Abstract
Background
Coronavirus disease 2019 (COVID-19) has affected individuals worldwide, and patients with cancer are particularly vulnerable to COVID-19-related severe illness, respiratory failure, and mortality. The relationship between COVID-19 and cancer remains a critical concern, and a comprehensive investigation of the factors affecting survival among patients with cancer who develop COVID-19-related respiratory failure is warranted. We aim to compare the characteristics and outcomes of COVID-19-related acute respiratory failure in patients with and without underlying cancer, while analyzing factors affecting in-hospital survival among cancer patients.
Methods
We conducted a retrospective observational study at Taipei Veterans General Hospital in Taiwan from May to September 2022, a period during which the omicron variant of the severe acute respiratory syndrome coronavirus 2 was circulating. Eligible patients had COVID-19 and acute respiratory failure. Clinical data, demographic information, disease severity markers, treatment details, and outcomes were collected and analyzed.
Results
Of the 215 enrolled critically ill patients with COVID-19, 65 had cancer. The patients with cancer were younger and had lower absolute lymphocyte counts, higher ferritin and lactate dehydrogenase (LDH) concentrations, and increased vasopressor use compared with those without cancer. The patients with cancer also received more COVID-19 specific treatments but had higher in-hospital mortality rate (61.5% vs 36%, P = 0.002) and longer viral shedding (13 vs 10 days, P = 0.007) than those without cancer did. Smoking [odds ratio (OR): 5.804, 95% confidence interval (CI): 1.847–39.746], elevated LDH (OR: 1.004, 95% CI: 1.001–1.012), vasopressor use (OR: 5.437, 95% CI: 1.202–24.593), and new renal replacement therapy (OR: 3.523, 95% CI: 1.203–61.108) were independent predictors of in-hospital mortality among patients with cancer and respiratory failure.
Conclusion
Critically ill patients with cancer experiencing COVID-19-related acute respiratory failure present unique clinical features and worse clinical outcomes compared with those without cancer. Smoking, elevated LDH, vasopressor use, and new renal replacement therapy were risk factors for in-hospital mortality in these patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-02850-z.
Keywords: Acute respiratory failure, Coronavirus disease 2019 (COVID-19), Malignancy, Vasopressor, Inflammatory marker
Background
The COVID-19 pandemic posed a considerable global challenge. The pandemic resulted in up to 6.9 million deaths (till November 2023, according to the data from World Health Organization) [1] and placed substantial burdens on health-care systems worldwide. Individuals with certain pre-existing conditions are particularly susceptible to COVID-19, and the relationship between COVID-19 and cancer has become a crucial and concerning issue. Patients with cancer are more susceptible to severe illness from COVID-19 than are those without cancer, which may be due to the presence of concurrent comorbidities, the inherent immunosuppressive characteristics of cancer, and the immunosuppression induced by systemic cancer treatments [2], with mortality rates as high as 25% being reported for patients with solid organ malignancies [3]. Respiratory failure is a severe complication of COVID-19 that typically occurs approximately 1 week after the onset of symptoms. Respiratory failure is usually accompanied by thrombosis and acute renal failure [4]. Treatment strategies for COVID-19-related respiratory failure are similar to those established for acute respiratory distress syndrome (ARDS) [5], and include oxygen therapy; lung-protective ventilation; prone positioning; supportive care; and administration of specific medications, such as corticosteroids, antiviral agents, immunomodulators, and anticoagulants [4–6]. Treatment for COVID-19-related respiratory failure among patients with cancer requires a multidisciplinary approach. The risk of death from COVID-19 among cancer patients is influenced by age; male sex; performance status; comorbidities; and hematological malignancies [7–10]. Whether recent cancer treatment influence survival remains controversial [2, 11, 12]. Understanding the factors that increase the risk of death from COVID-19 is crucial for optimizing patient management and improving outcomes. This study aims to investigate and compare the characteristics and outcomes among patients experiencing COVID-19-related acute respiratory failure between individuals with and without underlying cancer, while further analyzing the factors influencing in-hospital survival among cancer patients.
Methods
This retrospective observational study was conducted at Taipei Veterans General Hospital, a tertiary medical center in Taiwan, between May and September 2022. During this period, the omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was circulating in Taiwan. Patients were included in this study if they were infected with SARS-CoV-2 and experienced acute respiratory failure, defined as requiring high-flow nasal cannula (HFNC), or noninvasive ventilation (NIV), or mechanical ventilation (MV). SARS-CoV-2 infection was confirmed through reverse transcription polymerase chain reaction (RT-PCR) by using the Roche Cobas 6800 system (Roche Diagnostics, Rotkreuz, Switzerland).
