Abstract
Purpose
The use of a hydrogel spacer inserted into recto-vaginal fossa is a valuable strategy to mitigate radiation exposure to the rectum during radiation therapy for female pelvic malignancies. However, when the sigmoid colon is in proximity to the cervix, radiation exposure to the sigmoid colon cannot be adequately mitigated with a hydrogel spacer injected into the recto-vaginal fossa. Here, we presented a case, in which a hydrogel spacer was injected into the meso-sigmoid to protect the sigmoid colon.
Material and methods
A 73-year-old female diagnosed with T3b stage IIIC2r uterine cervical cancer (FIGO 2018) underwent high-dose-rate interstitial brachytherapy consisting of 24 Gy in 4 fractions, following concurrent chemoradiotherapy with external beam radiation therapy of 50 Gy in 25 fractions of whole pelvic radiation therapy. In the initial brachytherapy, the sigmoid colon was in close contact with the uterine cervix. In the second brachytherapy, attempts to create a space between the sigmoid colon and uterine cervix using injected artificial ascites were unsuccessful due to rapid absorption of fluid. In the third and fourth brachytherapy fractions, 5 mL of hydrogel was injected into the meso-sigmoid through a pouch of Douglas under trans-rectal ultrasonography guidance. Dose ratio of sigmoid colon D2cc and high-risk clinical target volume (HR-CTV) D90 of each brachytherapy were evaluated.
Results
Dose ratio of the sigmoid colon D2cc to HR-CTV D90 was 1.03, 0.43, 0.56, and 0.47 in each respective brachytherapy session, indicating dose escalation to HR-CTV whilst achieving acceptable sigmoid dose with hydrogel spacer injected into the meso-sigmoid.
Conclusions
The dose ratio of the sigmoid colon to HR-CTV D90 was decreased by introducing a hydrogel spacer into the meso-sigmoid. In cases where the sigmoid colon is in proximity to the cervical tumor, this novel technique can be considered to achieve better clinical outcomes.
Keywords: brachytherapy, spacer, hydrogel spacer, gynecologic brachytherapy, sigmoid colon protection
Purpose
High-dose-rate interstitial brachytherapy (HDR-ISBT) is an effective treatment modality in the field of female pelvic malignancies to control lesions and protect organs at risk (OARs). Although acute and late gastrointestinal (GI) complications are a major concern during and after radiation therapy (RT), perforation of the sigmoid colon is not well-known compared with other GI complications, such as proctitis and enteritis, because it has been rarely reported. However, in cases where the sigmoid colon is proximal to the uterine cervix, radiation exposure to the sigmoid from HDR-ISBT can be high. In such circumstances, artificial ascites injection, a treatment choice we previously proposed [1, 2], could be used to float the sigmoid colon and maintain a distance from gross tumor volume (GTV) to the sigmoid colon. However, in some patients, the fluid is re-distributed within the peritoneal cavity in a short time, and therefore, despite injecting saline into peritoneal cavity, the liquid soon disappears. Consequently, the distance between GTV and sigmoid colon remains dangerously close during HDR-ISBT treatment.
Here, we presented a case of a patient injected with a hydrogel spacer into the meso-sigmoid to address the issue of artificial ascites mentioned above. The material remained in place for a longer duration, and protective features of the spacer endured. The hydrogel spacer in the meso-sigmoid could achieve a higher dose to the tumor with a lower dose to the sigmoid colon during HDR-ISBT treatment.
Material and methods
A 73-year-old female presented to the Department of Gynecology with a main complaint of irregular genital bleeding. Cervical cancer was diagnosed, and a biopsy revealed adenocarcinoma. The tumor was classified as T3b, indicating para-aortic and bilateral pelvic lymph node swelling by computed tomography (CT), placing it at stage IIIC2r according to the FIGO 2018 staging system.The patient underwent concurrent chemoradiotherapy (CCRT) and four sessions of brachytherapy.
CCRT
The patient received weekly cisplatin (40 mg/m2) combined with external beam radiation therapy (EBRT) and brachytherapy. EBRT consisted of 50 Gy in 25 fractions of whole pelvic radiation therapy (WPRT) using a 4-field box technique. After WPRT, HDR-ISBT was performed in 1-2 sessions per week.
