Abstract
Brief summary
In early symptomatic COVID-19 treatment, high dose oral favipiravir did not accelerate viral clearance.
Background
Favipiravir, an anti-influenza drug, has in vitro antiviral activity against SARS-CoV-2. Clinical trial evidence to date is inconclusive. Favipiravir has been recommended for the treatment of COVID-19 in some countries.
Methods
In a multicentre open-label, randomised, controlled, adaptive platform trial, low-risk adult patients with early symptomatic COVID-19 were randomised to one of ten treatment arms including high dose oral favipiravir (3.6g on day 0 followed by 1.6g daily to complete 7 days treatment) or no study drug. The primary outcome was the rate of viral clearance (derived under a linear mixed-effects model from the daily log10 viral densities in standardised duplicate oropharyngeal swab eluates taken daily over 8 days [18 swabs per patient]), assessed in a modified intention-to-treat population (mITT). The safety population included all patients who received at least one dose of the allocated intervention. This ongoing adaptive platform trial was registered at ClinicalTrials.gov (NCT05041907) on 13/09/2021.
Results
In the final analysis, the mITT population contained data from 114 patients randomised to favipiravir and 126 patients randomised concurrently to no study drug. Under the linear mixed-effects model fitted to all oropharyngeal viral density estimates in the first 8 days from randomisation (4,318 swabs), there was no difference in the rate of viral clearance between patients given favipiravir and patients receiving no study drug; a -1% (95% credible interval: -14 to 14%) difference. High dose favipiravir was well-tolerated.
Interpretation
Favipiravir does not accelerate viral clearance in early symptomatic COVID-19. The viral clearance rate estimated from quantitative measurements of oropharyngeal eluate viral densities assesses the antiviral efficacy of drugs in vivo with comparatively few studied patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08835-3.
Keywords: Favipiravir, SARS-CoV-2, COVID-19, Early treatment, Antiviral efficacy, Pharmacometrics
Research in context
Evidence before this study
• The in vivo antiviral effect of favipiravir in patients with early symptomatic COVID-19 was not known.
Added value of this study
• High-dose favipiravir did not demonstrate antiviral activity in early symptomatic COVID-19.
• The rate of viral clearance derived from frequent oropharyngeal swabbing in early COVID-19 can be used to characterise in vivo antiviral efficacy.
Implications of all available evidence
• In vivo antiviral activity of COVID-19 therapeutics should be used to inform policies and practices.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08835-3.
Introduction
Favipiravir was developed in 2002 as an anti-influenza medication [1]. It is a pyrazinecarboxamide derivative, a prodrug that is metabolised within cells to its active antiviral form, favipiravir-ribofuranosyl-5'-triphosphate (favipiravir-RTP). Favipiravir-RTP is a nucleoside analogue which selectively inhibits viral RNA-dependent RNA polymerase and has shown in vitro activity against many RNA viruses [2]. Favipiravir has been licensed in Japan for influenza, and in China for investigational use, but it has not been licensed elsewhere. Favipiravir has been used in influenza at two doses- an initial dose of 3.2g (D0) followed by 1.2g daily thereafter, and a higher dose of 3.6g D0 and 1.6g daily (which is the dose used in this study). A trial using much higher doses of favipiravir (6g D0, and 2.4g daily D1-9) was conducted in patients with Ebola virus disease in Guinea, although the study had no control arm and could not reach conclusions on efficacy [3].
Favipiravir was identified as having antiviral activity against the SARS-CoV-2 virus through early in vitro screening [4–6], albeit at concentrations up to 1,000 fold higher than those required to inhibit influenza in vitro [7]. Studies in hamsters have demonstrated a beneficial antiviral effect against SARS-CoV-2 although only at very large doses, suggesting that high exposures might be needed to achieve beneficial effects in treating COVID-19 [8, 9]. Therapeutic recommendations for the treatment of early COVID-19 still vary widely. Favipiravir has been recommended and was widely used as a treatment for COVID-19 in some countries, including Thailand (https://ddc.moph.go.th/viralpneumonia/eng/file/guidelines/g_treatment.pdf). Although some observational studies have suggested benefit from favipiravir [10–14], and a large clinical benefit was reported in one open-label randomised controlled trial (with shortening of time to clinical improvement from 14 to 2 days in hospitalised patients) [15], the other reported randomised trials have either shown no benefit, or the evidence of clinical efficacy has been marginal or unconvincing [16–29]. However, several of these studies were conducted in hospitalised patients, in whom the window of opportunity for antivirals to benefit may have closed. Antiviral drugs work better in early illness than in later infections in hospitalised patients where inflammatory pathology dominates. Dosing has also varied between the favipiravir studies. Given the lower antiviral activity of favipiravir against SARS-CoV-2 relative to influenza, high doses are probably necessary for optimal in vivo antiviral efficacy. Reassuringly no significant safety or tolerability issues have been identified in these clinical studies, although concerns have been raised regarding the risk to the fetus if potentially mutagenic antiviral nucleoside analogues are given to pregnant women [30].
Overall, the available evidence still leaves considerable uncertainty whether or not high-dose favipiravir is a useful antiviral treatment of early COVID-19 in outpatients. We present the results from a randomised platform trial assessing the in vivo antiviral activity of favipiravir in adults with acute early COVID-19.