Electronic medical records were reviewed to collect clinical information. Patients with advanced stage or metastatic cancer and those without remission were included. Other demographic data, including age, sex, body mass index (BMI), smoking and vaccination history, underlying diseases, do not resuscitate (DNR) code status, laboratory results on admission, and severity, were also obtained. Severity was assessed on the day of respiratory failure, including sequential organ failure assessment (SOFA) scores, Mean arterial pressure (MAP) scores (derived from the SOFA score, accounted for the administration of vasoactive agents, rating as 0 (no hypotension), 1 (mean arterial pressure < 70 mmHg), 2 (dopamine ≤5 mcg/kg/min or any dose of dobutamine), 3 (dopamine > 5 mcg/kg/min, epinephrine ≤0.1 mcg/kg/min, or norepinephrine ≤0.1 mcg/kg/min), and 4 (dopamine > 15 mcg/kg/min, epinephrine > 0.1 mcg/kg/min, or norepinephrine > 0.1 mcg/kg/min) [13], Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score [14], Glasgow coma scale [15], vasopressor usage, PaO2/FiO2 ratio (estimated as the ratio of arterial oxygen partial pressure [PaO2 in mmHg] to fractional inspired oxygen) [16] were collected upon the day of respiratory failure. Treatment information, including receiving corticosteroids, tocilizumab, remdesivir, nirmatrelvir/ritonavir, molnupiravir, and enoxaparin; surgery; and new renal replacement therapy during admission, was also reviewed. Cytomegalovirus (CMV) infection, gastrointestinal bleeding, and thromboembolism were included as disease-related complications. Clinical courses and outcomes, such as the use of MV and ECMO, in-hospital mortality, and duration from the onset of symptoms until the day the cycle threshold (Ct) value exceeded 30, were also recorded [17]. Studies revealed a Ct value of 30 or higher to be non-infectious, with no virus isolated from culture [18]. In addition, a Ct value of at 30 or higher is the threshold for isolation release set by the Taiwan Center for Disease Control [19].
Statistical analysis
The baseline characteristics were summarized using descriptive statistics, and continuous variables were expressed as medians and interquartile ranges. The Mann-Whitney U test was employed to assess differences in distribution between two independent groups for non-normally distributed continuous variables. Pearson’s chi-square test or Fisher’s exact test were used to examine variations in the distribution of categorical variables across different groups. In-hospital survival time and time to reach Ct > 30 among the patients with and without cancer were plotted using the Kaplan-Meier method and compared using a log-rank test. Cox proportional hazard models were used to assess the factors associated with in-hospital mortality, and factors with P < 0.1 in univariable analysis were incorporated into multivariable analysis. Statistical significance was indicated by P < 0.05. Statistical analyses were performed using IBM SPSS Statistics, version 26.0 (IBM, Armonk, NY, USA).
Results
In total, 215 patients with COVID-19-related acute respiratory failure were enrolled. Among these patients, 65 had cancer. The patient characteristics, laboratory results, disease severity on the day of respiratory failure, treatment, complications, and outcomes are summarized in Table 1.
Table 1.
Characteristics between COVID-19 patients with respiratory failure with and without cancer
| All cases (n = 215) |
Cancer (n = 65) |
No Cancer (n = 150) |
P value*** | |
|---|---|---|---|---|
| Demographics | ||||
| Age, year, median | 80 | 73 | 82 | 0.001 |
| Male | 145(67.4) | 43(66.2) | 102(68) | 0.791 |
| Body mass index, kg/m2, median | 21.9 | 22.84 | 21.71 | 0.521 |
| BMIa < 18 | 36(18.3) | 18.5(20.3) | 24(16) | 0.624 |
| BMI > 24 | 62(31.5) | 23(35.4) | 39(26) | 0.138 |
| Vaccination doses, median | 2 | 2 | 2 | 0.392 |
| Ever vaccinated | 143(66.5) | 45(69.2) | 98(65.3) | 0.730 |
| Fully vaccinated (> = 3 doses) | 93(43.3) | 32(49.2) | 61(40.7) | 0.244 |
| Smoker | 53(24.7) | 20(30.8) | 33(22) | 0.171 |
| DNRa | 146(67.9) | 47(72.3) | 99(66) | 0.363 |
| Comorbidity | ||||
| Cerebrovascular disease | 37(17.2) | 6(9.2) | 31(20.7) | 0.041 |
| Dementia | 21(14.4) | 5(7.7) | 26(17.3) | 0.065 |
| Heart failure | 22(10.2) | 1(1.5) | 21(14%) | 0.003 |
| Myocardial infarction | 3(1.4) | 0 | 3(2) | 0.338 |
| Peripheral vascular disease | 11(5.1) | 2(3.1) | 9(6) | 0.372 |
| Diabetes mellitus | 86(40) | 22(33.8) | 64(42.7) | 0.225 |
| Chronic kidney disease | 50(23.3) | 12(19.5) | 34(24) | 0.539 |
| End stage renal disease | 24(11.2) | 5(7.7) | 19(12.7) | 0.287 |
