Abstract
Bicyclo[1.1.0]butanes (BCBs) have gained growing popularity in “strain release” chemistry for the synthesis of four-membered-ring systems and para- and meta-disubstituted arene bioisosteres as well as applications in chemoselective bioconjugation. However, functionalization of the bridge position of BCBs can be challenging due to the inherent strain of the ring system and reactivity of the central C–C bond. Here we report the first late-stage bridge cross-coupling of BCBs, mediated by directed metalation/palladium catalysis.
Bicyclo[1.1.0]butanes (BCBs) (Figure 1a) are a class of highly strained hydrocarbons that have become valuable tools for “strain release” chemistry.1 These reagents possess the impressive ability to react with nucleophiles,2 radicals,3 electrophiles,4 and transition metal catalysts,5 with applications ranging from the synthesis of natural products2d and para-(6) and meta-substituted7 arene bioisosteres to use as cystine-selective bioconjugation agents.2b,8 Access to these building blocks has been streamlined with the recent developments of late-stage bridgehead and bridge metalation protocols that deliver a broad portfolio of BCBs,9 including a convenient one-pot sulfone-based reaction sequence that affords exceptional diversity.10
Figure 1.
(a) Applications of BCBs in cyclobutane and bioisostere synthesis. (b) Previous work toward the construction of aryl-substituted BCBs. (c) Bridge directed metalation and cross-coupling of BCBs. DMG = directing metalation group.
BCBs that are aryl-substituted at the bridge carbon atoms are attractive targets due to their potential use in accessing arene-functionalized products upon ring opening. Specifically, access to these products would open up new avenues for medicinal chemists in bicyclo[1.1.1]pentane, -[2.1.1]hexane, and -[3.1.1]heptane synthesis for reaching novel chemical space.7b Recent methodologies developed for the synthesis of aryl-substituted BCBs include (1) biocatalytic double diazo–alkyne condensation that introduces two identical endo/exo bridge ester substituents (bridgehead aryl, Figure 1b);11 (2) asymmetric intramolecular diazo insertion into styrenes, catalyzed by rhodium(II) (bridge aryl);2e,12 and (3) bridgehead-directed metalation and cross-coupling (bridgehead aryl).9a The methods outlined in each case address different challenges, such as the latter providing a divergent synthesis of bicyclopentylation reagents, and the asymmetric diazo insertion facilitating a route toward the total synthesis of piperarborenine B.2d However, although intramolecular diazo insertion offers a powerful method for asymmetric bridge-arylated BCB synthesis, it suffers from the drawback of synthetic linearity rather than late-stage diversification.
We previously developed a method that enables late-stage bridge functionalization through directed metalation/electrophilic quench,9b although this tactic did not enable the introduction of aryl and alkenyl substituents. We questioned whether we might be able to extend this approach to bridge cross-coupling by transmetalation of the intermediate organolithium, enabling the rapid delivery of bridge aryl-substituted strain release reagents (Figure 1c). Notably, a similar strategy has been employed in the elegant polyfunctionalization of cubanes.13
Reaction development began by employing three potential BCB organometallic coupling partners, boronic acid 1a, stannane 1b, and organozinc 1c (prepared from metalation of BCB 2a with organolithiums and electrophilic quench (1a/b) or transmetalation to ZnCl2 (1c)), in Suzuki, Stille, and Negishi coupling protocols, respectively (Table 1, entries 1–3). Interestingly, the former two strategies led only to complete decomposition of the starting material with no observable product, while entry 3 returned 2a with no sign of degradation. This was surprising given previous reports on cyclopropylzinc Negishi couplings as well as our own work on BCB bridgehead Negishi reactivity,9a,14 and it was hypothesized that TMEDA might be interfering with the reaction. To our delight, the use of TMEDA-free metalation in the generation of 3a (t-BuLi in THF) and submission to equivalent coupling conditions (Pd(dba)2/2PPh3) achieved cross-coupling in 28% yield (as determined by 1H NMR spectroscopy; entry 4). A screen of 13 phosphine ligands was conducted, with the Buchwald-based ligands producing the highest yields and CyJPhos being optimal (48%; entry 5).15 A temperature and solvent screen identified THF at 65 °C as being crucial for this transformation (entries 6 and 7). Increasing the equivalents of iodobenzene led to a further increase in the yield (60%; entry 8). While a useful result, the conversion could be further enhanced by increasing the catalyst loading to 15 mol %, giving 3a in 71% yield (entry 9). On scale-up, it became apparent that stirring the reaction mixture for 1 h at room temperature was crucial; otherwise, the reaction would fail due to Pd black formation.
