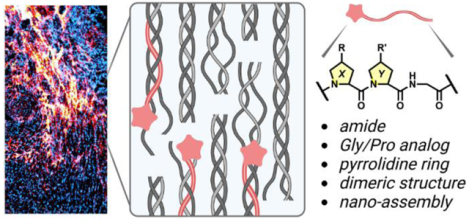
Abstract
Collagen provides mechanical and biological support for virtually all human tissues in the extracellular matrix (ECM). Its defining molecular structure, the triple-helix, could be damaged and denatured in disease and injuries. To probe collagen damage, the concept of collagen hybridization has been proposed, revised, and validated through a series of investigations reported as early as 1973: a collagen-mimicking peptide strand may form a hybrid triple-helix with the denatured chains of natural collagen but not the intact triple-helical collagen proteins, enabling assessment of proteolytic degradation or mechanical disruption to collagen within a tissue-of-interest. Here we describe the concept and development of collagen hybridization, summarize the decades of chemical investigations on rules underlying the collagen triple-helix folding, and discuss the growing biomedical evidence on collagen denaturation as a previously-overlooked ECM signature for an array of conditions involving pathological tissue remodeling and mechanical injuries. Finally, we propose a series of emerging questions regarding the chemical and biological nature of collagen denaturation and highlight the diagnostic and therapeutic opportunities from its targeting.
Keywords: denatured collagen, hybridization, MMP, triple-helix, peptidomimetics, mechanical injury
Graphical Abstract
1. Introduction
As the most abundant protein and the predominant component of the extracellular matrix (ECM), collagen forms the living environment with structural and biological support for cell adhesion, migration, differentiation, and function in almost all human organs.1 In many diseases and pathological conditions, collagen in the lesions can suffer substantial damage, much like buildings crumbled by natural disasters. For instance, collagen degradation, commonly mediated by matrix metalloproteases (MMPs), is intrinsically involved in cancer metastasis.2 In atherosclerosis, the proteolytic weakening of the collagenous fibrous cap renders the plaques susceptible to rupture, leading to myocardial infarction and sudden cardiac death.3 Additionally, as many fibrotic disorders progress, collagen synthesis and breakdown are often simultaneously up-regulated.4,5 Collagen is also the primary load-bearing component of connective tissues, providing tensile strength to bone, cartilage, ligaments, tendons, muscles, skin, corneas, and blood vessels. Injuries to these tissues largely involve mechanical destruction of the hierarchical collagen structures at various scales.6 For example, osteoarthritis, a prevalent disabling condition in the aged population, is considered to be heavily driven by the overuse of the articular cartilage in joints, which is rich in type II collagen.
All 28 collagen subtypes share an iconic structural motif in which three protein chains intertwine into a triple-helix, forming stabilizing inter-chain hydrogen bonds.1,7 This triple-helical structure protects the collagen molecules in the ECM from degradation by most proteases except certain collagenases, such as MMP1, 3, 8, 13, 14, and Cathepsin K.8,9 Following the initial cleavage of collagen I with MMP1 for instance, the fragmented collagen triple-helices become thermally unstable and spontaneously unfold at body temperature, leaving the cleaved and denatured collagen chains cross-linked within the partially degraded matrix.10–12 Meanwhile, biomechanical studies showed that the collagen molecules in connective tissues (e.g., tendon) can become susceptible to trypsin digestion following tissue overloading or cyclic fatigue, indicating that mechanical disruption can also compromise the collagen triple-helix structure in tissues.13,14 Furthermore, cells can readily recognize collagen denaturation: receptors DDR1 and DDR2,15,16 as well as integrins α2β1, α1β1, α10β1, and α11β1, only recognize their target collagen domains in the triple-helical conformation;17 yet the denatured collagen single chains can expose cryptic sites that are otherwise inactive in the triple-helical form, including numerous RGD sequences that are recognized by another integrin subset.18 It can be envisioned that collagen denaturation could be a crucial feature of tissues undergoing pathological remodeling or mechanical injury, which can be explored for diagnostic and therapeutic purposes. In this regard, an array of scientific questions regarding the structure, cellular effects, and downstream degradation pathways of the denatured collagen matrices needs to be answered for both fundamental and biomedical goals.
Even though advanced microscopic techniques, such as second-harmonic generation (SHG), atomic force microscopy (AFM), and transmission electron microscopy (TEM) can indicate structural alteration of collagen at the fiber scale (Fig. 1a),19,20 they cannot reveal denatured collagen molecules in situ. Therefore, a targeting agent that specifically recognizes the denatured collagen molecules could be the first step to approach the above-mentioned questions by identifying and localizing collagen damage in the body. Likewise, while the upstream proteolytic activities leading to collagen denaturation can be readily probed using fluorescence-based MMP substrates (e.g., MMPSense),21 the denatured collagen itself is typically undetectable within the tissue-of-interest (Fig. 1b). This is because most common small-molecule dyes (Picrosirius red and Masson’s trichrome) and antibodies bind to collagen regardless of its triple-helical folding.22,23 To this end, at least two classes of monoclonal antibodies (mAbs) against denatured collagens have been developed. (i) From the late 1980s, Poole and coworkers developed a series of mAbs for assessing collagen II damage. Using cyanogen-bromide-treated collagen II as immunizing peptides, they produced mAb COL2–3/4m which reacts with denatured collagen II.24,25 Further development using peptide epitopes at the MMP-cleavage sites yielded mAbs C1,2C (epitope: GGEGPOGPQG, O: hydroxyproline) and C2C (epitope: GPOGQG) against the MMP-cleaved collagen II.26,27 Efficacies of these antibodies were nicely demonstrated in assays of body fluids and immuno-stains of human articular cartilage for the detection of collagen II breakdown in both osteo- and rheumatoid-arthritis.24–28 (ii) Through subtractive immunization using thermally denatured collagens, Brooks and coworkers developed mAbs targeting the cryptic epitopes within proteolyzed or denatured collagens in the 2000s.29,30 Among them, mAb HU177 recognizes the PGxPG epitope (x: multiple amino acid residues) in the unfolded chains of type I-V collagens,31,32 and was shown to inhibit tumor angiogenesis and growth by disrupting cellular interactions with the denatured collagen.31–33 A phase-I clinical trial towards evaluating the antitumor activity of the humanized version of HU177 yielded encouraging results in 2010.34 Additionally, elevated levels of soluble HU177 epitopes were detected in the sera of melanoma patients with worse prognoses.35,36 These works well demonstrate the distinctive biological activities of denatured collagens as well as their diagnostic and therapeutic potentials.
Figure 1. Targeting denatured collagen using the concept of collagen hybridization.
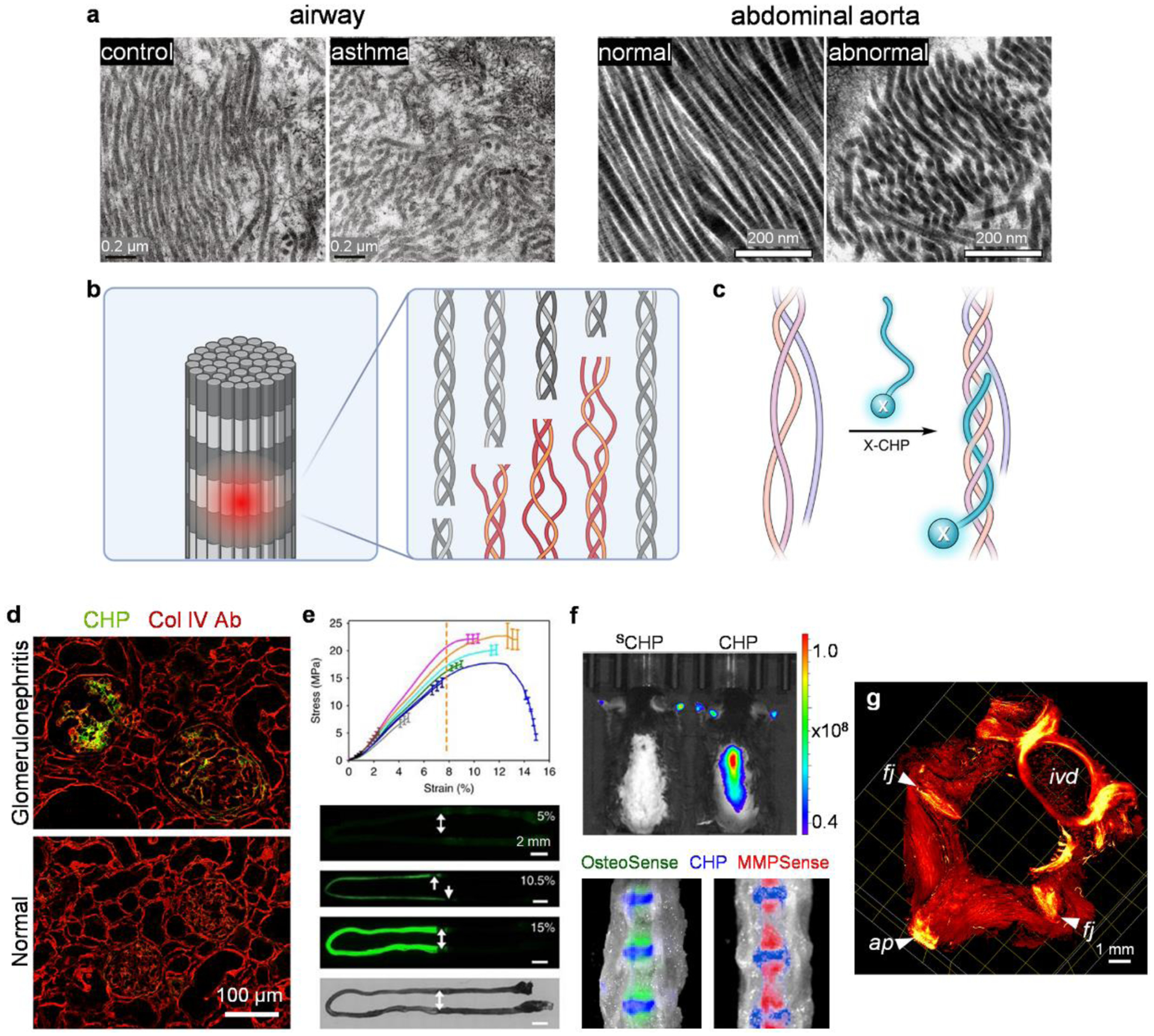
(a) TEM images showing characteristic collagen fibril structures of normal human large airways and mice abdominal aorta compared to disorganized and truncated collagen fibrils from the asthmatic airways and abdominal aorta aneurysm. Adapted with permission from ref 153 (Copyright 2019 American Thoracic Society) and 154 (Copyright 2020 Elsevier Ltd.). (b) The triple-helix, the hallmark structure of the collagen molecule, may be denatured due to protease degradation (e.g., by MMPs) or mechanical disruption to the collagen fibrils. (c) The Collagen Hybridizing Peptide (CHP), often conjugated with a functional moiety (X, e.g., a fluorophore) can specifically target a denatured collagen molecule through the formation of a hybrid triple-helix. (d) The fluorescently-labeled CHP, but not the anti-Col IV antibody, detects collagen degradation histologically within the glomeruli of nephritic rats. (c) and (d) adapted with permission from ref 41. Copyright 2017 American Chemical Society. (e) The fluorescence from the CHP staining increased with incremental strain levels within the stretched center of the rat tail tendon fascicles, reflecting molecular denaturation of collagen from mechanical damage. Adapted with permission under a Creative Commons Attribution 4.0 License from ref 6. Copyright 2017 Springer Nature. (f) In vivo binding: CHP targets denatured collagen within normal mice intervertebral discs sandwiched between the vertebral bodies marked by OsteoSense and MMPSense. SCHP: sequence-scrambled control peptide. (g) Light sheet microscopy 3D fluorescence imaging showing denatured collagen marked by in-vivo-administered CHP in the intervertebral disc (ivd), facet joints (fj), and the attachment point (ap) of the supraspinous ligament on a spinous process of a rat lumbar spine. (f) and (g) adapted with permission from ref 49. Copyright 2021 American Chemical Society.
Over the past two decades, we and others have developed a collagen-mimetic peptide to target denatured collagen strands in a fashion of triple-helix hybridization, which enabled in situ detection of collagen denaturation in an array of pathological tissues and investigations into its biological nature within these lesions. In this perspective, we introduce the concept and development of collagen hybridization and summarize the decades of ongoing chemical research on the structural principles underlying collagen triple-helical folding. We also discuss the current novel biomedical discoveries on collagen denaturation in a wide range of diseases and injuries aiming at potential diagnostic and therapeutic applications.
2. Collagen Hybridizing Peptide
The triple-helix is a super-secondary structure virtually exclusive to collagens.1,37 The Collagen Hybridizing Peptide (CHP) can specifically hybridize with denatured collagen strands by re-forming the triple-helical structure, in a fashion similar to a primer binding to melted DNA strands during PCR (Fig. 1c). The close-packing of a collagen triple-helix requires every third residue be Gly, creating a sequence of repeating GlyXaaYaa triplets.1 In the design of the first-generation of CHPs, there are 6–10 repeating units of glycine(G)–proline(P)–hydroxyproline(O). The GPO triplet possesses the strongest triple-helix propensity among all GlyXaaYaa units found in native collagens,38 allowing the CHPs to hybridize tightly with the denatured collagen strands. Presumably, the hybrid is stabilized through multiple hydrogen bonds formed among the backbones of the CHP and collagen strands, similar to a native collagen triple-helix.