| Peptic ulcer | 8(3.7) | 0 | 8(5.3) | 0.057 |
| Hepatobiliary disease | 21(9.8) | 11(16.9) | 10(6.7) | 0.051 |
| Chronic obstructive pulmonary disease | 14(6.5) | 4(6.2) | 10(6.7) | 0.889 |
| Bronchiectasis | 1(0.5) | 0 | 1(0.7) | 0.696 |
| Interstitial lung disease | 2(0.9) | 1(1.5) | 1(0.7) | 0.516 |
| Chronic oxygen use | 9(4.2) | 3(4.6) | 6(4) | 0.546 |
| Laboratory data on the day of respiratory failure (median) | ||||
| White blood cells, 109/L | 11,150 | 10,060 | 11,640 | 0.800 |
| Absolute lymphocyte count, 109/L | 708 | 546.8 | 781.6 | 0.003 |
| Albumin, g/dL | 3.05 | 3.1 | 3 | 0.795 |
| C-reactive protein, mg/dL | 6.1 | 6.89 | 5.91 | 0.331 |
| Procalcitonin, ng/mL | 0.72 | 0.68 | 0.75 | 0.909 |
| Ferritin, ng/mL | 668 | 1035 | 529 | 0.002 |
| Lactic dehydrogenase, U/L | 363 | 423 | 339 | 0.010 |
| Lactate, mg/dL | 23.3 | 26.4 | 23.15 | 0.274 |
| D-dimer, ug/mL | 2.472 | 2.35 | 2.62 | 0.600 |
| Fibrinogen, mg/dL | 381 | 378.1 | 390.7 | 0.453 |
| Platelet count, /uL | 182,000 | 159,000 | 186,500 | 0.090 |
| Severity on the day of respiratory failure | ||||
| PaO2/FiO2 ratio, median | 140 | 134.9 | 144 | 0.437 |
| SOFAa score, median | 8 | 8 | 8 | 0.705 |
| APACHE IIa score, median | 24 | 24 | 24 | 0.612 |
| MAP scoreb, median | 1 | 1 | 0.5 | 0.022 |
| Vasopressor use | 70(32.6) | 28(43.1) | 42(28) | 0.030 |
| GCSa, median | 7 | 8 | 7 | 0.286 |
| Treatment | ||||
| Mechanical ventilation | 131(60.9) | 42(64.6) | 89(59.3) | 0.466 |
| Surgery | 60(27.9) | 16(24.6) | 44(29.3) | 0.479 |
| New renal replacement therapy during admission | 21(9.8) | 10(15.4) | 11(7.3) | 0.068 |
| Extracorporeal membrane oxygenation | 8(3.7) | 5(7.7) | 3(2) | 0.056 |
| Tocilizumab | 76(35.3) | 30(46.2) | 46(30.7) | 0.029 |
| Remdesivir | 169(78.6) | 59(90.8) | 110(73.3) | 0.004 |
| Nirmatrelvir/ritonavir | 6(2.8) | 4(6.2) | 2(1.3) | 0.117 |
| Molnupiravir | 11(5.1) | 2(3.1) | 9(6) | 0.511 |
| Enoxaparin | 72(33.5) | 22(33.8) | 50(33.3) | 0.942 |
| Corticosteroid | 184(85.6) | 61(93.8) | 123(82) | 0.023 |
| Complications | ||||
| CMVa infection | 38(17.7) | 16(24.6) | 22(14.7) | 0.198 |
| Gastrointestinal bleeding | 62(28.8) | 17(26.2) | 45(30) | 0.567 |
| Thromboembolism | 13(6.0) | 7(10.8) | 6(4) | 0.084 |
| Outcomes | ||||
| ICUa admission | 159(77.6) | 48(73) | 111(74) | 0.987 |
| Hospital length of stay, days, median | 27 | 28 | 25 | 0.971 |
| In-hospital mortality | 94(43.7) | 40(61.5) | 54(36) | 0.002 |
| 28 days mortality | 69(30.7) | 24(36.9) | 42(28) | 0.193 |
| Time from symptoms onset to 1st Cta > 30, days, median | 11 | 13 | 10 | 0.007 |
aBMI, Body mass index; DNR, Do not resuscitate; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation; MAP, Mean arterial pressure; GCS, Glasgow coma scale; CMV, Cytomegalovirus; ICU, Intensive Care Unit; Ct, cycle threshold
bMAP score is defined from the calculation of SOFA score, with inotropic doses as mcg/kg/min: 0, No hypotension; 1, MAP < 70 mmHg; 2, Dopamine ≤5 or Dobutamine (any dose); 3, Dopamine > 5, Epinephrine ≤0.1, or norepinephrine ≤0.1; 4, Dopamine > 15, Epinephrine > 0.1, or Norepinephrine > 0.1
*** Between patients with and without malignancy
The patients with cancer were younger than those without cancer (median age 73 vs 82 years, P = 0.001). Furthermore, the patients with cancer had lower prevalence rates of cerebrovascular accidents (9.2% vs 20.7%, P = 0.041) and heart failure (1.5% vs 14%, P = 0.003) than did the patients without cancer.
The patients with cancer had lower absolute lymphocyte counts (median 546.8 vs 781.6 × 109/L, P = 0.003) and higher concentrations of ferritin (1035 vs 529 ng/mL, P = 0.002) and lactate dehydrogenase (LDH; median 423 vs 339 U/L, P = 0.01) on the day of respiratory failure than did the patients without cancer. The patients with cancer also had higher mean arterial pressure scores (median 1 vs 0.5, P = 0.022) and a higher prevalence of vasopressor use (43.1% vs 28%, P = 0.03) on the day of respiratory failure than did the patients without cancer.
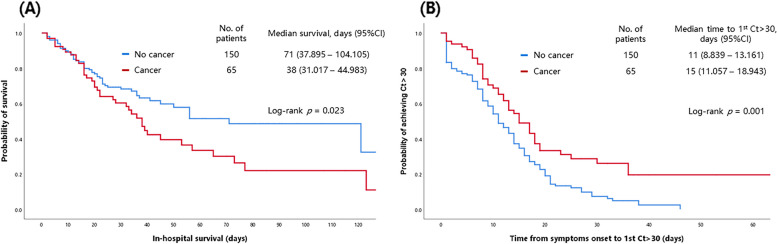
The patients with cancer were more likely to receive remdesivir (90.8% vs 73.3%, P = 0.004), tocilizumab (46.2% vs 30.7%, P = 0.029), and corticosteroids (93.8% vs 82%, P = 0.023) than were the patients without cancer. In terms of outcomes, the patients with cancer were significantly more likely to die in hospital (in-hospital mortality rate 61.5% vs 36%, P = 0.002) and took longer to reach Ct > 30 (median 13 vs 10 days, P = 0.007) than did the patients without cancer. The in-hospital survival and time to reach Ct > 30 in patients with and without cancer are illustrated in Fig. 1.
Fig. 1.
