl, AgNO3, CH2Cl2, −5 °C → reflux, 48 h, 16%; (d) PCl3, CH2Cl2, rt, 1 h, 70%; (e) H2SO4, H2O, THF, 85 °C, 15 h, 95%; (f) 1. SOCl2, CH2Cl2, rt, 5 h; 2. 5-substituted-1,3,4-oxadiazole-2-thiol, Et3N, THF, rt, overnight, 48–74%; (g) 5-aryl-1,3,4-oxadiazole-2-thiol, Et3N, CH3CN, rt, 30 min, 43–73%.
Chemistry Part B
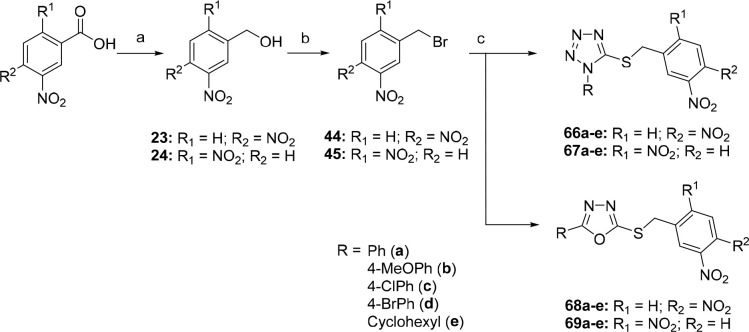
The synthesis of compounds with a shifted nitro group (66–69) is shown in Scheme 6. First, the appropriate dinitro-substituted benzyl alcohols 23 and 24 were prepared via the borane-mediated reductions of commercially available 3,4-dinitro or 2,5-dinitrobenzoic acids, respectively. These benzyl alcohols were converted to the corresponding benzyl bromides 44 and 45 by their reactions with NBS and PPh3 in dichloromethane.24,26 Benzyl bromides 44 and 45 were used to alkylate 1-substituted 1H-tetrazole-5-thiols and 5-substituted 1,3,4-oxadiazole-2-thiols to provide the final compounds of series 66–69 in high yields (61–88%). The alkylation was carried out in acetonitrile with triethylamine as a base (Scheme 6).
Scheme 6. Synthesis of Final 3,4-Dinitrobenzylsulfanyl Tetrazoles 66a–e and Oxadiazoles 68a–e and 2,5-Dinitrobenzylsulfanyl Tetrazoles 67a–e and Oxadiazoles 69a–e.
Reagents and conditions: (a) BH3·THF, THF, −20 °C → rt, overnight, 63–85%; (b) NBS, Ph3P, CH2Cl2, 0 °C → rt, 1 h, 92–93%; (c) 1-substituted 1H-tetrazole-5-thiol or 5-substituted 1,3,4-oxadiazole-2-thiol, Et3N, CH3CN, 0.5–1 h, rt, 61–88%.
Chemistry Part C
To prepare the 2-nitro-5-(trifluoromethyl)benzyl (70a–e and 72a–e) or 5-nitro-2-(trifluoromethyl)benzyl (71a–e and 73a–e) derivatives, commercially available 2-nitro-5-(trifluoromethyl) or 5-nitro-2-(trifluoromethyl)benzoic acids were used as the starting materials, respectively. They were first reduced to the benzyl alcohols 25 and 26 and then converted to benzyl bromides 46 and 47 by their reactions with NBS and PPh3 in dichloromethane. In the last step of synthesis, alkylations of the corresponding tetrazole-5-thiols or 1,3,4-oxadiazol-2-thiols resulted in the target compounds of series 70–73 in high yields (67–98%) (Scheme 7).
Scheme 7. Synthesis of the Series of Final 2-Nitro-5-(trifluoromethyl)benzylsulfanyl Tetrazoles 70a–e and Oxadiazoles 72a–e and 5-Nitro-2-(trifluoromethyl)benzylsulfanyl Tetrazoles 71a–e and Oxadiazoles 73a–e.
Reagents and conditions: (a) BH3·THF, THF, −20 °C → rt, overnight, 93%; (b) NBS, Ph3P, CH2Cl2, 0 °C → rt, 1 h, 78–80%; (c) 1-substituted 1H-tetrazole-5-thiol or 5-substituted 1,3,4-oxadiazole-2-thiol, Et3N, CH3CN, rt, 1–2 h, 67–98%.
Chemistry Part D
The synthesis of final compounds with an additional methyl or methoxy group on the key 3,5-dinitrobenzyl part of the molecule (74–81) started from commercially available 2-methyl-, 4-methyl-, 2-hydroxy, or 4-hydroxy-3,5-dinitrobenzoic acids (Scheme 8). 4-Hydroxy-3,5-dinitrobenzoic acid and 3,5-dinitrosalicylic acid were methylated using dimethyl sulfate in the presence of potassium carbonate to obtain methyl esters of methoxy acids 9 and 10, respectively. These esters were converted to acids 11 and 12 using sodium methoxide.27 4-Methoxy- or 2-methoxy-3,5-dinitrobenzoic acids 11 and 12, as well as 4-methyl- or 2-methyl-3,5-dinitrobenzoic acids were reduced to the corresponding benzyl alcohols 27–30 and then converted to benzyl bromides 48–51.26 The alkylation of the corresponding 1-substituted-1H-tetrazole-5-thiols or 5-substitued-1,3,4-oxadiazol-2-thiols provided the target tetrazole-based compounds of series 74–77 and oxadiazole-based compounds of series 78–81 in 66–95% yield (Scheme 8).
Scheme 8. Synthesis of 4-Methoxy- and 2-Methoxy-3,5-dinitrobenzylbromides and 4-Methyl- and 2-Methyl-3,5-dinitrobenzylbromides 48, 49, 50, and 51, respectively, and Their Use in the Synthesis of Final Tetrazoles of Series 74–77 and Oxadiazoles of Series 78–81.
Reagents and conditions: (a) CH3ONa, CH3OH, reflux, 2 h, 40–60%; (b) BH3·THF, THF, −20 °C → rt, overnight, 71–83%; (c) NBS, Ph3P, CH2Cl2, 0 °C → rt, 1–12 h, 70–85%; (d) Et3N, CH3CN, 0.5–1 h, rt, 66–95%; (e) Et3N, CH3CN, 0.5–1 h, rt, 68–91%.
In Vitro Antimycobacterial Activity
In vitro antimycobacterial activity of all final compounds of series 52–83 were evaluated against M.tb. CNCTC My 331/88 (H37Rv) and against nontuberculous mycobacterial strains of M. avium CNCTC My 330/88 and M. kansasii CNCTC My 235/80 and compared with in vitro antimycobacterial activity of lead compounds of series 1 and 2. The antimycobacterial activities of all compounds were evaluated after 7, 14, or 21 days of incubation and are expressed as minimum inhibitory concentration (MIC) in micromolar.
The first aim of this work was to explore the possibility of the replacement of one nitro group for another electron-withdrawing and/or (bio)isosteric group in 3,5-dinitrobenzylsulfanyl tetrazole 1 and/or oxadiazole 2 antitubercular agents (Part A). Therefore, derivatives with trifluoromethyl- (52a–e, 57a–e), chloro- (53a–e, 58a–e), fluoro- (54a–e, 59a–e), bromo- (55a–e, 60a–e), and cyano- (56a–e, 61a–e) groups instead of one nitro group in the 3,5-dinitrobenzyl part were prepared. In the case of oxadiazole lead compounds 2a–e, which displayed outstanding activities, additional analogues with methoxycarbonyl- (62a–e), carbamoyl- (63a–e, 64a–e), and pyrrole (65a–e) groups were prepared. However, a strong decrease of the antimycobacterial activity was observed in all cases, regardless of the introduced functional group or heterocycle involved; indeed the majority of compounds completely lost their antitubercular activity (Tables 2 and 3). Among the prepared analogues, 3-cyano-5-nitrobenzyl derivatives of series 56 and 61 were slightly effective against M.tb. 5-((3-Cyano-5-nitrobenzyl)sulfanyl)-1-(4-chlorophenyl)-1H-tetrazole (56c) and 2-((3-cyano-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (61b) showed the best activities with MIC values of 2 μM and 4 μM, respectively. Nonetheless, these MIC values were substantially lower than those of the parent oxadiazoles 2 or INH.
Table 2. In Vitro Antimycobacterial Activities of the Final Tetrazole-Based Compounds of Series 52–56 Expressed as MIC (μM) and Their Comparison with Those of Parent Tetrazoles 1a–e12.

| X | M. tuberculosis My 331/88a | M. avium My 330/88a | M. kansasii My 235/80b | |
|---|---|---|---|---|
| INH | 0.5/1 | 250/250 | 250/250/250 | |
| pretomanid | 0.125/0.25 | >32/>32 | >32/>32/>32 | |
| 1a | NO2 | 4/4 | 62/62 | 2/4/16 |
| 1b | NO2 | 2/4 | 16/32 | 1/4/4 |
| 1c | NO2 | 1/2 | 125/125 | 2/4/4 |
| 1d | NO2 | 1/1 | 125/125 | 1/2/2 |
| 1e | NO2 | 1/1 | 16/32 | 4/4/4 |
| 52a | CF3 | 32/64 | 250/250 | 125/250/250 |
| 52b | CF3 | 32/64 | 250/250 | 125/250/250 |
| 52c | CF3 | 32/32 | 250/250 | 125/250/250 |
| 52d | CF3 | 64/125 | 250/250 | 125/250/250 |
| 52e | CF3 | 64/64 | 250/250 | 64/64/64 |
| 53a | Cl | 64/125 | 250/250 | 125/250/250 |
| 53b | Cl | 250/250 | 250/250 | 125/250/250 |
| 53c | Cl | 250/250 | 250/250 | 250/250/250 |
| 53d | Cl | 250/250 | 250/250 | 125/250/250 |
| 53e | Cl | 250/250 | 250/250 | 125/250/250 |
| 54a | F | 250/250 | 250/250 | 125/250/250 |
| 54b | F | 250/250 | 250/250 | 125/250/250 |
| 54c | F | 125/250 | 250/250 | 125/250/250 |
| 54d | F | 64/64 | 250/250 | 64/125/250 |
| 54e | F | 64/125 | 250/250 | 125/250/250 |
| 55a–55e | Br | >250 | >250 | >250 |
| 56a | CN | 250/250 | 250/250 | 250/250/250 |
| 56b | CN | 32/32 | 250/250 | 16/32/32 |
| 56c | CN | 2/4 | 250/250 | 4/8/16 |
| 56d | CN | 32/32 | 250/250 | 16/32/32 |
| 56e | CN | 250/250 | 250/250 | 250/250/250 |
14/21 days.
7/14/21 days.
Table 3. In Vitro Antimycobacterial Activities of the Final Oxadiazole-Based Compounds of Series 57–65 Expressed as MICs (μM) and Their Comparison with Those of Parent Oxadiazoles 2a–e13.

| X | M. tuberculosis My 331/88a | M. avium My 330/88a | M. kansasii My 235/80b | |
|---|---|---|---|---|
| INH | 0.5/1 | 250/250 | 250/250/250 | |
| pretomanid | 0.125/0.25 | >32/>32 | >32/>32/>32 | |
| 2a | NO2 | 0.06/0.06 | 16/32 | 0.5/1/1 |
| 2b | NO2 | 0.125/0.125 | 16/32 | 0.125/0.25/0.25 |
| 2c | NO2 | 0.125/0.125 | >125/>125 | 0.125/0.25/0.25 |
| 2d | NO2 | 0.125/0.125 | 250/250 | 0.125/0.25/0.5 |
| 2e | NO2 | ≤0.03/≤0.03 | >32/>32 | 0.06/0.125/0.25 |
| 57a | CF3 | 64/64 | 250/250 | 32/64/125 |
| 57b | CF3 | 64/125 | 250/250 | 64/64/125 |
| 57c | CF3 | 250/250 | 250/250 | 250/250/250 |
| 57d | CF3 | 250/250 | 250/250 | 250/250/250 |
| 57e | CF3 | 125/125 | 250/250 | 64/125/250 |
| 58a–58e | Cl | >250 | >250 | >125 |
| 59a–59c | F | 250/250 | 250/250 | 250/250/250 |
| 59d | F | 250/250 | 250/250 | 125/250/250 |
| 59e | F | 125/125 | 250/250 | 64/125/125 |
| 60a | Br | 125/250 | 250/250 | 64/125/250 |
| 60b | Br | 125/250 | 250/250 | 64/125/250 |
| 60c | Br | 125/125 | 125/125 | 125/125/125 |
| 60d | Br | 250/250 | 250/250 | 250/250/250 |
| 60e | Br | 32/32 | 250/250 | 64/64/64 |
| 61a | CN | 16/16 | 250/250 | 4/8/2016 |
| 61b | CN | 4/4 | 250/250 | 2/4/2008 |
| 61c | CN | 8/16 | 250/250 | 4/8/2016 |
| 61d | CN | 250/250 | 250/250 | 250/250/250 |
| 61e | CN | 32/32 | 250/250 | 16/32/32 |
| 62a–62e | COOCH3 | >250 | >250 | >125 |
| 63a–63e | CONH2 | >250 | >250 | >125 |
| 64a–64e | CONHBn | >250 | >250 | >250 |
| 65a–65e | pyrrol-1-yl | >250 | >250 | >250 |
14/21 days.
7/14/21 days.
Another possibility to reduce the number of nitro groups in the lead compounds was the replacement of the 3,5-dinitrobenzyl fragment with heterocyclic (5-nitropyridin-3-yl)methyl and (5-nitrofuran-2-yl)methyl moieties, especially the latter, since the 5-nitrofuran-2-yl group has previously been identified as a key moiety responsible for high antimycobacterial effect of several series of potent anti-TB agents.28,29 Thus, oxadiazole-type series 82a–e and 83a–e were prepared. Despite good antimycobacterial activity found with some of the prepared analogues, especially in the case of 5-nitrofuran-2-yl analogue 83e, lead compounds of series 2 were always in excess of 10 times more active (Table 4).
Table 4. In Vitro Antimycobacterial Activities of the Compounds with (5-Nitropyridin-3-yl)methyl (82a–e) and (5-Nitrofuran-2-yl)methyl (83a–e) Groups Expressed as MIC (μM) and Their Comparison with Those of Parent Oxadiazoles 2a–e13.
| M. tuberculosis My 331/88a | M. avium My 330/88a | M. kansasii My 235/80b | |
|---|---|---|---|
| INH | 0.5/1 | 250/250 | 250/250/250 |
| pretomanid | 0.125/0.25 | >32/>32 | >32/>32/>32 |
| 2a | 0.06/0.06 | 16/32 | 0.5/1/1 |
| 2b | 0.125/0.125 | 16/32 | 0.125/0.25/0.25 |
| 2c | 0.125/0.125 | >125/>125 | 0.125/0.25/0.25 |
| 2d | 0.125/0.125 | 250/250 | 0.125/0.25/0.5 |
| 2e | ≤0.03/≤0.03 | >32/>32 | 0.06/0.125/0.25 |
| 82a | 125/250 | 250/250 | 125/250/250 |
| 82b | 16/16 | 250/250 | 16/16/32 |
| 82c | 4/8 | >1000/>1000 | 8/8/16 |
| 82d | 16/16 | >1000/>1000 | 8/16/16 |
| 82e | 16/16 | 1000/1000 | 8/16/16 |
| 83a | 16/32 | 32/62 | 32/>32/>32 |
| 83b | >32/>32 | 64/64 | >32/>32/>32 |
| 83c | 16/32 | 32/32 | >32/>32/>32 |
| 83d | 8/16 | 16/32 | 16/16/32 |
| 83e | 0.5/1 | 32/32 | 4/8/16 |
14/21 days.
7/14/21 days.
Because all the efforts to remove or replace one nitro group in the lead compounds 1 and 2 resulted in substantial decrease of antimycobacterial activity, we decided to explore more deeply the role of the position of both nitro groups in antimycobacterial activity. In our previous work, we proved that 2,4-dinitrobenzyl analogues showed lower antimycobacterial activity compared to their 3,5-dinitro counterparts.12,13,15 Therefore, 3,5-dinitro-substituted compounds served as the lead compounds in following studies.11,30 Thus, in Part B we focused on the remaining variants with a nitro group in position 3 (or 5), i.e. 2,5-dinitro and 3,4-dinitro analogues. Positive hits could open a new path to further structural modifications and the possibility of nitro group replacement, which was not the case with 3,5-dinitrobenzyl lead compounds. In the case of 3,4-dinitrobenzyl analogues of series 66 and 68, we found a decrease in antimycobacterial activity when compared to those of the lead compounds of series 1 and 2. Nonetheless, 2,5-dinitro analogues of series 67 and 69 showed very good activities comparable to that of INH, i.e., comparable to those of lead compounds 1a–e but lower than oxadiazole-based lead compounds 2a–e. Interestingly, activities of 3,4-dinitro and especially 2,5-dinitro analogues were not influenced by the type of the heterocycle. Tetrazole-based and oxadiazole-based compounds 67a–e and 69a–e, respectively, showed very similar activities. As 2,5-dinitrobenzylsulfanyl maintained high antimycobacterial activities, we preliminarily checked the possibility of replacing one nitro group for another electron-withdrawing group: trifluoromethyl. Thus, in Part C, 2-nitro-5-(trifluoromethyl) derivatives 70a–e and 72a–e and 5-nitro-2-(trifluoromethyl) derivatives 71a–e and 73a–e were prepared and evaluated for their antimycobacterial efficacy. Unfortunately, significant decrease of activity or its complete loss was observed for both tetrazole and oxadiazole series, similarly to the case of trifluoromethyl analogues of lead compounds 1 and 2 (Tables 5 and 6).
Table 5. In Vitro Antimycobacterial Activities of the Tetrazole-Based Compounds 66a–e and 67a–e with 3,4-Dinitrobenzyl and 2,5-Dinitrobenzyl Groups, Respectively, and Trifluoromethyl Analogues of the Latter with 5-Trifluoromethyl and 2-Trifluoromethyl Groups 70a–e and 71a–e, Respectively, Expressed as MIC (μM) and Their Comparison with Those of Parent Tetrazoles 1a–e12.

| X | M. tuberculosis My 331/88a | M. avium My 330/88a | M. kansasii My 235/80b | |
|---|---|---|---|---|
| INH | 0.5/1 | 250/250 | 250/250/250 | |
| pretomanid | 0.125/0.25 | >32/>32 | >32/>32/>32 | |
| 1a | 3-NO2 | 4/4 | 62/62 | 2/4/16 |
| 1b | 3-NO2 | 2/4 | 16/32 | 1/4/4 |
| 1c | 3-NO2 | 1/2 | 125/125 | 2/4/4 |
| 1d | 3-NO2 | 1/1 | 125/125 | 1/2/2 |
| 1e | 3-NO2 | 1/1 | 16/32 | 4/4/4 |
| 66a | 4-NO2 | 16/16 | 32/32 | 16/32/32 |
| 66b | 4-NO2 | 32/32 | 125/125 | 16/32/32 |
| 66c | 4-NO2 | 4/8 | 250/250 | 2/4/8 |
| 66d | 4-NO2 | 16/16 | 250/250 | 8/16/16 |
| 66e | 4-NO2 | 32/64 | >1000/>1000 | 32/64/125 |
| 67a | 2-NO2 | 1/1 | 250/250 | 2/4/4 |
| 67b | 2-NO2 | 0.5/1 | 250/250 | 1/1/1 |
| 67c | 2-NO2 | 0.5/1 | 250/250 | 1/1/2 |
| 67d | 2-NO2 | 1/1 | 250/250 | 1/1/2 |
| 67e | 2-NO2 | 1/1 | 250/250 | 1/1/1 |
| 70a | - | 250/250 | 250/250 | 125/250/250 |
| 70b–70e | - | 250/250 | 250/250 | 250/250/250 |
| 71a | 2-CF3 | 32/32 | 250/250 | 64/132/250 |
| 71b | 2-CF3 | 8/8 | 250/250 | 32/32/32 |
| 71c | 2-CF3 | 250/250 | 250/250 | 250/250/250 |
| 71d | 2-CF3 | 250/250 | 250/250 | 250/250/250 |
| 71e | 2-CF3 | 32/32 | 250/250 | 64/64/64 |
14/21 days.
7/14/21 days.
Table 6. In Vitro Antimycobacterial Activities of the Oxadiazole-Based Compounds 68a–e and 69a–e with 3,4-Dinitrobenzyl and 2,5-Dinitrobenzyl Group, Respectively, and Trifluoromethyl Analogues of the Latter with 5-Trifluoromethyl and 2-Trifluoromethyl Groups 72a–e and 73a–e, Respectively, Expressed as MIC (μM) and Their Comparison with Those of Parent Oxadiazoles 2a-e(13).

| X | M. tuberculosis My 331/88a | M. avium My 330/88a | M. kansasii My 235/80b | |
|---|---|---|---|---|
| INH | 0.5/1 | 250/250 | 250/250/250 | |
| pretomanid | 0.125/0.25 | >32/>32 | >32/>32/>32 | |
| 2a | 3-NO2 | 0.06/0.06 | 16/32 | 0.5/1/1 |
| 2b | 3-NO2 | 0.125/0.125 | 16/32 | 0.125/0.25/0.25 |
| 2c | 3-NO2 | 0.125/0.125 | >125/>125 | 0.125/0.25/0.25 |
| 2d | 3-NO2 | 0.125/0.125 | 250/250 | 0.125/0.25/0.5 |
| 2e | 3-NO2 | ≤0.03/≤0.03 | >32/>32 | 0.06/0.125/0.25 |
| 68a | 4-NO2 | 8/16 | 250/250 | 16/32/32 |
| 68b | 4-NO2 | 16/16 | 125/125 | 16/32/32 |
| 68c | 4-NO2 | 8/8 | 64/64 | 8/16/16 |
| 68d | 4-NO2 | 4/8 | 250/250 | 2/4/8 |
| 68e | 4-NO2 | 125/250 | 250/250 | 250/250/250 |
| 69a | 2-NO2 | 1/1 | 250/250 | 1/2/4 |
| 69b | 2-NO2 | 1/1 | 250/250 | 1/1/2 |
| 69c | 2-NO2 | 1/1 | >1000/>1000 | 2/2/4 |
| 69d | 2-NO2 | 1/1 | 250/250 | 2/4/4 |
| 69e | 2-NO2 | 1/1 | >1000/>1000 | 1/1/1 |
| 72a | - | 250/250 | 250/250 | 125/250/250 |
| 72b–72e | - | 250/250 | 250/250 | 250/250/250 |
| 73a–73e | 2-CF3 | 250/250 | 250/250 | 250/250/250 |
14/21 days.
7/14/21 days.
Another modification of the lead compounds in Part D, i.e., the introduction of a methyl or methoxy group at position 2 or 4 of the 3,5-dinitrobenzyl fragment, caused a slight to significant decrease of antimycobacterial activities (Tables 7 and 8). Antimycobacterial activities of 4-methoxy, 2-methoxy, or 2-methyl-substituted 3,5-dinitrobenzylsulfanyl tetrazoles 74a–e, 75a–e, and 77a–e, respectively, were considerably lower than those of parent compounds 1a–e. However, 4-methyl-3,5-dinitrobenzylsulfanyl analogous 76a–e maintained high efficacy with MIC values of 2–4 μM only slightly lower than those of tetrazoles 1a–e. Moreover, these compounds showed good activity against M. kansasii My 235/80 (Table 7).
Table 7. In Vitro Antimycobacterial Activities of the 3,5-Dinitrobenzysulfanyl Tetrazoles with Additional Methoxy Group (74a–e and 75a–e) or Methyl Group (76a–e and 77a–e) on 3,5-Dinitrophenyl Moiety Expressed as MICs (μM) and Their Comparison with Those of Parent Tetrazoles 1a–e12.

| R1 | R2 | M. tuberculosis My 331/88a | M. avium My 330/88a | M. kansasii My 235/80b | |
|---|---|---|---|---|---|
| INH | 0.5/1 | 250/250 | 250/250/250 | ||
| pretomanid | 0.125/0.25 | >32/>32 | >32/>32/>32 | ||
| 1a | H | H | 4/4 | 62/62 | 2/4/16 |
| 1b | H | H | 2/4 | 16/32 | 1/4/4 |
| 1c | H | H | 1/2 | 125/125 | 2/4/4 |
| 1d | H | H | 1/1 | 125/125 | 1/2/2 |
| 1e | H | H | 1/1 | 16/32 | 4/4/4 |
| 74a | OCH3 | H | 250/250 | 250/250 | 250/250/250 |
| 74b | OCH3 | H | 16/32 | 250/250 | 250/250/250 |
| 74c | OCH3 | H | 16/32 | 250/250 | 250/250/250 |
| 74d | OCH3 | H | >32/>32 | 250/250 | >32/>32/>32 |
| 74e | OCH3 | H | 32/32 | >1000/>1000 | 16/32/32 |
| 75a | H | OCH3 | >32/>32 | 250/250 | >32/>32/>32 |
| 75b | H | OCH3 | 16/32 | 250/250 | 8/16/32 |
| 75c | H | OCH3 | 8/16 | 250/250 | 8/16/32 |
| 75d | H | OCH3 | 8/16 | 250/250 | 8/16/32 |
| 75e | H | OCH3 | 32/64 | 250/250 | 32/64/125 |
| 76a | CH3 | H | 4/8 | 250/250 | 2/4/8 |
| 76b | CH3 | H | 4/4 | 500/500 | 8/8/16 |
| 76c | CH3 | H | 2/2 | 250/250 | 1/2/4 |
| 76d | CH3 | H | 2/2 | 500/500 | 2/4/4 |
| 76e | CH3 | H | 4/8 | 250/250 | 8/16/32 |
| 77a | H | CH3 | >32/>32 | 250/250 | 32/>32/>32 |
| 77b | H | CH3 | 16/16 | 250/250 | 8/16/32 |
| 77c | H | CH3 | 16/32 | 250/250 | 8/16/32 |
| 77d | H | CH3 | 32/32 | 64/64 | 32/32/32 |
| 77e | H | CH3 | 250/250 | 250/250 | 250/250/250 |
14/21 days.
7/14/21 days.
Table 8. In Vitro Antimycobacterial Activities of the 3,5-Dinitrobenzysulfanyl Oxadiazoles with Additional Methoxy Group (78a–e and 79a–e) or Methyl Group (80a–e and 81a–e) on 3,5-Dinitrophenyl Moiety Expressed as MICs (μM) and Their Comparison with Those of Parent Oxadiazoles 2a–e13.

