Abstract
Objective
Suicide risk in bipolar disorder (BD) is estimated to be up to 20 times higher than in the general population. While there is a large body of evidence suggesting that increased sympathetic activation is associated with disease and death, there is a paucity of research on the role of autonomic nervous system (ANS) dysfunction in patients with BD who have attempted suicide.
Methods
Fifty-three participants with BD used a wearable device to assess the association between history of suicide attempt, current suicidal ideation, and ANS dysfunction, including measures of heart rate variability (HRV) and respiratory rate. Data were analyzed in a series of unadjusted and adjusted bivariate models of association controlling for relevant variables.
Results
A history of suicide attempts was significantly associated with an increase in respiratory rate (p < 0.01). These results remained significant after adjusting for age, BMI, and current mood state. There was no association between current suicidal ideation and heart rate or respiratory rate. In the frequency domain, HRV parameters suggest reduced parasympathetic (i.e., vagal) activity in participants with a history of suicide attempts and in those with current suicidality, suggesting changes in sympathicovagal balance in BD.
Conclusions
Our results suggest that changes in the ANS in patients with BD and a history of suicide attempt are not restricted to pure vagally mediated HRV parameters, but rather signal a general ANS dysregulation. This ANS imbalance may be contributing to illness burden and cardiovascular disease. Further research on the relationship between ANS and suicidality in BD is needed.
Keywords: bipolar disorder, autonomic nervous system, suicidal behavior
Abrégé
Objectif
Le risque de suicide dans le trouble bipolaire (TB) est estimé être jusqu’à 20 fois plus élevé que dans la population générale. Bien qu’il y ait un vaste ensemble de données probantes suggérant que l’activation sympathique accrue est associée à la maladie et au décès, il y a une pénurie de recherche sur le rôle du dysfonctionnement du système nerveux autonome (SNA) chez les patients du TB qui ont tenté de se suicider.
Méthodes
53 participants souffrant du TB ont utilisé un appareil portable pour évaluer l’association entre les antécédents de tentatives de suicide, l’idéation suicidaire actuelle, et le dysfonctionnement du SNA, y compris les mesures de variabilité de la fréquence cardiaque (VFC) et de la fréquence respiratoire. Les données ont été analysées dans une série de modèles d'association bivariés non ajustés et ajustés contrôlant les variables pertinentes.
Résultats
Des antécédents de tentatives de suicide étaient significativement associés à une augmentation de e la fréquence respiratoire (p < 0,01). Ces résultats sont demeurés significatifs après ajustement pour l’âge, l’IMC, l’état actuel de l’humeur. Il n’y avait pas d’association entre l’idéation suicidaire actuelle et la fréquence cardiaque ou respiratoire. Dans le domaine de la fréquence, les paramètres de la VFC suggèrent une activité parasympathique réduite (c.-à-d., vagale) chez les participants ayant des antécédents de tentatives de suicide et chez ceux ayant une suicidabilité actuelle, ce qui suggère des changements de l’équilibre sympathico-vagal dans le TB.
Conclusions
Nos résultats suggèrent que les changements du SNA chez les patients souffrant du TB et les antécédents de tentatives de suicide ne sont pas restreints aux paramètres de la VFC à médiation purement vagale, mais signalent plutôt un dysfonctionnement général du SNA. Ce déséquilibre du SNA peut contribuer à la charge de la maladie et à la maladie cardiovasculaire. Il faut plus de recherche sur la relation entre le SNA et la suicidabilité dans le TB.
Introduction
While predictive models for suicide have been successfully developed at the level of populations, current demographic and clinical variables are neither sensitive nor specific enough for making individual clinical predictions. A recent meta-analysis concluded that the ability to predict suicide has not improved much over the past 50 years, 1 partially because analyses have only used static (e.g., demographic) variables. This is particularly important in bipolar disorder (BD), a mood disorder that has been associated with premature death due to both cardiovascular disease2–5 and suicide.6–8Strategies to improve the treatment of patients with BD have only partially decreased the incidence of suicide.9,10 This is in part because it is difficult to identify individual patients who are at the highest risk for suicide and to intervene to prevent it. New analytical approaches coupled with new technologies that passively capture densely sampled data may allow us to address this problem, which has been intractable so far.
There is a large body of evidence suggesting that autonomic nervous system (ANS) dysfunction (i.e., typically there is increased activation in the sympathetic system compared to the parasympathetic system) is associated with disease and death.11,12 Heart rate variability (HRV) has been used as an index of autonomic function, and its predictive power for morbidity and mortality in patients with or without cardiovascular disease has been confirmed. 13 Healthy people exhibit a high degree of HRV, reflecting the ability to quickly adapt to the physical or psychological demands of the environment 14 ; conversely, low HRV has been associated with arrhythmias, myocardial infarction, and sudden death. 13 In BD, previous studies have shown that illness burden is associated with lower HRV15–19 and that those BD patients with a family history of suicide present with HRV measures associated with lower parasympathetic activation. 20
HRV is regulated in part by respiratory rate.21,22 Different respiratory patterns have been associated with increased sympathetic (e.g., hyperventilation) or parasympathetic stimulation (e.g., slow, “deep” diaphragmatic breathing). 23 In turn, these different respiratory patterns can modify gas exchange, intracellular cascades,24,25 and brain activity, 26 which can further influence other components of the ANS, including heart rate and HRV, in a feedback loop. Moreover, recent studies have described an association between ANS dysfunction and suicidal ideation,27–34 reinforcing the notion that ANS dysfunction is a maladaptive mechanism that might be compromising the ability of the individual to adequately respond to the demands of the environment. Furthermore, baseline respiratory abnormalities have been described in patients with panic disorder35,36 and BD37,38 and more so in those with both diagnoses.39–41 In parallel, the prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease, asthma, and bronchitis is between 44 and 80%,42–46 with suicide risk almost 4 times higher in patients with 3 or more markers of chronic hypoxia, 47 despite antidepressant treatment. 48 These findings have led some authors to conceptualize the association of pulmonary disease and suicide as a function of hypoxia.49,50
Based on these studies, we conducted a pilot study to assess whether integrating demographic and clinical data with densely sampled physiologic measures of sympathetic and parasympathetic activation (i.e., heart rate, respiratory rate, and HRV) may differentiate BD patients with or without a history of suicide attempt. We hypothesize that patients with BD and a history of a suicide attempt would have higher sympathetic activation (i.e., both higher heart rate and respiratory rate and lower HRV) than patients with BD without a history of a suicide attempt.
