Abstract
The hypothalamus is part of the diencephalon and has several nuclei, one of which is the arcuate nucleus. The arcuate nucleus of hypothalamus (ARH) consists of neuroendocrine neurons and centrally-projecting neurons. The ARH is the center where the homeostasis of nutrition/metabolism and reproduction are maintained. As such, dysfunction of the ARH can lead to disorders of nutrition/metabolism and reproduction. Here, we review various types of neurons in the ARH and several genetic disorders caused by mutations in the ARH.
Keywords: Arcuate nucleus, Hypothalamus, Metabolic disease, Central nervous system disease, Obesity
INTRODUCTION
The hypothalamus is a component of the diencephalon located inferior to the thalamus and superior to the midbrain. It serves as the highest regulator of the autonomic nervous system and plays a crucial role in maintaining glucose homeostasis and regulating the secretion of insulin, glucagon and various hormones. Although Claudius Galen in the second century and Andreas Vesalius in the 16th century described the brain region corresponding to the part of the hypothalamus, it was Wilhelm His who coined the term “hypothalamus” in 1893. The hypothalamus has several nuclei, which are aggregations of neurons: paraventricular nucleus (PVH), ventromedial nucleus (VMH), dorsomedial nucleus (DMH), preoptic nucleus, supraoptic nucleus, suprachiasmatic nucleus, lateral hypothalamic area (LHA) and arcuate nucleus. These hypothalamic nuclei are connected to each other and various surrounding brain regions, regulating the secretion of various peptides and neurotransmitters. The arcuate nucleus is also referred to as the infundibular nucleus or the arcuate nucleus of the hypothalamus (ARH) to distinguish it from another arcuate nucleus in the medulla oblongata (MO). The ARH was first described as nucleus infundibularis in 1948 by Hugo Spatz and colleagues, and is located in the mediobasal hypothalamus, adjacent to the third ventricle and the median eminence (ME) [1-4].
The ARH consists of various neurons that have diverse physiological roles ranging from cardiovascular regulation, feeding, energy expenditure, and fertility to metabolism. These neurons can be classified into two groups: neuroendocrine neurons and centrally-projecting neurons, which are not mutually exclusive. The neuroendocrine neurons release various neurotransmitters and/or neuropeptides, such as neuropeptide Y (NPY), agouti-related peptide (AgRP), cocaine- and amphetamine-regulated transcript (CART), dopamine, gonadotropin-releasing hormone (GnRH), growth hormone–releasing hormone (GHRH), kisspeptin (Kiss1), neurokinin B (NKB), dynorphin A, proopiomelanocortin (POMC) and substance P (SP). The centrally-projecting neurons transmit information to other hypothalamic nuclei or other brain regions outside the hypothalamus [2, 5-7].
This review describes the physiological and molecular functions and genetic disorders of various neurons in the ARH.
NEUROENDOCRINE NEURONS
NPY-expressing neurons
NPY, a 36-amino-acid orexigenic neuropeptide [8], was first identified from extracts of porcine brains without the cerebellum and pituitary gland by Tatemoto et al. [9]. In 1984, Clark and colleagues reported that intraventricular administration of NPY to ovariectomized rats pretreated with estradiol benzoate plus progesterone stimulated feeding behavior [10] (Table 1). Intraventricular infusion of NPY suppresses pulsatile GH release in rats [11-13]. However, genetic ablation of NPY in mice does not alter food intake and body weight, suggesting a functional redundancy of NPY [14, 15].
Table 1.
Types of neurons in the ARH
| Types of neurons | References numbers | |
|---|---|---|
| Neuroendocrine neurons in ARH | NPY-expressing neurons | [8-10] |
| AgRP-expressing neurons | [16-18] | |
| CART-expressing neurons | [37-39] | |
| Dopamine-expressing neurons | [47-50] | |
| GnRH-expressing neurons | [55-59] | |
| GHRH-expressing neurons | [68-75] | |
| Kiss1-expressing neurons | [79, 80, 82-86] | |
| NKB-expressing neurons | [93-98] | |
| Dynorphin A-expressing neurons | [99-102] | |
| POMC-expressing neurons | [105-109] | |
| SP-expressing neurons | [117-125] | |
| Centrally-projecting neurons in ARH | ARH neurons projecting to the PVH | [16, 51, 126-129] |
| ARH neurons projecting to the LHA | [132, 133] | |
| ARH neurons projecting to the DMH | [127, 134] | |
| ARH neurons projecting to the aBNST | [133, 135] | |
| ARH neurons projecting to the PVT | [136] | |
| ARH neurons projecting to the CEA | [24, 137-139] | |
| ARH neurons projecting to the PAG | [142] | |
| ARH neurons projecting to the PBN | [145, 146] | |
| ARH neurons projecting to the VTA | [147] | |
| ARH neurons projecting to the nucleus raphe obscurus | [148] | |
| ARH neurons projecting to the NTS | [148] | |
| ARH neurons projecting to the NAc | [149-151] | |
| Projections from the PVHTRH/PACAP+ to the ARHAgRP+ neurons | [152] | |
| Projections from the vDMH to the ARHAgRP+ neurons | [153] | |
| Projections from the NTS to the ARHPOMC+ neurons | [154] |
AgRP-expressing neurons
In 1997, the Barsh group showed that AgRP is a selective antagonist of the melanocortin receptors MC3R and MC4R and that transgenic mice expressing human AgRP develop obesity [16]. AgRP is an orexigenic peptide consisting of 132 amino acids – its mature form has 112 amino acids [17]. The Schwartz group demonstrated in 1998 that NPY and AgRP are co-expressed in fasting-activated ARH neurons [18]. ARH neurons expressing NPY/AgRP (ARHNPY/AgRP+) are GABAergic [19, 20]. They are activated by ghrelin [21, 22], and inactivated by leptin [19, 23], insulin, and glucose in the blood [24, 25], thus regulating energy balance and food intake [24-27]. Knockout of AgRP in mice show normal food intake and body weight, implying its functional redundancy [15], and have an extended life span with their point estimate of median survival exceeding that of their littermates by 9.8% [28].
Optogenetic activation of AgRP neurons in mice triggers voracious feeding within minutes [29]. In addition, chemical activation of these neurons in mice evoked food consumption, decreased energy expenditure, and enhanced fat stores [30]. Either ablation or suppression of ARHAgRP+ neurons causes aphagia [17, 30-32]. In vivo Ca2+ imaging using GCaMP6s has shown that sensory detection of food inhibits mice AgRP neurons very rapidly [33]. The Knight group showed that food intake stimulates mechanoreceptors in the intestinal vagal sensory neurons, which in turn inhibits ARHAgRP+ neurons [34]. The Horvath, Nitsch, and Vogt groups proposed that fasting evoked activation of ARHAgRP+ neurons elevates lysophosphatidic acid (LPA) species in the blood and cerebrospinal fluid, which subsequently elevates cortical excitability leading to hyperphagia [35]. The Anderman group showed that preemptive photostimulation of AgRP neurons in a home cage, but not in a threat-containing task arena, induces conditioned food seeking under threat [36].
CART-expressing neurons
The Douglass group identified in the rat brain an mRNA that was induced four- to fivefold by the administration of cocaine or amphetamine and named it CART in 1995. Human proCART has 89 amino acids. ARH neurons express CART [37], which functions as an anorexigenic peptide [38, 39]. Mice lacking CART exhibit an obesity phenotype [40, 41]. CART is also implicated in immunity, fluid balance, reproduction, learning and memory, sleep, stress, addiction and depression [42, 43]. G-protein coupled receptor (GPR) 160 was reported as a CART receptor [44, 45]. Single-cell RNA sequencing revealed that of three POMC clusters, ARHCART+ neurons overlap most abundantly with POMC/Anxa2 cluster neurons, yet showed little to no overlap with POMC/Ttr cluster and POMC/Glipr1 cluster neurons [46].