The CHP-collagen hybridization involves several key features (Fig. 1). (1) Structural selectivity: the CHPs were shown to bind strongly to collagen chains denatured by heat, MMPs,39 and mechanical damage,6 but have much lower affinity to intact collagen due to the absence of binding sites. (2) Collagen types: as the triple-helix structure is ubiquitous in all types of collagens in mammals, the CHPs bind to unfolded collagen chains across animal species (e.g., human, rat, mouse) and collagen types (e.g., type I, II, III, IV).40,41 (3) Binding specificity: without charged and hydrophobic amino acids in the sequences, the CHPs exhibit virtually no non-specific binding to non-collagenous biomolecules.40 This is also because the triple-helix structure is essentially exclusive to the collagens in mammals, except a group of secreted proteins involved in host defense that contain triple-helical domains (e.g., C1q, surfactant protein A and D).42 Meanwhile, collagen-like proteins with G-X-Y repeating sequences (e.g., streptococcal collagen-like proteins)43 are also widely present in bacteria, which could also be potential targets of the CHPs.44 (4) Serum stability: the CHPs are highly stable in serum against a full panel of enzymes including proteases and esterases, thanks to their unique neutral, hydrophilic, Pro-rich, and repetitive sequences.45 (5) Histopathology and in vivo capacity: the fluorescently tagged CHPs allowed histological assessment of collagen damage in osteoarthritis and intervertebral disc degeneration, as well as collagen remodeling in myocardial, glomerular (Fig. 1d), liver, and pulmonary fibrosis.41,46–49 Also, the intravenously administered CHPs were shown to target pathological collagen remodeling (e.g., tumor xenografts39 and osteolytic bone lesions50) and mechanical destruction (e.g., intervertebral disc,49 Fig. 1f–g) in vivo.
Targeting denatured collagen by the triple-helix hybridization is a unique concept in structure binding, particularly compared to conventional library-based approaches for ligand discovery such as phage display and antibody production.24,29 Unlike antibody-epitope recognition based on interactions of hydrophobic and charged amino acid sidechains, the collagen triple-helix forms predominantly via hydrogen bonding among the chain backbones. The self-assembled CHP triple-helices have been studied as a structural model for collagen since the 1960s,51 but its power for probing the molecular structure of collagen had not been harnessed until recent decades.
3. Development of the Collagen Hybridizing Peptides
The hybrid formation between bovine collagen α chains and synthetic polypeptides [e.g., (ProAlaGly)n, (ProProGly)n] was first reported in 1973 by Heidemann, Harrap, and Schiele.52 After cooling the heated collagen-peptide mixture, they isolated the hybrid by molecular sieve chromatography and identified it by amino acid analysis. Optical rotation data supported that a common triple-helix structure was adopted by the hybrid.52
The Yu group reported in 2005 that a collagen mimetic peptide (CMP) with a strong triple-helical propensity [e.g., (ProHypGly)10] may adhere to intact collagen protein presumably through a “strand exchange” process to form a hybrid triple-helix, especially within the thermally labile domains of the collagen sequence,53,54 a process analogous to the spontaneous invasion and strand displacement of a DNA’s double helix by a peptide nucleic acid.55 Similar notions were also proposed by other researchers whose focus was on exploiting the single-stranded collagen peptides to invade synthetic collagen mimetics rather than the actual collagen protein.56–58 Initially, Yu and colleagues tested the hybridization by directly adding heated solutions of CMP monomers onto collagen films, and believed that denaturation of the collagen protein is not required for CMP binding.53 This idea prompted our early efforts during the 2000s to anchor therapeutic drugs (e.g., growth factors) to living tissues and collagenous biomaterials for tissue engineering applications.54
To disengage the factor of collagen denaturation from the CMP hybridization, we designed a caged, monomeric CMP whose triple-helical folding can be photo-triggered so that the hybridization experiments could be performed without heating.39 When the binding assays were performed at 4 °C with exposing the caged CMP to UV light directly on collagen and gelatin films, Li found that the UV-activated peptide has almost negligible binding on intact collagen, but exhibits a level of adhesion over an order of magnitude higher for gelatin, as well as for collagen denatured by MMP1 degradation.39 These results decisively changed the original hypothesis that the monomeric CMP can spontaneously hybridize with intact collagen molecules through strand invasion and displacement,53 and re-directed the research towards targeting denatured collagen in pathological tissues for the detection, imaging, and treatment of conditions associated with collagen remodeling.37,50,59–69 In 2016, we coined the term “collagen hybridizing peptide” for these rationally designed synthetic peptides that target denatured collagens via triple-helical folding,70 and started using the acronym CHP in subsequent reports. Compared to the term collagen mimetic peptide (CMP),54 the term CHP emphasizes the peptide’s hybridization function and implies its single-strand structure in action. We soon found that, besides the MMP-degraded collagen, CHPs can also target mechanically damaged collagen in connective tissues,6,71,72 as well as unfolded collagen molecules in ECM scaffolds decellularized with different agents.73 These findings have helped expand the concept of collagen hybridization into the fields of biomechanics and tissue engineering.
4. Chemistry of Collagen Hybridization
4.1. The backbone
The structural base of collagen hybridization is the CHP’s strong propensity to form the collagen triple-helix. For a typical GlyProHyp-based CHP, this propensity lies in the peptide backbone and is dominated by several structural elements, including a glycine residue that is small enough to fit inside the tight triple-helix, a network of interchain hydrogen bonds, as well as abundant proline and hydroxyproline residues whose pyrrolidine rings impose the backbone conformation. Here we summarize the guiding principles of triple-helical folding from decades of chemical research based on the GPO triplet unit and focus on how they can be applied to new CHP designs for hybridization. Related topics, such as the triple-helix folding of the CMP sequences modified with natural amino acids,74 as well as the charge-pairing-based CMP heterotrimer self-assemblies,75 have been extensively reviewed elsewhere, and thus not included in this perspective.
The amides.
Like most folded proteins, interchain hydrogen bonds between the backbone amides are directly responsible for the structural integrity of collagen. Within the (GlyXaaYaa)n triple-helix, the N-H(Gly)…O=C(Xaa) hydrogen bonds (most often Xaa: Pro) are specifically formed in between chains (Fig. 2, panel i). By replacing the central Pro-Gly amide of (ProProGly)10 with a hydrogen-lacking ester (1, Fig. 2a), Raines and colleagues estimated that an interchain hydrogen bond contributes approximately −2.0 kcal/mol to the triple-helix stabilization.76 They also found that the triple-helix is stabilized when the hydrogen-bonding amide at the Pro-Gly position is substituted with a thioamide (2, Fig. 2a), a stronger hydrogen bond donor than the natural oxoamide.77
Figure 2.
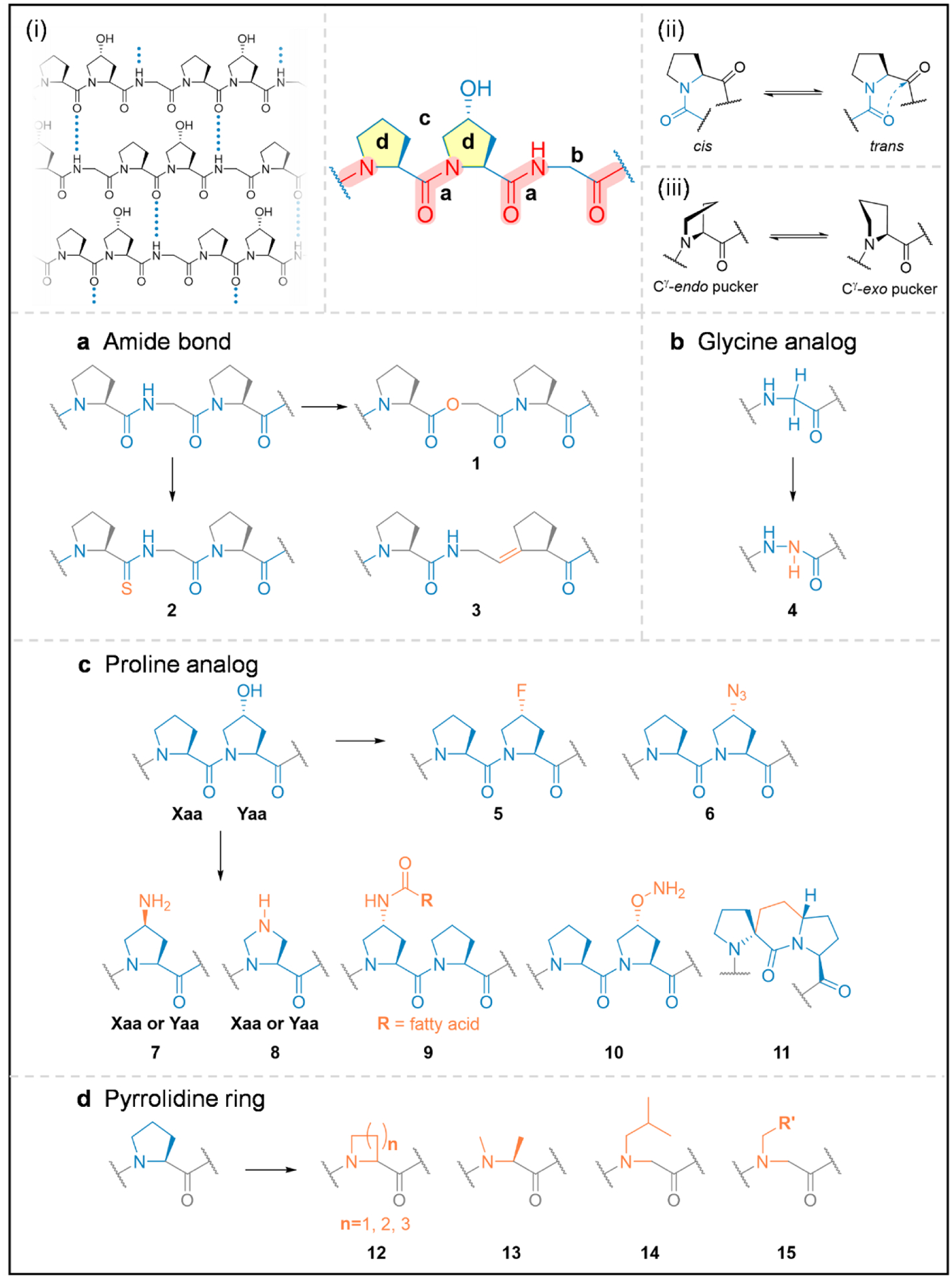
The structural model of the collagen hybridizing peptides, whose triple-helix folding is governed by the interstrand N-H(Gly)…O=C hydrogen bonding (i), the trans/cis conformation of the Pro-amide bond (ii), and the pucker of the pyrrolidine ring (iii). To gain insights into the peptide’s propensity to form the collagen triple-helix, extensive chemical modifications (in orange) have been made to its various structural sub-units (blue) including the amide bond (a), the Gly and Hyp residues (b,c), as well as the pyrrolidine ring (d).
The peptide amide bond has a partial double bond character resulting in two distinct conformations: cis and trans (Fig. 2, panel ii). In the folded collagen triple-helix, all amides must be the trans isomer.78 Because the amide bond preceding the dialkylated Pro residue has a higher cis-conformation propensity than common amino acids, Etzkorn and colleagues envisioned that locking the Gly-Pro amide in trans-conformation with an alkene isostere (3, Fig. 2a) would promote triple-helical folding by reducing the entropy cost for cis-trans isomerization. However, the resulting trans-alkene isostere was strongly destabilizing.79 This discrepancy highlights the contribution from structural factors other than the amide bond isomerization, such as the n→π* interactions between the backbone amides.80
Glycine analogs.
Every 3rd residue within a collagen triple-helical domain is occupied by Gly, the only amino acid residue that can be sterically accommodated inside the closely packed helix. Substituting Gly to any other canonical amino acid would substantially destabilize the triple-helix. Creatively, the Chenoweth group replaced one Gly residue in a collagen peptide with aza-glycine (azGly, 4, Fig. 2b):81 due to the possible extra hydrogen bond between azGly’s NH group and an interchain carbonyl, the thermal stability of the triple-helix increased by as much as 10 °C.82 They further validated that introducing multiple azGly residues stabilizes the structure synergistically while placing azGly near the center of the collagen peptide has a stronger stabilizing effect than that at both termini.83 The team also reported the first crystal structure of a triple-helical collagen model peptide featuring an azGly substitution.84 Together, their azGly studies demonstrated a critical structural basis for the triple-helix formation with Gly modification: whether it brings new stabilizing interactions, it must preserve the strict steric and conformational requirements for Gly in the triple-helix.84
Proline analogs.