Materials and Methods
The study protocol has been previously described 19 and is briefly summarized below.
Participants
The protocol was approved by local research ethics board (REB # 151/2018). After providing informed consent in writing, each participant was interviewed to collect demographic and clinical information. Outpatients were recruited in 2 academic psychiatric hospitals (the Centre for Addiction and Mental Health, Toronto, Canada, and the Royal Ottawa Hospital, Ottawa, Canada) between April 2016 and December 2019. Participants were men or women, 18 years or older, with a primary diagnosis of BD I or II according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) 5 criteria, confirmed with the Schedule for Affective Disorders and Schizophrenia – Lifetime version (SADS-L), 51 in any phase of the illness. Exclusion criteria included active substance abuse or dependence according to DSM 5, mood disorder secondary to a general medical condition, and conditions that may confound HRV measurements including atrial fibrillation or flutter, high-grade heart block, QTc > 500 ms, permanent pacemaker implantation, hemodynamically significant valvular or congenital heart disease, angina, history of acute myocardial infarction, bypass, angioplasty, diabetes mellitus type 1 or 2, or cardiac medications –including β-blockers, calcium channel blockers, and thiazides.
Participants received treatment as usual, according to standard local practices. We classified current psychotropic medications into 4 groups (lithium carbonate, antipsychotics, anticonvulsants, others); 1 participant was not taking any psychotropic medication at the time of measurements.
Psychiatric Measures
We obtained the following clinical information from the SADS-L to determine 12 factors characterizing burden of illness, as described in 19 : (1) lifetime number of depressive episodes; (2) lifetime number of manic, hypomanic, or mixed episodes; (3) lifetime number of all types of episodes; durations (in weeks) of the most severe (4) depressive and (5) hypomanic/manic episode; (6) if applicable, duration (in weeks) of current episode; (7) illness duration calculated by subtracting from the current age their age at onset (defined as the age at which the participant first experienced a depressive, manic, or hypomanic episode, according to DSM 5 criteria); history of (8) suicide attempts or (9) psychotic symptoms; (10) co-morbid anxiety disorders; family history of (11) mood disorders (MDD, BD); or (12) completed suicide. A history of suicide attempt was defined based on the SADS-L section on suicidal behavior, including questions on lifetime history of suicide attempts, intent, and their lethality. Durations of the most severe episodes and of the current episode were transformed logarithmically to better accommodate the assumption of linearity. An index of burden of illness (IBI) was calculated using a weighted geometric mean of the 12 factors characterizing illness burden, which is appropriate when comparing different items, and each item has multiple properties that have numeric ranges. 52 Upon enrolling in the study, clinicians completed the Montgomery–Asberg Depression Rating Scale (MADRS) 53 and the Young Mania Rating Scale (YMRS). 54 Euthymia was defined as both a MADRS score of <7 and YMRS score of <5 for at least 4 weeks.
Data Collection
Cardiovascular and Respiratory Measures
Each participant was provided with a compression shirt fitted with a battery-charged BioModule™ from Zephyr Technology 55 that they wore for 1 period of 24 h. Participants were instructed on how to turn on and off the device and wore it for 24 h, including while sleeping at night. We asked participants not to take a shower during these 24 h, as the device is not waterproof. The BioModule™ is worn against the skin, weighs 35 g, and is attached to the front of a shirt, acting as a data logger. It simultaneously measures 5 physiological and activity-related parameters in real time: respiratory rate, ECG, skin temperature, tri-axial accelerometry, and posture. Data were stored onto the BioModule™ and later transferred to a computer via USB port. The ECG was recorded at 250 Hz and stored onto the BioModule™ and later transferred to a computer via USB port. All ECG data were uploaded into and analyzed using Kubios HRV Premium software. 56
Respiratory rate was detected by a pressure sensor in the strap, which detects torso expansion and contraction due to breathing, and it is reported as the number of breaths per minute. As part of our data preprocessing, a trained rater identified time periods during which the data were very noisy due to movement artifacts. These data segments were not used in the HRV analysis to prevent noisy data from distorting time-varying analysis results. The program we used to analyze the data provided individual streams for each type of data (i.e., ECG data and breathing data), and it has a built-in QRS detector. Once the ECG was imported into the program, the R-wave time instants are automatically detected by applying the built-in QRS algorithm. This detection algorithm is based on the Pan–Tompkins detection algorithm, 57 which consists of applying a series of filters to highlight the frequency content of rapid heart depolarization and removes background noise of any kind. Subsequently, it squares the signal to amplify the QRS. Finally, it applies adaptive thresholds to detect the peaks of the filtered signal. The detector consisted of a preprocessing component, including bandpass filtering of the ECG (to reduce power line noise, baseline wander, and other noise components) and squaring the data samples (to highlight peaks and moving average filtering, thus smoothing close by peaks). The decision rules in this algorithm include amplitude threshold and comparison to expected value between adjacent R-waves. Both these results are adjusted every time a new R-wave is detected. Before R-wave time instant extraction, the R-wave is interpolated at 2,000 Hz to improve the time resolution of the detection. Finally, we used the automatic feature of the “artifact correction algorithm,” in which artifacts are detected from a time series consisting of differences between successive RR intervals. This time series provides a robust way to separate misplaced beats from the normal sinus rhythm. To ensure optimal correction of artifacts, the threshold for correction is estimated from the time-varying distribution of this time series. Detected ectopic beats were corrected by replacing these with interpolated RR values; extra beats were corrected by removing extra R-wave detection and recalculating RR interval series.
