Abstract
Gender is an important risk factor for adverse drug reactions. Women report significantly more adverse drug reactions than men. There is a growing consensus that gender differences in drug PK is a main contributor to higher drug toxicity in women. These differences stem from physiological differences (body composition, plasma protein concentrations, and liver and kidney function), drug interactions, and comorbidities. Contrast agents are widely used to enhance diagnostic performance in computed tomography and magnetic resonance imaging. Despite their broad use, these contrast agents can lead to important adverse reactions including hypersensitivity reactions, nephropathy, and hyperthyroidism. Importantly, female gender is one of the main risk factors for contrast agent toxicity. As these adverse reactions may be related to gender differences in PK, this perspective aims to describe distribution and elimination pathways of commonly used contrast agents and to critically discuss gender differences in these processes.
Keywords: contrast agent, computed tomography, magnetic resonsance imaging, pharmacokinetics, albumin, gender medicine
Gender is an important risk factor for adverse drug reactions. When compared to men, women reported 10.2% more adverse drug reactions worldwide in 20191 and, according to Eurostat, they were prescribed 9.1% more drugs in all European countries. The higher medication use among women has been explained with the higher rate of drug toxicity in women, which require additional drugs to treat the adverse reactions.1,2 Drug pharmacokinetics (PK) is a key factor of gender differences of adverse drug reactions. In a retrospective study of 86 drugs, 88% had higher drug exposure (area-under-the-curve) and maximum plasma concentrations in women than men, and 96% of these drugs induced a higher incidence of adverse events in women.2 Recently, gender differences in PK has led to a dosing adjustment. The FDA approved zolpidem dose is 5 mg in women and 5–10 mg in men because of slower zolpidem metabolism and higher risk of morning drowsiness in women.3 This gender bias in the pharmaceutical industry is partially attributable to the fact that, historically, clinical trials had a higher enrollment of male subjects because of higher potential risks for women (e.g., pregnancy, breast feeding) and the potential impact of the menstrual cycle on efficacy.2,4 The underrepresentation of women has raised concern in drug agencies including the FDA, which implemented the Safety and Innovation Act in 2012 to ensure a better representation of gender, ethnicity, and age in drug development. Despite those measures, gender differences in drug PK and pharmacodynamics remain underinvestigated, and drug dosing is rarely adjusted to gender.
Contrast agents are a drug class where adverse reactions show important gender differences (Table 1). Contrast agents are chemical substances that enhance the differences between tissues on images. They enhance the signal intensity of a targeted tissue by altering the way that electromagnetic radiation waves pass through the body. These substances can be administered to the patient orally, rectally, or intravenously.5,6 Multiple studies demonstrate that the incidence of contrast media-related adverse reactions were higher in women after the administration of iodinated contrast agents or gadolinium-based.7 For instance, compared to men, women are 140% more likely to develop non-immunologic anaphylaxis8 and 50% to 68.7% more likely to develop hypersensitivity reactions9,10 under iodinated and gadolinium-based contrast agents, respectively. They are also 149% more likely to develop contrast-induced nephropathy11 when administered iodixanol. Additionally, the extravasation of contrast agents, defined by the distribution of contrast agents out of blood vessels and in unwanted tissues, causes side effects that are predominant in women with 63% to 65% of reported extravasation-related events.12,13 The risk of thyroid dysfunction after iodinated contrast agent administration is increased 96% in women.14 Indeed, female gender is one of the main risk factors for acute adverse reactions to contrast agents.15−17
Table 1. Contrast Agent-Related Adverse Reactions with Higher Incidence in Female Gender.
| X-ray/CT contrast agents | MRI contrast |
|---|---|
| nonimmunologic anaphylaxis | hypersensitivity reactions |
| contrast-induced nephropathy | extravasation of contrast agents |
| extravasation of contrast agents | |
| thyroid dysfunction |
Several studies highlight differences in PK of drugs between men and women. These differences stem from physiological differences in body composition, plasma protein concentrations, and liver and kidney function that affect the drug distribution and elimination.2,4,18,19 Other factors leading to gender-associated PK differences are differences in drug use such as polypharmacy and hormone levels that increase the risk of drug interactions, and comorbidities with effects of elimination organ function.2 These PK differences can impact contrast agent toxicity. Prolonged exposure due to depot formation in fat tissue or reduced elimination can lead to toxicity, as observed with gadolinium-containing contrast agents in patients with kidney failure (nephrogenic systemic fibrosis).20,21 In this report, we are listing distribution and elimination pathways of the most widely used computed tomography (CT) and magnetic resonance imaging (MRI) contrast agents, and critically discuss gender differences in drug distribution and elimination with a potential bearing on contrast agent PK (Figure 1). To avoid ambiguity, the use of the term gender in the article refers to cisgendered women and men while the term sex to male or female animals.
Figure 1.
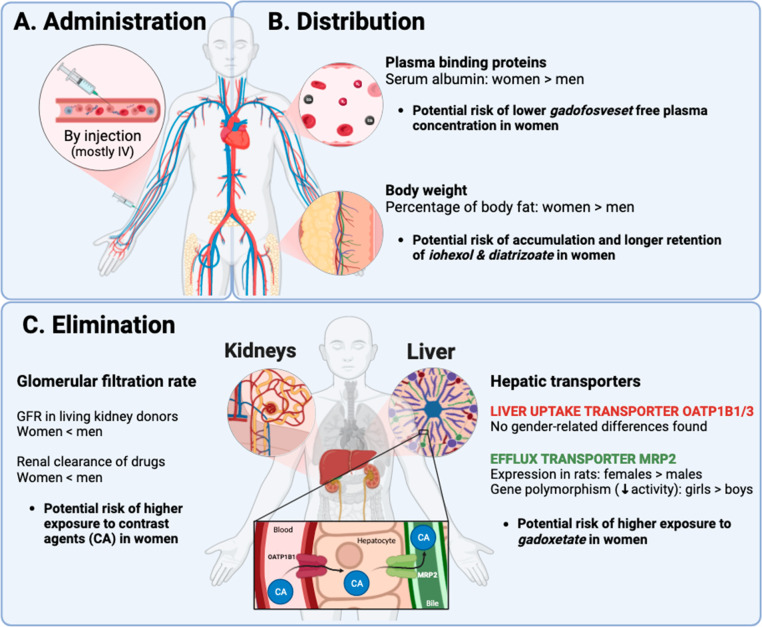
Contrast agent PK and physiological gender differences. After injection (A), contrast agents enter the bloodstream, bind to plasma proteins, and distribute throughout the body (B). These processes may be impacted by gender differences in plasma protein concentrations and body fat content. Contrast agents are eliminated by the kidney and the liver (C). Gender differences in glomerular filtration rate and hepatic uptake and efflux transporter expression may influence the kinetics of elimination. Gender differences on contrast agent PK could alter the plasma concentrations of contrast agents and their distribution in target tissues (e.g., liver-specific contrast agents), and thus influence the risk of adverse reactions.