The in-hospital survival and time to reach Ct > 30 in patients with and without cancer. CI, confidence interval; Ct, cycle threshold
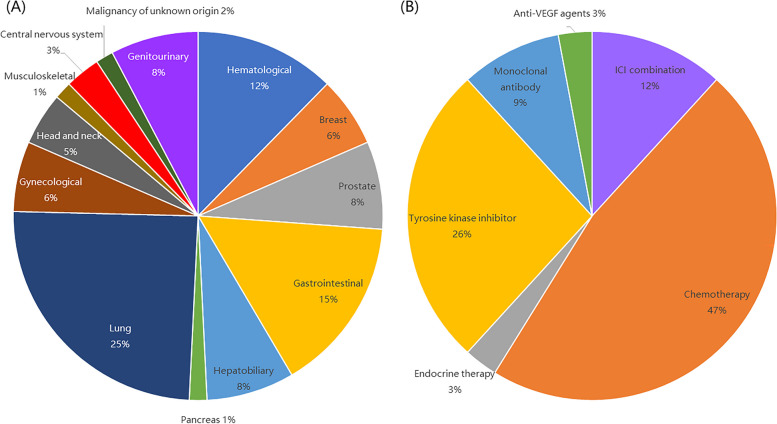
The characteristics of the 65 patients with cancer are summarized in Table 2 and Fig. 2.
Table 2.
Additional characteristics among COVID-19 cancer patients with respiratory failure. (n = 65)
| n (%) | |
|---|---|
| Cancer site | |
| Hematological | 8 (12.3) |
| Lymphoma | 7(10.8) |
| Myeloproliferative neoplasm | 1(1.5) |
| Solid tumors | 57 (87.7) |
| Breast | 4(6.2) |
| Prostate | 5(7.7) |
| Gastrointestinal | 10(15.4) |
| Hepatocellular carcinoma | 4(6.2) |
| Biliary tract | 1(1.5) |
| Pancreas | 1(1.5) |
| Lung | 16(24.6) |
| Gynecological | 4(6.2) |
| Head and neck | 3(4.6) |
| Genitourinary | 5(7.7) |
| Musculoskeletal | 1(1.5) |
| Central nervous system | 2(3.1) |
| Malignancy of unknown origin | 1(1.5) |
| Cancer treatment within 4 weeks of COVID-19 diagnosis | 34 (52.3) |
| ICI* combination | 4 (6.2) |
| ICI + chemotherapy | 3 (4.6) |
| ICI + TKI* | 1(1.5) |
| Cytotoxic chemotherapy | 16 (24.6) |
| Intravenous chemotherapy | 12 (18.5) |
| Oral chemotherapy | 4 (6.2) |
| Endocrine therapy | 1 (1.5) |
| TKI | 9 (13.8) |
| Monoclonal antibody | 3 (4.6) |
| Anti-VEGF* agents | 1 (1.5) |
*ICI immune checkpoint inhibitor, TKI tyrosine kinase inhibitor, VEGF vascular endothelial growth factor
Fig. 2.
Cancer sites and recent treatment category among COVID-19 cancer patients with respiratory failure. VEGF, vascular endothelial growth factor
Most (87.7%) patients with cancer had solid tumors, with lung cancer (24.6%) and gastrointestinal tumors (15.4%) being the most common, followed by hematological malignancies (12.3%). In total, 34 (52.3%) patients received cancer-related treatment within 4 weeks before receiving a COVID-19 diagnosis, with approximately half receiving cytotoxic chemotherapy.
In-hospital mortality among the patients with cancer was 61.5%, with 25 survivors and 40 nonsurvivors (Table 3). The nonsurvivors were more likely to be smokers (42.5% vs 12%, P = 0.024) than were the survivors. Furthermore, the nonsurvivors had higher white blood cell counts (median 12,450 vs 8500 × 109/L, P = 0.006) and concentrations of ferritin (median 3220 vs 673.5 ng/mL, P < 0.001), LDH (median 534.5 vs 256 U/L, P < 0.001), lactate (median 33 vs 15.7 mg/dL, P = 0.005), and D-dimer (median 4.605 vs 1.570 μg/mL, P = 0.007) than did the survivors. Additionally, the nonsurvivors had a higher incidence of vasopressor use on the day of respiratory failure (55% vs 24%, P = 0.014) and new renal replacement therapy during admission (22.5% vs 4%, P = 0.044) than did the survivors. The difference in survival status was not statistically significant based on whether patients had undergone systemic treatment or received cytotoxic chemotherapy within the 4 weeks preceding their COVID-19 diagnosis.
Table 3.