| R1 | R2 | M. tuberculosis My 331/88a | M. avium My 330/88a | M. kansasii My 235/80b | |
|---|---|---|---|---|---|
| INH | 0.5/1 | 250/250 | 250/250/250 | ||
| pretomanid | 0.125/0.25 | >32/>32 | >32/>32/>32 | ||
| 2a | H | H | 0.06/0.06 | 16/32 | 0.5/1/1 |
| 2b | H | H | 0.125/0.125 | 16/32 | 0.125/0.25/0.25 |
| 2c | H | H | 0.125/0.125 | >125/>125 | 0.125/0.25/0.25 |
| 2d | H | H | 0.125/0.125 | 250/250 | 0.125/0.25/0.5 |
| 2e | H | H | ≤0.03/≤0.03 | >32/>32 | 0.06/0.125/0.25 |
| 78a | OCH3 | H | 16/16 | 250/250 | 4/8/16 |
| 78b | OCH3 | H | 16/16 | 250/250 | 4/8/16 |
| 78c | OCH3 | H | 8/16 | 250/250 | 8/16/16 |
| 78d | OCH3 | H | 250/250 | 250/250 | 250/250/250 |
| 78e | OCH3 | H | 250/250 | 250/250 | 250/250/250 |
| 79a | H | OCH3 | 1/2 | 250/250 | 2/8/16 |
| 79b | H | OCH3 | 2/2 | 500/500 | 1/2/4 |
| 79c | H | OCH3 | 2/2 | 500/500 | 1/2/2 |
| 79d | H | OCH3 | 0.5/1 | 250/250 | 2/2/4 |
| 79e | H | OCH3 | 1/1 | 250/250 | 1/1/1 |
| 80a | CH3 | H | >32/>32 | 250/250 | 32/>32/>32 |
| 80b | CH3 | H | 250/250 | 250/250 | 250/250/250 |
| 80c | CH3 | H | 32/>32 | 250/250 | 16/32/>32 |
| 80d | CH3 | H | >32/>32 | 250/250 | >32/>32/>32 |
| 80e | CH3 | H | 125/250 | 250/250 | 250/250/250 |
| 81a | H | CH3 | 2/2 | 250/250 | 1/2/4 |
| 81b | H | CH3 | 2/4 | 250/250 | 2/4/8 |
| 81c | H | CH3 | 2/2 | 250/250 | 2/4/8 |
| 81d | H | CH3 | 2/2 | 250/250 | 2/4/8 |
| 81e | H | CH3 | 2/2 | 250/250 | 8/16/32 |
14/21 days.
7/14/21 days.
For methyl- and methoxy-substituted 3,5-dinitrobenzylsulfanyl oxadiazoles 78–81, it was found that the substitution in position 2 is more beneficial, while 2-methoxy and 2-methyl oxadiazoles 79a–e and 81a–e, respectively, were more active compared to their 4-substituted counterparts 78a–e and 80a–e (Table 8). This is the opposite phenomenon than what was found in the tetrazole series, where 4-substituted derivatives 76a–e showed the highest antimycobacterial activities within tetrazole series 74–77. Antimycobacterial activities of oxadiazoles 79a–e and 81a–e were comparable to those of tetrazoles 1a–e and INH but still significantly lower compared to the most efficient 3,5-dinitrobenzylsulfanyl oxadiazoles 2a–e (Table 8).
To further inspect the antimycobacterial activities of the most active derivatives prepared in this study, 14 compounds, tetrazoles 56c, 67a, 67b, 67c, and 67e and oxadiazoles 61b, 69a, 69b, 69c, 69e, 79a, 79e, 81a, and 81e, were selected, and their activity against seven clinically isolated MDR/XDR M.tb. strains was evaluated (Table 9). The activities of studied compounds against these resistant strains were comparable with those against the standard M.tb. strain indicating that these derivatives acted through a Ddn-activation pathway similar to the parent oxadiazoles 2. Consistently, the highest activities were found in the series of 2,5-dinitrobenzylsulfanyl derivatives 67 and 69, regardless of the substituent R on the tetrazole or oxadiazole, respectively.
Table 9. Antimycobacterial Activities of Compounds 56c, 61b, 67a-67c, 67e, 69a–69c, 69e, 79a, 79e, 81a, and 81e and Standard Anti-TB Drugs against Seven Clinically Isolated MDR/XDR-TB Strains Expressed as MIC (μM)a.
| MDR/XDR M.tb. strain |
|||||||
|---|---|---|---|---|---|---|---|
| Praha 1 | Praha 4 | Praha 131 | 9449/2007 | 234/2005 | 7357/1998 | 8666/2010 | |
| 56c | 4/8 | 4/8 | 4/8 | 4/8 | 4/8 | 4/8 | 4/8 |
| 61b | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 |
| 67a | 1/2 | 0.5/1 | 0.5/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| 67b | 0.5/1 | 0.25/0.5 | 0.25/0.5 | 0.25/0.5 | 0.5/1 | 0.5/1 | 0.5/1 |
| 67c | 0.5/1 | 0.25/0.5 | 0.25/0.5 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/0.5 |
| 67e | 1/2 | 1/1 | 1/1 | 1/1 | 0.5/1 | 0.5/1 | 0.5/0.5 |
| 69a | 1/2 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/0.5 |
| 69b | 1/1 | 0.5/1 | 0.5/1 | 1/1 | 0.5/1 | 0.5/1 | 0.5/0.5 |
| 69c | 1/1 | 0.5/1 | 0.5/1 | 1/2 | 0.5/1 | 0.5/1 | 0.5/0.5 |
| 69e | 1/1 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/1 | 0.5/1 | 0.25/0.5 |
| 79a | 2/4 | 1/2 | 1/2 | 2/2 | 1/2 | 1/2 | 1/2 |
| 79e | 1/2 | 0.5/1 | 0.5/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| 81a | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 |
| 81e | 2/4 | 1/2 | 2/2 | 4/4 | 2/2 | 1/2 | 1/2 |
| streptomycin | 16 (R) | >32 (R) | >32 (R) | >32 (R) | 32 (R) | >32 (R) | >32 (R) |
| isoniazid | 16 (R) | 16 (R) | 16 (R) | 64 (R) | 16 (R) | 16 (R) | 32 (R) |
| ethambutol | 32 (R) | 16 (R) | 32 (R) | 8 (S) | 16 (R) | 16 (R) | 16 (R) |
| rifampin | >8 (R) | >8 (R) | >8 (R) | >8 (R) | >8 (R) | >8 (R) | >8 (R) |
| ofloxacin | 1 (S) | >16 (R) | 16 (R) | 2 (S) | 0.5 (S) | 8 (R) | 8 (R) |
| gentamicin | 1 (S) | 0.5 (S) | >8 (R) | 1 (S) | 0.25 (S) | 1 (S) | 2 (S) |
| clofazimine | 0.5 (R) | 0.5 (R) | 0.25 (S) | 0.125 (S) | 0.125 (S) | 0.125 (S) | 2 (R) |
| amikacin | 0.5 (S) | 1 (S) | >32 (R) | 0.5 (S) | 0.5 (S) | 1 (S) | 2 (S) |
| pretomanid | n.d. | n.d. | 0.25 | 0.125 | n.d. | n.d. | 0.25 |
S, Strain susceptible to the given antibiotic drug. R, Strain resistant to the given antibiotic drug. n.d., not determined.
Mode of Action of 2,5-Dinitrobenzylsulfanyl Tetrazoles 67a–e and Oxadiazoles 69a–e
Due to the very small difference in the structure of 2,5-dinitro- and 3,5-dinitrobenzylsulfanyl derivatives, we first checked whether their mechanism of action is consistent. However, in contrast to the parent 3,5-dinitrobenzylsulfanyl derivatives T6030 and T6053, selected 2,5-dinitrobenzylsulfanyl tetrazoles 67b and 67c and oxadiazoles 69c and 69e showed the same inhibitory activity against wild-type M.tb. H37Rv as against Ddn- and FbiC-deficient mutants indicating that 2,5-dinitro compounds of series 67 and 69 acted via a Ddn-independent pathway. Thus, we turned our attention to DprE1, another important target of nitro-group-containing anti-TB agents including 3,5-dinitrophenyl-containing entities.11,14 First, we inspected the effects of 2,5-dinitrobenzylsulfanyl tetrazoles 67b and 67c and oxadiazoles 69c and 69e on the biosynthesis of lipids of M.tb. H37Rv via the [14C]acetate radiolabeling experiments in the presence of 10 times or 100 times the MIC of selected compound. The effects of parent T6030 and T6053 were also reassessed (11i and 14g in ref (13), respectively) as the reference. As shown in Figure 4, tetrazole 67b and oxadiazole 69e caused accumulation of trehalose monomycolates (TMMs) and trehalose dimycolates (TDMs) in mycobacteria, which is a typical phenomenon for DprE1 inhibitors including BTZ-043.11 Treatment of mycobacteria with derivatives 67c and 69c led to the accumulation of TMM only. As expected, treatment with 3,5-dinitrobenzylsulfanyl derivatives T6030 and T6053 did not affect the [14C]-labeled lipid profiles in mycobacteria (Figure 4). To confirm that the antimycobacterial activity of 2,5-dinitrobenzylsulfanyl heterocycles of series 67 and 69 is related to DprE1 inhibition, we determined their MIC values in M.tb. H37Ra overproducing DprE1/2, with BTZ-043, one of the most efficient DprE1 inhibitors, used as a control. As shown in Table 10, the activity of 2,5-dinitrobenzylsulfanyl tetrazole 67b and oxadiazole 69e against mycobacteria overproducing DprE1/2 dropped more than 10 times, while the activity of tetrazole 67c and oxadiazole 69c was not significantly affected. As expected, the activity of BTZ-043 dropped significantly, while the original 3,5-dinitro compounds T6030 and T6053 showed similar activity regardless of the level of DprE1/2 production.
Figure 4.
Evaluation of DprE1 inhibition by T6030, T6053, 67b, 67c, 69c, and 69e using metabolic radiolabeling via TLC analysis of the lipids from radiolabeled M.tb. H37Rv. Mycobacteria were co-incubated with [14C]acetate and tested compounds at 10 times or 100 times the MIC for 24 h. TMM, trehalose monomycolates; TDM, trehalose dimycolates; PE, phosphatidylethanolamine; CL, cardiolipin.
Table 10. Antimycobacterial Activities of Compounds T6030, T6053, 67b, 67c, 69c, and 69e against M.tb. H37Ra Overproducing DprE1/2 Expressed as MIC.
| BTZ-043 (ng/mL) | T6030 (μM) | T6053 (μM) | 67b (μM) | 67c (μM) | 69c (μM) | 69e (μM) | |
|---|---|---|---|---|---|---|---|
| pVV2 | 1 | 0.3 | 0.18 | 0.125 | 0.5–1.5 | 3 | 0.25–0.5 |
| pVV2-dprE1/2 | >30 | 0.09–0.3 | 0.18 | 1.5 | 1.5 | 1–3 | 3 |
In Vitro Effects of Studied Compounds on Mammalian Cell Viability
The effects of selected final compounds on mammalian cell viability were tested using HepG2 (human hepatocellular carcinoma) cells. In the cases when the IC50 exceeded 30 μM, the data are presented as the relative viability at a concentration of 30 μM compared to control vehicle-treated samples (100% viability). All 2,5-dinitrobenzylsulfanyl tetrazoles (67b, 67c, 67e) and oxadiazoles (69a–c, 69e) that showed the highest antimycobacterial activities within compounds in this SAR study showed the highest toxicity/antiproliferative activity to HepG2 cells (Table 11), which was not the case for parent 3,5-dinitrobenzylsulfanyl tetrazoles 1(12) and mainly oxadiazoles 2, which did not affect HepG2 cell viability at 50 μM concentrations after 48 h of incubation.13
Table 11. Viability of HepG2 Cell Line Determined by Viability Cell Assaysa after 48 h of Treatment with Compounds 56c, 61b, 67b, 67c, 67e, 69a, 69b, 69c, 69e, 79a, 79e, 81a, and 81e and Their Selectivity Indicesb.
| IC50 (μM) | viability at 30 μM (%) | SIc | (MIC for M.tb.) | |
|---|---|---|---|---|
| 56c | >30 | 92 ± 4 | >15 | (2) |
| 61b | >30 | 88 ± 6 | >7.5 | (4) |
| 67b | 10.18 ± 1.01 | 33 ± 4 | 20.4 | (0.5) |
| 67c | 23.28 ± 1.37 | 51 ± 16 | 46.6 | (0.5) |
| 67e | 12.47 ± 1.09 | 35 ± 5 | 12.5 | (1) |
| 69a | 8.3 ± 0.92 | 32 ± 9 | 8.3 | (1) |
| 69b | 5.94 ± 0.77 | 17 ± 1 | 5.9 | (1) |
| 69c | 13.41 ± 1.13 | 29 ± 15 | 13.4 | (1) |
| 69e | 10.16 ± 1.01 | 30 ± 12 | 10.2 | (1) |
| 79a | >30 | 93 ± 4 | >30 | (1) |
| 79e | >30 | 54 ± 2 | >30 | (1) |
| 81a | >30 | 98 ± 5 | >15 | (2) |
| 81e | >30 | 55 ± 5 | >15 | (2) |
CellTiter96 assay.
Vehicle-treated control viability was set to 100%. SDS-treated cell viability was set to 0%.
Selectivity index (SI) was calculated using the formula: SI = (IC50 for HepG2)/(MIC for M.tb).
Conclusions
The presence of nitro groups has often discouraged further development of hit compounds as drugs, because nitro groups can increase the risk of toxicity (mainly genotoxicity/mutagenicity), decrease the solubility of these compounds, and lead to their rapid metabolization.16 However, 3,5-dinitrobenzylsulfanyl-substituted heterocycles have been identified by us and others as readily accessible compounds with excellent antimycobacterial activities and acceptable toxicity profiles.12,13,15,20 Here, we first examined the role of the nitro groups in the mode of antimycobacterial action of these compounds. Whole genome sequencing of spontaneously resistant colonies showed that they harbored mutations in the fgd1 (Rv0407) gene encoding FGD1. Mutations in FGD1 disrupt the reduction of cofactor F420 to F420-H2, which inhibits the function of Ddn and blocks the reductive activation of nitro-group-containing drugs like pretomanid or delamanid.17 Decreased activity of 3,5-dinitrobenzylsulfanyl derivatives T6030 and T6053 toward Ddn- and FbiC-deficient M.tb. mutants proved that 3,5-dinitrobenzylsulfanyl heterocycles have a nitro-group-dependent mode of action that relies on Ddn-reductive activation. In the second part of this work, we have thoroughly investigated the structure–activity relationships of 3,5-dinitrobenzylsulfanyl tetrazoles and 1,3,4-oxadiazoles to see if we can replace/relocate one of the two nitro groups. Thus, various electron-withdrawing groups were attached instead of one nitro group. Moreover, the isosteric pyrrol-1-yl group, which has been successfully used to replace nitro group in various types of anti-TB agents,31 was utilized. Finally, the entire 3,5-dinitrophenyl group was replaced by nitro-substituted heterocyclic groups. However, the majority of the prepared compounds had significantly decreased activity as compared to their parent tetrazole and especially oxadiazole compounds. Thus, in the next step, we investigated the role of the relative position of the two nitro groups to possibly open the way for further structural optimization. We found that 2,5-dinitrobenzylsulfanyl tetrazoles 67a–e and oxadiazoles 69a–e showed consistently high antimycobacterial activity with MIC values around 1 μM against drug-susceptible and also MDR/XDR clinically isolated strains, i.e., activities comparable to those of parent tetrazoles 1a–e but lower compared to oxadiazoles 2a–e. Interestingly, shifting the nitro group from position 3 to position 2 led to a change in the dominant mechanism of antimycobacterial action. 2,5-Dinitrobenzylsulfanyl tetrazoles of series 67 and oxadiazoles of series 69 acted as DprE1 inhibitors as demonstrated by the accumulation of TMMs and TDMs in treated mycobacteria and by decreased activity of these compounds in mycobacteria overproducing DprE1/2. However, all 2,5-dinitro analogues showed significant toxicity to HepG2 cells, which was not the case for the parent 3,5-dinitro compounds. The replacement of one nitro group for a trifluoromethyl group in 2,5-dinitrobenzyl derivatives also led to a significant decrease or complete loss of antimycobacterial activity. The last attempt to modify the structure of compounds 1 and 2 was the introduction of an additional methyl or methoxy substituent adjacent to the 3,5-dinitrophenyl group, which can sterically hinder one or both nitro groups. However, these modifications also led to a significant decrease in antimycobacterial activity.
In conclusion, both nitro groups in 3,5-dinitrobenzylsulfanyl-containing antimycobacterial agents remain essential for their high efficacy. Further efforts should therefore be directed at fine-tuning the activity/toxicity ratios and finding ways to address the solubility issues, for example, by targeted delivery, rather than avoiding nitro groups.
Experimental Section
General
The prepared compounds were characterized using 1H NMR and 13C NMR spectroscopy. The purity of all prepared compounds was >95% as determined using elemental analysis (fluorine-free compounds) or HPLC–HRMS experiments (fluorine-containing compounds and oily compounds). All chemicals used in the syntheses were obtained from Sigma-Aldrich (Schnelldorf, Germany) and PENTA s.r.o. (Prague, Czech Republic) and were used as received. TLC separations were performed on Merck aluminum plates with silica gel 60 F254. Merck Kieselgel 60 (0.040–0.063 mm) was used for column chromatography. Melting points were recorded with a Büchi B-545 apparatus (BUCHI Labortechnik AG, Flawil, Switzerland) and are uncorrected. 1H and 13C NMR spectra were recorded using Varian Mercury Vx BB 300, VNMR S500 NMR (Varian, Palo Alto, CA, USA) or Jeol JNM-ECZ600R (JEOL Ltd., Akishima, Tokyo, Japan) spectrometers. Chemical shifts are reported as δ values in parts per million (ppm) and were indirectly referenced to tetramethylsilane (TMS) via the solvent signal. Elemental analyses were performed on an Automatic Microanalyzer EA1110CE (Fisons Instruments S.p.A., Milano, Italy). HPLC–HRMS (ESI) experiments were performed using an HRMS system Acquity UPLC I-class and a Synapt G2Si Q-TOF mass spectrometer (Waters, Milford, MA, USA).
General Method for the Synthesis of Final Compounds 52–81, 83
The corresponding alkyl halide 35–51 (1 mmol) was added to the solution of 1-substituted tetrazole-5-thiol or 5-substituted 1,3,4-oxadiazole-2-thiol (1.1 mmol) and triethylamine (1.2 mmol) in acetonitrile (5–10 mL). The reaction mixture was stirred at rt upon complete consumption of alkyl halide as determined by TLC. Then, the solvent was evaporated under reduced pressure, and the residue was dissolved in EtOAc (50 mL) and washed with 5% aqueous Na2CO3 (2 × 30 mL) and water (1 × 30 mL). The organic phase was separated, dried over anhydrous sodium sulfate, and evaporated under reduced pressure. The crude product was purified using column chromatography (mobile phase: hexane/EtOAc).
1-Alkyl/Aryl-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazoles 52a–52e
3-Nitro-5-(trifluoromethyl)benzyl bromide (35) was used as the alkylating agent. The reactions were completed in 1 h.
5-((3-Nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1-phenyl-1H-tetrazole (52a)
Yield: 93% as a yellowish solid; mp 112–113 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.63 (t, J = 2.1 Hz, 1H), 8.35 (t, J = 2.1 Hz, 1H), 8.31 (t, J = 1.9 Hz, 1H), 7.61–7.55 (m, 5H), 4.75 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.08, 148.63, 141.96, 133.43, 132.75, 131.23, 130.74 (q, J = 33.4 Hz), 130.53, 128.48, 125.11, 123.43 (d, J = 273.1 Hz), 120.18 (d, J = 4.3 Hz), 35.57. HRMS (ESI+) calcd for (C15H10F3N5O2S + H+) m/z: 382.05801(100%), 383.06136 (16%); found: 382.0588 (100%), 383.0610 (18%).
1-(4-Methoxyphenyl)-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (52b)
Yield: 80% as a yellowish solid; mp 104–105 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.61 (t, J = 1.9 Hz, 1H), 8.35 (s, 1H), 8.30 (s, 1H), 7.46 (d, J = 9.0 Hz, 2H), 7.10 (d, J = 9.0 Hz, 2H), 4.73 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.18, 154.18, 148.61, 142.04, 132.71 (q, J = 3.6 Hz), 130.74 (q, J = 33.4 Hz), 128.43, 126.88, 126.01, 123.43 (q, J = 273.1 Hz), 120.14 (d, J = 3.6 Hz), 115.52, 56.22, 35.52. HRMS (ESI+) calcd for (C16H12F3N5O3S + H+) m/z: 412.06857 (100%), 413.07193 (17%); found: 412.0688 (100%), 413.0711 (18%).
1-(4-Chlorophenyl)-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (52c)
Yield: 96% as a white solid; mp 125–126 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.62 (t, J = 1.9 Hz, 1H), 8.35 (s, 1H), 8.30 (s, 1H), 7.67 (d, J = 8.9 Hz, 2H), 7.62 (d, J = 8.9 Hz, 2H), 4.75 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.20, 148.61, 141.94, 135.86, 132.76 (q, J = 3.7 Hz), 132.26, 130.75 (q, J = 33.6 Hz), 130.54, 128.46, 127.03, 123.42 (q, J = 272.7 Hz), 120.16 (d, J = 3.9 Hz), 35.70. HRMS (ESI+) calcd for (C15H9ClF3N5O2S + H+) m/z: 416.01903 (100%), 418.01608 (32%); found: 416.0197 (100%), 418.0159 (38%).
1-(4-Bromophenyl)-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (52d)
Yield: 62% as a white solid; mp 121–123 °C.1H NMR (600 MHz, DMSO-d6) δ 8.62 (t, J = 1.9 Hz, 1H), 8.35 (s, 1H), 8.30 (s, 1H), 7.80 (d, J = 8.8 Hz, 2H), 7.55 (d, J = 8.7 Hz, 2H), 4.74 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.15, 148.61, 141.93, 133.50, 132.76 (d, J = 3.5 Hz), 132.68, 130.74 (q, J = 33.2 Hz), 128.46, 127.18, 124.42, 123.42 (q, J = 273.1 Hz), 120.16 (d, J = 4.0 Hz), 35.71. HRMS (ESI+) calcd for (C15H9BrF3N5O2S + H+) m/z: 459.96852 (100%), 461.96647 (97%); found: 461.9672 (100%), 459.9691 (97%).
1-Cyclohexyl-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (52e)
Yield: 91% as a white solid; mp 57–59 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.61 (t, J = 1.9 Hz, 1H), 8.35 (s, 1H), 8.28 (s, 1H), 4.73 (s, 2H), 4.21 (tt, J = 11.5, 3.9 Hz, 1H), 1.86–1.80 (m, 2H), 1.77–1.72 (m, 2H), 1.70–1.64 (m, 2H), 1.61–1.54 (m, 1H), 1.38–1.30 (m, 2H), 1.22–1.14 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 152.09, 148.63, 142.21, 132.64 (d, J = 3.6 Hz), 130.80 (q, J = 33.5 Hz), 128.35, 123.41 (q, J = 272.4 Hz), 120.14 (d, J = 4.1 Hz), 58.00, 35.54, 32.13, 24.94, 24.88. HRMS (ESI+) calcd for (C15H16F3N5O2S + H+) m/z: 388.10496 (100%), 389.10831 (16%); found: 388.1056 (100%), 389.1079 (18%).
1-Alkyl/Aryl-5-((3-chloro-5-nitrobenzyl)sulfanyl)-1H-tetrazoles 53a–53e
3-Chloro-5-nitrobenzyl chloride (36) was used as the alkylating agent. The reactions were stirred overnight.
5-((3-Chloro-5-nitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (53a)
Yield: 85% as a yellow solid; mp 112–114 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.30 (t, J = 1.8 Hz, 1H), 8.14 (t, J = 2.1 Hz, 1H), 8.00 (t, J = 1.8 Hz, 1H), 7.64–7.54 (m, 5H), 4.67 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.14, 148.96, 141.85, 135.99, 134.34, 133.44, 131.23, 130.53, 125.12, 123.38, 123.13, 35.56. Elem. Anal. Calcd for C14H10ClN5O2S: C, 48.35; H, 2.90; N, 20.14; S, 9.22. Found: C, 48.44; H, 2.60; N, 20.10; S, 9.39.
5-((3-Chloro-5-nitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (53b)
Yield: 89% as a white solid; mp 124–126 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.28 (t, J = 1.8 Hz, 1H), 8.14 (t, J = 2.0 Hz, 1H), 7.98 (t, J = 1.8 Hz, 1H), 7.48 (d, J = 8.8 Hz, 2H), 7.11 (d, J = 9.0 Hz, 2H), 4.64 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.18, 154.24, 148.95, 141.93, 135.95, 134.32, 126.92, 126.02, 123.33, 123.10, 115.53, 56.23, 35.50. Elem. Anal. Calcd for C15H12ClN5O3S: C, 47.69; H, 3.20; N, 18.54; S, 8.49. Found: C, 47.78; H, 2.88; N, 18.71; S, 8.60.
5-((3-Chloro-5-nitrobenzyl)sulfanyl)-1-(4-chlorophenyl)-1H-tetrazole (53c)
Yield: 80% as a white solid; mp 142–144 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.32 (dd, J = 2.1, 1.6 Hz, 1H), 8.17 (t, J = 2.0 Hz, 1H), 8.02 (t, J = 1.7 Hz, 1H), 7.71 (d, J = 8.9 Hz, 2H), 7.66 (d, J = 8.8 Hz, 2H), 4.69 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 154.16, 148.86, 141.72, 135.90, 135.78, 134.26, 132.18, 130.47, 126.97, 123.28, 123.04, 35.62. Elem. Anal. Calcd for C14H9Cl2N5O2S: C, 43.99; H, 2.37; N, 18.32; S, 8.39. Found: C, 44.16; H, 2.01; N, 18.49; S, 8.77.