Ectopic beats were corrected by linear interpolation with the adjacent complexes; ECG tracings with more than 1% premature beats were eliminated from the analysis. The remaining ECG samples were analyzed with an R-peak detection algorithm and thus produced RR interval (milliseconds) data for each participant, with an accuracy of 98%. The RR data were detrended using the smoothness priors approach (lambda = 500), and an automatic artifact correction algorithm was applied to the RR data, resulting in 1.9% of the beats corrected in the sample.
Based on our prior work,19,20 we analyzed the following HRV measures: (a) 3 HRV measures in the time domain – (i) the root mean square of successive differences (RMSSD), which is associated with short-term, rapid changes in heart rate; (ii) the standard deviation of all the normal RR intervals (SDNN), which is considered the gold standard for medical stratification of cardiac risk when recorded over a 24-h period58,59; (iii) the standard deviation of the average RR intervals (SDANN), which is calculated across 5 min blocks of time, instead of 24-h series – (b) 3 HRV measures in the frequency domain: high frequency (HF), associated with parasympathetic activity; low frequency (LF), associated with sympathetic activity; and LF/HF ratio.
Statistical Analysis
Due to the small sample size and large number of variables, our analysis should be considered hypothesis generating. Thus, we focused on the interpretation of patterns of the bivariate association and their 95% confidence intervals. We calculated odds ratio (OR) between history of suicide attempt (exposure) and HRV and respiratory rate parameters (outcome) using logistic regression and nonparametric tests in SPSS. 60 We conducted unadjusted and adjusted analyses (accounting for age, current mood state, and body mass index (BMI)) in the model. 95% confidence intervals for correlation coefficients were calculated using the inverse hyperbolic tangent transformation of the correlation coefficient, which has approximate normal distribution with variance 1.06/ n – 3, where n is the sample size. 61 We also explored the interaction between HRV and respiratory rate, being mindful of the small number of events and possible instability of these models.
Considering the precision of the estimates, a sample of 50 subjects, the width of a 95% confidence interval would be approximately 0.57 for a Spearman rank correlation coefficient of size 0. This width decreases to around 0.52 for a correlation of 0.30 and to 0.38 for a correlation of 0.60. Thus, if statistical tests for single correlation coefficient are considered, a sample of 50 subjects provides 80% power to detect correlation coefficients of size 0.38 assuming 2 tailed tests, significance level of 0.05, and no adjustment for multiple tests.
Results
Fifty-three participants were enrolled in the study, and none dropped out. Table 1 presents their demographic and clinical characteristics: 32 (60%) were euthymic, 14 (26%) were in a depressive episode, 6 (11%) were in a hypomanic or manic episode, and 1 (2%) was in a mixed episode. Mean (SD) for respiratory rate in the sample was 17.4 (5.1), which is in keeping with normal parameters in adults (12–20 breaths per minute). Almost half of the sample (47%) smoked cigarettes, but none had been diagnosed with a primary pulmonary disease. See Table S1 in the Supplementary Material for details on intent and lethality.
Table 1.
Demographic and Clinical Characteristics of the Sample.
| Variable | N (%) or mean ± SD |
|---|---|
| BD I | 24 (45) |
| Women gender | 35 (66) |
| Age | 44.7 ± 13.1 |
| Marital status | |
| Married/common law | 27 (51) |
| Single | 17 (32) |
| Divorced | 9 (17) |
| Race/ethnicity | |
| Caucasian | 48 (90) |
| Hispanic | 2 (4) |
| Asian | 2 (4) |
| Black Canadian | 1 (2) |
| Education (years) | 15.4 ± 2.7 |
| Employment status | |
| Work full/part time | 26 (49) |
| On disability | 10 (19) |
| Retired | 6 (11) |
| Student | 4 (7) |
| Home maker | 4 (7) |
| Unemployed | 3 (6) |
| Smokers | 26 (47) |
| Family history of BD | 19 (36) |
| Family history of MDD | 21 (39) |
| Family history of suicide | 19 (36) |
| History of suicide attempt | 11 (21) |
| Both family history of suicide and history of suicide attempt | 3 (6) |
| Illness phase at the time of HRV measurement | |
| Euthymic | 32 (60) |
| Depressive episode | 14 (26) |
| Manic or hypomanic episode | 6 (11) |
| Mixed episode | 1 (2) |
| Pharmacotherapy at the time of HRV measurement | |
| On no treatment at the time of entry to the study | 1 (2) |
| ECT | 2 (4) |
| Lithium monotherapy | 3 (6) |
| AAP monotherapy | 4 (7) |
| Anticonvulsant monotherapy | 6 (11) |
| Any combination of mood stabilizers | 20 (38) |
| Any combination of mood stabilizers + antidepressants | 17 (32) |
AAP, atypical antipsychotic; BD, bipolar disorder; ECT, electroconvulsive therapy; HRV, heart rate variability; MDD, major depressive disorder; SD, standard deviation
Table 2.