1. Distribution
After intravenous administration, contrast agents bind to plasma proteins in the bloodstream and distribute into different tissues at various degrees as seen in Tables 2 and 3. The extent of plasma protein binding and distribution within the body influences the free plasma concentration and the elimination rate of drugs.22 In this section, we are discussing gender-related differences in protein binding and distribution, and potential effects on contrast agents PK.
Table 2. Pharmacokinetic Properties of Iodinated Contrast Agentsa.
| contrast agents | generic name | elimination | metabolism | distribution | others |
|---|---|---|---|---|---|
| Accupaque (Omnipaque) (IV, IA, IT & in body cavities) | Iohexol (Iohexolum) | 90% by kidneys: Glomerular filtration ≫ Tubular secretion | No metabolites quantified in humans | Vd: 559 mL/kg | Protein binding: < 2% (no clinical relevance) |
| Mean CLrenal: 111 mL/min | Not a CYP450 or UGT substrate except for UGT1A9 | ||||
| Bile at 1–2% | |||||
| Mean CL: 119 mL/min | |||||
| Gastrografin (Oral or rectal) | Amidotrizoic acid (Diatrizoate) | >75% by kidneys | Not metabolized | Vd: 600 mL/kg | Only about 3% is resorbed from the stomach and intestine |
| Mean CLrenal: 1.7 mL/min/kg | |||||
| Hexabrix (Intravascular) | Ioxaglic acid (Ioxaglate) | 90% by kidneys: Possible tubular reabsorption (based on preclinical data) | Not metabolized | Little binding to plasma proteins | |
| Small intestin and liver: < 10% | |||||
| Heterogeneous excretion in saliva, sweat and colon | |||||
| CL: 245 mL/min | |||||
| Iomeron (IV or IA) | Iomeprol | >75% by kidneys | Not metabolized | Vd: 289 mL/kg (Extracellular fluid volume) | No binding to plasma proteins |
| Iopamiro (or Scanlux) | Iopamidol | >75% by kidneys | No significant metabolism | Vd: 220 mL/kg (Extracellular fluid volume) | Binds neither to plasma nor to serum proteins |
| <0.1% of the total amount of iodine administered is eliminated as inorganic iodide | |||||
| Lipiodol (Lymphatic injection or in the hepatic artery) | Ethyl esters of iodinated fatty acids | 25–50% by kidneys | Eliminated as iodine | Injection into lymphatic vessels: Transport in blood, liver and lungs (droplet distribution in alveoli, spleen and adipose tissue) | |
| <25% by liver | |||||
| <25% by pancreas | Phagocyted by Kupffer cells and eliminated by the lymphatic system | Hepatic artery injection: distribution in neovascular and extravascular tissues of hepatocellular carcinoma | |||
| Opitray | Ioversol | 86% by kidneys | Not metabolized | Distribution in the intravascular and interstitial space | Protein binding: 9%–13% |
| Partially heterotopic elimination by biliary route in renal insufficency | |||||
| Ultravist | Iopromide | 90% by kidneys: GFR | Not metabolized | Vdss: 220 mL/kg | Protein binding: 0.9 ± 0.2% |
| CL: 110 mL/min at low doses and 103 mL/min at high doses | |||||
| 3% in feces | |||||
| Visipaque | Iodixanol | 97% by kidneys: GFR | Not significantly metabolized | Vd: 260 mL/kg | Protein binding: < 2% |
| 1.2% in feces | |||||
| Xenetix | Iobitridol | 100% by kidneys (after 24 h) | Not metabolized | Vd: 200 mL/kg (Exclusively extracellular) | Very little binding to plasma proteins |
Pharmacokinetic propreties according to Health Canada’s Drug Product Database and Compendium as of March 2023.
Table 3. Pharmacokinetic Properties of Gadolinium-Based Contrast Agentsa.
| contrast agents | generic name | elimination | metabolism | distribution | others |
|---|---|---|---|---|---|
| Ablavar (or Vasovist) | Gadofosveset trisodium | 94% by kidneys | Not significantly metabolized | Vd: 150 mL/kg (2) | Significantly bound to plasma proteins: 79.8%–87.4% (between 0.05 and 4 h after injection) |
| 4.7% in feces | |||||
| CL: 6.57 ± 0.97 mL/h/kg (after injection of 0.03 mmol/kg) | |||||
| Artirem (or Clariscan/Dotagraf/Dotarem) | Gadoteric acid | 95% by kidneys: GFR | Not metabolized | Distribution in extracellular fluids | Does not bind to albumin |
| (Gadoterate, Gd-DOTA) | |||||
| Eovist (or Primovist) | Gadoxetic acid (gadoxetate) | 50% by kidneys | Not metabolized | Vdss: 210 mL/kg (extracellular volume) | Protein binding: < 10% |
| Gd-EOB-DTPA | 50% in bile | Transport influx in hepatocytes: OATP1B1 and B3 of the sinusoidal membrane82 | |||
| CL: 250 mL/min | Biliary excretion: MRP2 of the canalicular membrane82 | ||||
| Gadovist (or Gadavist) | Gadobutrol | 90% by kidneys | Not metabolized | Rapid distribution in extracellular fluids | No binding to plasma proteins |
| <0.1% in feces | Post-mortem: Traces of gadolinium in brain, bone, skin, liver, other organs and tissues (clinical relevance unknown) | Gender has no effect on gadobutrol PK | |||
| Magnevist (IV) | Gadopentetate dimeglumine (Gadopenthetic acid dimeglumine) | >93% by kidneys | Not metabolized | Vd: 266 mL/kg (Extracellular volume) | No binding to plasma proteins |
| <0.1% in feces | |||||
| May be secreted into the gastrointestinal tract | |||||
| Multihance | Gadobenic acid (gadobenate) | 78%–96% by kidneys: GFR | Not metabolized | Vd: between 170 and 248 mL/kg (Extracellular volume) | Little binding to plasmaproteins |
| <25% by liver | Transport influx in hepatocytes: OATP1B1 and B3 of the sinusoidal membrane58 | ||||
| 0.6–4% in bile and feces | Biliary excretion: MRP2 of the canalicular membrane58 | ||||
| Omniscan | Gadodiamide | 95.4 ± 5.5% by kidneys | Not metabolized | Vd: 200 ± 61 mL/kg | No binding to plasma proteins |
| 0.03–0.06% in feces | |||||
| Optimark | Gadoverset-amide | 95.5 ± 17.4% by kidneys | Not metabolized | Vd: 162 ± 25 mL/kg (Extracellular volume) | No binding to plasma proteins |
| Prohance | Gadoteridol | 95% by kidneys: GFR | Not metabolized | Vd: 129 mL/kg (Extracellular volume) | No binding to plasma proteins |
Pharmacokinetic propreties according to Health Canada’s Drug Product Database and Compendium as of March 2023.