Dopamine-expressing neurons
In the 1960s and 1970s, dopamine released from tuberoinfundibular dopaminergic (TIDA) neurons in the ARH was reported to regulate prolactin secretion [47-50]. The Murakami group found that Neuromedin U inhibits prolactin secretion via activation of TIDA neurons [51]. Zufall, Leinders-Zufall and colleagues demonstrated that deletion of transient receptor potential (TRP) channel Trpc5 enhances dopamine release from TIDA neurons, which in turn causes hypoprolactinemia [52]. Approximately half of TIDA neurons are GABAergic and do not participate in the regulation of prolactin secretion [53].
Optogenetic activation of mouse ARH neurons expressing tyrosine hydroxylase (TH) suppresses POMC neurons and induces hyperphagia. TH is an enzyme that converts L-tyrosine to L-DOPA, a precursor of dopamine. Conversely, ablation of ARHTH+ neurons decreases body weight [54].
GnRH-expressing neurons
GnRH, a decaneuropeptide, stimulates the biosynthesis and release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary gland. The primary structure of mammalian GnRH was characterized in the early 1970s [55, 56]. In mammals, GnRH-expressing neurons originate in the developing olfactory pit, and migrate towards the hypothalamus, encompassing the primarily preoptic area and ARH, during early embryogenesis [57-59]. Estradiol feedback and Kiss1 modulate the excitability of ARHGnRH+ neurons [60]. ARH neurons that co-express Kiss1, NKB and dynorphin A (dubbed KNDy neurons) act in coordination to facilitate pulsatile secretion of GnRH, which is critical to reproductive endocrine function [61-64]. Mice carrying homozygous null GnRH mutation show hypogonadotropic hypogonadism [65-67].
GHRH-expressing neurons
GHRH, a 44-amino acid peptide hormone, binds to the its cognate receptor in the anterior pituitary gland, thereby stimulating the secretion of growth hormone (GH) [68-70]. The primary structure of human GHRH was elucidated in 1982 [71, 72]. In mammals, GHRH is synthesized in ARH neurons [73-75]. The knockout of GHRH in mice results in reduced body weight, increased insulin sensitivity and a prolonged lifespan compared to their littermates [76-78].
Kiss1-expressing neurons
Initially identified as a protein that suppresses the metastasis of human malignant melanoma [79], Kiss1 is synthesized in hypothalamic nuclei including the ARH [80]. Kiss1+ neurons exhibit electrophysiological properties characteristic of pacemaker neurons [81], and synthesize NKB and dynorphin as well [82]. Upon binding to its cognate receptor, GPR54 (also known as Kiss1 receptor [Kiss1R]), Kiss1 stimulates GnRH neurons triggering pulsatile GnRH release into the portal circulation. This induces the secretion of LH and FSH from the anterior pituitary gland [83-86]. Prolactin binds to prolactin receptors in ARHKiss+ neurons and thus decreases Kiss1 expression in female rats, leading to suppression of LH secretion and subsequent infertility [87].
Mice with a Kiss1 hypomorph mutation exhibit sexually dimorphic reproductive phenotypes: male mutants are fertile, whereas female mutants show impaired fertility and ovulation [88, 89]. The genetic ablation of Kiss1+ neurons results in fertile mice with smaller ovaries compared to their littermates, with no impact on the timing of female puberty onset [90]. ARH-specific deletion of Kiss1 leads to arrested folliculogenesis, hypogonadism and infertility in female mice, and hypogonadism, and variable, defective spermatogenesis, and subfertility in male mice [91]. Furthermore, ARHKiss1+ neurons are a necessary component of the hypothalamic circadian oscillator circuit [92].
NKB-expressing neurons
NKB, identified as a decaneuropeptide in 1983 [93-95], is generated through the proteolytic cleavage of a preproprotein encoded by TAC3. Its primary receptor is neurokinin 3 receptor (NK3R), also known as tachykinin receptor 3 (TACR3), which is a GPCR. TACR3 is not only found in the central nervous system (CNS), but also in the uterus, mesenteric vein, gut neurons, and placenta [96, 97]. Upon binding to NK3R, NKB stimulates GnRH neurons to release GnRH into the portal circulation [98].
Dynorphin A-expressing neurons
Dynorphin A is a potent opioid peptide consisting of 13 amino acids and its amino acid sequence was determined by the Avram Goldstein laboratory in 1981 [99, 100]. It activates the κ-opioid receptor (KOR), which is expressed throughout the brain and spinal cord [101], resulting in the modulation of pain, addiction and mood [102]. In mice lacking dynorphin, corticotropin releasing factor is unable to activate KOR in the basolateral amygdala, dorsal hippocampus and bed nucleus of the stria terminalis (BNST), all of which are brain regions associated with fear and anxiety [103, 104].
POMC-expressing neurons
Proteolytic cleavage of POMC results in the formation of various biologically active peptides including adrenocorticotropic hormone (ACTH), N-POMC, β–endorphin, α-, β– and γ-melanocyte-stimulating hormones (MSH). While POMC is primarily synthesized in the anterior pituitary, some ARH neurons also express POMC. Once secreted, POMC undergoes cleavage into α-MSH that binds to MC3/4R in the PVH neurons, thereby activating satiety signals [105-107]. POMC neurons regulate food intake and energy expenditure by responding to circulating blood glucose levels [108, 109]. Optogenetic activation of POMC neurons in mice has reduces food intake and body weight [29]. In vivo Ca2+ imaging using GCaMP6s revealed that food presentation to fasted mice activates POMC neurons very rapidly [33]. Glucagon-like peptide-1 (GLP-1) binds to the GLP-1 receptor in ARHPOMC+ neurons, thus suppressing food intake [110-112]. The Horvath group reported that activation of cannabinoid receptor 1 (CB1R) in the presynaptic terminals of POMC neurons triggers β–endorphin release and drives feeding [113]. The Yu group discovered that β–endorphin in the ARH contributes to antinociception in rats with inflammation [114]. The Low group observed that conditional knockout of POMC in the mouse ARH elicited hyperphagia, insulin resistance, obesity and improved glucose tolerance [115, 116].
SP-expressing neurons
SP was named by Gaddum and Schild [117] in 1934 and its 11-amino acid long sequence was determined in 1971 [118]. This neurokinin peptide is encoded by preprotachykinin A (PPTA or Tac1). PPTA also generates neurokinin A through alternate slicing [119]. SP is widely expressed in the brain including the ARH [120] and is implicated in nociception, respiration, inflammation, thermoregulation, the cardiovascular function and emotional and anxiety-related behaviors [121-123]. Glutamate induces SP release from the ARH and ME, thereby stimulating the secretion of gonadotropins [124]. The expression of SP and its receptor in the ARH peaks before mice puberty, and SP-/- mice exhibit delayed puberty and female subfertility [125].
CENTRALLY-PROJECTING NEURONS
The ARH receives signals from various sources, including hormonal and nutrient signals through the ME, afferent inputs from the vagus nerve and other brain nuclei, coordinates them and sends feedback responses via centrally-projecting neurons.
ARH neurons projecting to the PVH
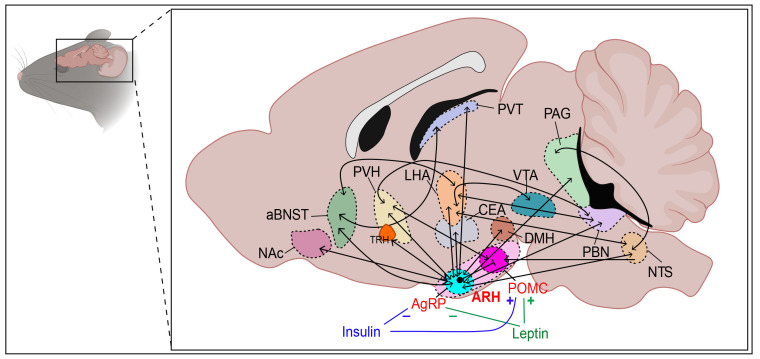
ARH neurons expressing orexigenic (appetite-stimulating) peptides AgRP and NPY project to the PVH, where they bind to MC3/4R and NPY Y1 receptor (NPY1R), respectively. The binding of AgRP to MC3/4R, a receptor for α-MSH, suppresses the anorexigenic effect of α-MSH in the PVH [16]. In addition, the binding of NPY to NPY1R activates GABAergic neurons in the intermediate and parvicellular reticular nuclei of the MO possibly via the nucleus tractus solitarius (NTS) in the MO. This results in the stimulation of feeding behavior through the activation of the masticatory motor region and a decrease in energy expenditure via reduced sympathetic output to the brown adipose tissue (BAT) thermogenesis [51, 126-128].