Adjusted and Unadjusted Models for the Association Between History of Suicidal Ideation and Respiratory Rate.
| Variable | Unadjusted coefficient | Adjusted coefficient | Wald chi-square (unadjusted) | Wald chi-square (adjusted) | p- Value (unadjusted) | p-Value (adjusted) | Odds ratio (unadjusted) | Odds ratio (adjusted) | Lower bound (unadjusted) | Lower bound (adjusted) | Upper bound (unadjusted) | Upper bound (adjusted) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean HR | −0.04 | −0.04 | 1.56 | 1.47 | 0.21 | 0.22 | 0.95 | 0.95 | 0.89 | 0.89 | 1.02 | 1.02 |
| RMSSD | −0.003 | -.009 | 0.24 | 1.51 | 0.62 | 0.21 | 0.99 | 0.99 | 0.98 | 0.97 | 1.01 | 1.00 |
| SDANN | −0.004 | 0.0 | 0.39 | 0.002 | 0.52 | 0.95 | 0.99 | 1.00 | 0.98 | 0.98 | 1.00 | 1.01 |
| SDNN | −0.002 | −0.006 | 0.09 | 0.48 | 0.75 | 0.48 | 0.99 | 0.99 | 0.97 | 0.97 | 1.01 | 1.01 |
| LF | −0.41 | −0.02 | 0.22 | 0.0 | 0.63 | 0.97 | 0.65 | 0.97 | 0.11 | 0.22 | 3.68 | 4.19 |
| HF | 2.48 | 1.13 | 0.25 | 1.16 | 0.61 | 0.28 | 11.99 | 3.11 | 0.0 | 0.39 | 18.86 | 24.58 |
| LF/HF | 0.51 | 0.61 | 3.37 | 4.19 | 0.06 | 0.04 | 1.67 | 1.82 | 0.96 | 1.02 | 2.91 | 3.24 |
| Respiratory rate | 0.58 | 0.64 | 14.09 | 10.95 | 0.001 | 0.009 | 1.81 | 1.91 | 1.32 | 1.31 | 2.45 | 2.79 |
Table 3.
Adjusted and Unadjusted Models for the Association Between Current Suicidal Ideation and Respiratory Rate.
| Variable | Unadjusted coefficient | Adjusted coefficient | Wald chi-square (unadjusted) | Wald chi-square (adjusted) | p- Value (unadjusted) | p-Value (adjusted) | Odds ratio (unadjusted) | Odds ratio (adjusted) | Lower bound (unadjusted) | Lower bound (adjusted) | Upper bound (unadjusted) | Upper bound (adjusted) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean HR | −0.00 | −0.00 | 0.06 | 0.04 | 0.79 | 0.83 | 0.99 | 0.99 | 0.94 | 0.94 | 1.04 | 1.04 |
| RMSSD | −0.00 | −0.006 | 0.27 | 0.66 | 0.61 | 0.41 | 0.99 | 0.99 | 0.98 | 0.97 | 1.01 | 1.01 |
| SDANN | −0.01 | −0.01 | 2.53 | 1.06 | 0.11 | 0.15 | 0.98 | 0.98 | 0.97 | 0.97 | 1.00 | 1.00 |
| SDNN | −0.00 | −0.00 | 0.01 | 0.16 | 0.96 | 0.89 | 0.99 | 0.99 | 0.98 | 0.97 | 1.01 | 1.01 |
| LF | −0.82 | −0.19 | 0.88 | 0.09 | 0.34 | 0.76 | 0.43 | 0.81 | 0.07 | 0.22 | 2.43 | 2.96 |
| HF | 0.05 | −0.17 | 0.00 | 0.01 | 0.96 | 0.89 | 1.05 | 0.83 | 0.09 | 0.05 | 12.16 | 12.33 |
| LF/HF | 0.44 | 0.51 | 3.63 | 3.84 | 0.05 | 0.04 | 1.55 | 1.67 | 0.98 | 1.00 | 2.44 | 2.79 |
| Respiratory rate | −0.08 | −0.14 | 1.67 | 1.01 | 0.19 | 0.31 | 0.91 | 0.86 | 0.80 | 0.65 | 1.04 | 1.14 |
Table 4.
Adjusted and Unadjusted Models for the Interaction Between History of Suicidal Ideation, HRV, and Respiratory Rate.
| Variable | B (unadjusted) | B (adjusted) | SE B (unadjusted) | SE B (adjusted) | β (unadjusted) | β (adjusted) | p-Value (unadjusted) | p-Value (adjusted) | |
|---|---|---|---|---|---|---|---|---|---|
| Mean HR | Main effect | 0.12 | 0.08 | 0.19 | 0.84 | 0.66 | 0.09 | 0.51 | 0.92 |
| Interaction | 0.18 | 0.14 | 0.21 | 0.86 | 0.89 | 0.16 | 0.38 | 0.87 | |
| RMSSD | Main effect | −0.05 | −0.04 | 0.08 | 0;11 | −0.63 | −0.41 | 0.53 | 0.68 |
| Interaction | −0.05 | −0.05 | 0.08 | 0.11 | −0.68 | −0.45 | 0.49 | 0.65 | |
| SDANN | Main effect | 0.04 | 0.04 | 0.06 | 0.p09 | 0.73 | 0.45 | 0.46 | 0.65 |
| Interaction | 0.06 | 0.06 | 0.06 | 0.09 | 0.95 | 0.64 | 0.35 | 0.52 | |
| SDNN | Main effect | 0.03 | 0.07 | 0.15 | 0.17 | 0.23 | 0.43 | 0.81 | 0.66 |
| Interaction | 0.03 | 0.07 | 0.16 | 0.17 | 0.23 | 0.41 | 0.81 | 0.68 | |
| LF | Main effect | 8.27 | 9.38 | 1.59 | 3.00 | 5.21 | 3.12 | 0.001 | 0.005 |
| Interaction | 8.12 | 8.69 | 1.79 | 3.11 | 4.52 | 2.79 | 0.001 | 0.01 | |
| HF | Main effect | −7.85 | −14.74 | 5.11 | 10.69 | −1.53 | −1.37 | 0.13 | 0.18 |
| Interaction | −9.47 | −15.75 | 6.35 | 11.08 | −1.48 | −1.42 | 0.14 | 0.17 | |
| LF/HF | Main effect | 1.64 | 1.54 | 2.11 | 3/06 | 0.77 | 0.51 | 0.44 | 0.62 |
| Interaction | 2.15 | 1.95 | 2.29 | 3.12 | 0.94 | 0.62 | 0.35 | 0.53 | |
Table 5.