The thermal stability of triple-helical collagen is dramatically enhanced through hydroxylation of Pro,85 a post-translational modification step before the chains fold into a triple-helix. The first crystal structure of the collagen triple-helix showed that the OH group on the pyrrolidine ring does not form direct interchain hydrogen bonds.7 To understand the chemical nature of its stabilizing effect, seminal work by Raines and colleagues in 1998 demonstrated that replacing every Hyp with a 4(R)-fluoroproline residue (Flp, 5, Fig. 2c) in (ProHypGly)10 drastically increased the stability of the triple helix (Tm: 69 → 91 °C).86 Since fluorine does not form hydrogen bonds, this result de-emphasized the contribution from the previously proposed hydrogen bonds formed among Hyp’s OH groups and bridging water molecules.7 A series of further studies from the Raines group suggested that the stabilization of Flp or Hyp comes from the inductive effect of the electronegative fluorine or oxygen atom, which favors the Cγ-exo ring pucker (Fig. 2, panel iii) of the pyrrolidine ring and the trans-isomer of the amide bond, both of which promote the correct peptide backbone conformation for the triple-helix formation.87
Raines’ studies on Hyp and Flp’s stabilizing stereoelectronic properties opened an avenue for designing collagen mimetic peptides featuring synthetic proline derivatives with electron-withdrawing moieties. Particularly, the Wennemers group substituted Hyp with (4R)azido-proline (Azp, 6, Fig. 2c), showcasing that (4R)Azp has a stabilizing stereoelectronic effect similar to Hyp while allowing facile functionalization via “click” chemistry.88 They also investigated the switchable triple-helix assemblies mediated by amino-proline and γ-azaproline residues (7, 8, Fig. 2c), whose ring puckering and hydrogen bond formation/release can be controlled by pH changes.89–91 Furthermore, the group recently attached hydrophobic moieties such as fatty acids through N-acylation of amino-proline (9, Fig. 2c), and found that the pendant lipids promote and accelerate the triple-helix formation,92 and even more so for the longer and more flexible fatty acids.93 Lastly, more exotic electron-withdrawing groups such as aminooxy (10, Fig. 2c) and oxime moieties were attached to proline, which enabled chemo-selective crosslinking within collagen triple-helices by oxime ligation94 as well as targeted imaging of lysyl oxidase-mediated collagen crosslinking in vivo.95
One key principle of almost all proline derivatization studies is preorganization: if the propensity of the free peptide strands to adopt the required conformation (i.e., a polyproline II type helix, PPII) is enhanced, the entropy cost for triple-helix formation is reduced, thereby leading to structural stability.96 In seeking new preorganization strategies different from 4-substitution on proline, Baumann, Schmalz, and coworkers developed a “proline-stapling” approach, where two adjacent proline rings are covalently connected via a C2 bridge to freeze the PPII-helix conformation (11, Fig. 2c), which produced triple-helices with stabilities close to the parent collagen model peptide.97
The pyrrolidine ring.
The high proline content in human collagen (~22%)98 is crucial for its triple-helical structure. As the only ribosomally-encoded N-substituted amino acid, proline promotes distinct secondary protein structures, due to its unique pyrrolidine ring with the restricted backbone dihedral angles and its tertiary amide group lacking the ability to donate a hydrogen-bond. Replacing Pro in the GlyProHyp triplet with any of the natural amino acids will destabilize the triple-helix;38 thus, for over five decades, proline and its derivatives have been considered compulsory for designing synthetic collagen mimetics. To appreciate the uniqueness of proline’s pyrrolidine ring, the Wennemers group explored the effect of four-and six-membered ring-size analogs of proline on the collagen triple-helix (12, Fig. 2d):99 they showed that both analogs destabilize the structure because of the inappropriate trans/cis conformation or dihedral angles. Additionally, the Raines group found that converting Pro to N-methyl-L-alanine (13, Fig. 2d, i.e., removing only the γ-carbon and opening the ring structure), substantially destabilizes the triple-helix.100 These studies highlighted Pro’s perfectly-adjusted conformational feature for the collagen triple-helix.
Alternatively, from a chemical perspective, proline can also be considered an N-substituted α-amino acid. In an attempt to produce collagen triple-helices without Pro, Goodman and colleagues conducted the pioneering studies showing that replacing Pro with N-isobutylglycine (Nleu, 14, Fig. 2d), a synthetic peptoid residue within CMPs results in stable triple-helices.101–105 They attributed the stabilization to the interchain interactions between the hydrophobic side chains of Nleu and the adjacent Pro.102–104 Recently, we confirmed that besides Nleu, many other peptoid residues exhibit a general triple-helical propensity similar to or greater than Pro in CMPs (15, Fig. 2d).106 Supported by atomic-resolution crystal structures and computational analysis, we reasoned that the stability is less involved with hydrophobic interchain interactions, but primarily because the peptoid residues can sterically preorganize individual CMP chains into the required PPII conformation.106 Since there are hundreds of synthetically available peptoid residues, we anticipated this structural principle of peptoids to enable the syntheses of stable triple-helical CMPs with extraordinary side-chain diversity and open up opportunities for a new generation of collagen-inspired therapeutics and materials.
4.2. Supramolecular designs for collagen hybridization
Non-self-trimerizing CHP monomers.
For CHP-collagen hybridization, the strong triple-helical propensity of a CHP strand is both the driving force and a barrier at the same time. This is because the single-stranded CHPs (monomers) can spontaneously and gradually form peptide homotrimers in solution, thereby losing their propensity for collagen hybridization. Consequently, a CHP solution needs to be heated (e.g., at 80 °C) to dissociate the peptide into monomers immediately before a hybridization application (e.g., tissue staining).41 Although the pre-heated solution can be quickly quenched to room temperature to avoid thermal impairment to tissues, this pre-heating process complicates the histological and in vivo applications of the CHPs, and poses a challenge for fully quantitative analysis of the imaging results, due to the uncertainty over the active concentration of the CHP monomers. For these reasons, various strategies have been explored to inhibit the CHP self-trimerization while maintaining its collagen-hybridizing capacity.
One ad hoc strategy is to introduce unfavorable interactions (e.g., electrostatic, steric) that weaken the self-trimerization more than the hybridization. For instance, the Xiao group attached multiple Asp residues to (GPO)7 to destabilize the peptide homotrimer by electrostatic repulsion, allowing histological recognition of denatured collagen without preheating.107 However, it remains to be further tested whether these charges may reduce the peptide’s binding specificity. The team also created a monomeric CHP by attaching a fluorescein dye to a Y-positioned (2S,4S)-aminoproline residue in the peptide’s center,60 to introduce inter-strand steric repulsion from the bulky fluorescein dye (Fig. 3a). Utilizing unnatural proline derivatives, the Raines group discovered that (flpFlpGly)7 [flp: (4S)-fluoroproline, Flp: (4R)-fluoroproline] does not self-trimerize due to the steric hindrance between the fluorine atoms from neighboring chains but can from stable heterotrimeric helices with (ProProGly)7.108,109 Similarly, (flpHypGly)n or (GlyflpHyp)n [(GfO)n] should not self-assemble into a stable homotrimeric helix because flp is sterically repulsed by every neighboring Hyp residue (Fig. 3a).110 However, Bennink et al. reported that the sequence maintains the ability to hybridize with denatured collagen,50 probably because Hyp only occurs in about 34% of the Gly-X-Y triplets within natural collagen chains [compared to 100% for (GfO)9].38 Without the pre-heating requirement, this CHP sequence has greatly promoted the in vivo imaging of collagen damage.49
Figure 3. Supramolecular designs of collagen hybridizing peptides.
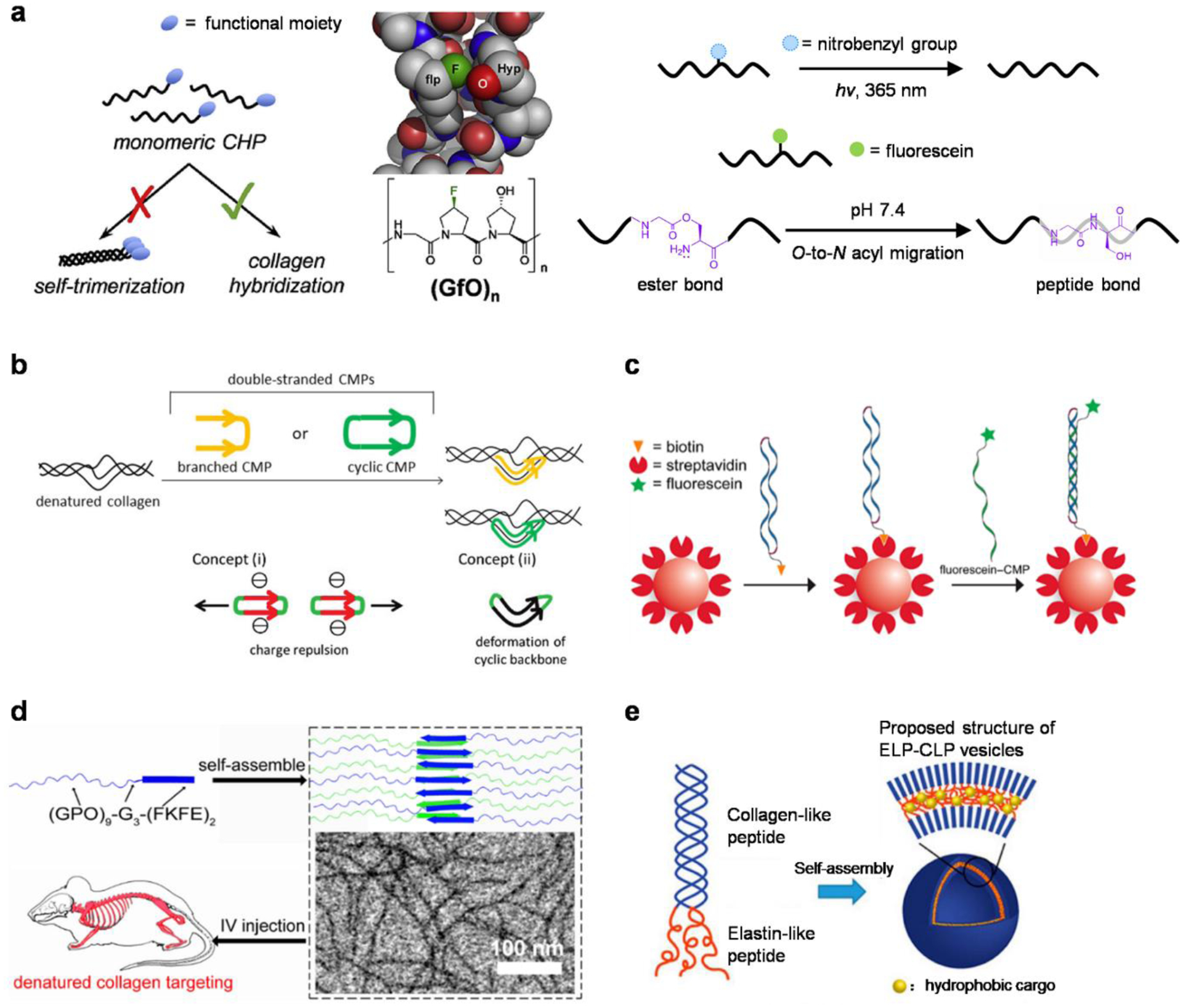
(a) Designs of monomeric CHPs that do not self-trimerize but can inherently or be triggered to hybridize with denatured collagen. Adapted with permission from ref 50. Copyright 2018 Elsevier Ltd. (b) Concepts of the dimeric CHP designs to promote the hybridizing affinity and to limit the peptide self-association. Adapted with permission from ref 61 (Copyright 2019 Royal Society of Chemistry) and 115 (Copyright 2018 Wiley-VCH). (c) A cyclic dimeric CMP was designed to mimic the damaged collagen molecules for assessing triple-helix hybridization. Adapted with permission from ref 116. Copyright 2020 American Chemical Society. (d) CHP strands displayed on anti-parallel β-sheet nanofibers at distance were sterically restricted from self-trimerizing, but they provided the nanofibers the capacity to target denatured collagen in vivo. Adapted with permission from ref 66. Copyright 2017 American Chemical Society. (e) Design of the self-assembled elastin- and collagen-like peptide conjugate (ELP-CLP) nanovesicle. Adapted with permission from ref 67. Copyright 2017 American Chemical Society.
Another approach involves CHP precursors with designed chemical modifications which can be removed when collagen-hybridization is demanded. A caged CHP can be designed by attaching a photo-cleavable nitrobenzyl (NB) group to the central Gly residue (Fig. 3a). This abolishes the CHP’s triple-helical folding, yet the NB cage can be released by UV exposure to trigger the CHP to hybridize.39 This photo-triggered hybridization allows photo-patterning of gelatin hydrogels111 as well as the first in vivo study of collagen hybridization.39 Recently, the Koide group developed a monomeric CHP precursor featuring a central O-acyl isopeptide unit to disrupt the triple-helical folding (Fig. 3a); upon changing from acidic to physiological pH, the inserted ester bond is converted to a peptide bond via an O-to-N acyl migration, allowing the peptide to regain its folding and collagen-binding ability.112
Dimeric CHPs.