Upon nutrient ingestion, ARH neurons expressing POMC (ARHPOMC+) that project to the PVH release α-MSH, activating MC4R on PVH neurons (Fig. 1). As a result, food intake is suppressed [129, 130]. ARH neurons expressing TH project to the PVH and the optogenetic activation of ARHTH+ axons releases both dopamine and GABA, thus inhibiting PVH neurons [54]. Projections from ARHAgRP/NPY+ to PVH can be remodeled both morphologically and functionally by fasting in mice [131].
Fig. 1.
Schematic illustration of projections to and from ARH neurons. aBNST, anterior bed nucleus of the stria terminalis; ARH, arcuate nucleus of hypothalamus; CEA, central nucleus of the amygdala; DMH, dorsomedial nucleus; LHA, lateral hypothalamic area; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; PAG, periaqueductal gray; PBN, parabrachial nucleus; POMC, proopiomelanocortin; PVH, paraventricular nucleus; PVT, paraventricular thalamic nucleus; TRH, thyrotropin-releasing hormone; VTA, ventral tegmental area.
ARH neurons projecting to the LHA
ARH neurons expressing both AgRP and NPY project to the LHA [132], and optogenetic stimulation of this projection can evoke feeding behavior in mice [133].
ARH neurons projecting to the DMH
ARHKiss1+ neurons project to DMH neurons. When activated optogenetically, they release glutamate, thus regulating energy expenditure in female mice [134]. Moreover, ARHNPY+ neurons project to DMH neurons expressing NPY1R. These in turn project to the nucleus raphe pallidus, resulting in the inhibition of sympathetic outputs for BAT thermogenesis, mean arterial pressure, and heart rate [127].
ARH neurons projecting to the anterior BNST (aBNST)
ARHAgRP+ neurons project to two distinct parts of the aBNST: the dorsomedial part (aBNSTdm) and the ventrolateral part (aBNSTvl). ARHAgRP+ projections to the aBNSTdm and aBNSTvl induce feeding and peripheral insulin resistance, respectively [133, 135].
ARH neurons projecting to the paraventricular thalamic nucleus (PVT)
ARHNPY+ and ARHCART+ neurons project to the PVT. As NPY and CART are orexigenic and anorexigenic, respectively, these two antagonistic circuits suggest that the PVT may integrate orexigenic and anorexigenic inputs [136].
ARH neurons projecting to the central nucleus of the amygdala (CEA)
The amygdala is involved in emotional responses including fear, anxiety, and aggression as well as the regulation of energy balance [137, 138]. Reciprocal projections exist between ARHNPY+/CART+ neurons and CEA neurons [24, 139]. Infusion of insulin into the CEA increases the immunoreactivity of c-Fos, a neuronal activity marker, in the ARH, suggesting that insulin mediates anorexia via this circuit [137, 140]. Alcohol activates ARHPOMC+ neurons projecting primarily to the amygdala, which may be implicated in rewarding effect responsible for alcohol use disorders [141].
ARH neurons projecting to the periaqueductal gray (PAG) in the midbrain
Retrograde labeling has revealed the projection of POMC neurons in the ARH to the PAG [142]. During electroacupuncture, glutamatergic reciprocal projections between ARH neurons and ventrolateral PAG neurons become activated [143]. Galanin activates projections from ARHβ–endorphin+ neurons to PAG neurons, thereby triggering anti-nociceptive effects [144].
ARH neurons projecting to the parabrachial nucleus (PBN)
ARH neurons co-expressing AgRP, NPY and GABA project to the PBN in the pons, thus inhibiting the GABAA receptor in neurons expressing calcitonin gene-related peptide (CGRP). This inhibition delays meal termination [145, 146].
ARH neurons projecting to the ventral tegmental area (VTA)
ARHPOMC+ neurons project to the VTA, inhibiting dopamine neurons in this region. In mice under chronic restraint stress, optogenetic inhibition of this circuit increases body weight and food intake, and suppresses depression-like behaviors and anhedonia [147].
ARH neurons projecting to the nucleus raphe obscurus
Optogenetic stimulation of aBNST neurons expressing glutamate decarboxylase 2 (aBNSTGad2+) in mice activates the nucleus raphe obscurus in the MO via projections from ARHGad2+ neurons, thereby mobilizing glucose rapidly [148].
ARH neurons projecting to the NTS
Activation of ARHGad2+ neurons through projections from aBNSTGad2+ neurons in mice stimulates projections to the NTS, eliciting anxiety-like behavior [148].
ARH neurons projecting to the nucleus accumbens (NAc)
ARHβ-endorphin+ neurons project to the GABAergic neurons in the NAc, which are associated with ethanol reinforcement [149, 150]. Chang et al. [151] demonstrated that acupuncture stimulates this projection and thus attenuates alcohol dependence in rats.
Projections from the PVHTRH/PACAP+ to the ARHAgRP+ neurons
PVH afferent neurons expressing thyrotropin-releasing hormone (TRH) and pituitary adenylate cyclase-activating polypeptide (PACAP), provide excitatory input to ARHAgRP+ neurons, thereby inducing intense feeding [152].
Projections from the ventral compartment of the DMH (vDMH) to the ARHAgRP+ neurons
In the fasted state, food detection rapidly activates GABAergic vDMHLepR/pDYN+ → ARHAgRP+ neurons, which in turn inhibit ARHAgRP+ neurons [153].
Projections from the NTS to the ARHPOMC+ neurons
GLP-1 neurons in the NTS project to ARHPOMC+ neurons expressing GLP-1R. This circuit is involved in the suppression of food intake [154].
ARH ASSOCIATED GENETIC DISEASES
Hypothalamic obesity syndrome (HOS)
Obesity, a chronic inflammation disorder, is associated with diverse diseases such as cardiovascular diseases, type 2 diabetes mellitus, certain types of cancer, and CNS diseases [155]. Characterized by overweight and disrupted energy homeostasis, obesity results from an imbalance between energy storage and expenditure, and excessive food intake [156]. The hypothalamus is the main brain region controlling energy homeostasis [157]. Specifically, neurons in the ARH including ARHAgRP/NPY+, ARHPOMC+ and ARHCART+ play a critical role in energy homeostasis [158]. In obese condition, hyperglycemia and insulin resistance can lead to hypothalamic inflammation, POMC neuronal loss and microglia activation in the ARH [159, 160]. While various factors including anatomic lesions, can caused HOS [161, 162], here we focus solely on genetic causes in the ARH.
Leptin, an anorexigenic hormone, is primarily produced in adipose cells and binds to its cognate receptor, the leptin receptor. This binding in AgRP/NPY neurons reduces expression and release of AgRP and NPY. However, this binding in CART/POMC neurons increases expression and release of CART and POMC. As a result, appetite reduction is suppressed, and locomotion, thermogenesis, and lipolysis are enhanced [163, 164]. Mutations in the leptin receptor are known to cause HOS [165, 166].
MC4R mutations negate the satiating effect of α-MSH in the ARH, and thus elicit hyperphagia and a higher satiety threshold. Once stimulated by leptin, ARHPOMC+ neurons produce α-MSH, which in turn binds to MC4R in PVH neurons. These mutations are the most common cause of monogenic obesity [167-170].
A heterozygous missense mutation in the CART gene (Leu34Phe) in ARHCART+ neurons is responsible for obesity with a reduced metabolic rate [171, 172].