1.1. Body Weight and Composition
As discussed previously, women and men differ in their body composition. Women have a higher percentage of body fat than men.4,18,19 The percentage of adipose tissue impacts the distribution of lipophilic molecules. Contrast agents with a high volume of distribution such as the intravenous contrast agents iohexol (559 mL/kg) and diatrizoate (600 mL/kg) tend to extravasate from blood vessels12,23 to distribute into the fat tissue to a greater extent. Such an extravasation reduces plasma concentrations and increases their retention in the body. Lower plasma concentrations generally lead to a lower signal-to-noise ratio. The smaller vessel size in women24 and the higher needle/vein size ratio may also contribute to a higher risk of extravasation in women. Furthermore, the lower blood volume in women25 will likely increase plasma contrast agent levels. Interestingly, the dose for certain indications of iohexol is adjusted to body weight to account for differences in body composition but gender-adjusted dosing is not required. Indeed, a correction for body weight or body surface area only eliminates a minority of sex-dependent pharmacokinetic differences.2 Additionally, ethiodized oil has a very distinct PK profile because of its lipophilicity. After intralymphatic administration, the contrast agent enters the lymphatic system and drains into the systemic compartment via the subclavian vein.26 According to its monography, when administered in lymphatic vessels, this contrast agent can be retained from several weeks to months after its lipid droplets are broken down in the pulmonary alveoli, the spleen, and adipose tissues. Similarly to iohexol, ethiodized oil could have lower lymphatic concentrations due to higher accumulation in fat tissue and may be retained longer in women.
1.2. Protein Binding
Most contrast agents listed in Table 2 exhibit low plasma protein binding except for the extracellular gadolinium-containing MRI contrast agent gadofosveset whose plasma protein binding is about 80% according to the monography. For drugs with high plasma protein binding, plasma protein concentrations strongly influence free drug concentrations in plasma, the volume of distribution, and the elimination half-life.22 As serum albumin is the most important plasma protein, its concentration may impact the PK of gadofosveset. Serum albumin was higher in women than men in an age range of 16 to 75.27 This gender difference may increase the plasma protein concentrations of gadofosveset and prolong its half-life in women. Serum albumin also has important effects on the relaxivity of gadolinium-containing contrast agents, and differences in albumin levels may change the signal-to-noise ratio of all gadolinium-containing contrast agents.28,29 Of note is that gadofosveset is currently withdrawn in the United States and Europe but continues to be used in research.30−33
Furthermore, drugs with high plasma protein binding compete for binding sites on plasma proteins.34 These drug interactions could increase free gadofosveset concentrations, and lead to stronger drug effects and toxicity, and faster elimination.34 Examples of drugs that could potentially introduce such interactions with gadofosveset include proton pump inhibitors, antidepressants, and nonsteroidal anti-inflammatory drugs (NSAIDs), all of which are more frequently prescribed to women.35,36 Most proton pumps inhibitors and antidepressants are bound to plasma protein at more than 80%.37,38 According to their respective monographs, ibuprofen, celecoxib, and naproxen are also highly bound to plasma proteins, ranging from 90% to 99%. As these interactions could increase the free concentrations and accelerate the elimination of gadofosveset or proton pumps inhibitors, antidepressants, and NSAIDs, more studies are warranted that investigate potential differences in contrast agent PK in patients under these treatments.
Comorbidities may also impact contrast agent PK. Diseases such as obesity may alter drug distribution for lipophilic contrast agents iohexol and diatrizoate, and hypoalbuminemia for the highly plasma protein bound contrast agent gadofosveset. From 1999 to 2018, a cross-sectional survey made by the National Center for Health Statistics established that the age-adjusted prevalence of obesity in adults was not statistically different between men and women.39 However, gender differences were established when race is taken into consideration. In non-Hispanic black adults, the prevalence of obesity was significantly higher in women than men.39 In these adults, the plasma concentrations of iohexol and diatrizoate may be lower. Hypoalbuminemia is another comorbidity plausible of influencing gadofosveset’s PK. It occurs in pregnancy and liver disease such as primary biliary cirrhosis, which has a higher occurrence in women.40 Hence, more studies on gadofosveset PK in pregnancy and liver disease are warranted.
2. Elimination
2.1. Renal Pathway
The prevailing elimination pathway of contrast agents is the renal pathway. Growing clinical evidence suggests that drugs which are mainly or exclusively eliminated unchanged by renal elimination were cleared faster in men.41 These drugs include digoxine, aminoglycosides, cephalosporins, and fluoroquinolones.41 A decreased renal clearance in women has also been reported for antibiotics that are mainly renally excreted such as vancomycin, ceftazidime, and cefepime.42 Therefore, it is likely that renally eliminated contrast agents are cleared less rapidly in women than in men. As studies on gender differences on the PK of renally cleared contrast agents could not be retrieved from the literature, more clinical studies on potential differences in contrast agent clearance between men and women are warranted.
Gender differences in drug clearance may be related to physiological differences between men and women. A retrospective multicenter study was conducted on a total of 2974 living kidney donors. The results showed that males had higher GFR than females (92.0 vs 88.1 mL/min/1.73m2, P < 0.0001) and the linear decline of measured GFR (mGFR) was faster in females compared to males over a period of up to 12 years.43 Women have higher renal vascular resistance and lower renal plasma flow, which contribute to their reduced GFR.44,45 A hypothesis for these results was that the loss of estrogen during aging can impact the renal hemodynamics and structure,43 due to the hormone’s effects on the glomerular mesangial cells including direct antigrowth and inhibition of extracellular matrix accumulation.46 It was also hypothesized that, in women, the GFR is masked by scaling to their body surface area which increases more rapidly over time than in men.