Table 1. BCB Cross-Coupling Optimizationa.
| entry | [M] | ligand, variation of conditions | yield (%)b |
|---|---|---|---|
| 1 | B(OH)2 (1a) | PPh3 | 0 |
| 2 | Sn(n-Bu)3 (1b) | AsPh3 | 0 |
| 3 | ZnCl·LiCl (1c) | PPh3, TMEDA (1.1 equiv) | 0 |
| 4 | ZnCl·LiCl (1c) | PPh3 | 28 |
| 5 | ZnCl·LiCl (1c) | CyJPhos | 48 |
| 6 | ZnCl·LiCl (1c) | CyJPhos, 20 °C | 6 |
| 7 | ZnCl·LiCl (1c) | CyJPhos, DMF/Tol/1,4-dioxanec | –d |
| 8 | ZnCl·LiCl (1c) | CyJPhos, PhI (4.0 equiv) | 60 |
| 9 | ZnCl·LiCl (1c) | CyJPhos2 (30 mol %) and Pd(dba)2 (15 mol %)e | 71 |
Reactions were conducted on a 0.1 mmol scale. See the Supporting Information for details of metalation protocols in the synthesis of 1a–1c.
Determined by 1H NMR spectroscopic analysis of the crude reaction mixtures using 1,3,6-trimethoxybenzene as an internal standard.
Heated at 110 °C.
Extensive decomposition was observed in these solvents.
Stirred for 1 h at room temperature before heating.
With optimized metalation and cross-coupling conditions in hand, we then examined the scope of the reaction (Scheme 1). A selection of aryl iodides bearing electron-withdrawing and -donating groups at the para position was first investigated. To our delight, these couplings proceeded in good to excellent yields (3a–3e, 60–84%). Reaction efficiency was maintained with ortho-substituted aryl iodide derivatives (3f and 3g). The introduction of biorelevant functionality was also possible, for example, incorporating a galactose-bearing side chain in excellent yield (3h, 91%, 1:1 dr due to the racemic generation of 1c).
Scheme 1. Synthesis of Aryl Bridge-Substituted BCBs.
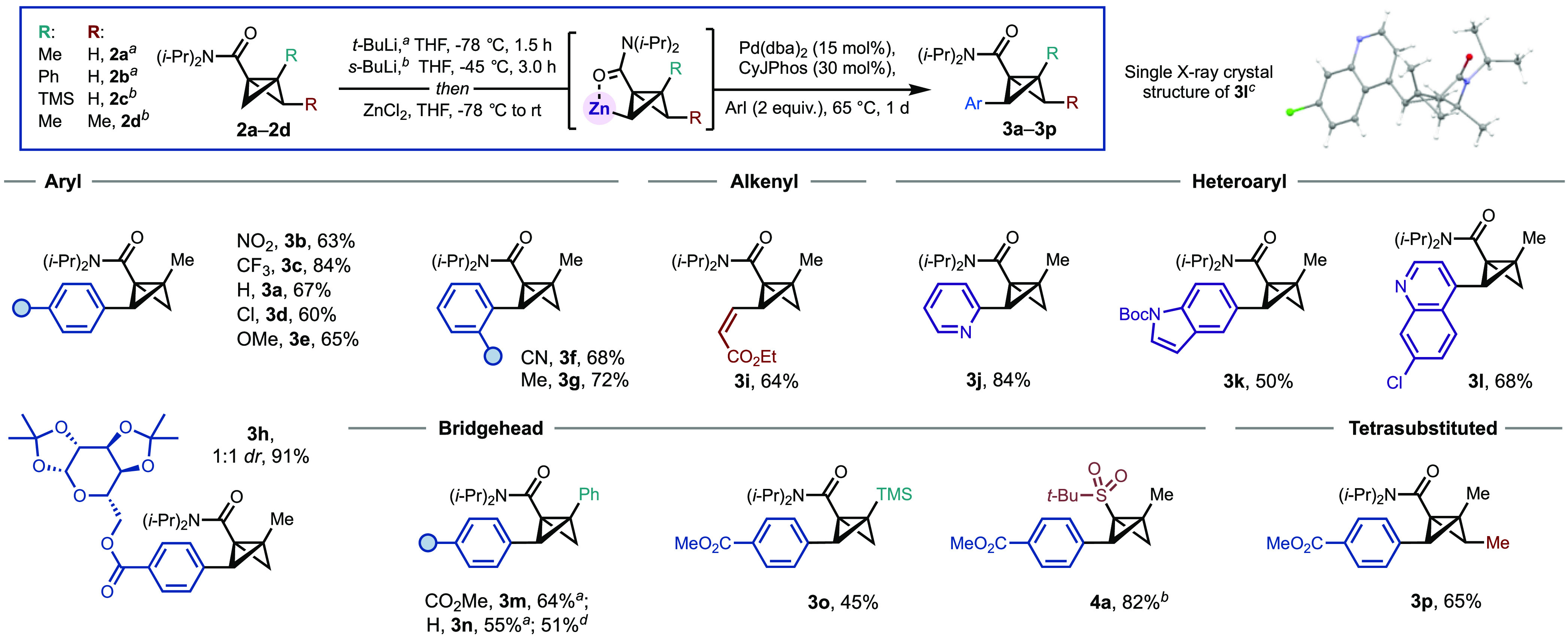
Reaction conditions, unless stated otherwise: 2a or 2b (0.20 mmol, 1.0 equiv); 1. Lithiation: at-BuLi (0.22 mmol, 1.1 equiv, 1.1–1.3 M in pentane, THF, −78 °C, 1.5 h; bs-BuLi (0.22 mmol, 1.1 equiv, 1.1–1.5 M in cyclohexane), THF, −45 °C, 3.0 h; 2. Transmetalation/coupling: ZnCl2 (0.22 mmol, 1.1 equiv), THF, −78 °C (or −45 °C) to rt; Pd(dba)2 (15 mol %), CyJPhos (30 mol %), R–I (0.4 mmol, 2.0 equiv) THF, 1 h, 20 °C; 1 d, 65 °C. cStructure of 3l from single-crystal X-ray diffraction studies (displacement ellipsoids drawn at 50% probability). dThe reaction was conducted on a 1 mmol scale.