Homozygous or compound heterozygous mutations in the POMC gene in ARHPOMC+ neurons cause early-onset obesity, hyperphagia, blunted satiety, secondary adrenal insufficiency, and pigmentary changes [173, 174].
Prohormone convertase 1 (PC1, also referred to as proprotein convertase subtilisin/kexin type 1 [PCSK1]) splices POMC in ARHPOMC+ neurons to liberate various biologically active peptides including ACTH and α-MSH. Compound heterozygous mutations in PC1 lead to early-onset obesity, hypoadrenalism, and reactive hypoglycemia [175, 176].
Age-related progressive weight gain typically develops in middle age, which is followed by anorexia (sarcopenia and/or cachexia) in old age. This phenomenon is primarily linked to a decrease and increase in ARHPOMC+ neuronal tone in middle age and old age, respectively [177-179].
Neurogenin 3 (Ngn3 or Neurog3) is a basic helix-loop-helix transcription factor implicated in the development of pancreatic β-cells and the hypothalamus. The conditional knockout of hypothalamic Ngn3 in mice elicits hyperphagia and reduced energy expenditure leading to obesity. This is primarily due to a decrease in the number of ARHPOMC+ neurons and an increase in the number of ARHNPY+ neurons [180, 181].
Nescient helix-loop-helix 2 (NHLH2) is a transcription factor that promotes the transcription of PC1/3 [182], and is a downstream target gene of leptin signaling [183]. Mutations in NHLH2 are associated with obesity [184-186].
Islet 1 (ISL1), a LIM-homeodomain transcription factor, augments expression of POMC, thereby promoting the terminal differentiation of ARHPOMC+ neurons in the developing hypothalamus. Conditional ISL1 knockout in mice ARHPOMC+ neurons induces hyperphagia and obesity [187, 188].
Tubby (Tub) is expressed in the human hypothalamus including the ARH and adipose tissue. Homozygous mutations in human Tub are associated with retinal dystrophy and early-onset obesity. However, the molecular function of Tub is still under debate [189, 190].
Orthopedia homeobox (Otp), a homeodomain transcription factor, is expressed in several hypothalamic nuclei including the ARH, and plays an important role in the development of hypothalamic neuroendocrine cell lineages in mice. Heterozygous missense mutations in Otp results in obesity, glucose intolerance and anxious behavior in mice [31, 191].
Given that obesity can ensue from various genetic mutations in the ARH neurons, investigation of genes associated with HOS followed by editing of a causative mutation(s) may offer a way to alleviate obesity.
Puberty and sexual maturation disorders
Homozygous or compound heterozygous mutations in Kiss1 or Kiss1R (also called GPR54) lead to hypogonadotropic hypogonadism, resulting in pubertal failure [85, 192-195]. Activating mutations in Kiss1 or Kiss1R cause central precocious puberty (CPP) [194, 196]. Mutations in NHLH2 also lead to hypogonadotropic hypogonadism [185, 186]. Furthermore, homozygous mutations in TAC3 or TACR3 elicit hypogonadotropic hypogonadism [197-200]. Loss-of-function mutations in the makorin ring finger protein 3 (MKRN3) and deletion mutations in the delta-like 1 homolog (DLK1) result in CPP [201, 202].
CONCLUSION
Various types of ARH neurons, their neuropeptides, and their projections play an important role in the regulation of nutrition/metabolism and reproduction. However, their precise roles remain unclear. Advanced manipulation of these neuropeptides and their cognate receptors in the ARH through genetics, optogenetics and chemogenetics could shed more light on the molecular mechanism by which the ARH regulates nutrition/metabolism and reproduction.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Ministry of Science and ICT (MSIT)/National Research Foundation of Korea (NRF) (2021R1A2C2012951).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Anderson E, Haymaker W. Breakthroughs in hypothalamic and pituitary research. Prog Brain Res. 1974;41:1–60. doi: 10.1016/S0079-6123(08)61898-1. [DOI] [PubMed] [Google Scholar]
- 2.Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6 Suppl 2:1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- 3.Lechan RM, Toni R. Functional anatomy of the hypothalamus and pituitary. In: Feingold KR, Anawalt B, Blackman MR, editors. Endotext. MDText.com, Inc.; South Dartmouth, MA: 2016. [Google Scholar]
- 4.Toni R, Malaguti A, Benfenati F, Martini L. The human hypothalamus: a morpho-functional perspective. J Endocrinol Invest. 2004;27(6 Suppl):73–94. [PubMed] [Google Scholar]
- 5.Joly-Amado A, Cansell C, Denis RG, Delbes AS, Castel J, Martinez S, Luquet S. The hypothalamic arcuate nucleus and the control of peripheral substrates. Best Pract Res Clin Endocrinol Metab. 2014;28:725–737. doi: 10.1016/j.beem.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 7.Uwaifo GI. The human hypothalamus: anatomy, dysfunction and disease management. Humana; Cham: 2021. [DOI] [Google Scholar]
- 8.Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10:679–689. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 10.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 11.Pierroz DD, Catzeflis C, Aebi AC, Rivier JE, Aubert ML. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinology. 1996;137:3–12. doi: 10.1210/endo.137.1.8536627. [DOI] [PubMed] [Google Scholar]
- 12.Sainsbury A, Herzog H. Inhibitory effects of central neuropeptide Y on the somatotropic and gonadotropic axes in male rats are independent of adrenal hormones. Peptides. 2001;22:467–471. doi: 10.1016/S0196-9781(01)00342-4. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki N, Okada K, Minami S, Wakabayashi I. Inhibitory effect of neuropeptide Y on growth hormone secretion in rats is mediated by both Y1- and Y2-receptor subtypes and abolished after anterolateral deafferentation of the medial basal hypothalamus. Regul Pept. 1996;65:145–151. doi: 10.1016/0167-0115(96)00085-7. [DOI] [PubMed] [Google Scholar]
- 14.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 15.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 17.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 18.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 19.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 20.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/S0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 21.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/S0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 22.Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- 23.Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148:2251–2263. doi: 10.1210/en.2006-1240. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell CS, Begg DP. The regulation of food intake by insulin in the central nervous system. J Neuroendocrinol. 2021;33:e12952. doi: 10.1111/jne.12952. [DOI] [PubMed] [Google Scholar]
- 25.Vohra MS, Benchoula K, Serpell CJ, Hwa WE. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur J Pharmacol. 2022;915:174611. doi: 10.1016/j.ejphar.2021.174611. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein N, McKnight AD, Carty JRE, Arnold M, Betley JN, Alhadeff AL. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 2021;33:676–687.e5. doi: 10.1016/j.cmet.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morselli LL, Claflin KE, Cui H, Grobe JL. Control of energy expenditure by AgRP neurons of the arcuate nucleus: neurocircuitry, signaling pathways, and angiotensin. Curr Hypertens Rep. 2018;20:25. doi: 10.1007/s11906-018-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redmann SM, Jr, Argyropoulos G. AgRP-deficiency could lead to increased lifespan. Biochem Biophys Res Commun. 2006;351:860–864. doi: 10.1016/j.bbrc.2006.10.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13:2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, Madisen L, Zeng H, Krasnow MA, Knight ZA. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179:1129–1143.e23. doi: 10.1016/j.cell.