1-(4-Bromophenyl)-5-((3-chloro-5-nitrobenzyl)sulfanyl)-1H-tetrazole (53d)
Yield: 86% as a yellow solid; mp 155–157 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.28 (t, J = 1.8 Hz, 1H), 8.14 (t, J = 2.1 Hz, 1H), 7.98 (t, J = 1.7 Hz, 1H), 7.81 (d, J = 9.0 Hz, 2H), 7.56 (d, J = 9.0 Hz, 2H), 4.66 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.20, 148.95, 141.81, 135.98, 134.34, 133.50, 132.70, 127.21, 124.43, 123.37, 123.12, 35.71. Elem. Anal. Calcd for C14H9BrClN5O2S: C, 39.41; H, 2.13; N, 16.41; S, 7.51. Found: C, 39.56; H, 1.70; N, 16.54; S, 7.61.
5-((3-Chloro-5-nitrobenzyl)sulfanyl)-1-cyclohexyl-1H-tetrazole (53e)
Yield: 81% as a white solid; mp 110–112 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.28 (t, J = 1.8 Hz, 1H), 8.15 (t, J = 2.1 Hz, 1H), 7.97 (t, J = 1.7 Hz, 1H), 4.64 (s, 2H), 4.23–4.18 (m, 1H), 1.86–1.79 (m, 2H), 1.80–1.73 (m, 2H), 1.68 (qd, J = 12.4, 3.6 Hz, 2H), 1.65–1.55 (m, 1H), 1.35 (qt, J = 12.9, 3.5 Hz, 2H), 1.18 (qt, J = 12.8, 3.6 Hz, 1H). 13C NMR (151 MHz, DMSO-d6) δ 152.14, 148.98, 142.09, 135.92, 134.38, 123.25, 123.10, 58.01, 35.57, 32.16, 24.96, 24.91. Elem. Anal. Calcd for C14H16ClN5O2S: C, 47.52; H, 4.56; N, 19.79; S, 9.06. Found: C, 47.32; H, 4.25; N, 19.86; S, 9.36.
1-Alkyl/Aryl-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1H-tetrazoles 54a–54e
3-Fluoro-5-nitrobenzyl chloride (37) was used as the alkylating agent. The reactions were stirred overnight.
5-((3-Fluoro-5-nitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (54a)
Yield: 79% as a white solid; mp 120–122 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.21 (s, 1H), 7.97 (dt, J = 8.6, 2.4 Hz, 1H), 7.81 (dt, J = 9.1, 1.9 Hz, 1H), 7.64–7.53 (m, 5H), 4.68 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 161.89 (d, J = 248.4 Hz), 154.13, 149.10 (d, J = 9.6 Hz), 142.16 (d, J = 8.3 Hz), 133.46, 131.21, 130.52, 125.10, 123.41 (d, J = 22.4 Hz), 120.85 (d, J = 2.9 Hz), 110.95 (d, J = 26.8 Hz), 35.70. HRMS (ESI+) calcd for (C14H10FN5O2S + H+) m/z: 332.0618; found: 332.0622.
5-((3-Fluoro-5-nitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (54b)
Yield: 81% as a white solid; mp 113–114 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.19 (t, J = 1.8 Hz, 1H), 7.97 (dt, J = 8.6, 2.2 Hz, 1H), 7.80 (dt, J = 9.1, 2.1 Hz, 1H), 7.48 (d, J = 9.0 Hz, 2H), 7.11 (d, J = 8.9 Hz, 2H), 4.65 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.85 (d, J = 248.5 Hz), 161.19, 154.26, 149.09 (d, J = 9.7 Hz), 142.24 (d, J = 8.0 Hz), 126.92, 126.03, 123.37 (d, J = 22.4 Hz), 120.82 (d, J = 3.0 Hz), 115.54, 110.94 (d, J = 26.8 Hz), 56.23, 35.63. HRMS (ESI+) calcd for (C15H12FN5O3S + H+) m/z: 362.0723; found: 362.0727.
1-(4-Chlorophenyl)-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1H-tetrazole (54c)
Yield: 90% as a white solid; mp 108–110 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.20 (t, J = 1.9 Hz, 1H), 7.97 (dt, J = 8.6, 2.3 Hz, 1H), 7.80 (dt, J = 8.8., 1.9 Hz, 1H), 7.68 (d, J = 8.8 Hz, 2H), 7.63 (d, J = 8.8 Hz, 2H), 4.67 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 161.89 (d, J = 248.4 Hz), 154.26, 149.09 (d, J = 9.5 Hz), 142.12 (d, J = 8.1 Hz), 135.86, 132.28, 130.56, 127.06, 123.40 (d, J = 22.4 Hz), 120.85 (d, J = 3.0 Hz), 110.95 (d, J = 26.8 Hz), 35.82. HRMS (ESI+) calcd for (C14H9ClFN5O2S + H+) m/z: 366.0228; found: 366.0232.
1-(4-Bromophenyl)-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1H-tetrazole (54d)
Yield: 89% as a white solid; mp 123–125 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.20 (t, J = 1.7 Hz, 1H), 7.97 (dt, J = 8.6, 2.3 Hz, 1H), 7.83–7.78 (m, 3H), 7.56 (d, J = 8.7 Hz, 2H), 4.67 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 161.89 (d, J = 248.4 Hz), 154.21, 149.09 (d, J = 9.5 Hz), 142.12 (d, J = 8.0 Hz), 133.51, 132.71, 127.20, 124.42, 123.40 (d, J = 22.5 Hz), 120.85 (d, J = 3.0 Hz), 110.96 (d, J = 26.8 Hz), 35.83. HRMS (ESI+) calcd for (C14H9BrFN5O2S + H+) m/z: 409.9718 (100%), 411.9697 (97%); found: 409.9721 (97%), 411.9701 (100%).
1-Cyclohexyl-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1H-tetrazole (54e)
Yield: 87% as a white solid; mp 83–85 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.19 (t, J = 1.8 Hz, 1H), 7.98 (dt, J = 8.6, 2.3 Hz, 1H), 7.79 (dt, J = 9.1, 1.9 Hz, 1H), 4.65 (s, 2H), 4.25–4.18 (m, 1H), 1.88–1.82 (m, 2H), 1.79–1.73 (m, 2H), 1.73–1.64 (m, 2H), 1.62–1.57 (m, 1H), 1.40–1.30 (m, 2H), 1.23–1.12 (m, 1H). 13C NMR (151 MHz, DMSO-D6) δ 161.91 (d, J = 248.5 Hz), 152.15, 149.13 (d, J = 9.6 Hz), 142.41 (d, J = 8.0 Hz), 123.34 (d, J = 22.4 Hz), 120.74 (d, J = 3.1 Hz), 110.94 (d, J = 26.7 Hz), 58.01, 35.71, 32.15, 24.96, 24.90. HRMS (ESI+) calcd for (C14H16FN5O2S + H+) m/z: 338.1087; found: 338.1092.
1-Alkyl/Aryl-5-((3-bromo-5-nitrobenzyl)sulfanyl)-1H-tetrazoles 55a–55e
3-Bromo-5-nitrobenzyl chloride (38) was used as the alkylating agent. The reactions were stirred overnight.
5-((3-Bromo-5-nitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (55a)
Yield: 86% as a white solid; mp 100–101 °C. 1H NMR (500 MHz, DMSO-d6): δ 8.37 (t, J = 1.8 Hz, 1H), 8.28 (t, J = 1.9 Hz, 1H), 8.16 (t, J = 1.7 Hz, 1H), 7.53–7.60 (m, 5H), 4.69 (s, 2H). 13C NMR (126 MHz, DMSO-d6): δ 153.77, 148.60, 141.63, 138.48, 133.07, 130.86, 130.16, 125.49, 124.76, 123.36, 121.92, 35.12. Elem. Anal. Calcd for C14H10BrN5O2S: C, 42.87; H, 2.57; N, 17.86; S, 8.17. Found: C, 43.09; H, 2.20; N, 18.04; S, 8.18.
5-((3-Bromo-5-nitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (55b)
Yield: 96% as a white solid; mp 130–131 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.31 (t, J = 1.8 Hz, 1H), 8.24 (t, J = 1.9 Hz, 1H), 8.11 (t, J = 1.6 Hz, 1H), 7.47 (d, J = 9.0 Hz, 2H), 7.11 (d, J = 9.0 Hz, 2H), 4.63 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.19, 154.24, 148.97, 142.08, 138.81, 126.93, 126.02, 125.83, 123.68, 122.28, 115.53, 56.24, 35.44. Elem. Anal. Calcd for C15H12BrN5O3S: C, 42.67; H, 2.86; N, 16.59; S, 7.59. Found: C, 42.36; H, 2.55; N, 16.48; S, 7.66.
5-((3-Bromo-5-nitrobenzyl)sulfanyl)-1-(4-chlorophenyl)-1H-tetrazole (55c)
Yield: 70% as a white solid; mp 182–183 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.32 (t, J = 2.1, 1H), 8.24 (t, J = 2.0 Hz, 1H), 8.11 (t, J = 1.7 Hz, 1H), 7.68 (d, J = 8.9 Hz, 2H), 7.63 (d, J = 8.9 Hz, 2H), 4.65 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.25, 148.96, 141.97, 138.85, 135.86, 132.27, 130.55, 127.06, 125.85, 123.72, 122.30, 35.63. Elem. Anal. Calcd for C14H9BrClN5O2S: C, 39.41; H, 2.13; N, 16.41; S, 7.51. Found: C, 39.58; H, 1.87; N, 16.55; S, 7.53.
5-((3-Bromo-5-nitrobenzyl)sulfanyl)-1-(4-bromophenyl)-1H-tetrazole (55d)
Yield: 70% as a yellowish solid; mp 187–188 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.32 (t, J = 2.0 Hz, 1H), 8.24 (t, J = 2.0 Hz, 1H), 8.11 (t, J = 1.7 Hz, 1H), 7.81 (d, J = 8.7 Hz, 2H), 7.55 (d, J = 8.7 Hz, 2H), 4.65 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.20, 148.97, 141.96, 138.85, 133.50, 132.70, 127.22, 125.85, 124.43, 123.72, 122.30, 35.65. Elem. Anal. Calcd for C14H9Br2N5O2S: C, 35.69; H, 1.93; N, 14.87; S, 6.80. Found: C, 35.61; H, 1.71; N, 14.74; S, 6.68.
5-((3-Bromo-5-nitrobenzyl)sulfanyl)-1-cyclohexyl-1H-tetrazole (55e)
Yield: 88% as a white solid; mp 112–113 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.31 (t, J = 1.8 Hz, 1H), 8.25 (t, J = 2.0 Hz, 1H), 8.10 (t, J = 1.7 Hz, 1H), 4.63 (s, 2H), 4.20 (tt, J = 11.5, 3.9 Hz, 1H), 1.86–1.82 (m, 2H), 1.79–1.72 (m, 2H), 1.71–1.65 (m, 2H), 1.63–1.56 (m, 1H), 1.41–1.28 (m, 2H), 1.25–1.11 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 152.13, 148.98, 142.24, 138.78, 125.82, 123.59, 122.33, 58.01, 35.52, 32.16, 24.96, 24.92. Elem. Anal. Calcd for C14H16BrN5O2S: C, 42.22; H, 4.05; N, 17.58; S, 8.05. Found: C, 42.61; H, 3.91; N, 17.87; S, 8.07.
1-Alkyl/Aryl-((3-cyano-5-nitrobenzyl)sulfanyl)-1H-tetrazoles 56a–56e
3-Cyano-5-nitrobenzyl chloride (39) was used as the alkylating agent. The reactions were stirred overnight.
5-((3-Cyano-5-nitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (56a)
Yield: 88% as a white solid; mp 140–142 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.63 (t, J = 1.9 Hz, 1H), 8.62–8.60 (m, 1H), 8.36 (t, J = 1.5 Hz, 1H), 7.64–7.55 (m, 5H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.07, 148.44, 141.64, 139.39, 133.44, 131.25, 130.54, 129.03, 127.15, 125.13, 117.38, 113.26, 35.32. Elem. Anal. Calcd for C15H10N6O2S: C, 53.25; H, 2.98; N, 24.84; S, 9.48. Found: C, 53.11; H, 2.95; N, 24.52; S, 9.87.
5-((3-Cyano-5-nitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (56b)
Yield: 75% as a yellowish solid; mp 121–122 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.62–8.60 (m, 2H), 8.34 (t, J = 1.5 Hz, 1H), 7.48 (d, J = 9.0 Hz, 2H), 7.11 (d, J = 9.0 Hz, 2H), 4.68 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.19, 154.17, 148.42, 141.72, 139.34, 128.98, 127.12, 126.93, 126.01, 117.38, 115.53, 113.24, 56.23, 35.28. Elem. Anal. Calcd for C16H12N6O3S: C, 52.17; H, 3.28; N, 22.81; S, 8.70. Found: C, 52.34; H, 3.31; N, 22.6; S, 8.77.
1-(4-Chlorophenyl)-5-((3-cyano-5-nitrobenzyl)sulfanyl)-1H-tetrazole (56c)
Yield: 90% as a white solid; mp 143–144 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.62 (t, J = 1.9 Hz, 1H), 8.61 (t, J = 1.8 Hz, 1H), 8.35 (t, J = 1.5 Hz, 1H), 7.68 (d, J = 8.8 Hz, 2H), 7.64 (d, J = 8.8 Hz, 2H), 4.70 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.20, 148.42, 141.61, 139.37, 135.88, 132.27, 130.57, 129.02, 127.14, 127.07, 117.37, 113.26, 35.44. Elem. Anal. Calcd for C15H9ClN6O2S: C, 48.33; H, 2.43; N, 22.54; S, 8.60. Found: C, 48.11; H, 2.17; N, 22.59; S, 8.80.
1-(4-Bromophenyl)-5-((3-cyano-5-nitrobenzyl)sulfanyl)-1H-tetrazole (56d)
Yield: 70% as a yellow solid; mp 159–160 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.63–8.60 (m, 2H), 8.35 (t, J = 1.6 Hz, 1H), 7.82 (d, J = 8.7 Hz, 2H), 7.57 (d, J = 8.7 Hz, 2H), 4.70 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.15, 148.42, 141.61, 139.37, 133.52, 132.70, 129.02, 127.23, 127.14, 124.45, 117.37, 113.26, 35.45. Elem. Anal. Calcd for C15H9BrN6O2S: C, 43.18; H, 2.17; N, 20.14; S, 7.68. Found: C, 43.39; H, 1.92; N, 20.05; S, 7.62.
5-((3-Cyano-5-nitrobenzyl)sulfanyl)-1-cyclohexyl-1H-tetrazole (56e)
Yield: 86% as a white solid; mp 121–123 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.63–8.61 (m, 2H), 8.35 (t, J = 1.5 Hz, 1H), 4.69 (s, 2H), 4.22 (tt, J = 11.5, 3.9 Hz, 1H), 1.89–1.84 (m, 2H), 1.79–1.74 (m, 2H), 1.72–1.64 (m, 2H), 1.62–1.58 (m, 1H), 1.41–1.31 (m, 2H), 1.22–1.14 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 152.13, 148.46, 141.86, 139.38, 128.94, 127.15, 117.34, 113.27, 58.02, 35.27, 32.15, 24.96, 24.90. Elem. Anal. Calcd for C15H16N6O2S: C, 52.31; H, 4.68; N, 24.40; S, 9.31. Found: C, 52.13; H, 4.55; N, 24.34; S, 9.78.
2-Alkyl/Aryl-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazoles 57a–57e
3-Nitro-5-(trifluoromethyl)benzyl bromide (35) was used as the alkylating agent. The reactions were completed in 1 h.
2-((3-Nitro-5-(trifluoromethyl)benzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (57a)
Yield: 90% as a yellowish solid; 89–91 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.69 (s, 1H), 8.36 (s, 2H), 7.92–7.87 (m, 2H), 7.60–7.56 (m, 1H), 7.55–7.50 (m, 2H), 4.75 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.00, 163.33, 148.69, 142.44, 132.65, 130.82 (q, J = 33.2 Hz), 129.91, 128.46, 128.38, 126.94, 123.44, 123.44 (q, J = 273.1 Hz), 120.20, 34.82. HRMS (ESI+) calcd for (C16H10F3N3O3S + H+) m/z: 382.04677 (100%), 383.05013 (17%); found: 382.0472 (100%); 383.0498 (18%).
2-(4-Methoxyphenyl)-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (57b)
Yield: 82% as a yellowish solid; 105–107 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.71 (t, J = 1.9 Hz, 1H), 8.39 (s, 1H), 8.37 (s, 1H), 7.85 (d, J = 8.9 Hz, 2H), 7.09 (d, J = 8.9 Hz, 2H), 4.76 (s, 2H), 3.83 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.89, 162.56, 162.39, 148.58, 142.37, 132.53 (q, J = 3.5 Hz), 130.73 (q, J = 33.2 Hz), 128.73, 128.30, 123.34 (d, J = 273.2 Hz), 120.08 (q, J = 4.1 Hz), 115.68, 115.25, 55.98, 34.76. HRMS (ESI+) calcd for (C17H12F3N3O4S + H+) m/z: 412.05734 (100%), 413.06069 (18%); found: 412.0580 (100%), 413.0604 (18%).
2-(4-Chlorophenyl)-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (57c)
Yield: 82% as a white solid; mp 118–120 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.71 (t, J = 1.9 Hz, 1H), 8.39 (s, 2H), 7.93 (d, J = 8.6 Hz, 2H), 7.62 (d, J = 8.6 Hz, 2H), 4.78 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.17, 163.55, 148.58, 142.25, 137.26, 132.57 (q, J = 3.6 Hz), 130.73 (q, J = 33.3 Hz), 129.97, 128.66, 128.33, 123.34 (q, J = 272.8 Hz), 122.25, 120.12 (q, J = 3.8 Hz), 34.72. HRMS (ESI+) calcd for (C16H9ClF3N3O3S + H+) m/z: 416.00780 (100%), 418.00485 (32%); found: 416.0088 (100%), 418.0055 (38%).
2-(4-Bromophenyl)-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (57d)
Yield: 83% as a white solid; 126–127 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.72 (t, J = 1.9 Hz, 1H), 8.41–8.37 (m, 2H), 7.86 (d, J = 8.6 Hz, 2H), 7.77 (d, J = 8.6 Hz, 2H), 4.78 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.29, 163.57, 148.59, 142.25, 132.91, 132.57 (q, J = 3.6 Hz), 130.73 (q, J = 33.3 Hz), 128.78, 128.34, 126.16, 123.35 (d, J = 273.0 Hz), 122.59, 120.13 (d, J = 4.0 Hz), 34.72. HRMS (ESI+) calcd for (C16H9BrF3N3O3S + H+) m/z: 459.95729 (100%), 461.95524 (97%); found: 461.9563 (100%), 459.9581 (97%).
2-Cyclohexyl-5-((3-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (57e)
Yield: 98% as a colorless oil. 1H NMR (500 MHz, DMSO-d6) δ 8.65 (t, J = 1.9 Hz, 1H), 8.40 (t, J = 1.9 Hz, 1H), 8.32 (s, 1H), 4.68 (s, 2H), 2.91–2.85 (m, 1H), 1.95–1.86 (m, 2H), 1.71–1.58 (m, 3H), 1.47–1.10 (m, 5H). 13C NMR (126 MHz, DMSO-d6) δ 171.44, 162.30, 148.57, 142.42, 132.48 (q, J = 3.5 Hz), 130.73 (q, J = 33.4 Hz), 128.26, 123.35 (d, J = 272.8 Hz), 120.07 (q, J = 3.9 Hz), 34.67, 34.60, 29.79, 25.51, 25.05. HRMS (ESI+) calcd for (C16H16F3N3O3S + H+) m/z: 388.09372 (100%), 389.09708 (17%); found: 388.0941 (100%), 389.0970 (18%).
2-Alkyl/Aryl-5-((3-chloro-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 58a–58e
3-Chloro-5-nitrobenzyl chloride (36) was used as the alkylating agent. The reactions were stirred overnight.
2-((3-Chloro-5-nitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (58a)
Yield: 86% as a white solid; mp 95–97 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.39 (t, J = 1.8 Hz, 1H), 8.18 (t, J = 2.1 Hz, 1H), 8.08 (t, J = 1.7 Hz, 1H), 7.96–7.89 (m, 2H), 7.65–7.55 (m, 3H), 4.69 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.62, 163.03, 148.63, 141.94, 135.63, 134.01, 132.27, 129.58, 126.58, 123.10, 122.99, 122.80, 34.46. Elem. Anal. Calcd for C15H10ClN3O3S: C, 51.81; H, 2.90; N, 12.08; S, 9.22. Found: C, 51.44; H, 2.63; N, 12.07; S, 9.24.
2-((3-Chloro-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (58b)
Yield: 87% as a yellowish solid; mp 129–131 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.35 (t, J = 1.9 Hz, 1H), 8.15 (t, J = 2.1 Hz, 1H), 8.04 (t, J = 1.7 Hz, 1H), 7.84 (d, J = 8.9 Hz, 2H), 7.08 (d, J = 8.8 Hz, 2H), 4.64 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.96, 162.66, 162.53, 149.01, 142.34, 135.96, 134.37, 128.84, 123.32, 123.14, 115.80, 115.39, 56.08, 34.86. Elem. Anal. Calcd for C16H12ClN3O4S: C, 50.87; H, 3.20; N, 11.12; S, 8.49. Found: C, 50.58; H, 2.92; N, 11.16; S, 8.88.
2-((3-Chloro-5-nitrobenzyl)sulfanyl)-5-(4-chlorophenyl)-1,3,4-oxadiazole (58c)
Yield: 89% as a white solid; mp 140–142 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.39 (t, J = 1.9 Hz, 1H), 8.18 (t, J = 2.1 Hz, 1H), 8.08 (t, J = 1.7 Hz, 1H), 7.94 (d, J = 8.7 Hz, 2H), 7.64 (d, J = 8.7 Hz, 2H), 4.69 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.88, 163.33, 148.63, 141.86, 136.98, 135.63, 134.02, 129.74, 128.40, 122.99, 122.82, 122.00, 34.44. Elem. Anal. Calcd for C15H9Cl2N3O3S: C, 47.14; H, 2.37; N, 10.99; S, 8.39. Found: C, 47.49; H, 2.31; N, 10.79; S, 8.0.
2-(4-Bromophenyl)-5-((3-chloro-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (58d)
Yield: 90% as a white solid; mp 132–134 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.35 (t, J = 1.9 Hz, 1H), 8.15 (t, J = 2.1 Hz, 1H), 8.05 (t, J = 1.8 Hz, 1H), 7.83 (d, J = 8.6 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 4.66 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.36, 163.71, 149.01, 142.21, 135.98, 134.39, 133.02, 128.87, 126.24, 123.35, 123.18, 122.70, 34.82. Elem. Anal. Calcd for C15H9BrClN3O3S: C, 42.23; H, 2.13; N, 9.85; S, 7.51. Found: C, 42.35; H, 1.90; N, 9.86; S, 7.78.
2-((3-Chloro-5-nitrobenzyl)sulfanyl)-5-cyclohexyl-1,3,4-oxadiazole (58e)
Yield: 91% as a white solid; mp 78–80 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.31 (t, J = 1.8 Hz, 1H), 8.19 (t, J = 2.0 Hz, 1H), 8.01 (t, J = 1.8 Hz, 1H), 4.59 (s, 2H), 2.90 (tt, J = 11.0, 3.7 Hz, 1H), 2.00–1.88 (m, 2H), 1.73–1.57 (m, 3H), 1.49–1.40 (m, 2H), 1.38–1.29 (m, 2H), 1.27–1.17 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 171.15, 162.04, 148.61, 142.00, 135.56, 134.00, 122.91, 122.74, 34.42, 34.32, 29.53, 25.25, 24.78. Elem. Anal. Calcd for C15H16ClN3O3S: C, 50.92; H, 4.56; N, 11.88; S, 9.06. Found: C, 50.56; H, 4.32; N, 11.91; S, 9.42.
2-Alkyl/Aryl-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 59a–59e
3-Fluoro-5-nitrobenzyl chloride (37) was used as the alkylating agent. The reactions were stirred overnight.
2-((3-Fluoro-5-nitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (59a)
Yield: 87% as a white solid; mp 138–139 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.27 (t, J = 1.8 Hz, 1H), 7.99 (dt, J = 8.5, 2.3 Hz, 1H), 7.92–7.88 (m, 2H), 7.86 (dt, J = 9.2, 2.0 Hz, 1H), 7.59–7.55 (m, 1H), 7.58–7.48 (m, 2H), 4.67 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.99, 163.39, 161.95 (d, J = 248.2 Hz), 149.13 (d, J = 9.5 Hz), 142.57 (d, J = 8.0 Hz), 132.62, 129.93, 126.94, 123.46 (d, J = 4.6 Hz), 123.30, 120.82 (d, J = 2.9 Hz), 111.01 (d, J = 26.8 Hz), 35.00. HRMS (ESI+) calcd for (C15H10FN3O3S + H+) m/z: 332.0505; found: 332.0505.
2-((3-Fluoro-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (59b)
Yield: 85% as a white solid; mp 115–117 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.25 (t, J = 1.8 Hz, 1H), 7.98 (dt, J = 8.6, 2.3 Hz, 1H), 7.88–7.79 (m, 3H),7.07 (d, J = 8.7 Hz, 2H), 4.65 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.96, 162.65, 162.52, 161.92 (d, J = 247.9 Hz), 149.13 (d, J = 9.5 Hz), 142.61 (d, J = 8.2 Hz), 128.82, 123.34 (d, J = 22.3 Hz), 120.79 (d, J = 2.9 Hz), 115.80, 115.38, 110.98 (d, J = 26.8 Hz), 56.06, 35.02. HRMS (ESI+) calcd for (C16H12FN3O4S + H+) m/z: 362.0605; found: 362.0618.
2-(4-Chlorophenyl)-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (59c)
Yield: 82% as a white solid; mp 141–142 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.26 (t, J = 1.9 Hz, 1H), 7.99 (dt, J = 8.6, 2.3 Hz, 1H), 7.91 (d, J = 8.6 Hz, 2H), 7.85 (dt, J = 9.1, 2.0 Hz, 1H), 7.61 (d, J = 8.5 Hz, 2H), 4.67 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.25, 163.68, 161.94 (d, J = 248.3 Hz), 149.13 (d, J = 9.3 Hz), 142.49 (d, J = 8.1 Hz), 137.34, 130.10, 128.76, 123.37 (d, J = 22.4 Hz), 122.37, 120.82 (d, J = 3.1 Hz), 111.03 (d, J = 26.8 Hz), 34.98. HRMS (ESI+) calcd for (C15H9ClFN3O3S + H+) m/z: 366.0115; found: 366.0116.