HDR-ISBT
Due to large tumor size, a total of 24 Gy in 4 fractions to clinical target volume (CTV) was delivered using CT-based image-guided adaptive brachytherapy. EBRT was not administered on the same day as brachytherapy.
The patient was placed in lithotomy position after saddle block anesthesia or local anesthesia and intravenous sedation. Before inserting applicators guided by trans-rectal ultrasonography (TRUS), 19 G needle (disposable ultrasonography compatible puncture needles; Create Medic Co., Ltd., Kanagawa, Japan) was advanced to the recto-vaginal fossa and vesico-uterine fossa through posterior and anterior vaginal wall, respectively. The position of the needle tip was confirmed using sagittal image of TRUS [3, 4]. After final confirmation of the needle with axial image, hydrogel was injected into the spaces. Hydrogel consisted of five vials of Suvenyl (Chugai Pharmaceutical Co., Tokyo, Japan), 5 ml of saline, and 2 to 4 ml of contrast enhancement agent (Oiparomin 370; Fuji Pharmaceutical Company, Toyama, Japan). The gel was placed for every fraction, and it was absorbed within two to three days.
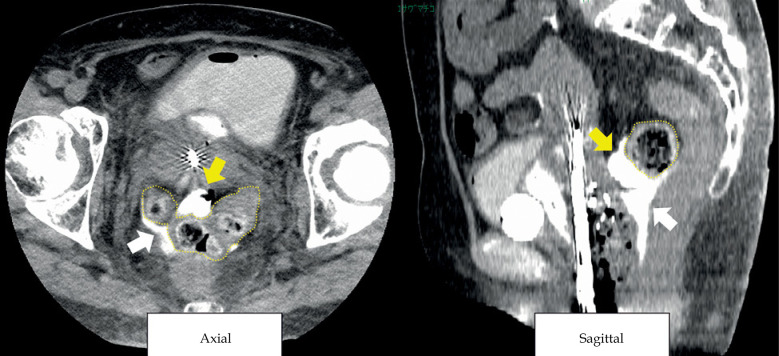
In the first brachytherapy fraction, tandem and ovoids were used as applicators. A part of the sigmoid colon was included in 100% isodose line of a high-risk clinical target volume (HR-CTV) due to its proximity to the cervix. The dose to the tumor was reduced to protect the sigmoid colon from high radiation exposure. In the second brachytherapy, a Rotte endometrial applicator (Elekta, Veenendaal, the Netherlands) was used due to uterine body involvement that could not be adequately covered by a tandem. A 500 ml saline was administered as artificial ascites trans-vaginally towards Douglas pouch to elevate the sigmoid colon and maintain a distance from GTV. However, CT planning revealed only a small amount of ascitic fluid around the sigmoid colon, and the distance between the sigmoid colon and the cervix did not change significantly. The injected fluid could have been re-distributed intra-peritoneally in a short time. The position of the sigmoid colon did not change during the infusion of fluid, suggesting adhesion of the sigmoid colon due to endometriosis or other factors. In the third brachytherapy session, a hydrogel spacer was injected into the meso-sigmoid through mid-transvaginal to create a space and protect the sigmoid colon. Under TRUS guidance, 5 ml of the hydrogel was injected into the meso-sigmoid through the pouch of Douglas after injecting a hydrogel spacer into the recto-vaginal fossa, avoiding penetration of the colon wall (Figure 1). After the insertion of transperineal free-hand interstitial needles and ICBT applicators, the Rotte endometrial applicator, a CT image of 2 mm slice thickness was taken using large-bore CT simulator (Aquilion™, Canon Medical Systems, Tochigi, Japan) situated in a brachytherapy suite with the patient lying in lithotomy position with applicators in place. The hydrogel spacer was confirmed to be positioned in the meso-sigmoid on CT images (Figure 2). The same techniques were applied in the fourth brachytherapy session. Before injecting hydrogel in the fourth fraction, no residual hydrogel from the third session was observed.
Fig. 1.