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endle H, Horta G, Stutz B, Muthuraman M, Tegeder I, Schreiber Y, Snodgrass IF, Gurke R, Liu ZW, Sestan-Pesa M, Radyushkin K, Streu N, Fan W, Baumgart J, Li Y, Kloss F, Groppa S, Opel N, Dannlowski U, Grabe HJ, Zipp F, Rácz B, Horvath TL, Nitsch R, Vogt J. AgRP neurons control feeding behaviour at cortical synapses via peripherally derived lysophospholipids. Nat Metab. 2022;4:683–692. doi: 10.1038/s42255-022-00589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jikomes N, Ramesh RN, Mandelblat-Cerf Y, Andermann ML. Preemptive stimulation of AgRP neurons in fed mice enables conditioned food seeking under threat. Curr Biol. 2016;26:2500–2507. doi: 10.1016/j.cub.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15(3 Pt 2):2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 39.Lau J, Farzi A, Qi Y, Heilbronn R, Mietzsch M, Shi YC, Herzog H. CART neurons in the arcuate nucleus and lateral hypothalamic area exert differential controls on energy homeostasis. Mol Metab. 2018;7:102–118. doi: 10.1016/j.molmet.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Köster A. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–4400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- 41.Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahrén B, Sundler F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept. 2005;129:203–211. doi: 10.1016/j.regpep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Lau J, Herzog H. CART in the regulation of appetite and energy homeostasis. Front Neurosci. 2014;8:313. doi: 10.3389/fnins.2014.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A, de Araujo AM, Krieger JP, Vergara M, Ip CK, de Lartigue G. Demystifying functional role of cocaine- and amphetamine-related transcript (CART) peptide in control of energy homeostasis: a twenty-five year expedition. Peptides. 2021;140:170534. doi: 10.1016/j.peptides.2021.170534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haddock CJ, Almeida-Pereira G, Stein LM, Hayes MR, Kolar GR, Samson WK, Yosten GLC. Signaling in rat brainstem via Gpr160 is required for the anorexigenic and antidipsogenic actions of cocaine- and amphetamine-regulated transcript peptide. Am J Physiol Regul Integr Comp Physiol. 2021;320:R236–R249. doi: 10.1152/ajpregu.00096.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yosten GL, Harada CM, Haddock C, Giancotti LA, Kolar GR, Patel R, Guo C, Chen Z, Zhang J, Doyle TM, Dickenson AH, Samson WK, Salvemini D. GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. J Clin Invest. 2020;130:2587–2592. doi: 10.1172/JCI133270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, Rosen ED, Lowell BB, Tsai LT. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20:484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birge CA, Jacobs LS, Hammer CT, Daughaday WH. Catecholamine inhibition of prolactin secretion by isolated rat adenohypophyses. Endocrinology. 1970;86:120–130. doi: 10.1210/endo-86-1-120. [DOI] [PubMed] [Google Scholar]
- 48.Björklund A, Moore RY, Nobin A, Stenevi U. The organization of tubero-hypophyseal and reticulo-infundibular catecholamine neuron systems in the rat brain. Brain Res. 1973;51:171–191. doi: 10.1016/0006-8993(73)90371-5. [DOI] [PubMed] [Google Scholar]
- 49.Dahlstroem A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl Suppl. 1964;232:1–55. [PubMed] [Google Scholar]
- 50.MacLeod RM, Fontham EH, Lehmeyer JE. Prolactin and growth hormone production as influenced by catecholamines and agents that affect brain catecholamines. Neuroendocrinology. 1970;6:283–294. doi: 10.1159/000121933. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura Y, Yanagawa Y, Morrison SF, Nakamura K. Medullary reticular neurons mediate neuropeptide Y-induced metabolic inhibition and mastication. Cell Metab. 2017;25:322–334. doi: 10.1016/j.cmet.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blum T, Moreno-Pérez A, Pyrski M, Bufe B, Arifovic A, Weissgerber P, Freichel M, Zufall F, Leinders-Zufall T. Trpc5 deficiency causes hypoprolactinemia and altered function of oscillatory dopamine neurons in the arcuate nucleus. Proc Natl Acad Sci U S A. 2019;116:15236–15243. doi: 10.1073/pnas.1905705116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown RS, Kokay IC, Phillipps HR, Yip SH, Gustafson P, Wyatt A, Larsen CM, Knowles P, Ladyman SR, LeTissier P, Grattan DR. Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J Neurosci. 2016;36:9173–9185. doi: 10.1523/JNEUROSCI.1471-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, van den Pol AN. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat Neurosci. 2016;19:1341–1347. doi: 10.1038/nn.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, Guillemin R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) (LH-hypothalamus-LRF-gas chromatography-mass spectrometry-decapeptide-Edman degradation) Proc Natl Acad Sci U S A. 1972;69:278–282. doi: 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/S0006-291X(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 57.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 58.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wray S, Nieburgs A, Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res Dev Brain Res. 1989;46:309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- 60.Adams C, Stroberg W, DeFazio RA, Schnell S, Moenter SM. Gonadotropin-releasing hormone (GnRH) neuron excitability is regulated by estradiol feedback and kisspeptin. J Neurosci. 2018;38:1249–1263. doi: 10.1523/JNEUROSCI.2988-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114:E10216–E10223. doi: 10.1073/pnas.1713897114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 63.Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci U S A. 2015;112:13109–13114. doi: 10.1073/pnas.1512243112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plant TM. The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus. 2019;F1000Res 8:F1000 Faculty Rev-982. doi: 10.12688/f1000research.18356.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adelman JP, Mason AJ, Hayflick JS, Seeburg PH. Isolation of the gene and hypothalamic cDNA for the common precursor of gonadotropin-releasing hormone and prolactin release-inhibiting factor in human and rat. Proc Natl Acad Sci U S A. 1986;83:179–183. doi: 10.1073/pnas.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- 67.Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- 68.Frohman LA, Szabo M. Ectopic production of growth hormone-releasing factor by carcinoid and pancreatic islet tumors associated with acromegaly. Prog Clin Biol Res. 1981;74:259–271. [PubMed] [Google Scholar]
- 69.Gaylinn BD. Molecular and cell biology of the growth hormone-releasing hormone receptor. Growth Horm IGF Res. 1999;9 Suppl A:37–44. doi: 10.1016/S1096-6374(99)80008-2. [DOI] [PubMed] [Google Scholar]
- 70.Reichlin S. Growth hormone content of pituitaries from rats with hypothalamic lesions. Endocrinology. 1961;69:225–230. doi: 10.1210/endo-69-2-225. [DOI] [PubMed] [Google Scholar]
- 71.Guillemin R, Brazeau P, Böhlen P, Esch F, Ling N, Wehrenberg WB. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982;218:585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- 72.Rivier J, Spiess J, Thorner M, Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982;300:276–278. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- 73.Bloch B, Gaillard RC, Brazeau P, Lin HD, Ling N. Topographical and ontogenetic study of the neurons producing growth hormone-releasing factor in human hypothalamus. Regul Pept. 1984;8:21–31. doi: 10.1016/0167-0115(84)90025-9. [DOI] [PubMed] [Google Scholar]
- 74.Frohman LA, Jansson JO. Growth hormone-releasing hormone. Endocr Rev. 1986;7:223–253. doi: 10.1210/edrv-7-3-223. [DOI] [PubMed] [Google Scholar]
- 75.Frohman LA, Nernardis LL, Kant KJ. Hypothalamic stimulation of growth hormone secretion. Science. 1968;162:580–582. doi: 10.1126/science.162.3853.580. [DOI] [PubMed] [Google Scholar]
- 76.Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: a new model of isolated growth hormone deficiency. Endocrinology. 2004;145:4134–4143. doi: 10.1210/en.2004-0119. [DOI] [PubMed] [Google Scholar]