Because of their negative impact on renal function, some of the drugs that are more commonly used by women such as NSAIDs, oral contraceptives, and oral estrogen therapy might alter the elimination rate of renally cleared contrast agents.35,36,47 While the risk of consuming anti-inflammatory and antirheumatic drugs is 27% higher in women than in men, these drugs have also been shown to increase the risk of nephrotoxicity.48 The administration of NSAIDs is associated with papillary necrosis and intrarenal vasoconstriction, due to the inhibition of prostaglandin synthesis, which lowers the GFR.49 As for contraceptives, while proof of their protective outcome on the renal pathway had been demonstrated in former studies,50 new evidence established the deleterious effects of contraceptives on renal function. These include a decrease in GFR51 as well as an increase in renal vascular resistance,52 which also leads to reduced GFR in healthy subjects.45 Similarly, findings on the effect of hormonal therapy on kidney functions show conflicting results. While some studies found no change in GFR,53 others found an association between hormonal therapy in postmenopausal women and the loss of kidney function as well as a decline in GFR.54 Exogenous estrogen increases the activity of the renin-angiotensin system55 which negatively impacts renal function.54 The administration of contraceptives, hormonal therapy or NSAIDs might therefore decrease the renal elimination of contrast agents. More studies are needed to determine the clinical relevance of these potential interactions.
Renal diseases may also alter the renal elimination of contrast agents. For instance, women have a higher prevalence for predialysis chronic kidney disease than men.11 Complications of diabetes, including chronic kidney disease as well as end-stage kidney disease, are more severe in women.56 Contrast agent administration can also lead to kidney disease, especially with iodinated contrast agents.15 Women are at higher risk for acute renal failure, contrast-induced nephropathy,11 as well as developing overall renal complications after contrast agent exposure.57 These diseases may increase plasma contrast agent levels and prolong their half-life, which could lead to a higher risk of contrast agent toxicity.
2.2. Hepatic Pathway
While the renal pathway is the most common elimination pathway of iodinated and gadolinium-based contrast agents, the hepatic pathway is an important elimination route for the liver-specific MRI contrast agents gadoxetate and gadobenate. Ethiodized oil is also eliminated by the liver as it is cleared by the macrophage phagocytic system. Changes in the expression of contrast agent uptake and/or efflux transporters, liver pathologies with hepatocyte loss, drug interactions, and diet can impact the pharmacokinetics and biodistribution of these contrast agents.
Gadoxetate and gadobenate are taken up by organic anion transporter peptides OATP1B1 and OATP1B3 into hepatocytes and subsequently excreted into bile by the multidrug resistance-associated protein efflux transporter MRP2.58 Potential sex and gender differences in OATP expression are poorly understood, and may impact the uptake of gadoxetate and gadobenate into the liver. This could influence the pharmacokinetics of gadoxetate particularly due to its high uptake by hepatocytes (about 50% of gadoxetate dose and 3–5% of gadobenate dose are taken up by hepatocytes59). In preclinical studies with rats, differences in mRNA expression were not observed for liver OATP1.60,61 A retrospective clinical study analyzed the PK of an endogenous substrate of OATP1B (Coproporphyrin I) in men and women and did not find differences between genders.62 A prospective clinical study of 356 healthy volunteers analyzed the endogenous OATP1B1 substrates glycochenodeoxycholate and glycodeoxycholate 3-O-glucuronides (GCDCA-3G and GDCA-3G). These substrats showed lower plasma concentrations in women, suggesting a higher OATP1B1 activity in women compared to men. This conclusion is however most likely due to differences in bile acid synthesis rate resulting in quantitively different GCDCA-3G and GDCA-3G synthesis between genders, and thus in lower plasma concentrations for these substrates in women rather than higher OATP activity.63 Other clinical data established that OATP expression had no direct relationship with gender64 and that liver accumulation of gadoxetate did not differ between genders.65 Moreover, neither genetic variant of OATP1B (SCLO1B1 521T > C), associated with impaired uptake activity of numerous drug substrates,66 or any other OATP1B1 genotypes67 differ between women and men. It is thus unlikely that OATP expression is different between genders and impacts the PK profile of gadoxetate and gadobenate in women.
Evidence of sex and gender differences in MRP2 expression has been established in both preclinical and clinical studies. In a study on an MRP2 substrate (emodin) in rat liver, a significantly higher MRP2 efflux transporter expression with a lower hepatotoxicity of emodin was found in male rats compared to females.68 In another rat study, MRP2 mRNA levels were significantly lower and MRP2 substrate (α-naphthylisothiocyanate) concentration significantly higher in female livers than male livers under high-fat diet, while no differences were observed in normal diet.69 Hence, nutritional status may also significantly affect the PK of gadoxetate and gadobenate. In a prospective clinical study in a pediatric population, a gene polymorphism of MRP2 (C-24T) was significantly more prevalent in girls and led to an increased exposure of the MRP2 substrate (methotrexate) in those subjects.70 Therefore, MRP2 activity seems to be reduced in girls, and possibly in women, and may lead to increased gadoxetate and gadobenate exposure.
Certain drugs and sex hormones predominantly used by women are eliminated by OATP1B or MRP2 which could impact the elimination of gadoxetate and gadobenate. For instance, women are 76.5% more likely to be prescribed thyroid hormones than men35 and 84% of adverse events related to levothyroxine, the most prescribed synthetic thyroid hormone, are reported by women.71 Levothyroxine is mainly degraded in the liver and is a substrate for both OATP1B1 and OATP1B3, potentially leading to lower liver uptake and slower elimination of these contrast agents.35,72 Hormonal therapy may also impact the hepatic elimination of contrast agents. Estrogens including estrone sulfate, 17β-estradiol, and estradiol sulfate are among OATP1B1’s known endogenous73 and exogenous74 substrates, which, following their metabolism, are transported out of hepatocytes by efflux transporters such as MRP2.75 Although these substances may produce drug–drug interactions with gadoxetate and gadobenate in women, there are no studies that investigate potential increases in contrast agents exposure in patients under thyroid or hormonal therapy.
Liver disease such as liver cirrhosis and failure decreases the clearance of liver-specific MRI contrast agents.20,21,76−78 Therefore, gender differences in liver disease prevalence need to be considered. For instance, autoimmune liver diseases, benign liver tumors, advanced fibrosis, and graft loss in hepatitis C virus-related disease and the hepatic form of metabolic liver disease all display a higher prevalence in women.79 Furthermore, primary biliary cirrhosis is ten times more likely to occur in women, which can impact the enterohepatic cycle and thus the elimination of liver-cleared contrast agents.79 Systemic lupus erythematosus,80 the most common type of lupus, is also more present in women. This disease is characterized by decreased mRNA levels of OATP1B1 and OATP1B3, and OATP1B1 protein expression in human hepatocytes due to increases in plasmatic TNF-α and IL-6.81 Therefore, these differences in hepatic and extrahepatic pathologies could impact the blood circulation time of liver-specific contrast agents.