Alkenyl iodides were also compatible with the coupling conditions (3i, 64%); however, alkenes bearing an electron-withdrawing group were essential for the product stability. Heterocycle cross-coupling is also highly appealing from a medicinal chemistry stance due to the application of BCBs in para- and meta-arene bioisostere synthesis. We were therefore delighted to find that a representative range of azacycles could be installed in good yields (50–84%), including 2-substituted pyridine (3j), indole (3k), and quinoline (3i). Pleasingly, these conditions could also be applied to BCB 2b, which is more sterically demanding at the bridgehead position (Ph substituent), giving 3m (64%) and 3n (55%). The latter coupling was also carried out on a 1 mmol scale without significant detriment to the yield (51%).
Cross-coupling on other BCBs, such as trimethylsilyl-substituted BCB 2c and trisubstituted BCB 2d would demonstrate the feasibility of constructing more complex derivatives, including a tetrasubstituted product. However, no product was observed when 2c and 2d were subjected to the developed metalation and cross-coupling conditions, with neither undergoing productive metalation with t-BuLi at −78 °C in THF. Fortunately, TMEDA-free conditions for directed lithiation were identified (s-BuLi at −45 °C in THF)15 that could be applied to 2c and 2d. These substrates were then subjected to the cross-coupling conditions and, to our delight, produced silyl-substituted BCB 3o in 45% yield and tetrasubstituted BCB 3p in 65% yield. Resolving the cross-coupling issue of 2c and 2d inspired us to examine sulfone substrates; pleasingly, 4a could be obtained in an excellent yield of 82% with the s-BuLi metalation conditions.