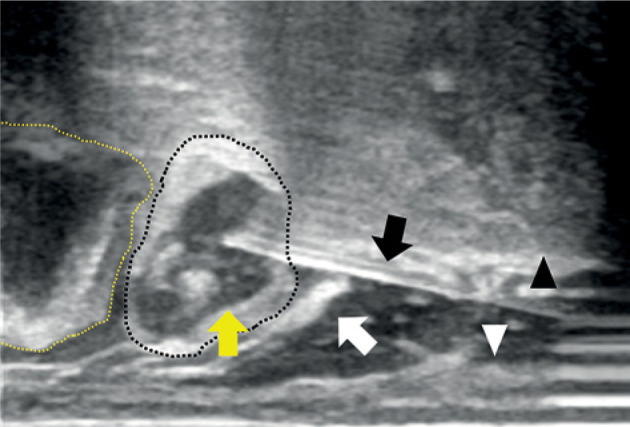
Trans-rectal ultrasound (TRUS) sagittal image of hydrogel (yellow arrow) injected into the meso-sigmoid (wavy black line) through pouch of Douglas (white arrow). Wavy yellow line – sigmoid colon, black arrow – needle, white arrowhead – rectal wall, and black arrowhead – uterine cervix
Fig. 2.
CT image of hydrogel (yellow arrow) injected into the meso-sigmoid. White arrow - hydrogel injected into pouch of Douglas, wavy yellow line – sigmoid colon
HR-CTV at HDR-ISBT was delineated on CT with applicators in place using magnetic resonance imaging (MRI) taken immediately before the first HDR-ISBT and TRUS performed during applicator placement as a reference. Treatment planning was executed with planning software Oncentra® (Elekta, Veenendaal, the Netherlands). Dose calculation of HDR-ISBT was based on CT. Doses were calculated for each brachytherapy session to achieve a total dose of 85 Gy for HR-CTV D90, including the four sessions and external radiation dose. This was done while adhering to dose constraints for OARs, as recommended by guidelines. All brachytherapy treatment was carried out using iridium-192 remote afterloading system (RALS, Flexitron HDR™, Elekta, Stockholm, Sweden). No statistical analysis was conducted, as this study exclusively focused on a single patient.
Results
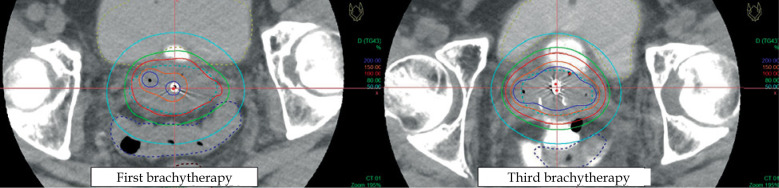
The dose evaluation of each brachytherapy physical dose and equivalent dose in 2 Gy fractions (EQD2) for CTV and OAR doses, calculated based on linear-quadratic model, is shown in Table 1. In comparison with the first brachytherapy session, the dose administered to the sigmoid colon was reduced in the subsequent three sessions of brachytherapy (Figure 3). The dose ratio of the sigmoid colon D2cc (physical dose) to HR-CTV D90 (physical dose) also decreased from the second to fourth brachytherapy sessions compared with the first one. The spacer allowed dose escalation to HR-CTV whilst achieving acceptable sigmoid dose.
Table 1.
Dose evaluation
| Fraction | HR-CTV V100% (%) | HR-CTV D90 (PD/ED10) (Gy) |
Sigmoid colon D2cc (PD/ED3) (Gy) |
Rectum D2cc (PD/ED3) (Gy) |
Bladder D2cc (PD/ED3) (Gy) |
Small bowel D2cc (PD/ED3) (Gy) | Sigmoid colon D2cc (PD)/ HR-CTV D90 (PD) |
|---|---|---|---|---|---|---|---|
| 1 | 76.3 | 4.2/4.9 | 4.3/6.3 | 3.6/4.8 | 5.0/7.9 | 2.1/2.1 | 1.03 |
| 2 | 91.4 | 6.1/8.2 | 2.7/3.0 | 1.8/1.7 | 3.4/4.4 | 2.4/2.6 | 0.43 |
| 3 | 96.7 | 7.4/10.7 | 4.1/5.8 | 1.5/1.4 | 3.7/5.0 | 3.7/4.9 | 0.56 |
| 4 | 96.2 | 7.0/9.9 | 3.3/4.2 | 1.7/1.6 | 3.1/3.9 | 0.0/0.0 | 0.47 |
| Sub-total | 24.7/33.7 | 13.8/19.3 | 8.6/9.5 | 15.2/21.2 | 8.2/9.6 | ||
| Total | 74.7/83.7 | 63.8/69.3 | 58.6/59.5 | 65.2/71.2 | 58.2/59.7 |
PD – physical dose, ED10 – EQD2 dose, α/β = 10 Gy, ED3 – EQD2 dose, α/β = 3 Gy, sub-total is the sum of dose in each brachytherapy. Total is the sum of doses of external beam radiation therapy and brachytherapy.