3. Conclusions
There are considerable gender differences in the risk of contrast agent adverse reactions. In this perspective article, we described distribution and elimination pathways of commonly used CT and MRI contrast agents and critically discussed gender differences in these processes. While data on gender effects on contrast agent PK are scarce, the gender differences in organ function, comorbidities, and drug interaction risk we retrieved from the literature are likely to impact the blood concentration and half-life of contrast agents. Further preclinical and clinical studies are warranted in animal models and cis, trans, and nonbinary individuals to elucidate the role of gender in the processes (physiological differences, co-morbidities, drug interactions) influencing contrast agent PK in order to understand the impact of gender in contrast agent adverse reactions. Such studies will inform possibly needed gender-specific dosing adjustments in contrast agents. The findings will contribute to reducing gender biases in contrast-enhanced radiological evaluations.
Glossary
Abbreviations Used
- CT
Computed tomography
- CL
Clearance
- FDA
Food and Drug Administration
- GCDCA-3G
Glycochenodeoxycholate
- GDCA-3G
Glycodeoxycholate 3-O-glucuronides
- IA
Intra-arterial
- IL-6
Interleukine 6
- IT
Intrathecal
- IV
Intravenous
- mGFR
Measured glomerular filtration rate
- MRI
Magnetic resonance imaging
- MRP
Multidrug resistance-associated proteins
- NSAID
Nonsteroidal anti-inflammatory drug
- OATP
Organic anion transporters polypeptide
- PK
Pharmacokinetic
- SLCO1B1
Solute carrier organic anion transporter family member 1B1
- TNF-α
Tumor necrosis factor alpha
- Vd
Distribution volume
- Vdss
Distribution volume at steady state
The authors declare no competing financial interest.
References
- Watson S., Caster O., Rochon P. A., den Ruijter H.. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. eClinicalMedicine 2019, 17, 100188. 10.1016/j.eclinm.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I.; Prendergast B. J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ 2020, 11, 32. 10.1186/s13293-020-00308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J.; Harmatz J. S.; Singh N. N.; Steinberg F.; Roth T.; Moline M. L.; Harris S. C.; Kapil R. P. Gender differences in pharmacokinetics and pharmacodynamics of zolpidem following sublingual administration. Journal of Clinical Pharmacology 2014, 54, 282–290. 10.1002/jcph.220. [DOI] [PubMed] [Google Scholar]
- Madla C. M.; Gavins F. K. H.; Merchant H. A.; Orlu M.; Murdan S.; Basit A. W. Let’s talk about sex: Differences in drug therapy in males and females. Adv. Drug Deliv Rev. 2021, 175, 113804 10.1016/j.addr.2021.05.014. [DOI] [PubMed] [Google Scholar]
- Kaller M. O.; An J.. Contrast Agent Toxicity, In StatPearls; StatPearls Publishing, LLC.: Treasure Island, FL, 2023. [PubMed] [Google Scholar]
- Pomara C.; Pascale N.; Maglietta F.; Neri M.; Riezzo I.; Turillazzi E. Use of contrast media in diagnostic imaging: medico-legal considerations. Radiol Med. 2015, 120, 802–809. 10.1007/s11547-015-0549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen H. S. Contrast media safety-an update. Eur. J. Radiol 2011, 80, 77–82. 10.1016/j.ejrad.2010.12.104. [DOI] [PubMed] [Google Scholar]
- Lang D. M.; Alpern M. B.; Visintainer P. F.; Smith S. T. Gender risk for anaphylactoid reaction to radiographic contrast media. J. Allergy Clin Immunol 1995, 95, 813–817. 10.1016/S0091-6749(95)70123-0. [DOI] [PubMed] [Google Scholar]
- Jung J. W.; Kang H. R.; Kim M. H.; Lee W.; Min K. U.; Han M. H.; Cho S. H. Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 2012, 264, 414–422. 10.1148/radiol.12112025. [DOI] [PubMed] [Google Scholar]
- Ahn Y. H.; Kang D. Y.; Park S. B.; Kim H. H.; Kim H. J.; Park G. Y.; Yoon S. H.; Choi Y. H.; Lee S. Y.; Kang H. R. Allergic-like Hypersensitivity Reactions to Gadolinium-based Contrast Agents: An 8-year Cohort Study of 154 539 Patients. Radiology 2022, 303, 329–336. 10.1148/radiol.210545. [DOI] [PubMed] [Google Scholar]
- Lucreziotti S.; Centola M.; Salerno-Uriarte D.; Ponticelli G.; Battezzati P. M.; Castini D.; Sponzilli C.; Lombardi F. Female gender and contrast-induced nephropathy in primary percutaneous intervention for ST-segment elevation myocardial infarction. Int. J. Cardiol 2014, 174, 37–42. 10.1016/j.ijcard.2014.03.087. [DOI] [PubMed] [Google Scholar]
- Niv G.; Costa M.; Kicak P.; Richman K. Vascular extravasation of contrast medium in radiological examinations: University of California San Diego Health System Experience. J. Patient Saf 2014, 10, 105–110. 10.1097/PTS.0000000000000114. [DOI] [PubMed] [Google Scholar]
- Shaqdan K.; Aran S.; Thrall J.; Abujudeh H. Incidence of contrast medium extravasation for CT and MRI in a large academic medical centre: a report on 502,391 injections. Clin Radiol 2014, 69, 1264–1272. 10.1016/j.crad.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Hsieh M. S.; Chiu C. S.; Chen W. C.; Chiang J. H.; Lin S. Y.; Lin M. Y.; Chang S. L.; Sheu M. L.; Hu S. Y. Iodinated Contrast Medium Exposure During Computed Tomography Increase the Risk of Subsequent Development of Thyroid Disorders in Patients Without Known Thyroid Disease: A Nationwide Population-Based, Propensity Score-Matched, Longitudinal Follow-Up Study. Medicine (Baltimore) 2015, 94, e2279 10.1097/MD.0000000000002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett K. R.; Moriarity A. K.; Langer J. M. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics 2015, 35, 1738–1750. 10.1148/rg.2015150033. [DOI] [PubMed] [Google Scholar]