Methods
PLATCOV is an ongoing phase 2 open label, randomised, controlled adaptive platform trial (ClinicalTrials.gov: NCT05041907 registered 13/09/2021) [31]. It provides a standardised quantitative comparative method for in vivo assessment of potential antiviral treatments in low-risk adults with early symptomatic COVID-19. Daily oropharyngeal viral densities are measured by qPCR. The primary outcome measure in PLATCOV is the viral clearance rate derived from the slope of the log10 oropharyngeal viral clearance curve over the next 7 days following randomisation, estimated under a linear model [32]. The treatment effect is defined as the multiplicative change in viral clearance rate estimate relative to the contemporaneous no study drug arm (detailed below). The trial was conducted in Bangkok: Faculty of Tropical Medicine (FTM), Mahidol University, Bangplee hospital, Samut Prakarn; and Vajira hospital, Navamindradhiraj University, Bangkok, all in Thailand and in Belo Horizonte, Minas Gerais, Brazil (see Supplementary materials). All patients provided fully informed written consent. All methods were approved and carried out in accordance with local and national research boards in Thailand, the Mahidol University Faculty of Tropical Medicine Ethics Committee, the Central Research Ethics Committee, Thailand, the National Research Ethics Commission of Brazil, and the Oxford University Tropical Research Ethics Committee (see Supplementary materials). The PLATCOV trial was coordinated and monitored by the Mahidol Oxford Tropical Medicine Research Unit (MORU) in Bangkok, and overseen by a trial steering committee (TSC). Interim results were reviewed regularly by a data and safety monitoring board (DSMB). The funders had no role in the design, conduct, analysis or interpretation of the trial.
Participants and procedures
Previously healthy adults aged between 18 and 50 years were eligible for the trial if they had early symptomatic COVID-19 (i.e., reported symptoms for ≤ 4 days), oxygen saturation ≥ 96%, were unimpeded in activities of daily living, and gave fully informed consent to study participation. SARS-CoV-2 positivity was defined either as a nasal lateral flow antigen test which became positive within two minutes (STANDARD® Q COVID-19 Ag Test, SD Biosensor, Suwon-si, Korea) or a positive PCR test within the previous 24h with a cycle threshold value (Ct) < 25 (all viral gene targets), both of which suggest high pharyngeal viral densities. The latter was added on 25 November 2021 to include those patients with recent PCRs confirming high viral loads. This was the only change to the pre-trial pre-specified inclusion/exclusion criteria. Exclusion criteria included taking any potential antivirals or pre-existing concomitant medications, chronic illness or significant comorbidity, haematological or biochemical abnormalities, pregnancy (a urinary pregnancy test was performed in females), breastfeeding, or contraindication or known hypersensitivity to any of the study drugs [31].
Block randomisation was performed for each site via a centralised web-app designed by MORU software engineers using RShiny®, hosted on a MORU webserver. At enrollment, after obtaining fully informed consent and entering the patient details, the app provided the randomised allocation. The no study drug arm comprised a minimum proportion of 20% of patients at all times, with uniform randomisation ratios applied across the active treatment arms. The study was open-label (no placebos). Enrolled patients were either admitted to the study ward (in Thailand), consistent with National recommendations at the time, or followed as outpatients at home (in Brazil). After randomisation and baseline procedures (see Supplementary materials) oropharyngeal swabs (two swabs from each tonsil) were taken as follows. Each flocked swab (Thermo Fisher MicroTest® and later COPAN FLOQSwabs®) was rotated against the tonsil through 360° four times and placed in Thermo Fisher M4RT™ viral transport medium (3mL). Swabs were transferred at 4–8°C, aliquoted, and then frozen at -80°C within 48h. Separate swabs from each tonsil were taken once daily from day 0 to day 7, and again on day 14. Each swab was processed and tested separately. Vital signs were recorded three times daily and symptoms and any adverse effects were recorded daily [31].
Patients allocated to favipiravir received 1800mg on an empty stomach, (nine 200mg tablets; Favir®, Government Pharmaceutical Organization in Thailand, n = 100; or Avigan®, FUJIFILM Toyama Chemical Co., Ltd. in Brazil n = 16), at the start of treatment followed 12 h later by a further 1800mg. Thereafter the patients took 800mg twice daily for a further 6 days totalling 13.2g over 7 days. All patients received standard symptomatic treatment excluding antivirals.
The TaqCheck® SARS-CoV-2 Fast PCR Assay (Applied Biosystems, Thermo Fisher Scientific, Waltham, Massachusetts) quantitated viral densities (SARS-CoV-2 RNA copies per mL). This multiplexed real-time PCR method detects the SARS-CoV-2 N and S genes, and human RNase P in a single reaction. RNase P was used to correct for variation in human cell content in samples. Viral densities were quantified against ATCC heat-inactivated SARS-CoV-2 (VR-1986HK strain 2019-nCoV/USA-WA1/2020) standards. Viral variants were identified using Whole Genome Sequencing (see Supplementary materials).