2-(4-Bromophenyl)-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (59d)
Yield: 87% as a white solid; mp 149–151 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.30 (t, J = 1.8 Hz, 1H), 8.03 (dt, J = 8.7, 2.3 Hz, 1H), 7.92–7.82 (m, 3H), 7.78 (d, J = 8.5 Hz, 2H), 4.70 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.01, 163.36, 161.58 (d, J = 248.3 Hz), 148.77 (d, J = 9.6 Hz), 142.14 (d, J = 8.1 Hz), 132.68, 128.52, 125.89, 123.04 (d, J = 22.4 Hz), 122.35, 120.49 (d, J = 3.0 Hz), 110.70 (d, J = 26.7 Hz), 34.60. HRMS (ESI+) calcd for (C15H9BrFN3O3S + H+): 409.9605 (100%), 411.9585 (97%); found: 409.9609 (97%), 411.9590 (100%).
2-Cyclohexyl-5-((3-fluoro-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (59e)
Yield: 88% as a white solid; mp 64–66 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.22 (t, J = 1.8 Hz, 1H), 8.03 (dt, J = 8.7, 2.3 Hz, 1H), 7.86–7.79 (m, 1H), 4.60 (s, 2H), 2.94–2.85 (m, 1H), 1.96–1.87 (m, 2H), 1.73–1.64 (m, 2H), 1.67–1.57 (m, 1H), 1.50–1.40 (m, 2H), 1.42–1.22 (m, 2H), 1.25–1.15 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 171.43, 162.35, 161.86 (d, J = 248.5 Hz), 149.00 (d, J = 9.7 Hz), 142.57 (d, J = 7.9 Hz), 123.27 (d, J = 22.4 Hz), 120.68 (d, J = 2.9 Hz), 110.89 (d, J = 26.8 Hz), 34.83, 34.58, 29.79, 25.53, 25.05. HRMS (ESI+) calcd for (C15H16FN3O3S + H+) m/z: 338.0975; found: 338.0982.
2-Alkyl/Aryl-5-((3-bromo-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 60a–60e
3-Bromo-5-nitrobenzyl chloride (38) was used as the alkylating agent. The reactions were stirred overnight.
2-((3-Bromo-5-nitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (60a)
Yield: 73% as a white solid; mp 100–101 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.43 (t, J = 1.8 Hz, 1H), 8.29 (t, J = 2.0 Hz, 1H), 8.22 (t, J = 1.7 Hz, 1H), 7.96–7.91 (m, 2H), 7.64–7.55 (m, 3H), 4.68 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.60, 163.01, 148.65, 142.10, 138.47, 132.25, 129.56, 126.58, 125.50, 123.32, 123.09, 121.95, 34.38. Elem. Anal. Calcd for C15H10BrN3O3S: C, 45.93; H, 2.57; N, 10.71; S, 8.17. Found: C, 45.95; H, 2.29; N, 10.73; S, 8.38.
2-((3-Bromo-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (60b)
Yield: 73% as a white solid; mp 116–118 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.41 (t, J = 1.8 Hz, 1H), 8.29 (t, J = 2.0 Hz, 1H), 8.20 (t, J = 1.7 Hz, 1H), 7.87 (d, J = 8.9 Hz, 2H), 7.11 (d, J = 8.9 Hz, 2H), 4.66 (s, 2H), 3.84 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.56, 162.26, 162.14, 148.63, 142.13, 138.44, 128.46, 125.46, 123.28, 121.93, 115.41, 115.00, 55.70, 34.39. Elem. Anal. Calcd for C16H12BrN3O4S: C, 45.51; H, 2.86; N, 9.95; S, 7.59. Found: C, 45.90; H, 3.03; N, 9.56; S, 7.20.
2-((3-Bromo-5-nitrobenzyl)sulfanyl)-5-(4-chlorophenyl)-1,3,4-oxadiazole (60c)
Yield: 70% as a white solid; mp 157–158 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.39 (t, J = 1.8 Hz, 1H), 8.25 (t, J = 2.0 Hz, 1H), 8.18 (t, J = 1.7 Hz, 1H), 7.91 (d, J = 8.6 Hz, 2H), 7.61 (d, J = 8.6 Hz, 2H), 4.65 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.24, 163.69, 149.03, 142.40, 138.85, 137.35, 130.11, 128.77, 125.89, 123.70, 122.37, 122.33, 34.75. Elem. Anal. Calcd for C15H9BrClN3O3S: C, 42.23; H, 2.13; N, 9.85; S, 7.51. Found: C, 41.84; H, 1.80; N, 9.78; S, 7.46.
2-((3-Bromo-5-nitrobenzyl)sulfanyl)-5-(4-bromophenyl)-1,3,4-oxadiazole (60d)
Yield: 68% as a yellowish solid; mp 152–153 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.39 (t, J = 1.8 Hz, 1H), 8.26 (t, J = 1.5 Hz, 1H), 8.18 (t, J = 1.7 Hz, 1H), 7.85–7.81 (m, 2H), 7.77–7.72 (m, 2H), 4.65 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.36, 163.71, 149.03, 142.40, 138.85, 133.03, 128.88, 126.24, 125.89, 123.70, 122.71, 122.34, 34.74. Elem. Anal. Calcd for C15H9Br2N3O3S: C, 38.24; H, 1.93; N, 8.92; S, 6.81. Found: C, 38.3; H, 1.61; N, 8.95; S, 6.95.
2-((3-Bromo-5-nitrobenzyl)sulfanyl)-5-cyclohexyl-1,3,4-oxadiazole (60e)
Yield: 75% as a white solid; mp 67–68 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.31 (t, J = 1.8 Hz, 1H), 8.26 (t, J = 2.0 Hz, 1H), 8.10 (t, J = 1.7 Hz, 1H), 4.54 (s, 2H), 2.87 (tt, J = 11.0, 3.7 Hz, 1H), 1.92–1.86 (m, 2H), 1.69–1.63 (m, 2H), 1.61–1.54 (m, 1H), 1.46–1.37 (m, 2H), 1.36–1.26 (m, 2H), 1.23–1.17 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.53, 162.40, 149.00, 142.54, 138.78, 125.83, 123.62, 122.31, 34.71, 34.69, 29.91, 25.61, 25.15. Elem. Anal. Calcd for C15H19BrN3O3S: C, 45.24; H, 4.05; N, 10.55; S, 8.05. Found: C, 45.09; H, 3.93; N, 10.50; S, 8.04.
2-Alkyl/Aryl-5-((3-cyano-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 61a–61e
3-Cyano-5-nitrobenzyl chloride (39) was used as the alkylating agent. The reactions were stirred overnight.
2-((3-Cyano-5-nitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (61a)
Yield: 79% as a yellowish solid; mp 125–126 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.69 (t, J = 2.0 Hz, 1H), 8.62 (t, J = 1.8 Hz, 1H), 8.41 (t, J = 1.6 Hz, 1H), 7.92–7.87 (m, 2H), 7.61–7.51 (m, 3H), 4.70 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.00, 163.29, 148.50, 142.06, 139.40, 132.63, 129.93, 128.99, 127.18, 126.95, 123.47, 117.36, 113.32, 34.63. Elem. Anal. Calcd for C16H10N4O3S: C, 56.80; H, 2.98; N, 16.56; S, 9.48. Found: C, 56.49; H, 2.80; N, 16.31; S, 9.46.
2-((3-Cyano-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (61b)
Yield: 80% as a white solid; mp 147–148 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.68 (t, J = 1.9 Hz, 1H), 8.63–8.62 (m, 1H), 8.40 (t, J = 1.6 Hz, 1H), 7.83 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 9.0 Hz, 2H), 4.68 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.97, 162.65, 162.44, 148.49, 142.09, 139.38, 128.96, 128.84, 127.16, 117.37, 115.80, 115.38, 113.30, 56.07, 34.65. Elem. Anal. Calcd for C17H12N4O4S: C, 55.43; H, 3.28; N, 15.21; S, 8.70. Found: C, 55.29; H, 3.10; N, 15.14; S, 8.79.
2-(4-Chlorophenyl)-5-((3-cyano-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (61c)
Yield: 77% as a white solid; mp 166–168 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.72 (t, J = 1.9 Hz, 1H), 8.66 (t, J = 1.8 Hz, 1H), 8.44 (t, J = 1.5 Hz, 1H), 7.94 (d, J = 8.6 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 4.73 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.88, 163.21, 148.11, 141.59, 139.03, 136.96, 129.72, 128.62, 128.39, 126.83, 122.01, 117.00, 112.94, 34.24. Elem. Anal. Calcd for C16H9ClN4O3S: C, 51.55; H, 2.43; N, 15.03; S, 8.60. Found: C, 51.65; H, 2.36; N, 14.87; S, 8.65.
2-(4-Bromophenyl)-5-((3-cyano-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (61d)
Yield: 71% as a yellow solid; mp 175–176 °C. 1H NMR (500 MHz, CDCl3) δ 8.63 (t, J = 2.0 Hz, 1H), 8.45 (t, J = 1.8 Hz, 1H), 8.19 (t, J = 1.6 Hz, 1H), 7.85 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.6 Hz, 2H), 4.61 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 165.77, 162.47, 148.37, 140.51, 138.00, 132.51, 128.06, 126.77, 126.53, 122.03, 116.19, 114.37, 34.72. Elem. Anal. Calcd for C16H9BrN4O3S: C, 46.06; H, 2.17; N, 13.43; S, 7.68. Found: C, 46.45; H, 2.21; N, 13.06; S, 7.29.
2-((3-Cyano-5-nitrobenzyl)sulfanyl)-5-cyclohexyl-1,3,4-oxadiazole (61e)
Yield: 91% as a white solid; mp 91–92 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.63–8.61 (m, 2H), 8.34 (t, J = 1.6 Hz, 1H), 4.59 (s, 2H), 2.86 (tt, J = 10.9, 3.7 Hz, 1H), 1.91–1.85 (m, 2H), 1.70–1.63 (m, 2H), 1.61–1.56 (m, 1H), 1.46–1.36 (m, 2H), 1.36–1.25 (m, 2H), 1.24–1.14 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.53, 162.33, 148.49, 142.14, 139.35, 128.94, 127.15, 117.32, 113.28, 34.68, 34.56, 29.90, 25.60, 25.14. Elem. Anal. Calcd for C16H16N4O3S: C, 55.80; H, 4.68; N, 16.27; S, 9.31. Found: C, 55.68; H, 4.59; N, 16.27; S, 9.61.
2-Alkyl/Aryl-5-((3-(methoxycarbonyl)-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 62a–62e
Methyl 3-(bromomethyl)-5-nitrobenzoate (40) was used as the alkylating agent. The reactions were stirred overnight.
2-((3-(Methoxycarbonyl)-5-nitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (62a)
Yield: 98% as a white solid; mp 106–107 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.68 (t, J = 2.0 Hz, 1H), 8.53 (t, J = 1.6 Hz, 1H), 8.51 (dd, J = 2.3, 1.5 Hz, 1H), 7.95–7.89 (m, 2H), 7.63–7.52 (m, 3H), 4.77 (s, 2H), 3.89 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.58, 164.46, 163.07, 148.09, 141.14, 135.90, 132.27, 131.41, 129.57, 128.36, 126.58, 123.08, 123.06, 53.08, 34.54. Elem. Anal. Calcd for C17H13N3O5S: C, 54.98; H, 3.53; H, 11.32; S, 8.63. Found: C, 55.08; H, 3.49; N, 11.23; S, 8.81.
2-((3-(Methoxycarbonyl)-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (62b)
Yield: 91% as a yellow solid; mp 107–108 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.66 (t, J = 2.0 Hz, 1H), 8.52–8.49 (m, 2H), 7.86 (d, J = 8.9 Hz, 2H), 7.09 (d, J = 8.9 Hz, 2H), 4.75 (s, 2H), 3.90 (s, 3H), 3.83 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.55, 164.46, 162.27, 162.21, 148.08, 141.17, 135.87, 131.40, 128.47, 128.33, 123.04, 115.42, 115.00, 55.72, 53.09, 34.56. Elem. Anal. Calcd for C18H15N3O6S: C, 53.86; H, 3.77; N, 10.47; S, 7.99. Found: C, 53.85; H, 3.52; N, 10.27; S, 7.98.
2-(4-Chlorophenyl)-5-((3-(methoxycarbonyl)-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (62c)
Yield: 86% as a white solid; mp 154–155 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.67 (t, J = 1.9 Hz, 1H), 8.52 (t, J = 1.6 Hz, 1H), 8.50 (dd, J = 2.2, 1.5 Hz, 1H), 7.93 (d, J = 8.6 Hz, 2H), 7.63 (d, J = 8.7 Hz, 2H), 4.77 (s, 2H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 164.84, 164.45, 163.37, 148.07, 141.04, 136.97, 135.90, 131.41, 129.72, 128.39, 128.36, 123.07, 121.98, 53.09, 34.52. Elem. Anal. Calcd for C17H12ClN3O5S: C, 50.32; H, 2.98; N, 10.35; S, 7.90. Found: C, 50.33; H, 2.99; N, 10.12; S, 7.91.
2-(4-Bromophenyl)-5-((3-(methoxycarbonyl)-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (62d)
Yield: 81% as a yellowish solid; mp 145–146 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.67 (t, J = 2.0 Hz, 1H), 8.53 (t, J = 1.6 Hz, 1H), 8.51 (dd, J = 2.3, 1.5 Hz, 1H), 7.86 (d, J = 8.6 Hz, 2H), 7.77 (d, J = 8.6 Hz, 2H), 4.77 (s, 2H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 164.96, 164.46, 163.39, 148.08, 141.04, 135.90, 132.64, 131.41, 128.50, 128.36, 125.87, 123.07, 122.31, 53.09, 34.52. Elem. Anal. Calcd for C17H12BrN3O5S: C, 45.35; H, 2.69; N, 9.33; S, 7.12. Found: C, 45.39; H, 2.52; N, 9.11; S, 7.31.
2-Cyclohexyl-5-((3-(methoxycarbonyl)-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (62e)
Yield: 97% as a yellowish oil, which crystallized over time; mp 51–53 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.60 (t, J = 2.0 Hz, 1H), 8.51 (dd, J = 2.3, 1.5 Hz, 1H), 8.46 (t, J = 1.6 Hz, 1H), 4.67 (s, 2H), 3.92 (s, 3H), 2.90–2.85 (m, 1H), 1.93–1.88 (m, 2H), 1.70–1.58 (m, 3H), 1.47–1.18 (m, 5H). 13C NMR (126 MHz, DMSO-d6) δ 171.12, 164.46, 162.10, 148.06, 141.23, 135.84, 131.41, 128.30, 123.01, 53.11, 34.49, 34.32, 29.53, 25.25, 24.80. HRMS (ESI+) calcd for (C17H19N3O5S + H+) m/z: 378.11182 (100%); found: 378.1123 (100%).
2-Alkyl/aryl-5-((3-(carbamoyl)-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 63a–63e
3-(Bromomethyl)-5-nitrobenzamide (41) was used as the alkylating agent. The reactions were completed in 30 min. The final products 63a–63d had low solubility. Therefore, upon reaction completion, the solvent was evaporated, and the residue was washed with 5% Na2CO3 (2 × 15 mL), water (2 × 20 mL), and EtOAc (7 mL) to give the final product. Compound 63e was purified using column chromatography (mobile phase: hexane/EtOAc, 4:1).
2-((3-(Carbamoyl)-5-nitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (63a)
Yield: 94% as a white solid; mp 192–193 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.59 (t, J = 1.9 Hz, 1H), 8.53 (t, J = 1.9 Hz, 1H), 8.44 (t, J = 1.6 Hz, 1H), 8.32 (s, 1H, NH-H), 7.91–7.86 (m, 2H), 7.68 (s, 1H, NH-H), 7.61–7.50 (m, 3H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.03, 165.94, 163.47, 148.30, 140.58, 136.50, 135.29, 132.60, 129.93, 126.96, 126.87, 123.45, 121.88, 35.22. Elem. Anal. Calcd for C16H12N4O4S: C, 53.93; H, 3.39; N, 15.72; S, 9.0. Found: C, 53.98; H, 3.45; N, 15.76; S, 9.36.
2-((3-(Carbamoyl)-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (63b)
Yield: 80% as a white solid; mp 157–158 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.59 (t, J = 1.9 Hz, 1H), 8.51 (t, J = 1.9 Hz, 1H), 8.43 (t, J = 1.6 Hz, 1H), 8.32 (s, 1H, NH-H), 7.83 (d, J = 8.9 Hz, 2H), 7.68 (s, 1H, NH-H), 7.06 (d, J = 8.9 Hz, 2H), 4.69 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 166.01, 165.90, 162.62, 148.30, 140.61, 136.50, 135.28, 128.85, 126.84, 121.85, 115.80, 115.37, 56.06, 35.24. Elem. Anal. Calcd for C17H14N4O5S: C, 52.85; H, 3.65; N, 14.50; S, 8.30. Found: C, 53.24; H, 3.54; N, 14.56; S, 8.48.
2-((3-(Carbamoyl)-5-nitrobenzyl)sulfanyl)-5-(4-chlorophenyl)-1,3,4-oxadiazole (63c)
Yield: 73% as a white solid; mp 217–218 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.59 (t, J = 1.9 Hz, 1H), 8.53 (t, J = 1.9 Hz, 1H), 8.43 (t, J = 1.6 Hz, 1H), 8.32 (s, 1H, NH-H), 7.91 (d, J = 8.6 Hz, 2H), 7.68 (s, 1H, NH-H), 7.60 (d, J = 8.6 Hz, 2H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.03, 165.19, 163.77, 148.32, 140.53, 137.31, 136.48, 135.30, 130.09, 128.79, 126.89, 122.36, 121.88, 35.20. Elem. Anal. Calcd for C16H11ClN4O4S: C, 49.18; H, 2.84; N, 14.34; S, 8.2. Found: C, 48.79; H, 2.63; N, 14.24; S, 8.0.
2-(4-Bromophenyl)-5-((3-(carbamoyl)-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazole (63d)
Yield: 77% as a white solid; mp 222–223 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.59 (t, J = 1.9 Hz, 1H), 8.53 (t, J = 2.0 Hz, 1H), 8.43 (t, J = 1.6 Hz, 1H), 8.32 (s, 1H, NH-H), 7.83 (d, J = 8.6 Hz, 2H), 7.74 (d, J = 8.6 Hz, 2H), 7.69 (s, 1H, NH-H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.02, 165.30, 163.79, 148.33, 140.53, 136.49, 135.31, 133.01, 128.90, 126.89, 126.20, 122.70, 121.88, 35.20. Elem. Anal. Calcd for C16H11BrN4O4S: C, 44.15; H, 2.55; N, 12.87; S, 7.37. Found: C, 43.77; H, 2.57; N, 12.74; S, 7.18.
2-((3-(Carbamoyl)-5-nitrobenzyl)sulfanyl)-5-cyclohexyl-1,3,4-oxadiazole (63e)
Yield: 93% as a white solid; mp 119–120 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.58 (t, J = 2.0 Hz, 1H), 8.44 (t, J = 1.9 Hz, 1H), 8.35 (t, J = 1.9 Hz, 1H), 8.30 (s, 1H, NH-H), 7.68 (s, 1H, NH-H), 4.60 (s, 2H), 2.85 (tt, J = 11.0, 3.7 Hz, 1H), 1.93–1.82 (m, 2H), 1.67–1.62 (m, 2H), 1.60–1.54 (m, 1H), 1.45–1.35 (m, 2H), 1.34–1.24 (m, 2H), 1.21–1.13 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.50, 165.96, 162.43, 148.26, 140.62, 136.49, 135.24, 126.79, 121.80, 35.20, 34.68, 29.87, 25.59, 25.14. Elem. Anal. Calcd for C16H18N4O4S: C, 53.03; H, 5.01; N, 15.46; S, 8.85. Found: C, 52.99; H, 5.14; N, 15.09; S, 8.59.
2-Alkyl/Aryl-5-((3-(N-benzylcarbamoyl)-5-nitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 64a–64e
N-Benzyl-3-(bromomethyl)-5-nitrobenzamide (42) was used as the alkylating agent. The reactions were completed in 1 h. The final products 64a–64e had low solubility. Therefore, upon reaction completion, the solvent was evaporated, and the residue was washed with 5% Na2CO3 (2 × 15 mL), water (2 × 20 mL, and EtOAc (7 mL) to give the final product.
2-((3-(N-Benzylcarbamoyl)-5-nitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (64a)
Yield: 81% as a white solid; mp 162–163 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.41 (t, J = 5.9 Hz, 1H), 8.64 (t, J = 1.9 Hz, 1H), 8.55 (t, J = 1.9 Hz, 1H), 8.47 (t, J = 1.6 Hz, 1H), 7.93–7.86 (m, 2H), 7.60–7.53 (m, 1H), 7.54–7.47 (m, 2H), 7.32–7.25 (m, 4H), 7.25–7.18 (m, 1H), 4.73 (s, 2H), 4.47 (d, J = 5.8 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.94, 164.36, 163.48, 148.31, 140.71, 139.62, 136.38, 135.20, 132.60, 129.93, 128.88, 127.96, 127.46, 126.96, 126.90, 123.46, 121.66, 43.48, 35.21. Elem. Anal. Calcd for C23H18N4O4S: C, 61.87; H, 4.06; N, 12.55; S, 7.18. Found: C, 62.09; H, 4.05; N, 12.69; S, 7.51.
2-((3-(N-Benzylcarbamoyl)-5-nitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (64b)
Yield: 60% as a white solid; mp 169–170 °C. 1H NMR (500 MHz, DMSO-d6) δ 9.44 (t, J = 5.9 Hz, 1H), 8.67 (t, J = 1.7 Hz, 1H), 8.56 (t, J = 2.3 Hz, 1H), 8.49 (t, J = 2.0 Hz, 1H), 7.87 (d, J = 8.9 Hz, 2H), 7.34–7.30 (m, 4H), 7.28–7.21 (m, 1H), 7.09 (d, J = 8.8 Hz, 2H), 4.74 (s, 2H), 4.50 (d, J = 5.8 Hz, 2H), 3.83 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.53, 163.98, 162.24, 147.91, 140.35, 139.24, 135.99, 134.80, 128.50, 128.47, 127.55, 127.07, 126.49, 121.26, 115.43, 115.00, 55.69, 43.09, 34.86. Elem. Anal. Calcd for C24H20N4O5S: C, 60.50; H, 4.23; N, 11.76; S, 6.73. Found: C, 60.12; H, 4.22; N, 11.44; S, 6.85.
2-((3-(N-Benzylcarbamoyl)-5-nitrobenzyl)sulfanyl)-5-(4-chlorophenyl)-1,3,4-oxadiazole (64c)
Yield: 81% as a white solid; mp 165–166 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.40 (t, J = 5.9 Hz, 1H), 8.64 (t, J = 1.9 Hz, 1H), 8.54 (t, J = 1.9 Hz, 1H), 8.47 (t, J = 1.6 Hz, 1H), 7.90 (d, J = 8.6 Hz, 2H), 7.58 (d, J = 8.6 Hz, 2H), 7.31–7.24 (m, 4H), 7.27–7.16 (m, 1H), 4.73 (s, 2H), 4.47 (d, J = 5.8 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.20, 164.36, 163.77, 148.31, 140.64, 139.62, 137.31, 136.36, 135.21, 130.08, 128.88, 128.77, 127.94, 127.46, 126.90, 122.36, 121.66, 43.48, 35.20. Elem. Anal. Calcd for C23H17ClN4O4S: C, 57.44; H, 3.56; N, 11.65; S, 6.67. Found: C, 57.09; H, 3.49; N, 11.64; S, 6.86.
2-((3-(N-Benzylcarbamoyl)-5-nitrobenzyl)sulfanyl)-5-(4-bromophenyl)-1,3,4-oxadiazole (64d)
Yield: 83% as a white solid; mp 185–186 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.40 (t, J = 5.9 Hz, 1H), 8.64 (t, J = 1.9 Hz, 1H), 8.54 (t, J = 1.9 Hz, 1H), 8.46 (t, J = 1.6 Hz, 1H), 7.83 (d, J = 8.6 Hz, 2H), 7.72 (d, J = 8.6 Hz, 2H), 7.31–7.25 (m, 4H), 7.25–7.18 (m, 1H), 4.73 (s, 2H), 4.47 (d, J = 5.9 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.31, 164.36, 163.79, 148.31, 140.64, 139.61, 136.36, 135.21, 133.00, 128.88, 127.94, 127.47, 126.91, 126.20, 122.70, 121.66, 43.48, 35.20. Elem. Anal. Calcd for C23H17BrN4O4S: C, 52.58; H, 3.26; N, 10.66; S, 6.10. Found: C, 52.21; H, 3.20; N, 10.57; S, 6.46.
2-((3-(N-Benzylcarbamoyl)-5-nitrobenzyl)sulfanyl)-5-cyclohexyl-1,3,4-oxadiazole (64e)
Yield: 85% as a yellowish solid; mp 100–101 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.40 (t, J = 5.9 Hz, 1H), 8.63 (t, J = 1.9 Hz, 1H), 8.46 (t, J = 2.0 Hz, 1H), 8.39 (t, J = 1.7 Hz, 1H), 7.31–7.29 (m, 4H), 7.25–7.18 (m, 1H), 4.61 (s, 2H), 4.47 (d, J = 5.8 Hz, 2H), 2.84 (tt, J = 10.9, 3.7 Hz, 1H), 1.90–1.83 (m, 2H), 1.68–1.51 (m, 3H), 1.44–1.35 (m, 2H), 1.34–1.21 (m, 2H), 1.21–1.13 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.49, 164.31, 162.44, 148.26, 140.73, 139.63, 136.37, 135.13, 128.87, 127.96, 127.46, 126.81, 121.59, 43.47, 35.19, 34.67, 29.87, 25.59, 25.13. Elem. Anal. Calcd for C23H24N4O4S: C, 61.05; H, 5.35; N, 12.38; S, 7.08. Found: C, 60.80; H, 5.30; N, 12.40; S, 7.46.
2-Alkyl/Aryl-5-((3-nitro-5-(1H-pyrrol-1-yl)benzyl)sulfanyl)-1,3,4-oxadiazoles 65a–65e
3-Nitro-5-(1H-pyrrol-1-yl)benzyl chloride (43) was used as the alkylating agent. The reactions were stirred overnight.