Because the collagen-hybridization requires the formation of heterotrimeric helices, numerous constructs featuring covalently-tethered parallel CHP strands (dimeric CHPs) have emerged as novel designs to target single collagen chains.113,114 Koide and coworkers synthesized such dimeric CHPs using an N-terminal Lys branching unit and cyclic CHPs by combining Lys and Cys linkages at both termini (Fig. 3b).115 They revealed that the cyclic CHP could form a more stable hybridized product with collagen than the single-strand.115 Surprisingly, even the cyclic CHPs showed a tendency to self-assemble into homotrimers.61 Therefore, to avoid the pre-heating and disassembling step in detecting denatured collagen, the team optimized the cyclic design by introducing charged residues and incorporating two CHP chains with different lengths (Fig. 3b).61 Interestingly, the cyclic CHP construct featuring two (ProProGly)10 strands made by the Raines group was shown to be monomeric in solution and was utilized as a polypeptide model of naturally damaged collagen for evaluating CHP hybridization at the molecular level (Fig. 3c).116 Meanwhile, Yu and coworkers reported that the dimeric CHPs are particularly effective in capturing trace-levels of degraded collagen fragments from biological fluids (e.g., urine from osteopenic mice) and enable peptidomic analyses towards biomarker discovery and disease detection.117 Because only one collagen chain is needed to form a triple-helix with a dimeric CHP, it could be inferred that its hybridization with denatured collagen may be faster than a single-strand one. However, this and other advantages of the dimeric CHPs remain to be further verified in direct comparison to the single-strand counterparts, especially in tissues and in vivo.
Self-assembled nanostructures.
Nanomaterials have also been created to target denatured collagen via assembling CHPs into precise structures that limit their triple-helical self-association. By conjugating an anti-parallel β-sheet peptide motif to a CHP strand, the Yu group fabricated a self-assembled, water-soluble nanofiber that displays CHP single-strands at a fixed distance (Fig. 3d), thereby preventing their trimerization.66 This fiber maintained a high affinity to denatured collagen and enabled direct in vivo targeting of collagen remodeling without any pre-injection treatment.66 Furthermore, the Kiick group produced a set of thermoresponsive conjugates of elastin-collagen-like peptides (ELP-CLP) (Fig. 3e), which self-assembled into nanoscale vesicles displaying CHPs at the surface and showed strong retention on a type II collagen film.67 By adjusting the composition and length of the ELP and CLP domains, various spherical or rectangular nanostructures were further assembled and exhibited.118 These nano-assemblies provide new opportunities for controlled drug delivery to the matrices in pathological tissues.119
5. Biology of Collagen Hybridization
The collagen matrix is present in virtually all tissues (Table 1) and undergoes constant remodeling. Controlled collagen remodeling is essential to tissue morphogenesis during development, while its dysregulation can lead to pathological conditions, including fibrosis, inflammation, and cancer.8 The remodeling process mediated by specific proteases (e.g., metalloproteinases) may disrupt the collagen triple-helix. Besides, mechanical damages to connective tissues can also render the collagen triple-helix in a denatured state. Our understanding of the biological significance of collagen denaturation in disease and injury is still in its infancy, although evidence for the presence and disease-correlation of denatured collagen is emerging from dozens of recent studies120–123 enabled by the CHP-collagen hybridization (Table 1).
Table 1.
Selected examples of collagen denaturation reported in various diseases and conditions.
| Tissue | Conditions | Factors associated with collagen denaturation | ref | ||
|---|---|---|---|---|---|
| Biomechanics | Tissue remodeling | Other factors | |||
| bone | osteogenesis imperfecta | misfolding due to mutation | 146 | ||
| cartilage | rheumatoid arthritis | protease degradation, inflammation | 62,133 | ||
| osteoarthritis | |||||
| spine | intervertebral disc degeneration | mechanical damage | aging, inflammation, accumulation of AGEs | 48,49,136,137 | |
| heart | myocardial infarction | mechanical tension | immune response, inflammation, protease degradation | 130 | |
| tendon | in vitro testing | mechanical loading and cyclic fatigue damage | 72,124–128 | ||
| muscle | quadriceps muscle atrophy | fibrosis | 155 | ||
| eye | scleritis | protease degradation, inflammation | 139 | ||
| skin | chronic ultraviolet radiation | repair and remodeling | 156,157 | ||
| burn wounds | thermal burns | ||||
| artery | in vitro testing | mechanical stretching | 71 | ||
| liver | biliary atresia | progressive fibrosis | 47 | ||
| lung | fibrosis (bleomycin injury) | cathepsin K-mediated degradation | 144 | ||
Collagen denaturation related to biomechanics
In 2017, utilizing a fluorescent CHP, the Weiss and Yu groups reported that denaturation of collagen molecules can occur and accumulate during a monotonic stretch of a rat tail tendon fascicle once the stress-strain curve departs the linear region.6 This was the first report of visual detection of mechanical damage to collagen at the molecular level, which demonstrated that the unwinding of the collagen triple-helix is an important mechanism of mechanical damage to connective tissues.6 Subsequent research on tendon biomechanics72,124–128 not only described the mechanical properties of tendons with different anatomical functions (i.e., positional vs. energy-storing) from the molecular level,125,126 but also suggested that the unfolded collagen triple-helices can accumulate during cyclic fatigue loadings (Fig. 4a)72,124,127,128 and may precede changes in the local tissue mechanics.128 Interestingly, it was noted that there lacks a correlation between the levels of collagen fiber kinking and the molecular denaturation,128 which seemed to suggest that the structures at the fibrillar and molecular scales have different mechanical functions.124
Figure 4. Mechanically denatured collagen revealed via collagen hybridization.
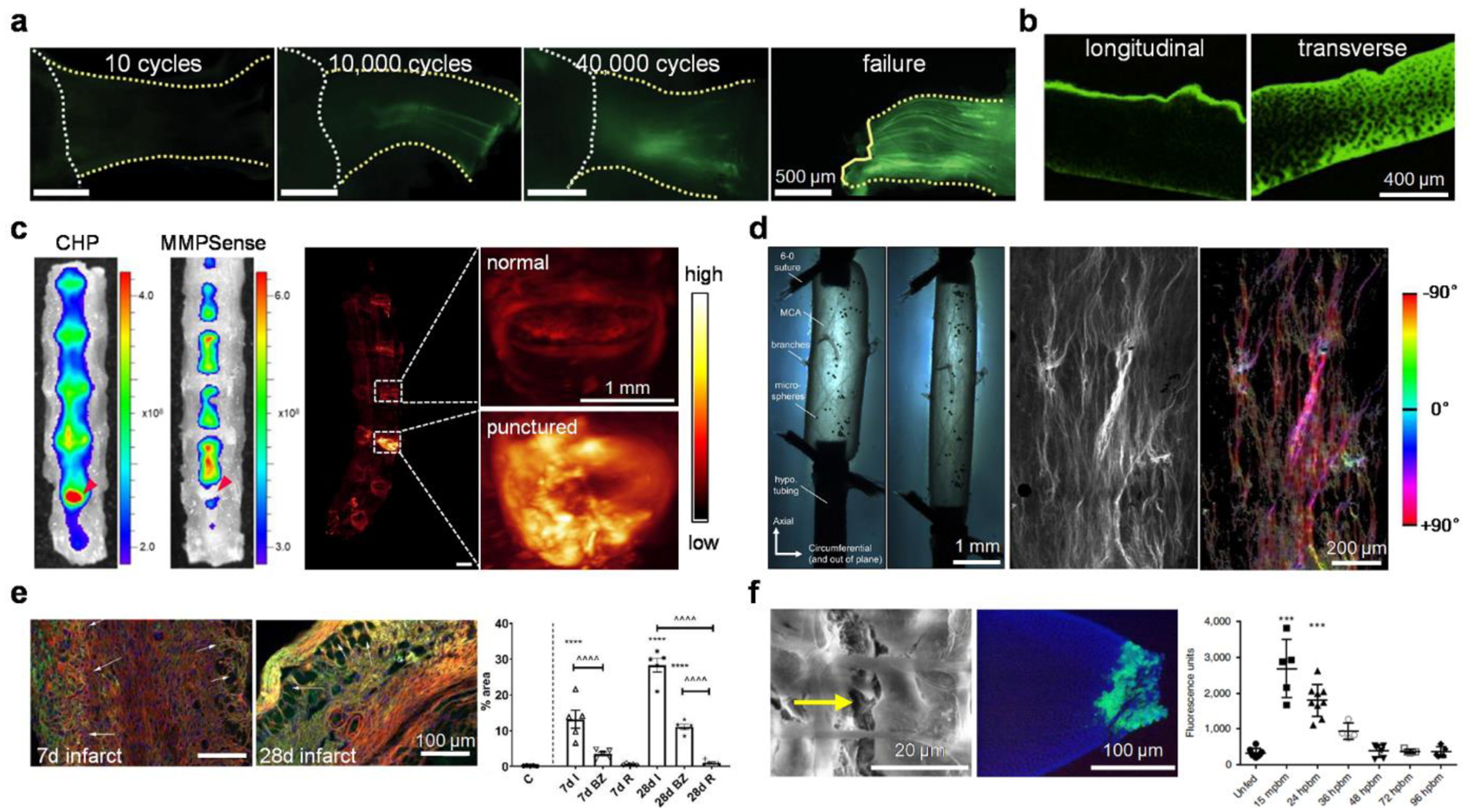
(a) Collagen damage accumulation in the mouse supraspinatus tendon, indicated by CHP fluorescence, increased with the number of loading cycles. CHP intensity was initially concentrated in a few fibers near the tendon mid-substance and ultimately propagated down the entire tendon in concentrated bands. Adapted with permission from ref 124. Copyright 2021 American Association for the Advancement of Science. (b) Bovine condylar cartilage specimens worn in the transverse direction have higher CHP staining than those worn in the longitudinal direction, suggesting more collagen damage. Adapted with permission from ref 129. Copyright 2020 Elsevier Ltd. (c) Left: Fluorescence imaging of lumbar spine specimens collected from a needle-punctured mouse model of intervertebral disc degeneration intravenously injected with CHP and MMPSense, showing prominent CHP binding to the damaged disc but the absence of MMPSense signals (red arrowheads). Right: 3D fluorescence scan of the lumbar spine after tissue clearing showed strong CHP signals in the punctured disc. Adapted with permission from ref 49. Copyright 2021 American Chemical Society. (d) Configuration for axial overstretch of a sheep middle cerebral artery (left); CHP imaging and quantitative analysis showed that the damaged collagen fibers were mainly aligned with the loading direction in the axial orientation (±90 degrees, right). Adapted with permission from ref 71. Copyright 2017 Acta Materialia. (e) During the proliferative phase of cardiac repair (7d after coronary occlusion), denatured collagen was concentrated pericellularly in the highly cellular healing infarct areas (arrows). During the maturation phase (28d infarct), although the scar has a low cellular content, it exhibited a marked increase in collagen denaturation in the infarct zoom (arrows), likely reflecting mechanical tension (red: wheat germ agglutinin, green: CHP, blue: DAPI; bottom: quantitative image analysis, BZ: border zone, I: infarct, R: remote myocardium). Adapted with permission from ref 130. Copyright 2021 Elsevier. (f) Left: Engorged A. aegypti mosquito midguts with micro-perforations in the basal lamina (yellow arrow) with clear signs of epithelial infection (green: Zika virus antigen) post-blood-meal (pbm). Right: high degrees of CHP binding to the midguts within 15 min and up to 36 h pbm. Adapted with permission from ref 131. Copyright 2019 Springer Nature.
Apart from the tendon studies, Wagner and coworkers discovered that the bovine cartilage specimens worn with loading in the direction orthogonal (transverse) to the collagen fiber orientation at the articular surface had collagen damage extended through greater depth of the tissue than those worn in the directions parallel (longitudinal) to the collagen fibers (Fig. 4b).129 This study revealed a new anisotropic mechanical behavior of articular cartilage. Moreover, the Li group reported the live animal imaging and 3D visualization of collagen destruction to the soft tissues in rodent spines with fluorescent CHP probes.49 The study revealed that the collagen destruction was localized to the load-bearing anatomical components including the annulus fibrosus and facet joints in normal spines, where aging, tensile force, and disc degeneration (e.g., Fig. 4c) can escalate the collagen damage. Interestingly, prominent CHP binding was noted in the degenerated intervertebral disc (IVD) in regions where no MMP activities were detected (Fig. 4c),49 seemingly suggesting that the observed collagen destruction within the torn IVDs in this needle-puncture mouse model is more likely to correlate with mechanical disruption rather than pathological proteolysis.
