Abstract
Current therapy for primary amoebic meningoencephalitis (PAM), a highly lethal brain infection in humans caused by Naegleria fowleri amoeba, is restricted to repurposed drugs with limited efficacy and success. Discovery of an antiamoebic benzylamine scaffold 2 precipitated a medicinal chemistry effort to improve potency, cytotoxicity profile, and drug-like properties. Thirty-four compounds were prepared, leading to compound 28 with significant gains in potency (EC50 = 0.92 μM), solubility, and microsomal stability and a demonstrated absence of cytotoxicity in SH-SY5Y human neuroblastoma cells (CC50 > 20 μM). The compounds demonstrated excellent blood–brain barrier permeability in an in vitro assay, thereby providing a new structural scaffold that inhibits N. fowleri viability and permits the investigation of therapeutic interventions in an understudied neglected disease.
Keywords: Naegleria fowleri, Brain-eating amoeba, Inhibitor, Encephalitis
Naegleria fowleri is a thermophilic protozoan that does not require a host to survive; however, the amoeba can cause an almost always fatal brain infection in humans called primary amoebic meningoencephalitis (PAM).1 The pathogen is typically found in freshwater, soil, and poorly treated recreational and tap water. The amoeba exists in a cyst, trophozoite, or flagellate life stage, and human infection occurs when trophozoites are introduced into the nasal cavity, where the amoeba infiltrates the olfactory mucosa and travels to the frontal lobes of the brain, where it causes tissue damage.1,2 PAM is usually associated with swimming in freshwater rivers or lakes during warm months when humans encounter the amoeba.1 The infection progresses rapidly and has a reported fatality rate of greater than 97%.1,3 Infection often results in death within 1 to 18 days of symptom onset. Out of 154 reported PAM cases in the United States between 1962 and 2021, there have been only four survivors.1
Current treatment for PAM typically involves the administration of amphotericin B and miltefosine, which can be used in combination with other drugs such as azithromycin, fluconazole, and rifampin.2,4,5 The efficacy of these drugs is unclear, as very few treated patients have survived. Additionally, the cocktail components have limitations, including toxicity, undesirable side effects, or limited blood–brain barrier (BBB) permeability.2,4 Successful treatment of PAM is attributed to early detection, which allows for expedient therapeutic intervention.3 However, this infection is not routinely tested for, and the symptoms are often mistaken for viral or bacterial meningitis.6
These factors underscore the need for effective and safe PAM treatments. Recent research has described N. fowleri inhibitors, though some discoveries are relatively new7,8 and progress is slow to yield high-caliber leads with properties that are developmentally suitable.6,9−14 To identify potential chemical matter that impacts N. fowleri, we surveyed compounds from our lab compound library in a previously described N. fowleri cell viability assay conducted initially at a single 10 μM compound concentration.15 For compounds demonstrating >50% inhibition, the assay was repeated in a dose–response format, and half-maximal effective inhibitory concentration (EC50) values were resolved. The screen included a series of picolinic acid derivatives, including benzamide 1, that we investigated as Toxoplasma gondii inhibitors (Figure 1).16
Figure 1.
Structures of T. gondii inhibitor 1 and N. fowleri hit 2.