- 77.Icyuz M, Fitch M, Zhang F, Challa A, Sun LY. Physiological and metabolic features of mice with CRISPR/Cas9-mediated loss-of-function in growth hormone-releasing hormone. Aging (Albany NY) 2020;12:9761–9780. doi: 10.18632/aging.103242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife. 2013;2:e01098. doi: 10.7554/eLife.01098.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 80.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Rønnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152:4298–4309. doi: 10.1210/en.2011-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 84.Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311:126–134. doi: 10.1016/j.mce.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20:485–500. doi: 10.1093/humupd/dmu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 87.Araujo-Lopes R, Crampton JR, Aquino NS, Miranda RM, Kokay IC, Reis AM, Franci CR, Grattan DR, Szawka RE. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014;155:1010–1020. doi: 10.1210/en.2013-1889. [DOI] [PubMed] [Google Scholar]
- 88.Kavanagh GS, Tadi J, Balkenhol SM, Kauffman AS, Maloney SK, Smith JT. Kisspeptin impacts on circadian and ultradian rhythms of core body temperature: evidence in kisspeptin receptor knockout and kisspeptin knockdown mice. Mol Cell Endocrinol. 2022;542:111530. doi: 10.1016/j.mce.2021.111530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Popa SM, Moriyama RM, Caligioni CS, Yang JJ, Cho CM, Concepcion TL, Oakley AE, Lee IH, Sanz E, Amieux PS, Caraty A, Palmiter RD, Navarro VM, Chan YM, Seminara SB, Clifton DK, Steiner RA. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154:2784–2794. doi: 10.1210/en.2013-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. doi: 10.1038/nn.2818. [DOI] [PubMed] [Google Scholar]
- 91.Nandankar N, Negrón AL, Wolfe A, Levine JE, Radovick S. Deficiency of arcuate nucleus kisspeptin results in postpubertal central hypogonadism. Am J Physiol Endocrinol Metab. 2021;321:E264–E280. doi: 10.1152/ajpendo.00088.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Padilla SL, Perez JG, Ben-Hamo M, Johnson CW, Sanchez REA, Bussi IL, Palmiter RD, de la Iglesia HO. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr Biol. 2019;29:592–604.e4. doi: 10.1016/j.cub.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kangawa K, Minamino N, Fukuda A, Matsuo H. Neuromedin K: a novel mammalian tachykinin identified in porcine spinal cord. Biochem Biophys Res Commun. 1983;114:533–540. doi: 10.1016/0006-291X(83)90813-6. [DOI] [PubMed] [Google Scholar]
- 94.Maggio JE. Tachykinins. Annu Rev Neurosci. 1988;11:13–28. doi: 10.1146/annurev.ne.11.030188.000305. [DOI] [PubMed] [Google Scholar]
- 95.Nawa H, Hirose T, Takashima H, Inayama S, Nakanishi S. Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursor. Nature. 1983;306:32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- 96.Buell G, Schulz MF, Arkinstall SJ, Maury K, Missotten M, Adami N, Talabot F, Kawashima E. Molecular characterisation, expression and localisation of human neurokinin-3 receptor. FEBS Lett. 1992;299:90–95. doi: 10.1016/0014-5793(92)80107-R. [DOI] [PubMed] [Google Scholar]
- 97.Huang RR, Cheung AH, Mazina KE, Strader CD, Fong TM. cDNA sequence and heterologous expression of the human neurokinin-3 receptor. Biochem Biophys Res Commun. 1992;184:966–972. doi: 10.1016/0006-291X(92)90685-E. [DOI] [PubMed] [Google Scholar]
- 98.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chavkin C. Dynorphin--still an extraordinarily potent opioid peptide. Mol Pharmacol. 2013;83:729–736. doi: 10.1124/mol.112.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci U S A. 1981;78:7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wagner JJ, Evans CJ, Chavkin C. Focal stimulation of the mossy fibers releases endogenous dynorphins that bind kappa 1-opioid receptors in guinea pig hippocampus. J Neurochem. 1991;57:333–343. doi: 10.1111/j.1471-4159.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 102.Ferré G, Czaplicki G, Demange P, Milon A. Structure and dynamics of dynorphin peptide and its receptor. Vitam Horm. 2019;111:17–47. doi: 10.1016/bs.vh.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cawley NX, Li Z, Loh YP. 60 Years of POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol. 2016;56:T77–T97. doi: 10.1530/JME-15-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 107.Toda C, Santoro A, Kim JD, Diano S. POMC neurons: from birth to death. Annu Rev Physiol. 2017;79:209–236. doi: 10.1146/annurev-physiol-022516-034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- 109.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 110.Biglari N, Gaziano I, Schumacher J, Radermacher J, Paeger L, Klemm P, Chen W, Corneliussen S, Wunderlich CM, Sue M, Vollmar S, Klöckener T, Sotelo-Hitschfeld T, Abbasloo A, Edenhofer F, Reimann F, Gribble FM, Fenselau H, Kloppenburg P, Wunderlich FT, Brüning JC. Functionally distinct POMC-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat Neurosci. 2021;24:913–929. doi: 10.1038/s41593-021-00854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E, Fekete C, Frederiksen KS, Helms HCC, Jeppesen JF, John LM, Pyke C, Nøhr J, Lu TT, Polex-Wolf J, Prevot V, Raun K, Simonsen L, Sun G, Szilvásy-Szabó A, Willenbrock H, Secher A, Knudsen LB, Hogendorf WFJ. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5:e133429. doi: 10.1172/jci.insight.133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koch M, Varela L, Kim JG, Kim JD, Hernández-Nuño F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun YG, Lundeberg T, Yu LC. Involvement of endogenous beta-endorphin in antinociception in the arcuate nucleus of hypothalamus in rats with inflammation. Pain. 2003;104:55–63. doi: 10.1016/S0304-3959(02)00464-5. [DOI] [PubMed] [Google Scholar]
- 115.Bumaschny VF, Yamashita M, Casas-Cordero R, Otero-Corchón V, de Souza FS, Rubinstein M, Low MJ. Obesity-programmed mice are rescued by early genetic intervention. J Clin Invest. 2012;122:4203–4212. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chhabra KH, Adams JM, Fagel B, Lam DD, Qi N, Rubinstein M, Low MJ. Hypothalamic POMC deficiency improves glucose tolerance despite insulin resistance by increasing glycosuria. Diabetes. 2016;65:660–672. doi: 10.2337/db15-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gaddum JH, Schild H. Depressor substances in extracts of intestine. J Physiol. 1934;83:1–14. doi: 10.1113/jphysiol.1934.sp003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang MM, Leeman SE, Niall HD. Amino-acid sequence of Substance P. Nat New Biol. 1971;232:86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- 119.Harmar AJ, Hyde V, Chapman K. Identification and cDNA sequence of delta-preprotachykinin, a fourth splicing variant of the rat Substance P precursor. FEBS Lett. 1990;275:22–24. doi: 10.1016/0014-5793(90)81429-R. [DOI] [PubMed] [Google Scholar]
- 120.Ribeiro-da-Silva A, Hökfelt T. Neuroanatomical localisation of Substance P in the CNS and sensory neurons. Neuropeptides. 2000;34:256–271. doi: 10.1054/npep.2000.0834. [DOI] [PubMed] [Google Scholar]
- 121.Harrison S, Geppetti P. Substance P. Int J Biochem Cell Biol. 2001;33:555–576. doi: 10.1016/S1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 122.Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 123.Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 124.Caruso C, Durand D, Watanobe H, Lasaga M. NMDA and group I metabotropic glutamate receptors activation modulates Substance P release from the arcuate nucleus and median eminence. Neurosci Lett. 2006;393:60–64. doi: 10.1016/j.neulet.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 125.Simavli S, Thompson IR, Maguire CA, Gill JC, Carroll RS, Wolfe A, Kaiser UB, Navarro VM. Substance P regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156:2313–2322. doi: 10.1210/en.2014-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, Enriquez RF, Baldock PA, Zhang L, Sainsbury A, Herzog H, Lin S. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 127.Shi Z, Bonillas AC, Wong J, Padilla SL, Brooks VL. Neuropeptide Y suppresses thermogenic and cardiovascular sympathetic nerve activity via Y1 receptors in the paraventricular nucleus and dorsomedial hypothalamus. J Neuroendocrinol. 2021;33:e13006. doi: 10.1111/jne.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yokosuka M, Kalra PS, Kalra SP. Inhibition of neuropeptide Y (NPY)-induced feeding and c-Fos response in magnocellular paraventricular nucleus by a NPY receptor antagonist: a site of NPY action. Endocrinology. 1999;140:4494–4500. doi: 10.1210/endo.140.10.7058. [DOI] [PubMed] [Google Scholar]