More interestingly, collagen denaturation caused by mechanical factors outside the musculoskeletal soft tissues were also interrogated recently. The Frangogiannis group uncovered the distinct spatiotemporal patterns and molecular mechanisms of collagen denaturation throughout the myocardial infarction process (Fig. 4e):130 while the denatured collagen molecules are found near the macrophages and myofibroblasts with upregulated MMP14 during the early inflammatory and proliferative phases, in the maturation phase of infarct healing, extensive denaturation of the collagen fibers is noted in the hypocellular infarct, the border zone, and the mitral valve annulus where MMP14 are absent. They, therefore, reasoned that instead of proteolysis, this collagen denaturation may be caused by the mechanical tension from the contraction of the viable cardiomyocytes and the increased intraventricular pressure.130 This is the first study proposing a new biomechanical, non-inflammatory mechanism of collagen damage in myocardial scarring. Interestingly, by labeling the disrupted collagen IV in the mosquito midgut with a fluorescently-tagged CHP, Armstrong, Brackney, and colleagues found that virus dissemination within mosquitoes can be facilitated by the expansion of and micro-perforations in the midgut basal lamina following successive blood acquisitions (Fig. 4f).131
Collagen denaturation related to remodeling
Collagen denaturation related to biological tissue remodeling (e.g., due to inflammation, Fig. 5) can be prevalent in the musculoskeletal or connective tissues thanks to their high collagen contents. For example, while studying how bone fracture healing is delayed by obesity and prediabetic hyperglycemia, Elbarbary and colleagues discovered that unfolded collagen molecules could pathologically accumulate in the diet-induced obesity (DIO) mice with fracture healing defects during endochondral ossification.132 They reasoned that the DIO-related changes in the fibrillar collagen structure can be partially attributed to the accumulation of advanced glycation end products (AGEs) that increase collagen-fiber crosslinking. Also, Haqqi and colleagues showed that mitochondrial dysfunction in chondrocytes and cartilage explants from patients with OA increased the mitochondrial superoxide production, the expression of inflammatory factors, and the degradation of type II collagen in the cartilage matrix.133 The correlation of intervertebral disc (IVD) degeneration with aging,48 inflammatory factors,48 dysregulated osmoadaptation,134 accumulation of AGEs (Fig. 5a),135,136 and ex vivo mechanical culturing conditions137 have been assessed histologically with CHPs to show the denatured collagen in the annulus fibrosus of the IVDs. The cornea and sclera are connective tissues formed by networks of collagen fibrils. The in vivo collagen degradation and disruption in mouse corneas caused by an inflammatory response to virus infection was readily visualized by CHPs.138 Similarly, another mouse study showed that the inflammatory stimulation of interleukin 1-β (1L1-β) results in significant scleral collagen degradation, which can be rescued by a dexamethasone treatment (Fig. 5b).139
Figure 5. Collagen denaturation associated with pathological remodeling revealed via collagen hybridization.
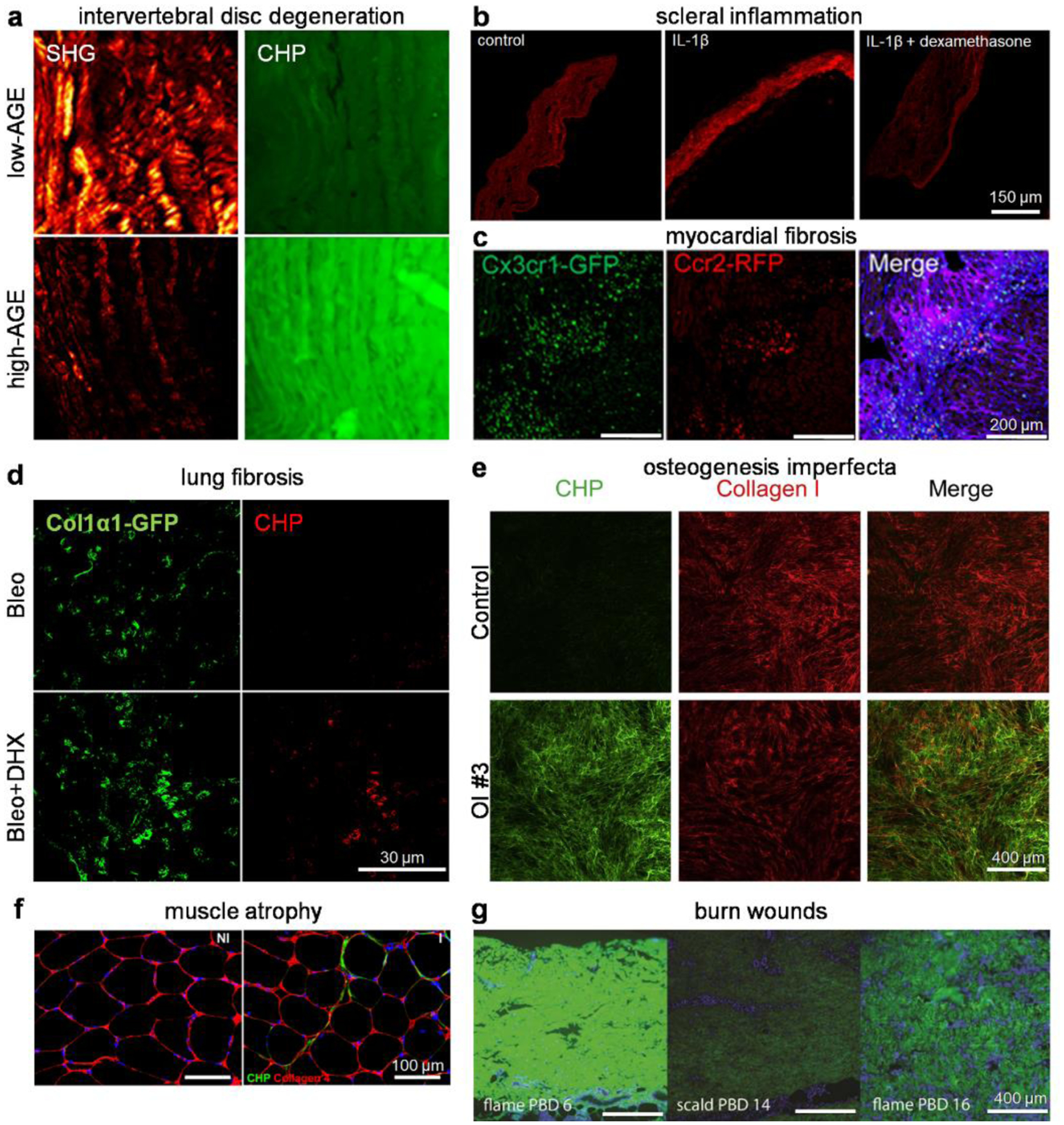
(a) High advanced glycation end product (AGE) diets caused collagen degradation in the central anterior annulus fibrosus of mouse intervertebral disks, as shown by a marked decrease in SHG intensity and an increase in CHP staining. Adapted with permission under a Creative Commons Attribution 4.0 License from ref 135. Copyright 2020 Wiley Periodicals LLC. (b) The IL1-β-induced collagen degradation in organotypic cultured mouse scleral tissue was abolished with dexamethasone treatment. Adapted with permission from ref 139. Copyright 2021 Springer Nature. (c) Histological images of ischemia-reperfusion injured hearts of mice that received a bone marrow mononuclear cell treatment showed localization of CCR2+ and CX3CR1+ macrophages within areas of active collagen remodeling (CHP: purple) in the infarct border zone. Adapted with permission from ref 142. Copyright 2019 Springer Nature. (d) Dihydrexidine (DHX) treatment promoted fibroblast-mediated collagen degradation, evidenced by an enhancement in CHP staining that colocalized with Col1α1-GFP+ fibroblasts in fibrotic lungs of mice treated with DHX in addition to bleomycin. Adapted with permission from ref 144. Copyright 2020 The Company of Biologists Ltd. (e) The ECM produced by cultured dermal fibroblasts isolated from patients with osteogenesis imperfecta (OI) contained a heavy portion of misfolded collagen I, estimated by the fluorescence ratio between the CHP and collagen I stains. Adapted with permission under a Creative Commons Attribution 4.0 License from ref 146. Copyright 2020 Elsevier Inc. (f) Enhanced CHP staining indicative of greater ECM turnover was noted in the injured (I) limb quadriceps muscle compared to the non-injured (NI) ones. Adapted with permission from ref 155. Copyright 2019 SAGE Publications. (g) Collagen denaturation assessed by CHP staining on human burn wound eschar after scalding or flame burn at different post-burn days (PBD). Adapted with permission from ref 156. Copyright 2020 Wound Healing Society.
Collagen denaturation has been linked to tissue remodeling during development and healing.140–142 The Yutzey team measured the level of collagen remodeling in postnatal mouse hearts, revealing a cardiac ECM transition from fibronectin to fibrillar collagen during heart maturation after birth.141 The Molkentin group showed that intracardiac injection of stem cells induces the accumulation of CX3CR1+ and CCR2+ macrophages in the active remodeling region of the heart (Fig. 5c), which can reduce fibrosis and enhance the mechanical properties of the injured area by regulating the activity of local fibroblasts.142 In a recent article about tendon healing, Huang and colleagues marked the persistent collagen damage with CHPs in the transected and unhealed Achilles tendon from neonatal mice with regulatory T cell (Treg) ablation, suggesting that the neonatal Tregs are critical for promoting tendon regeneration.143
Collagen denaturation has also been implicated in the resolution of organ fibrosis. The hallmark of pulmonary fibrosis, a progressive and devastating interstitial lung disorder, is the excessive deposition of ECM proteins, primarily type I collagen, by activated fibroblasts. Through probing the fibrotic lung tissues from the bleomycin mouse model with CHPs and a hydroxyproline antibody, Song et al. followed the disease progression with extended durations and located large amounts of hydroxyproline-rich, denatured collagen fragments accumulating intracellularly 10–16 weeks post the bleomycin-treatment. This study strongly supported that the fibrotic condition of the bleomycin mouse model undergoes spontaneous resolution following the peak of fibrogenesis (4 weeks post-bleo), a notion that had been under debate.46 In the lung tissues from the Col1α1-GFP+ mice with the bleomycin-induced injury, Haak and colleagues discovered that those CHP-labeled intracellular collagen fragments highly colocalize with the GFP+ fibroblasts when the mice had been treated with dihydrexidine, an agonist of dopamine receptor D1 (DRD1). This study indicated that the DRD1 agonism can stimulate a cathepsin K-mediated fibrosis resorption in the lung fibroblasts in vivo (Fig. 5d),144 implying a therapeutic potential of this pathway.
Collagen denaturation related to other factors
The collagen triple-helix has been found to be unfolded by different means other than biomechanics and tissue remodeling. While developing new vision-correction laser approaches, Huang et al. visualized the photo-induced denaturation of collagen in the rabbit corneal stroma caused by femtosecond laser through fluorescent CHP staining.145 Inspiringly, Takeyari et al. revealed that the collagen deposited by fibroblasts derived from patients of osteogenesis imperfecta, a bone disorder mainly caused by genetic mutations of collagen I, contains excessive misfolded triple-helices, while treatment with the chemical chaperone 4-phenylbutyric acid normalizes the collagen misfolding (Fig. 5e).146 Besides, the collagen hybridization technique has become a widely adopted quality-control method for measuring collagen denaturation in the decellularized ECM scaffolds prepared by various detergents and methods.73,147,148 It has also been creatively utilized in various investigations of cell-matrix interactions inside 3D collagen matrices149–151 and the biology of non-mammalian organisms.44,152
6. Outlook
For decades, the scientific interrogations of the pathological changes to the ECM have been focused on the abundance, composition, and mechanical strength (e.g., in fibrosis),8 whereas less attention has been paid to the finest underlying structural changes at the molecular level.153,154 As the collagen hybridization methodology becomes widely adopted, there is growing evidence of the extensive collagen denaturation as a structural signature of the ECMs in a broad range of diseases and injuries. Accordingly, a series of fundamental questions regarding the chemical and biological nature of collagen denaturation are starting to emerge. For instance, what are the exact proteases, cell phenotypes, and cytokines that mediate the collagen degradation and denaturation in each pathological remodeling event? What are the differences in the biological role between intact and denatured collagen in tissues? Specifically, how does denatured collagen affect the essential physiological or pathological processes, such as wound healing, immunoregulation, and responding to mechanical stimuli, through specific receptor-mediated signaling pathways? What are the recognition sites for these cellular receptors in the denatured collagen? Are they different from the ones found in the intact collagen? What is the structure of the denatured collagen and how is it different from the intact triple-helix depicted by crystallography? What is the biological fate of denatured collagen in health and disease? How is the denatured collagen catabolized extracellularly and intracellularly? Do collagen peptide fragments in body fluids exhibit distinctive profiles under various pathological conditions?
As our understanding of the principles governing the folding of the collagen triple-helix advances, new peptides and peptidomimetics with strong collagen-hybridizing capacities will be developed for improved affinity with limited self-trimerization. Furthermore, CHP libraries can be built to screen for candidates that target the denatured chains from specific collagen types or tissues (e.g., type II collagen in cartilage). We need these novel molecular tools to answer the above-mentioned fundamental questions of matrix biology and to further clarify the biomedical significance of the damaged collagen molecules as a biomarker. These scientific explorations and technological developments will lead to novel diagnostic and therapeutic strategies for unmet medical needs. For instance, a new class of contrast agents and biochemical assays targeting collagen remodeling can be created for the non-invasive detection, prognostic monitoring, and therapeutic efficacy assessment of fibrosis and degenerative diseases. Meanwhile, the collagen hybridization approach is now being employed to deliver therapeutic agents including small molecules, peptides, and antibodies to targeted lesions.62,64 Moreover, with the expansion of the “alphabet” of collagen sequences by unnatural residues,95,106 new CHPs with desired biological activities can be screened from billions of sequences to not only anneal to the damaged collagen in lesions, but also interact with specific cellular receptors or enzymes for therapeutic goals including wound management, bone regeneration, and fibrosis treatment. Therefore, we anticipate the long-awaited new era of collagens as targets for emerging biomedical technologies.