Adjusted and Unadjusted Models for the Interaction Between Current Suicidal Ideation, HRV, and Respiratory Rate.
| Variable | B (unadjusted) | B (adjusted) | SE B (unadjusted) | SE B (adjusted) | β (unadjusted) | β (adjusted) | p-Value (unadjusted) | p-Value (adjusted) | |
|---|---|---|---|---|---|---|---|---|---|
| Mean HR | Main effect | −2.23 | −1.89 | 6.78 | 3.44 | −0.32 | −0.55 | 0.74 | 0.59 |
| Interaction | −2.56 | −2.1 | 6.78 | 3.46 | −0.37 | −0.61 | 0.71 | 0.54 | |
| RMSSD | Main effect | 0.12 | −0.27 | 1.53 | 7.67 | 0.08 | −0.03 | 0.93 | 0.97 |
| Interaction | 0.19 | −0.26 | 1.54 | 7.67 | 0.12 | −0.03 | 0.89 | 0.97 | |
| SDANN | Main effect | 0.48 | 3.92 | 2.15 | 5.93 | 0.22 | 0.66 | 0.82 | 0.51 |
| Interaction | 0.23 | 3.58 | 2.18 | 5.95 | 0.10 | 0.61 | 0.91 | 0.55 | |
| SDNN | Main effect | −0.37 | −3.56 | 2.23 | 7.11 | −0.16 | −0.51 | 0.86 | 0.62 |
| Interaction | −0.42 | 3.63 | 2.23 | 7.11 | −0.19 | −0.51 | 0.84 | 0.61 | |
| LF | Main effect | −0.22 | −1.02 | 0.84 | 0.63 | −0.26 | −1.61 | 0.79 | 0.12 |
| Interaction | −0.58 | −1.35 | 0.88 | 0.66 | −0.65 | −2.02 | 0.51 | 0.05 | |
| HF | Main effect | −0.12 | −0.87 | 0.55 | 1.35 | −0.22 | −0.64 | 0.82 | 0.52 |
| Interaction | 0.13 | −0.73 | 0.58 | 1.37 | 0.22 | −0.53 | 0.82 | 0.61 | |
| LF/HF | Main effect | 0.06 | 0.57 | 0.37 | 1.24 | 0.17 | 0.45 | 0.86 | 0.65 |
| Interaction | −0.21 | 0.38 | 0.43 | 1.27 | −0.46 | 0.1 | 0.64 | 0.76 | |
HR, heart rate; HRV, heart rate variability; LF, low frequency; HF, high frequency; RMSSD, root mean square of successive differences; SDANN, standard deviation of the average RR intervals; SDNN, standard deviation of all the normal RR intervals.
A history of suicide attempts was significantly associated with a 26% increase in respiratory rate (U = 14.09; p < 0.01). These results remained significant after adjusting for age, current mood state, and BMI. There was no association between current suicidal ideation and heart rate or respiratory rate (p > 0.05).
A history of suicide attempts was also associated with decreased global HRV in all time domain variables (RMSSD, SDANN, and SDNN); none of these variables were significant in the adjusted or unadjusted models (p > 0.05). See Table 2 and Figure 1 for further details. However, in the frequency domain, after adjusting for age, current mood state, and BMI, a history of suicide attempt was associated with an 82% increase in LF/HF ratio (Wald = 4.1; p = 0.04; [1.02, 3.24]; OR = 1.82). Similarly, after controlling for age, BMI and current mood state, participants with current suicidal ideation showed a 67% increase in LF/HF ratio (Wald X2 = 3.84; p <0.05; [1.00, 2.79]; OR = 1.67). See Table 3 for details.
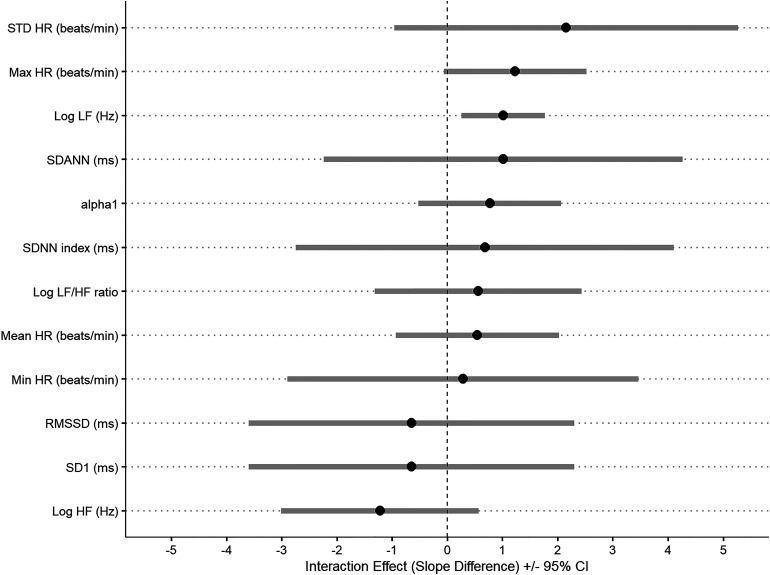
Figure 1.
Interaction between HRV, RR, and history of suicidal behavior in 53 patients with BD. Legend: Error bars are the estimates of the difference in association between HRV and RR between those participants with a history of suicide attempt compared to those without. Estimates higher than 0 indicate that the association is higher among those participants with a history of suicide. HR, heart rate; HF, high frequency; LF, low frequency; Min, minimum; Max, maximum; RR, respiratory rate; SDANN, standard deviation of the average RR intervals; SDNN, standard deviation of all the normal RR intervals; SE, standard error; STD: standard.