Outcome measures
The primary outcome measure was the rate of viral clearance, expressed as a slope coefficient [32], and estimated under a Bayesian hierarchical linear model (mixed-effects model) fitted to the daily log10 oropharyngeal swab eluate viral density measurements between days 0 and 7 (18 measurements per patient). Before model fitting, Ct values were transformed to RNA copies per mL using a random effects linear model fit to the ATCC controls (random slope and intercept for each plate with additional fixed effects for each laboratory). Viral load measurements below the limit of quantification (Ct values ≥ 40) were treated as left-censored under the model. A non-linear model (allowing an initial log-linear increase in viral loads followed by a log-linear decrease in some patients) was also fitted to the data as a sensitivity analysis. All models included slope and intercept covariate effects for the virus variant, expressed as the major sub-lineages). Additional models included slope and intercept covariate effects for age, vaccination status, and days since symptom onset. The estimated individual viral clearance rates (i.e., slope coefficients from the model fit) can be expressed as clearance half-lives (t1/2 = log10 0.5/slope). The treatment effect was defined as the multiplicative change (%) in the mean viral clearance rate relative to the no study drug arm (i.e., how much the test treatment accelerates on average the viral clearance) [32]. Thus, a 50% increase in clearance rate equals a 33% reduction in clearance half-life. All-cause hospitalisation for clinical deterioration (until day 28) was a secondary endpoint. For each studied intervention the sample size was adaptive based on prespecified futility and success stopping rules. Initially the futility stopping rule was set as a probability > 0.9 that the acceleration in viral clearance was < 5%, but at the prespecified open first interim analysis performed after 50 patients had been enrolled, the futility threshold was increased to 12.5%.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events v.5.0 (CTCAE). Summaries were generated if the adverse event was ≥ grade 2 and was new or had increased in intensity. Serious adverse events were recorded separately and reported to the DSMB.
Statistical analysis
All analyses were done in a prespecified modified intention-to-treat (mITT) population, comprising patients who had ≥ 3 days follow-up data. A series of linear and non-linear Bayesian hierarchical models were fitted to the viral quantitative PCR (qPCR) data (Supplementary materials). Model fits were compared using approximate leave-one-out comparison as implemented in the package loo. All data analysis was done in R version 4.0.2. Model fitting was done in Stan via the RStan interface. All code and data are openly accessible via GitHub: https://github.com/jwatowatson/PLATCOV-Favipiravir.
Results
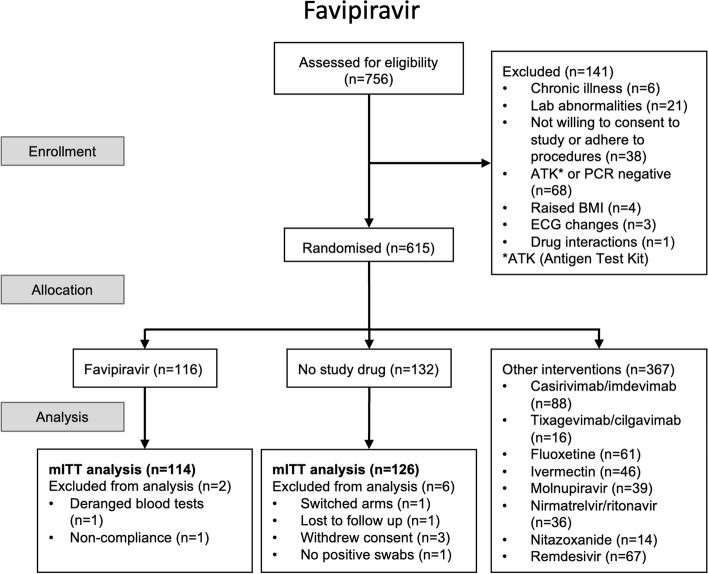
The trial began recruitment on 30 September 2021. On 31 October 2022, the favipiravir arm of the trial was stopped and favipiravir was removed from the randomisation lists in Thailand and Brazil following a recommendation from the DSMB as the prespecified futility margin had been reached. This decision was based on PCR data from 102 patients randomised to favipiravir and 104 concurrent controls. Of the 615 patients enrolled by that time, 116 patients had been randomised to receive favipiravir, 132 had been randomised to no study drug, and the remainder (n = 367) were randomised to other interventions (casirivimab/imdevimab, tixagevimab/cilgavimab, remdesivir, ivermectin, nitazoxanide, fluoxetine, molnupiravir, or nirmatrelvir/ritonavir).