2-((3-Nitro-5-(1H-pyrrol-1-yl)benzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (65a)
Yield: 61% as a yellow solid; mp 110–111 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.25–8.23 (m, 1H), 8.23–8.21 (m, 2H), 7.91–7.86 (m, 2H), 7.60–7.54 (m, 1H), 7.5–7.49 (m, 2H), 7.48 (t, J = 2.2 Hz, 2H), 6.30 (t, J = 2.2 Hz, 2H), 4.69 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.99, 163.48, 149.38, 141.66, 141.06, 132.61, 129.90, 126.94, 126.49, 123.47, 120.58, 119.85, 113.38, 112.17, 35.37. Elem. Anal. Calcd for C19H14N4O3S: C, 60.31; H, 3.73; N, 14.81; S, 8.47. Found: C, 60.06; H, 3.59; N, 14.82; S, 8.74.
2-(4-Methoxyphenyl)-5-((3-nitro-5-(1H-pyrrol-1-yl)benzyl)sulfanyl)-1,3,4-oxadiazole (65b)
Yield: 65% as a yellow solid; mp 124–125 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.24 (t, J = 2.2 Hz, 1H), 8.22–8.19 (m, 2H), 7.82 (d, J = 8.9 Hz, 2H), 7.48 (t, J = 2.2 Hz, 2H), 7.03 (d, J = 8.9 Hz, 2H), 6.30 (t, J = 2.2 Hz, 2H), 4.67 (s, 2H), 3.79 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.94, 162.62, 149.38, 141.71, 141.04, 128.82, 126.49, 120.55, 119.85, 115.80, 115.35, 113.35, 112.17, 56.06, 35.40. Elem. Anal. Calcd for C20H16N4O4S: C, 58.82; H, 3.95; N, 13.72; S, 7.85. Found: C, 58.66; H, 4.06; N, 13.36; S, 7.73.
2-(4-Chlorophenyl)-5-((3-nitro-5-(1H-pyrrol-1-yl)benzyl)sulfanyl)-1,3,4-oxadiazole (65c)
Yield: 58% as a brownish solid; mp 155–157 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.24 (t, J = 2.1 Hz, 1H), 8.23–8.19 (m, 2H), 7.90 (d, J = 8.4 Hz, 2H), 7.58 (d, J = 8.2 Hz, 2H), 7.48 (t, J = 2.2 Hz, 2H), 6.30 (s, 2H), 4.69 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.25, 163.77, 149.38, 141.59, 141.05, 137.32, 130.07, 128.76, 126.50, 122.37, 120.58, 119.86, 113.39, 112.17, 35.37. Elem. Anal. Calcd for C19H13ClN4O3S: C, 55.28; H, 3.17; N, 13.57; S, 7.77. Found: C, 55.03; H, 3.14; N, 13.23; S, 7.55.
2-(4-Bromophenyl)-5-((3-nitro-5-(1H-pyrrol-1-yl)benzyl)sulfanyl)-1,3,4-oxadiazole (65d)
Yield: 61% as a yellow solid; 156–158 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.24 (t, J = 2.1 Hz, 1H), 8.26–8.23 (m, 2H), 7.85 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 8.4 Hz, 2H), 7.51 (t, J = 2.2 Hz, 2H), 6.33 (t, J = 2.2 Hz, 2H), 4.72 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 164.96, 163.41, 148.99, 141.19, 140.66, 132.60, 128.47, 126.11, 125.82, 122.32, 120.19, 119.47, 113.00, 111.78, 34.97. Elem. Anal. Calcd for C19H13BrN4O3S: C, 49.90; H, 2.87; N, 12.25; S, 7.01. Found: C, 50.06; H, 2.66; N, 12.15; S, 7.11.
2-Cyclohexyl-5-((3-nitro-5-(1H-pyrrol-1-yl)benzyl)sulfanyl)-1,3,4-oxadiazole (65e)
Yield: 53% as a yellow solid; mp 82–84 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.24 (t, J = 2.1 Hz, 1H), 8.15–8.11 (m, 2H), 7.48 (t, J = 2.2 Hz, 2H), 6.30 (t, J = 2.3 Hz, 2H), 4.58 (s, 2H), 2.84 (tt, J = 10.9, 3.7 Hz, 1H), 1.89–1.81 (m, 2H), 1.65–1.53 (m, 3H), 1.41–1.34 (m, 2H), 1.30–1.22 (m, 2H), 1.18–1.09 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.56, 162.45, 149.34, 141.70, 141.05, 126.44, 120.48, 119.84, 113.31, 112.16, 35.37, 34.68, 29.86, 25.58, 25.11. Elem. Anal. Calcd for C19H20N4O3S: C, 59.36; H, 5.24; N, 14.57; S, 8.34. Found: C, 58.99; H, 5.48; N, 14.18; S, 8.10.
1-Alkyl/Aryl-5-((3,4-dinitrobenzyl)sulfanyl)-1H-tetrazoles 66a–66e
3,4-Dinitrobenzyl bromide (44) was used as the alkylating agent. The reactions were completed in 1 h.
5-((3,4-Dinitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (66a)
Yield: 72% as a yellow solid; mp 125–126 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.30 (d, J = 1.7 Hz, 1H), 8.16 (d, J = 8.3 Hz, 1H), 8.01 (dd, J = 8.4, 1.8 Hz, 1H), 7.67–7.53 (m, 5H), 4.72 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.00, 145.48, 142.46, 141.44, 135.36, 133.43, 131.27, 130.54, 126.49, 126.36, 125.14, 35.43. Elem. Anal. Calcd for C14H10N6O4S: C, 46.93; H, 2.81; N, 23.45; S, 8.95. Found: C, 46.90; H, 2.69; N, 23.17; S, 9.14.
5-((3,4-Dinitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (66b)
Yield: 78% as a yellow solid; mp 117–118 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.28 (d, J = 2.0 Hz, 1H), 8.16 (d, J = 8.3 Hz, 1H), 8.00 (dd, J = 8.3, 2.0 Hz, 1H), 7.49 (d, J = 9.0 Hz, 2H), 7.12 (d, J = 9.0 Hz, 2H), 4.68 (s, 2H), 3.81 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.22, 154.11, 145.56, 142.46, 141.42, 135.32, 126.95, 126.45, 126.36, 126.00, 115.54, 56.24, 35.38. Elem. Anal. Calcd for C15H12N6O5S: C, 46.39; H, 3.11; N, 21.64; S, 8.26. Found: C, 46.03; H, 2.98; N, 21.36; S, 8.29.
1-(4-Chlorophenyl)-5-((3,4-dinitrobenzyl)sulfanyl)-1H-tetrazole (66c)
Yield: 83% as a yellow solid; mp 157–158 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.29 (d, J = 1.9 Hz, 1H), 8.16 (d, J = 8.3 Hz, 1H), 8.00 (dd, J = 8.4, 1.9 Hz, 1H), 7.69 (d, J = 8.8 Hz, 2H), 7.64 (d, J = 8.8 Hz, 2H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.12, 145.44, 142.46, 141.44, 135.90, 135.36, 132.26, 130.57, 127.09, 126.48, 126.35, 35.55. Elem. Anal. Calcd for C14H9ClN6O4S: C, 42.81; H, 2.31; N, 21.40; S, 8.16. Found: C, 43.18; H, 2.19; N, 21.27; S, 8.29.
1-(4-Bromophenyl)-5-((3,4-dinitrobenzyl)sulfanyl)-1H-tetrazole (66d)
Yield: 88% as a yellow solid; mp 146–147 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.29 (d, J = 1.9 Hz, 1H), 8.16 (d, J = 8.4 Hz, 1H), 8.00 (dd, J = 8.4, 1.8 Hz, 1H), 7.82 (d, J = 8.7 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.07, 145.43, 142.46, 141.44, 135.36, 133.52, 132.68, 127.22, 126.48, 126.35, 124.47, 35.56. Elem. Anal. Calcd for C14H9BrN6O4S: C, 38.46; H, 2.07; N, 19.22; S, 7.33. Found: 38.82; H, 1.91; N, 19.33; S, 7.38.
1-Cyclohexyl-5-((3,4-dinitrobenzyl)sulfanyl)-1H-tetrazole (66e)
Yield: 63% as a yellow solid; mp 117–119 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.29 (d, J = 1.9 Hz, 1H), 8.17 (d, J = 8.3 Hz, 1H), 7.97 (dd, J = 8.3, 1.9 Hz, 1H), 4.69 (s, 2H), 4.21 (tt, J = 11.6, 3.9 Hz, 1H), 1.89–1.80 (m, 2H), 1.80–1.72 (m, 2H), 1.72–1.64 (m, 2H), 1.63–1.58 (m, 1H), 1.41–1.29 (m, 2H), 1.24–1.13 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 151.99, 145.73, 142.51, 141.39, 135.26, 126.43, 58.04, 35.49, 32.16, 24.96, 24.90. Elem. Anal. Calcd for C14H16N6O4S: C, 46.15; H, 4.43; N, 23.06; S, 8.80. Found: C, 46.53; H, 4.35; N, 23.35; S, 9.16.
1-Alkyl/Aryl-5-((2,5-dinitrobenzyl)sulfanyl)-1H-tetrazoles 67a–67e
2,5-Dinitrobenzyl bromide (45) was used as the alkylating agent. The reactions were completed in 1 h.
5-((2,5-Dinitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (67a)
Yield: 75% as a yellow solid; mp 120–121 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.68 (d, J = 2.5 Hz, 1H), 8.34 (dd, J = 8.9, 2.6 Hz, 1H), 8.27 (d, J = 9.0 Hz, 1H), 7.67–7.56 (m, 5H), 4.93 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 153.97, 151.84, 149.76, 134.49, 133.32, 131.17, 130.46, 128.08, 127.28, 125.05, 124.86, 33.69. Elem. Anal. Calcd for C14H10N6O4S: C, 46.93; H, 2.81; N, 23.45; S, 8.95. Found: C, 46.70; H, 2.7; N, 23.34; S, 9.25.
5-(2,5-Dinitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (67b)
Yield: 85% as a yellow solid; mp 124–125 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.67 (d, J = 2.5 Hz, 1H), 8.34 (dd, J = 8.9, 2.6 Hz, 1H), 8.27 (d, J = 8.9 Hz, 1H), 7.50 (d, J = 9.0 Hz, 2H), 7.13 (d, J = 9.0 Hz, 2H), 4.90 (s, 2H), 3.83 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 161.11, 154.09, 151.81, 149.75, 134.56, 128.03, 127.28, 126.85, 125.88, 124.84, 115.47, 56.15, 33.62. Elem. Anal. Calcd for C15H12N6O5S: C, 46.39; H, 3.11; N, 21.64; S, 8.26. Found: C, 46.43; H, 3.03; N, 21.60; S, 8.35.
1-(4-Chlorophenyl)-5-((2,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (67c)
Yield: 75% as a yellow solid; mp 144–145 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.66 (d, J = 2.5 Hz, 1H), 8.35 (dd, J = 8.9, 2.5 Hz, 1H), 8.27 (d, J = 9.0 Hz, 1H), 7.71 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.7 Hz, 2H), 4.91 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 153.77, 151.52, 149.45, 135.50, 134.17, 131.85, 130.19, 127.78, 126.99, 126.71, 124.56, 33.55. Elem. Anal. Calcd for C14H9ClN6O4S: C, 42.81; H, 2.31; N, 21.40; S, 8.16. Found: C, 42.88; H, 2.11; N, 21.46; S, 8.25.
1-(4-Bromophenyl)-5-((2,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (67d)
Yield: 80% as a yellow solid; mp 150–151 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.63 (d, J = 2.5 Hz, 1H), 8.31 (dd, J = 8.7, 2.5 Hz, 1H), 8.23 (d, J = 8.7 Hz, 1H), 7.80 (d, J = 8.7 Hz, 2H), 7.54 (d, J = 8.7 Hz, 2H), 4.87 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.10, 151.90, 149.83, 134.55, 133.52, 132.66, 128.17, 127.37, 127.24, 124.94, 124.46, 33.93. Elem. Anal. Calcd for C14H9BrN6O4S: C, 38.46; H, 2.07; N, 19.22; S, 7.33. Found: C, 38.33; H, 1.91; N, 19.18; S, 7.19.
1-Cyclohexyl-5-((2,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (67e)
Yield: 65% as yellowish solid; mp 98–99 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.61 (d, J = 2.5 Hz, 1H), 8.32 (dd, J = 8.9, 2.5 Hz, 1H), 8.26 (d, J = 8.9 Hz, 1H), 4.86 (s, 2H), 4.22 (tt, J = 11.5, 3.9 Hz, 1H), 1.88–1.86 (m, 2H), 1.77–1.74 (m, 2H), 1.72–1.66 (m, 2H), 1.62–1.59 (m, 1H), 1.39–1.31 (m, 2H), 1.22–1.18 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 152.11, 151.93, 149.80, 134.73, 128.05, 127.42, 124.97, 58.03, 33.77, 32.19, 24.97, 24.89. Elem. Anal. Calcd for C14H16N6O4S: C, 46.15; H, 4.43; N, 23.06; S, 8.80. Found: C, 46.33; H, 4.40; N, 23.07; S, 8.90.
2-Alkyl/Aryl-5-((3,4-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 68a–68e
3,4-Dinitrobenzyl bromide (44) was used as the alkylating agent. The reactions were completed in 1 h.
2-((3,4-Dinitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (68a)
Yield: 71% as a yellow solid; mp 84–85 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.35 (d, J = 1.7 Hz, 1H), 8.20 (d, J = 8.3 Hz, 1H), 8.05 (dd, J = 8.3, 1.9 Hz, 1H), 7.92–7.87 (m, 2H), 7.61–7.51 (m, 3H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.05, 163.19, 145.85, 142.51, 141.45, 135.31, 132.64, 129.95, 126.97, 126.50, 126.46, 123.47, 34.78. Elem. Anal. Calcd for C15H10N4O5S: C, 50.28; H, 2.81; N, 15.64; S, 8.95. Found: C, 50.65; H, 2.66; N, 15.69; S, 9.24.
2-((3,4-Dinitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (68b)
Yield: 62% as a yellow solid; mp 109–110 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.34 (d, J = 1.7 Hz, 1H), 8.19 (d, J = 8.3 Hz, 1H), 8.04 (dd, J = 8.4, 1.8 Hz, 1H), 7.83 (d, J = 8.9 Hz, 2H), 7.08 (d, J = 9.0 Hz, 2H), 4.69 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 166.01, 162.66, 162.34, 145.90, 142.51, 141.42, 135.28, 128.86, 126.49, 126.43, 115.80, 115.39, 56.08, 34.80. Elem. Anal. Calcd for C16H12N4O6S: C, 49.48; H, 3.11; N, 14.43; S, 8.26. Found: C, 49.87; H, 3.10; N, 14.45; S, 8.36.
2-(4-Chlorophenyl)-5-((3,4-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (68c)
Yield: 61% as a white solid; mp 115–116 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.35 (d, J = 1.9 Hz, 1H), 8.19 (d, J = 8.3 Hz, 1H), 8.05 (dd, J = 8.4, 1.8 Hz, 1H), 7.91 (d, J = 8.7 Hz, 2H), 7.61 (d, J = 8.6 Hz, 2H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.30, 163.49, 145.77, 142.50, 141.45, 137.35, 135.32, 130.11, 128.78, 126.49, 126.46, 122.38, 34.75. Elem. Anal. Calcd for C15H9ClN4O5S: C, 45.87; H, 2.31; N, 14.26; S, 8.16. Found: C, 45.91; H, 2.13; N, 14.23; S, 8.23.
2-(4-Bromophenyl)-5-((3,4-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (68d)
Yield: 72% as a yellow solid; mp 145–146 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.34 (d, J = 1.9 Hz, 1H), 8.19 (d, J = 8.3 Hz, 1H), 8.05 (dd, J = 8.4, 1.9 Hz, 1H), 7.83 (d, J = 8.6 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.41, 163.51, 145.76, 142.50, 141.45, 135.32, 133.03, 128.89, 126.49, 126.46, 126.24, 122.71, 34.75. Elem. Anal. Calcd for C15H9BrN4O5S: C, 41.21; H, 2.07; N, 12.81; S, 7.33. Found: C, 41.44; H, 1.89; N, 12.74; S, 7.34.
2-Cyclohexyl-5-((3,4-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (68e)
Yield: 66% as a yellowish solid; mp 114–115 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.28 (d, J = 1.9 Hz, 1H), 8.18 (d, J = 8.3 Hz, 1H), 7.99 (dd, J = 8.3, 1.9 Hz, 1H), 4.60 (s, 2H), 2.86 (tt, J = 10.9, 3.7 Hz, 1H), 1.91–1.85 (m, 2H), 1.68–1.62 (m, 2H), 1.61–1.54 (m, 1H), 1.45–1.35 (m, 2H), 1.34–1.25 (m, 2H), 1.22–1.14 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.58, 162.23, 145.90, 142.45, 141.42, 135.29, 126.47, 126.39, 34.72, 34.66, 29.86, 25.60, 25.12. Elem. Anal. Calcd for C15H16N4O5S: C, 49.44; H, 4.43; N, 15.38; S, 8.80. Found: C, 49.80; H, 4.35; N, 15.42; S, 9.12.
2-Alkyl/Aryl-5-((2,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 69a–69e
2,5-Dinitrobenzyl bromide (45) was used as the alkylating agent. The reactions were completed in 1 h.
2-((2,5-Dinitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (69a)
Yield: 80% as a yellow solid; mp 141–142 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.69 (d, J = 2.5 Hz, 1H), 8.34 (dd, J = 8.9, 2.5 Hz, 1H), 8.28 (d, J = 8.9 Hz, 1H), 7.94–7.87 (m, 2H), 7.62–7.57 (m, 1H), 7.56–7.51 (m, 2H), 4.88 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.18, 163.15, 151.78, 149.89, 134.89, 132.67, 129.92, 128.10, 127.54, 127.01, 125.06, 123.47, 33.27. Elem. Anal. Calcd for C15H10N4O5S: C, 50.28; H, 2.81; N, 15.64; S, 8.95. Found: C, 49.90; H, 2.58; N, 15.61; S, 9.04.
2-((2,5-Dinitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (69b)
Yield: 68% as a yellow solid; mp 125–126 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.67 (d, J = 2.6 Hz, 1H), 8.34 (dd, J = 8.9, 2.5 Hz, 1H), 8.27 (d, J = 8.9 Hz, 1H), 7.83 (d, J = 8.9 Hz, 2H), 7.07 (d, J = 8.9 Hz, 2H), 4.86 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 166.15, 162.68, 162.30, 151.78, 149.87, 134.93, 128.89, 128.06, 127.51, 125.03, 115.79, 115.37, 56.07, 33.26. Elem. Anal. Calcd for C16H12N4O6S: C, 49.48; H, 3.11; N, 14.43; S, 8.26. Found: C, 49.09; H, 3.02; N, 14.31; S, 8.27.
2-(4-Chlorophenyl)-5-((2,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (69c)
Yield: 71% as a yellow solid; mp 121–122 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.71 (d, J = 2.5 Hz, 1H), 8.37 (dd, J = 8.9, 2.5 Hz, 1H), 8.31 (d, J = 8.9 Hz, 1H), 7.88 (d, J = 8.6 Hz, 2H), 7.79 (d, J = 8.6 Hz, 2H), 4.92 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.17, 163.10, 151.40, 149.52, 134.44, 132.65, 128.57, 127.73, 127.17, 125.92, 124.71, 122.33, 32.86. Elem. Anal. Calcd for C15H9ClN4O5S: C, 45.87; H, 2.31; N, 14.26; S, 8.16. Found: C, 45.77; H, 2.68; N, 14.32; S, 8.23.
2-(4-Bromophenyl)-5-((2,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (69d)
Yield: 88% as a yellow solid; mp 148–149 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.68 (d, J = 2.5 Hz, 1H), 8.34 (dd, J = 8.8, 2.5 Hz, 1H), 8.28 (d, J = 9.0 Hz, 1H), 7.84 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 8.5 Hz, 2H), 4.88 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.54, 163.48, 151.77, 149.89, 134.80, 133.01, 128.92, 128.10, 127.53, 126.29, 125.07, 122.70, 33.24. Elem. Anal. Calcd for C15H9BrN4O5S: C, 41.21; H, 2.07; N, 12.81; S, 7.33. Found: C, 40.88; H, 1.88; N, 12.78; S, 7.34.
2-Cyclohexyl-5-((2,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (69e)
Yield: 67% as a yellow solid; mp 100–101 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.59 (d, J = 2.6 Hz, 1H), 8.34 (dd, J = 8.9, 2.5 Hz, 1H), 8.27 (d, J = 9.0 Hz, 1H), 4.78 (s, 2H), 2.85 (tt, J = 11.0, 3.7 Hz, 1H), 1.92–1.85 (m, 2H), 1.70–1.53 (m, 3H), 1.45–1.36 (m, 2H), 1.35–1.25 (m, 2H), 1.23–1.16 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.71, 162.23, 151.79, 149.82, 134.92, 128.03, 127.52, 125.01, 34.70, 33.11, 29.87, 25.61, 25.15. Elem. Anal. Calcd for C15H16N4O5S: C, 49.44; H, 4.43; N, 15.38; S, 8.80. Found: C, 49.07; H, 4.36; N, 15.42; S, 9.05.
1-Alkyl/Aryl-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazoles 70a–70e
2-Nitro-5-(trifluoromethyl)benzyl bromide (46) was used as the alkylating agent. The reactions were completed in 1 h.
5-((2-Nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1-phenyl-1H-tetrazole (70a)
Yield: 90% as a beige solid; mp 100–101 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.12 (d, J = 1.9 Hz, 1H), 8.08 (d, J = 8.3 Hz, 1H), 7.99 (dd, J = 8.2, 1.9 Hz, 1H), 7.64–7.55 (m, 5H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.05, 146.90, 144.09, 135.38, 133.43, 131.23, 130.50, 129.32 (d, J = 5.1 Hz), 126.27, 125.12, 122.54 (d, J = 273.1 Hz), 121.90 (q, J = 33.6 Hz), 35.63. HRMS (ESI+) calcd for (C15H10F3N5O2S + H+) m/z: 382.05801 (100%), 383.06136 (16.2%); found: 382.0584 (100%), 383.0611 (17%).
1-(4-Methoxyphenyl)-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (70b)
Yield: 69% as a white solid; mp 100–101 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.11 (d, J = 1.8 Hz, 1H), 8.08 (d, J = 8.6 Hz, 1H), 7.97 (dd, J = 8.4, 1.9 Hz, 1H), 7.47 (d, J = 9.0 Hz, 2H), 7.11 (d, J = 9.0 Hz, 2H), 4.68 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.19, 154.15, 146.88, 144.17, 135.33, 129.29, 126.91, 126.26, 126.01, 122.55 (d, J = 273.1 Hz), 121.89 (d, J = 33.2 Hz), 115.51 (d, J = 15.9 Hz), 56.21, 35.59. HRMS (ESI+) calcd for (C16H12F3N5O3S + H+) m/z: 412.06857 (100%), 413.07193 (17.3%); found: 412.0688 (100%), 413.0716 (8%).
1-(4-Chlorophenyl)-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (70c)
Yield: 83% as a white solid; mp 127–128 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.11 (d, J = 1.9 Hz, 1H), 8.08 (d, J = 8.3 Hz, 1H), 7.97 (dd, J = 8.4, 1.9 Hz, 1H), 7.68 (d, J = 8.8 Hz, 2H), 7.63 (d, J = 8.8 Hz, 2H), 4.70 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 154.17, 146.90, 144.05, 135.87, 135.38, 132.26, 130.54, 129.33 (d, J = 5.4 Hz), 127.06, 126.26, 122.54 (d, J = 273.1 Hz), 121.89 (d, J = 33.6 Hz), 35.77. HRMS (ESI+) calcd for (C15H9ClF3N5O2S + H+) m/z: 416.01903 (100%), 418.01609 (32%); found: 416.0193 (100%), 418.0167 (35%).
1-(4-Bromophenyl)-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (70d)
Yield: 88% as a white solid; mp 118–119 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.15 (d, J = 1.8 Hz, 1H), 8.12 (d, J = 8.3 Hz, 1H), 8.01 (dd, J = 8.3, 1.9 Hz, 1H), 7.84 (d, J = 8.8 Hz, 2H), 7.59 (d, J = 8.8 Hz, 2H), 4.73 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 154.04, 146.81, 143.97, 135.30, 133.41, 132.60, 129.25 (q, J = 5.2 Hz), 127.13, 126.18, 124.35, 122.46 (q, J = 273.2 Hz), 121.81 (q, J = 33.4 Hz), 35.69. HRMS (ESI+) calcd for (C15H9BrF3N5O2S + H+) m/z: 459.96852 (100%), 461.96648 (97.3%); found: 461.9674 (100%), 459.9696 (97%).
1-Cyclohexyl-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (70e)
Yield: 93% as a white solid; mp 158–159 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.15–8.10 (m, 2H), 7.97 (dd, J = 8.4, 1.9 Hz, 1H), 4.71 (s, 2H), 4.22 (tt, J = 11.5, 3.9 Hz, 1H), 1.90–1.58 (m, 7H), 1.36 (qt, J = 12.8, 3.4 Hz, 2H), 1.29–1.12 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 151.89, 146.79, 144.27, 135.22, 129.09 (q, J = 5.2 Hz), 126.23, 122.44 (q, J = 273.0 Hz), 121.85 (q, J = 33.4 Hz), 57.92, 35.68, 32.07, 24.86, 24.82. HRMS (ESI+) calcd for (C15H16F3N5O2S + H+) m/z: 388.10496 (100%), 389.10831 (16.2%); found: 388.1054 (100%), 389.1080 (17%).
1-Alkyl/Aryl-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazoles 71a–71e
5-Nitro-2-(trifluoromethyl)benzyl bromide (47) was used as the alkylating agent. The reactions were completed in 1 h.
5-((5-Nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1-phenyl-1H-tetrazole (71a)
Yield: 98% as a yellowish solid; mp 64–65 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.58 (d, J = 2.4 Hz, 1H), 8.27 (dd, J = 8.6, 2.4, 1H), 8.01 (d, J = 8.6 Hz, 1H), 7.63–7.54 (m, 5H), 4.80 (d, J = 1.4 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 153.64, 150.40, 137.65, 133.42, 132.86 (d, J = 30.5 Hz), 131.24, 130.52, 129.04 (d, J = 5.6 Hz), 127.16, 125.18, 124.06, 123.74 (d, J = 274.5 Hz), 33.97. HRMS (ESI+) calcd for (C15H10F3N5O2S + H+) m/z: 382.05801 (100%), 383.06136 (16.2%); found: 382.0589 (100%), 383.0611 (17%).