Fig. 3.
Dose distribution of the first and third brachytherapy sessions. Hydrogel in the meso-sigmoid was used in the third and fourth brachytherapy fractions
In the second brachytherapy session, the patient received only 6 Gy to HR-CTV D90 despite low-dose delivered to the sigmoid colon. This was because the boundary between the injected artificial ascites and the sigmoid colon was difficult to identify, and HR-CTV dose was kept to a minimum in order to avoid delivering a high-dose to the sigmoid colon due to an inadequate assessment of dose in the sigmoid colon. The capacity to deliver high doses to HR-CTV in the third and fourth brachytherapy sessions brought the total dose from external irradiation and the four brachytherapy session much closer to the intended dose. No severe complication was associated with the spacer injection procedure. One month after completion of radiotherapy, the patient did not report any pelvic symptoms.
Discussion
The efficacy of hydrogel spacers has been well-established in prostate radiotherapy [5], and has been extended to female pelvic malignancies [3, 4, 6-11], although its application remains limited compared with prostate radiotherapy. As the rectum and uterine cervix are in proximity, a hydrogel spacer is placed in the recto-vaginal fossa to decrease rectal dose. However, when the sigmoid colon is adjacent to the cervix, hydrogel spacers into the recto-vaginal fossa are ineffective in safeguarding the sigmoid colon, because it is located in the peritoneal cavity. While the use of solid spacers, such as mesh or polyglycolic acid spacers (Neskeep®, Alfresa Pharma Corporation, Osaka, Japan) [12-15] between tumor and sigmoid colon can be beneficial, this method requires open abdominal surgery, and is much more invasive. Another technique involves trans-vaginally injected artificial ascites into the peritoneal cavity to float the sigmoid colon and create separation from tumor [1, 2]. Nevertheless, the effectiveness of artificial ascites varies among patients due to increased fluid absorption within the peritoneal cavity in a short time, supposedly because of peritoneal inflammation. In the current case, artificial ascites was applied, but they were unable to float the sigmoid colon for a meaningful period. Therefore, we attempted to protect the sigmoid colon by injecting a hydrogel spacer into the meso-sigmoid. This spacer did not spread laterally in the meso-sigmoid, but only in the direction of needle penetration (antero-posterior), thereby increasing the distance to the cervix even with a small amount remained in place much longer than artificial ascites. The slow absorption of hyaluronic acid, Suvenyl, likely contributed to this prolonged stability, as it has a high weight-average molecular weight of 2,700 kDa [16], and is highly viscous. We achieved a high-dose delivery to CTV while ensuring a safe dose to the sigmoid colon using the spacer in the meso-sigmoid, resulting in the total dose nearly reaching the target. To our knowledge, this case report represents the first instance of using a spacer in the meso-sigmoid.
This report includes only one patient, and presents the concept and technique of avoiding radiation exposure to the sigmoid colon. However, not all patients may be eligible for this technique. For example, applying this technique to a thin individual may be challenging due confined peritoneal cavity space and thin meso-sigmoid, posing a higher risk of injuring the sigmoid colon wall during needle insertion. It is also necessary to ensure no direct tumor infiltration in the needle route and mesentery of the sigmoid colon to be injected to avoid dissemination. In the case presented here, although sigmoid colon adhesion was suspected at the left parametrial infiltration, no direct tumor infiltration in the needle route and the meso-sigmoid was confirmed through gynecological examination, TRUS, CT, and MRI findings.
Complications or adverse effects related to this technique require further exploration, including peritoneal dissemination rate, iatrogenic implantation, bowel injury, bleeding, and infections.
While we applied this novel technique for definitive radiotherapy for uterine cervical cancer patients, it can also be employed in post-hysterectomy vaginal recurrence, or re-irradiation settings. Future investigation will focus on examining the late effects of the sigmoid colon, and determining the specific patient population and conditions for optimal application of this technique to minimize late effects.