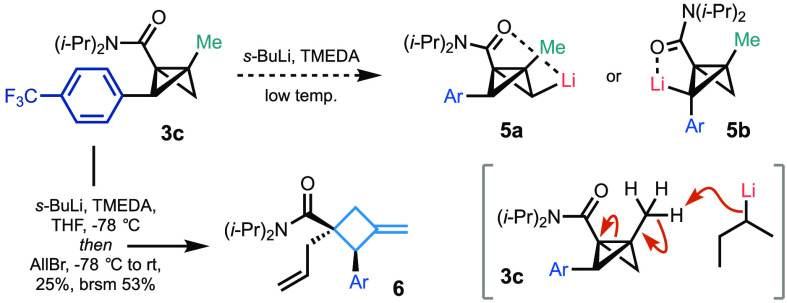
Having successfully demonstrated cross-coupling with trisubstituted BCB 2d, we questioned whether a complementary approach could be established through a second directed bridge metalation after bridge arylation (Scheme 2). BCB 3c was chosen as a candidate, as the bridge arene possesses a para electron-withdrawing group (CF3) that is tolerant of organolithiums. This substrate presents a regioselectivity challenge: the possibility of directed metalation (5a, Scheme 2) or benzylic deprotonation (5b), both of which would provide a useful class of novel BCBs. Surprisingly, when 3c was subjected to the optimized conditions, neither the unsubstituted bridge nor the benzylic bridge underwent lithiation. Instead, s-BuLi was directed to the bridgehead methyl group, which then underwent BCB ring opening to give the corresponding enolate; quenching with allyl bromide afforded polysubstituted exocyclic cyclobutene 6. This observation of this alternative metalation pathway may relate to restricted rotation of the directing group in substrate 3c, which prevents access to the unsubstituted bridge, as observed in the X-ray crystal structure of 3l and 1H NMR spectrum of the bridge aryl-BCB derivatives.15
Scheme 2. Unexpected Fragmentation in the Metalation of Bridge-Arylated BCB 3c.
The reaction was run on a 0.12 mmol scale using optimized conditions with allyl bromide (0.6 mmol, 5.0 equiv) quench.
In summary, we have developed a convenient and general late-stage Negishi cross-coupling strategy to access sp2-bridge-substituted BCBs. This approach enables the introduction of arenes, heteroarenes, and alkenes with broad functional group tolerance with respect to the arene: nitro, ester, halide, silyl, nitrile, ether, and acetal groups are all accommodated, which can allow for further manipulation. This approach enables the rapid delivery of new strain release reagents, which we expect to be of use to the wider chemical community for small-ring and bioisostere construction.
Acknowledgments
R.E.M. thanks the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine for a studentship (EP/L015838/1) generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Jannsen, Novartis, Pfizer, Syngenta, Takeda, UCB, and Vertex. E.A.A. and A.D.G. thank the EPSRC for support (EP/S013172/1).
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c04030.