Comparison of patient characteristics between COVID-19 survivors and non-survivors among patients with cancer during hospital stay. (n = 65)
| Survivor (n = 25) | Nonsurvivor (n = 40) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years, median | 75 | 72.5 | 0.212 |
| Male | 13(52) | 30(75) | 0.057 |
| Body mass index, kg/m2, median | 21.04 | 23.17 | 0.229 |
| BMI* < 18 | 7(28) | 5(14.7) | 0.210 |
| BMI* > 24 | 9(36) | 14(41.2) | 0.687 |
| Vaccination doses, median | 2 | 2 | 0.890 |
| Ever vaccinated | 17(68) | 28(70) | 0.865 |
| Full vaccination (> = 3 doses) | 12(48) | 12(48) | 0.875 |
| DNR* | 15(60) | 32(80) | 0.080 |
| Smoker | 3(12) | 17(42.5) | 0.024 |
| Cerebrovascular disease | 4(16) | 2(5) | 0.194 |
| Dementia | 2(8) | 3(7.5) | 0.941 |
| Heart failure | 1(4) | 0(0) | 0.202 |
| Peripheral vascular disease | 1(4) | 1(2.5) | 0.733 |
| Diabetes mellitus | 9(36) | 13(32.5) | 0.772 |
| Chronic kidney disease | 3(12) | 9(22.5) | 0.288 |
| End stage renal disease | 1(4) | 4(10) | 0.377 |
| Chronic obstructive pulmonary disease | 2(8) | 2(5) | 0.624 |
| Chronic oxygen use | 2(8) | 1(2.5) | 0.304 |
| Admitted due to COVID-19 | 12(48) | 20(50) | 0.875 |
| Infected during hospitalization | 3(12) | 9(22.5) | 0.344 |
| Hematological malignancy | 1(4) | 6(15) | 0.235 |
| Laboratory data on the day of respiratory failure (median) | |||
| White blood cells, 109/L | 8500 | 12,450 | 0.006 |
| Absolute neutrophil count, 109/L | 6318.7 | 7105.45 | 0.345 |
| Hemoglobin, g/dL | 11.5 | 10.5 | 0.153 |
| Absolute lymphocyte count, 109/L | 639.58 | 531.40 | 0.571 |
| Albumin, g/dL | 3.1 | 3.1 | 0.349 |
| C-reactive protein, mg/dL | 4.78 | 7.16 | 0.157 |
| Procalcitonin, ng/mL | 0.51 | 1.42 | 0.375 |
| Ferritin, ng/mL | 673.5 | 3220 | < 0.001 |
| Lactic dehydrogenase, U/L | 256 | 534.5 | < 0.001 |
| Lactate, mg/dL | 15.7 | 33 | 0.005 |
| D-dimer, ug/mL | 1.570 | 4.605 | 0.007 |
| Fibrinogen, mg/dL | 435.6 | 358 | 0.188 |
| Platelet count, /uL | 159,000 | 154,000 | 0.422 |
| Severity on the day of respiratory failure | |||
| PaO2/FiO2 ratio, median | 148 | 125.39 | 0.364 |
| SOFA* score, median | 7 | 10 | 0.071 |
| APACHE* II score, median | 22 | 25.5 | 0.160 |
| MAP score**, median | 1 | 3 | 0.048 |
| GCS*, median | 9 | 7.5 | 0.995 |
| Vasopressor use | 6(24) | 22(55) | 0.014 |
| Treatment | |||
| Cancer treatment in 4 weeks prior to COVID-19 diagnosis | 12(48) | 22(55) | 0.583 |
| Cytotoxic chemotherapy in 4 weeks prior to COVID-19 diagnosis | 8(32) | 11(27.5) | 0.698 |
| Mechanical ventilation | 18(72) | 24(60) | 0.325 |
| Re-application of MV* after weaning | 2(8) | 2(5) | 0.624 |
| Tracheostomy | 3(12) | 1(2.5) | 0.121 |
| New renal replacement therapy during admission | 1(4) | 9(22.5) | 0.044 |
| Extracorporeal membrane oxygenation | 0(0) | 5(12.5) | 0.066 |
| Tocilizumab | 9(36) | 21(52.5) | 0.194 |
| Remdesivir | 22(88) | 37(92.5) | 0.542 |
| Nirmatrelvir/ritonavir | 1(4) | 3(7.5) | 0.568 |
| Molnupiravir | 1(4) | 1(2.5) | 0.733 |
| Enoxaparin | 9(36) | 13(32.5) | 0.772 |
| Corticosteroid | 22(88) | 39(97.5) | 0.121 |
| Complications | |||
| CMV* infection | 2(8) | 14(35) | 0.048 |
| Gastrointestinal bleeding | 6(24) | 11(27.5) | 0.755 |
| Thromboembolism | 3(12) | 4(10) | 0.800 |
| Outcome | |||
| ICU* admission | 20(80) | 28(70) | 0.372 |
| Hospital length of stay, days, median | 33 | 21.5 | 0.082 |
| Ventilator days, median | 10 | 5.5 | 0.710 |
| Time from symptoms onset to 1st Ct* > 30, days | 14 | 11.5 | 0.721 |
| Prolonged shredding (> 10 days) | 18(72) | 21(52.5) | 0.118 |
*BMI Body mass index, DNR Do not resuscitate, SOFA Sequential Organ Failure Assessment, APACHE Acute Physiology and Chronic Health Evaluation, MAP Mean arterial pressure, GCS Glasgow coma scale, CMV Cytomegalovirus, ICU Intensive Care Unit, Ct cycle threshold
**MAP score is defined from the calculation of SOFA score, with inotropic doses as mcg/kg/min: 0, No hypotension; 1, MAP < 70 mmHg; 2, Dopamine ≤5 or Dobutamine (any dose); 3, Dopamine > 5, Epinephrine ≤0.1, or norepinephrine ≤0.1; 4, Dopamine > 15, Epinephrine > 0.1, or Norepinephrine > 0.1
The comparison of the characteristics, laboratory data, treatment, complications, and outcomes between cancer patients who have undergone recent systemic treatment and those who have not received it was summarized in Supplemental Table 1. The patients who have underwent cancer treatment were younger (median 71.5 vs 79 years old, P = 0.029), had lower absolute lymphocyte count on the day of respiratory failure (median 657.6 vs 440.28 × 109/L, P = 0.030), higher LDH level (median 536 vs 342 U/L, P = 0.016), and took shorter to reach Ct > 30 (median 8.5 vs 17 days, P = 0.033) than did the patients without treatment.