- Lee H.; Song S.; Oh Y. K.; Kang W.; Kim E. Is gender still a predisposing factor in contrast-media associated adverse drug reactions? A systematic review and meta-analysis of randomized trials and observational studies. Eur. J. Radiol 2017, 89, 81–89. 10.1016/j.ejrad.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Fraum T. J.; Ludwig D. R.; Bashir M. R.; Fowler K. J. Gadolinium-based contrast agents: A comprehensive risk assessment. J. Magn Reson Imaging 2017, 46, 338–353. 10.1002/jmri.25625. [DOI] [PubMed] [Google Scholar]
- Soldin O. P.; Chung S. H.; Mattison D. R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 2011, 187103 10.1155/2011/187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regitz-Zagrosek V.; Seeland U. Sex and gender differences in clinical medicine. Handb Exp Pharmacol 2013, 214, 3–22. 10.1007/978-3-642-30726-3_1. [DOI] [PubMed] [Google Scholar]
- Kukuk G. M.; Schaefer S. G.; Fimmers R.; Hadizadeh D. R.; Ezziddin S.; Spengler U.; Schild H. H.; Willinek W. A. Hepatobiliary magnetic resonance imaging in patients with liver disease: correlation of liver enhancement with biochemical liver function tests. Eur. Radiol 2014, 24, 2482–2490. 10.1007/s00330-014-3291-x. [DOI] [PubMed] [Google Scholar]
- Matoori S.; Froehlich J. M.; Breitenstein S.; Pozdniakova V.; Reischauer C.; Kolokythas O.; Koh D.-M.; Gutzeit A. Serum albumin, total bilirubin, and patient age are independent confounders of hepatobiliary-phase gadoxetate parenchymal liver enhancement. European Radiology 2019, 29, 5813–5822. 10.1007/s00330-019-06179-8. [DOI] [PubMed] [Google Scholar]
- Schmidt S.; Gonzalez D.; Derendorf H. Significance of Protein Binding in Pharmacokinetics and Pharmacodynamics. J. Pharm. Sci. 2010, 99, 1107–1122. 10.1002/jps.21916. [DOI] [PubMed] [Google Scholar]
- Cohan R. H.; Bullard M. A.; Ellis J. H.; Jan S. C.; Francis I. R.; Garner W. L.; Dunnick N. R. Local reactions after injection of iodinated contrast material: detection, management, and outcome. Acad. Radiol 1997, 4, 711–718. 10.1016/S1076-6332(97)80073-6. [DOI] [PubMed] [Google Scholar]
- Aqeel S.; Amjad S.; Tauqir J.; Fatima M. Sonographic correlation of portal vein diameter with gender in population of Lahore. TPMJ 2022, 29, 607–612. 10.29309/TPMJ/2022.29.05.6543. [DOI] [Google Scholar]
- Diaz-Canestro C.; Pentz B.; Sehgal A.; Montero D. Sex differences in cardiorespiratory fitness are explained by blood volume and oxygen carrying capacity. Cardiovasc. Res. 2022, 118, 334–343. 10.1093/cvr/cvab028. [DOI] [PubMed] [Google Scholar]
- Phang K.; Bowman M.; Phillips A.; Windsor J. Review of thoracic duct anatomical variations and clinical implications. Clinical Anatomy 2014, 27, 637–644. 10.1002/ca.22337. [DOI] [PubMed] [Google Scholar]
- Weaving G.; Batstone G. F.; Jones R. G. Age and sex variation in serum albumin concentration: an observational study. Ann. Clin Biochem 2016, 53, 106–111. 10.1177/0004563215593561. [DOI] [PubMed] [Google Scholar]
- Goetschi S.; Froehlich J. M.; Chuck N. C.; Curcio R.; Runge V. M.; Andreisek G.; Nanz D.; Boss A. The protein and contrast agent-specific influence of pathological plasma-protein concentration levels on contrast-enhanced magnetic resonance imaging. Invest Radiol 2014, 49, 608–619. 10.1097/RLI.0000000000000061. [DOI] [PubMed] [Google Scholar]
- Rohrer M.; Bauer H.; Mintorovitch J.; Requardt M.; Weinmann H. J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005, 40, 715–724. 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- Shahrouki P.; Khan S. N.; Yoshida T.; Iskander P. J.; Ghahremani S.; Finn J. P. High-resolution three-dimensional contrast-enhanced magnetic resonance venography in children: comparison of gadofosveset trisodium with ferumoxytol. Pediatric Radiology 2022, 52, 501–512. 10.1007/s00247-021-05225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer M. A.; Keeling I. M.; Salvan-Schaschl C. V.; Knez I.; Binder B.; Raggam R. B.; Trantina-Yates A. E. Gadofosveset-Trinatrium-Enhanced MR Angiography and MR Venography in the Diagnosis of Venous Thromboembolic Disease: A Single-Center Cohort Study. Diseases 2022, 10, 122. 10.3390/diseases10040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L.-C.; Landmesser U.; Abdelwahed Y. S.; Gigengack K.; Wurster T.; Manes C.; Skurk C.; Lauten A.; Schuster A.; Noutsias M.; Hamm B.; Botnar R. M.; Bigalke B.; Makowski M. R. In vivo assessment of endothelial permeability of coronary lesions with variable degree of stenosis using an albumin-binding MR probe. International Journal of Cardiovascular Imaging 2021, 37, 3049–3055. 10.1007/s10554-021-02293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H. M. C.; Karanicolas P. J.; Coburn N.; Law C.; Milot L. Late Gadolinium Hyperintensity of Suspected Colorectal Liver Metastases on Gadofosveset-Enhanced Magnetic Resonance Imaging: A Predictor of Benignity and a Potential Problem-Solving Tool. Canadian Association of Radiologists Journal 2019, 70, 239–245. 10.1016/j.carj.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Bohnert T.; Gan L.-S. Plasma protein binding: From discovery to development. J. Pharm. Sci. 2013, 102, 2953–2994. 10.1002/jps.23614. [DOI] [PubMed] [Google Scholar]
- Orlando V.; Mucherino S.; Guarino I.; Guerriero F.; Trama U.; Menditto E. Gender Differences in Medication Use: A Drug Utilization Study Based on Real World Data. International journal of environmental research and public health 2020, 17, 3926. 10.3390/ijerph17113926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe C. M.; McNamara A. M.; Motheral B. R. Gender- and Age-Related Prescription Drug Use Patterns. Annals of Pharmacotherapy 2002, 36, 30–39. 10.1345/aph.1A113. [DOI] [PubMed] [Google Scholar]
- Hagymási K.; Müllner K.; Herszényi L.; Tulassay Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2011, 12, 873–888. 10.2217/pgs.11.4. [DOI] [PubMed] [Google Scholar]
- Palleria C.; Roberti R.; Iannone L. F.; Tallarico M.; Barbieri M. A.; Vero A.; Manti A.; De Sarro G.; Spina E.; Russo E. Clinically relevant drug interactions between statins and antidepressants. Journal of Clinical Pharmacy and Therapeutics 2020, 45, 227–239. 10.1111/jcpt.13058. [DOI] [PubMed] [Google Scholar]