Virological responses
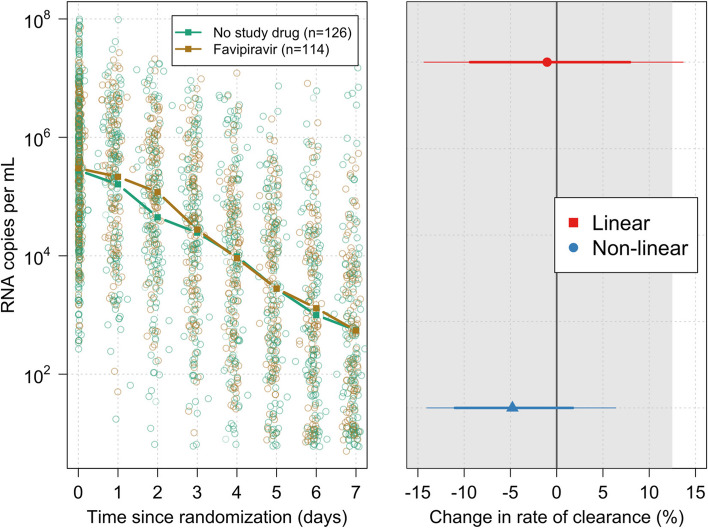
The mITT population included 114 patients randomised to favipiravir and 126 patients randomised to no study drug (Fig. 1). The baseline geometric mean (GM) oropharyngeal swab eluate viral load was 5.5×105 RNA copies/mL (IQR 4.7×105 to 6.3×105), (Table 1, Fig. 2a). Rates of viral clearance were estimated under a linear mixed-effects model fit to all PCR data taken up to day 7 after randomisation in the mITT population (4,318 swabs in 240 patients, of which 3,839 were above the lower limit of quantification, 89%). A non-linear model was used as a sensitivity analysis. Under the linear model, there was no evidence of a difference in viral clearance rates between the favipiravir treated patients and those receiving no study drug (mean difference: –1%; 95%CI: -14% to 14%). The posterior probability that the effect was less than the pre-specified futility margin of 12.5% was 0.97 (Fig. 2b). The non-linear model gave very similar estimates (mean difference: -5%; 95%CI: -14% to 6%; probability less than 12.0% equal to 1).
Fig. 1.
CONSORT diagram
Table 1.
Baseline demographic characteristics in the mITT population
| Baseline demographic characteristics in mITT population | ||||
|---|---|---|---|---|
| No study drug | Favipiravir | Total | ||
| Total | 126 | 114 | 240 | |
| Site n (%) | Brazil site | 14 (11.1) | 16 (14.0) | 30 (12.5) |
| Thailand FTM site | 107 (84.9) | 96 (84.2) | 203 (84.6) | |
| Thailand Vajira site | 3 (2.4) | 2 (1.8) | 5 (2.1) | |
| Thailand Bangplee site | 2 (1.6) | 0 (0.0) | 2 (0.8) | |
| Age (years) | Mean (SD) | 30.0 (7.3) | 30.2 (7.5) | 30.1 (7.4) |
| Sex n (%) | Female | 81 (64.3) | 71 (62.3) | 152 (63.3) |
| Male | 45 (35.7) | 43 (37.7) | 88 (36.7) | |
| BMI (kg/m2) | Mean (SD) | 23.0 (3.8) | 23.1 (3.7) | 23.0 (3.8) |
| Weight (kg) | Mean (SD) | 62.8 (13.1) | 63.0 (13.6) | 62.9 (13.3) |
| Baseline viral load (log10 copies per mL) | Mean (SD) | 5.4 (1.2) | 5.5 (1.0) | 5.5 (1.1) |
| SARS-CoV-2 Variant/ subvariants | Delta | 10 (7.9) | 11 (9.6) | 21 (8.8) |
| BA. 1 | 15 (11.9) | 21 (18.4) | 36 (15.0) | |
| BA. 2 | 58 (46.0) | 47 (41.2) | 105 (43.8) | |
| BA. 3 | 1 (0.8) | 0 (0.0) | 1 (0.4) | |
| BA. 4 | 2 (1.6) | 3 (2.6) | 5 (2.1) | |
| BA. 5 | 40 (31.7) | 32 (28.1) | 72 (30.0) | |
| Symptoms duration (days) | Mean (SD) | 2.2 (0.8) | 2.1 (0.7) | 2.2 (0.7) |
| Vaccinated (%) | Yes | 122 (96.8) | 112 (98.2) | 234 (97.5) |
| No | 4 (3.2) | 2 (1.8) | 6 (2.5) | |
Fig. 2.
a (left): qPCR estimates of oropharyngeal swab eluate viral densities (all measurements) with the daily median values graphed by treatment arm (green: no study drug; brown: favipiravir). b (right): Estimated change in the rate of viral clearance under the linear (red) and non-linear (blue) models (median posterior estimates and corresponding 80% (thick line) and 95% (thin line) credible intervals are shown)
Under the linear model, patients treated with favipiravir had an estimated median viral clearance half-life of 16.6 h (range 6.7 to 48.0) and patients randomised to the no study drug arm had an estimated median viral clearance half-life of 15.7 h (range 3.4 to 42.1), (Fig. 3a). In patients receiving favipiravir, there was no association between body weight (i.e., mg/kg dose of favipiravir) and the estimated viral clearance (p = 0.2) (Fig. 3b).
Fig. 3.