Furthermore, when we investigated the interaction between HRV and respiratory rate among those with and without history of suicide attempts, we found a significant interaction for LF (t = 4.52; df = 23, p < 0.001), indicating that the association was significantly higher among those with a history of suicide attempts (i.e., an increase of 1 unit in LF was associated with an increase of 8.28 (SE = 1.60; t = 5.21; p < 0.001) units in respiratory rate among those participants with a history of suicide attempt). These results remained significant after adjusting for age, current mood state, and BMI (t = 2.79; df = 20, p = 0.01; 1 unit increase in LF was associated with 8.52 unit increase in respiratory rate (SE = 3.24; t = 2.62; p = 0.01) see Table 4 for details. Conversely, there was no significant difference when comparing participants with current suicidal ideation and those without (Table 5).
While our study was not powered to analyze the potential additive effects of both family history of suicide and a history of suicide attempts on ANS dysfunction, participants with both conditions showed the lowest RMSSD, SD1, and HF values, all indicative of decreased parasympathetic activation (p > 0.05).
Discussion
The aim of this hypothesis-generating study was to examine differences in ANS in patients with BD with and without a history of suicide attempt and with and without current suicidal ideation. In accordance with our previous findings, 20 our results indicate that individuals with BD have lower HRV. Lower RMSSD and SDNN suggest reduced parasympathetic (i.e., vagal) activity and total variability, respectively, in individuals with BD. Higher LF/HF ratio suggests changes in sympathicovagal balance in BD, and increased HR and respiratory rate may be indicative of higher sympathetic activity. Our results suggest that changes in the ANS in individuals with a history of suicide attempt or with current suicidal ideation are not restricted to pure vagally mediated HRV parameters, but rather indicate a general ANS dysregulation.
While our sample size is not large enough to draw conclusions on the association between family history of suicide, history of suicide attempts, and ANS parameters, we consider that our results confirm prior hypotheses related to maladaptive physiological responses to stressors in suicide attempters.34,62 We hypothesize that there is a withdrawal of an already “weakened” parasympathetic branch (e.g., in those with family history of suicide) via decreased RSA, contributing to decreased inhibition of the sympathetic response. This autonomic imbalance is further stimulated by an increased respiratory rate. Our work adds new knowledge to the field by identifying the role of respiratory rate and the potential implications of modulating it to improve mood regulation.
Further research is needed to understand the mechanistic implications of respiratory rate on ANS regulation (other than the importance of RSA 63 ) and the role of hypocapnia (as a result of higher respiratory rate) in BD. Several studies have shown that changes in carbon dioxide levels change the intracellular and extracellular concentrations of H+ very quickly, a change that can only be normalized after 120 h of compensatory (renal) mechanisms. 24 Thus, changes in acidity affect the charge on enzymes, the rate of metabolic reactions, the binding of molecules, as well as pharmacokinetic reactions, pulmonary perfusion, and cardiac activity.64,65 Although a thorough review of acid-base disturbances is outside the scope of this paper, it is important to mention that alkalosis (as a result of hypocapnia) has been associated with sudden death in the context of arrhythmias. 66
Our main limitation is the modest sample size. Other limitations include that this is a cross-sectional analysis and the lack of a control group. While we did not control for changes in metabolic requirements or posture in this study, both of which can affect ANS activity, this is a hypothesis-generating study that sheds light on respiratory rate as an important aspect to be considered in BD. Moreover, although an increase in LF (associated with standing) is often cited as evidence for its association with sympathetic activity, only one-third of healthy subjects show this change, 67 further strengthening our findings. Similarly, while we excluded participants treated with medications that could potentially affect ANS markers, many psychotropics have targets at different receptor levels. Finally, while there is still some controversy between HRV indices and its relationship with the sympathetic and parasympathetic systems, 68 our findings add new knowledge to the field by describing the association between ANS dysfunction and a history of suicide attempt in patients with BD.
Conclusion
Our findings add to the body of knowledge showing that a history of suicide attempt and current suicidal ideation is associated with ANS dysfunction. Together with our previous results on sympathetic activation in BD patients with higher illness burden, our results provide a plausible mechanistic hypothesis to explain the association between increased respiratory rate and further increase in sympathetic stimulation in patients with a history of suicide attempt. Longitudinal studies are needed to understand the role of slow (diaphragmatic) breathing on parasympathetic stimulation and its repercussions on mood regulation in patients with BD.
Supplemental Material
Supplemental material, sj-docx-1-cpa-10.1177_07067437231194334 for A History of Suicide Attempt Is Associated with Increased Sympathetic Activation in Bipolar Disorder by Abigail Ortiz, Yunkyung Park, Stephane MacLean, M. Ishrat Husain, Marcos Sanches, Arun Ravindran and Benoit H. Mulsant in The Canadian Journal of Psychiatry
Acknowledgments
We would like to thank Ms. Pooja Moorti for her help with data preprocessing and harmonization.
Footnotes
Data Availability Statement: The data that support these findings are not publicly available due to ethical restrictions (i.e., containing information that could compromise the privacy of research participants).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was funded by a grant from the University of Ottawa Department of Psychiatry Research Funds (AO) and was supported in part by Academic Scholar Awards from the Department of Psychiatry, University of Toronto (AO, MIH) and by the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto (BHM).