- Hales C. M.; Carroll M. D.; Fryar C. D.; Ogden C. L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020, 1–8. [PubMed] [Google Scholar]
- Tan A. C.; Mulder C. J. Increased survival in advanced primary biliary cirrhosis patients with regular albumin infusions?. Eur. J. Gastroenterol. Hepatol. 1999, 11, 927–930. 10.1097/00042737-199908000-00021. [DOI] [PubMed] [Google Scholar]
- Schwartz J. B. The influence of sex on pharmacokinetics. Clin Pharmacokinet 2003, 42, 107–121. 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- Anderson G. D. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J. Womens Health (Larchmt) 2005, 14, 19–29. 10.1089/jwh.2005.14.19. [DOI] [PubMed] [Google Scholar]
- Fenton A.; Montgomery E.; Nightingale P.; Peters A. M.; Sheerin N.; Wroe A. C.; Lipkin G. W. Glomerular filtration rate: new age- and gender- specific reference ranges and thresholds for living kidney donation. BMC Nephrol 2018, 19, 336. 10.1186/s12882-018-1126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F.; Berthold H. K.; Campesi I.; Carrero J.-J.; Dhakal S.; Franconi F.; Gouni-Berthold I.; Heiman M. L.; Kautzky-Willer A.; Klein S. L.; Murphy A.; Regitz-Zagrosek V.; Reue K.; Rubin J. B. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021, 73, 730–762. 10.1124/pharmrev.120.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päivärinta J.; Oikonen V.; Räisänen-Sokolowski A.; Tolvanen T.; Löyttyniemi E.; Iida H.; Nuutila P.; Metsärinne K.; Koivuviita N. Renal vascular resistance is increased in patients with kidney transplant. BMC Nephrology 2019, 20, 437. 10.1186/s12882-019-1617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg U. B. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant 2006, 21, 2577–2582. 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- Loikas D.; Wettermark B.; von Euler M.; Bergman U.; Schenck-Gustafsson K. Differences in drug utilisation between men and women: a cross-sectional analysis of all dispensed drugs in Sweden. BMJ. Open 2013, 3, e002378 10.1136/bmjopen-2012-002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu D.; Paul E.; Madaio M.. Rheumatology and the Kidney; Oxford University Press: Oxford, U.K., 2012 10.1093/med/9780199579655.001.0001. [DOI] [Google Scholar]
- Naidoo S.; Meyers A. M. Drugs and the kidney. S. Afr. Med. J. 2015, 105, 322. 10.7196/SAMJ.9537. [DOI] [PubMed] [Google Scholar]
- Brändle E.; Gottwald E.; Melzer H.; Sieberth H. G. Influence of oral contraceptive agents on kidney function and protein metabolism. Eur. J. Clin Pharmacol 1992, 43, 643–646. 10.1007/BF02284965. [DOI] [PubMed] [Google Scholar]
- Atthobari J.; Gansevoort R. T.; Visser S. T.; De Jong P. E.; De Jong-van den Berg L. T. W. The impact of hormonal contraceptives on blood pressure, urinary albumin excretion and glomerular filtration rate. Br. J. Clin. Pharmacol. 2007, 63, 224–231. 10.1111/j.1365-2125.2006.02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S. B.; Vitek W. S.; Holley J. L. Fertility, Contraception, and Novel Reproductive Technologies in Chronic Kidney Disease. Semin Nephrol 2017, 37, 327–336. 10.1016/j.semnephrol.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Campesi I.; Montella A.; Sotgiu G.; Saderi L.; Tonolo G.; Seghieri G.; Franconi F. Smoking and combined oral contraceptives should be considered as an independent variable in sex and gender-oriented studies. Toxicol. Appl. Pharmacol. 2022, 457, 116321 10.1016/j.taap.2022.116321. [DOI] [PubMed] [Google Scholar]
- Ahmed S. B.; Culleton B. F.; Tonelli M.; Klarenbach S. W.; Macrae J. M.; Zhang J.; Hemmelgarn B. R. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008, 74, 370–376. 10.1038/ki.2008.205. [DOI] [PubMed] [Google Scholar]
- Safari T.; Nematbakhsh M.; Evans R. G.; Denton K. M. High-Dose Estradiol-Replacement Therapy Enhances the Renal Vascular Response to Angiotensin II via an AT2-Receptor Dependent Mechanism. Adv. Pharmacol Sci. 2015, 2015, 682745 10.1155/2015/682745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard B. D. Sex differences in diabetes and kidney disease: mechanisms and consequences. Am. J. Physiol Renal Physiol 2019, 317, F456–F462. 10.1152/ajprenal.00249.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll R. G.; Yelavarthy P.; Menees D. S.; Sutton N. R. Predicting Contrast-Induced Renal Complications. Interv Cardiol Clin 2020, 9, 321–333. 10.1016/j.iccl.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Lee Y.-H.; Wu M.-R.; Hsiao J.-K. Organic Anion Transporting Polypeptide 1B1 Is a Potential Reporter for Dual MR and Optical Imaging. International journal of molecular sciences 2021, 22, 8797. 10.3390/ijms22168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich J. M.; Moussa L.; Guirguis N.; Gutzeit A.; Wu D.; Sartoretti-Schefer S.; Koh D.-M.; Kolokythas O.; Matoori S. Comparison of gadolinium-based contrast agents for MR cholangiography in saline, blood and bile: a phantom study. European Radiology Experimental 2023, 7, 21. 10.1186/s41747-023-00331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami Riedmaier A.; Nies A. T.; Schaeffeler E.; Schwab M. Organic anion transporters and their implications in pharmacotherapy. Pharmacol Rev. 2012, 64, 421–449. 10.1124/pr.111.004614. [DOI] [PubMed] [Google Scholar]
- Buist S. C.; Klaassen C. D. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1–3; Slc22a6–8) mRNA levels. Drug Metab. Dispos. 2004, 32, 620–625. 10.1124/dmd.32.6.620. [DOI] [PubMed] [Google Scholar]