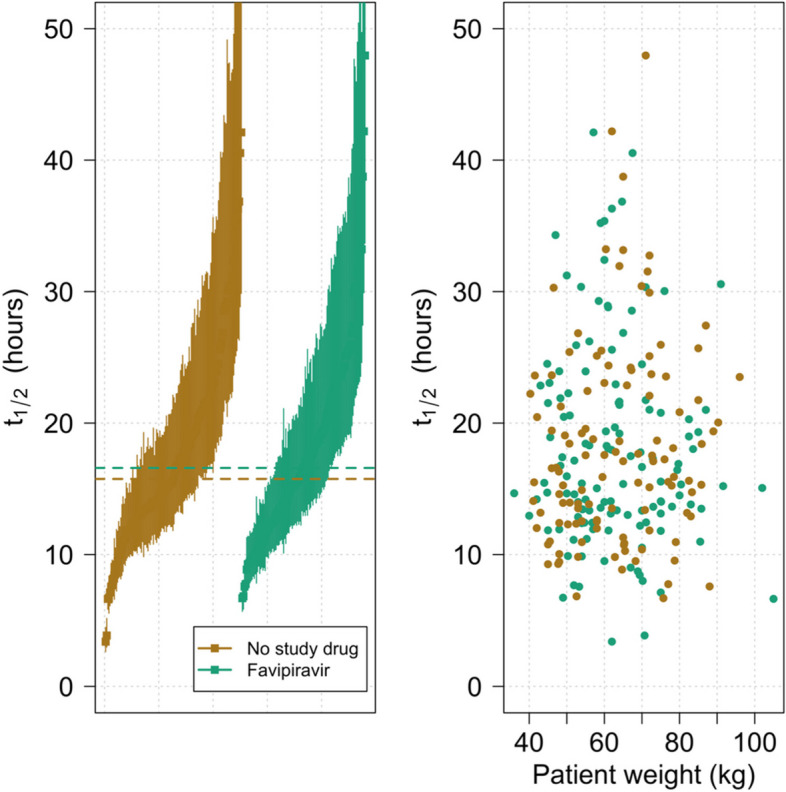
a (left): Estimated viral clearance half-lives ordered by increasing median estimate (lines show 80% credible intervals). b (right): Relationship between body weight and median estimated viral clearance half-life. As the individual doses were all the same, body weight is a surrogate for dose/kg and thus exposure
Adverse effects
The oropharyngeal swabbing procedures and all treatments were well-tolerated. There were three serious adverse events (SAEs) in the no study drug arm and two in the favipiravir arm, all resulting in the secondary endpoint of clinical deterioration leading to hospitalisation for medical reasons (three patients with raised creatinine phosphokinase (CPK) were already inpatients for isolation reasons; two no study drug and one favipiravir). In the favipiravir arm, a patient was readmitted 2 days after completing the 7-day course of favipiravir with fever and a maculopapular rash over the face, trunk, back, and extremities with sparing of the palms and soles. The rash was reviewed by a dermatologist who diagnosed a viral exanthem not related to the study drug. Two patients in the no study drug arm and one in the favipiravir arm had raised creatinine phosphokinase (CPK) levels (> 10 times ULN) attributed to COVID-19-related skeletal muscle damage. These improved with fluids and supportive management and were considered unrelated to study treatment. One patient in the no study drug arm was readmitted one day after discharge due to chest pain and lethargy. All clinical and laboratory investigations were normal and the patient was discharged the following day. There were no treatment related serious adverse events.
Discussion
Continued uncertainty over the value of different COVID-19 treatments has resulted in substantial variation in therapeutic guidelines and clinical practices across the world. In the absence of other affordable and available oral antiviral treatments favipiravir has been recommended for the treatment of uncomplicated COVID-19 in several countries including Japan, Russia, Saudi Arabia, Turkey, Hungary, Kenya and Thailand (where it was recommended for patients with mild COVID-19 pneumonia from May 2020 until December 2022) (https://ddc.moph.go.th/viralpneumonia/eng/file/guidelines/g_treatment.pdf).
Knowing definitively if an antiviral drug has antiviral efficacy in vivo should be a prerequisite for its deployment. But the urgency and gravity of the spreading pandemic in 2020 meant that many drugs were recommended without clear evidence of clinical benefit. In this fourth year of the COVID-19 pandemic, increasingly mild clinical presentations resulting from immune protection from vaccines and previous infections, declining viral virulence, and availability in some regions of newly developed oral antivirals with proven efficacy (notably molnupiravir and nirmatrelvir/ritonavir) [33, 34], have all contributed to favipiravir being no-longer recommended for COVID-19. For the same reason use of other repurposed drugs has also decreased. This has left substantial uncertainty as to their clinical benefit in COVID-19, and their potential use in future pandemics caused by novel viruses.
This comparative in vivo pharmacodynamic assessment conducted in “low risk” adults with early symptomatic COVID-19 infections shows that favipiravir, given at relatively high oral doses, does not have measurable antiviral activity in vivo and is, therefore, very unlikely to be clinically beneficial. The lack of demonstrable in vivo activity contrasts with the approximate 30 to 40% acceleration in viral clearance rate observed for remdesivir and molnupiravir in this trial platform [31]. The main limitation of our study that it is open label, which may have led to more withdrawals in the no study drug arm.