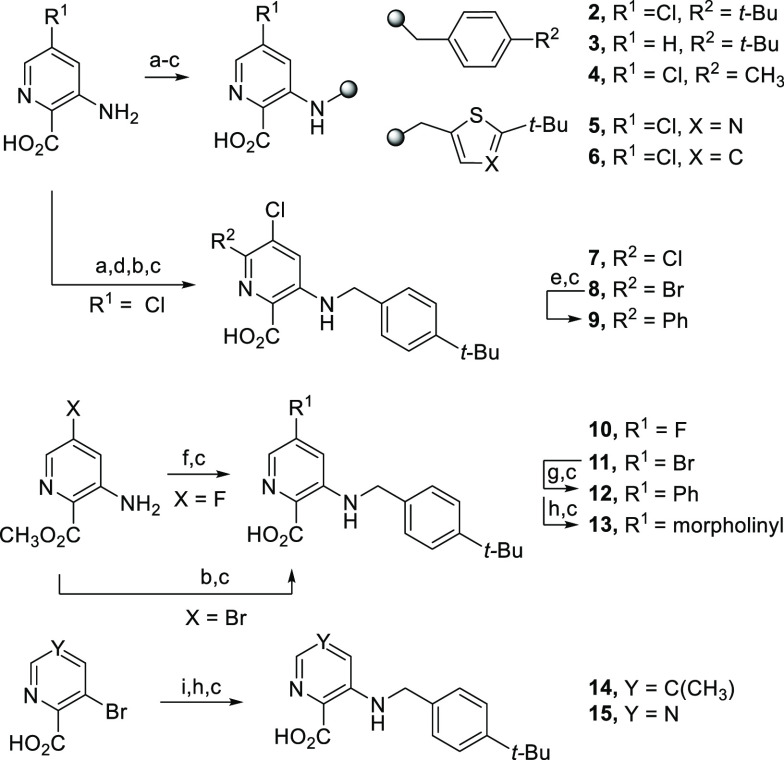
While benzamide 1 was inactive at 10 μM, the benzylamine analog 2 exhibited promising N. fowleri trophozoite inhibition (EC50 = 6.0 μM). We further examined the compound cytotoxicity in human neuroblastoma cells (SH-SY5Y) as a species-relevant cell model17 for assessing neurotoxicology in the primary site of PAM infection. Evaluation at two independent concentrations revealed that analog 2 demonstrated a mean 55% cell viability at 20 μM, which was reduced from a mean 86% at a concentration of 1 μM. Given the results, we explored structure–activity relationships (SARs) governing N. fowleri inhibition and sought an improved cytotoxicity profile. Analogs were designed to examine benzylamine linker changes exclusive from or in combination with picolinic core substituent changes or terminal phenyl ring modifications. Methyl picolinates, available commercially or prepared via Fischer esterification or by using SOCl2, were alkylated on the core-appended amine (analogs 2–6, Scheme 1). Halogenation provided additional core modifications, including those produced through Buchwald–Hartwig or Suzuki couplings (analogs 7–15).
Scheme 1. Synthesis of Picolinic Acid Analogs with Core Substituent or Terminal Phenyl Modifications.
Reagents and conditions: (a) 1:4 H2SO4/CH3OH, 70 °C, 24 h, 42–69%; (b) BrCH2R, DIPEA, CH3CN, 70 °C, 18–24 h, 22–41%; (c) 1.3 M LiOH, CH3OH or THF, RT to 50 °C, 4–24 h, 20–83%; (d) NBS or NCS, CH3CN, 70 °C, 18 h, 59%; (e) phenylboronic acid, Pd(PPh3)2Cl2, Na2CO3, 10:1 CH3CN/H2O, 100 °C MW, 3.5 h, 23%; (f) 4-tert-butylbenzyl bromide, KI, K2CO3, CH3CN, 70 °C, 18 h, 47%; (g) phenylboronic acid, Pd(PPh3)4, Cs2CO3, 4:1 dioxane/H2O, 85 °C, 18 h, 83%; (h) morpholine or H2NR, Pd2(dba)3, xantphos, Cs2CO3, toluene or dioxane, 85–100 °C, 9–18 h, 22–88%; (i) SOCl2, CH3OH, 70 °C, 4 h, 69–92%.
Carboxylic acid and aminoalkyl linker changes were also surveyed (Scheme 2). Linker-modified analogs were prepared, after hydrolysis, through N-acylation to give 16, N-sulfonation to provide 17, and deprotonation of 5-chloro-3-methylpicolinic acid with LDA followed by alkylation to afford 18. Buchwald–Hartwig coupling between an aryl bromide and the corresponding amine, followed by hydrolysis, afforded analogs 19–27. An SNAr reaction between 3-bromo-5-fluoropicolinonitrile and sodium methoxide provided a coupling partner to afford 28 and 29. Nitrile 30 and amide 31 were derived from the same starting material, and chloropyridine 32 was delivered through the coupling of the corresponding aryl bromide starting material.
Scheme 2. Synthesis of Linker- and Acid-Modified Analogs.