1-(4-Methoxyphenyl)-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (71b)
Yield: 98% as a white solid; mp 107–108 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.56 (d, J = 2.3 Hz, 1H), 8.27 (dd, J = 8.6, 2.4 Hz, 1H), 8.01 (d, J = 8.7 Hz, 1H), 7.47 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 9.0 Hz, 2H), 4.77 (s, 2H), 3.79 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.19, 153.75, 150.38, 137.76, 132.82 (d, J = 30.7 Hz), 129.04 (d, J = 5.8 Hz), 127.11, 126.94, 125.97, 124.03, 123.73 (d, J = 275.3 Hz), 115.52, 56.21, 33.88. HRMS (ESI+) calcd for (C16H12F3N5O3S + H+) m/z: 412.06857 (100%), 413.07193 (17.3%); found: 412.0688 (100%), 413.0717 (17%).
1-(4-Chlorophenyl)-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (71c)
Yield: 95% as a white solid; mp 144–145 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.60 (d, J = 2.3 Hz, 1H), 8.31 (dd, J = 8.6, 1,5 Hz, 1H), 8.04 (d, J = 8.7 Hz, 1H), 7.70 (d, J = 8.9 Hz, 2H), 7.66 (d, J = 8.9 Hz, 2H), 4.82 (d, J = 1.3 Hz, 2H). 13C NMR (126 MHz, DMSO-d6) δ 153.38, 150.01, 137.29, 135.53, 132.45 (q, J = 30.6 Hz), 131.86, 130.17, 128.68 (q, J = 5.6 Hz), 126.81, 126.75, 123.68, 123.35 (q, J = 274.9 Hz), 33.75 (d, J = 2.3 Hz). HRMS (ESI+) calcd for (C15H9ClF3N5O2S + H+) m/z: 416.01903 (100%), 418.01609 (32%); found: 416.0202 (100%), 418.0172 (38%).
1-(4-Bromophenyl)-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (71d)
Yield: 90% as a white solid; mp 148–150 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.60 (d, J = 2.4 Hz, 1H), 8.31 (dd, J = 8.6, 1.5 Hz, 1H), 8.04 (d, J = 8.7 Hz, 1H), 7.84 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 8.9 Hz, 2H), 4.82 (d, J = 1.3 Hz, 2H). 13C NMR (126 MHz, DMSO-d6) δ 153.62, 150.30, 137.58, 133.42, 132.74 (d, J = 30.5 Hz), 132.57, 128.98 (d, J = 5.6 Hz), 127.20, 127.10, 124.39, 123.97, 123.64 (d, J = 275.1 Hz), 34.05. HRMS (ESI+) calcd for (C15H9BrF3N5O2S + H+) m/z: 459.96852 (100%), 461.96648 (97.3%); found: 461.9673 (100%), 459.9691 (97%).
1-Cyclohexyl-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1H-tetrazole (71e)
Yield: 93% as a white solid; mp 83–84 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.59 (d, J = 2.4 Hz, 1H), 8.33 (dd, J = 8.7, 2.4 Hz, 1H), 8.07 (d, J = 8.7 Hz, 1H), 4.81 (d, J = 1.2 Hz, 2H), 4.29 (tt, J = 11.3, 3.9 Hz, 1H), 1.93–1.85 (m, 2H), 1.82–1.68 (m, 4H), 1.68–1.57 (m, 1H), 1.47–1.30 (m, 2H), 1.28–1.13 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 151.62, 150.30, 137.97, 132.68 (d, J = 30.8 Hz), 129.07 (q, J = 5.5 Hz), 127.01, 123.96, 123.67 (d, J = 275.1 Hz), 58.01, 33.89 (d, J = 2.2 Hz), 32.15, 24.87, 24.83. HRMS (ESI+) calcd for (C15H16F3N5O2S + H+) m/z: 388.10496 (100%), 389.10831 (16.2%); found: 388.1058 (100%), 389.1084 (16%).
2-Alkyl/Aryl-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazoles 72a–72e
2-Nitro-5-(trifluoromethyl)benzyl bromide (46) was used as the alkylating agent. The reactions were completed in 1 h.
2-((2-Nitro-5-(trifluoromethyl)benzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (72a)
Yield: 78% as a white solid; mp 91–92 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.21 (d, J = 1.9 Hz, 1H), 8.16 (d, J = 8.4 Hz, 1H), 8.07 (dd, J = 8.4, 1.9 Hz, 1H), 7.95–7.88 (m, 2H), 7.65–7.53 (m, 3H), 4.74 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.94, 163.19, 146.82, 144.43, 135.28, 132.55, 129.84, 129.15 (q, J = 5.2 Hz), 126.85, 126.33, 123.36, 122.47 (d, J = 273.2 Hz), 121.86 (q, J = 33.4 Hz), 34.85. HRMS (ESI+) calcd for (C16H10F3N3O3S + H+) m/z: 382.04677 (100%), 383.05013 (17.3%); found: 382.0478 (100%), 383.0502 (17%).
2-(4-Methoxyphenyl)-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (72b)
Yield: 79% as a white solid; mp 112–113 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.15 (d, J = 1.8 Hz, 1H), 8.12 (d, J = 8.3 Hz, 1H), 8.02 (dd, J = 8.4, 1.9 Hz, 1H), 7.82 (d, J = 8.9 Hz, 2H), 7.07 (d, J = 8.9 Hz, 2H), 4.68 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.99, 162.65, 162.41, 146.89, 144.53, 135.32, 129.20, 128.82, 126.39, 122.55 (d, J = 273.1 Hz), 121.95 (d, J = 33.4 Hz), 115.78, 115.65–115.07 (m), 56.07, 34.96. HRMS (ESI+) calcd for (C17H12F3N3O4S + H+) m/z: 412.05734 (100%), 413.0607 (18.4%); found: 412.0577 (100%), 413.0602 (19%).
2-(4-Chlorophenyl)-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-2-phenyl-1,3,4-oxadiazole (72c)
Yield: 67% as a white solid; mp 156–157 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.20 (d, J = 1.9 Hz, 1H), 8.15 (d, J = 8.3 Hz, 1H), 8.07 (dd, J = 8.3, 1.9 Hz, 1H), 7.93 (d, J = 8.7 Hz, 2H), 7.64 (d, J = 8.7 Hz, 2H), 4.74 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.19, 163.49, 146.81, 144.34, 137.27, 135.29, 130.01, 129.16 (q, J = 5.2 Hz), 128.66, 126.31, 122.46 (q, J = 273.2 Hz), 122.27, 121.85 (d, J = 33.4 Hz), 34.82. HRMS (ESI+) calcd for (C16H9ClF3N3O3S + H+) m/z: 416.00780 (100%), 418.00485 (32%); found: 416.0085 (100%), 418.0052 (37%).
2-(4-Bromophenyl)-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-2-phenyl-1,3,4-oxadiazole (72d)
Yield: 71% as a white solid; mp 153–154 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.20 (d, J = 1.9 Hz, 1H), 8.15 (d, J = 8.3 Hz, 1H), 8.07 (dd, J = 8.4, 1.9 Hz, 1H), 7.86 (d, J = 8.6 Hz, 2H), 7.78 (d, J = 8.6 Hz, 2H), 4.74 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.31, 163.52, 146.82, 144.33, 135.30, 132.94, 129.16 (q, J = 5.2 Hz), 128.77, 126.31, 126.16, 122.61, 122.47 (q, J = 272.8 Hz), 121.85 (d, J = 33.3 Hz), 34.82. HRMS (ESI+) calcd for (C16H9BrF3N3O3S + H+) m/z: 459.95729 (100%), 461.95524 (97.3%); found: 461.9561 (100%), 459.9578 (97%).
2-Cyclohexyl-5-((2-nitro-5-(trifluoromethyl)benzyl)sulfanyl)-2-phenyl-1,3,4-oxadiazole (72e)
Yield: 69% as a white solid; mp 104–105 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.14 (d, J = 8.3 Hz, 1H), 8.13 (d, J = 1.9 Hz, 1H), 8.01 (dd, J = 8.3, 1.9 Hz, 1H), 4.63 (s, 2H), 2.91–2.85 (m, 1H), 1.95–1.86 (m, 2H), 1.70–1.58 (m, 3H), 1.47–1.27 (m, 4H), 1.27–1.13 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 171.47, 162.23, 146.79, 144.46, 135.23, 129.04 (q, J = 5.1 Hz), 126.28, 122.46 (q, J = 273.2 Hz), 121.81 (q, J = 33.3 Hz), 34.81, 34.57, 29.77, 25.51, 25.03. HRMS (ESI+) calcd for (C16H16F3N3O3S + H+) m/z: 388.09372 (100%), 389.09708 (17.3%); found: 388.0943 (100%), 389.0969 (17%).
2-Alkyl/Aryl-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadizaoles 73a–73e
5-Nitro-2-(trifluoromethyl)benzyl bromide (47) was used as the alkylating agent. The reactions were completed in 1 h.
2-((5-Nitro-2-(trifluoromethyl)benzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (73a)
Yield: 92% as a white solid; mp 124–125 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.67 (d, J = 2.3 Hz, 1H), 8.35–8.31 (m, 1H), 8.08 (d, J = 8.7 Hz, 1H), 7.97–7.91 (m, 2H), 7.65–7.53 (m, 3H), 4.83 (d, J = 1.4 Hz, 2H). 13C NMR (126 MHz, DMSO-d6) δ 166.17, 162.67, 150.32, 138.06, 132.79 (q, J = 30.5 Hz), 132.63, 129.87, 129.09 (q, J = 5.5 Hz), 127.11, 126.90, 124.01, 123.72 (q, J = 274.9 Hz), 123.34, 33.19 (d, J = 2.2 Hz). HRMS (ESI+) calcd for (C16H10F3N3O3S + H+) m/z: 382.04677 (100%), 383.05013 (17.3%); found: 382.0470 (100%), 383.0497 (18%).
2-(4-Methoxyphenyl)-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (73b)
Yield: 88% as a white solid; mp 134–135 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.62 (d, J = 2.4 Hz, 1H), 8.30 (dd, J = 8.7, 2.3 Hz, 1H), 8.04 (d, J = 8.7 Hz, 1H), 7.83 (d, J = 8.9 Hz, 2H), 7.08 (d, J = 8.8 Hz, 2H), 4.77 (s, 2H), 3.81 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 166.24, 162.73, 161.89, 150.41, 138.24, 132.85 (d, J = 31.0 Hz), 129.16 (d, J = 5.6 Hz), 128.88, 127.17, 124.06, 123.80 (d, J = 275.3 Hz), 115.75, 115.41, 56.08, 33.31. HRMS (ESI+) calcd for (C17H12F3N3O4S + H+) m/z: 412.05734 (100%), 413.0607 (18.4%); found: 412.0578 (100%), 413.0605 (19%).
2-(4-Chlorophenyl)-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (73c)
Yield: 87% as a white solid; mp 101–103 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.66 (d, J = 2.4 Hz, 1H), 8.33 (dd, J = 8.7, 2.4 Hz, 1H), 8.07 (d, J = 8.7 Hz, 1H), 7.94 (d, J = 8.6 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 4.84 (d, J = 1.3 Hz, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.40, 162.96, 150.32, 137.96, 137.36, 132.79 (q, J = 30.8 Hz), 130.04, 129.10 (q, J = 5.6 Hz), 128.71, 127.10, 124.03, 123.71 (q, J = 274.7 Hz), 122.23, 33.16. HRMS (ESI+) calcd for (C16H9ClF3N3O3S + H+) m/z: 416.00780 (100%), 418.00485 (32%); found: 416.0082 (100%), 418.0052 (32%).
2-(4-Bromophenyl)-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (73d)
Yield: 98% as a white solid; mp 92–93 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.66 (d, J = 2.4 Hz, 1H), 8.33 (dd, J = 8.6, 2.4 Hz, 1H), 8.07 (d, J = 8.7 Hz, 1H), 7.87 (d, J = 8.6 Hz, 2H), 7.78 (d, J = 8.5 Hz, 2H), 4.83 (s, 2H). 13C NMR (126 MHz, DMSO-d6) δ 165.51, 162.99, 150.32, 137.95, 132.96, 132.79, (q, J = 31.0 Hz), 129.10 (q, J = 5.5 Hz), 128.81, 127.10, 126.26, 124.03, 123.71 (q, J = 275.1 Hz), 122.56, 33.16 (d, J = 2.2 Hz). HRMS (ESI+) calcd for (C16H9BrF3N3O3S + H+) m/z: 459.95729 (100%), 461.95524 (97.3%); found: 461.9558 (100%), 459.9578 (97%).
2-Cyclohexyl-5-((5-nitro-2-(trifluoromethyl)benzyl)sulfanyl)-1,3,4-oxadiazole (73e)
Yield: 96% as a white solid; mp 102–103 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.57 (d, J = 2.3 Hz, 1H), 8.33 (dd, J = 8.6, 2.4 Hz, 1H), 8.07 (d, J = 8.7 Hz, 1H), 4.73 (d, J = 1.3 Hz, 2H), 2.91 (tt, J = 11.0, 3.7 Hz, 1H), 2.00–1.88 (m, 2H), 1.74–1.57 (m, 3H), 1.53–1.17 (m, 5H). 13C NMR (126 MHz, DMSO-d6) δ 171.77, 161.76, 150.27, 138.17, 132.77 (q, J = 30.9 Hz), 129.10 (q, J = 5.6 Hz), 127.02, 123.96, 123.68 (q, J = 274.7 Hz), 34.63, 33.12 (d, J = 2.5 Hz), 29.78, 25.53, 25.04. HRMS (ESI+) calcd for (C16H16F3N3O3S + H+) m/z: 388.09372 (100%), 389.09708 (17.3%); found: 388.0941 (100%), 389.0967 (18%).
1-Alkyl/Aryl-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazoles 74a–74e
4-Methoxy-3,5-dinitrobenzyl bromide (48) was used as the alkylating agent. The reactions were completed in 30 min.
5-((4-Methoxy-3,5-dinitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (74a)
Yield: 70% as a yellowish solid; mp 119–120 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.41 (s, 2H), 7.62–7.58 (m, 5H), 4.66 (s, 2H), 3.89 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 154.11, 146.22, 144.61, 135.12, 133.46, 131.24, 130.65, 130.54, 125.10, 64.89, 35.00. Elem. Anal. Calcd for C15H12N6O5S: C, 46.39; H, 3.11; N, 21.64; S, 8.26. Found: C, 46.76; H, 2.90; N, 21.27; S, 8.30.
5-((4-Methoxy-3,5-dinitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (74b)
Yield: 83% as a yellowish solid; mp 107–108 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.40 (s, 2H), 7.50 (d, J = 8.8 Hz, 2H), 7.12 (d, J = 8.9 Hz, 2H), 4.63 (s, 2H), 3.90 (s, 3H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.19, 154.22, 146.20, 144.60, 135.21, 130.61, 126.90, 126.03, 115.54, 64.89, 56.24, 34.95. Elem. Anal. Calcd for C16H14N6O6S: C, 45.93; H, 3.37; N, 20.09; S, 7.66. Found: C, 46.15; H, 3.58; N, 19.98; S, 7.77.
1-(4-Chlorophenyl)-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (74c)
Yield: 83% as a white solid; mp 138–139 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.40 (s, 2H), 7.69 (d, J = 9.0 Hz, 2H), 7.65 (d, J = 9.0 Hz, 2H), 4.65 (s, 2H), 3.90 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 154.23, 146.20, 144.59, 135.87, 135.10, 132.29, 130.63, 130.58, 127.03, 64.90, 35.12. Elem. Anal. Calcd for C15H11ClN6O5S: C, 42.61; H, 2.62; N, 19.88; S, 7.58. Found: C, 42.64; H, 2.31; N, 19.90; S, 7.73.
1-(4-Bromophenyl)-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (74d)
Yield: 94% as a white solid; mp 151–153 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.40 (s, 2H), 7.83 (d, J = 8.7 Hz, 2H), 7.58 (d, J = 8.7 Hz, 2H), 4.65 (s, 2H), 3.90 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 154.18, 146.21, 144.60, 135.09, 133.53, 132.71, 130.63, 127.18, 124.43, 64.90, 35.12. Elem. Anal. Calcd for C15H11BrN6O5S: C, 38.56; H, 2.37; N, 17.99; S, 6.86. Found: C, 38.20; H, 2.23; N, 17.64; S, 6.72.
1-Cyclohexyl-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (74e)
Yield: 66% as yellow oil, which crystallized over time; mp 67–69 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.39 (s, 2H), 4.63 (s, 2H), 4.21 (tt, J = 11.5, 3.9 Hz, 1H), 3.89 (s, 3H), 1.90–1.84 (m, 2H), 1.79–1.56 (m, 5H), 1.44–1.33 (m, 2H), 1.28–1.19 (m, 1H). 13C NMR (126 MHz, DMSO-d6) δ 152.04, 146.08, 144.55, 135.29, 130.46, 64.84, 57.95, 34.98, 32.09, 24.89, 24.83. Elem. Anal. Calcd for C15H18N6O5S: C, 45.68; H, 4.60; N, 21.31; S, 8.13. Found: C, 46.02; H, 4.56; N, 21.08; S, 8.02. HRMS (ESI+) calcd for (C15H18N6O5S + H+) m/z: 395.11322 (100%), 396.11657 (16.2%); found: 395.1138 (100%), 396.1158 (17%).
1-Alkyl/Aryl-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazoles 75a–75e
2-Methoxy-3,5-dinitrobenzyl bromide (49) was used as the alkylating agent. The reactions were completed in 1 h.
5-((2-Methoxy-3,5-dinitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (75a)
Yield: 87% as a white solid; mp 144–145 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.71 (d, J = 2.9 Hz, 1H), 8.69 (d, J = 2.9 Hz, 1H), 7.70–7.51 (m, 5H), 4.73 (s, 2H), 3.93 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 156.25, 153.64, 142.43, 141.80, 134.59, 133.10, 130.89, 130.18, 129.97, 124.84, 121.43, 63.45, 31.51. Elem. Anal. Calcd for C15H12N6O5S: C, 46.39; H, 3.11; N, 21.64; S, 8.26. Found: C, 46.56; H, 3.02; N, 21.74; S, 8.63.
5-((2-Methoxy-3,5-dinitrobenzyl)sulfanyl)-1-(4-methoxyphenyl)-1H-tetrazole (75b)
Yield: 95% as a beige solid; mp 166–168 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.70 (d, J = 2.9 Hz, 1H), 8.67 (d, J = 2.9 Hz, 1H), 7.51 (d, J = 9.0 Hz, 2H), 7.13 (d, J = 9.0 Hz, 2H), 4.70 (s, 2H), 3.92 (s, 3H), 3.83 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 160.82, 156.24, 153.75, 142.44, 141.80, 134.68, 129.92, 126.63, 125.67, 121.40, 115.17, 63.46, 55.89, 31.45. Elem. Anal. Calcd for C15H14N6O6S: C, 45.93; H, 3.37; N, 20.09; S, 7.66. Found: C, 45.94; H, 3.28; N, 20.03; S, 8.01.
1-(4-Chlorophenyl)-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (75c)
Yield: 93% as a brownish solid; mp 136–139 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.71 (d, J = 2.9 Hz, 1H), 8.68 (d, J = 2.8 Hz, 1H), 7.71 (d, J = 8.9 Hz, 2H), 7.66 (d, J = 8.8 Hz, 2H), 4.72 (s, 2H), 3.93 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 156.24, 153.76, 142.41, 141.78, 135.52, 134.57, 131.92, 130.21, 129.97, 126.78, 121.42, 63.46, 31.63. Elem. Anal. Calcd for C15H11ClN6O5S: C, 42.61; H, 2.62; N, 19.88; S, 7.58. Found: C, 42.93; H, 2.48; N, 19.97; S, 7.92.
1-(4-Bromophenyl)-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (75d)
Yield: 80% as a brownish solid; mp 153–155 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.71 (d, J = 2.8 Hz, 1H), 8.67 (d, J = 2.9 Hz, 1H), 7.84 (d, J = 8.7 Hz, 2H), 7.59 (d, J = 8.7 Hz, 2H), 4.72 (s, 2H), 3.93 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 156.20, 153.66, 142.40, 141.78, 134.54, 133.12, 132.33, 129.92, 126.89, 124.06, 121.36, 63.43, 31.64. Elem. Anal. Calcd for C15H11BrN6O5S: C, 38.56; H, 2.37; N, 17.99; S, 6.86. Found: C, 38.72 H, 2.21; N, 17.91; S, 7.08.
1-Cyclohexyl-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (75e)
Yield: 80% as a yellow solid; mp 96–97 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.69 (d, J = 2.9 Hz, 1H), 8.63 (d, J = 2.9 Hz, 1H), 4.68 (s, 2H), 4.24 (tt, J = 11.5, 3.9 Hz, 1H), 3.93 (s, 3H), 1.92–1.85 (m, 2H), 1.81–1.66 (m, 4H), 1.67–1.56 (m, 1H), 1.40–1.31 (m, 2H), 1.23–1.16 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 156.55, 152.10, 142.91, 142.19, 135.25, 130.13, 121.69, 63.84, 58.06, 32.20, 31.85, 24.97, 24.92. Elem. Anal. Calcd for C15H18N6O5S: C, 45.68; H, 4.60; N, 21.31; S, 8.13. Found: C, 46.05; H, 4.57; N, 21.25; S, 8.28.
1-Alkyl/Aryl-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazoles 76a–76e
4-Methyl-3,5-dinitrobenzyl bromide (50) was used as the alkylating agent. The reactions were completed in 1 h.
5-((4-Methyl-3,5-dinitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (76a)
Yield: 70% as a beige solid; mp 112–113 °C. 1H NMR (500 MHz, acetone-d6) δ 8.38 (s, 2H), 7.72–7.61 (m, 5H), 4.85 (s, 2H), 2.52 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 154.26, 152.39, 139.51, 134.55, 131.46, 130.92, 129.16, 126.70, 125.26, 35.70, 14.74. Elem. Anal. Calcd for C15H12N6O4S: C, 48.38; H, 3.25; N, 22.57; S, 8.61. Found: C,48.48; H, 3.50; N, 22.81; S, 8.90.
1-(4-Methoxyphenyl)-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (76b)
Yield: 71% as a white solid; 144–145 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.30 (s, 2H), 7.49 (d, J = 8.9 Hz, 2H), 7.11 (d, J = 9.0 Hz, 2H), 4.64 (s, 2H), 3.80 (s, 3H), 2.39 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.19, 154.12, 151.24, 138.89, 128.81, 126.91, 126.02, 125.98, 115.52, 56.23, 35.04, 14.79. Elem. Anal. Calcd for C16H14N6O5S: C, 47.76; H, 3.51; N, 20.89; S, 7.97. Found: C, 47.72; H, 3.22; N, 21.09; S, 8.18.
1-(4-Chlorophenyl)-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl(-1H-tetrazole (76c)
Yield: 94% as a beige solid; mp133–134 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.30 (s, 2H), 7.68 (d, J = 8.7 Hz, 2H), 7.63 (d, J = 8.9 Hz, 2H), 4.66 (s, 2H), 2.39 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 154.12, 151.24, 138.78, 135.86, 132.27, 130.55, 128.83, 127.05, 125.99, 35.24, 14.78. Elem. Anal. Calcd for C15H11ClN6O4S: C, 44.29; H, 2.73; N, 20.66; S, 7.88. Found: C, 44.20; H, 2.48; N, 20.67; S, 8.04.
1-(4-Bromophenyl)-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (76d)
Yield: 94% as a white solid; mp 124–125 °C. 1H NMR (500 MHz, acetone-d6) δ 8.35 (s, 2H), 7.85 (d, J = 8.9 Hz, 2H), 7.61 (d, J = 8.8 Hz, 2H), 4.83 (s, 2H), 2.50 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 154.25, 152.29, 139.34, 133.97, 133.65, 129.06, 127.07, 126.61, 124.79, 35.71, 14.64. Elem. Anal. Calcd for C15H11BrN6O4S: C, 39.93; H, 2.46; N, 18.62; S, 7.10. Found: 40.23; H, 2.31; N, 18.40; S, 7.11.
1-Cyclohexyl-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (76e)
Yield: 73% as a white solid; mp 121–122 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.31 (s, 2H), 4.65 (s, 2H), 4.21 (tt, J = 11.5, 3.9 Hz, 1H), 2.39 (s, 3H), 1.90–1.81 (m, 2H), 1.79–1.73 (m, 2H), 1.74–1.64 (m, 2H), 1.65–1.53 (m, 1H), 1.41–1.30 (m, 2H), 1.23–1.13 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 152.05, 151.28, 139.02, 128.77, 125.98, 58.02, 35.08, 32.16, 24.97, 24.91, 14.76. Elem. Anal. Calcd for C15H18N6O4S: C, 47.61; H, 4.79; N, 22.21; S, 8.47. Found: C, 47.78; H, 4.69; N, 22.26; S, 8.46.
1-Alkyl/Aryl-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazoles 77a–77e
2-Methyl-3,5-dinitrobenzyl bromide (51) was used as the alkylating agent. The reactions were completed in 1 h.
5-((2-Methyl-3,5-dinitrobenzyl)sulfanyl)-1-phenyl-1H-tetrazole (77a)
Yield: 76% as a yellow solid; mp 128–129 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.58 (s, 2H), 7.61–7.54 (m, 5H), 4.81 (s, 2H), 2.46 (s, 3H, overlap with solvent). 13C NMR (151 MHz, DMSO-d6) δ 153.68, 151.31, 145.58, 140.13, 138.67, 133.42, 131.23, 130.50, 128.57, 125.16, 119.13, 34.92, 15.56. Elem. Anal. Calcd for C15H12N6O4S: C, 48.38; H, 3.25; N, 22.57; S, 8.61. Found: C, 48.19; H, 3.32; N, 22.75; S, 8.82
1-(4-Methoxyphenyl)-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (77b)
Yield: 80% as a white solid; mp 168–168 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.57 (d, J = 2.5 Hz, 1H), 8.55 (d, J = 2.5 Hz, 1H), 7.46 (d, J = 9.0 Hz, 2H), 7.10 (d, J = 9.0 Hz, 2H), 4.77 (s, 2H), 3.80 (s, 3H), 2.45 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 161.18, 153.76, 151.30, 145.56, 140.21, 138.63, 128.50, 126.93, 126.00, 119.10, 115.50, 56.22, 34.88, 15.55. Elem. Anal. Calcd for C16H14N6O5S: C, 47.76; H, 3.51; N, 20.89; S, 7.97. Found: C, 48.09; H, 3.46; N, 20.93; S, 7.95.