Favipiravir was well-tolerated at the high doses used in this study. Favipiravir has complex non-linear pharmacokinetic properties [32]. It is metabolised primarily in the liver by aldehyde oxidase and excreted via the kidneys. Because of dose and time dependent auto-inhibition of aldehyde oxidase, favipiravir boosts its own plasma concentrations. This can result in exposures over twice the SARS-CoV-2 in vitro EC90 [6], although there is substantial inter-patient variability in achieved plasma concentrations, and lower exposures have been noted in certain populations, e.g. those from the United States compared to Japan and China [35]. Despite pharmacokinetic modelling suggesting that exposures sufficient for an antiviral effect can be achieved, the relationship between ex vivo SARS-CoV-2 inhibitory concentrations and consequent therapeutic effects in COVID-19 in vivo is uncertain. This study does not exclude therapeutic benefit from even higher oral or parenteral doses of favipiravir, although there was no evidence of a dose response relationship in this study derived from the variation in weight adjusted doses.
Similar negative results have been reported recently with ivermectin [36], which also fails to halt disease progression when given to outpatients [37]. In contrast, the antiviral remdesivir clearly does accelerate viral clearance [38], and in clinical trials it does prevent disease progression [39]. The association between accelerated viral clearance and improved clinical outcomes in early COVID-19 has been confirmed in studies with monoclonal antibodies as well as the newly developed antiviral drugs [33, 34, 36, 40–42]. In contrast, the reported lack of demonstrable antiviral effect in the PINETREE study of remdesivir, despite demonstration of a clear clinically beneficial effect, likely resulted from too infrequent nasopharyngeal viral density measurements and from the statistical analysis approach used to assess differences in viral loads. All these studies were completed in largely unvaccinated populations at a time when a higher proportion of COVID-19 infections progressed to hospitalisation and severe outcomes. If repeated today such studies would need to be substantially, and perhaps prohibitively, larger in order to detect clinical benefit. For example, molnupiravir was shown to provide clinical benefit in studies conducted over two years ago [33], but in the more recent community based PANORAMIC study [43] conducted in the UK there was no clear effect of molnupiravir on hospitalisation or death, despite recruiting 26,411 patients. However, molnupiravir was associated with a reduced time to recovery (although it was an open-label study) and faster reduction in viral loads. Given the very low event rate for the primary endpoint, despite its size, the PANORAMIC study was still underpowered.
The time and expense required to conduct large phase III studies in vaccinated populations and the difficulty of demonstrating efficacy using clinical end-points in early infections suggests that other approaches are needed for therapeutic assessment in COVID-19 (and other viral respiratory infections). The simple methodology described in this study provides one possible solution. It is readily performed anywhere which can perform accurate qPCR viral quantitation and it gives a rapid comparative assessment with much lower patient numbers than clinical trials with currently used viral endpoints (e.g. time-to-clearance) [44]. Duplicate daily oropharyngeal swabs are well-tolerated (whereas daily nasopharyngeal swabbing is not). The pharmacometric assessment can be used to characterise in vivo antiviral efficacy in real-time and thereby inform choice of drugs for large trials and therapeutic practice. Regulatory authority and treatment guideline decisions should be based upon evidence of in vivo antiviral efficacy, as well as in vitro evidence.
Supplementary Information
Additional file 1: Supplementary Table 1. Summary of Adverse Events (grade 3 and above) for favipiravir. Supplementary Table 2. Summary of Serious Adverse Events. Figure S1. Genotyped SARS CoV2 variants over time (combined Thai and Brazilian sites). Figure S2. Covariate effects on intercept (left) and slope (right) for the linear model with additional covariate adjustment.
Acknowledgements
We thank all the patients with COVID-19 who volunteered to be part of the study. We thank the data safety and monitoring board (DSMB) (Tim Peto, André Siqueira, and Panisadee Avirutnan); the trial steering committee (TSC) (Nathalie Strub-Wourgaft, Martin Llewelyn, Deborah Waller, and Attavit Asavisanu); Sompob Saralamba and Tanaphum Wichaita for developing the RShiny randomisation app; and Mavuto Mukaka for invaluable statistical support. We also thank all the staff of the Clinical Trials Unit (CTU) at MORU, PCR Expert group (Janjira Thaipadungpanit, Audrey Dubot-Pérès and Clare Ling), Thermo Fisher for their excellent support with this project, and all the hospital staff at the Hospital of Tropical Diseases, Faculty of Tropical Medicine, Bangplee (BP) and Vajira (VJ) hospitals, as well as those involved in sample processing in MORU and the processing and analysis at the Faculty of Tropical Medicine (FTM), molecular genetics laboratory. We thank the MORU Clinical Trials Support Group (CTSG) for data management, monitoring, ethics and regulatory submissions and logistics, and the purchasing, administration and support staff at MORU, and those at the Brazil site who provided expert help in managing patients (Joseane Fratari, Josiane Vaz, Fátima Brant and Lísia Esper).
Authors’ contributions
V.L., J.A.W., S.B., W.H.K.S., and N.J.W wrote the first draft of the manuscript. P.J., V.L., T.S., T.N., B.H., S.S., K.P., P.B., V.C., P.J.A., M.M., S.P., W.P., W.P. were responsible for collection of clinical data. T.N. was responsible for data curation. J.A.W. was responsible for statistical analysis and the figures. S.K., W.M., M.Y.A., R.A.S., F.M.S., R.T., M.I., K.C. were responsible for laboratory testing and analysis. V.K., J.T., were responsible for trial set-up and monitoring. C.C. was responsible for coordination of the study in Brazil and J.J.C. and S.B. for safety monitoring and document preparation. W.R.J.T., A.M.D, N.P.J.D, N.J.W supervised the study and gave scientific input. All authors reviewed the manuscript.