Reagents and conditions: (a) 1:4 H2SO4/CH3OH, 70 °C, 24 h, 42–69%; (b) 2-(4-tert-butylphenyl)acetyl chloride, DIPEA, CH3CN, RT, 24 h, 18%; (c) 1.3 M LiOH, CH3OH or THF, RT to 50 °C, 4–24 h, 20–83%; (d) 4-tert-butylbenzenesulfonyl chloride, pyridine, and cat. DMAP, CH2Cl2, RT, 2 h, 29%; (e) LDA, TEA, then 4-tert-butylbenzyl bromide, THF, −78 °C to RT, 24 h, 60%; (f) SOCl2, CH3OH, 70 °C, 4 h, 69–92%; (g) H2NR, Pd2(dba)3, xantphos, Cs2CO3, toluene, 85 °C or dioxane, 100 °C, 9–18 h, 22–88%; (h) NaOMe, CH3OH, 0 to 40 °C, 24 h, 77%; (i) 2.5:1 EtOH/50% aq NaOH, 100 °C, 2 h, 84%; (j) 2.5 M aq NaOH, 30% H2O2, acetone, RT, 2 h, 71%.
Reductive amination was employed to generate analogs bearing other heterocyclic cores to the picolinic acid (Scheme 3). Thiazole acid 33 and nicotinate 34 resulted directly or after hydrolysis of the corresponding ester intermediate.
Scheme 3. Synthesis of Analogs with Other Heterocyclic Cores.
Reagents and conditions: (a) 4-tert-butylbenzaldehyde, CF3CO2H, STAB, toluene, RT, 2 h, 60%; (b) 1.3 M LiOH, CH3OH or THF, RT to 50 °C, 4–24 h, 54–60%; (c) 1:4 H2SO4/CH3OH, 70 °C, 24 h, 42–69%; (d) 4-tert-butylbenzaldehyde, AcOH, STAB, CH2Cl2, RT, 24 h, 22%.
Compounds in this chemical series were tested against N. fowleri trophozoites (Nf69 strain) at a single compound concentration of 10 μM.15 Compounds that inhibited amoeba viability by ≥50% were subsequently tested in a dose–response format to obtain EC50 values for comparison. The compounds were also assessed for cytotoxicity in human neuroblastoma cells (SH-SY5Y) at concentrations of 1 and 20 μM (Table 1). Miltefosine, included as a control, afforded an EC50 value of 37.5 μM in the amoeba assay.
Table 1. N. fowleri and Mammalian Cell Viability Data for Key Substituent Changes at R1, R2, and R3.

| structure |
N. fowleri dataa |
percent cell viabilityb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| cmpd | R1 | R2 | R3 | % growth inhibition | EC50 (μM) | 1 μM | 20 μM | ||
| 2 | Cl | H | CO2H | 98.8 ± 0.3 | 6.0 ± 0.3 | 87 | 85 | 57 | 53 |
| 3 | H | H | CO2H | 99.2 ± 0.35 | 3.0 ± 0.7 | 100 | 100 | 89 | 91 |
| 7 | Cl | Cl | CO2H | 12.3 ± 4.9 | >10 | 100 | 100 | 100 | 100 |
| 8 | Cl | Br | CO2H | 17.6 ± 4.6 | >10 | 100 | 100 | 100 | 100 |
| 9 | Cl | Ph | CO2H | 20.4 ± 4.9 | >10 | 100 | 100 | 86 | 82 |
| 10 | F | H | CO2H | 99.5 ± 0.3 | 5–10c | 91 | 93 | 75 | 73 |
| 11 | Br | H | CO2H | 84.4 ± 2.0 | 7.2 ± 0.3 | 99 | 99 | 85 | 85 |
| 12 | Ph | H | CO2H | 99.3 ± 0.4 | 5–10c | 100 | 100 | 81 | 85 |
| 13 | morpholinyl | H | CO2H | 52.8 ± 8.1 | 11.5 ± 1.9 | 89 | 85 | 75 | 77 |
| 14 | CH3 | H | CO2H | 100.4 ± 0.02 | 1.4 ± 0.2 | 96 | 98 | 98 | 98 |
| 28 | OCH3 | H | CO2H | 100.3 ± 0.2 | 0.92 ± 0.03 | 98 | 98 | 99 | 100 |
| 30 | Cl | H | CN | 22.2 ± 1.6 | >10 | 96 | 98 | 57 | 64 |
| 31 | Cl | H | CONH2 | 27.7 ± 6.3 | >10 | 100 | 100 | 77 | 81 |
| 32 | Cl | H | H | 17.6 ± 7.4 | >10 | 100 | 100 | 88 | 88 |
Data are reported as averages of triplicate runs (n = 3) with ±SEM at 10 μM and with half-maximal effective concentration (EC50) against N. fowleri (Nf69 strain, ATCC 30215) trophozoites; miltefosine (positive control) N. fowleri EC50 = 35.7 ± 9.1 μM.