Conclusions
The dose ratio of the sigmoid colon D2cc to HR-CTV D90 was reduced by injecting a hydrogel spacer into the meso-sigmoid while achieving dose escalation to HR-CTV D90. In cases where the sigmoid colon is in proximity to the cervical tumor, this spacer provides an opportunity to improve HR-CTV D90 doses.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (No.: 23K07171) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
We would also like to thank all of the staff involved in radiotherapy.
Disclosure
There is no conflict of interest to declare, except for the following:
Dr. Murakami reports grants from Chiyoda Technol Corporation and Boston Scientific outside the submitted work.
Dr. Kashihara reports grants from the Foundation for Promotion of Cancer Research in Japan outside the submitted work.
Dr. Okamoto reports grants from Accuray and Item Corporation outside the submitted work.
Dr. Igaki reports grants from Elekta KK and personal fees from VARIAN outside the submitted work.
References
- 1.Karube M, Murakami N, Okamoto Het al. Transvaginal artificial ascites infusion as a spacer in gynecological brachytherapy: a novel technique. J Contemp Brachytherapy 2020; 12: 487-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami N, Shima S, Okuma Ket al. Artificial ascites for organs at risk sparing in intrapelvic brachytherapy: a case report of recurrent uterine cervical carcinoma adjacent to the bowel. BJR Case Rep 2019; 5: 20180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami N, Nakamura S, Kashihara Tet al. Hyaluronic acid gel injection in rectovaginal septum reduced incidence of rectal bleeding in brachytherapy for gynecological malignancies. Brachytherapy 2020; 19: 154-161. [DOI] [PubMed] [Google Scholar]
- 4.Kashihara T, Murakami N, Tselis Net al. Hyaluronate gel injection for rectum dose reduction in gynecologic high-dose-rate brachytherapy: initial Japanese experience. J Radiat Res 2019; 60: 501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariados N, Sylvester J, Shah Det al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2015; 92: 971-977. [DOI] [PubMed] [Google Scholar]
- 6.Murakami N, Okuma K, Kato T, Igaki H. Now is it time to implement spacers in cervical cancer brachytherapy? J Radiat Res 2022; 63: 696-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi R, Murakami N, Chiba Tet al. Effect of hyaluronate acid injection on dose-volume parameters in brachytherapy for cervical cancer. Adv Radiat Oncol 2022; 7: 100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iijima K, Murakami N, Nakamura Set al. Configuration analysis of the injection position and shape of the gel spacer in gynecologic brachytherapy. Brachytherapy 2021; 20: 95-103. [DOI] [PubMed] [Google Scholar]
- 9.Murakami N, Shima S, Kashihara Tet al. Hyaluronic gel injection into the vesicovaginal septum for high-dose-rate brachytherapy of uterine cervical cancer: an effective approach for bladder dose reduction. J Contemp Brachytherapy 2019; 11: 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viswanathan AN, Damato AL, Nguyen PL. Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol 2013; 31: e446–e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishi K, Mabuchi Y, Sonomura Tet al. Eradicative brachytherapy with hyaluronate gel injection into pararectal space in treatment of bulky vaginal stump recurrence of uterine cancer. J Radiat Res 2012; 53: 601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyata Y, Murakami N, Okuma Ket al. Technical Note: High-dose-rate interstitial brachytherapy for pelvic sidewall recurrence using intraperitoneal spacers. Adv Radiat Oncol 2022; 8: 101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akasaka H, Sasaki R, Miyawaki Det al. Preclinical evaluation of bioabsorbable polyglycolic acid spacer for particle therapy. Int J Radiat Oncol Biol Phys 2014; 90: 1177-1185. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki R, Demizu Y, Yamashita Tet al. First-in-human phase 1 study of a nonwoven fabric bioabsorbable spacer for particle therapy: Space-making particle therapy (SMPT). Adv Radiat Oncol 2019; 4: 729-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serizawa I, Kusano Y, Kano Ket al. Three cases of retroperitoneal sarcoma in which bioabsorbable spacers (bioabsorbable polyglycolic acid spacers) were inserted prior to carbon ion radiotherapy. J Radiat Res 2022; 63: 296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano T, Takahashi Y. Hyaluronan for the treatment of osteoarthritis and rheumatoid arthritis. In: Arthritis: Pathophysiology, Prevention, and Therapeutics. 2016. 10.1201/b10852-18. [DOI] [Google Scholar]