Additional experimental discussion, experimental procedures, and copies of 1H and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Golfmann M.; Walker J. C. L. Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis. Commun. Chem. 2023, 6, 9. 10.1038/s42004-022-00811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kelly C. B.; Milligan J. A.; Tilley L. J.; Sodano T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci. 2022, 13, 11721–11737. 10.1039/D2SC03948F. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Fawcett A. Recent advances in the chemistry of bicyclo- and 1-azabicyclo[1.1.0]butanes. Pure Appl. Chem. 2020, 92, 751–765. 10.1515/pac-2019-1007. [DOI] [Google Scholar]; d Turkowska J.; Durka J.; Gryko D. Strain release – an old tool for new transformations. Chem. Commun. 2020, 56, 5718–5734. 10.1039/D0CC01771J. [DOI] [PubMed] [Google Scholar]
- a Guo L.; Noble A.; Aggarwal V. K. α-Selective Ring-Opening Reactions of Bicyclo[1.1.0]butyl Boronic Ester with Nucleophiles. Angew. Chem., Int. Ed. 2021, 60, 212–216. 10.1002/anie.202011739. [DOI] [PubMed] [Google Scholar]; b Lopchuk J. M.; Fjelbye K.; Kawamata Y.; Malins L. R.; Pan C.-M.; Gianatassio R.; Wang J.; Prieto L.; Bradow J.; Brandt T. A.; Collins M. R.; Elleraas J.; Ewanicki J.; Farrell W.; Fadeyi O. O.; Gallego G. M.; Mousseau J. J.; Oliver R.; Sach N. W.; Smith J. K.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity. J. Am. Chem. Soc. 2017, 139, 3209–3226. 10.1021/jacs.6b13229. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Gianatassio R.; Lopchuk J. M.; Wang J.; Pan C.; Malins L. R.; Prieto L.; Brandt T. A.; Collins M. R.; Gallego G. M.; Sach N. W.; Spangler J. E.; Zhu H.; Zhu J.; Baran P. S. Strain-release amination. Science 2016, 351, 241. 10.1126/science.aad6252. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Panish R. A.; Chintala S. R.; Fox J. M. A Mixed-Ligand Chiral Rhodium(II) Catalyst Enables the Enantioselective Total Synthesis of Piperarborenine B. Angew. Chem., Int. Ed. 2016, 55, 4983–4987. 10.1002/anie.201600766. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Panish R.; Chintala S. R.; Boruta D. T.; Fang Y.; Taylor M. T.; Fox J. M. Enantioselective Synthesis of Cyclobutanes via Sequential Rh-catalyzed Bicyclobutanation/Cu-catalyzed Homoconjugate Addition. J. Am. Chem. Soc. 2013, 135, 9283–9286. 10.1021/ja403811t. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Gaoni Y. New bridgehead-substituted 1-(arylsulfonyl)bicyclo[1.1.0]butanes and some novel addition reactions of the bicyclic system. Tetrahedron 1989, 45, 2819–2840. 10.1016/S0040-4020(01)80112-5. [DOI] [Google Scholar]; g Gaoni Y.; Tomazic A. Bridgehead reactivity, nucleophilic and radical additions, and lithium aluminum hydride reduction of 1-(arylsulfonyl)bicyclobutanes: general access to substituted, functionalized cyclobutanes. Syntheses of (±)-citrilol acetate, (±)-junionone, and the tricyclo[3.3.0.01,4]octane and tricyclo[4.3.0.01,7]nonane ring systems. J. Org. Chem. 1985, 50, 2948–2957. 10.1021/jo00216a028. [DOI] [Google Scholar]
- a Pratt C. J.; Aycock R. A.; King M. D.; Jui N. T. Radical α-C–H Cyclobutylation of Aniline Derivatives. Synlett 2020, 31, 51–54. 10.1055/s-0039-1690197. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Silvi M.; Aggarwal V. K. Radical Addition to Strained σ-Bonds Enables the Stereocontrolled Synthesis of Cyclobutyl Boronic Esters. J. Am. Chem. Soc. 2019, 141, 9511–9515. 10.1021/jacs.9b03653. [DOI] [PubMed] [Google Scholar]