Funding
“Finding treatments for COVID-19: A phase 2 multi-centre adaptive platform trial to assess antiviral pharmacodynamics in early symptomatic COVID-19 (PLAT-COV)” is supported by the Wellcome Trust Grant ref: 223195/Z/21/Z through the COVID-19 Therapeutics Accelerator.
Availability of data and materials
All code and data are openly accessible via GitHub: https://github.com/jwatowatson/PLATCOV-Favipiravir. The final datasets will be stored locally and securely at the Mahidol Oxford Research Unit for long-term storage and access. Additional anonymised participant data can be made available by request on a case-by-case basis from the MORU Data Access Committee at datasharing@tropmedres.ac and can be made available by request to the corresponding author.
Declarations
Ethics approval and consent to participate
All patients provided fully informed written consent. The trial was approved by local and national research ethics boards in Thailand (Faculty of Tropical Medicine Ethics Committee, Mahidol University, FTMEC Ref: TMEC 21–058) and the Central Research Ethics Committee (CREC, Bangkok, Thailand, CREC Ref: CREC048/64BP-MED34), in Brazil by the Research Ethics Committee of the Universidade Federal de Minas Gerais (COEP-UFMG, Minas Gerais, Brazil, COEP-UFMG) and National Research Ethics Commission- (CONEP, Brazil, COEP-UFMG and CONEP Ref: CAAE:51593421.1.0000.5149), and by the Oxford University Tropical Research Ethics Committee (OxTREC, Oxford, UK, OxTREC Ref: 24–21). All methods were performed in accordance with the relevant guidelines and regulations (e.g. Declaration of Helsinki).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
William H. K. Schilling and Podjanee Jittamala equally contributed to this work.
Contributor Information
William H. K. Schilling, Email: william@tropmedres.ac
Nicholas J. White, Email: nickw@tropmedres.ac
References
- 1.Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sissoko D, Laouenan C, Folkesson E, M'Lebing AB, Beavogui AH, Baize S, et al. Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13:e1001967. doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A, et al. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell. 2020;182:417–428. doi: 10.1016/j.cell.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75:2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleeman K, Mishin VP, Deyde VM, Furuta Y, Klimov AI, Gubareva LV. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrob Agents Chemother. 2010;54:2517–2524. doi: 10.1128/AAC.01739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaptein SJF, Jacobs S, Langendries L, et al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci USA. 2020;117:26955–26965. doi: 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driouich JS, Cochin M, Lingas G, Moureau G, Touret F, Petit PR, et al. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat Commun. 2021;12:1735. doi: 10.1038/s41467-021-21992-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy PK, Patil S, Khobragade A, Balki A, Raj A, Kalikar M, et al. Evaluation of the safety and efficacy of favipiravir in adult Indian patients with mild-to-moderate COVID-19 in a real-world setting. Int J Gen Med. 2022;15:4551–4563. doi: 10.2147/IJGM.S349241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitasuwan T, Phisalprapa P, Srivanichakorn W, Washirasaksiri C, Auesomwang C, Tinmanee R, et al. Early antiviral and supervisory dexamethasone treatment improve clinical outcomes of nonsevere COVID-19 patients. Medicine (Baltimore) 2022;101:e31681. doi: 10.1097/MD.0000000000031681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi Y, Ishihara T, Banno S, Ando M, Kondo M. Favipiravir Observational Study. Favipiravir for symptomatic COVID-19: a nationwide observational cohort study. J Infect Chemother. 2022:S1341–321X(22)00291–4. [DOI] [PMC free article] [PubMed]
- 14.Abdulrahman B, Mady A, Odat MA, Tayar AA, Rana MA, Alharthy A, et al. Favipiravir efficacy and safety for the treatment of severe coronavirus disease 2019: a retrospective study. J Ayub Med Coll Abbottabad. 2022;34(3):397–402. doi: 10.55519/JAMC-03-10305. [DOI] [PubMed] [Google Scholar]
- 15.Sirijatuphat R, Manosuthi W, Niyomnaitham S, Owen A, Copeland KK, Charoenpong L, et al. Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study. Emerg Microbes Infect. 2022;11:2197–2206. doi: 10.1080/22221751.2022.2117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, et al. Prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64:e01897–20. doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khamis F, Al Naabi H, Al Lawati A, Ambusaidi Z, Al Sharji M, Al Barwani U, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis. 2021;102:538–543. doi: 10.1016/j.ijid.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase ii/iii multicenter randomized clinical trial. Clin Infect Dis. 2021;73:531–534. doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Zhang C, Zhu Q, Chen X, Chen G, Sun W, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: a multicenter, open-label, randomized trial. Int Immunopharmacol. 2021;97:107702. [DOI] [PMC free article] [PubMed]