1-(4-Chlorophenyl)-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (77c)
Yield: 80% as a yellow solid; mp 131–133 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.61 (d, J = 2.5 Hz, 1H), 8.59 (d, J = 2.5 Hz, 1H), 7.70 (d, J = 8.8 Hz, 2H), 7.65 (d, J = 8.8 Hz, 2H), 4.83 (s, 2H), 2.49 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 153.39, 150.92, 145.18, 139.72, 138.28, 135.50, 131.86, 130.14, 128.17, 126.70, 118.74, 34.71, 15.19. Elem. Anal. Calcd for C15H11ClN6O4S: C, 44.29; H, 2.73; N, 20.66; S, 7.88. Found: C, 43.95; H, 2.45; N, 20.66; S, 7.79.
1-(4-Bromophenyl)-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (77d)
Yield: 94% as a yellow solid; mp 145–146 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.57 (d, J = 2.4 Hz, 1H), 8.55 (d, J = 2.5 Hz, 1H), 7.80 (d, J = 8.7 Hz, 2H), 7.54 (d, J = 8.8 Hz, 2H), 4.79 (s, 2H), 2.45 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 153.73, 151.30, 145.57, 140.10, 138.67, 133.48, 132.66, 128.55, 127.24, 124.45, 119.12, 35.08, 15.56. Elem. Anal. Calcd for C15H11BrN6O4S: C, 39.93; H, 2.46; N, 18.62; S, 7.10. Found: C, 40.27; H, 2.28; N, 18.74; S, 7.15.
1-Cyclohexyl-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1H-tetrazole (77e)
Yield: 79% as a white solid; mp 97–98 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.59 (d, J = 2.4 Hz, 1H), 8.51 (d, J = 2.5 Hz, 1H), 4.79 (s, 2H), 4.23 (tt, J = 11.5, 3.9 Hz, 1H), 2.51 (s, 3H), 1.88–1.81 (m, 2H), 1.78–1.74 (m, 2H), 1.71–1.64 (m, 2H), 1.62–1.59 (m, 1H), 1.40–1.31 (m, 2H), 1.26–1.09 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 151.75, 151.42, 145.56, 140.43, 138.57, 128.32, 119.03, 58.09, 34.90, 32.19, 24.95, 24.92, 15.54. Elem. Anal. Calcd for C15H18N6O4S: C, 47.61; H, 4.79; N, 22.21; S, 8.47. Found: C, 47.62; H, 4.57; N, 22.37; S, 8.60.
2-Alkyl/Aryl-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 78a–78e
4-Methoxy-3,5-dinitrobenzyl bromide (48) was used as the alkylating agent. The reactions were completed in 30 min.
2-((4-Methoxy-3,5-dinitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (78a)
Yield: 75% as a yellow solid; mp 99–101 °C. 1H NMR (500 MHz, acetone-d6) δ 8.51 (s, 2H), 8.02–7.98 (m, 2H), 7.65–7.55 (m, 3H), 4.80 (s, 2H), 4.05 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.79, 163.71, 147.15, 145.82, 136.05, 132.71, 130.71, 130.10, 127.31, 124.49, 64.98, 34.91. Elem. Anal. Calcd for C16H12N4O6S: C, 49.48; H, 3.11; N, 14.43; S, 8.26. Found: C, 49.52; H, 2.97; N, 14.47; S, 8.61.
2-((4-Methoxy-3,5-dinitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (78b)
Yield: 70% as a yellowish solid; mp 128–131 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.49 (s, 2H), 7.88 (d, J = 9.0 Hz, 2H), 7.11 (d, J = 9.0 Hz, 2H), 4.66 (s, 2H), 3.93 (s, 3H), 3.84 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.59, 162.26, 162.09, 145.89, 144.28, 135.18, 130.24, 128.48, 115.45, 114.99, 64.51, 55.70, 33.96. Elem. Anal. Calcd for: C17H14N4O7S: C, 48.80; H, 3.37; N, 13.39; S, 7.66. Found: C, 49.09; H, 3.41; N, 13.35; S, 7.97.
2-(4-Chlorophenyl)-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (78c)
Yield: 81% as a yellow solid; mp 105–107 °C. 1H NMR (500 MHz, acetone-d6) δ 8.50 (s, 2H), 8.01 (d, J = 8.9 Hz, 2H), 7.63 (d, J = 8.9 Hz, 2H), 4.80 (s, 2H), 4.04 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.02, 164.05, 147.17, 145.80, 138.24, 135.97, 130.72, 130.35, 128.98, 123.27, 64.99, 34.90. Elem. Anal. Calcd for C16H11ClN4O6S: C, 45.45; H, 2.62; N, 13.25; S, 7.58. Found: C, 45.80; H, 2.69; N, 13.23; S, 7.93.
2-(4-Bromophenyl)-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (78d)
Yield: 91% as a yellow solid; mp 142–145 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.50 (s, 2H), 7.88 (d, J = 8.6 Hz, 2H), 7.78 (d, J = 8.6 Hz, 2H), 4.68 (s, 2H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.02, 163.30, 145.97, 144.30, 135.10, 132.66, 130.34, 128.54, 125.89, 122.38, 64.56, 33.92. Anal. Calcd for C16H11BrN4O6S: C, 41.13; H, 2.37; N, 11.99; S, 6.86. Found: C, 41.36; H, 2.26; N, 11.9; S, 7.23.
2-Cyclohexyl-5-((4-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (78e)
Yield: 75% as a yellow solid; 125–126 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.39 (s, 2H), 4.54 (s, 2H), 3.90 (s, 3H), 2.87 (tt, J = 11.1, 3.7 Hz, 1H), 1.92–1.86 (m, 2H), 1.70–1.63 (m, 2H), 1.62–1.55 (m, 1H), 1.46–1.36 (m, 2H), 1.35–1.26 (m, 2H), 1.24–1.16 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.52, 162.37, 146.24, 144.64, 135.61, 130.58, 64.90, 34.69, 34.25, 29.88, 25.60, 25.15. Anal. Calcd for C16H18N4O6S: C, 48.73; H, 4.60; N, 14.21; S, 8.13. Found: C, 49.12; H, 4.67; N, 14.01; S, 8.16.
2-Alkyl/Aryl-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 79a–79e
2-Methoxy-3,5-dinitrobenzyl bromide (49) was used as the alkylating agent. The reactions were completed in 30 min.
2-((2-Methoxy-3,5-dinitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (79a)
Yield: 68% as a yellow solid; mp 92–95 °C. 1H NMR (500 MHz, acetone-d6) δ 8.85 (d, J = 2.8 Hz, 1H), 8.72 (d, J = 2.8 Hz, 1H), 8.03–7.97 (m, 2H), 7.66–7.54 (m, 3H), 4.82 (s, 2H), 4.15 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.80, 163.73, 157.53, 143.00, 136.07, 132.72, 130.49, 130.11, 127.32, 124.48, 122.06, 63.86, 31.45. Elem. Anal. Calcd for C, 49.48; H, 3.11; N, 14.43; N, 8.26. Found: C, 49.73; H, 3.12; N, 14.48; S, 8.62.
2-((2-Methoxy-3,5-dinitrobenzyl)sulfanyl)-5-(4-methoxyphenyl)-1,3,4-oxadiazole (79b)
Yield: 78% as a yellowish solid; mp 115–117 °C. 1H NMR (500 MHz, acetone-d6) δ 8.83 (d, J = 2.8 Hz, 1H), 8.72 (d, J = 2.9 Hz, 1H), 7.93 (d, J = 8.8 Hz, 2H), 7.12 (d, J = 8.9 Hz, 2H), 4.79 (s, 2H), 4.14 (s, 3H), 3.91 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 165.93, 162.63, 162.00, 156.65, 142.60, 142.14, 135.30, 129.60, 128.30, 121.17, 115.93, 114.65, 63.00, 55.07, 30.60. Elem. Anal. Calcd for C17H14N4O7S: C, 48.80; H, 3.37; N, 13.39; S, 7.66. Found: C, 48.85; H, 3.27; N, 13.14; S, 7.43.
2-(4-Chlorophenyl)-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (79c)
Yield: 76% as a yellowish solid; 109–112 °C. 1H NMR (500 MHz, acetone-d6) δ 8.84 (d, J = 2.8 Hz, 1H), 8.72 (d, J = 2.9 Hz, 1H), 8.02 (d, J = 8.6 Hz, 2H), 7.63 (d, J = 8.6 Hz, 2H), 4.83 (s, 2H), 4.15 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.03, 164.08, 157.52, 143.44, 143.00, 138.25, 136.00, 130.49, 130.36, 128.99, 123.27, 122.08, 63.86, 31.45. Elem. Anal. Calcd for C16H11ClN4O6S: C, 45.45; H, 2.62; N, 13.25; S, 7.58. Found: C, 45.59; H, 2.40; N, 13.39; S, 7.65.
2-(4-Bromophenyl)-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (79d)
Yield: 76% as a beige solid; mp 114–116 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.76 (d, J = 2.9 Hz, 1H), 8.73 (d, J = 2.8 Hz, 1H), 7.89 (d, J = 8.6 Hz, 2H), 7.79 (d, J = 8.6 Hz, 2H), 4.74 (s, 2H), 3.98 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.34, 163.43, 156.54, 142.71, 142.11, 135.22, 132.94, 130.24, 128.82, 126.19, 122.62, 121.76, 63.91, 31.09. Anal. Calcd for C16H11BrN4O6S: C, 41.13; H, 2.37; N, 11.99; S, 6.86. Found: C, 41.44; H, 2.35; N, 11.92; S, 7.25.
2-Cyclohexyl-5-((2-methoxy-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (79e)
Yield: 90% as a yellowish oil. 1H NMR (600 MHz, DMSO-d6) δ 8.69 (d, J = 3.0 Hz, 1H), 8.63 (d, J = 2.9 Hz, 1H), 4.59 (s, 2H), 3.93 (s, 3H), 2.93–2.80 (m, 1H), 1.95–1.84 (m, 2H), 1.72–1.54 (m, 3H), 1.46–1.37 (m, 2H), 1.35–1.25 (m, 2H), 1.24–1.15 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.66, 162.25, 156.60, 142.86, 142.18, 135.46, 130.17, 121.74, 63.90, 34.72, 31.06, 29.89, 25.61, 25.15. HRMS (ESI+) calcd for (C16H18N4O6S + H+) m/z: 395.10198 (100%), 396.10533 (17.3%); found: 395.1033 (100%), 396.1055 (17%).
2-Alkyl/Aryl-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 80a–80e
4-Methyl-3,5-dinitrobenzyl bromide (50) was used as the alkylating agent. The reactions were completed in 1 h.
2-((4-Methyl-3,5-dinitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (80a)
Yield: 86% as a beige solid; mp 116–117 °C. 1H NMR (500 MHz, acetone-d6) δ 8.44 (s, 2H), 8.01–7.97 (m, 2H), 7.64–7.56 (m, 3H), 4.82 (s, 2H), 2.53 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.79, 163.67, 152.35, 139.69, 132.70, 130.09, 129.01, 127.30, 126.69, 124.47, 34.95, 14.67. Elem. Anal. Calcd for C16H12N4O6S: C, 51.61; H, 3.25; N, 15.05; S, 8.61. Found: 51.50; H, 3.07; N, 15.12; S, 8.77.
2-(4-Methoxyphenyl)-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (80b)
Yield: 70% as a white solid; mp 124–125 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.37 (s, 2H), 7.83 (d, J = 9.0 Hz, 2H), 7.07 (d, J = 9.0 Hz, 2H), 4.64 (s, 2H), 3.80 (s, 3H), 2.39 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.98, 162.65, 162.41, 151.32, 139.24, 128.85, 128.82, 126.10, 115.81, 115.36, 56.07, 34.40, 14.83. Elem. Anal. Calcd for C15H16N4O5S: C, 50.74; H, 3.51; N, 13.92; S, 7.97. Found: C, 50.64; H, 3.34; N, 13.91; S, 7.79.
2-(4-Chlorophenyl)-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (80c)
Yield: 81% as a beige solid; mp 159–160 °C. 1H NMR (500 MHz, acetone-d6) δ 8.43 (s, 2H), 8.00 (d, J = 8.6 Hz, 2H), 7.63 (d, J = 8.6 Hz, 2H), 4.82 (s, 2H), 2.53 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.02, 164.01, 152.36, 139.63, 138.23, 130.34, 129.02, 128.98, 126.72, 123.26, 34.95, 14.67. Elem. Anal. Calcd for C16H11ClN4O5S: C, 47.24; H, 2.73; N, 13.77; S, 7.88. Found: C, 47.31; H, 2.4; N, 13.88; S, 7.89.
2-(4-Bromophenyl)-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (80d)
Yield: 75% as a white solid; mp 160–161 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.37 (s, 2H), 7.83 (d, J = 8.6 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 4.66 (s, 2H), 2.39 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.39, 163.59, 151.33, 139.14, 133.01, 128.89, 128.85, 126.23, 126.14, 122.72, 34.37, 14.83. Elem. Anal. Calcd for C16H11BrN4O5S: C, 42.59; H, 2.46; N, 12.42; S, 7.10. Found: C, 42.58; H, 2.23; N, 12.36; S, 7.22.
2-Cyclohexyl-5-((4-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (80e)
Yield: 70% as a white solid; mp 96–97 °C. 1H NMR (600 MHz, DMSO-d6) δ 8.31 (s, 2H), 4.56 (s, 2H), 2.86 (tt, J = 11.0, 3.7 Hz, 1H), 2.40 (s, 3H), 1.92–1.85 (m, 2H), 1.71–1.54 (m, 3H), 1.49–1.36 (m, 2H), 1.36–1.25 (m, 2H), 1.23–1.12 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.55, 162.30, 151.30, 139.27, 128.80, 126.07, 34.69, 34.33, 29.88, 25.61, 25.16, 14.81. Elem. Anal. Calcd for C16H18N4O5S: C, 50.79; H, 4.79; N, 14.81; S, 8.47. Found: C, 50.80; H, 4.63; N, 14.90; S, 8.55.
2-Alkyl/Aryl-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazoles 81a–81e
2-Methyl-3,5-dinitrobenzyl bromide (51) was used as the alkylating agent. The reactions were completed in 1 h.
2-((2-Methyl-3,5-dinitrobenzyl)sulfanyl)-5-phenyl-1,3,4-oxadiazole (81a)
Yield: 87% as a yellow solid; mp 93–94 °C. 1H NMR (500 MHz, CDCl3) δ 8.67 (d, J = 2.4 Hz, 1H), 8.58 (d, J = 2.4 Hz, 1H), 8.05–7.92 (m, 2H), 7.56–7.45 (m, 3H), 4.70 (s, 2H), 2.69 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 166.42, 162.12, 151.24, 145.64, 139.06, 138.43, 131.99, 129.11, 128.01, 126.70, 123.15, 119.11, 34.09, 15.72. Elem. Anal. Calcd for C16H12N4O5S: C, 51.61; H, 3.25; N, 15.05; S, 8.61. Found: C, 51.62; H, 3.01; N, 15.19; S, 8.74.
2-(4-Methoxyphenyl)-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (81b)
Yield: 82% as a yellow solid; mp 118–120 °C. 1H NMR (500 MHz, acetone-d6) δ 8.75 (d, J = 2.4 Hz, 1H), 8.59 (d, J = 2.4 Hz, 1H), 7.90 (d, J = 8.9 Hz, 2H), 7.09 (d, J = 8.9 Hz, 2H), 4.90 (s, 2H), 3.89 (s, 3H), 2.70 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.37, 163.07, 162.04, 151.83, 146.07, 140.74, 138.80, 128.72, 128.38, 118.99, 116.31, 115.06, 55.49, 34.22, 15.22. Elem. Anal. Calcd for C17H1N4O6S: C, 50.74; H, 3.51; N, 13.92; S, 7.97. Found: C, 50.38; H, 3.36; N, 13.65; S, 8.18.
2-(4-Chlorophenyl)-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (81c)
Yield: 75% as a yellow solid; mp 120–121 °C. 1H NMR (500 MHz, CDCl3) δ 8.67 (d, J = 2.3 Hz, 1H), 8.58 (d, J = 2.4 Hz, 1H), 7.93 (d, J = 8.8 Hz, 2H), 7.49 (d, J = 8.7 Hz, 2H), 4.70 (s, 2H), 2.69 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 165.62, 162.42, 151.26, 145.65, 138.94, 138.41, 138.34, 129.54, 128.02, 127.96, 121.62, 119.16, 34.08, 15.73. Elem. Anal. Calcd for C16H11ClN4O5S: C, 47.24; H, 2.73; N, 13.77; S, 7.88. Found: C, 47.41; H, 2.50; N, 13.88; S, 8.26.
2-(4-Bromophenyl)-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (81d)
Yield: 79% as a yellow solid; mp 141–142 °C. 1H NMR (500 MHz, CDCl3) δ 8.67 (d, J = 2.4 Hz, 1H), 8.58 (d, J = 2.4 Hz, 1H), 7.86 (d, J = 8.7 Hz, 2H), 7.65 (d, J = 8.7 Hz, 2H), 4.70 (s, 2H), 2.69 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 165.71, 162.47, 151.26, 145.65, 138.93, 138.41, 132.50, 128.08, 128.02, 126.76, 122.05, 119.16, 34.08, 15.73. Elem. Anal. Calcd for C16H11BrN4O5S: C, 42.59; H, 2.46; N, 12.42; S, 7.10. Found: C, 42.23; H, 2.15; N, 12.29; S, 7.11.
2-Cyclohexyl-5-((2-methyl-3,5-dinitrobenzyl)sulfanyl)-1,3,4-oxadiazole (81e)
Yield: 75% as a colorless oil. 1H NMR (600 MHz, DMSO-d6) δ 8.60 (d, J = 2.4 Hz, 1H), 8.54 (d, J = 2.4 Hz, 1H), 4.72 (s, 2H), 2.88–2.84 (m, 1H), 2.50 (s, 3H), 1.93–1.85 (m, 2H), 1.74–1.57 (m, 3H), 1.44–1.37 (m, 2H), 1.34–1.27 (m, 2H), 1.25–1.14 (m, 1H). 13C NMR (151 MHz, DMSO-d6) δ 171.68, 161.98, 151.43, 145.56, 140.68, 138.62, 128.39, 119.09, 34.70, 34.10, 29.88, 25.60, 25.15, 15.57. HRMS (ESI+) calcd for (C16H18N4O5S + H+) m/z: 379.10707 (100%), 379.11042 (17.3%); found: 379.1080 (100%), 380.1106 (17%).
2-Alkyl/Aryl-5-((5-nitrofuran-2-yl)methylsulfanyl)-1,3,4-oxadiazoles 83a–83e
Commercially available 5-(bromomethyl)-2-nitrofuran was used as the alkylating agent. The reactions were completed in 30 min.
2-((5-Nitrofuran-2-yl)methylsulfanyl)-5-phenyl-1,3,4-oxadiazole (83a)
Yield: 73% as a yellow solid; mp 102–104 °C. 1H NMR (500 MHz, acetone-d6) δ 8.08–8.00 (m, 2H), 7.67–7.56 (m, 3H), 7.48 (d, J = 3.7 Hz, 1H), 6.9 (d, J = 3.7, 1H), 4.80 (s, 2H). 13C NMR (126 MHz, aceton-D6) δ 166.95, 163.19, 155.07, 132.75, 130.11, 127.37, 124.51, 113.78, 113.73, 29.25. Elem. Anal. Calcd for C13H9N3O4S: C, 51.48; H, 2.99; N, 13.85; S, 10.57. Found: C, 51.65; H, 3.02; N, 13.77; S, 10.21.
2-(4-Methoxyphenyl)-5-((5-nitrofuran-2-yl)methylsulfanyl)-1,3,4-oxadiazole (83b)
Yield: 56% as a yellow solid; mp 108–110 °C. 1H NMR (500 MHz, acetone-d6) δ 7.97 (d, J = 8.4 Hz, 2H), 7.48 (dd, J = 3.7, 0.5 Hz, 1H), 7.13 (d, J = 8.5 Hz, 2H), 6.88 (dd, J = 3.7, 0.6 Hz, 1H), 4.77 (s, 2H), 3.91 (s, 3H). 13C NMR (126 MHz, acetone-d6) δ 166.94, 163.51, 162.31, 155.16, 129.21, 116.82, 115.51, 113.73, 55.93, 29.28. Elem. Anal. Calcd for C14H11N3O5S: C, 50.45; H, 3.33; N, 12.61; S, 9.62. Found: C, 50.06; H 3.47; N, 12.50; S 9.24.
2-(4-Chlorophenyl)-5-((5-nitrofuran-2-yl)methylsulfanyl)-1,3,4-oxadiazole (83c)
Yield: 61% as a beige solid; mp 125–127 °C. 1H NMR (500 MHz, acetone-d6) δ 8.05 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 7.48 (dd, J = 3.7, 0.5 Hz, 1H), 6.89 (dd, J = 3.8, 0.7 Hz, 1H), 4.80 (s, 2H). 13C NMR (126 MHz, acetone-d6) δ 166.17, 163.53, 155.00, 138.27, 130.36, 129.04, 123.27, 113.81, 113.73, 29.23. Elem. Anal. Calcd for C13H8ClN3O4S: C, 46.23; H, 2.39; N, 12.44; S, 9.49. Found: C, 46.46; H, 2.49; N, 12.05; S, 9.88.
2-(4-Bromophenyl)-5-((5-nitrofuran-2-yl)methylsulfanyl)-1,3,4-oxadiazole (83d)
Yield: 43% as a white solid; mp 143–145 °C. 1H NMR (500 MHz, acetone-d6) δ 7.98 (d, J = 8.3 Hz, 2H), 7.81 (d, J = 8.3 Hz, 2H), 7.48 (dd, J = 3.7, 0.6 Hz, 1H), 6.89 (dd, J = 3.8, 0.6 Hz, 1H), 4.81 (s, 2H). 13C NMR (126 MHz, acetone-d6) δ 166.28, 163.57, 155.00, 133.38, 129.17, 126.71, 123.69, 113.82, 113.73, 29.24. Elem. Anal. Calcd for C13H8BrN3O4S: C, 40.86; H, 2.11; N, 10.99; S, 8.39. Found: C, 40.89; H, 1.94; N, 10.92; S, 8.48.
2-Cyclohexyl-5-((5-nitrofuran-2-yl)methylsulfanyl)-1,3,4-oxadiazole (83e)
Yield: 57% as a beige oil. 1H NMR (600 MHz, acetone-d6) δ 7.42 (d, J = 3.7 Hz, 1H), 6.78 (d, J = 3.7 Hz, 1H), 4.64 (s, 2H), 2.91 (tt, J = 11.1, 3.7 Hz, 1H), 2.02–1.97 (m, 2H), 1.78–1.72 (m, 2H), 1.67–1.64 (m, 1H), 1.59–1.49 (m, 2H), 1.44–1.35 (m, 2H), 1.32–1.24 (m, 1H). 13C NMR (151 MHz, acetone-d6) δ 171.53, 161.52, 154.40, 112.92, 112.85, 34.93, 29.81, 28.32, 25.45, 25.03. HRMS (ESI+) calcd for (C13H15N3O4S + H+) m/z: 310.08560 (100%); found: 310.0860 (100%).
Synthesis of 2-Alkyl/Aryl-5-((5-nitropyridin-3-yl)methylsulfanyl)-1,3,4-oxadiazoles 82a–82e
Thionyl chloride (0.52 g, 0.32 mL, 4.37 mmol) was added to a stirred solution of 3-hydroxymethyl-5-nitropyrydine 34 (0.17 g, 1.1 mmol) in CH2Cl2 (5 mL) under argon at −5 °C. The reaction mixture was removed from the cooling bath and stirred for 7 h at rt. Then the reaction mixture was concentrated under reduced pressure, and THF (5 mL) was added to the reaction residue. The resulting suspension was added to a solution of 5-substituted 1,3,4-oxadiazole-2-thiol (1.2 mmol) and Et3N (0.33 g, 0.46 mL, 3.3 mmol) in THF (15 mL), and the resulting mixture was stirred at rt overnight. Then, the solvent was evaporated under reduced pressure; the residue was dissolved in EtOAc (50 mL) and washed with 5% aqueous Na2CO3 (2 × 25 mL) and water (1 × 30 mL). The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was suspended in Et2O (15 mL) and filtered off to give final compounds in high purity.
2-((5-Nitropyridin-3-yl)methylsulfanyl)-5-phenyl-1,3,4-oxadiazole (82a)
Yield: 63% as a yellowish solid; mp 145–147 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.24 (d, J = 2.5 Hz, 1H), 9.06 (d, J = 2.0 Hz, 1H), 8.75 (t, J = 2.3 Hz, 1H), 7.91–7.89 (m, 2H), 7.61–7.51 (m, 3H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 166.02, 163.32, 155.95, 144.60, 144.23, 135.39, 132.64, 132.22, 129.95, 126.96, 123.47, 32.54. Elem. Anal. Calcd for C14H10N4O3S: C, 53.50; H, 3.21; N, 17.83; S, 10.2. Found: C, 53.75; H, 3.26; N, 17.42. S, 10.55.
2-(4-Methoxyphenyl)-5-((5-nitropyridin-3-yl)methylsulfanyl)-1,3,4-oxadiazole (82b)
Yield: 68% as a yellowish solid; mp 117–118 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.24 (d, J = 2.5 Hz, 1H), 9.05 (d, J = 1.8 Hz, 1H), 8.73 (t, J = 2.3 Hz, 1H), 7.83 (d, J = 8.7 Hz, 2H), 7.07 (d, J = 8.9 Hz, 2H), 4.69 (s, 2H), 3.80 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 165.98, 162.66, 162.46, 155.92, 144.60, 144.22, 135.43, 132.20, 128.85, 115.79, 115.40, 56.08, 32.55. Elem. Anal. Calcd for C15H12N4O4S: C, 52.32; H, 3.51; N, 16.27; S, 9.31. Found: C, 52.31; H, 3.40; N, 16.03; N, 9.7.