Acknowledgments
The authors gratefully acknowledge support from the National Natural Science Foundation of China (92059104 and 82071977), the 2018 High-level Health Team of Zhuhai to Y.L., as well as support from the National Institutes of Health (R01OD026618 and R01AR071358 to S.M.Y.). Figure 1b was created by Biorender.com.
Footnotes
Conflict of interest
S.M.Y. and Y.L. are founders of 3Helix Inc, which commercializes collagen hybridizing peptides.
Reference
- (1).Shoulders MD; Raines RT Collagen structure and stability. Annu. Rev. Biochem 2009, 78 (1), 929–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Liotta LA; Kohn EC The microenvironment of the tumour–host interface. Nature 2001, 411 (6835), 375–379. [DOI] [PubMed] [Google Scholar]
- (3).Finn AV; Nakano M; Narula J; Kolodgie FD; Virmani R Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol 2010, 30 (7), 1282–1292. [DOI] [PubMed] [Google Scholar]
- (4).Jenkins RG; Simpson JK; Saini G; Bentley JH; Russell A-M; Braybrooke R; Molyneaux PL; McKeever TM; Wells AU; Flynn A; Hubbard RB; Leeming DJ; Marshall RP; Karsdal MA; Lukey PT; Maher TM Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: An analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015, 3 (6), 462–472. [DOI] [PubMed] [Google Scholar]
- (5).Decaris ML; Li KW; Emson CL; Gatmaitan M; Liu S; Wang Y; Nyangau E; Colangelo M; Angel TE; Beysen C; Cui J; Hernandez C; Lazaro L; Brenner DA; Turner SM; Hellerstein MK; Loomba R Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. Hepatology 2017, 65 (1), 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Zitnay JL; Li Y; Qin Z; San BH; Depalle B; Reese SP; Buehler MJ; Yu SM; Weiss JA Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat Commun. 2017, 8, 14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bella J; Eaton M; Brodsky B; Berman HM Crystal and molecular structure of a collagen-like peptide at 1.9 Å resolution. Science 1994, 266 (5182), 75–81. [DOI] [PubMed] [Google Scholar]
- (8).Bonnans C; Chou J; Werb Z Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014, 15 (12), 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Fields GB Interstitial collagen catabolism. J. Biol. Chem 2013, 288 (13), 8785–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Danielsen CC Thermal stability of human-fibroblast-collagenase-cleavage products of type-I and type-III collagens. Biochem. J 1987, 247 (3), 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chung L; Dinakarpandian D; Yoshida N; Lauer-Fields JL; Fields GB; Visse R; Nagase H Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004, 23 (15), 3020–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Fisher GJ; Kang S; Varani J; Bata-Csorgo Z; Wan Y; Datta S; Voorhees JJ Mechanisms of photoaging and chronological skin aging. Arch. Dermatol 2002, 138 (11), 1462–1470. [DOI] [PubMed] [Google Scholar]
- (13).Willett TL; Labow RS; Avery NC; Lee JM Increased proteolysis of collagen in an in vitro tensile overload tendon model. Ann. Biomed. Eng 2007, 35 (11), 1961–1972. [DOI] [PubMed] [Google Scholar]
- (14).Veres SP; Harrison JM; Lee JM Mechanically overloading collagen fibrils uncoils collagen molecules, placing them in a stable, denatured state. Matrix Biol. 2014, 33, 54–59. [DOI] [PubMed] [Google Scholar]
- (15).Sun X; Wu B; Chiang HC; Deng H; Zhang X; Xiong W; Liu J; Rozeboom AM; Harris BT; Blommaert E; Gomez A; Garcia RE; Zhou Y; Mitra P; Prevost M; Zhang D; Banik D; Isaacs C; Berry D; Lai C; Chaldekas K; Latham PS; Brantner CA; Popratiloff A; Jin VX; Zhang N; Hu Y; Pujana MA; Curiel TJ; An Z; Li R Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 2021, 599 (7886), 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Di Martino JS; Nobre AR; Mondal C; Taha I; Farias EF; Fertig EJ; Naba A; Aguirre-Ghiso JA; Bravo-Cordero JJ A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer 2022, 3 (1), 90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).An B; Lin Y-S; Brodsky B Collagen interactions: Drug design and delivery. Adv. Drug Del. Rev 2016, 97, 69–84. [DOI] [PubMed] [Google Scholar]
- (18).Barczyk MM; Carracedo S; Gullberg D Integrins. Cell Tissue Res. 2009, 339, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chen X; Nadiarynkh O; Plotnikov S; Campagnola PJ Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc 2012, 7 (4), 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zeldin L; Mosley GE; Laudier D; Gallate ZS; Gansau J; Hoy RC; Poeran J; Iatridis JC Spatial mapping of collagen content and structure in human intervertebral disk degeneration. JOR SPINE 2020, 3 (4), e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bremer C; Tung C-H; Weissleder R In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat. Med 2001, 7 (6), 743–748. [DOI] [PubMed] [Google Scholar]
- (22).Nielsen LF; Moe D; Kirkeby S; Garbarsch C Sirius red and acid fuchsin staining mechanisms. Biotech. Histochem 1998, 73 (2), 71–77. [DOI] [PubMed] [Google Scholar]
- (23).Dayan D; Hiss Y; Hirshberg A; Bubis J; Wolman M Are the polarization colors of Picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 2004, 93, 27–29. [DOI] [PubMed] [Google Scholar]
- (24).Dodge GR; Poole AR Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J. Clin. Invest 1989, 83 (2), 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Hollander AP; Heathfield TF; Webber C; Iwata Y; Bourne R; Rorabeck C; Poole AR Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J. Clin. Invest 1994, 93 (4), 1722–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Billinghurst RC; Dahlberg L; Ionescu M; Reiner A; Bourne R; Rorabeck C; Mitchell P; Hambor J; Diekmann O; Tschesche H; Chen J; Van Wart H; Poole AR Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest 1997, 99 (7), 1534–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Poole AR; Ionescu M; Fitzcharles M-A; Billinghurst RC The assessment of cartilage degradation in vivo: Development of an immunoassay for the measurement in body fluids of type II collagen cleaved by collagenases. J. Immunol. Methods 2004, 294 (1–2), 145–153. [DOI] [PubMed] [Google Scholar]
- (28).Hollander AP; Pidoux I; Reiner A; Rorabeck C; Bourne R; Poole AR Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J. Clin. Invest 1995, 96 (6), 2859–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Xu J; Rodriguez D; Kim JJ; Brooks PC Generation of monoclonal antibodies to cryptic collagen sites by using subtractive immunization. Hybridoma 2000, 19 (5), 375–385. [DOI] [PubMed] [Google Scholar]
- (30).Han X; Caron JM; Brooks PC Cryptic collagen elements as signaling hubs in the regulation of tumor growth and metastasis. J. Cell. Physiol 2020, 235 (12), 9005–9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cretu A; Roth JM; Caunt M; Akalu A; Policarpio D; Formenti S; Gagne P; Liebes L; Brooks PC Disruption of endothelial cell interactions with the novel HU177 cryptic collagen epitope inhibits angiogenesis. Clin. Cancer. Res 2007, 13 (10), 3068–3078. [DOI] [PubMed] [Google Scholar]
- (32).Freimark B; Clark D; Pernasetti F; Nickel J; Myszka D; Baeuerle PA; Van Epps D Targeting of humanized antibody D93 to sites of angiogenesis and tumor growth by binding to multiple epitopes on denatured collagens. Mol. Immunol 2007, 44 (15), 3741–3750. [DOI] [PubMed] [Google Scholar]
- (33).Caron JM; Ames JJ; Contois L; Liebes L; Friesel R; Muggia F; Vary CPH; Oxburgh L; Brooks PC Inhibition of ovarian tumor growth by targeting the HU177 cryptic collagen epitope. Am. J. Pathol 2016, 186 (6), 1649–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Robert F; Gordon MS; Rosen LS; Mendelson DS; Mulay M; Adams BJ; Alvarez D; Theuer CP; Leigh BR Final results from a phase I study of TRC093 (humanized anti-cleaved collagen antibody) in patients with solid cancer. J. Clin. Oncol 2010, 28 (15_suppl), 3038–3038. [Google Scholar]
- (35).Ng B; Zakrzewski J; Warycha M; Christos PJ; Bajorin DF; Shapiro RL; Berman RS; Pavlick AC; Polsky D; Mazumdar M; Montgomery A; Liebes L; Brooks PC; Osman I Shedding of distinct cryptic collagen epitope (HU177) in sera of melanoma patients. Clin. Cancer. Res 2008, 14 (19), 6253–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hamilton HK; Rose AE; Christos PJ; Shapiro RL; Berman RS; Mazumdar M; Ma MW; Krich D; Liebes L; Brooks PC; Osman I Increased shedding of HU177 correlates with worse prognosis in primary melanoma. J. Transl. Med 2010, 8 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Li Y; Yu SM Targeting and mimicking collagens via triple helical peptide assembly. Curr. Opin. Chem. Biol 2013, 17 (6), 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Persikov AV; Ramshaw JAM; Kirkpatrick A; Brodsky B Amino acid propensities for the collagen triple-helix. Biochemistry 2000, 39 (48), 14960–14967. [DOI] [PubMed] [Google Scholar]
- (39).Li Y; Foss CA; Summerfield DD; Doyle JJ; Torok CM; Dietz HC; Pomper MG; Yu SM Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (37), 14767–14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Li Y; Ho D; Meng H; Chan TR; An B; Yu H; Brodsky B; Jun AS; Michael Yu S Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconjug. Chem 2013, 24 (1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Hwang J; Huang Y; Burwell TJ; Peterson NC; Connor J; Weiss SJ; Yu SM; Li Y In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS nano 2017, 11 (10), 9825–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Casals C; García-Fojeda B; Minutti CM Soluble defense collagens: Sweeping up immune threats. Mol. Immunol 2019, 112, 291–304. [DOI] [PubMed] [Google Scholar]
- (43).Lukomski S; Bachert BA; Squeglia F; Berisio R Collagen-like proteins of pathogenic streptococci. Mol. Microbiol 2017, 103 (6), 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Ellison AJ; Dempwolff F; Kearns DB; Raines RT Role for cell-surface collagen of streptococcus pyogenes in infections. ACS Infect Dis. 2020, 6 (7), 1836–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Bennink LL; Smith DJ; Foss CA; Pomper MG; Li Y; Yu SM High serum stability of collagen hybridizing peptides and their fluorophore conjugates. Mol. Pharm 2017, 14 (6), 1906–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Song S; Fu Z; Guan R; Zhao J; Yang P; Li Y; Yin H; Lai Y; Gong G; Zhao S; Yu J; Peng X; He Y; Luo Y; Zhong N; Su J Intracellular hydroxyproline imprinting following resolution of bleomycin-induced pulmonary fibrosis. Eur. Respir. J 2022, 59 (5), 2100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Jaramillo C; Guthery SL; Lowichik A; Stoddard G; Kim T; Li Y; Jensen MK Quantitative liver fibrosis using collagen hybridizing peptide to predict native liver survival in biliary atresia: A pilot study. J. Pediatr. Gastroenterol. Nutr 2020, 70 (1), 87–92. [DOI] [PubMed] [Google Scholar]
- (48).Xiao L; Majumdar R; Dai J; Li Y; Xie L; Shen FH; Jin L; Li X Molecular detection and assessment of intervertebral disc degeneration via a collagen hybridizing peptide. ACS Biomater Sci Eng. 2019, 5 (4), 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Liu L; Huang K; Li W; Qiu R; Fang Y; Huang Y; Zhao S; Lv H; Zhang K; Shan H; Li Y Molecular imaging of collagen destruction of the spine. ACS Nano 2021, 15 (12), 19138–19149. [DOI] [PubMed] [Google Scholar]
- (50).Bennink LL; Li Y; Kim B; Shin IJ; San BH; Zangari M; Yoon D; Yu SM Visualizing collagen proteolysis by peptide hybridization: From 3D cell culture to in vivo imaging. Biomaterials 2018, 183, 67–76. [DOI] [PubMed] [Google Scholar]
- (51).Shumpei S; Yasuo K; Yasuo K; Rume S; Kinji K Synthesis of poly-(L-prolyl-L-prolylglycyl) of defined molecular weights. Bull. Chem. Soc. Jpn 1968, 41 (5), 1273–1273. [Google Scholar]
- (52).Heidemann ER; Harrap BS; Schiele HD Hybrid formation between collagen and synthetic polypeptides. Biochemistry 1973, 12 (16), 2958–2963. [DOI] [PubMed] [Google Scholar]
- (53).Wang AY; Mo X; Chen CS; Yu SM Facile modification of collagen directed by collagen mimetic peptides. J. Am. Chem. Soc 2005, 127 (12), 4130–4131. [DOI] [PubMed] [Google Scholar]