- 20.Solaymani-Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, Hassan Saadat S, et al. Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol. 2021;95:107522. doi: 10.1016/j.intimp.2021.107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi: 10.1016/j.ijid.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Qahtani M, Kumar N, Aljawder D, Abdulrahman A, Mohamed MW, Alnashaba F, et al. Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease. Sci Rep. 2022;12(1):4925. doi: 10.1038/s41598-022-08794-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassaniazad M, Farshidi H, Gharibzadeh A, Bazram A, Khalili E, Noormandi A, Fathalipour M. Efficacy and safety of favipiravir plus interferon-beta versus lopinavir/ritonavir plus interferon-beta in moderately ill patients with COVID-19: a randomized clinical trial. J Med Virol. 2022;94:3184–3191. doi: 10.1002/jmv.27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon JH, Lau JSY, Coldham A, Roney J, Hagenauer M, Price S, et al. Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial. EClinicalMedicine. 2022;54:101703. doi: 10.1016/j.eclinm.2022.101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe DM, Brown LK, Chowdhury K, Davey S, Yee P, Ikeji F, et al. Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): a randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med. 2022;19:e1004120. doi: 10.1371/journal.pmed.1004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golan Y, Campos JAS, Woolson R, Cilla D, Hanabergh R, Gonzales-Rojas Y, et al. Favipiravir in patients with early mild-to-moderate COVID-19: a randomized controlled trial. Clin Infect Dis. 2022;6:ciac712. doi: 10.1093/cid/ciac712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosaeed M, Alharbi A, Mahmoud E, Alrehily S, Bahlaq M, Gaifer Z, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect. 2022;28:602–608. doi: 10.1016/j.cmi.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuah CH, Chow TS, Hor CP, Cheng JT, Ker HB, Lee HG, et al. Efficacy of early treatment with favipiravir on disease progression among high-risk patients with coronavirus disease 2019 (COVID-19): a randomized open-label clinical trial. Clin Infect Dis. 2022;75:e432–e439. doi: 10.1093/cid/ciab962. [DOI] [PubMed] [Google Scholar]
- 29.Holubar M, Subramanian A, Purington N, Hedlin H, Bunning B, Walter KS, et al. Favipiravir for treatment of outpatients with asymptomatic or uncomplicated coronavirus disease 2019: a double-blind, randomized, placebo-controlled, phase 2 trial. Clin Infect Dis. 2022;75:1883–1892. doi: 10.1093/cid/ciac312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters MD, Warren S, Hughes C, Lewis P, Zhang F. Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir. Environ Mol Mutagen. 2022;63:37–63. doi: 10.1002/em.22471. [DOI] [PubMed] [Google Scholar]
- 31.Schilling WHK, Jittamala P, Watson JA, Boyd S, Luvira V, Siripoon T, et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial. Lancet Infect Dis. 2023:S1473-3099(23)00493-0. 10.1016/S1473-3099(23)00493-0. Epub ahead of print. Erratum in: Lancet Infect Dis. 2023;23(12):e511. [DOI] [PMC free article] [PubMed]
- 32.Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020;108:242–247. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 33.Jayk Bernal A, da Gomes Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386:509–20. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen TH, Guedj J, Anglaret X, Laouénan C, Madelain V, Taburet AM, et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis. 2017;11:e0005389. doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schilling WHK, Jittamala P, Watson JA, Ekkapongpisit M, Siripoon T, Ngamprasertchai T, et al. Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, controlled adaptive platform trial (PLATCOV). Elife. 2023;12:e83201. 10.7554/eLife.83201. [DOI] [PMC free article] [PubMed]
- 37.Reis G, Silva EASM, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with ivermectin among patients with Covid-19. N Engl J Med. 2022;386:1721–1731. doi: 10.1056/NEJMoa2115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jittamala P, Schilling WHK, Watson JA, Luvira V, Siripoon T, et al. Clinical antiviral efficacy of remdesivir in COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV) J Infect Dis. 2023;228(10):1318–1325. doi: 10.1093/infdis/jiad275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al. Covid-19 phase 3 prevention trial team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer WA, 2nd, Eron JJ, Jr, Holman W, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14:eabl7430. doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler CC, Hobbs DR, Gbinigie OA, Rahman NM, Hayward G, Richards DB, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401:281–293. doi: 10.1016/S0140-6736(22)02597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson JA, Kissler SM, Day NPJ, Grad YH, White NJ. Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies. Antimicrob Agents Chemother. 2022;66:e0019222. doi: 10.1128/aac.00192-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Summary of Adverse Events (grade 3 and above) for favipiravir. Supplementary Table 2. Summary of Serious Adverse Events. Figure S1. Genotyped SARS CoV2 variants over time (combined Thai and Brazilian sites). Figure S2. Covariate effects on intercept (left) and slope (right) for the linear model with additional covariate adjustment.
Data Availability Statement
All code and data are openly accessible via GitHub: https://github.com/jwatowatson/PLATCOV-Favipiravir. The final datasets will be stored locally and securely at the Mahidol Oxford Research Unit for long-term storage and access. Additional anonymised participant data can be made available by request on a case-by-case basis from the MORU Data Access Committee at datasharing@tropmedres.ac and can be made available by request to the corresponding author.