- a Guin A.; Bhattacharjee S.; Harariya M. S.; Biju A. T. Lewis acid-catalyzed diastereoselective carbofunctionalization of bicyclobutanes employing naphthols. Chem. Sci. 2023, 14, 6585–6591. 10.1039/D3SC01373A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kerner M. J.; Wipf P. Semipinacol-Type Rearrangements of [3-(Arylsulfonyl)bicyclo[1.1.0]butan-1-yl]alkanols. Org. Lett. 2021, 23, 3615–3619. 10.1021/acs.orglett.1c01004. [DOI] [PubMed] [Google Scholar]; c Bennett S. H.; Fawcett A.; Denton E. H.; Biberger T.; Fasano V.; Winter N.; Aggarwal V. K. Difunctionalization of C–C σ-Bonds Enabled by the Reaction of Bicyclo[1.1.0]butyl Boronate Complexes with Electrophiles: Reaction Development, Scope, and Stereochemical Origins. J. Am. Chem. Soc. 2020, 142, 16766–16775. 10.1021/jacs.0c07357. [DOI] [PubMed] [Google Scholar]
- a Wölfl B.; Winter N.; Li J.; Noble A.; Aggarwal V. K. Strain-Release Driven Epoxidation and Aziridination of Bicyclo[1.1.0]butanes via Palladium Catalyzed σ-Bond Nucleopalladation. Angew. Chem., Int. Ed. 2023, 62, e202217064 10.1002/anie.202217064. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang Z.; Gevorgyan V. Palladium Hydride-Enabled Hydroalkenylation of Strained Molecules. J. Am. Chem. Soc. 2022, 144, 20875–20883. 10.1021/jacs.2c09045. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pinkert T.; Das M.; Schrader M. L.; Glorius F. Use of Strain-Release for the Diastereoselective Construction of Quaternary Carbon Centers. J. Am. Chem. Soc. 2021, 143, 7648–7654. 10.1021/jacs.1c03492. [DOI] [PubMed] [Google Scholar]; d Ociepa M.; Wierzba A. J.; Turkowska J.; Gryko D. Polarity-Reversal Strategy for the Functionalization of Electrophilic Strained Molecules via Light-Driven Cobalt Catalysis. J. Am. Chem. Soc. 2020, 142, 5355–5361. 10.1021/jacs.0c00245. [DOI] [PubMed] [Google Scholar]; e Fawcett A.; Biberger T.; Aggarwal V. K. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem. 2019, 11, 117–122. 10.1038/s41557-018-0181-x. [DOI] [PubMed] [Google Scholar]; f Walczak M. A. A.; Krainz T.; Wipf P. Ring-Strain-Enabled Reaction Discovery: New Heterocycles from Bicyclo[1.1.0]butanes. Acc. Chem. Res. 2015, 48, 1149–1158. 10.1021/ar500437h. [DOI] [PubMed] [Google Scholar]; g Walczak M. A. A.; Wipf P. Rhodium(I)-Catalyzed Cycloisomerizations of Bicyclobutanes. J. Am. Chem. Soc. 2008, 130, 6924–6925. 10.1021/ja802906k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Stepan A. F.; Subramanyam C.; Efremov I. V.; Dutra J. K.; O’Sullivan T. J.; DiRico K. J.; McDonald W. S.; Won A.; Dorff P. H.; Nolan C. E.; Becker S. L.; Pustilnik L. R.; Riddell D. R.; Kauffman G. W.; Kormos B. L.; Zhang L.; Lu Y.; Capetta S. H.; Green M. E.; Karki K.; Sibley E.; Atchison K. P.; Hallgren A. J.; Oborski C. E.; Robshaw A. E.; Sneed B.; O’Donnell C. J. Application of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem. 2012, 55, 3414–3424. 10.1021/jm300094u. [DOI] [PubMed] [Google Scholar]; b Measom N. D.; Down K. D.; Hirst D. J.; Jamieson C.; Manas E. S.; Patel V. K.; Somers D. O. Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an LpPLA2 inhibitor. ACS Med. Chem. Lett. 2017, 8, 43–48. 10.1021/acsmedchemlett.6b00281. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bychek R. M.; Hutskalova V.; Bas Y. P.; Zaporozhets O. A.; Zozulya S.; Levterov V. V.; Mykhailiuk P. K. Difluoro-Substituted Bicyclo[1.1.1]pentanes for Medicinal Chemistry: Design, Synthesis, and Characterization. J. Org. Chem. 2019, 84, 15106–15117. 10.1021/acs.joc.9b01947. [DOI] [PubMed] [Google Scholar]