According to multivariable analysis (Table 4), smoking (OR: 5.804, 95% CI: 1.847–39.746, P = 0.043), an elevated concentration of LDH (OR: 1.004, 95% CI: 1.001–1.012, P = 0.025), vasopressor use on the day of respiratory failure (OR: 5.437, 95% CI: 1.202–24.593, P = 0.028), and new renal replacement therapy during admission (OR: 3.523, 95% CI: 1.203–61.108, P = 0.034) were significantly associated with in-hospital mortality among patients with cancer and COVID-19-related respiratory failure.
Table 4.
Factors associated with in-hospital survival among COVID-19 cancer patients with respiratory failure (n = 65)
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variables | Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value |
| Male | 2.769 | 0.958–8.009 | 0.060 | 1.050 | 0.090–12.266 | 0.969 |
| DNR | 2.667 | 0.876–8.122 | 0.084 | 11.605 | 0.478–281.509 | 0.132 |
| Smoker | 5.420 | 1.392–21.107 | 0.015 | 5.804 | 1.847–39.746 | 0.043 |
| Lactic dehydrogenase, U/L | 1.004 | 1.001–1.007 | 0.008 | 1.004 | 1.001–1.012 | 0.025 |
| Lactate, mg/dL | 1.022 | 0.999–1.045 | 0.064 | 1.032 | 0.969–1.100 | 0.321 |
| D-dimer, ug/mL | 1.254 | 1.022–1.540 | 0.030 | 1.189 | 0.826–1.526 | 0.174 |
| SOFA score | 1.110 | 0.991–1.243 | 0.070 | 0.792 | 0.513–1.222 | 0.292 |
| MAP score | 1.415 | 1.028–1.948 | 0.033 | 0.330 | 0.037–2.936 | 0.320 |
| Vasopressor use | 3.870 | 1.276–11.735 | 0.017 | 5.437 | 1.202–24.593 | 0.028 |
| New renal replacement therapy during admission | 6.968 | 0.825–58.844 | 0.075 | 3.523 | 1.203–61.108 | 0.034 |
| CMV infection | 0.889 | 0.780–1.014 | 0.080 | 1.264 | 0.840–1.901 | 0.260 |
CI confidence interval, DNR do not resuscitate, SOFA sequential organ failure assessment, MAP mean arterial pressure, CMV cytomegalovirus
Discussion
This study revealed the characteristics and factors that influence in-hospital mortality among patients with cancer and COVID-19-related respiratory failure during the period in which the omicron variant of SARS-CoV-2 was circulating in Taiwan. The patients with cancer and COVID-19-related respiratory failure exhibited distinct clinical characteristics, including lower lymphocyte counts, higher ferritin and LDH concentrations, and increased vasopressor use than did the patients without cancer. Additionally, the patients with cancer received COVID-19-related treatments more frequently than did the patients without cancer; however, in-hospital mortality was higher among the patients with cancer than among those without cancer. Smoking, an elevated LDH concentration, vasopressor use, and new renal replacement therapy were independent predictors of in-hospital mortality among this population.
The patients with cancer were generally younger and less likely to have histories of cerebrovascular accidents and heart failure than were the patients without cancer. This finding indicates that comorbidities other than advanced stage cancer contributed to the development of severe disease. The patients with cancer had lower absolute lymphocyte counts and higher ferritin and LDH concentrations on the day of respiratory failure than did the patients without cancer. Other biomarkers, such as C-reactive protein (CRP), lactate, fibrinogen, D-dimer, and procalcitonin, did not significantly differ between the patients with and without cancer. In Cai et al., among patients with COVID-19, those with cancer had higher concentrations of inflammatory markers and cytokines (high-sensitivity C-reactive protein, procalcitonin, interleukin (IL)-2 receptor, IL-6, and IL-8) and fewer immune cells than did those without cancer, indicating that patients with cancer are more susceptible to immune dysregulation [17]. Lymphopenia is a marker of COVID-19 severity and may be used to detect respiratory failure [20–22]. Patients with COVID-19 who are critically ill often exhibit hyperferritinemia; however, ferritin concentration is not a reliable predictor of patient outcomes [23–25]. An elevated LDH concentration has also been associated with mortality among patients with COVID-19 with severe disease and acute respiratory distress syndrome [22, 26–28].
In the present study, we discovered that the patients with cancer were more frequently treated with remdesivir, tocilizumab, and corticosteroids than were those without cancer. Use of enoxaparin and oral antivirals (nirmatrelvir/ritonavir and molnupiravir) did not significantly differ between the patients with and without cancer. Interleukin (IL)-6, known to be associated with adverse clinical outcomes in patients with COVID-19 [29], is also a key cytokine in the tumor microenvironment. IL-6, present in high concentrations in various cancer types, correlates with cancer progression and therapeutic resistance [30, 31]. IL-6 deregulation participates in the systemic hyperactivated immune response commonly referred to as the cytokine storm. Corticosteroids modulate inflammation-mediated lung injury and thereby reduce the likelihood of short-term mortality and the need for mechanical ventilation [6, 32]. Tocilizumab, a monoclonal antibody against IL-6 receptor, reduces the likelihood of progression to mechanical ventilation or death in patients hospitalized with COVID-19 and is effective among patients with COVID-19 with various cancer types [33–35]. We propose that corticosteroids and tocilizumab were used more frequently among the patients with cancer than among those without cancer due to the hyperinflammatory status of the patients with cancer, whose inflammatory status was confirmed by their elevated concentrations of inflammatory markers (ferritin and LDH). The immunocompromised status of the patients with cancer may have led to active viral replication; therefore, although remdesivir was used more frequently among the patients with cancer than among those without cancer, the patients with cancer took longer to reach Ct > 30. The patients with cancer exhibited prolonged nasopharyngeal viral RNA shedding. Longer viral shedding is associated with older age, distant metastasis, and more severe COVID-19 disease [36].