2-(4-Chlorophenyl)-5-((5-nitropyridin-3-yl)methylsulfanyl)-1,3,4-oxadiazole (82c)
Yield: 70% as a white solid; mp 144–145 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.24 (d, J = 2.5 Hz, 1H), 9.06 (d, J = 2.0 Hz, 1H), 8.74 (t, J = 2.2 Hz, 1H), 7.91 (d, J = 8.7 Hz, 2H), 7.61 (d, J = 8.7 Hz, 2H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.28, 163.62, 155.95, 144.60, 144.24, 137.36, 135.33, 132.23, 130.12, 128.78, 122.37, 32.52. Elem. Anal. Calcd for C14H9ClN4O3S: C, 48.21; H, 2.60; N, 16.06; S, 9.19. Found: C, 48.36; H, 2.48; N, 15.92; S, 9.58.
2-(4-Bromophenyl)-5-((5-nitropyridin-3-yl)methylsulfanyl)-1,3,4-oxadiazole (82d)
Yield: 74% as a beige solid; mp 145–147 °C. 1H NMR (600 MHz, DMSO-d6) δ 9.24 (d, J = 2.5 Hz, 1H), 9.06 (d, J = 2.0 Hz, 1H), 8.74 (t, J = 2.3 Hz, 1H), 7.83 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 4.71 (s, 2H). 13C NMR (151 MHz, DMSO-d6) δ 165.39, 163.64, 155.95, 144.59, 144.24, 135.31, 133.04, 132.22, 128.88, 126.25, 122.70, 32.52. Elem. Anal. Calcd for C14H9BrN4O3S: C, 42.76; H, 2.31; N, 14.25; S, 8.15. Found: C, 43.07; H, 2.23; N, 14.02; S, 8.53.
2-Cyclohexyl-5-((5-nitropyridin-3-yl)methylsulfanyl)-1,3,4-oxadiazole (82e)
Yield: 48% as a beige solid; mp 98–99 °C. 1H NMR (600 MHz, DMSO-D6) δ 9.24 (d, J = 2.5 Hz, 1H), 9.00 (d, J = 1.9 Hz, 1H), 8.68 (t, J = 2.3 Hz, 1H), 4.61 (s, 2H), 2.92–2.80 (m, 1H), 1.94–1.84 (m, 2H), 1.71–1.60 (m, 2H), 1.63–1.53 (m, 1H), 1.47–1.37 (m, 2H), 1.37–1.24 (m, 2H), 1.25–1.09 (m, 1H). 13C NMR (151 MHz, DMSO-D6) δ 171.55, 162.37, 155.93, 144.55, 144.18, 135.44, 132.15, 34.66, 32.44, 29.88, 25.61, 25.13. Elem. Anal. Calcd for C14H16N4O3S: C, 52.49; H, 5.03; N, 17.49; S, 10.01. Found: 52.66; H, 4.64; N, 17.38; S, 10.38.
In Vitro Antimycobacterial Assay
The in vitro antimycobacterial activities of all compounds were evaluated against M. tuberculosis CNCTC My 331/88 (H37Rv), M. avium CNCTC My 330/88, and M. kansasii CNCTC My 235/80 from the Czech National Collection of Type Cultures (CNCTC). The in vitro antimycobacterial activities of selected compounds were evaluated against clinically isolated drug-resistant strains M.tb. 7357/1998, M.tb. 234/2005, M.tb. 9449/2007, M.tb. 8666/2010, M.tb. Praha 1, M.tb. Praha 4, and M.tb. Praha 131. Basic suspensions of the mycobacterial strains were prepared according to a 1.0 McFarland standard. Subsequent dilutions of each strain from the basic suspension were made: M. tuberculosis, 10–3; M. avium, 10–5; and M. kansasii, 10–4. The appropriate dilutions of the strains were prepared, and 0.1 mL of the appropriate solution was added to each well of the microtiter plates containing the compounds. The activities of the compounds were determined via the micromethod for the determination of the MIC in Šula’s semisynthetic medium (SEVAC, Prague). The compounds were dissolved in DMSO and added to the medium at concentrations of 250, 125, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.06, and 0.03 μM for M. tuberculosis and M. kansasii strains and at concentrations of 1000, 500, 250, 125, 64, 32, 16, 8, 4, 2, 1 for M. avium strain. The MIC values, i.e., the lowest concentration of a substance at which mycobacterial growth inhibition occurred (the concentration that inhibited >99% of the mycobacterial population), were determined after incubation at 37 °C for 14 and 21 days for M. tuberculosis and M. avium strains and for 7, 14, and 21 days for M. kansasii strains. Isoniazid (INH) was used as the standard drug.
Cell Proliferation/Viability Assay
HepG2 cells were cultivated in DMEM supplemented with 10% fetal bovine serum and sodium pyruvate (1 mM). The viability assay was carried out using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s protocol. Briefly, the cells were seeded onto the 96-well plates at the density of 30 000 cells/well and allowed to attach for 24 h. After that, the cells were treated with the tested compounds that were predissolved in DMSO to a 1000× concentration and then dissolved in cultivation medium to 1× concentration and vehicle control (0.1% DMSO). The cells were treated for 48 h. After that, the reagent was added to the wells, and the plates were incubated in 37 °C, 5% CO2 for 1 h. After incubation, the absorbance was measured at 490 nm using the Synergy 2 Biotek plate reader (Biotek, Winooski, VT).
Isolation and Characterization of M. tuberculosis Erdman Mutants Resistant to T6030 or T6053
3,5-Dinitrobenzylsulfanyl oxadiazole mutants of M. tuberculosis H37Rv were isolated from 7H9 cultures over 5 passages with increasing concentrations of T6030 or T6053 starting from 2×, 5×, and 10× MIC to final concentrations of 50× and 100× MIC. Single colonies were obtained from three independent cultures by streaking on 7H10 agar plates, and resistance to T6030 and T6053 was measured by REMA. Genomic DNA extraction was performed using the QiaAMP UCP pathogen minikit (Qiagen) as per the manufacturer’s instructions. Whole-genome sequencing was performed using Illumina technology with sequencing libraries prepared using the KAPA HyperPrep kit (Roche) and sequenced on an Illumina HiSeq 2500 instrument. All raw reads were adapter and quality trimmed with Trimmomatic v0.3332 and mapped onto the M. tuberculosis H37Rv reference genome (RefSeq no. NC_000962.3) using Bowtie2 v2.2.5.33 The bamleftalign program from the FreeBayes package v0.9.20–1834 was used to left-align indels. Reads with a mapping quality below 8 and duplicate reads were omitted.
Variant Analysis
Variant calling was done using VarScan v2.3.935 using the following cutoffs: minimum overall coverage of 10 nonduplicated reads, minimum of 5 nonduplicated reads supporting the SNP, base quality score of >15, and an SNP frequency above 30%. The rather low thresholds, especially the SNP frequency, were deliberately chosen to avoid missing potential variants in regions where alignment was difficult or in the case of a mixed population. All putative variants unique to the mutant strains were manually checked by inspecting the alignments.
Testing Antimycobacterial Activity of the Selected Target Compounds in Ddn- and FbiC-deficient M.tb. H37Rv Strains
The parental M.tb. H37Rv strain harboring an integrative gfp-encoding plasmid carrying hygromycin resistance cassette, as well as the derived Ddn– (DdnL49P) and FbiC– (fbicSTOP, F420−) mutant strains were grown shaking (120 rpm) at 37 °C in 7H9 medium supplemented with 10% ADC, 0.05% Tween 80, and hygromycin (40 μg/mL) to early logarithmic phase. Each culture was then diluted to OD600 = 0.1 and 2.5 μL was spotted on 7H11-agar plates supplemented with 10% OADC and the tested compounds in concentrations corresponding to 0×, 0.5×, 1×, 3×, 10×, and 30× MIC. The plates were incubated at 37 °C, and the growth was recorded after 12 days.
Evaluation of the Effects of the Selected Target Compounds on M.tb. H37Rv Lipids by [14C]Acetate Metabolic Labeling
M.tb. H37Rv was grown shaking (120 rpm) at 37 °C in 7H9 medium supplemented with 10% ADC and 0.05% Tween 80 until OD600 = 0.18. The culture aliquots (100 μL) were transferred to Eppendorf tubes containing the tested compounds (2 μL of the stock solutions in DMSO) to achieve the final concentrations corresponding to 10× and 100× MIC. At the same time [14C]acetate (specific activity: 110 mCi/mmol, American Radiolabeled Chemicals, Inc.) was added at 0.5 μCi/mL, and the cultures were incubated statically at 37 °C for 24 h. The lipids were then extracted with CHCl3/CH3OH (2:1), analyzed by TLC [Silica Gel TLC plate (Merck), CHCl3/CH3OH/H2O (40:8:1)] as described,11 and visualized by Amersham Typhoon 5 phosphorimager (GE Healthcare).
Examination of DprE1 Target Specificity of the Selected Compounds in M. tb. H37Ra
The experiment was performed as recently described, using M. tb. H37Ra overproducing DprE1 and DprE2 proteins from M. tb. H37Rv.36 Briefly, the early log cultures of M. tb. H37Ra transformed with pVV2-dprE1-dprE2 plasmid37 and the empty plasmid strain M. tb. H37Ra/pVV2 were diluted to OD600 = 0.5 and 0.25 with the growth medium, 7H9 medium supplemented with 10% ADC, 0.05% Tween 80, and kanamycin (20 μg/mL). Aliquots of 3 μL of each culture were spotted on 7H11-agar plates supplemented with 10% OADC, kanamycin (20 μg/mL), and the tested compounds at 0×, 0.25×, 0.5×, 1×, 3×, 10×, and 30× MIC. The plates were incubated at 37 °C, and the growth was evaluated after 12 days.
Acknowledgments
This study was supported by Czech Health Research Council (grant NU21-05-00446); by the project National Institute of virology and bacteriology (Programme EXCELES, ID Project No. LX22NPO5103) - Funded by the European Union - Next Generation EU; the Slovak Research and Development Agency [grant n. APVV-19-0189] and the OPII, ACCORD, ITMS2014+: 313021X329, cofinanced by ERDF.
Glossary
Abbreviations
- DMSO
dimethyl sulfoxide
- CNCTC
Czech National Collection of Type Cultures
- CL
cardiolipin
- Ddn
deazaflavin-dependent nitroreductase
- DprE1
decaprenylphosphoryl-β-D-ribose 2′-oxidase
- FbiC
7,8-didemethyl-8-hydroxy-5-deazariboflavin (FO) synthase
- FGD1
F420-dependent glucose-6-phosphate dehydrogenase
- HRMS
high-resolution mass spectrometry
- INH
isoniazid
- mCPBA
meta-chloroperoxybenzoic acid
- MDR
multidrug-resistant
- MIC
minimum inhibitory concentration
- PE
phosphatidylethanolamine
- SDS
sodium dodecyl sulfate
- RIF
rifampicin
- TB
tuberculosis
- TMM
trehalose monomycolates
- TDM
trehalose dimycolates
- THF
tetrahydrofuran
- TLC
thin layer chromatography
- XDR
extensively drug-resistant
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.3c00925.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Global tuberculosis report 2022. World Health Organization, Geneva: 2022. Licence: CC BY-NC-SA 3.0 IGO.
- Gler M. T.; Skripconoka V.; Sanchez-Garavito E.; Xiao H. P.; Cabrera-Rivero J. L.; Vargas-Vasquez D. E.; Gao M. Q.; Awad M.; Park S. K.; Shim T. S.; Suh G. Y.; Danilovits M.; Ogata H.; Kurve A.; Chang J.; Suzuki K.; Tupasi T.; Koh W. J.; Seaworth B.; Geiter L. J.; Wells C. D. Delamanid for Multidrug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2012, 366, 2151–2160. 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- Singh R.; Manjunatha U.; Boshoff H. I. M.; Ha Y. H.; Niyomrattanakit P.; Ledwidge R.; Dowd C. S.; Lee I. Y.; Kim P.; Zhang L.; Kang S.; Keller T. H.; Jiricek J.; Barry C. E. III PA-824 Kills Nonreplicating Mycobacterium tuberculosis by Intracellular NO Release. Science 2008, 322, 1392–1395. 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellitti S. E.; Shaffer J.; Jones D. H.; Mukherjee T.; Gurumurthy M.; Bursulaya B.; Boshoff H. I.; Choi I.; Nayyar A.; Lee Y. S.; Cherian J.; Niyomrattanakit P.; Dick T.; Manjunatha U. H.; Barry C. E.; Spraggon G.; Geierstanger B. H. Structure of Ddn, the Deazaflavin-Dependent Nitroreductase from Mycobacterium tuberculosis Involved in Bioreductive Activation of PA-824. Structure 2012, 20, 101–112. 10.1016/j.str.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov V.; Manina G.; Mikusova K.; Mollmann U.; Ryabova O.; Saint-Joanis B.; Dhar N.; Pasca M. R.; Buroni S.; Lucarelli A. P.; Milano A.; De Rossi E.; Belanova M.; Bobovska A.; Dianiskova P.; Kordulakova J.; Sala C.; Fullam E.; Schneider P.; McKinney J. D.; Brodin P.; Christophe T.; Waddell S.; Butcher P.; Albrethsen J.; Rosenkrands I.; Brosch R.; Nandi V.; Bharath S.; Gaonkar S.; Shandil R. K.; Balasubramanian V.; Balganesh T.; Tyagi S.; Grosset J.; Riccardi G.; Cole S. T. Benzothiazinones Kill Mycobacterium tuberculosis by Blocking Arabinan Synthesis. Science 2009, 324, 801–804. 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trefzer C.; Skovierova H.; Buroni S.; Bobovska A.; Nenci S.; Molteni E.; Pojer F.; Pasca M. R.; Makarov V.; Cole S. T.; Riccardi G.; Mikusova K.; Johnsson K. Benzothiazinones Are Suicide Inhibitors of Mycobacterial Decaprenylphosphoryl-beta-D-ribofuranose 2’-Oxidase DprE1. J. Am. Chem. Soc. 2012, 134, 912–915. 10.1021/ja211042r. [DOI] [PubMed] [Google Scholar]
- Trefzer C.; Rengifo-Gonzalez M.; Hinner M. J.; Schneider P.; Makarov V.; Cole S. T.; Johnsson K. Benzothiazinones: Prodrugs That Covalently Modify the Decaprenylphosphoryl-beta-D-ribose 2’-epimerase DprE1 of Mycobacterium tuberculosis. J. Am. Chem. Soc. 2010, 132, 13663–13665. 10.1021/ja106357w. [DOI] [PubMed] [Google Scholar]
- Working Group on New TB Drugs: Clinical Pipeline. https://www.newtbdrugs.org/pipeline/clinical (accessed Oct 5, 2023).
- Němeček J.; Sychra P.; Macháček M.; Benková M.; Karabanovich G.; Konečná K.; Kavková V.; Stolaříková J.; Hrabálek A.; Vávrová K.; Soukup O.; Roh J.; Klimešová V. Structure-Activity Relationship Studies on 3,5-Dinitrophenyl Tetrazoles as Antitubercular Agents. Eur. J. Med. Chem. 2017, 130, 419–432. 10.1016/j.ejmech.2017.02.058. [DOI] [PubMed] [Google Scholar]
- Karabanovich G.; Němeček J.; Valášková L.; Carazo A.; Konečná K.; Stolaříková J.; Hrabálek A.; Pavliš O.; Pávek P.; Vávrová K.; Roh J.; Klimešová V. S-Substituted 3,5-Dinitrophenyl 1,3,4-Oxadiazole-2-thiols and Tetrazole-5-thiols as Highly Efficient Antitubercular Agents. Eur. J. Med. Chem. 2017, 126, 369–383. 10.1016/j.ejmech.2016.11.041. [DOI] [PubMed] [Google Scholar]
- Karabanovich G.; Dušek J.; Savková K.; Pavliš O.; Pávková I.; Korábečný J.; Kučera T.; KočováVlčková H.; Huszár S.; Konyariková Z.; Konečná K.; Jand’ourek O.; Stolaříková J.; Korduláková J.; Vávrová K.; Pávek P.; Klimešová V.; Hrabálek A.; Mikušová K.; Roh J. Development of 3,5-Dinitrophenyl-Containing 1,2,4-Triazoles and Their Trifluoromethyl Analogues as Highly Efficient Antitubercular Agents Inhibiting Decaprenylphosphoryl-β-d-ribofuranose 2′-Oxidase. J. Med. Chem. 2019, 62, 8115–8139. 10.1021/acs.jmedchem.9b00912. [DOI] [PubMed] [Google Scholar]
- Karabanovich G.; Roh J.; Smutný T.; Němeček J.; Vicherek P.; Stolaříková J.; Vejsová M.; Dufková I.; Vávrová K.; Pávek P.; Klimešová V.; Hrabálek A. 1-Substituted-5-[(3,5-Dinitrobenzyl)sulfanyl]-1H-Tetrazoles and Their Isosteric Analogs: A New Class of Selective Antitubercular Agents Active against Drug-Susceptible and Multidrug-Resistant Mycobacteria. Eur. J. Med. Chem. 2014, 82, 324–340. 10.1016/j.ejmech.2014.05.069. [DOI] [PubMed] [Google Scholar]
- Karabanovich G.; Zemanova J.; Smutny T.; Szekely R.; Sarkan M.; Centarova I.; Vocat A.; Pavkova I.; Conka P.; Nemecek J.; Stolarikova J.; Vejsova M.; Vavrova K.; Klimesova V.; Hrabalek A.; Pavek P.; Cole S. T.; Mikusova K.; Roh J. Development of 3,5-Dinitrobenzylsulfanyl-1,3,4-oxadiazoles and Thiadiazoles as Selective Antitubercular Agents Active Against Replicating and Nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 2362–2380. 10.1021/acs.jmedchem.5b00608. [DOI] [PubMed] [Google Scholar]
- Christophe T.; Jackson M.; Jeon H. K.; Fenistein D.; Contreras-Dominguez M.; Kim J.; Genovesio A.; Carralot J. P.; Ewann F.; Kim E. H.; Lee S. Y.; Kang S.; Seo M. J.; Park E. J.; Skovierova H.; Pham H.; Riccardi G.; Nam J. Y.; Marsollier L.; Kempf M.; Joly-Guillou M. L.; Oh T.; Shin W. K.; No Z.; Nehrbass U.; Brosch R.; Cole S. T.; Brodin P. High Content Screening Identifies Decaprenyl-Phosphoribose 2’ Epimerase as a Target for Intracellular Antimycobacterial Inhibitors. PLoS Pathog. 2009, 5, e1000645 10.1371/journal.ppat.1000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanovich G.; Roh J.; Soukup O.; Pávková I.; Pasdiorová M.; Tambor V.; Stolaříková J.; Vejsová M.; Vávrová K.; Klimešová V.; Hrabálek A. Tetrazole Regioisomers in the Development of Nitro Group-Containing Antitubercular Agents. Med. Chem. Commun. 2015, 6, 174–181. 10.1039/C4MD00301B. [DOI] [Google Scholar]
- Nepali K.; Lee H.-Y.; Liou J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. 10.1021/acs.jmedchem.8b00147. [DOI] [PubMed] [Google Scholar]
- Haver H. L.; Chua A.; Ghode P.; Lakshminarayana S. B.; Singhal A.; Mathema B.; Wintjens R.; Bifani P. Mutations in Genes for the F420 Biosynthetic Pathway and a Nitroreductase Enzyme Are the Primary Resistance Determinants in Spontaneous In Vitro-Selected PA-824-Resistant Mutants of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 5316–5323. 10.1128/AAC.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadura S.; King N.; Nakhoul M.; Zhu H.; Theron G.; Köser C. U.; Farhat M. Systematic Review of Mutations Associated with Resistance to the New and Repurposed Mycobacterium tuberculosis Drugs Bedaquiline, Clofazimine, Linezolid, Delamanid and Pretomanid. J. Antimicrob. Chemother. 2020, 75, 2031–2043. 10.1093/jac/dkaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M.; Kawasaki M.; Hariguchi N.; Liu Y.; Matsumoto M. Mechanisms of Resistance to Delamanid, a Drug for Mycobacterium tuberculosis. Tuberculosis 2018, 108, 186–194. 10.1016/j.tube.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Jian Y.; Forbes H. E.; Hulpia F.; Risseeuw M. D. P.; Caljon G.; Munier-Lehmann H.; Boshoff H. I. M.; Van Calenbergh S. 2-((3,5-Dinitrobenzyl)thio)quinazolinones: Potent Antimycobacterial Agents Activated by Deazaflavin (F420)-Dependent Nitroreductase (Ddn). J. Med. Chem. 2021, 64, 440–457. 10.1021/acs.jmedchem.0c01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon N. M.; Pak C. S.; Brown H. C.; Krishnamurthy S.; Stocky T. P. Selective Reductions.19. Rapid Reaction of Carboxylic-Acids with Borane-Tetrahydrofuran - Remarkably Convenient Procedure for Selective Conversion of Carboxylic-Acids to Corresponding Alcohols in Presence of Other Functional Groups. J. Org. Chem. 1973, 38, 2786–2792. 10.1021/jo00956a011. [DOI] [Google Scholar]
- Rajesh K.; Somasundaram M.; Saiganesh R.; Balasubramanian K. K. Bromination of Deactivated Aromatics: A Simple and Efficient Method. J. Org. Chem. 2007, 72, 5867–5869. 10.1021/jo070477u. [DOI] [PubMed] [Google Scholar]
- Weissman S. A.; Zewge D.; Chen C. Ligand-Free Palladium-Catalyzed Cyanation of Aryl Halides. J. Org. Chem. 2005, 70, 1508–1510. 10.1021/jo0481250. [DOI] [PubMed] [Google Scholar]
- Nagano H.; Hamana M.; Nawata Y. The Mechanism of the Reaction of Nicotinic Acid 1 Oxide with Acetic Anhydride. Chem. Pharm. Bull. 1987, 35, 4068–4077. 10.1248/cpb.35.4068. [DOI] [Google Scholar]
- Ashimori A.; Ono T.; Uchida T.; Ohtaki Y.; Fukaya C.; Watanabe M.; Yokoyama K. Novel 1,4-Dihydropyridine Calcium Antagonists. I. Synthesis and Hypotensive Activity of 4-(Substituted pyridyl)-1,4-dihydropyridine Derivatives. Chem. Pharm. Bull. 1990, 38, 2446–2458. 10.1248/cpb.38.2446. [DOI] [PubMed] [Google Scholar]
- Gribble A. D.; Dolle R. E.; Shaw A.; McNair D.; Novelli R.; Novelli C. E.; Slingsby B. P.; Shah V. P.; Tew D.; Saxty B. A.; Allen M.; Groot P. H.; Pearce N.; Yates J. ATP-Citrate Lyase as a Target for Hypolipidemic Intervention. Design and Synthesis of 2-Substituted Butanedioic Acids as Novel, Potent Inhibitors of the Enzyme. J. Med. Chem. 1996, 39, 3569–3584. 10.1021/jm960167w. [DOI] [PubMed] [Google Scholar]
- Jones P. R.; Rothenberger S. D. Nucleophilic Aromatic Substitution with Elimination in a Dinitrosalicylic Lactone or Ester via Meisenheimer Intermediates. J. Org. Chem. 1986, 51, 3016–3023. 10.1021/jo00365a031. [DOI] [Google Scholar]
- Rakesh; Bruhn D. F.; Scherman M. S.; Woolhiser L. K.; Madhura D. B.; Maddox M. M.; Singh A. P.; Lee R. B.; Hurdle J. G.; McNeil M. R.; Lenaerts A. J.; Meibohm B.; Lee R. E. Pentacyclic Nitrofurans with In Vivo Efficacy and Activity against Nonreplicating Mycobacterium tuberculosis. PLoS One 2014, 9, e87909 10.1371/journal.pone.0087909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh; Bruhn D.; Madhura D. B.; Maddox M.; Lee R. B.; Trivedi A.; Yang L.; Scherman M. S.; Gilliland J. C.; Gruppo V.; McNeil M. R.; Lenaerts A. J.; Meibohm B.; Lee R. E. Antitubercular Nitrofuran Isoxazolines with Improved Pharmacokinetic Properties. Bioorg. Med. Chem. 2012, 20, 6063–6072. 10.1016/j.bmc.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.; Karabanovich G.; Vlckova H.; Carazo A.; Nemecek J.; Sychra P.; Valaskova L.; Pavlis O.; Stolarikova J.; Klimesova V.; Vavrova K.; Pavek P.; Hrabalek A. Development of Water-Soluble 3,5-Dinitrophenyl Tetrazole and Oxadiazole Antitubercular Agents. Bioorg. Med. Chem. 2017, 25, 5468–5476. 10.1016/j.bmc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Makarov V.; Neres J.; Hartkoorn R. C.; Ryabova O. B.; Kazakova E.; Šarkan M.; Huszár S.; Piton J.; Kolly G. S.; Vocat A.; Conroy T. M.; Mikušová K.; Cole S. T. The 8-Pyrrole-Benzothiazinones Are Noncovalent Inhibitors of DprE1 from Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 4446–4452. 10.1128/AAC.00778-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M.; Lohse M.; Usadel B. Trimmomatic: a Flexible Trimmer for Illumina Sequence Data. Bioinform. 2014, 30, 2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B.; Salzberg S. L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison E.; Marth G.. Haplotype-Based Variant Detection from Short-Read Sequencing. arXiv 1207.3907v2 [q-bio.GN], 2012, https://arxiv.org/abs/1207.3907. [Google Scholar]
- Koboldt D. C.; Zhang Q.; Larson D. E.; Shen D.; McLellan M. D.; Lin L.; Miller C. A.; Mardis E. R.; Ding L.; Wilson R. K. VarScan 2: Somatic Mutation and Copy Number Alteration Discovery in Cancer by Exome Sequencing. Genome Res. 2012, 22, 568–576. 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflégr V.; Stolaříková J.; Pál A.; Korduláková J.; Krátký M. Novel Pyrimidine-1,3,4-Oxadiazole Hybrids and Their Precursors as Potential Antimycobacterial Agents. Future Med. Chem. 2023, 15, 1049–1067. 10.4155/fmc-2023-0096. [DOI] [PubMed] [Google Scholar]
- Brecik M.; Centárová I.; Mukherjee R.; Kolly G. S.; Huszár S.; Bobovská A.; Kilacsková E.; Mokošová V.; Svetlíková Z.; Šarkan M.; Neres J.; Korduláková J.; Cole S. T.; Mikušová K. DprE1 Is a Vulnerable Tuberculosis Drug Target Due to Its Cell Wall Localization. ACS Chem. Biol. 2015, 10, 1631–1636. 10.1021/acschembio.5b00237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.