- 129.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 130.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24:2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cabral A, Fernandez G, Tolosa MJ, Rey Moggia Á, Calfa G, De Francesco PN, Perello M. Fasting induces remodeling of the orexigenic projections from the arcuate nucleus to the hypothalamic paraventricular nucleus, in a growth hormone secretagogue receptor-dependent manner. Mol Metab. 2020;32:69–84. doi: 10.1016/j.molmet.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Broberger C, De Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. doi: 10.1002/(SICI)1096-9861(19981228)402:4<460::AID-CNE3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 133.Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stincic TL, Qiu J, Connors AM, Kelly MJ, Rønnekleiv OK. Arcuate and preoptic kisspeptin neurons exhibit differential projections to hypothalamic nuclei and exert opposite postsynaptic effects on hypothalamic paraventricular and dorsomedial nuclei in the female mouse. eNeuro. 2021;8:ENEURO.0093-21.2021. doi: 10.1523/ENEURO.0093-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, Paeger L, Bremser S, Klein AC, Morgan DA, Frommolt P, Brinkkötter PT, Hammerschmidt P, Benzing T, Rahmouni K, Wunderlich FT, Kloppenburg P, Brüning JC. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell. 2016;165:125–138. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee JS, Lee EY, Lee HS. Hypothalamic, feeding/arousal-related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Res. 2015;1598:97–113. doi: 10.1016/j.brainres.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 137.Boghossian S, Lemmon K, Park M, York DA. High-fat diets induce a rapid loss of the insulin anorectic response in the amygdala. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1302–R1311. doi: 10.1152/ajpregu.00252.2009. [DOI] [PubMed] [Google Scholar]
- 138.Ressler KJ. Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry. 2010;67:1117–1119. doi: 10.1016/j.biopsych.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hwang YG, Lee HS. Neuropeptide Y (NPY) or cocaine- and amphetamine-regulated transcript (CART) fiber innervation on central and medial amygdaloid neurons that project to the locus coeruleus and dorsal raphe in the rat. Brain Res. 2018;1689:75–88. doi: 10.1016/j.brainres.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 140.Loh K, Zhang L, Brandon A, Wang Q, Begg D, Qi Y, Fu M, Kulkarni R, Teo J, Baldock P, Brüning JC, Cooney G, Neely GG, Herzog H. Insulin controls food intake and energy balance via NPY neurons. Mol Metab. 2017;6:574–584. doi: 10.1016/j.molmet.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leyrer-Jackson JM, Hood LE, Olive MF. Alcohol consumption preferentially activates a subset of pro-opiomelanocortin (POMC) producing neurons targeting the amygdala. Neuropharmacology. 2021;195:108674. doi: 10.1016/j.neuropharm.2021.108674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Metz MJ, Daimon CM, King CM, Rau AR, Hentges ST. Individual arcuate nucleus proopiomelanocortin neurons project to select target sites. Am J Physiol Regul Integr Comp Physiol. 2021;321:R982–R989. doi: 10.1152/ajpregu.00169.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo ZL, Longhurst JC. Activation of reciprocal pathways between arcuate nucleus and ventrolateral periaqueductal gray during electroacupuncture: involvement of VGLUT3. Brain Res. 2010;1360:77–88. doi: 10.1016/j.brainres.2010.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun YG, Gu XL, Yu LC. The neural pathway of galanin in the hypothalamic arcuate nucleus of rats: activation of beta-endorphinergic neurons projecting to periaqueductal gray matter. J Neurosci Res. 2007;85:2400–2406. doi: 10.1002/jnr.21396. [DOI] [PubMed] [Google Scholar]
- 145.Campos CA, Bowen AJ, Schwartz MW, Palmiter RD. Parabrachial CGRP neurons control meal termination. Cell Metab. 2016;23:811–820. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qu N, He Y, Wang C, Xu P, Yang Y, Cai X, Liu H, Yu K, Pei Z, Hyseni I, Sun Z, Fukuda M, Li Y, Tian Q, Xu Y. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol Psychiatry. 2020;25:1006–1021. doi: 10.1038/s41380-019-0506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jia X, Chen S, Li X, Tao S, Lai J, Liu H, Huang K, Tian Y, Wei P, Yang F, Lu Z, Chen Z, Liu XA, Xu F, Wang L. Divergent neurocircuitry dissociates two components of the stress response: glucose mobilization and anxiety-like behavior. Cell Rep. 2022;41:111586. doi: 10.1016/j.celrep.2022.111586. [DOI] [PubMed] [Google Scholar]
- 149.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 150.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Chang S, Kim DH, Jang EY, Yoon SS, Gwak YS, Yi YJ, Lee JY, Ahn SH, Kim JM, Ryu YH, Kim SN, Roh HS, Lee MY, Kim SC, Lee BH, Kim HY, Yang CH. Acupuncture attenuates alcohol dependence through activation of endorphinergic input to the nucleus accumbens from the arcuate nucleus. Sci Adv. 2019;5:eaax1342. doi: 10.1126/sciadv.aax1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, Tannous BA, Myers MG, Jr, Andermann ML, Krashes MJ, Lowell BB. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19:1628–1635. doi: 10.1038/nn.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Singh I, Wang L, Xia B, Liu J, Tahiri A, El Ouaamari A, Wheeler MB, Pang ZP. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci. 2022;12:178. doi: 10.1186/s13578-022-00914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):S176–S185. [PubMed] [Google Scholar]
- 156.Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126:126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rossi MA. Control of energy homeostasis by the lateral hypothalamic area. Trends Neurosci. 2023;46:738–749. doi: 10.1016/j.tins.2023.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 159.Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, Guzmán-Ruiz M, Layritz C, Legutko B, Zinser E, García-Cáceres C, Buijs RM, Woods SC, Kalsbeek A, Seeley RJ, Nawroth PP, Bidlingmaier M, Tschöp MH, Yi CX. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab. 2017;6:897–908. doi: 10.1016/j.molmet.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Babcock Gilbert S, Roth LW. Hypothalamic obesity. Minerva Endocrinol. 2015;40:61–70. [PubMed] [Google Scholar]
- 162.Hochberg I, Hochberg Z. Expanding the definition of hypothalamic obesity. Obes Rev. 2010;11:709–721. doi: 10.1111/j.1467-789X.2010.00727.x. [DOI] [PubMed] [Google Scholar]
- 163.Nakagawa T, Hosoi T. Recent progress on action and regulation of anorexigenic adipokine leptin. Front Endocrinol (Lausanne) 2023;14:1172060. doi: 10.3389/fendo.2023.1172060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, Gojobori T, Isenovic ER. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne) 2021;12:585887. doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 166.Kakar N, Ahmad J, Kubisch C, Borck G. Exon skipping and severe childhood-onset obesity caused by a leptin receptor mutation. Am J Med Genet A. 2013;161A:2672–2674. doi: 10.1002/ajmg.a.36125. [DOI] [PubMed] [Google Scholar]
- 167.Stäubert C, Tarnow P, Brumm H, Pitra C, Gudermann T, Grüters A, Schöneberg T, Biebermann H, Römpler H. Evolutionary aspects in evaluating mutations in the melanocortin 4 receptor. Endocrinology. 2007;148:4642–4648. doi: 10.1210/en.2007-0138. [DOI] [PubMed] [Google Scholar]
- 168.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest. 2000;106:253–262. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 170.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 171.del Giudice EM, Santoro N, Cirillo G, D'Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes. 2001;50:2157–2160. doi: 10.2337/diabetes.50.9.2157. [DOI] [PubMed] [Google Scholar]