- (54).Yu SM; Li Y; Kim D Collagen mimetic peptides: Progress towards functional applications. Soft Matter 2011, 7 (18), 7927–7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Nielsen PE; Egholm M; Berg RH; Buchardt O Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254 (5037), 1497–1500. [DOI] [PubMed] [Google Scholar]
- (56).Raines RT: Collagen structure and stability. National Institutes of Health: University of Wisconsin-Madison, 2000. [Google Scholar]
- (57).Hodges JA: Strand invasion of the collagen triple helix. National Institutes of Health: University of Wisconsin-Madison, 2001. [Google Scholar]
- (58).Kotch FW: Invasion and association of tethered collagen mimetics. National Institutes of Health: University of Wisconsin-Madison, 2004. [Google Scholar]
- (59).Chattopadhyay S; Murphy CJ; McAnulty JF; Raines RT Peptides that anneal to natural collagen in vitro and ex vivo. Org. Biomol. Chem 2012, 10 (30), 5892–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Cai X; Liu Z; Zhao S; Song C; Dong S; Xiao J A single stranded fluorescent peptide probe for targeting collagen in connective tissues. Chem. Commun 2017, 53 (87), 11905–11908. [DOI] [PubMed] [Google Scholar]
- (61).Takita KK; Fujii KK; Ishii K; Koide T Structural optimization of cyclic peptides that efficiently detect denatured collagen. Org. Biomol. Chem 2019, 17 (31), 7380–7387. [DOI] [PubMed] [Google Scholar]
- (62).Arlotta KJ; San BH; Mu H-H; Yu SM; Owen SC Localization of therapeutic Fab-CHP conjugates to sites of denatured collagen for the treatment of rheumatoid arthritis. Bioconjug. Chem 2020, 31 (8), 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Chattopadhyay S; Teixeira LBC; Kiessling LL; McAnulty JF; Raines RT Bifunctional peptide that anneals to damaged collagen and clusters TGF-β receptors enhances wound healing. ACS Chem. Biol 2022, 17 (2), 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Chattopadhyay S; Guthrie KM; Teixeira L; Murphy CJ; Dubielzig RR; McAnulty JF; Raines RT Anchoring a cytoactive factor in a wound bed promotes healing. J. Tissue Eng. Regen. Med 2016, 10 (12), 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Ellison AJ; Raines RT A pendant peptide endows a sunscreen with water-resistance. Org. Biomol. Chem 2018, 16 (39), 7139–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).San BH; Hwang J; Sampath S; Li Y; Bennink LL; Yu SM Self-assembled water-soluble nanofibers displaying collagen hybridizing peptides. J. Am. Chem. Soc 2017, 139 (46), 16640–16649. [DOI] [PubMed] [Google Scholar]
- (67).Luo T; David MA; Dunshee LC; Scott RA; Urello MA; Price C; Kiick KL Thermoresponsive elastin-b-collagen-like peptide bioconjugate nanovesicles for targeted drug delivery to collagen-containing matrices. Biomacromolecules 2017, 18 (8), 2539–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Jin H-E; Farr R; Lee S-W Collagen mimetic peptide engineered M13 bacteriophage for collagen targeting and imaging in cancer. Biomaterials 2014, 35 (33), 9236–9245. [DOI] [PubMed] [Google Scholar]
- (69).San BH; Li Y; Tarbet EB; Yu SM Nanoparticle assembly and gelatin binding mediated by triple helical collagen mimetic peptide. ACS Appl Mater Interfaces 2016, 8 (31), 19907–19915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Wahyudi H; Reynolds AA; Li Y; Owen SC; Yu SM Targeting collagen for diagnostic imaging and therapeutic delivery. J. Control. Release 2016, 240, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Converse MI; Walther RG; Ingram JT; Li Y; Yu SM; Monson KL Detection and characterization of molecular-level collagen damage in overstretched cerebral arteries. Acta Biomater. 2018, 67, 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Zitnay JL; Jung GS; Lin AH; Qin Z; Li Y; Yu SM; Buehler MJ; Weiss JA Accumulation of collagen molecular unfolding is the mechanism of cyclic fatigue damage and failure in collagenous tissues. Sci Adv. 2020, 6 (35), eaba2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Hwang J; San BH; Turner NJ; White LJ; Faulk DM; Badylak SF; Li Y; Yu SM Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomater. 2017, 53, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Brodsky B; Persikov AV Molecular structure of the collagen triple helix. In Adv. Protein Chem, Academic Press: 2005; Vol. 70, pp 301–339. [DOI] [PubMed] [Google Scholar]
- (75).Hulgan SAH; Hartgerink JD Recent advances in collagen mimetic peptide structure and design. Biomacromolecules 2022, 23 (4), 1475–1489. [DOI] [PubMed] [Google Scholar]
- (76).Jenkins CL; Vasbinder MM; Miller SJ; Raines RT Peptide bond isosteres: Ester or (E)-alkene in the backbone of the collagen triple helix. Org. Lett 2005, 7 (13), 2619–2622. [DOI] [PubMed] [Google Scholar]
- (77).Newberry RW; VanVeller B; Raines RT Thioamides in the collagen triple helix. Chem. Commun 2015, 51 (47), 9624–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Engel J; Bächinger HP Structure, stability and folding of the collagen triple helix. In Collagen: Primer in Structure, Processing and Assembly, Brinckmann J; Notbohm H; Müller PK, Eds. Springer Berlin Heidelberg: Berlin, Heidelberg, 2005; pp 7–33. [Google Scholar]
- (79).Dai N; Wang XJ; Etzkorn FA The effect of a trans-locked Gly-Pro alkene isostere on collagen triple helix stability. J. Am. Chem. Soc 2008, 130 (16), 5396–5397. [DOI] [PubMed] [Google Scholar]
- (80).Newberry RW; Raines RT The n→π* interaction. Acc. Chem. Res 2017, 50 (8), 1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Melton SD; Smith MS; Chenoweth DM Incorporation of aza-glycine into collagen peptides. J Org Chem. 2020, 85 (3), 1706–1711. [DOI] [PubMed] [Google Scholar]
- (82).Zhang Y; Malamakal RM; Chenoweth DM Aza-glycine induces collagen hyperstability. J. Am. Chem. Soc 2015, 137 (39), 12422–12425. [DOI] [PubMed] [Google Scholar]
- (83).Zhang Y; Herling M; Chenoweth DM General solution for stabilizing triple helical collagen. J. Am. Chem. Soc 2016, 138 (31), 9751–9754. [DOI] [PubMed] [Google Scholar]
- (84).Kasznel AJ; Zhang Y; Hai Y; Chenoweth DM Structural basis for aza-glycine stabilization of collagen. J. Am. Chem. Soc 2017, 139 (28), 9427–9430. [DOI] [PubMed] [Google Scholar]
- (85).Berg RA; Prockop DJ The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem. Biophys. Res. Commun 1973, 52 (1), 115–120. [DOI] [PubMed] [Google Scholar]
- (86).Holmgren SK; Taylor KM; Bretscher LE; Raines RT Code for collagen’s stability deciphered. Nature 1998, 392 (6677), 666–667. [DOI] [PubMed] [Google Scholar]
- (87).Holmgren SK; Bretscher LE; Taylor KM; Raines RT A hyperstable collagen mimic. Chem. Biol 1999, 6 (2), 63–70. [DOI] [PubMed] [Google Scholar]
- (88).Erdmann RS; Wennemers H Functionalizable collagen model peptides. J. Am. Chem. Soc 2010, 132 (40), 13957–13959. [DOI] [PubMed] [Google Scholar]
- (89).Aronoff MR; Egli J; Schmitt A; Wennemers H Alkylation of γ-azaproline creates conformationally adaptable proline derivatives for pH-responsive collagen triple helices. Chem. Eur. J 2020, 26 (22), 5070–5074. [DOI] [PubMed] [Google Scholar]
- (90).Aronoff MR; Egli J; Menichelli M; Wennemers H γ-Azaproline confers pH responsiveness and functionalizability on collagen triple helices. Angew. Chem. Int. Ed. Engl 2019, 58 (10), 3143–3146. [DOI] [PubMed] [Google Scholar]
- (91).Siebler C; Erdmann RS; Wennemers H Switchable proline derivatives: Tuning the conformational stability of the collagen triple helix by pH changes. Angew. Chem. Int. Ed. Engl 2014, 53 (39), 10340–10344. [DOI] [PubMed] [Google Scholar]
- (92).Egli J; Siebler C; Köhler M; Zenobi R; Wennemers H Hydrophobic moieties bestow fast-folding and hyperstability on collagen triple helices. J. Am. Chem. Soc 2019, 141 (14), 5607–5611. [DOI] [PubMed] [Google Scholar]
- (93).Egli J; Esposito C; Müri M; Riniker S; Wennemers H Influence of lipidation on the folding and stability of collagen triple helices-an experimental and theoretical study. J. Am. Chem. Soc 2021, 143 (15), 5937–5942. [DOI] [PubMed] [Google Scholar]
- (94).Hentzen NB; Smeenk LEJ; Witek J; Riniker S; Wennemers H Cross-linked collagen triple helices by oxime ligation. J. Am. Chem. Soc 2017, 139 (36), 12815–12820. [DOI] [PubMed] [Google Scholar]
- (95).Aronoff MR; Hiebert P; Hentzen NB; Werner S; Wennemers H Imaging and targeting LOX-mediated tissue remodeling with a reactive collagen peptide. Nat. Chem. Biol 2021, 17 (8), 865–871. [DOI] [PubMed] [Google Scholar]
- (96).Bella J Collagen structure: New tricks from a very old dog. Biochem. J 2016, 473 (8), 1001–1025. [DOI] [PubMed] [Google Scholar]
- (97).Maaßen A; Gebauer JM; Theres Abraham E; Grimm I; Neudörfl JM; Kühne R; Neundorf I; Baumann U; Schmalz HG Triple-helix-stabilizing effects in collagen model peptides containing PPII-helix-preorganized diproline modules. Angew. Chem. Int. Ed. Engl 2020, 59 (14), 5747–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Ramshaw JA; Shah NK; Brodsky B Gly-X-Y tripeptide frequencies in collagen: A context for host-guest triple-helical peptides. J. Struct. Biol 1998, 122 (1–2), 86–91. [DOI] [PubMed] [Google Scholar]
- (99).Egli J; Schnitzer T; Dietschreit JCB; Ochsenfeld C; Wennemers H Why proline? Influence of ring-size on the collagen triple helix. Org. Lett 2020, 22 (2), 348–351. [DOI] [PubMed] [Google Scholar]
- (100).Kersteen EA; Raines RT Contribution of tertiary amides to the conformational stability of collagen triple helices. Biopolymers 2001, 59 (1), 24–28. [DOI] [PubMed] [Google Scholar]
- (101).Goodman M; Melacini G; Feng Y Collagen-like triple helices incorporating peptoid residues. J. Am. Chem. Soc 1996, 118 (44), 10928–10929. [Google Scholar]
- (102).Feng Y; Melacini G; Goodman M Collagen-based structures containing the peptoid residue N-isobutylglycine (Nleu): Synthesis and biophysical studies of Gly-Nleu-Pro sequences by circular dichroism and optical rotation. Biochemistry 1997, 36 (29), 8716–8724. [DOI] [PubMed] [Google Scholar]
- (103).Melacini G; Feng Y; Goodman M Collagen-based structures containing the peptoid residue N-isobutylglycine (Nleu): Conformational analysis of Gly-Nleu-Pro sequences by 1H-NMR and molecular modeling. Biochemistry 1997, 36 (29), 8725–8732. [DOI] [PubMed] [Google Scholar]
- (104).Goodman M; Bhumralkar; Jefferson EA; Kwak J; Locardi E Collagen mimetics. Biopolymers 1998, 47 (2), 127–142. [DOI] [PubMed] [Google Scholar]
- (105).Johnson G; Jenkins M; McLean KM; Griesser HJ; Kwak J; Goodman M; Steele JG Peptoid-containing collagen mimetics with cell binding activity. J. Biomed. Mater. Res 2000, 51 (4), 612–624. [DOI] [PubMed] [Google Scholar]
- (106).Kessler JL; Kang G; Qin Z; Kang H; Whitby FG; Cheatham TE; Hill CP; Li Y; Yu SM Peptoid residues make diverse, hyperstable collagen triple-helices. J. Am. Chem. Soc 2021, 143 (29), 10910–10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Cai X; Wei W; Liu Z; Bai Z; Lei J; Xiao J In situ imaging of pathological collagen by electrostatic repulsion-destabilized peptide probes. ACS Applied Bio Materials 2020, 3 (11), 7492–7499. [DOI] [PubMed] [Google Scholar]
- (108).Hodges JA; Raines RT Stereoelectronic and steric effects in the collagen triple helix: Toward a code for strand association. J. Am. Chem. Soc 2005, 127 (45), 15923–15932. [DOI] [PubMed] [Google Scholar]
- (109).Newberry RW; Raines RT 4-Fluoroprolines: Conformational analysis and effects on the stability and folding of peptides and proteins. Top. Heterocycl. Chem 2017, 48, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Barth D; Milbradt AG; Renner C; Moroder LA (4R)- or a (4S)-fluoroproline residue in position Xaa of the (Xaa-Yaa-Gly) collagen repeat severely affects triple-helix formation. ChemBioChem 2004, 5 (1), 79–86. [DOI] [PubMed] [Google Scholar]
- (111).Li Y; San BH; Kessler JL; Kim JH; Xu Q; Hanes J; Yu SM Non-covalent photo-patterning of gelatin matrices using caged collagen mimetic peptides. Macromol. Biosci 2015, 15 (1), 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Kanai S; Machida K; Masuda R; Koide T Peptide precursors that acquire denatured collagen-hybridizing ability by O-to-N acyl migration at physiological pH. Org. Biomol. Chem 2020, 18 (15), 2823–2827. [DOI] [PubMed] [Google Scholar]