Percent viability (n = 2), 72 h, human neuronal SH-SY5Y cells, 1 μM or 20 μM compound concentration.
The Hill slope of the dose–response assay was steep, leading to a lack of resolution of EC50 but a clear range as indicated.
Compared to the hit benzylamine 2, the addition of an adjacent substituent at R2 resulted in weaker antiamoebic activity (7–9, Table 1). Exchange of the R1 chlorine atom for a hydrogen atom (analog 3) gave a similar potency to 2, but an improved cytotoxicity profile was observed at both low and high compound concentrations. Analogously, substitution of the chlorine atom for a phenyl ring in 12, a fluorine or bromine atom (analogs 10 and 11, respectively), or a morpholino substituent in 13 resulted in similar potency to hit 2; however, comparable erosion of mammalian cell viability was also observed. Also, fluorine analog 10 and phenyl analog 12 uniquely and consistently demonstrated steep Hill slopes in the amoeba dose–response assay, thereby limiting a sharp determination of EC50 beyond that of 5–10 μM. Better results were obtained when the chlorine atom of 2 was replaced with a methyl or methoxy group (analogs 14 and 28, respectively), as a 4- to 7-fold improvement in potency was observed without cytotoxicity at the highest tested concentration of 20 μM. Alterations to the carboxylic acid moiety, such as replacement with a nitrile (30), amide (31), or hydrogen atom (32), were not tolerated in terms of antiamoebic activity.
Examination of the 4-tert-butylphenyl moiety of hit 2 revealed poor tolerance for changes (Table 2). Exchange of the tert-butyl group for methyl (3), methoxy (19), fluorine (20), trifluoromethyl (21), or a hydrogen atom (22) all resulted in inferior amoeba inhibition and EC50 values >10 μM. Thiazole (4) or thiophene (5) rings inserted in place of the phenyl ring led to a complete loss of activity. As such, linker region changes were evaluated while preserving the tert-butylphenyl component. Analogs with an extended amide (16), a sulfonamide (17), or the aniline-like nitrogen atom replaced with a methylene (18) were not active. Methylation of the linker methylene group or excision of it altogether also led to inactive analogs (23 and 24). Lengthening the benzyl linker by one methylene unit afforded compound 25 and restored activity to what was observed for compound 2 with an EC50 of 3.3 μM and no notable cytotoxicity. Assessment showed that tert-butyl group changes in combination with the extended linker were not tolerated, as observed with previous analogs. Evaluation of compound 28 had revealed that the incorporation of a C5 methoxy group was beneficial, so this change was integrated into an analog with the extended chain, resulting in compound 29 with an EC50 of 1.1 μM but with little improvement compared to compound 2 with regard to cytotoxicity. Picolinic acid core changes were investigated; however, pyrimidine 15, thiazole 33, and nicotinic acid 34 all afforded N. fowleri EC50 values >10 μM (Figure 2).
Table 2. N. fowleri and Mammalian Cell Viability Data for Analogs with Linker and tert-Butylphenyl Group Changes.

Data are reported as averages of triplicate runs (n = 3) with ±SEM at 10 μM and with half-maximal effective concentration (EC50) against N. fowleri (ATCC 30215) trophozoites; miltefosine (positive control) N. fowleri EC50 = 35.7 ± 9.1 μM.