- 172.Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology. 2006;147:39–43. doi: 10.1210/en.2005-0812. [DOI] [PubMed] [Google Scholar]
- 173.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 174.Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, Grüters A. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab. 2003;88:4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- 175.Jackson RS, Creemers JW, Farooqi IS, Raffin-Sanson ML, Varro A, Dockray GJ, Holst JJ, Brubaker PL, Corvol P, Polonsky KS, Ostrega D, Becker KL, Bertagna X, Hutton JC, White A, Dattani MT, Hussain K, Middleton SJ, Nicole TM, Milla PJ, Lindley KJ, O'Rahilly S. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112:1550–1560. doi: 10.1172/JCI200318784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O'Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 177.Füredi N, Mikó A, Gaszner B, Feller D, Rostás I, Tenk J, Solymár M, Balaskó M, Pétervári E. Activity of the hypothalamic melanocortin system decreases in middle-aged and increases in old rats. J Gerontol A Biol Sci Med Sci. 2018;73:438–445. doi: 10.1093/gerona/glx213. [DOI] [PubMed] [Google Scholar]
- 178.Gruenewald DA, Matsumoto AM. Age-related decrease in proopiomelanocortin gene expression in the arcuate nucleus of the male rat brain. Neurobiol Aging. 1991;12:113–121. doi: 10.1016/0197-4580(91)90049-P. [DOI] [PubMed] [Google Scholar]
- 179.Pétervári E, Garami A, Soós S, Székely M, Balaskó M. Age-dependence of alpha-MSH-induced anorexia. Neuropeptides. 2010;44:315–322. doi: 10.1016/j.npep.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 180.Anthwal N, Pelling M, Claxton S, Mellitzer G, Collin C, Kessaris N, Richardson WD, Gradwohl G, Ang SL. Conditional deletion of neurogenin-3 using Nkx2.1iCre results in a mouse model for the central control of feeding, activity and obesity. Dis Model Mech. 2013;6:1133–1145. doi: 10.1242/dmm.011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pelling M, Anthwal N, McNay D, Gradwohl G, Leiter AB, Guillemot F, Ang SL. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev Biol. 2011;349:406–416. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 182.Jing E, Nillni EA, Sanchez VC, Stuart RC, Good DJ. Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides alpha melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology. 2004;145:1503–1513. doi: 10.1210/en.2003-0834. [DOI] [PubMed] [Google Scholar]
- 183.Al Rayyan N, Zhang J, Burnside AS, Good DJ. Leptin signaling regulates hypothalamic expression of nescient helix-loop-helix 2 (Nhlh2) through signal transducer and activator 3 (Stat3) Mol Cell Endocrinol. 2014;384:134–142. doi: 10.1016/j.mce.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carraro RS, Nogueira GA, Sidarta-Oliveira D, Gaspar RS, Dragano NR, Morari J, Bobbo VCD, Araujo EP, Mendes NF, Zanesco AM, Tobar N, Ramos CD, Toscaro JM, Bajgelman MC, Velloso LA. Arcuate nucleus overexpression of NHLH2 reduces body mass and attenuates obesity-associated anxiety/depression-like behavior. J Neurosci. 2021;41:10004–10022. doi: 10.1523/JNEUROSCI.0222-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Good DJ, Porter FD, Mahon KA, Parlow AF, Westphal H, Kirsch IR. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat Genet. 1997;15:397–401. doi: 10.1038/ng0497-397. [DOI] [PubMed] [Google Scholar]
- 186.Topaloglu AK, Simsek E, Kocher MA, Mammadova J, Bober E, Kotan LD, Turan I, Celiloglu C, Gurbuz F, Yuksel B, Good DJ. Inactivating NHLH2 variants cause idiopathic hypogonadotropic hypogonadism and obesity in humans. Hum Genet. 2022;141:295–304. doi: 10.1007/s00439-021-02422-9. [DOI] [PubMed] [Google Scholar]
- 187.Loid P, Mustila T, Mäkitie RE, Viljakainen H, Kämpe A, Tossavainen P, Lipsanen-Nyman M, Pekkinen M, Mäkitie O. Rare variants in genes linked to appetite control and hypothalamic development in early-onset severe obesity. Front Endocrinol (Lausanne) 2020;11:81. doi: 10.3389/fendo.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nasif S, de Souza FS, González LE, Yamashita M, Orquera DP, Low MJ, Rubinstein M. Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood. Proc Natl Acad Sci U S A. 2015;112:E1861–E1870. doi: 10.1073/pnas.1500672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Borman AD, Pearce LR, Mackay DS, Nagel-Wolfrum K, Davidson AE, Henderson R, Garg S, Waseem NH, Webster AR, Plagnol V, Wolfrum U, Farooqi IS, Moore AT. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum Mutat. 2014;35:289–293. doi: 10.1002/humu.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nies VJM, Struik D, Wolfs MGM, Rensen SS, Szalowska E, Unmehopa UA, Fluiter K, van der Meer TP, Hajmousa G, Buurman WA, Greve JW, Rezaee F, Shiri-Sverdlov R, Vonk RJ, Swaab DF, Wolffenbuttel BHR, Jonker JW, van Vliet-Ostaptchouk JV. TUB gene expression in hypothalamus and adipose tissue and its association with obesity in humans. Int J Obes (Lond) 2018;42:376–383. doi: 10.1038/ijo.2017.214. [DOI] [PubMed] [Google Scholar]
- 191.Moir L, Bochukova EG, Dumbell R, Banks G, Bains RS, Nolan PM, Scudamore C, Simon M, Watson KA, Keogh J, Henning E, Hendricks A, O'Rahilly S, Barroso I, Sullivan AE, Bersten DC, Whitelaw ML, Kirsch S, Bentley E, Farooqi IS, Cox RD UK10K consortium, author. Disruption of the homeodomain transcription factor orthopedia homeobox (Otp) is associated with obesity and anxiety. Mol Metab. 2017;6:1419–1428. doi: 10.1016/j.molmet.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brioude F, Bouligand J, Francou B, Fagart J, Roussel R, Viengchareun S, Combettes L, Brailly-Tabard S, Lombès M, Young J, Guiochon-Mantel A. Two families with normosmic congenital hypogonadotropic hypogonadism and biallelic mutations in KISS1R (KISS1 receptor): clinical evaluation and molecular characterization of a novel mutation. PLoS One. 2013;8:e53896. doi: 10.1371/journal.pone.0053896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hu KL, Chen Z, Li X, Cai E, Yang H, Chen Y, Wang C, Ju L, Deng W, Mu L. Advances in clinical applications of kisspeptin-GnRH pathway in female reproduction. Reprod Biol Endocrinol. 2022;20:81. doi: 10.1186/s12958-022-00953-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Silveira LG, Noel SD, Silveira-Neto AP, Abreu AP, Brito VN, Santos MG, Bianco SD, Kuohung W, Xu S, Gryngarten M, Escobar ME, Arnhold IJ, Mendonca BB, Kaiser UB, Latronico AC. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95:2276–2280. doi: 10.1210/jc.2009-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 196.Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358:709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Francou B, Bouligand J, Voican A, Amazit L, Trabado S, Fagart J, Meduri G, Brailly-Tabard S, Chanson P, Lecomte P, Guiochon-Mantel A, Young J. Normosmic congenital hypogonadotropic hypogonadism due to TAC3/TACR3 mutations: characterization of neuroendocrine phenotypes and novel mutations. PLoS One. 2011;6:e25614. doi: 10.1210/endo-meetings.2011.PART1.OR6.OR05-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95:2857–2867. doi: 10.1210/jc.2009-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–2295. doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]
- 201.Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, Cukier P, Thompson IR, Navarro VM, Gagliardi PC, Rodrigues T, Kochi C, Longui CA, Beckers D, de Zegher F, Montenegro LR, Mendonca BB, Carroll RS, Hirschhorn JN, Latronico AC, Kaiser UB. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368:2467–2475. doi: 10.1056/NEJMoa1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dauber A, Cunha-Silva M, Macedo DB, Brito VN, Abreu AP, Roberts SA, Montenegro LR, Andrew M, Kirby A, Weirauch MT, Labilloy G, Bessa DS, Carroll RS, Jacobs DC, Chappell PE, Mendonca BB, Haig D, Kaiser UB, Latronico AC. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab. 2017;102:1557–1567. doi: 10.1210/jc.2016-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]