- (113).Tanrikulu IC; Westler WM; Ellison AJ; Markley JL; Raines RT Templated collagen “double helices” maintain their structure. J. Am. Chem. Soc 2020, 142 (3), 1137–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Tanrikulu IC; Raines RT Optimal interstrand bridges for collagen-like biomaterials. J. Am. Chem. Soc 2014, 136 (39), 13490–13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Takita KK; Fujii KK; Kadonosono T; Masuda R; Koide T Cyclic peptides for efficient detection of collagen. ChemBioChem 2018, 19 (15), 1613–1617. [DOI] [PubMed] [Google Scholar]
- (116).Ellison AJ; Tanrikulu IC; Dones JM; Raines RT Cyclic peptide mimetic of damaged collagen. Biomacromolecules 2020, 21 (4), 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Kessler JL; Li Y; Fornetti J; Welm AL; Yu SM Enrichment of collagen fragments using dimeric collagen hybridizing peptide for urinary collagenomics. J. Proteome Res 2020, 19 (8), 2926–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Qin J; Sloppy JD; Kiick KL Fine structural tuning of the assembly of ECM peptide conjugates via slight sequence modifications. Sci Adv. 2020, 6 (41), eabd3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Hwang J; Sullivan MO; Kiick KL Targeted drug delivery via the use of ECM-mimetic materials. Front Bioeng Biotechnol. 2020, 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Jones B; Debski A; Hans CP; Go MR; Agarwal G Structurally abnormal collagen fibrils in abdominal aortic aneurysm resist platelet adhesion. J. Thromb. Haemost 2022, 20 (2), 470–477. [DOI] [PubMed] [Google Scholar]
- (121).Viiklepp K; Nissinen L; Ojalill M; Riihila P; Kallajoki M; Meri S; Heino J; Kahari VM C1r upregulates production of matrix metalloproteinase-13 and promotes invasion of cutaneous squamous cell carcinoma. J. Invest. Dermatol 2022, 142 (5), 1478–1488. [DOI] [PubMed] [Google Scholar]
- (122).Novais EJ; Tran VA; Johnston SN; Darris KR; Roupas AJ; Sessions GA; Shapiro IM; Diekman BO; Risbud MV Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021, 12 (1), 5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Humeres C; Shinde AV; Hanna A; Alex L; Hernandez SC; Li R; Chen B; Conway SJ; Frangogiannis NG Smad7 effects on TGF-beta and ErbB2 restrain myofibroblast activation and protect from postinfarction heart failure. J. Clin. Invest 2022, 132 (3), e146926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Golman M; Abraham AC; Kurtaliaj I; Marshall BP; Hu YJ; Schwartz AG; Guo XE; Birman V; Thurner PJ; Genin GM; Thomopoulos S Toughening mechanisms for the attachment of architectured materials: The mechanics of the tendon enthesis. Sci Adv. 2021, 7 (48), eabi5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Lin AH; Allan AN; Zitnay JL; Kessler JL; Yu SM; Weiss JA Collagen denaturation is initiated upon tissue yield in both positional and energy-storing tendons. Acta Biomater. 2020, 118, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Quigley AS; Bancelin S; Deska-Gauthier D; Légaré F; Kreplak L; Veres SP In tendons, differing physiological requirements lead to functionally distinct nanostructures. Sci. Rep 2018, 8 (1), 4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Saini K; Tewari M; Cho S; Vashisth M; Jalil A; Andrechak JC; Kasznel A; Chenoweth D; Yamamoto K; Discher DE Heterogeneous strains in tissue suppress collagen degradation by collagenases. Biophys. J 2022, 121 (3), 492a. [Google Scholar]
- (128).Szczesny SE; Aeppli C; David A; Mauck RL Fatigue loading of tendon results in collagen kinking and denaturation but does not change local tissue mechanics. J. Biomech 2018, 71, 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Hossain MJ; Noori-Dokht H; Karnik S; Alyafei N; Joukar A; Trippel SB; Wagner DR Anisotropic properties of articular cartilage in an accelerated in vitro wear test. J. Mech. Behav. Biomed. Mater 2020, 109, 103834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Hanna A; Shinde AV; Li R; Alex L; Humeres C; Balasubramanian P; Frangogiannis NG Collagen denaturation in the infarcted myocardium involves temporally distinct effects of MT1-MMP-dependent proteolysis and mechanical tension. Matrix Biol. 2021, 99, 18–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Armstrong PM; Ehrlich HY; Magalhaes T; Miller MR; Conway PJ; Bransfield A; Misencik MJ; Gloria-Soria A; Warren JL; Andreadis TG; Shepard JJ; Foy BD; Pitzer VE; Brackney DE Successive blood meals enhance virus dissemination within mosquitoes and increase transmission potential. Nat Microbiol. 2020, 5 (2), 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Khajuria DK; Soliman M; Elfar JC; Lewis GS; Abraham T; Kamal F; Elbarbary RA Aberrant structure of fibrillar collagen and elevated levels of advanced glycation end products typify delayed fracture healing in the diet-induced obesity mouse model. Bone 2020, 137, 115436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Ansari MY; Ahmad N; Voleti S; Wase SJ; Novak K; Haqqi TM Mitochondrial dysfunction triggers a catabolic response in chondrocytes via ROS-mediated activation of the JNK/AP1 pathway. J. Cell Sci. Ther 2020, 133 (22), jcs247353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Tessier S; Doolittle AC; Sao K; Rotty JD; Bear JE; Ulici V; Loeser RF; Shapiro IM; Diekman BO; Risbud MV Arp2/3 inactivation causes intervertebral disc and cartilage degeneration with dysregulated TonEBP-mediated osmoadaptation. JCI Insight 2020, 5 (4), e131382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Hoy RC; D’Erminio DN; Krishnamoorthy D; Natelson DM; Laudier DM; Illien-Jünger S; Iatridis JC Advanced glycation end products cause RAGE-dependent annulus fibrosus collagen disruption and loss identified using in situ second harmonic generation imaging in mice intervertebral disk in vivo and in organ culture models. JOR SPINE 2020, 3 (4), e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Krishnamoorthy D; Hoy RC; Natelson DM; Torre OM; Laudier DM; Iatridis JC; Illien-Junger S Dietary advanced glycation end-product consumption leads to mechanical stiffening of murine intervertebral discs. Dis. Model. Mech 2018, 11 (12), dmm036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Xing Y; Zhang P; Zhang Y; Holzer L; Xiao L; He Y; Majumdar R; Huo J; Yu X; Ramasubramanian MK; Jin L; Wang Y; Li X; Oberholzer J A multi-throughput mechanical loading system for mouse intervertebral disc. J. Mech. Behav. Biomed. Mater 2020, 105, 103636. [DOI] [PubMed] [Google Scholar]
- (138).Filiberti A; Gmyrek GB; Montgomery ML; Sallack R; Carr DJJ Loss of osteopontin expression reduces HSV-1-induced corneal opacity. Invest. Ophthalmol. Vis. Sci 2020, 61 (10), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Atta G; Schroedl F; Kaser-Eichberger A; Spitzer G; Traweger A; Heindl LM; Tempfer H Scleraxis expressing scleral cells respond to inflammatory stimulation. Histochem. Cell Biol 2021, 156 (2), 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Hortells L; Meyer EC; Thomas ZM; Yutzey KE Periostin-expressing Schwann cells and endoneurial cardiac fibroblasts contribute to sympathetic nerve fasciculation after birth. J. Mol. Cell. Cardiol 2021, 154, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Hortells L; Valiente-Alandi I; Thomas ZM; Agnew EJ; Schnell DJ; York AJ; Vagnozzi RJ; Meyer EC; Molkentin JD; Yutzey KE A specialized population of Periostin-expressing cardiac fibroblasts contributes to postnatal cardiomyocyte maturation and innervation. Proc. Natl. Acad. Sci. U. S. A 2020, 117 (35), 21469–21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Vagnozzi RJ; Maillet M; Sargent MA; Khalil H; Johansen AKZ; Schwanekamp JA; York AJ; Huang V; Nahrendorf M; Sadayappan S; Molkentin JD An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020, 577 (7790), 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Arvind V; Howell KL; Huang AH Reprogramming adult tendon healing using regenerative neonatal regulatory T cells. bioRxiv; 2021, May 12, 10.1101/2021.05.12.443424. (accessed 2021-05-14) [DOI] [Google Scholar]
- (144).Diaz-Espinosa AM; Link PA; Sicard D; Jorba I; Tschumperlin DJ; Haak AJ Dopamine D1 receptor stimulates cathepsin K-dependent degradation and resorption of collagen I in lung fibroblasts. J. Cell Sci. Ther 2020, 133 (23), jcs248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Huang R; Yu D; Savage D; Wozniak K; Zheleznyak L; Knox W; Huxlin K The Blue-LIRIC in the rabbit cornea: Efficacy, tissue effects and repetition rate scaling. Biomed Opt Express 2022, 13 (4), 2346–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Takeyari S; Kubota T; Ohata Y; Fujiwara M; Kitaoka T; Taga Y; Mizuno K; Ozono K 4-Phenylbutyric acid enhances the mineralization of osteogenesis imperfecta iPSC-derived osteoblasts. J. Biol. Chem 2021, 296, 100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Motoike S; Kajiya M; Komatsu N; Horikoshi S; Ogawa T; Sone H; Matsuda S; Ouhara K; Iwata T; Mizuno N; Fujita T; Ikeya M; Kurihara H Clumps of mesenchymal stem cell/extracellular matrix complexes generated with xeno-free conditions facilitate bone regeneration via direct and indirect osteogenesis. Int. J. Mol. Sci 2019, 20 (16), 3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Kobayashi M; Ohara M; Hashimoto Y; Nakamura N; Fujisato T; Kimura T; Kishida A Effect of luminal surface structure of decellularized aorta on thrombus formation and cell behavior. PLoS One 2021, 16 (5), e0246221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Wang WY; Jarman EH; Lin D; Baker BM Dynamic endothelial stalk cell-Matrix interactions regulate angiogenic sprout diameter. Front Bioeng Biotechnol. 2021, 9, 620128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Yang G; Mahadik B; Choi JY; Yu JR; Mollot T; Jiang B; He X; Fisher JP Fabrication of centimeter-sized 3D constructs with patterned endothelial cells through assembly of cell-laden microbeads as a potential bone graft. Acta Biomater. 2021, 121, 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Goudar VS; Koduri MP; Ta Y-NN; Chen Y; Chu L-A; Lu L-S; Tseng F-G Impact of a desmoplastic tumor microenvironment for colon cancer drug sensitivity: A study with 3D chimeric tumor spheroids. ACS Appl Mater Interfaces 2021, 13 (41), 48478–48491. [DOI] [PubMed] [Google Scholar]
- (152).Wyatt RA; Crawford BD Post-translational activation of Mmp2 correlates with patterns of active collagen degradation during the development of the zebrafish tail. Dev. Biol 2021, 477, 155–163. [DOI] [PubMed] [Google Scholar]
- (153).Mostaço-Guidolin LB; Osei ET; Ullah J; Hajimohammadi S; Fouadi M; Li X; Li V; Shaheen F; Yang CX; Chu F; Cole DJ; Brandsma C-A; Heijink IH; Maksym GN; Walker D; Hackett T-L Defective fibrillar collagen organization by fibroblasts contributes to airway remodeling in asthma. Am. J. Respir. Crit. Care Med 2019, 200 (4), 431–443. [DOI] [PubMed] [Google Scholar]
- (154).Jones B; Tonniges JR; Debski A; Albert B; Yeung DA; Gadde N; Mahajan A; Sharma N; Calomeni EP; Go MR; Hans CP; Agarwal G Collagen fibril abnormalities in human and mice abdominal aortic aneurysm. Acta Biomater. 2020, 110, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Peck BD; Brightwell CR; Johnson DL; Ireland ML; Noehren B; Fry CS Anterior cruciate ligament tear promotes skeletal muscle myostatin expression, fibrogenic cell expansion, and a decline in muscle quality. Am. J. Sports Med 2019, 47 (6), 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Kwa KAA; van Haasterecht L; Elgersma A; Breederveld RS; Groot ML; van Zuijlen PPM; Boekema B Effective enzymatic debridement of burn wounds depends on the denaturation status of collagen. Wound Repair Regen. 2020, 28 (5), 666–675. [DOI] [PubMed] [Google Scholar]
- (157).Rognoni E; Goss G; Hiratsuka T; Sipilä KH; Kirk T; Kober KI; Lui PP; Tsang VSK; Hawkshaw NJ; Pilkington SM; Cho I; Ali N; Rhodes LE; Watt FM Role of distinct fibroblast lineages and immune cells in dermal repair following UV radiation-induced tissue damage. eLife 2021, 10, e71052. [DOI] [PMC free article] [PubMed] [Google Scholar]