ORCID iDs: Abigail Ortiz https://orcid.org/0000-0001-6886-767X
M. Ishrat Husain https://orcid.org/0000-0001-5771-5750
Arun Ravindran https://orcid.org/0000-0002-1655-2753
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Franklin JC, Ribeiro JD, Fox KR, et al. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol Bull. 2017;143(2):187–232. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein BI, Carnethon MR, Matthews KA, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American heart association. Circulation. 2015;132(10):965–986. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein BI, Schaffer A, Wang S, et al. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J Clin Psychiatry. 2015;76(2):163–169. [DOI] [PubMed] [Google Scholar]
- 4.Prieto ML, Schenck LA, Kruse JL, et al. Long-term risk of myocardial infarction and stroke in bipolar I disorder: a population-based cohort study. J Affect Disord. 2016;194:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westman J, Hallgren J, Wahlbeck K, et al. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open. 2013;3(4):e002373. doi: 10.1136/bmjopen-2012-002373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon GE, Hunkeler E, Fireman B, et al. Risk of suicide attempt and suicide death in patients treated for bipolar disorder. Bipolar Disord. 2007;9(5):526–530. [DOI] [PubMed] [Google Scholar]
- 7.Rihmer Z, Gonda X, Döme P. The assessment and management of suicide risk in bipolar disorder. In The treatment of bipolar disorder: Integrative clinical strategies and future directions. Oxford University Press; 2017. [Google Scholar]
- 8.Dome P, Rihmer Z, Gonda X. Suicide Risk in Bipolar Disorder: A Brief Review. Medicina (Kaunas. 2019;55(8):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deisenhammer EA, Behrndt EM, Kemmler G, et al. A comparison of suicides in psychiatric in-patients, after discharge and in not recently hospitalized individuals. Compr Psychiatry. 2016;69:100–105. [DOI] [PubMed] [Google Scholar]
- 10.Müller-Oerlinghausen B, Felber W, Berghöfer A, et al. The impact of lithium long-term medication on suicidal behavior and mortality of bipolar patients. Arch Suicide Res. 2005;9(3):307–319. [DOI] [PubMed] [Google Scholar]
- 11.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131. [DOI] [PubMed] [Google Scholar]
- 12.Drury RL, Porges S, Thayer J, et al. Editorial: heart rate variability, health and well-being: a systems perspective. Front Public Health. 2019;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villareal RP, Liu BC, Massumi A. Heart rate variability and cardiovascular mortality. Curr Atheroscler Rep. 2002;4(2):120–127. [DOI] [PubMed] [Google Scholar]
- 14.Peng CK, Havlin S, Hausdorff JM, et al. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdnown with disease. J Electrocardiol. 1995;28(Suppl):59–65. [DOI] [PubMed] [Google Scholar]
- 15.Fiedorowicz JG. Depression and cardiovascular disease: an update on how course of illness may influence risk. Curr Psychiatry Rep. 2014;16(10):492–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiedorowicz JG, Coryell WH, Rice JP, et al. Vasculopathy related to manic/hypomanic symptom burden and first-generation antipsychotics in a sub-sample from the collaborative depression study. Psychother Psychosom. 2012;81(4):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray DP, Metz NS, Haynes WG, et al. Vascular function is not impaired early in the course of bipolar disorder. J Psychosom Res. 2012;72(3):195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray DP, Weiner M, Prabhakar M, et al. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475–480. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz A, Bradler K, Moorti P, et al. Reduced heart rate variability is associated with higher illness burden in bipolar disorder. J Psychosom Res. 2021;145:110478. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz A, Bradler K, Moorti P, et al. Increased sympathetic tone is associated with illness burden in bipolar disorder. J Affect Disord. 2021;471-476. [DOI] [PubMed] [Google Scholar]
- 21.Betka S, Adler D, Similowski T, et al. Breathing control, brain, and bodily self-consciousness: toward immersive digiceuticals to alleviate respiratory suffering. Biol Psychol. 2022;171:108329. [DOI] [PubMed] [Google Scholar]
- 22.Fukushi I, Yokota S, Okada Y. The role of the hypothalamus in modulation of respiration. Respir Physiol Neurobiol. 2019;265:172–179. [DOI] [PubMed] [Google Scholar]
- 23.Sinha M, Sinha R, Ghate J, et al. Impact of altered breathing patterns on interaction of EEG and heart rate variability. Ann Neurosci. 2020;27(2):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikter A, Faludi G, Rihmer Z. The role of carbon dioxide (and intracellular pH) in the pathomechanism of several mental disorders. Are the diseases of civilization caused by learnt behaviour, not the stress itself? Neuropsychopharmacol Hung. 2009;11(3):161–173. [PubMed] [Google Scholar]
- 25.Sikter A, Rihmer Z, Guevara R. New aspects in the pathomechanism of diseases of civilization, particularly psychosomatic disorders. Part 1. Theoretical background of a hypothesis. Neuropsychopharmacol Hung. 2017;19(2):95–105. [PubMed] [Google Scholar]
- 26.Herrero JL, Khuvis S, Yeagle E, et al. Breathing above the brain stem: volitional control and attentional modulation in humans. J Neurophysiol. 2018;119(1):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowell SE, Beauchaine TP, McCauley E, et al. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Dev Psychopathol. 2005;17(4):1105–1127. doi: 10.1017/S0954579405050522 [DOI] [PubMed] [Google Scholar]
- 28.Giletta M, Calhoun CD, Hastings PD, et al. Multi-Level risk factors for suicidal ideation among at-risk adolescent females: the role of hypothalamic-pituitary-adrenal axis responses to stress. J Abnorm Child Psychol. 2015;43(5):807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James KM, Woody ML, Feurer C, et al. Disrupted physiological reactivity among children with a history of suicidal ideation: moderation by parental expressed emotion-criticism. Biol Psychol. 2017;130:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Daches S, George CJ, et al. Autonomic correlates of lifetime suicidal thoughts and behaviors among adolescents with a history of depression. Psychophysiology. 2019;56(8):e13378. doi: 10.1111/psyp.13378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsypes A, James KM, Woody ML, et al. Resting respiratory sinus arrhythmia in suicide attempters. Psychophysiology. 2018;55(2):e12978. doi: 10.1111/psyp.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forkmann T, Meessen J, Teismann T, et al. Resting vagal tone is negatively associated with suicide ideation. J Affect Disord. 2016;194:30–32. [DOI] [PubMed] [Google Scholar]