- Takita H.; Barnett S.; Zhang Y.; Ménochet K.; Shen H.; Ogungbenro K.; Galetin A. PBPK Model of Coproporphyrin I: Evaluation of the Impact of SLCO1B1 Genotype, Ethnicity, and Sex on its Inter-Individual Variability. CPT Pharmacometrics Syst. Pharmacol 2021, 10, 137–147. 10.1002/psp4.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuvonen M.; Hirvensalo P.; Tornio A.; Rago B.; West M.; Lazzaro S.; Mathialagan S.; Varma M.; Cerny M. A.; Costales C.; Ramanathan R.; Rodrigues A. D.; Niemi M. Identification of Glycochenodeoxycholate 3-O-Glucuronide and Glycodeoxycholate 3-O-Glucuronide as Highly Sensitive and Specific OATP1B1 Biomarkers. Clin Pharmacol Ther 2021, 109, 646–657. 10.1002/cpt.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badée J.; Achour B.; Rostami-Hodjegan A.; Galetin A. Meta-analysis of expression of hepatic organic anion-transporting polypeptide (OATP) transporters in cellular systems relative to human liver tissue. Drug Metab. Dispos. 2015, 43, 424–432. 10.1124/dmd.114.062034. [DOI] [PubMed] [Google Scholar]
- Verloh N.; Haimerl M.; Zeman F.; Teufel A.; Lang S.; Stroszczynski C.; Fellner C.; Wiggermann P. Multivariable Analysis of Clinical Influence Factors on Liver Enhancement of Gd-EOB-DTPA-Enhanced 3T MRI. Rofo 2014, 187, 29–35. 10.1055/s-0034-1385211. [DOI] [PubMed] [Google Scholar]
- Lee H. H.; Ho R. H. Interindividual and interethnic variability in drug disposition: polymorphisms in organic anion transporting polypeptide 1B1 (OATP1B1; SLCO1B1). Br. J. Clin. Pharmacol. 2017, 83, 1176–1184. 10.1111/bcp.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori D.; Kashihara Y.; Yoshikado T.; Kimura M.; Hirota T.; Matsuki S.; Maeda K.; Irie S.; Ieiri I.; Sugiyama Y.; Kusuhara H. Effect of OATP1B1 genotypes on plasma concentrations of endogenous OATP1B1 substrates and drugs, and their association in healthy volunteers. Drug Metabolism and Pharmacokinetics 2019, 34, 78–86. 10.1016/j.dmpk.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Wu L.; Han W.; Chen Y.; Zhang T.; Liu J.; Zhong S.; Liu H.; Han C.; Zhang Z.; Liu S.; Tang L. Gender Differences in the Hepatotoxicity and Toxicokinetics of Emodin: The Potential Mechanisms Mediated by UGT2B7 and MRP2. Mol. Pharmaceutics 2018, 15, 3931–3945. 10.1021/acs.molpharmaceut.8b00387. [DOI] [PubMed] [Google Scholar]
- Kong B.; Csanaky I. L.; Aleksunes L. M.; Patni M.; Chen Q.; Ma X.; Jaeschke H.; Weir S.; Broward M.; Klaassen C. D.; Guo G. L. Gender-specific reduction of hepatic Mrp2 expression by high-fat diet protects female mice from ANIT toxicity. Toxicol. Appl. Pharmacol. 2012, 261, 189–195. 10.1016/j.taap.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau T.; Erney B.; Göres R.; Eschenhagen T.; Beck J.; Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: Impact of ABCC2 polymorphisms on plasma concentrations. Clinical Pharmacology & Therapeutics 2006, 80, 468–476. 10.1016/j.clpt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- World Health Organization . VigiAccess; 2023http://www.vigiaccess.orgVigiaccess.org. [Google Scholar]
- Karlgren M.; Vildhede A.; Norinder U.; Wisniewski J. R.; Kimoto E.; Lai Y.; Haglund U.; Artursson P. Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J. Med. Chem. 2012, 55, 4740–4763. 10.1021/jm300212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anabtawi N.; Drabison T.; Hu S.; Sparreboom A.; Talebi Z. The role of OATP1B1 and OATP1B3 transporter polymorphisms in drug disposition and response to anticancer drugs: a review of the recent literature. Expert Opinion on Drug Metabolism & Toxicology 2022, 18, 459–468. 10.1080/17425255.2022.2113380. [DOI] [PubMed] [Google Scholar]
- Moyer A. M.; de Andrade M.; Faubion S. S.; Kapoor E.; Dudenkov T.; Weinshilboum R. M.; Miller V. M. SLCO1B1 genetic variation and hormone therapy in menopausal women. Menopause 2018, 25, 877–882. 10.1097/GME.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen E.; Deng F.; Kidron H.; Finel M. Efflux transport of estrogen glucuronides by human MRP2, MRP3, MRP4 and BCRP. J. Steroid Biochem Mol. Biol. 2018, 178, 99–107. 10.1016/j.jsbmb.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Gutzeit A.; Matoori S.; Froehlich J. M.; von Weymarn C.; Reischauer C.; Kolokythas O.; Goyen M.; Hergan K.; Meissnitzer M.; Forstner R.; Soyka J. D.; Doert A.; Koh D.-M. Reduction in respiratory motion artefacts on gadoxetate-enhanced MRI after training technicians to apply a simple and more patient-adapted breathing command. European Radiology 2016, 26, 2714–2722. 10.1007/s00330-015-4086-4. [DOI] [PubMed] [Google Scholar]
- Matoori S.; Froehlich J. M.; Breitenstein S.; Doert A.; Pozdniakova V.; Koh D.-M.; Gutzeit A. Age dependence of spleen- and muscle-corrected hepatic signal enhancement on hepatobiliary phase gadoxetate MRI. European Radiology 2016, 26, 1889–1894. 10.1007/s00330-015-3965-z. [DOI] [PubMed] [Google Scholar]
- Gutzeit A.; Matoori S.; Froehlich J.; Koh D. Reduction in Respiratory Motion Artifacts on Gadoxetate Acid–enhanced MR Images after Training Technicians. Radiology 2016, 279, 981–982. 10.1148/radiol.2016152605. [DOI] [PubMed] [Google Scholar]
- Guy J.; Peters M. G. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol. Hepatol. 2013, 9, 633. [PMC free article] [PubMed] [Google Scholar]
- Kattah A. G.; Suarez M. L. G.; Milic N.; Kantarci K.; Zeydan B.; Mosley T.; Turner S. T.; Ware E. B.; Kardia S. L. R.; Garovic V. D. Hormone therapy and urine protein excretion: a multiracial cohort study, systematic review, and meta-analysis. Menopause 2018, 25, 625. 10.1097/GME.0000000000001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari R. N.; de Oliveira R. D. R.; de Souza F. F. L.; Pippa L. F.; Nardotto G. H. B.; Rocha A.; Donadi E. A.; Lanchote V. L. Systemic Lupus Erythematosus Activity Affects the Sinusoidal Uptake Transporter OATP1B1 Evaluated by the Pharmacokinetics of Atorvastatin. Clin Transl Sci. 2020, 13, 1227–1235. 10.1111/cts.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beers B. E.; Pastor C. M.; Hussain H. K. Primovist, Eovist: What to expect?. Journal of Hepatology 2012, 57, 421–429. 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]