Percent viability (n = 2), 72 h, human neuronal SH-SY5Y cells, 1 μM or 20 μM compound concentration.
Figure 2.
Alternative cores to the picolinic acid hit structure.
Given the improvement in antiamoebic potency and cytotoxicity profile, analogs 14 and 28 were selected for comparison to hit 2 with respect to several physiochemical and drug-like properties (Table 3). Cytotoxicity for these analogs was determined in dose–response format, revealing CC50 values of >20 μM, though inspection of the concentration curves showed clear advantages for compounds 14 and 28, which showed no attenuation of cell viability in neuronal cells from 1 to 20 μM. All three compounds landed in the desirable logD range. Compared to hit 2, significant gains were made in solubility for both analogs 14 and 28, and mouse microsomal stability increased, with the greatest improvement observed for methoxy derivative 28. Subsequent evaluations compared 2 and 28 only, revealing excellent stability in mouse plasma, high protein binding, and excellent brain tissue permeability as assessed by a BBB parallel artificial membrane permeability assay (PAMPA),18 which showed that 2 and 28 are CNS-penetrant, an important consideration for treating PAM infection.
Table 3. Comparison of Physiochemical and Drug-like Parameters for Selected Compounds 2, 14, and 28.
Half-maximal effective concentration in inhibiting N. fowleri trophozoite viability.
Half-maximal cytotoxic concentration after 72 h in human neuronal SH-SY5Y cells.
Kinetic solubility, PBS buffer.
Sourced from CD-1 mouse.
2 μM protein concentration
10 μM compound concentration; porcine brain lipid extract; 4 h incubation; negative control (CNS−): nadalol, Pe < 0.1 nm/s; positive control (CNS+): carbamazepine, Pe = 66.3 nm/s. ND = not determined.
Naegleria fowleri causes a rare, devastating brain infection in patients who have acquired the pathogen through contaminated water entering the nasal cavity. With a lethality of >97%, improved treatments are needed that specifically and efficaciously address this infection. A screening effort directed at identifying new chemical matter with activity against N. fowleri revealed that benzylamines such as 2 reduced the amoeba viability. Optimization efforts focused on improvement of potency and cytotoxicity, understanding of pharmacophoric structural elements of the scaffold, and assessment of physiochemical and preliminary absorption, distribution, metabolism, and excretion (ADME) parameters to determine feasibility for further analysis of pharmacokinetics and efficacy in animal models. Through synthesis, 34 compounds were prepared, resulting in a 6.5-fold potency enhancement for compound 28 with a concomitant advancement at higher concentrations such that human neuronal cells showed no indication of cytotoxic effects. Analog 28 also featured a boost in aqueous solubility and metabolic stability compared to compound 2. The SAR efforts for the series revealed a strong reliance on the position of the picolinic acid nitrogen atom and the need for the carboxylic acid and tert-butylphenyl moieties, as no tolerance was observed for changes in these regions. The aminoalkyl linker was more amenable to alteration, and substituents on the picolinic acid core permitted tuning of physiochemical and ADME parameters. Importantly, the compounds were found to be CNS-penetrant as determined by a BBB permeability assay, which is a significant parameter in the consideration of any scaffold development plan for PAM. Further studies will focus on pharmacokinetic analysis in mice, sensitivity of other patient isolates besides Nf69, and a study of mechanism of action; however, this work demonstrates that benzylamines inhibit N. fowleri amoeba and provides useful chemical probes for additional studies, which may reveal potential therapeutic opportunity.