- a Kleinmans R.; Pinkert T.; Dutta S.; Paulisch T. O.; Keum H.; Daniliuc C. G.; Glorius F. Intermolecular [2π + 2σ]-photocycloaddition enabled by triplet energy transfer. Nature 2022, 605, 477–482. 10.1038/s41586-022-04636-x. [DOI] [PubMed] [Google Scholar]; b Guo R.; Chang Y.-C.; Herter L.; Salome C.; Braley S. E.; Fessard T. C.; Brown M. K. Strain-release [2π + 2σ] cycloadditions for the synthesis of bicyclo[2.1.1]hexanes Initiated by energy transfer. J. Am. Chem. Soc. 2022, 144, 7988–7994. 10.1021/jacs.2c02976. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Dhake K.; Woelk K. J.; Becica J.; Un A.; Jenny S. E.; Leitch D. C. Beyond Bioisosteres: Divergent Synthesis of Azabicyclohexanes and Cyclobutenyl Amines from Bicyclobutanes. Angew. Chem., Int. Ed. 2022, 61, e202204719 10.1002/anie.202204719. [DOI] [PubMed] [Google Scholar]; d Agasti S.; Beltran F.; Pye E.; Kaltsoyannis N.; Crisenza G. E. M.; Procter D. J. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Nat. Chem. 2023, 15, 535–541. 10.1038/s41557-023-01135-y. [DOI] [PubMed] [Google Scholar]
- a Tokunaga K.; Sato M.; Kuwata K.; Miura C.; Fuchida H.; Matsunaga N.; Koyanagi S.; Ohdo S.; Shindo N.; Ojida A. Bicyclobutane carboxylic amide as a cysteine-directed strained electrophile for selective targeting of proteins. J. Am. Chem. Soc. 2020, 142, 18522–18531. 10.1021/jacs.0c07490. [DOI] [PubMed] [Google Scholar]; b Zhang P.; Zhuang R.; Wang X.; Liu H.; Li J.; Su X.; Chen X.; Zhang X. Highly Efficient and Stable Strain-Release Radioiodination for Thiol Chemoselective Bioconjugation. Bioconjugate Chem. 2018, 29, 467–472. 10.1021/acs.bioconjchem.7b00790. [DOI] [PubMed] [Google Scholar]
- a McNamee R. E.; Haugland M. M.; Nugent J.; Chan R.; Christensen K. E.; Anderson E. A. Synthesis of 1,3-disubstituted bicyclo[1.1.0]butanes via directed bridgehead functionalization. Chem. Sci. 2021, 12, 7480–7485. 10.1039/D1SC01836A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b McNamee R. E.; Thompson A. L.; Anderson E. A. Synthesis and Applications of Polysubstituted Bicyclo[1.1.0]butanes. J. Am. Chem. Soc. 2021, 143, 21246–21251. 10.1021/jacs.1c11244. [DOI] [PubMed] [Google Scholar]
- Jung M.; Lindsay V. N. G. One-Pot Synthesis of Strain-Release Reagents from Methyl Sulfones. J. Am. Chem. Soc. 2022, 144, 4764–4769. 10.1021/jacs.2c00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.; Huang X.; Kan S. B. J.; Zhang R. K.; Arnold F. H. Enzymatic construction of highly strained carbocycles. Science 2018, 360, 71–75. 10.1126/science.aar4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C.; Davies H. M. L. Enantioselective Synthesis of 2-Arylbicyclo[1.1.0]butane Carboxylates. Org. Lett. 2013, 15, 310–313. 10.1021/ol303217s. [DOI] [PubMed] [Google Scholar]
- Okude R.; Mori G.; Yagi A.; Itami K. Programmable synthesis of multiply arylated cubanes through C–H metalation and arylation. Chem. Sci. 2020, 11, 7672–7675. 10.1039/D0SC01909G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Greszler S. N.; Halvorsen G. T.; Voight E. A. Synthesis of Substituted Cyclopropanecarboxylates via Room Temperature Palladium-Catalyzed α-Arylation of Reformatsky Reagents. Org. Lett. 2017, 19, 2490–2493. 10.1021/acs.orglett.7b00707. [DOI] [PubMed] [Google Scholar]; b Yasui M.; Ota R.; Tsukano C.; Takemoto Y. Synthesis of cis-/All-cis-Substituted Cyclopropanes through Stereocontrolled Metalation and Pd-Catalyzed Negishi Coupling. Org. Lett. 2018, 20, 7656–7660. 10.1021/acs.orglett.8b03390. [DOI] [PubMed] [Google Scholar]
- See the Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.