The patients with cancer had higher MAP scores and a greater likelihood of vasopressor use on the day of respiratory failure than did those without cancer, indicating greater hemodynamic instability among these patients. The patients with cancer were also demonstrated a significantly higher in-hospital mortality rate compared to those without cancer, which is consistent with the finding of another study [37].
Among the patients with cancer in our study, in-hospital mortality was associated with smoking; a higher white blood cell count; and elevated concentrations of ferritin, LDH, lactate, and D-dimer. These factors indicate that an active inflammatory process may have contributed to a poor prognosis. The nonsurvivors with cancer were also significantly more likely to use vasopressors and receive new renal replacement therapy during their admission than were the survivors.
Vaccination status, comorbidities, recent systemic cancer treatment, whether admitted due to COVID-19, whether infected during hospitalization, SOFA score and APACHE II score on the day of respiratory failure, and specific treatments for COVID-19 (including those involving corticosteroids, antiviral and anticoagulation agents, and tocilizumab) did not significantly affect mortality.
In multivariable analysis, we identified several factors that were associated with in-hospital mortality among the patients with cancer and COVID-19-related respiratory failure. These factors included smoking, elevated LDH concentrations on the day of respiratory failure, requiring vasopressor use on the day of respiratory failure, and undergoing new renal replacement therapy during admission.
Active smoking is considered as an independent predictor of severe disease and mortality among patients with COVID-19 [9, 38–40]. Current smokers had significantly increased ACE2 expression in airway epithelial cells compared with nonsmokers, which provided more entry points for the SARS-CoV-2 virus and potentially increased susceptibility to infection [41]. However, in one study, active smoking was not associated with COVID-19 severity [42]. Elevated LDH concentration has been identified as an independent risk factor for disease severity and mortality among patients with COVID-19 [27, 28, 43]. The requirement for mechanical ventilation, vasopressors, and renal replacement therapy were reported to be poor prognostic factors among patients with cancer who were admitted to the intensive care unit [44]. Patients with COVID-19 who are admitted to the intensive care unit frequently receive continuous vasopressor support [45], highlighting the importance of hemodynamic monitoring and fluid management.
Our study has several limitations. First, this was a single-center retrospective cohort study with a limited sample size. Second, the laboratory data and SARS-CoV-2 PCR follow-up intervals were not uniform, which potentially introduced bias. Third, some inflammatory biomarkers such as IL-6, IL-2R, IL-8 and antibody titers are either not routinely tested or have no available exam in our hospital, thus we do not have sufficient data to incorporate into our analysis. Treatment strategies may have also varied considerably by patient clinical status and clinician practice.
Conclusion
Patients with cancer who develop COVID-19-related respiratory failure exhibit distinct clinical characteristics and have a higher likelihood of receiving specific COVID-19 treatments, such as remdesivir and corticosteroids, than those without cancer do. Patients who develop COVID-19-related respiratory failure with cancer also experience unfavorable outcomes, including higher in-hospital mortality and longer duration of viral shedding, compared with those without cancer. Smoking, elevated LDH concentrations, vasopressor use, and new renal replacement therapy were identified as significant predictors of in-hospital mortality in this patient population. Further research is warranted to validate these findings, elucidate the underlying mechanisms, and explore tailored management strategies to improve outcomes in this vulnerable population.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus disease 2019
- LDH
Lactate dehydrogenase
- OR
Odds ratio
- CI
Confidence interval
- ARDS
Acute respiratory distress syndrome
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- HFNC
High-flow nasal cannula
- NIV
Noninvasive ventilation
- MV
Mechanical ventilation
- RT-PCR
Reverse transcription polymerase chain reaction
- BMI
Body mass index
- DNR
Do not resuscitate
- SOFA
Sequential organ failure assessment
- MAP
Mean arterial pressure
- APACHE
Acute Physiologic Assessment and Chronic Health Evaluation
- CMV
Cytomegalovirus
- Ct
Cycle threshold
- IL
Interleukin
Authors’ contributions
Conceptualization: Ying-Ting Liao, Hsiao-Chin Shen, Jhong-Ru Huang, Chuan-Yen Sun, Hung-Jui Ko, Chih-Jung Chang, Jia-Yih Feng, Wei-Chih Chen, Kuang-Yao Yang. Supervision: Wei-Chih Chen, Jia-Yih Feng, Kuang-Yao Yang, Yuh-Min Chen. Data Collection and/or Processing: Hsiao-Chin Shen, Chuan-Yen Sun, Jhong-Ru Huang, Ying-Ting Liao, Hung-Jui Ko, Chih-Jung Chang. Analysis and/or Interpretation: Ying-Ting Liao, Wei-Chih Chen, Kuang-Yao Yang. Writing – original draft: Ying-Ting Liao, Wei-Chih Chen, Kuang-Yao Yang. Writing – review and editing: Ying-Ting Liao, Wei-Chih Chen, Kuang-Yao Yang. All authors read and approved the final manuscript.
Funding
This research was funded by grants from Taipei Veterans General Hospital (V111C-050, V111B-024, V112C-068, V112B-031, V112D65–003-MY2–1, V112A-001, V113B-015, V113A-003), and National Science and Technology Council, Taiwan (MOST109–2314-B-010-051-MY3, NSTC 112–2314-B-075-050, NSTC 112–2314-B-A49–040). Additionally, this work was supported by grants from the Ministry of Education, Higher Education SPROUT Project for Cancer Progression Research Center(111 W31101) and Cancer and Immunology Research Center (112 W31101).