Acknowledgments
This work is submitted in commemoration of the 60th anniversary of the “MIKIW” regional medicinal chemistry meeting-in-miniature that is held annually between the Schools of Pharmacy at the Universities of Minnesota, Illinois at Chicago, Kansas, Iowa, and (as of 2018) Wisconsin-Madison. As a student alum of the University of Kansas and now an Associate Professor at Wisconsin, J.E.G. has had the honor of presenting in and now sending her students to contribute to this unique, research-diverse, and richly engaging Midwest tradition in the medicinal chemistry community. Many thanks to the students, faculty, companies and vendors, and the American Chemical Society who have supported and continue to support the mission of this meeting. J.E.G. and J.C.M. gratefully acknowledge support from the National Institutes of Health for the chemistry and screening efforts, respectively (R01AI156382). Work from the J.C.M. laboratory was also supported in part by R21AI171217. Synthesis work used instrumentation at the UW-Madison Analytical Instrumentation Center and the Medicinal Chemistry Center, both within the UW-Madison School of Pharmacy. We also thank the University of Wisconsin Carbone Cancer Center (UWCCC) Small Molecule Screening Facility, especially Song Guo and Spencer Erickson, for SH-SY5Y toxicity screening (Support Grant P30 CA014520).
Glossary
Abbreviations
- ADME
absorption, distribution, metabolism, and excretion
- BBB
blood–brain barrier
- CNS
central nervous system
- PAM
primary amoebic meningoencephalitis
- SAR
structure–activity relationship
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00440.
Assay, experimental details, synthetic procedures, and NMR spectra for final compounds 2–34 reported in this work (PDF)
Author Contributions
J.M.P. and M.M.K. synthesized compounds. J.E.G. guided the medicinal chemistry and synthesis efforts. J.E.M. and C.M.P. assessed compounds in the parasite and enzymatic assays. J.C.M. formulated the Naegleria viability assay and assisted with data analysis. The manuscript was written through contributions of all authors. All of the authors approved the final version of the manuscript.
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Medicinal Chemistry Lettersvirtual special issue “Celebrating the 60th Anniversary of the MIKIW Meeting-in-Miniature”.
Supplementary Material
References
- Naegleria fowleri–Primary Amebic Meningoencephalitis (PAM)–Amebic Encephalitis: Treatment. Centers for Disease Control and Prevention (CDC), last reviewed April 20, 2023. https://www.cdc.gov/parasites/naegleria/treatment-hcp.html#twelve.
- Güémez A.; García E. Primary Amoebic Meningoencephalitis by Naegleria fowleri: Pathogenesis and Treatments. Biomolecules 2021, 11 (9), 1320. 10.3390/biom11091320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope J. R.; Ali I. K. Primary Amebic Meningoencephalitis: What Have We Learned in the Last 5 Years?. Curr. Infect. Dis. Rep. 2016, 18 (10), 31. 10.1007/s11908-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace E.; Asbill S.; Virga K. Naegleria fowleri: Pathogenesis, Diagnosis, and Treatment Options. Antimicrob. Agents Chemother. 2015, 59 (11), 6677–6681. 10.1128/AAC.01293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope J. R.; Conrad D. A.; Cohen N.; Cotilla M.; DaSilva A.; Jackson J.; Visvesvara G. S. Use of the Novel Therapeutic Agent Miltefosine for the Treatment of Primary Amebic Meningoencephalitis: Report of 1 Fatal and 1 Surviving Case. Clin. Infect. Dis. 2016, 62 (6), 774–776. 10.1093/cid/civ1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon B. L.; Rice C. A.; Guy R. K.; Kyle D. E. Phenotypic Screens Reveal Posaconazole as a Rapidly Acting Amebicidal Combination Partner for Treatment of Primary Amoebic Meningoencephalitis. J. Infect. Dis. 2019, 219 (7), 1095–1103. 