- 33.Rottenberg J, Wilhelm FH, Gross JJ, et al. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. J Affect Disord. 2002;71(1-3):265–272. [DOI] [PubMed] [Google Scholar]
- 34.Miller AB, Eisenlohr-Moul TA. Biological responses to acute stress and suicide: a review and opportunities for methodological innovation. Curr Behav Neurosci Rep. 2019;6:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grassi M, Caldirola D, Di Chiaro NV, et al. Are respiratory abnormalities specific for panic disorder? A meta-analysis. Neuropsychobiology. 2014;70(1):52–60. [DOI] [PubMed] [Google Scholar]
- 36.Tunnell NC, Ritz T, Wilhelm FH, et al. Habituation or normalization? Experiential and respiratory recovery from voluntary hyperventilation in treated versus untreated patients with panic disorder. Behav Ther. 2021;52(1):124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKinnon DF, Craighead B, Hoehn-Saric R. Carbon dioxide provocation of anxiety and respiratory response in bipolar disorder. J Affect Disord. 2007;99(1-3):45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackinnon DF, Craighead B, Lorenz L. Carbon dioxide induces erratic respiratory responses in bipolar disorder. J Affect Disord. 2009;112(1-3):193–200. [DOI] [PubMed] [Google Scholar]
- 39.MacKinnon DF, Zandi PP, Cooper J, et al. Comorbid bipolar disorder and panic disorder in families with a high prevalence of bipolar disorder. Am J Psychiatry. 2002;159(1):30–35. [DOI] [PubMed] [Google Scholar]
- 40.MacKinnon DF, Zamoiski R. Panic comorbidity with bipolar disorder: what is the manic-panic connection? Bipolar Disord. 2006;8(6):648–664. [DOI] [PubMed] [Google Scholar]
- 41.Doughty CJ, Elisabeth Wells J, Joyce PR, et al. Bipolar-panic disorder comorbidity within bipolar disorder families: a study of siblings. Bipolar Disord. 2004;6(3):245–252. [DOI] [PubMed] [Google Scholar]
- 42.Asnaashari AM, Talaei A, Haghigh B. Evaluation of psychological status in patients with asthma and COPD. Iran J Allergy Asthma Immunol. 2012;11(1):65–71. [PubMed] [Google Scholar]
- 43.Decramer M, Rennard S, Troosters T, et al. COPD As a lung disease with systemic consequences–clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5(4):235–256. [DOI] [PubMed] [Google Scholar]
- 44.Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):170026. doi: 10.1183/16000617.0026-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanania NA, Mullerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604–611. [DOI] [PubMed] [Google Scholar]
- 46.Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205–1211. [DOI] [PubMed] [Google Scholar]
- 47.Riblet NB, Gottlieb DJ, Watts BV, et al. Hypoxia-related risk factors for death by suicide in a national clinical sample. Psychiatry Res. 2019;273:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleehart S, Fan VS, Nguyen HQ, et al. Prevalence and correlates of suicide ideation in patients with COPD: a mixed methods study. Int J Chron Obstruct Pulmon Dis. 2015;10:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilaire G, Voituron N, Menuet C, et al. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol. 2010;174(1-2):76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young SN. Elevated incidence of suicide in people living at altitude, smokers and patients with chronic obstructive pulmonary disease and asthma: possible role of hypoxia causing decreased serotonin synthesis. J Psychiatry Neurosci. 2013;38(6):423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35(7):837–844. [DOI] [PubMed] [Google Scholar]
- 52.Qi F, Zhang XJ, Li WH. An integral representation for the weighted geometric mean and its applications. Acta Math Sin. 2014;30:61–68. [Google Scholar]
- 53.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 54.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 55.Johnstone JA, Ford PA, Hughes G, et al. Bioharness() multivariable monitoring device: part. I: validity. J Sports Sci Med. 2012;11(3):400–408. [PMC free article] [PubMed] [Google Scholar]
- 56.Tarvainen MP, Niskanen JP, Lipponen JA, et al. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113(1):210–220. [DOI] [PubMed] [Google Scholar]
- 57.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32(3):230–236. doi: 10.1109/TBME.1985.325532 [DOI] [PubMed] [Google Scholar]
- 58.Quintana DS, Alvares GA, Heathers JA. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry. 2016;6(5):e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.IBM SPSS Statistics for Windows [computer program]. IBM Corp., Armonk, N.Y., U.S.A. 2020. [Google Scholar]
- 61.Fieller E, Herman OH, Pearson ES. Tests for rank correlation coefficients. Biometrika. 1957;44(3):470–481. [Google Scholar]
- 62.Adolph D, Teismann T, Forkmann T, et al. High frequency heart rate variability: evidence for a transdiagnostic association with suicide ideation. Biol Psychol. 2018;138:165–171. [DOI] [PubMed] [Google Scholar]
- 63.Bernardi L, Porta C, Gabutti A, et al. Modulatory effects of respiration. Auton Neurosci. 2001;90(1-2):47–56. [DOI] [PubMed] [Google Scholar]
- 64.Relman AS. Metabolic consequences of acid-base disorders. Kidney Int. 1972;1(5):347–359. [DOI] [PubMed] [Google Scholar]
- 65.Mitchell JH, Wildenthal K, Johnson RL. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972;1(5):375–389. [DOI] [PubMed] [Google Scholar]
- 66.Nakao K, Ohgushi M, Yoshimura M, et al. Hyperventilation as a specific test for diagnosis of coronary artery spasm. Am J Cardiol. 1997;80(5):545–549. [DOI] [PubMed] [Google Scholar]
- 67.Hayano J, Mukai S, Fukuta H, et al. Postural response of low-frequency component of heart rate variability is an increased risk for mortality in patients with coronary artery disease. Chest. 2001;120(6):1942–1952. [DOI] [PubMed] [Google Scholar]
- 68.Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol. 2019;38(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cpa-10.1177_07067437231194334 for A History of Suicide Attempt Is Associated with Increased Sympathetic Activation in Bipolar Disorder by Abigail Ortiz, Yunkyung Park, Stephane MacLean, M. Ishrat Husain, Marcos Sanches, Arun Ravindran and Benoit H. Mulsant in The Canadian Journal of Psychiatry