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Ethical Review Board of Taipei Veterans General Hospital (Approval No. 2022–11-002 AC). Written informed consent was waived by Institutional Ethical Review Board of Taipei Veterans General Hospital due to retrospective design of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei-Chih Chen and Kuang-Yao Yang contributed equally to this work.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. Accessed Nov 27, 2023. https://covid19.who.int/
- 2.Zhang JT, Zhong WZ, Wu YL. Cancer treatment in the coronavirus disease pandemic. Lung Cancer. 2021;152:98–103. doi: 10.1016/j.lungcan.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of Cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.Cd-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 5.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/s0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer consortium. Ann Oncol. 2021;32(6):787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboueshia M, Hussein MH, Attia AS, et al. Cancer and COVID-19: analysis of patient outcomes. Future Oncol. 2021;17(26):3499–3510. doi: 10.2217/fon-2021-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. The Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID-19 outcomes in patients with Cancer. J Clin Oncol. 2020;38(30):3538–3546. doi: 10.1200/jco.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. 2020;141:92–104. doi: 10.1016/j.ejca.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/bf01709751. [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 16.Kao HC, Lai TY, Hung HL, et al. Sequential oxygenation index and organ dysfunction assessment within the first 3 days of mechanical ventilation predict the outcome of adult patients with severe acute respiratory failure. Sci World J. 2013;2013:413216. doi: 10.1155/2013/413216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai G, Gao Y, Zeng S, et al. Immunological alternation in COVID-19 patients with cancer and its implications on mortality. Oncoimmunology. 2021;10(1):1854424. doi: 10.1080/2162402x.2020.1854424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young BE, Ong SWX, Ng LFP, et al. Viral Dynamics and Immune Correlates of Coronavirus Disease 2019 (COVID-19) Severity. Clin Infect Dis. 2021;73(9):e2932–e2942. doi: 10.1093/cid/ciaa1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CECC revises criteria for releasing COVID-19 patients with moderate and severe symptoms from isolation to preserve isolation care capacity 2022. Accessed Nov 27, 2023. https://covid19.mohw.gov.tw/en/cp-4868-69897-206.html
- 20.Illg Z, Muller G, Mueller M, Nippert J, Allen B. Analysis of absolute lymphocyte count in patients with COVID-19. Am J Emerg Med. 2021;46:16–19. doi: 10.1016/j.ajem.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elhadad D, Bronstein Y, Yana M, et al. Characteristics and outcomes of patients infected with SARS-CoV-2 in Israel: correlation between laboratory findings on admission to emergency department and subsequent respiratory failure. Isr Med Assoc J. 2020;22(10):605–611. [PubMed] [Google Scholar]
- 22.Malik P, Patel U, Mehta D, et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26(3):107–108. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feld J, Tremblay D, Thibaud S, Kessler A, Naymagon L. Ferritin levels in patients with COVID-19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2020;42(6):773–779. doi: 10.1111/ijlh.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gómez-Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID-19 patients - is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249–251. doi: 10.1016/j.cca.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushal K, Kaur H, Sarma P, et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care. 2022;67:172–181. doi: 10.1016/j.jcrc.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topp G, Bouyea M, Cochran-Caggiano N, et al. Biomarkers predictive of Extubation and survival of COVID-19 patients. Cureus. 2021;13(6):e15462. doi: 10.7759/cureus.15462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez Mesa A, Cabrera César E, Martín-Montañez E, et al. Acute lung injury biomarkers in the prediction of COVID-19 severity: Total thiol, ferritin and lactate dehydrogenase. Antioxidants (Basel). 2021;10(8) 10.3390/antiox10081221. [DOI] [PMC free article] [PubMed]
- 28.Li C, Ye J, Chen Q, et al. Elevated Lactate Dehydrogenase (LDH) level as an independent risk factor for the severity and mortality of COVID-19. Aging (Albany NY). 2020;12(15):15670–15681. doi: 10.18632/aging.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 31.Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–148. doi: 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24(s):696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer. 2021;20(1):76. doi: 10.1186/s12943-021-01363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2020;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(3):1620–1630. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 36.Goubet A-G, Dubuisson A, Geraud A, et al. Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis. Cell Death Different. 2021;28(12):3297–3315. doi: 10.1038/s41418-021-00817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauser MJ, Tabak J, Baier H. Survival of patients with Cancer in a medical critical care unit. Arch Intern Med. 1982;142(3):527–529. doi: 10.1001/archinte.1982.00340160107022. [DOI] [PubMed] [Google Scholar]
- 38.Espejo-Paeres C, Núñez-Gil IJ, Estrada V, et al. Impact of smoking on COVID-19 outcomes: a HOPE registry subanalysis. BMJ Nutr Prev Health. 2021;4(1):285–292. doi: 10.1136/bmjnph-2021-000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patanavanich R, Glantz SA. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith JC, Sausville EL, Girish V, et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. 2020;53(5):514–529.e3. doi: 10.1016/j.devcel.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur J Intern Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szarpak L, Ruetzler K, Safiejko K, et al. Lactate dehydrogenase level as a COVID-19 severity marker. Am J Emerg Med. 2021;45:638–639. doi: 10.1016/j.ajem.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35(3):808–814. doi: 10.1097/01.Ccm.0000256846.27192.7a. [DOI] [PubMed] [Google Scholar]
- 45.Michard F, Malbrain ML, Martin GS, et al. Haemodynamic monitoring and management in COVID-19 intensive care patients: an international survey. Anaesth Crit Care Pain Med. 2020;39(5):563–569. doi: 10.1016/j.accpm.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.