10.1093/infdis/jiy622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R.; El-Gamal M. I.; Boghossian A.; Saeed B. Q.; Oh C. H.; Abdel-Maksoud M. S.; Alharbi A. M.; Alfahemi H.; Khan N. A. Imidazothiazole Derivatives Exhibited Potent Effects against Brain-Eating Amoebae. Antibiotics (Basel) 2022, 11 (11), 1515. 10.3390/antibiotics11111515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrins L.; Buskes M. J.; Kapteyn M. M.; Engels H. N.; Enos S. E.; Lu C.; Klug D. M.; Singh B.; Quotadamo A.; Bachovchin K.; Tear W. F.; Spaulding A. E.; Forbes K. C.; Bag S.; Rivers M.; LeBlanc C.; Burchfield E.; Armand J. R.; Diaz-Gonzalez R.; Ceballos-Perez G.; García-Hernández R.; Pérez-Moreno G.; Bosch-Navarrete C.; Gómez-Liñán C.; Ruiz-Pérez L. M.; Gamarro F.; González-Pacanowska D.; Navarro M.; Mensa-Wilmot K.; Pollastri M. P.; Kyle D. E.; Rice C. A. Identification of novel anti-amoebic pharmacophores from kinase inhibitor chemotypes. Front. Microbiol. 2023, 14, 1149145. 10.3389/fmicb.2023.1149145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo-Liendo A.; Arberas-Jiménez I.; Sifaoui I.; Gkolfi D.; Santana Y.; Cotos L.; Tejedor D.; García-Tellado F.; Piñero J. E.; Lorenzo-Morales J. The therapeutic potential of novel isobenzofuranones against Naegleria fowleri. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 139–149. 10.1016/j.ijpddr.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. A.; Colon B. L.; Alp M.; Göker H.; Boykin D. W.; Kyle D. E. Bis-benzimidazole hits against Naegleria fowleri discovered with new high-throughput screens. Antimicrob. Agents Chemother. 2015, 59 (4), 2037–44. 10.1128/AAC.05122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungroo M. R.; Shahbaz M. S.; Anwar A.; Saad S. M.; Khan K. M.; Khan N. A.; Siddiqui R. Aryl Quinazolinone Derivatives as Novel Therapeutic Agents against Brain-Eating Amoebae. ACS Chem. Neurosci. 2020, 11 (16), 2438–2449. 10.1021/acschemneuro.9b00596. [DOI] [PubMed] [Google Scholar]
- Chao-Pellicer J.; Arberas-Jiménez I.; Delgado-Hernández S.; Sifaoui I.; Tejedor D.; García-Tellado F.; Piñero J. E.; Lorenzo-Morales J. Cyanomethyl Vinyl Ethers against Naegleria fowleri. ACS Chem. Neurosci. 2023, 14 (11), 2123–2133. 10.1021/acschemneuro.3c00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arberas-Jiménez I.; Nocchi N.; Chao-Pellicer J.; Sifaoui I.; Soares A. R.; Díaz-Marrero A. R.; Fernández J. J.; Piñero J. E.; Lorenzo-Morales J. Chamigrane-Type Sesquiterpenes from Laurencia dendroidea as Lead Compounds against Naegleria fowleri. Mar. Drugs 2023, 21 (4), 224. 10.3390/md21040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A.; Mungroo M. R.; Khan S.; Fatima I.; Rafique R.; Kanwal; Khan K. M.; Siddiqui R.; Khan N. A. Novel Azoles as Antiparasitic Remedies against Brain-Eating Amoebae. Antibiotics (Basel) 2020, 9 (4), 188. 10.3390/antibiotics9040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanes J. E.; Suryadi J.; Abendroth J.; Van Voorhis W. C.; Barrett K. F.; Dranow D. M.; Phan I. Q.; Patrick S. L.; Rozema S. D.; Khalifa M. M.; Golden J. E.; Morris J. C. Enzymatic and Structural Characterization of the Naegleria fowleri Glucokinase. Antimicrob. Agents Chemother. 2019, 63 (5), e02410-18. 10.1128/AAC.02410-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa M. M.; Martorelli Di Genova B.; McAlpine S. G.; Gallego-Lopez G. M.; Stevenson D. M.; Rozema S. D.; Monaghan N. P.; Morris J. C.; Knoll L. J.; Golden J. E. Dual-Stage Picolinic Acid-Derived Inhibitors of Toxoplasma gondii. ACS Med. Chem. Lett. 2020, 11 (12), 2382–2388. 10.1021/acsmedchemlett.0c00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Suarez L.; Awabdh S. A.; Coumoul X.; Chauvet C. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: Focus on organic pollutants. Neurotoxicology 2022, 92, 131–155. 10.1016/j.neuro.2022.07.008. [DOI] [PubMed] [Google Scholar]
- Kansy M.; Senner F.; Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41 (7), 1007–10. 10.1021/jm970530e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.